Abstract
Near infrared spectroscopy (NIRS) and functional magnetic resonance imaging (fMRI) both allow non‐invasive monitoring of cerebral cortical oxygenation responses to various stimuli. To compare these methods in elderly subjects and to determine the effect of age on cortical oxygenation responses, we determined motor‐task‐related changes in deoxyhemoglobin concentration ([HHb]) over the left motor cortex in six healthy young subjects (age 35 ± 9 years, mean ± SD) and five healthy elderly subjects (age 73 ± 3 years) by NIRS and blood‐oxygen‐level‐dependent (BOLD) fMRI simultaneously. The motor‐task consisted of seven cycles of 20‐sec periods of contralateral finger‐tapping at a rate as fast as possible alternated with 40‐sec periods of rest. Time‐locked averages over the seven cycles were used for further analysis. Task‐related decreases in [HHb] over the motor cortex were measured by NIRS, with maximum changes of −0.83 ± 0.38 μmol/L (P < 0.01) for the young and −0.32 ± 0.17 μmol/L (P < 0.05) for the elderly subjects. The BOLD‐fMRI signal increased over the cortex volume under investigation with NIRS, with maximum changes of 2.11 ± 0.72% (P < 0.01) for the young and 1.75 ± 0.71% (P < 0.01) for the elderly subjects. NIRS and BOLD‐fMRI measurements showed good correlation in the young (r = −0.70, r 2 = 0.48, P < 0.001) and elderly subjects (r = −0.82, r 2 = 0.67, P < 0.001). Additionally, NIRS measurements demonstrated age‐dependent decreases in task‐related cerebral oxygenation responses (P < 0.05), whereas fMRI measurements demonstrated smaller areas of cortical activation in the elderly subjects (P < 0.05). These findings demonstrate that NIRS and fMRI similarly assess cortical oxygenation changes in young subjects and also in elderly subjects. In addition, cortical oxygenation responses to brain activation alter with aging. Hum. Brain Mapping 16:14–23, 2002. © 2002 Wiley‐Liss, Inc.
Keywords: near infrared spectroscopy, magnetic resonance imaging, brain oxygenation, cerebrovascular circulation physiology, functional motor cortex activation, blood oxygen, aging
INTRODUCTION
Changes in cerebral hemodynamics and oxygenation can be measured by several techniques. For example, positron emission tomography (PET) or tracer methods such as 133Xe‐single photon emission computer tomography (SPECT) have been used to measure changes in regional cerebral blood flow (CBF). These methods, however, are expensive, not widely available, associated with injection of radioactive compounds, and have a low temporal resolution [Villringer, 1997]. Transcranial Doppler (TCD) sonography has been used to monitor changes in blood flow velocity in the large cerebral arteries. This method is relatively cheap, has a good temporal resolution, but does not provide information about regional changes in cerebral oxygen supply or metabolism [Hirth et al., 1997a; Vernieri et al., 1999]. Near infrared spectroscopy (NIRS) and functional magnetic resonance imaging (fMRI) are rapidly developing technologies that both allow non‐invasive monitoring of regional changes in cortical tissue oxygenation, particularly changes in deoxyhemoglobin concentration ([HHb]), in response to various stimuli including visual, auditory, and motor stimuli [Colier et al., 1997, 1999; Kleinschmidt et al., 1996; Mehagnoul‐Schipper et al., 2000; Obrig et al., 1996, 2000; Rao et al., 1993; van der Kallen et al., 1998].
Functional MRI and NIRS both have advantages and limitations. Functional MRI provides high‐spatial‐resolution imaging of cerebral [HHb] changes over time due to exploitation of the difference in magnetic susceptibility between oxyhemoglobin and deoxyhemoglobin. A decrease in paramagnetic [HHb] will increase the effective transverse relaxation rate T2* and fMRI signal. In T2* weighted imaging, this effect is called blood‐oxygen‐level‐dependent (BOLD) contrast imaging, and it can be used to determine relative changes in [HHb] but not oxyhemoglobin concentration ([O2Hb]). Disadvantages of the fMRI method include its high expenses, contraindications in cases of ferrous implants such as vascular clips and pacemakers, claustrophobia, liability to movement artifacts, inability to detect changes in oxygenation parameters other than [HHb], and limitations in monitoring changes in cerebral oxygenation during assuming a different position. Finally, fMRI availability in clinical centers for research purposes is often limited.
NIRS is a simple, inexpensive bedside technique that permits specified monitoring of changes in [O2Hb], [HHb], and total hemoglobin ([tHb]) with high temporal resolution. The NIRS method is based on near‐infrared light absorption changes that depend on concentration changes of the chromophores [O2Hb] and [HHb] in the tissue under investigation. Changes in [tHb], defined as the sum of the changes in [O2Hb] and [HHb], can be used as a measure of blood volume changes [Delpy et al., 1988]. Disadvantages of NIRS are the relatively low spatial resolution and low cerebral penetration depth. In addition, optode positioning over specific cerebral cortical areas depends on external anatomical landmarks and on determining the area of optimum signal responses by trial‐and‐error positioning.
NIRS and fMRI can be considered as complementary methods for cerebral cortical oxygenation monitoring. Because we often encounter cerebral symptomatology during posture changes or an inability to lie completely still in elderly patients, NIRS may be an easier method than fMRI for investigating cerebral oxygenation responses in an elderly population, e.g., for investigating cerebral oxygenation responses to orthostatic stress [Mehagnoul‐Schipper et al., 2000]. The agreement of NIRS and fMRI measurements for cortical oxygenation monitoring in elderly subjects is not fully known, however, because NIRS and fMRI measurements have only been compared in studies with young subjects [Kleinschmidt et al., 1996] or animals [Dunn et al., 1998; Kida et al., 1996; Punwani et al., 1997, 1998]. In addition, it is not clear whether NIRS and fMRI measurements are affected by aging to the same extent.
