Abstract
Quite a few studies in functional magnetic resonance imaging (fMRI) have tested that, even in a resting state, motor cortices constitute a network. It has never been investigated how the network modulates from the resting state to the motor task state. In this report, by a newly developed approach taking into account n‐to‐1 connectivity using 1‐to‐1 connectivity measures instead of conventional pairwise connectivity, we show the existence of a large organized functional connectivity network related to motor function in the resting brain with fMRI. More importantly, we found that such a network can be modulated from a conscious resting state to planning, initiation, coordination, guidance, and termination of voluntary movement state, exhibited by significant changes of functional connectivity of some brain regions in different brain activity. Moreover, a quantitative description of such a functional modulation has also been presented. Hum. Brain Mapp. 22:65–73, 2004. © 2004 Wiley‐Liss, Inc.
Keywords: fMRI, functional connectivity, resting state, modulation, motor system
INTRODUCTION
Functional magnetic resonance imaging (fMRI) has been used to study human brain activities for over a decade. Such studies traditionally focused on identifying activated regions of the brain during an experimental task. However, the studies about interaction among human brain regions may play a more important role in understanding brain functions because the human brain can accomplish a large quantity of perception, cognition, and emotion tasks by integrating a small quantity of brain regions. Therefore, researchers have made a great effort in exploring interregional connectivity in a given task [Buchel et al., 1999; Friston and Buchel, 2000; Liu et al., 1999], which is usually characterized in terms of functional connectivity [Friston et al., 1993a] or effective connectivity [Friston et al., 1993b]. It is a powerful way to characterize neural interactions among brain regions during some particular tasks using a functional or effective connectivity technique, e.g., attention modulation of visual cortex [Friston and Buchel, 2000], plastic changes of brain regions relating with individual learning performance [Buchel et al., 1999], and task‐related dynamic reconfiguration of large‐scale neural networks [Liu et al., 1999].
Recently, increasing attention has been focused on detecting interregional connectivity in a resting state, which is usually described in terms of functional connectivity. Some convincing evidence has demonstrated that there exists very low frequency fluctuations (LFFs; < 0.08 Hz) in MR signals measured in the resting brain [Biswal et al., 1995; Greicius et al., 2003; Lowe et al., 1998; Xiong et al., 1999;]. In functionally related regions of the brain, these fluctuations are synchronous and exhibit high temporal coherence, even in those regions located remotely, which implies the existence of neuronal connectivity coordinating activity in the human brain. So far, functional connectivity in the resting brain has been reported in motor regions [Biswal et al., 1995; Cordes et al., 2000, 2001; Lowe et al., 1998; Xiong et al., 1999], auditory regions [Cordes et al., 2001], visual regions [Biswal et al., 1995; Cordes et al., 2000; Lowe et al., 1998], and the language system [Hampson et al., 2002]. Most of these studies have focused on “seed” voxel(s) analysis, i.e., by selecting a voxel or voxels (so‐called “seed”) in a region of the brain as a reference and cross‐correlating the time course of the “seed” with that of all other voxels in the whole brain to produce functional connectivity maps. Using this technique, brain regions showing high temporal coherence with the seed are considered as functionally connected regions that characterize a network related to one certain brain function, e.g., a motor network [Biswal et al., 1995; Xiong et al., 1999]. Although seed voxel(s) analysis can identify brain regions functionally connected to the initially selected seed, it is unable to completely characterize the joint interactions among multiple brain regions. Moreover, it would be very interesting to ascertain how functional connectivity modulates from the resting state to the task state.
Recently, eigenimage analysis based on the multivariate technique has been used to describe functional connectivity patterns of the brain during rest [Piyaratna et al., 2001]. The elements corresponding to significant intensities in the eigenimages indicate the voxels having significant connectivity. However, it is difficult to interpret the connectivity patterns results in some of the eigeniamges and make statistical inferences for a group study. Thus, a new method measuring joint interaction among brain regions is necessary.
In this study, we propose a novel way to address the above‐mentioned problem. It can completely characterize the joint interactions among multiple motor regions of the brain. We found a large organized functional connectivity network related to motor function in the resting brain with fMRI in human. More notably, we show that such a network can be modulated during the rest state and the motor task state. In addition, we assigned a measure based on graph theory for functional modulation.
SUBJECTS AND METHODS
Participants in the fMRI experiments were eight healthy, right‐handed volunteers (4 women; age range 21–23, mean 22), claiming to be in good health with no history of neurological illness. Before subjects were imaged, informed consent was obtained according to the procedure approved by the Institutional Review Board at the Institute of Psychology, Chinese Academy of Sciences. All subjects underwent a one‐run scan, the former part of a resting state and the latter part of the movement state in a blocked design paradigm.
