Abstract
We used the combined technique of functional magnetic resonance imaging (fMRI) and transcranial magnetic stimulation (TMS) to observe changes that occur in adult brains after the practice of stringed musical instruments. We carried out fMRI on eight volunteers (aged 20–22 years): five novices and three individuals who had discontinued practice for more than 5 years. The motor paradigm contained a repetitive lift‐abduction/fall‐adduction movement of the left/right little finger, carried out with maximum efforts without pacing. The sensory paradigm was to stimulate the same little finger using a string. In parallel to the fMRI acquisition, TMS motor maps for the little finger were obtained using a frameless stereotactic neuronavigation system. After the baseline study, each participant began to learn a stringed instrument. Newly developed fMRI activations for the left little finger were observed 6 months after practice at multiple brain regions including inferior parietal lobule, premotor area (PMA), left precuneus, right anterior superior temporal gyrus, and posterior middle temporal gyrus. In contrast, new activations were rarely observed for the right little finger. The TMS study revealed new motor representation sites for the left little finger in the PMA or supplementary motor area (SMA). Unexpectedly, TMS motor maps for the right little finger were reduced significantly. Among new fMRI activations for sensory stimuli of the left little finger, the cluster of highest activation was located in the SMA. Collectively, these data provide insight into orchestrated reorganization of the sensorimotor and temporal association cortices contributing to the skillful fingering and musical processing after the practice of playing stringed instruments. Hum. Brain Mapping 23:188–199, 2004. © 2004 Wiley‐Liss, Inc.
Keywords: musical training, fMRI, TMS, string player, brain, plasticity
INTRODUCTION
Musicians have been an ideal subject pool for the study of learning and brain plasticity [Schlaug, 2001]. Significant effort has been put into research of various brain regions of professional musicians, particularly the auditory cortex [Pantev et al., 2001; Parsons, 2001; Rauschecker, 2001], somatosensory cortex [Pantev et al., 2001], and motor cortex [Jancke et al., 2000; Krings et al., 2000]. In addition to learning itself, functional differences exhibited by brains of professional musicians probably reflect their innate abilities, perhaps fostered by early exposure to musical training [Schlaug, 2001]. We studied amateur adult stringed‐instrument players who started learning musical instruments. Complex human behaviors such as musical performance would require multiple interconnected neural networks where information is transmitted via parallel pathways across different brain regions, offering redundancy [Parsons, 2001]. In defining activational changes that would occur after the practice of the instruments, we focused more on overall functional activities than on specific regional variations.
In the physical playing of stringed instruments, one of the important things is the execution of rapid fingerfall and fingerlift in a vertical plane and finger shift in a horizontal plane, without changing the overall hand position. As for the fingering, it is well known that the fifth digit is the weakest and requires the most training compared to other fingers for players to willfully control its movement [Mozart, 1948]. As a motor task that could reflect possible improvement in fingering skills after the practice of stringed instruments, we thus concluded that an appropriate task would be rapid repetitive lift‐abduction/fall‐adduction (LAFA) movement of the little finger, carried out with maximum efforts without pacing. Although the LAFA movement paradigm lacked explicit musical context, the movement was expected to show the brain areas relevant to musical training, if the movement was coupled extensively with music performance over a long time.
If a tapping frequency is high enough (>2 Hz), functional magnetic resonance imaging (fMRI) activation in the primary sensorimotor cortex can be saturated [Sadato et al., 1997]. In this case, motor representational changes after practice may not be reflected fully on fMRI. We therefore carried out transcranial magnetic stimulation (TMS) as a mapping tool complementary to fMRI, providing electrophysiologic information to supplement the hemodynamic data of fMRI [Krings et al., 1997]. Importantly, the combined techniques could also help determine whether a new fMRI activation is a mere coincidence of activation at areas in two parallel pathways or if it represents a newly developed connection with the peripheral motor system [Krings et al., 1997].
Using the combined techniques of fMRI and TMS, we tried to observe changes in adult brains induced by learning and practice of stringed musical instruments. Specifically, we set out to answer if: (1) the fMRI activation areas for the LAFA movement or sensory stimuli of the little finger would change after the practice; (2) if TMS motor output maps would correspond with fMRI data; and (3) if the newly activated areas would include neural structures, related to more skillful fingering or possible musical processing, such as parietal cortex, premotor cortex, and temporal association cortex. In addition, we tried to locate the areas showing reduced neural activation after practice, taking into account the decreased prefrontal activation associated with the motor memory consolidation [Shadmehr and Holcomb, 1997]. In answering these questions, we focused on examining the difference between the left and right hands, because it is only the left hand that gets used for the LAFA movement during the practice of conventional stringed instruments.
SUBJECTS AND METHODS
Participants
We carried out fMRI and TMS on eight volunteers (five men, three women; all right‐handed; age range 20–22 years) among the newcomers to Seoul National University Medical Orchestra's string section, who were scheduled to learn stringed musical instruments (violin, viola, and cello). Five subjects had not played the instruments previously. The other three had played previously for 3–4 years but had discontinued their practice for more than 5 years. Four novices and all those who had stopped playing had experience in piano performance (mean ± SD, 4.0 ± 3.6 years). Among these, five had stopped playing piano after age 13 years and two stopped after age 16 years. Follow‐up fMRI and TMS studies were carried out 6 months after commencement of practice on the stringed instruments. Subjects gave informed consent according to institutional guidelines. After completion of the fMRI and TMS studies, participants were compensated for their time. Data are presented as mean ± SD.
fMRI
Blood oxygen level‐dependent (BOLD) imaging was carried out on a 1.5‐T MR scanner (GE Signa whole‐body and standard RF coil) equipped with echo‐planar imaging (EPI). Twenty axial slices of 5 mm thickness, parallel to the line through the anterior and posterior commissure, were collected using a gradient echo EPI sequence (repetition time [TR] = 3 s; echo time [TE] = 60 ms; flip angle = 90 degrees; field of view [FOV] = 240 mm). For subsequent anatomic coregistration, T1‐weighted images (TR = 500 ms, TE = 12 ms, flip angle = 90 degrees) were acquired in axial planes using the same slice selection parameters as that used in the BOLD imaging.