We attempt to determine the extent of agreement between NIRS and fMRI monitoring of functional cortical oxygenation changes by performing simultaneous NIRS and fMRI measurements over the motor cortex in healthy elderly and young subjects during a contralateral finger‐tapping task. We compared the [HHb] changes measured by NIRS with the maximum changes in BOLD‐fMRI signal over the motor cortex, and also with the changes in BOLD‐fMRI signal over a banana‐shaped cortex volume underneath the NIRS optodes [Okada et al., 1995]. In addition, we aimed to determine whether the two methods give comparable results for the effects of aging on functional cortical oxygenation responses.
MATERIALS AND METHODS
Subjects
This study was performed in seven healthy elderly subjects and seven healthy young subjects. Two elderly subjects and one young subject were excluded from further analysis because of movement artifacts, leaving five elderly subjects (age 73 ± 3 years (mean ± SD), two males) and six young subjects (age 35 ± 9 years, three males). Pre‐set inclusion criteria were a medical history free of cardiovascular, pulmonary, renal, endocrinological and neurological disorders, no use of medications, and an active and independent life. Exclusion criteria were a distinct dominance of the left hand, the presence of claustrophobia, ferrous prostheses incompatible with high magnetic fields, and anemia. All subjects were in good physical and mental health and regularly performed physical exercise such as walking or cycling. All subjects provided written informed consent. The study was approved by the Ethics Committee for Research on Human Subjects of the University Medical Center Nijmegen, the Netherlands, and it complied with the standards established in the Declaration of Helsinki.
Materials
The NIRS technique has been described in detail elsewhere [Elwell et al., 1994]. We used the recently developed oxymon continuous wave near infrared spectrophotometer (University Medical Center Nijmegen, the Netherlands) [van der Sluijs et al., 1998]. Light with wavelengths of 775, 845, and 904 nm was guided to the subjects' heads through 8‐m long glass fiber bundles that contained no metal parts. The transmitter and receiver optodes were positioned over the left motor cortex enclosing C3, according to the modified international EEG 10‐20 system [American Electroencephalographic Society, 1994]. The optodes had an interoptode distance of 45 mm in a vertical line and were marked with vitamin E capsules to check their positions above the central sulcus on anatomical MRI scans. For absolute quantification of changes in [O2Hb], [HHb] and [tHb], a modified Lambert‐Beer law was used, which describes optical attenuation in a highly scattering medium: attenuation (OD) = log I0/I = αcLB + G, or Δc = ΔOD/αLB. In these equations, OD is the optical density, I0 is the incident light intensity, I is the detected light intensity, α is the absorption coefficient of the chromophore in (mmol/L)−1 · cm−1, c is the concentration of chromophores in mmol/L, L is the interoptode distance in cm, B is the differential optical pathlength factor that takes into account the scattering of light in tissue, and G is a factor related to the tissue geometry. B was calculated by the formula 4.99 + 0.067(age0.814) in the young subjects and B was set at 6.61 in the elderly subjects [Delpy et al., 1988; Duncan et al., 1996]. In the elderly subjects, B is a derived constant because precise formulas for B in elderly subjects over 50 years of age are currently unknown [Duncan et al., 1996]. NIRS measurements are considered to originate from a banana‐shaped tissue volume underneath the optodes [Okada et al., 1995]. The NIRS data were collected with a sample frequency of 10 Hz, and stored on disk for off‐line smoothing with a 25‐point Savitzky‐Golay filter and analysis by Oxysoft software (University Medical Center Nijmegen, the Netherlands).
The fMRI studies were performed on a commercial 1.5 Tesla scanner equipped with echo planar imaging (EPI) (Siemens Vision, Erlangen, Germany) using a standard circular polarized head coil. Scout images were acquired to position the anatomical and functional image series. The functional MRI images were acquired with single shot EPI measurements over 36 sagittally‐oriented, contiguous slices that covered the whole brain (sequence time/echo time 4,000/50 msec, thickness 4 mm interleaved, gap 0 mm, flip angle 90°, matrix 64 × 64, field of view 230 × 230 mm, in‐plane resolution 3.6 × 3.6 × 4.0 mm). The total scanning time was 7 min 40 sec, consisting of seven series of alternating 40‐sec rest periods and 20‐sec motor‐tasks. One imaging series consisted of 15 sequential gradient‐echo echo‐planar measurements with a sequence time of 4 sec. Overall, 118 sequential gradient‐echo echo‐planar measurements were run, but the first three measurements were excluded from further analysis because baseline signal equilibrium was only reached after this. The fMRI data were analyzed off‐line by Medx software (Sensor Systems, Inc., Sterling, VA).
Study protocol
Before the start of the study, the subjects were familiarized with the protocol. The NIRS optodes were placed at the subjects' left motor cortex area enclosing C3 of the modified international EEG 10‐20 system [American Electroencephalographic Society, 1994] and were fixed with elastic bands around the head. The optode positioning around the motor area for the hand was checked during a forefinger‐tapping task of the right hand to induce functional brain activation. If no oxygenation change was detected by NIRS in response to the finger‐tapping task, the optodes were moved over several millimeters by trial‐and‐error until a consistent oxygenation response was found. After a clear NIRS signal was detected, the subjects were placed in the MRI scanner. Three MRI sessions were performed. First, scout images in three orthogonal projections were acquired to localize the NIRS optodes and the EPI measurements in relation to the motor cortex. Then, simultaneous NIRS and fMRI‐EPI measurements were made during performance of the motor‐task of the right hand. The motor‐task involved seven cycles of 20‐sec periods of forefinger‐tapping on a switch as fast as possible, alternated with 40‐sec periods of rest. The total time of the measurement session was 7 min 40 sec. Instructions to start and stop the motor‐task were video projected via a backprojection screen located at the end of the MRI gantry, and they were viewed with a mirror mounted on the head coil. The finger‐tapping signal was recorded simultaneously with the NIRS and fMRI measurements to check on the subjects' performances. Finally, 3D high‐resolution anatomical MRI images were made in support of the fMRI data analysis and to determine the NIRS optode positioning marked with the vitamin E capsules (magnetization prepared rapid angle gradient‐echo sequences, repetition time 9.7 msec, echo time 4 msec, flip angle 12°, matrix 256 × 256, field of view 230 × 230 mm, in‐plane resolution 0.9 × 0.9 × 2.0 mm, total scanning time 4 min 37 sec).