During the resting state, subjects were instructed to keep their eyes closed, relax their mind, and remain motionless as much as possible. The scan lasted for 370 sec.
After that, subjects were told to open their eyes, and the motor task state began. There were two kinds of epochs, rest and finger tapping alternatively. Each epoch lasted for 20 sec excluding the first rest epoch, which lasted for 40 sec. During the rest epoch, subjects were instructed to stare at the digit “0” shown at the center of the screen. During the finger tapping epoch, instead of “0,” a digit “1,” “2,” “3,” or “4,” representing the right index, middle, ring, and little finger, respectively, appeared in a pseudorandom way at the center and lasted for 500 msec with an interval of 500 msec. Subjects were instructed to tap their finger with a frequency of 1 Hz when a corresponding digit appeared. The motor task state lasted for 280 sec.
Imaging Methods
Anatomical images were acquired axially on a 1.5 T scanner SIEMENS Sonata equipped with high‐speed gradients. The following parameters were used for T1 anatomical imaging: 442/15 msec (TR/TE), 20 slices, 256 × 256 matrix, 90° flip angle, 22‐cm field of view (FOV), 5‐mm section thickness, and 2‐mm gap. At the same locations to anatomical slices, functional images were acquired by using an echo‐planar imaging (EPI) sequence with the following technical parameters: 2,000/60 msec (TR/TE), 20 slices, 100‐msec inter‐slice acquisition delay, 64 × 64 matrix, 90° flip angle, 22‐cm FOV, 5‐mm section thickness, and 2‐mm gap. In addition, a 3D spoiled gradient‐recalled whole‐brain volume was acquired in the sagittal plane with the following parameters: 30/1.17 msec (TR/TE), 35° flip angle, 1.27 × 1.27 × 1.3 mm spatial resolution.
Data Pre‐Processing Analysis
The first 5 time points of the resting state were discarded because of the instability of the initial MRI signal leaving 180 time points. Similarly, the first 10 time points of the movement state were discarded for adaptation after the subject's opening of his/her eyes leaving 130 time points. The functional images' data were preprocessed and analyzed using AFNI [Cox, 1996]. Individual scans were corrected for head movement. Then, linear drift was removed. After being spatially normalized [Talairach and Tournoux, 1998], the data were re‐sampled at 3 mm3 and spatially smoothed by a 4‐mm FWHM Gaussian kernel to decrease spatial noise. For the resting state data, a low‐pass frequency filter (f < 0.08 Hz) was applied to remove physiological high‐frequency noise, e.g., respiratory and cardiac [Biswal et al., 1995], and LFFs were further analyzed. No temporal filtering was used for the movement state data.
Definition of Regions of Interest (ROIs)
In this study, the precise ROIs related to motor function are defined as follows.
Group ROIs.
For the movement task, statistical analysis was performed on individual data by cross‐correlation between the temporally smoothed boxcar reference function and time courses of voxels [Bandettini et al., 1993], and individual r‐map was obtained. Next, in order to extend inference based on the individual statistical analyses to the general population from which the subjects were drawn, a random‐effect analysis was then performed. This estimates the error variance for each condition of interest across subjects, rather than across scans [Holmes and Friston, 1998]. In this random‐effect analysis, one‐sample t test (d.f. = 7) at each voxel was performed across subjects on their individual r‐maps. Voxels with |t| > 3.498 (P < 0.01, uncorrected) and clusters with size > 20 voxels were superimposed on high‐resolution anatomical images (Fig. 1). The resulting map was used to determine those brain regions related to motor function, including bilateral superior cerebellum (SCb), supplement motor area (SMA, Brodman Area [BA] 6), and left primary motor cortex (M1, BA 4), etc. Other activated regions like those within occipital lobe were presumably less related to motor control and, hence, were excluded from further connectivity analysis. Some areas, like the posterior cingulate cortex (PCC), showing negative correlation to the reference were also excluded. Thus, 8 independent clusters survived. However, we found that some larger clusters are comprised of several different brain regions. As shown in Figure 2, there is a very large cluster in the left of the slice, and obviously it includes two different brain regions (i.e., anterior inferior cerebellum [AICb] and posterior inferior cerebellum [PICb]). We, therefore, partitioned it into two sub‐clusters, and two peak voxels were selected separately within each. Similarly, we repeated the process of partitioning to other large clusters. Finally, a total of 24 peak voxels were obtained. Each peak voxel and its nearest 26 neighbors were defined as a group ROI. These group ROIs were used to further define subject‐specific ROIs.
Figure 1.