Data analysis and statistics
All fMRI measurements were subjected to motion correction and ratio normalization by Medx software to de‐trend scanner drift over the imaged brain volume. After segmentation of skin and skull, the 3D anatomical MRI brain volume was realigned to the mean volume from all functional images. Subsequently, the functional images were separately overlaid on the matching realigned anatomic MRI images to determine the precise anatomic position of the fMRI and NIRS data. For analysis of the task‐related changes in BOLD‐fMRI signal intensity, the time courses of the BOLD‐fMRI signal in voxels were cross‐correlated to a phase‐shifted reference waveform [Bandettini et al., 1993]. Voxels in the primary sensory motor cortex passing a cross‐correlation z‐score value of 3.28 with P < 0. 0001 were considered significantly activated and were included in the determination of the size of the activated motor cortex area and the maximum BOLD‐fMRI signal change. The P‐value of the z‐score was chosen this low to implement a correction for the number of voxels (29545) in the imaged brain volume. Additionally, the fMRI measurements were processed to achieve an interoptode BOLD‐fMRI signal value from a banana‐shaped cortex volume underneath the NIRS optodes irrespective of the z‐score value. The banana‐shaped, interoptode cortex volume included the two voxels under the two NIRS optodes and the intervening voxels in a broadening cortex volume of at most 10.8 mm deep and 22.0 mm wide [Okada et al., 1995].
Changes in NIRS variables (in μmol/L) and BOLD‐fMRI signal (in %) were calculated from a baseline defined as the average value over the 12‐sec period just before the start of the motor‐task. The seven cycles of the finger‐tapping task were averaged time‐locked for all NIRS and fMRI measurements in each subject. To allow for comparison with the fMRI data, the NIRS changes were also averaged over 4‐sec periods. Grand‐average curves over the groups were obtained by averaging the measurements over the six young and five elderly subjects from 12 sec before until 32 sec after the 20‐sec motor‐task.
Statistical analysis was performed by SPSS 9.0 for Windows (SPSS Inc., 1999). The effects of time and age on cerebral oxygenation changes during the motor‐task were analyzed with repeated‐measures analysis of variance (ANOVA). Single measurement variables were compared between the groups with one‐way ANOVA. The correlation between NIRS and fMRI measurements was determined with Pearson's correlation tests and linear regression analysis. A P‐value <0.05 was considered to be statistically significant. All data are presented as mean ± SD.
RESULTS
The six young and five elderly subjects had normal brain MRI scans. The depth distance from the NIRS optodes to the cortical surface was 13.9 ± 1.3 mm in the young group and 14.2 ± 0.8 mm in the older group. The cerebrospinal fluid space was 1.5 ± 1.3 mm in the young subjects and 1.3 ± 0.5 mm in the elderly subjects. Mean resting BOLD‐fMRI signal intensities were not significantly different between the two groups. All subjects performed the finger‐tapping task at the fastest rate they could, and the performances were therefore effort‐matched. The actual tapping rate, however, was significantly lower in the elderly subjects (2.3 ± 0.9 sec−1) than in the young subjects (4.0 ± 0.7 sec−1, P < 0.01).
Figure 1 shows the imaging results as activation maps over the left motor cortex volume under investigation with both methods for a young subject (Panel 1–6) and an elderly subject (Panel 7–12). Figure 2 illustrates the time course of the changes in [HHb], [O2Hb], and [tHb] as measured by NIRS and the changes in the maximum BOLD‐fMRI signal over the left motor cortex area in a young subject during the seven cycles of contralateral finger‐tapping. Each period of finger‐tapping induced a pronounced decrease in [HHb] that disappeared in each period of rest.
Figure 1.
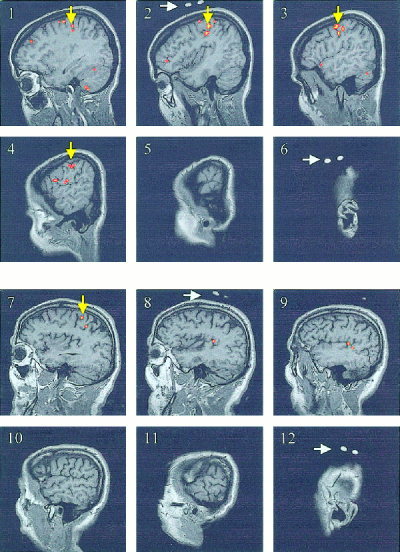
MRI activation maps showing the area of activation (yellow arrows) over the left motor cortex for a young subject (Panel 1–6, from medial to lateral) and for an elderly subject (Panel 7–12, from medial to lateral) during a 20‐sec contralateral finger‐tapping task. The NIRS optode positioning was marked with vitamin E capsules, as shown in Panel 2 and 6, and Panel 8 and 12 (white arrows).
Figure 2.