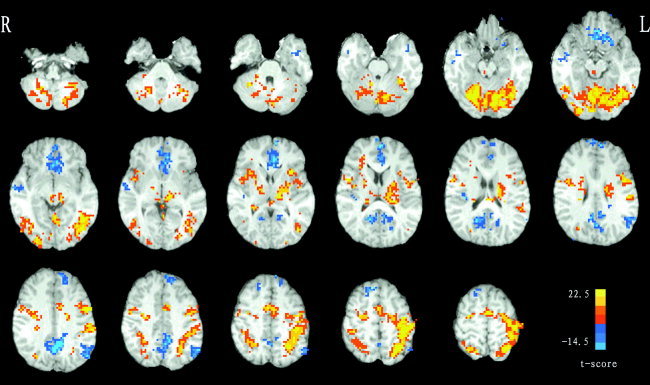
Group activation t‐map of the movement task (P < 0.01, uncorrected, clusters with size > 20 voxels). Some brain regions (e.g., the M1, SMA, SCb, PCC, etc.) show task‐related increases or decreases during the task. z‐Axis from Z = −39 to Z = +62 in standard Talairach and Tournoux coordinates. L indicates the left hemisphere of the brain.
Figure 2.
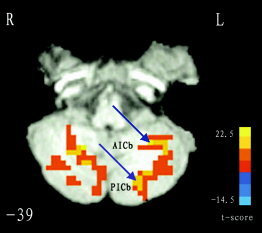
The partition of a large activated cluster. In the left of the slice (Z = −39), there is a large cluster, and obviously it includes two different brain regions (i.e., AICb and PICb). We, therefore, partitioned it into two sub‐clusters, and two peak voxels were selected separately within each. The blue arrows show their locations. Similarly, we dealt with other large clusters. L indicates the left hemisphere of the brain. For details, see Methods.
Subject‐specific ROIs.
Taking into account the anatomical variance across subjects, subject‐specific peak voxel and subject‐specific ROI were defined on his/her own r‐maps as follows. A given group ROI was first taken as a mask. Then, based on individual r‐maps, the voxel with the largest r‐value within this mask was taken as the subject‐specific peak voxel. This subject‐specific peak voxel together with its 6 nearest neighbors were taken as a subject‐specific ROI. Subject‐specific averaged time series were extracted by averaging the time series of 7 voxels in the subject‐specific ROI. For each of the resting and movement states, 24 such subject‐specific averaged time series were obtained leading to further functional connectivity analysis. A flow chart involved in the definition of ROIs is shown in Figure 3.
Figure 3.
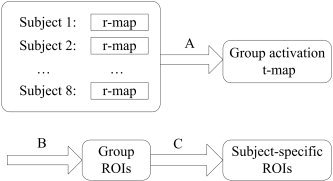
A flow chart involved in the definition of ROIs. A three‐step process was undertaken. First, a random‐effect analysis was performed across subjects on their individual r‐maps to produce a group activation t‐map (A). Then, each peak voxel and its nearest 26 neighbors in the t‐map were defined as a group ROI (B). Finally, subject‐specific ROIs were obtained on the basis of the group ROIs (C). For details, see Methods.
Functional Connectivity Analyses
In this study, we used a network model based on graph theory to describe functional connectivity. Thus, the nodes denote the brain regions and the links denote the connections or information flow among them. In order to measure the connectivity degree ηij between the node i and the node j, we used one of the simplest ways that is exponentially related to the distance between them [Lopez and Sanjuan, 2002], i.e., ηij = e , where ξ is a real positive constant, measuring how the strength of the relationship decreases with the distance between the two nodes (ξ is a subjective selection and discussed by Lopez and Sanjuan [2002] and is here fixed to ξ = 2), and d ij is the distance between the two nodes, calculated as a hyperbolic correlation measure [Golay et al., 1998]. This calculation is as follows: d ij = (1 − c ij)/(1 + c ij), where c ij represents the Pearson correlation coefficient between the two nodes (i.e., cross‐correlating two averaged time series of the above). In this way, we can define the total connectivity degree Γi of a node i in a graph as the sum of all the connectivity degrees between i and all other nodes, i.e., Γi = ∑ ηij. It describes the amount of information of the node i receiving from the particular network. In our context, a larger Γ means that this region is more functionally connected to other regions in the network. Obviously, instead of conventional pairwise connectivity [Lahaye et al., 2003], the Γ takes into account n‐to‐1 connectivity using 1‐to‐1 connectivity measures. Thus, it is possible to find the changes of the total functional connectivity degree for some regions by detecting Γ in different brain activity states.
In this study, as there are different time points and different pre‐processing between the two states, we normalized Γi of a node i, namely, 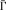 i = Γi/∑ Γj. The normality of the distribution of the
i = Γi/∑ Γj. The normality of the distribution of the 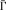 values for both states was tested using the kurtosis test. Differences of
values for both states was tested using the kurtosis test. Differences of 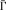 among the ROIs in the resting state and the movement state were tested, respectively, with ANOVA. The differences of
among the ROIs in the resting state and the movement state were tested, respectively, with ANOVA. The differences of 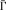 of each ROI between the two states were tested using paired Student's t‐test.
of each ROI between the two states were tested using paired Student's t‐test.
RESULTS
Task‐Related Brain Regions in Movement State
Brain regions showing task‐related increases or decreases during the movement task are presented in Figure 1. After occipital lobe and some deactivated regions (e.g., PCC, etc.) are excluded, the surviving regions are presented in Table I.
Table I.
Coordinate and t score of peak voxel within group ROIs for the movement task*
| Region | Hem | BA | Peak voxel location | t | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| FG | L | 18 | −10 | −64 | −12 | 22.290 |
| SCb | R | 16 | −58 | −15 | 21.244 | |
| M1 | L | 4 | −43 | −25 | 50 | 20.888 |
| Th | L | −10 | −19 | 11 | 20.465 | |
| SPL | L | 7 | −22 | −58 | 53 | 17.627 |
| FG | R | 18 | 1 | −70 | −12 | 16.522 |
| SMA | L | 6 | −1 | −1 | 53 | 15.328 |
| PMC | R | 6 | 28 | −7 | 50 | 12.203 |
| PMC | L | 6 | −49 | 1 | 35 | 11.479 |
| SCb | L | −25 | −55 | −15 | 8.761 | |
| SPL | R | 7 | 16 | −61 | 56 | 8.726 |
| DN | R | 19 | −55 | −30 | 8.713 | |
| PMC | R | 6 | 52 | 1 | 23 | 8.683 |
| AICb | L | −22 | −46 | −39 | 8.079 | |
| AICb | R | 16 | −46 | −39 | 7.707 | |
| PCG | R | 3 | 37 | −31 | 50 | 7.668 |
| PMC | L | 4 | −22 | −10 | 53 | 7.664 |
| BG | R | 22 | −1 | 11 | 7.400 | |
| BG | L | −25 | −13 | 8 | 7.331 | |
| PICb | L | −13 | −70 | −39 | 7.215 | |
| Th | R | 7 | −19 | 11 | 6.654 | |
| DN | L | −28 | −55 | −33 | 6.484 | |
| PICb | R | 19 | −70 | −48 | 5.680 | |
| Cu | R | 19 | 25 | −70 | 29 | 5.140 |
BA, Brodmann's area; Hem: hemisphere; R: right; L: left; FG: fusiform gyrus; SCb: superior cerebellum; M1: primary motor cortex; Th: thalamus; SPL: superior parietal lobule; SMA: supplement motor area; PMC: premotor cortex; DN: dentate nucleus; AICb: anterior inferior cerebellum; PCG: postcentral gyrus; BG: basal ganglia; PICb: posterior inferior cerebellum; Cu: cuneus.
Resting State Functional Connectivity Network
Figure 4 shows the total connectivity degree (Γ, see Methods) of each brain region in the resting state across all subjects. A larger Γ suggests that there exists significant functional connectivity between the brain region and others; thus, it is considered as an important node in the network. The top 10 in Γ are as follows: the bilateral SCb, bilateral fusiform gyrus (FG, BA 18), SMA (BA 6), left superior parietal lobule (SPL, BA 7), left thalamus (Th), right AICb, right premotor cortex (PMC, BA 6), and left dentate nucleus (DN).
Figure 4.
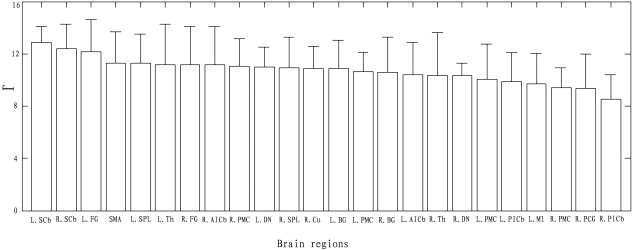
Plot shows Γ (±SD) of each brain region in the resting state across all subjects. Those brain regions with larger Γ are considered to be important nodes in the resting state functional connectivity network.