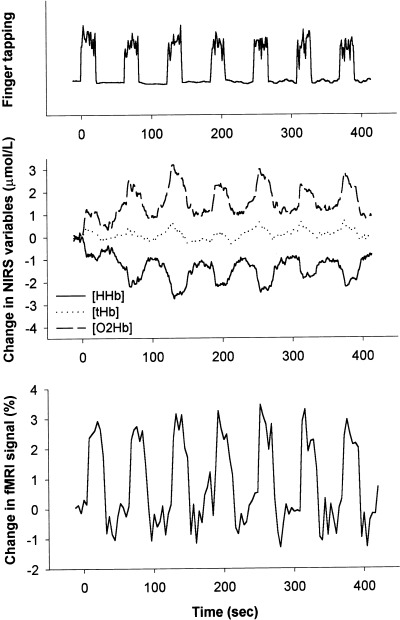
Time course of finger‐tapping activity represented by vertical peaks and periods of rest represented by horizontal lines (upper panel), time course of changes in deoxyhemoglobin ([HHb]), oxyhemoglobin ([O2Hb]), and total hemoglobin ([tHb]) concentrations as measured by NIRS (middle panel), and time course of changes in the maximum BOLD‐fMRI signal (lower panel) over the left motor cortex in a young subject during seven cycles of contralateral finger‐tapping for 20 sec and rest for 40 sec.
Figure 3 presents the time course of the grand‐average task‐related changes in [HHb], [O2Hb], and [tHb] over the left motor cortex area in the young and elderly subjects as measured by NIRS. Significant task‐related increases in [O2Hb] and decreases in [HHb] were present in the young and elderly subjects (P < 0.05). The slight increase in [tHb], as a reflection of an increase in blood volume, was significant in the young subjects (P < 0.01) but not in the elderly subjects (P = 0.08). Although the responses of [HHb], [O2Hb], and [tHb] were uniform in the two groups, the [HHb] and [O2Hb] tended to be less pronounced in the elderly subjects (P = 0.10 and P = 0.07, respectively). The averages of maximum individual changes in [HHb] and [O2Hb] were indeed significantly smaller in the elderly subjects than in the young subjects (P < 0.05; Table I).
Figure 3.
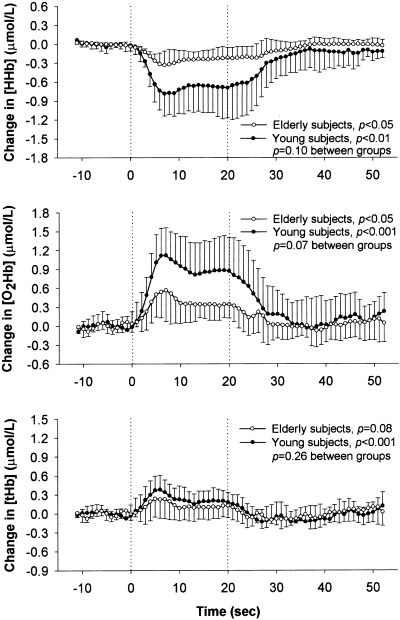
Grand‐average changes in deoxyhemoglobin ([HHb]), oxyhemoglobin ([O2Hb]), and total hemoglobin ([tHb]) concentrations as measured by NIRS over the left motor cortex in healthy young (n = 6, —•—) and elderly subjects (n = 5, —○—) during a 20‐sec contralateral finger‐tapping task. The individual changes were time‐locked averages over seven cycles of finger‐tapping. Data are presented as mean ± SD. P‐values representing the effect of time for both groups and P‐values representing the time‐by‐group interaction are shown (repeated‐measures ANOVA).
Table I.
Average maximum changes in NIRS and fMRI measurements over the left motor cortex area in six young and five elderly subjects during 20 sec of contralateral finger‐tapping
| Variable | Young subjects | Elderly subjects | P‐value between groups |
|---|---|---|---|
| NIRS measurements | |||
| Δ Deoxyhemoglobin [HHb] (μmol/L) | −0.83 ± 0.38** | −0.32 ± 0.17* | 0.04 |
| Δ Oxyhemoglobin [O2Hb] (μmol/L) | 1.17 ± 0.44** | 0.55 ± 0.41* | 0.02 |
| Δ Total hemoglobin [tHb] (μmol/L) | 0.36 ± 0.19** | 0.23 ± 0.29 | 0.39 |
| fMRI measurements | |||
| Δ Maximum BOLD‐fMRI signal (%) | 2.72 ± 0.72** | 3.08 ± 0.95** | 0.49 |
| Δ Interoptode BOLD‐fMRI signal (%) | 2.11 ± 0.72** | 1.75 ± 0.71** | 0.42 |
| Activated voxels (n) | 33 ± 25* | 6 ± 6*** | 0.05 |
NIRS data are average values over 4‐second periods to allow for comparison with the fMRI data. Data are presented as mean ± SD.
P < 0.05,
P < 0.01,
P = 0.08, vs. baseline.
Figure 4 presents the grand‐average changes over time in the BOLD‐fMRI signal due to changes in [HHb] over the left motor cortex area in the young and elderly subjects. The decrease in [HHb] as determined by NIRS, was mirrored by a simultaneous increase in maximum BOLD‐fMRI signal over the left primary motor cortex (P < 0.01; Fig. 4, upper panel) and a smaller increase in the BOLD‐fMRI signal over the banana‐shaped, interoptode motor cortex volume (P < 0.01; Fig. 4, lower panel) in both groups. The two groups showed comparable increases in the BOLD‐fMRI signals over time, and also the averages of maximum individual changes in BOLD‐fMRI signals were similar (Table I).
Figure 4.
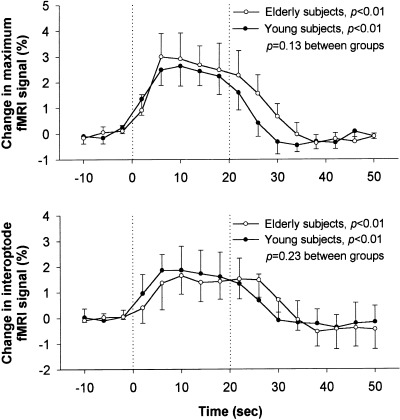
Grand‐average changes in BOLD‐fMRI signal due to deoxyhemoglobin concentration changes over the left motor cortex in healthy young (n = 6, —•—) and elderly subjects (n = 5, —○—) during a 20‐sec contralateral finger‐tapping task. The two graphs represent consecutively the BOLD‐fMRI signal changes in the cortex area with maximum activation (maximum fMRI) and in the banana‐shaped interoptode cortex volume underneath the NIRS optodes (interoptode fMRI). The individual changes were time‐locked averages over seven cycles of finger‐tapping. Data are presented as mean ± SD. P‐values representing the effect of time for both groups and P‐values representing the time‐by‐group interaction are shown (repeated‐measures ANOVA).