Modulation of Functional Connectivity Network
For both states, 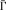 values had an approximate Gaussian distribution (kurtosis test: kurtosis = 3.27. For Gaussian distribution, kurtosis = 3). The Differences in
values had an approximate Gaussian distribution (kurtosis test: kurtosis = 3.27. For Gaussian distribution, kurtosis = 3). The Differences in 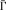 among the ROIs were significant in either the resting (F23, 168 = 2.25, P = 0.002) or the movement state (F23, 168 = 9.87, P = 0). The ranked
among the ROIs were significant in either the resting (F23, 168 = 2.25, P = 0.002) or the movement state (F23, 168 = 9.87, P = 0). The ranked 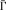 values are shown in Figure 5A for the resting state and Figure 5B for the movement state. Furthermore, with strict Bonferroni correction (i.e., 0.05/24 ≈ 0.002 as threshold), paired t‐test show significant difference in
values are shown in Figure 5A for the resting state and Figure 5B for the movement state. Furthermore, with strict Bonferroni correction (i.e., 0.05/24 ≈ 0.002 as threshold), paired t‐test show significant difference in 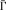 for some pairs of ROIs between the two states (Fig. 5C), e.g., the left M1 (P = 0.002, BA 4), the left SCb (P = 0.001). In addition, trends toward increases in the left PMC (P = 0.005, BA 4), the left SPL (P = 0.02, BA 7), and toward decreases in the left DN (P = 0.06), the right cuneus (P = 0.04, BA19), the left basal ganglia (BG, P = 0.02), were also observed during the movement state, in contrast to during the resting state (Fig. 5C).
for some pairs of ROIs between the two states (Fig. 5C), e.g., the left M1 (P = 0.002, BA 4), the left SCb (P = 0.001). In addition, trends toward increases in the left PMC (P = 0.005, BA 4), the left SPL (P = 0.02, BA 7), and toward decreases in the left DN (P = 0.06), the right cuneus (P = 0.04, BA19), the left basal ganglia (BG, P = 0.02), were also observed during the movement state, in contrast to during the resting state (Fig. 5C).
Figure 5.
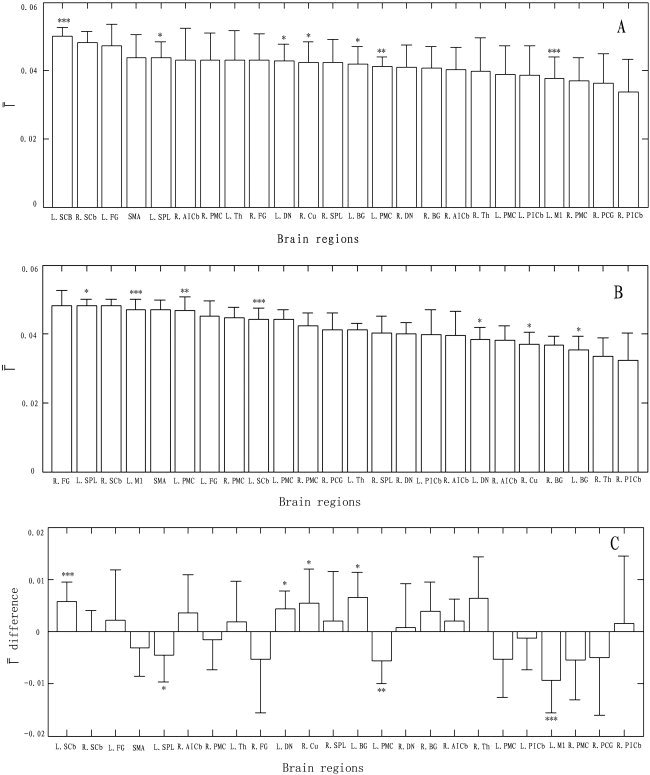
Ranking of the selected brain regions based on their 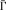 (±SD). A: Resting state. B: Movement state. C: Difference in
(±SD). A: Resting state. B: Movement state. C: Difference in 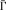 between two states. *P < 0.05; **P < 0.01; ***P < 0.001 (paired t‐test).
between two states. *P < 0.05; **P < 0.01; ***P < 0.001 (paired t‐test).
DISCUSSION
In this study, we demonstrated that a set of brain regions related to motor function exhibit high temporal coherence (i.e., significant functional connectivity) during the resting state. This indicates the existence of a large functional connectivity network related to motor function in the resting brain. More importantly, we demonstrated the changes of the network from the resting state to the movement state, which suggests that the functional connectivity network of human brain is dynamic and can be modulated in different brain activities.
Although the resting state connectivity network related to motor function has been shown with the functional connectivity MR imaging (fcMRI) technique based on “seed” voxel in previous studies [Biswal et al., 1995; Xiong et al., 1999], it just reflects the functional relationship between the selected seed region and other brain regions. Thus, the network characterized with fcMRI is incomplete. The present study overcame this problem by taking into account the joint interactions among multiple motor regions of the brain. Under this circumstance, some brain regions (e.g., the bilateral SCb, bilateral FG [BA 18], SMA [BA 6], left SPL [BA 7], left Th, right AICb, right PMC [BA 6], left DN, Fig. 4) with larger Γ values indicate stronger interactions with other brain regions and were considered as important nodes implicated in the resting state network. We found that these nodes included not only some cortical regions, but also some subcortical regions (e.g., the bilateral SCb and left Th, etc), which have not been detected with the fcMRI technique when M1 was selected as the seed [Biswal et al., 1995; Xiong et al., 1999]. Unexpectedly, some significantly activated brain regions showing larger t scores (Table I), such as the left M1 (BA 4) and left PMC (BA 4), were not considered as important nodes in the network (Fig. 4), although they are proverbial brain regions closely related to motor function. This is easy to understand because the contralateral (i.e., the left) M1 plays a dominant role in movement execution. When M1 was taken as the seed, like in previous studies [Biswal et al., 1995; Xiong et al., 1999], it should appear in the connectivity network. We speculate that the functional network related to motor function is in existence during resting and maintains a dynamic equilibrium; it may make the brain keep a state of readiness in executing a future task [Bressler and Kelso, 2001].