Table II shows the relations the NIRS and fMRI measurements, as time‐locked averages over seven cycles, in each subject. The maximum task‐related [HHb] and [tHb] changes measured by NIRS were very closely related to the maximum changes in BOLD‐fMRI signal and to a lesser degree to the BOLD‐fMRI values over the interoptode cortex volume in most subjects. In one elderly subject, however, the task‐related [HHb] changes were not pronounced, which was also expressed by the lower correlation coefficients in this subject (Table II). Linear regression analysis over all individual curves of the [HHb] changes as measured by NIRS and the maximum BOLD‐fMRI signal changes showed an excellent correlation between these variables with r = −0.70, r 2 = 0.48, p < 0.001 for the young subjects and r = −0.82, r 2 = 0.67, P < 0.001 for the elderly subjects (Fig. 5). In addition, the individual curves of the [HHb] changes and the interoptode BOLD‐fMRI signal changes were significantly correlated with r = −0.55, r 2 = 0.31, P < 0.001 for the young subjects and r = −0.53, r 2 = 0.28, P < 0.001 for the elderly subjects. We also found significant relations between the individual [tHb] responses as measured by NIRS and the changes in maximum and interoptode BOLD‐fMRI signal for the young subjects (r = −0.55, r 2 = 0.30, P < 0.001, and r = −0.20, r 2 = 0.04, P = 0.05, respectively). These relations, however, were not clear for the elderly subjects (r = −0.20, r 2 = 0.04, P = 0.07, and r = −0.19, r 2 = 0.04, P = 0.09, respectively). The lower correlation coefficients between [tHb] changes and the BOLD‐fMRI signal changes in the elderly subjects compared to the young subjects expressed this as well (Table II).
Table II.
Pearson's correlation coefficients between changes in [HHb] and [tHb] as measured by NIRS on the one hand, and changes in maximum BOLD‐fMRI signal (left columns) and changes in interoptode BOLD‐fMRI signal (right columns) on the other, over the left motor cortex area in young and elderly subjects during a 20‐sec contralateral finger‐tapping task
| Subject | Δ Maximum BOLD‐fMRI signal | Δ Interoptode BOLD‐fMRI signal | ||
|---|---|---|---|---|
| vs. [HHb] | vs. [tHb] | vs. [HHb] | vs. [tHb] | |
| Young subjects | ||||
| 1 | −0.78** | 0.83** | −0.89** | 0.68** |
| 2 | −0.59* | 0.94** | −0.59* | 0.93** |
| 3 | −0.88** | 0.78** | −0.58* | 0.35 |
| 4 | −0.63** | 0.54* | −0.53* | 0.47 |
| 5 | −0.88** | 0.62* | −0.63** | 0.23 |
| 6 | −0.96** | 0.74** | −0.96** | 0.65** |
| Mean | −0.79 ± 0.15 | 0.74 ± 0.14 | −0.70 ± 0.18 | 0.55 ± 0.25 |
| Elderly subjects | ||||
| 1 | −0.97** | −0.11 | −0.97** | −0.13 |
| 2 | −0.94** | 0.79** | −0.91** | 0.58* |
| 3 | −0.33 | 0.31 | 0.49 | −0.49 |
| 4 | −0.81** | 0.69** | −0.72** | 0.69** |
| 5 | −0.98** | −0.03 | −0.93** | −0.21 |
| Mean | −0.81 ± 0.27 | 0.09 ± 0.52 | ||
| All subjects (n = 11) | ||||
| Mean | −0.80 ± 0.20 | 0.55 ± 0.35 | −0.66 ± 0.42 | 0.34 ± 0.45 |
Data are presented as mean ± SD.
P < 0.05,
P < 0.01, Pearson's correlation.
Figure 5.
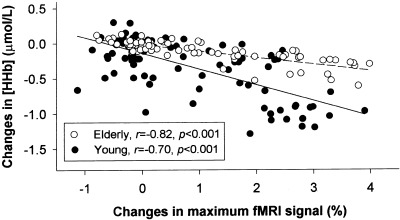
Correlation between changes in deoxyhemoglobin concentration ([HHb]) as measured by NIRS and changes in BOLD‐fMRI signal over the left motor cortex in healthy young (n = 6, —•—) and elderly subjects (n = 5, —○—) during a 60‐sec period involving 20 sec of contralateral finger‐tapping and 40 sec of rest. For each subject, the time‐locked average changes over 7 cycles of finger‐tapping are shown, including 15 points of 4‐sec periods.
The maximum [HHb] changes on NIRS and the number of activated voxels on fMRI were significantly correlated (r = 0.75, r 2 = 0.56, P = 0.01), when one young subject who showed a widespread area of activation on fMRI and relatively low changes in [HHb] was excluded. When we included this young outlier, the relation was not significant (r = 0.39, r 2 = 0.150, P = 0.23). The anatomical distance between the NIRS optodes and activated fMRI voxels (6.7 ± 6.0 mm and 6.4 ± 6.7 mm in the six young and five elderly subjects, respectively) was not related the maximum [HHb] changes (r = 0.03, r 2 = 0.001, P = 0.93) or the maximum [O2Hb] changes (r = 0.02, r 2 = 0.000, P = 0.95). This indicated that the age‐dependency of the NIRS changes could not be explained by a less‐accurate optode positioning over the motor cortex in the elderly subjects.
DISCUSSION
The present study showed remarkably comparable task‐related decreases in [HHb] as measured by NIRS and fMRI over the left motor cortex area during a contralateral finger‐tapping task, with good correlation between the two methods in both young and elderly subjects. In addition, an age‐dependent decrease in the cortical oxygenation response to the finger‐tapping task was found by NIRS, related to a smaller area of cortical activation by fMRI. These findings demonstrate that NIRS and fMRI measurements of cortical [HHb] changes are coherent in elderly subjects.
Previous studies have shown that both NIRS and fMRI can monitor cortical oxygenation changes and functional activation in adult subjects [Colier et al., 1997, 1999; Kleinschmidt et al., 1996; Obrig et al., 1996]. The quantitative linear relation between NIRS and BOLD‐fMRI measurements of cortical [HHb] changes during arterial oxygen saturation changes has been demonstrated in several animal studies [Dunn et al., 1998; Kida et al., 1996; Punwani et al., 1997, 1998]. Kleinschmidt et al. [1996] have described the qualitative relation between simultaneous NIRS and BOLD‐fMRI measurements of [HHb] changes over the motor cortex in young adults during a motor‐task. The present study showed an excellent time‐relationship between NIRS and BOLD‐fMRI measurements of [HHb] decreases over the motor cortex in elderly subjects during a contralateral motor‐task.
In addition to the regional task‐related decreases in [HHb] over the left motor cortex as determined by NIRS and fMRI in young and elderly subjects, simultaneous increases in [O2Hb] were demonstrated by NIRS in both groups. Such [O2Hb] increases have been reported before [Colier et al., 1999; Hirth et al., 1997a,b; Obrig et al., 1996]. The [HHb] changes were smaller than the [O2Hb] changes. Because the [HHb] changes most probably reflect the match between oxygen supply and oxygen demand, whereas the [O2Hb] changes reflect the alterations in CBF [Delpy et al., 1988; Elwell et al., 1994], an overshoot in cerebral oxygenation and uncoupling of CBF and metabolism seem to be present during activation [Obrig et al., 1996]. On the other hand, the higher CBF may in fact be necessary to meet an enhanced oxygen demand during cerebral activation when CBF and metabolism are considered to be not uncoupled [Buxton and Frank, 1997; Hyder et al., 1999]. Cerebral blood volume did increase during the motor task as reflected by a rise in [tHb].
A robust trend toward age‐dependent decreases in [HHb] and [O2Hb] responses was found. The [tHb] increase seemed to be more closely related to the brain activation in the young subjects than in the elderly subjects. These findings suggest an altered cerebrovascular response to cortical activation with aging, such as a lower CBF increase and less local (arteriolar) vasodilatation during activation [Hock et al., 1995; Ross et al., 1997]. The fMRI measurements, however, did not show smaller changes in maximum BOLD signal over the activated cortex area in the older group. Apparently, if a voxel was activated on fMRI, it was equally activated in young and elderly subjects. Indeed, D' Esposito et al. [1999] found a comparable BOLD signal, but also increased noise in elderly subjects compared to young subjects. Our method of fMRI data analysis implies that the signal‐to‐noise ratio of each voxel had to exceed a certain threshold to meet an activation change in BOLD‐fMRI signal intensity, disguising relatively smaller changes and emphasizing larger changes in BOLD‐fMRI signal intensity in voxels over the motor cortex area. Subtle differences in cerebrovascular responses between young and elderly subjects may be less clear per voxel by BOLD‐fMRI than over a larger cortex volume at once by NIRS. In addition, the BOLD‐fMRI signal may originate relatively more from stable larger vessels than from subtle activation changes at the microvasculature level [Kida et al., 1996], in contrast to NIRS measurements [Vernieri et al., 1999]. The activation changes in BOLD‐fMRI signal intensity might have differed more clearly between young and elderly subjects, if we had performed a fMRI study with higher field strength, thereby strengthening the reproduction of oxygenation changes at microvasculature level [Gati et al., 1997]. The extent of the cortical area and the number of voxels activated during the motor‐task, however, did decrease with aging on fMRI, as has been demonstrated previously [D'Esposito et al., 1999]. A close relation was present with the age‐dependent decrease in [HHb] response as measured by NIRS. This finding fits with an age‐dependent alteration in cerebrovascular response to cortical activation [Buckner et al., 2000; D'Esposito et al., 1999]. Changes in cerebral structure, blood volume, blood flow, or oxygen metabolism, neurodegeneration, or an altered coupling of CBF and cortical activation with advancing age may explain the age‐related difference [Hock et al., 1995; Leenders et al., 1990; Marchal et al., 1992; Ross et al., 1997].
The age‐related effects found may be confounded by several factors. First, the finger‐tapping performance was effort‐matched, and consequently it was not rate‐matched for the two groups [Rao et al., 1996]. We think, however, that effort matching is more important than rate matching in the present study. It is unknown whether a certain tapping rate is an equal stimulus for young and elderly subjects to induce cortical activation. In addition, the frequency‐dependent changes in cortical activation occur mainly at tapping rates lower than 2 Hz, not at higher rates [Sadato et al., 1997]. The lower tapping rate in the elderly subjects might even result from an altered cerebrovascular response. Second, changes in optical properties of the brain volume under investigation might confound our findings [Duncan et al., 1996], although we aimed to overcome this by applying an age‐dependent optical pathlength factor in our NIRS analyses [Duncan et al., 1996]. A less accurate NIRS optode positioning in the elderly subjects is not a likely explanation for the age‐effect found, because the distances from the NIRS optodes to the activated cortex area on fMRI was not significantly different between the young and elderly subjects. Additionally, the tissue volumes under investigation were unlikely to be smaller in the elderly subjects, because normal and similar brain MRI scans were found in both groups, without signs of cerebral atrophy.