If the network during rest can be modulated from the movement task, we expect that there exist significant differences in 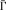 values for some specific brain regions between during the resting state and during the movement state. This hypothesis was strongly supported by statistical analysis in
values for some specific brain regions between during the resting state and during the movement state. This hypothesis was strongly supported by statistical analysis in 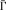 values (Fig. 5C). Among these brain regions,
values (Fig. 5C). Among these brain regions, 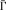 values of the left M1 (BA 4), left PMC (BA 4), and left SPL (BA 7) had significant increases or a trend toward increases, but those of the left SCb, left DN, right cuneus (BA 19), and left BG had significant decreases or a trend toward decrease during the movement state, compared with during the resting state. With these observations, understanding cognitive functions of the above brain regions may yield insight into the functions supported by the network and its transition from state to state.
values of the left M1 (BA 4), left PMC (BA 4), and left SPL (BA 7) had significant increases or a trend toward increases, but those of the left SCb, left DN, right cuneus (BA 19), and left BG had significant decreases or a trend toward decrease during the movement state, compared with during the resting state. With these observations, understanding cognitive functions of the above brain regions may yield insight into the functions supported by the network and its transition from state to state.
It is also essential to discuss what subserves the changes of the contralateral (i.e., the left) M1. It has been shown that M1 is involved in motor function of the human brain [Georgopoulos, 2000; Kawashima et al., 1994; Richter et al., 1997; Zang et al., 2003]. Functional connectivity associated with M1 in the resting brain has also been investigated [Biswal et al., 1995; Xiong et al., 1999]. However, in this study, our finding that the left M1 (BA 4) had a lower 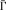 (Fig. 5A) in the resting state indicates that it may show less dependencies on most of the regions related to motor function of the human brain, compared with those important nodes (e.g., left SCb, etc.) implicated in the resting state network. During the movement state, a significant increase in
(Fig. 5A) in the resting state indicates that it may show less dependencies on most of the regions related to motor function of the human brain, compared with those important nodes (e.g., left SCb, etc.) implicated in the resting state network. During the movement state, a significant increase in 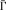 (P = 0.002, Fig. 5B,C) demonstrated that the left M1 had strong influence on the movement state connectivity network. It may be attributable to participating in movement execution [Georgopoulos et al., 2000; Kawashima et al., 1994; Richter et al., 1997; Zang et al., 2003].
(P = 0.002, Fig. 5B,C) demonstrated that the left M1 had strong influence on the movement state connectivity network. It may be attributable to participating in movement execution [Georgopoulos et al., 2000; Kawashima et al., 1994; Richter et al., 1997; Zang et al., 2003].
Similarly, we observed that the contralateral (i.e., the left) PMC (BA 4) was also precluded from those important nodes in the resting state connectivity network, but its 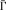 during the movement state had a trend toward increase (P = 0.005, Fig. 5C); presumably it is related with the motor function of the left PMC. Some verification has been shown that the PMC has potential roles in selection of movement [Deiber et al., 1991] and motor programming of sequential and rhythmic patterns [Halsband et al., 1993]. On the other hand, we found that the contralateral SCb had a significant decease in
during the movement state had a trend toward increase (P = 0.005, Fig. 5C); presumably it is related with the motor function of the left PMC. Some verification has been shown that the PMC has potential roles in selection of movement [Deiber et al., 1991] and motor programming of sequential and rhythmic patterns [Halsband et al., 1993]. On the other hand, we found that the contralateral SCb had a significant decease in 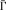 during the movement state (P = 0.001, Fig. 5C), compared with during the resting state. Although a large body of evidence has revealed that the SCb may play a significant role in motor control [Cui et al., 2000; Jancke et al., 1999], initiating and maintaining the coordination or ongoing updating of the entire retrieval process [Andreasen et al., 1999], such as autobiographical episodic memory retrieval [Fink et al., 1996], attribution or experience of emotion [Lane et al., 1997; Paradiso et al., 1999], and the formation of consciousness [Edelman 1989], it is still difficult to interpret for the decrease and needs to studied further. Besides the above, we also found the trends toward increase in the left SPL (P = 0.02, BA 7), and toward decreases in the left DN (P = 0.06), right cuneus (P = 0.04, BA19), left BG (P = 0.02) during the movement state, in contrast to during the resting state. Presumably, these observed changes between the two states might result from inhibition or excitation of the task.