Determination of the brain area contributing to the NIRS signal is based on modeling, incorporating an optical pathlength factor for the scattering of near infrared light in various human tissues [Delpy et al., 1988]. The NIRS signal is generally considered to originate from a banana‐shaped, interoptode cortex volume [Okada et al., 1995; Vernieri et al., 1999], although contributions from extracranial tissue cannot completely be ruled out [Kleinschmidt et al., 1996; Smielewski et al., 1995]. The good agreement between the NIRS and BOLD‐fMRI measurements of [HHb] changes over the motor cortex volume underscores the cortical origin of the NIRS signal. We aimed to reduce possible effects of age‐dependent tissue changes by applying a differential optical pathlength factor for age, extrapolated from subjects with a maximum age of 50 years [Duncan et al., 1996]. More precise optical pathlength factors for subjects over 50 years of age are currently unknown but highly needed.
The simple, easily applicable, and inexpensive NIRS method can be a reliable alternative to fMRI for assessment of cortical oxygenation changes in young and elderly subjects. Several limitations of NIRS, however, need to be pointed out. First, the spatial resolution and cerebral cortical penetration depth are limited in comparison with fMRI. In addition, the anatomical optode positioning over the motor cortex is less precise because this depends on external bony landmarks and trial‐and‐error optode positioning, unless anatomical brain imaging is available. Further, the NIRS signal might be influenced by changes in extracranial blood flow, although such contributions are generally regarded as minor [Smielewski et al., 1995]. We assumed that the differential optical pathlength factor did not change during the finger‐tapping task. Finally, we cannot obtain absolute baseline values of [O2Hb], [HHb] and [tHb], due to limitations of the continuous wave NIRS technique, e.g., the unknown exact optical pathlength of near infrared light in brain tissue for subjects over 50 years of age. In addition to the NIRS limitations, the small sample size is an important limitation of the study, particularly given the variability in behavior and physiology in elderly subjects. Larger numbers of subjects in each group might have elucidated the differences between young and elderly subjects more clearly. The present study results are merely restrictedly representative of changes in cerebral oxygenation responses in the population as a whole.
CONCLUSIONS
The present study showed a good agreement between NIRS and fMRI measurements of cortical oxygenation changes in young and elderly subjects during brain activation. Additionally, NIRS measurements demonstrated age‐dependent decreases in the task‐related cortical oxygenation responses, whereas fMRI measurements demonstrated smaller areas of cortical activation with aging. We conclude that NIRS is a reliable, non‐invasive, bedside tool to assess cortical oxygenation changes in elderly subjects, and that cortical oxygenation responses to brain activation alter with aging.
REFERENCES
- American Electroencephalographic Society (1994): Guideline thirteen: guidelines for standard electrode position nomenclature. J Clin Neurophysiol 11: 111–113. [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS (1993): Processing strategies for time‐course data sets in functional MRI of the human brain. Magn Reson Med 30: 161–173. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC (2000): Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci 12(Suppl): 24–34. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Frank LR (1997): A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J Cereb Blood Flow Metab 17: 64–72. [DOI] [PubMed] [Google Scholar]
- Colier WNJM, Quaresima V, Barattelli G, Cavallari P, van der Sluijs M, Ferrari M (1997): Detailed evidence of cerebral hemoglobin oxygenation changes in response to motor cortical activation revealed by a continuous wave spectrophotometer with 10 Hz temporal resolution. Proc SPIE 2979: 390–396. [Google Scholar]
- Colier WNJM, Quaresima V, Oeseburg B, Ferrari M (1999): Human motor‐cortex oxygenation changes induced by cyclic coupled movements of hand and foot. Exp Brain Res 129: 457–461. [DOI] [PubMed] [Google Scholar]
- Delpy DT, Cope M, van der Zee P, Arridge S, Wray S, Wyatt J (1988): Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol 33: 1433–1442. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Zarahn E, Aguirre GK, Rypma B (1999): The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. Neuroimage 10: 6–14. [DOI] [PubMed] [Google Scholar]
- Duncan A, Meek JH, Clemence M, Elwell CE, Fallon P, Tyszczuk L, Cope M, Delpy DT (1996): Measurement of cranial optical path length as a function of age using phase resolved near infrared spectroscopy. Pediatr Res 39: 889–894. [DOI] [PubMed] [Google Scholar]
- Dunn JF, Zaim‐Wadghiri Y, Pogue BW, Kida I (1998): BOLD MRI vs. NIR spectrophotometry. Will the best technique come forward? Adv Exp Med Biol 454: 103–113. [PubMed] [Google Scholar]
- Elwell CE, Cope M, Edwards AD, Wyatt JS, Delpy DT, Reynolds EOR (1994): Quantification of adult cerebral hemodynamics by near‐infrared spectroscopy. J Appl Physiol 77: 2753–2760. [DOI] [PubMed] [Google Scholar]
- Gati JS, Menon RS, Ugurbil K, Rutt BK (1997): Experimental determination of the BOLD field strength dependence in vessels and tissue. Magn Reson Med 38: 296–302. [DOI] [PubMed] [Google Scholar]
- Hirth C, Obrig H, Valdueza J, Dirnagl U, Villringer A (1997a): Simultaneous assessment of cerebral oxygenation and hemodynamics during a motor task. A combined near infrared and transcranial Doppler sonography study. Adv Exp Med Biol 411: 461–469. [DOI] [PubMed] [Google Scholar]
- Hirth C, Villringer K, Thiel A, Bernarding J, Muhlnickl W, Obrig H, Dirnagl U, Villringer A (1997b): Toward brain mapping combining near‐infrared spectroscopy and high resolution 3D MRI. Adv Exp Med Biol 413: 139–147. [DOI] [PubMed] [Google Scholar]
- Hock C, Muller Spahn F, Schuh Hofer S, Hofmann M, Dirnagl U, Villringer A (1995): ); Age dependency of changes in cerebral hemoglobin oxygenation during brain activation: a near‐infrared spectroscopy study. J Cereb Blood Flow Metab 15: 1103–1108. [DOI] [PubMed] [Google Scholar]
- Hyder F, Shulman RG, Rothman DL (1999): Regulation of cerebral oxygen delivery. Adv Exp Med Biol 471: 99–110. [DOI] [PubMed] [Google Scholar]
- Kida I, Yamamoto T, Tamura M (1996): Interpretation of BOLD MRI signals in rat brain using simultaneously measured near‐infrared spectrophotometric information. NMR Biomed 9: 333–338. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Obrig H, Requardt M, Merboldt KD, Dirnagl U, Villringer A, Frahm J (1996): Simultaneous recording of cerebral blood oxygenation changes during human brain activation by magnetic resonance imaging and near‐infrared spectroscopy. J Cereb Blood Flow Metab 16: 817–826. [DOI] [PubMed] [Google Scholar]
- Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJR, Gibbs JM, Wise RJS, Hatazawa J, Herold S, Beaney RP, Brooks DJ, Spinks T, Rhodes C, Frackowiak RSJ, Jones T (1990): ); Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 113: 27–47. [DOI] [PubMed] [Google Scholar]
- Marchal G, Rioux P, Petit‐Taboue MC, Sette G, Travere JM, Le Poec C, Courtheoux P, Derlon JM, Baron JC (1992): Regional cerebral oxygen consumption, blood flow, and blood volume in healthy human aging. Arch Neurol 49: 1013–1020. [DOI] [PubMed] [Google Scholar]
- Mehagnoul‐Schipper DJ, Vloet LCM, Colier WNJM, Hoefnagels WHL, Jansen RWMM (2000): Cerebral oxygenation declines in healthy elderly subjects in response to assuming the upright position. Stroke 31: 1615–1620. [DOI] [PubMed] [Google Scholar]
- Obrig H, Hirth C, Junge Hulsing JG, Doge C, Wolf T, Dirnagl U, Villringer A (1996): Cerebral oxygenation changes in response to motor stimulation. J Appl Physiol 81: 1174–1183. [DOI] [PubMed] [Google Scholar]
- Obrig H, Wenzel R, Kohl M, Horst S, Wobst P, Steinbrink J, Thomas F, Villringer A (2000): Near‐infrared spectroscopy: does it function in functional activation studies of the adult brain? Int J Psychophysiol 35: 125–142. [DOI] [PubMed] [Google Scholar]
- Okada E, Firbank M, Delpy DT (1995): The effect of overlying tissue on the spatial sensitivity profile of near‐infrared spectroscopy. Phys Med Biol 40: 2093–2108. [DOI] [PubMed] [Google Scholar]
- Punwani S, Cooper CE, Clemence M, Penrice J, Amess P, Thornton J, Ordidge RJ (1997): Correlation between absolute deoxyhemoglobin [dHb] measured by near infrared spectroscopy (NIRS) and absolute R2′ as determined by magnetic resonance imaging (MRI). Adv Exp Med Biol 413: 129–137. [DOI] [PubMed] [Google Scholar]
- Punwani S, Ordidge RJ, Cooper CE, Amess P, Clemence M (1998): MRI measurements of cerebral deoxyhemoglobin concentration [dHb]: correlation with near infrared spectroscopy (NIRS). NMR Biomed 11: 281–289. [DOI] [PubMed] [Google Scholar]
- Rao SM, Bandettini PA, Binder JR, Bobholz JA, Hammeke TA, Stein EA, Hyde JS (1996): Relationship between finger movement rate and functional magnetic resonance signal change in human primary motor cortex. J Cereb Blood Flow Metab 16: 1250–1254. [DOI] [PubMed] [Google Scholar]
- Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz A, Lisk LM, Morris GL, Mueller WM, Estkowski LD, Wong EC, Haughton VM, Hyde JS. (1993): Functional magnetic resonance imaging of complex human movements. Neurology 43: 2311–2318. [DOI] [PubMed] [Google Scholar]
- Ross MH, Yurgelun‐Todd DA, Renshaw PF, Maas LC, Mendelson JH, Mello NK, Cohen BM, Levin JM (1997): Age‐related reduction in functional MRI response to photic stimulation. Neurology 48: 173–176. [DOI] [PubMed] [Google Scholar]
- Sadato N, Ibanez V, Campbell G, Deiber MP, Le Bihan D, Hallett M (1997): Frequency‐dependent changes of regional cerebral blood flow during finger movements: functional MRI compared to PET. J Cereb Blood Flow Metab 17: 670–679. [DOI] [PubMed] [Google Scholar]
- Smielewski P, Kirkpatrick P, Minnas P, Pickard JD, Czosnyka M (1995): Can cerebrovascular reactivity be measured with near‐infrared spectroscopy? Stroke 26: 2285–2292. [DOI] [PubMed] [Google Scholar]
- van der Kallen BFW, van Erning LJTO, van Zuijlen MW, Merx H, Thijssen HOM (1998): Activation of the sensorimotor cortex at 1.0 T: comparison of echo‐planar and gradient‐echo imaging. Am J Neuroradiol 19: 1099–1104. [PMC free article] [PubMed] [Google Scholar]
- van der Sluijs MC, Colier WNJM, Houston RJF, Oeseburg B (1998): A new and highly sensitive continuous wave near infrared spectrophotometer with multiple detectors. Proc SPIE 3194: 63–72. [Google Scholar]
- Vernieri F, Rosato N, Pauri F, Tibuzzi F, Passarelli F, Rossini PM (1999): Near infrared spectroscopy and transcranial Doppler in monohemispheric stroke. Eur Neurol 41: 159–162. [DOI] [PubMed] [Google Scholar]
- Villringer A (1997): Understanding functional neuroimaging methods based on neurovascular coupling. Adv Exp Med Biol 413: 177–193. [DOI] [PubMed] [Google Scholar]