during the movement state (P = 0.001, Fig. 5C), compared with during the resting state. Although a large body of evidence has revealed that the SCb may play a significant role in motor control [Cui et al., 2000; Jancke et al., 1999], initiating and maintaining the coordination or ongoing updating of the entire retrieval process [Andreasen et al., 1999], such as autobiographical episodic memory retrieval [Fink et al., 1996], attribution or experience of emotion [Lane et al., 1997; Paradiso et al., 1999], and the formation of consciousness [Edelman 1989], it is still difficult to interpret for the decrease and needs to studied further. Besides the above, we also found the trends toward increase in the left SPL (P = 0.02, BA 7), and toward decreases in the left DN (P = 0.06), right cuneus (P = 0.04, BA19), left BG (P = 0.02) during the movement state, in contrast to during the resting state. Presumably, these observed changes between the two states might result from inhibition or excitation of the task.
It is important to emphasize that the changes of the interaction of the brain regions reflect changes of the functions of the network. In the present study, the resting‐state connectivity network seems to be much looser than its counterpart in the movement state. This difference can be identified with statistical difference of the brain regions in 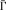 between in the resting state (ANOVA, F23, 168 = 2.25, P = 0.0017) and in the movement state (ANOVA, F23, 168 = 9.87, P = 0). This change from a loose connectivity network to a tight one implies the accomplishment of movement function, i.e., planning, initiation, coordination, guidance, and termination of voluntary movements.
between in the resting state (ANOVA, F23, 168 = 2.25, P = 0.0017) and in the movement state (ANOVA, F23, 168 = 9.87, P = 0). This change from a loose connectivity network to a tight one implies the accomplishment of movement function, i.e., planning, initiation, coordination, guidance, and termination of voluntary movements.
As shown above, the brain's functional connectivity is highly flexible and can be modulated for a given changing environment. The modulation is very important for the brain to execute different function in different brain states. So far, research in the connectivity changes of brain regions can be classified into two categories: change from one task state to another and change from the resting state to the task state. The investigations of modulation of functional connectivity from one task state to another include: using visual motion to examine attentional modulation of the visual cortex [Friston and Buchel, 2000], exploring the plastic changes of brain regions relating with individual learning performance [Buchel et al., 1999], and observing task‐related dynamic reconfiguration of large‐scale neural networks [Liu et al., 1999]. Recent evidence has demonstrated that disease also can lead to the changes of connectivity relationship among some brain regions [Li et al., 2000]. However, the investigations of modulation of functional connectivity from the resting state to the task state are very limited. The present study focuses on this category. We believe that these investigations can improve our understanding of how the brain works.
In summary, we have provided compelling evidence for the existence of a functional connectivity network related to motor function in the resting brain. We believe that the activity in this network is ongoing and maintains a dynamic equilibrium. Most importantly, we have shown that this network is modulated during a motor task that may alter the activities of a population of neurons by disturbing the balance of local mutual inhibition. Moreover, we provide a quantitative measure for such modulation taking into account n‐to‐1 connectivity using 1‐to‐1 connectivity measures instead of conventional pairwise connectivity. Thus, our study can be considered as fully assessing joint interaction among multiple brain regions in different states of brain activity, which may be helpful to understand basic neurophysiological mechanisms that operate in the baseline [Gusnard et al., 2001]. Of course, exact mental process supported by the connectivity network during rest due to its uncontrolled nature is essentially difficult and needs to be elucidated in the future.
Acknowledgements
We are grateful to both the anonymous referees for their significant and constructive comments and suggestions, which greatly improved the paper. T.J. also thanks his colleague, Mrs. Catherine Putonti, Department of Computer Sciences, University of Houston, for her careful checking of the manuscript.
REFERENCES
- Andreasen NC, O'Leary DS, Paradiso S, Cizadlo T, Arndt S, Watkins GL, Ponto LL, Hichwa RD (1999): The cerebellum plays a role in conscious episodic memory retrieval. Hum Brain Mapp 8: 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wang EC, Hyde JS (1993): Processing strategies for time‐course data sets in functional MRI of human brain. Magn Reson Med 30: 161–173. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34: 537–541. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Kelso JAS (2001): Cortical coordination dynamics and cognition. Trends Cogn Science 5: 26–36. [DOI] [PubMed] [Google Scholar]
- Buchel C, Coull JT, Friston KJ (1999): The predictive value of changes in effective connectivity for human learning. Science 283: 1538–1541. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, Quigley MA, Meyerand ME (2000): Mapping functionally related regions of brain with functional connectivity MRI (fcMRI). Am J Neuroradiol 21: 1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton V, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand E (2001): Frequencies contributing to functional connectivity in the cerebral cortex in “resting‐state” data. Am J Neuroradiol 22: 1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Cox, R.W (1996): AFNI software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Cui SZ, Li EZ, Zang YF, Weng XC, Jia FC, Ivry I, Wang JJ (2000): Both sides of human cerebellum involved in preparation and execution of sequential movements. NeuroReport 11: 3849–3853. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RSJ (1991): Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res 84: 393–402. [DOI] [PubMed] [Google Scholar]
- Edelman G.M (1989): The Remembered Present. New York: Basic Books. [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD (1996): Cerebral representation of one's own past: neural networks involved in autobiographical memory. J Neurosci 16: 4275–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buchel C (2000): Attentional modulation of effective connectivity from V2 to V5/MT in humans, Proc Natl Acad Sci 97: 7591–7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS (1993a): Functional connectivity: the principal component analysis of large (PET) data sets. J Cereb Blood Flow Metab 13: 5–14. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS (1993b): Time‐dependent changes in effective connectivity measured with PET. Hum Brain Mapp 1: 69–80. [Google Scholar]
- Georgopoulos AP (2000): Neural aspects of cognitive motor control. Curr Opin Neurobiol 10: 238–241. [DOI] [PubMed] [Google Scholar]
- Golay X, Kollias S, Stoll G, Merier D, Valavanis A, Boesiger PA (1998): New correlation‐based fuzzy logic clustering algorithm for fMRI. J Magn Res Med 40: 249–260. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis, Proc Natl Acad Sci 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME (2001): Searching for a baseline: function imaging and the resting human brain. Nat Rev Neurosci 2: 685–694. [DOI] [PubMed] [Google Scholar]
- Halsband U, Ito N, Tanji J, Freund HJ (1993): The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain 116: 243–266. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC (2002): Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp 15: 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ (1998): Generalisability, random effects and population inference. NeuroImage 7: 754. [Google Scholar]
- Jancke L, Specht K, Mirzazade S, Peters M (1999): The effect of finger‐movement speed of the dominant and the subdominant hand on cerebellar activation: a functional magnetic resonance imaging study. NeuroImage 9: 497–507. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Roland PE, O'Sullivan BT (1994): Fields in human motor areas involved in preparation for reaching, actual reaching, and visuomotor learning: a positron emission tomography. J Neurosci 14: 3462–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahaye PJ, Poline JB, Flandin G, Dodel S, Garnero L (2003): Functional connectivity: studying nonlinear, delayed interactions between BOLD signals. NeuroImage 20: 962–974. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE (1997): Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia 35: 1437–1444. [DOI] [PubMed] [Google Scholar]
- Li SJ, Biswal BB, Wang Y, Li Z, Risinger R, Rainey C, Cho JK, Salmeron BJ, Stein EA (2000): Cocaine administration decrease functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med 43: 45–51. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Gao JH, Liotti M, Pu Y, Fox PT (1999): Temporal dissociation of parallel processing in the human subcortical outputs. Nature 400: 364–367. [DOI] [PubMed] [Google Scholar]
- Lopez L, Sanjuan MAF (2002): Relation between structure and size in social networks. Phys Rev E 65: 036107. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA (1998): Functional connectivity in single and multislice echoplanar imaging using resting state fluctuations. Neuroimage 7: 119–132. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Johnson DL, Andreasen NC, O'Leary DS, Watkins GL, Ponto LL, Hichwa RD (1999): Cerebral blood flow changes associated with attribution of emotional valence to pleasant, unpleasant and neutral visual stimuli. Am J Psychiatry 156: 1618–1629. [DOI] [PubMed] [Google Scholar]
- Piyaratna J, Rajapakse JC, Hennig J (2001): Eigenimage analysis of resting brain fMR images. Proceedings of the 3rd International Conference on Information, Communications and Signal Processing (ICICS 01), October 15–18, 2001, Singapore.
- Richter W, Andersen PM, Georgopoulos AP, Kim SG (1997): Sequential activity in human motor areas during a delayed cued finger movement task studied by time resolved fMRI. NeuroReport 8: 1257–1261. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux PA (1988): Coplanar stereotactic atlas of the human brain. Stuttgart: Thieme. [Google Scholar]
- Xiong J, Parsons LM, Gao JH, Fox PT (1999): Interregional connectivity to primary motor cortex revealed using MRI resting state images. Hum Brain Mapp 8: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang YF, Jia FC, Weng XC, Li EZ, Wang YF, Hazeltine E, Ivry R (2003): Functional organization of the primary motor cortex characterized by event‐related fMRI during movement preparation and execution. Neurosci Lett 337: 69–72. [DOI] [PubMed] [Google Scholar]