In the first fMRI session, subjects carried out repetitive LAFA movement of the little finger with maximal efforts. In the second task, stimulation of the little finger was carried out by two of the authors. A slightly rough string was rubbed (middle 3‐cm portion of the string on the volar surface of the finger tip perpendicular to the long axis of the finger) back and forth at 12 cm/sec. In the follow‐up study after the practice period, one more imaging session was added to see whether changes of fMRI activation had resulted from the differences in maximum effort‐dependent fingering speeds, possibly induced by the 6 months of practice. In this added session, subjects were asked to carry out the LAFA movement at a slower speed, approximately half their maximum effort.
Each block paradigm consisted of 16 blocks (21 sec/block) alternating between rest and activation (rest‐right‐rest‐left, repeated for four cycles). Before the imaging, the subjects were instructed not to move any other parts of the body except the little finger during the motor task. They practiced the LAFA movement until they produced the requested abduction length of about 3 cm, which took approximately 1–2 min. During the scanning, all movements were monitored visually by two of the authors for consistent performance. The fingering frequency was also measured.
The fMRI data were analyzed using SPM99 (Wellcome Department of Cognitive Neurology, London, UK). Brain slices were aligned using an automated image registration algorithm (AIR package, v.3.0) [Woods et al., 1993] and normalized according to the space defined by Talairach and Tournoux [ 1988] for coregistration of images across subjects. The images were convolved with a Gaussian kernel of 8 mm full‐width at half maximum (FWHM) to enhance the signal‐to‐noise ratio. The hemodynamic response function was modeled by a boxcar function. Low‐frequency noise was removed by applying a high‐pass filter (1 cycle/168 sec) to the fMRI time series at each voxel.
Functional activation sites were identified on a voxel‐by‐voxel basis using a t‐test to look for the contrast differences between the task and rest states. The first four images of each task were discarded to allow MR images of the brain to reach the steady state. The threshold for the activation was set at a P < 0.05 (corrected, spatial extent ≥8 voxels/cluster). We also acquired the contrast defined by ([post‐practice task − matching rest] − [pre‐practice task − matching rest]) or ([pre‐practice task − matching rest] − [post‐practice task − matching rest]) in the little finger of each hand. In addition, the contrast to see effects of varying fingering frequency was obtained by calculating the following: ([post‐practice LAFA with maximal efforts − matching rest] − [post‐practice LAFA with half the efforts − matching rest]).
Registration of these activation images with anatomy was achieved by coregistering T1‐weighted images with the activation maps.
TMS
A tightly adherent elastic cap was placed on the subject's head. A 1 × 1 cm grid (16 cm in mediolateral direction and 12 cm in anterior–posterior direction) covering 140 cm2 was drawn on the cap. The center of it was 1 cm anterior from Cz toward the nose. TMS was carried out [Rossini et al., 1994] with a Magstim 220 stimulator (Magstim Ltd., UK) connected to a figure‐of‐eight–shaped coil (2 × 70‐mm diameter). Electromyography (EMG) recordings were made using a Viking II EMG device with a bandpass filter (2 Hz–10 kHz). Surface electrodes were placed over the relaxed abductor digiti minimi (ADM), one of the most important muscles needed to carry out the LAFA movement adopted for the fMRI motor task. The stimuli were applied with the junction of the coil loops held tangential to the scalp and the coil handle directed posteriorly. The minimum stimulator output producing three or more motor evoked potentials (MEP) larger than 50 μV in six consecutive stimuli were considered the motor threshold. The hot spot was defined as the scalp position from which a contralateral MEP of maximal amplitude and lowest threshold was obtained. The MEP map of each hemisphere was made by stimulating at 10% over the threshold of the registered hotspot while changing stimuli locations. The coil was moved from the hotspot over the skull in steps of 1–2 cm to identify all scalp positions at which stimulation produced more than three EMG responses larger than 50 μV in three to six consecutive stimuli. The investigators who carried out the TMS were blinded to the fMRI data. They were also blinded to the baseline TMS data during the follow‐up study, in which the TMS intensity was calibrated to the new threshold.
Registration of TMS Data on 3‐D–rendered MRI
A common coordinate system for the TMS and anatomic images was provided by the frameless stereotactic neuronavigation system [Paus et al., 1997] (Brainsight; Rogue Research, Canada). To register stimulation sites on T1‐weighted images obtained in the fMRI study, MRI data were transferred to the neuronavigation system. The system contained a pointer that transmitted the spatial position of its tip via infrared light to a Polaris camera, which was not connected mechanically to the subject. The information was then transferred to the attached computer, which calculated the position of the pointer tip and updated the anatomic views in real time. When the probe tip was moved around the head, its position was indicated graphically on the axial, coronal, and sagittal views of the MRI. The pointer was held perpendicularly to the skull surface on each point of the grid. TMS representation sites were overlaid on the volumetric MRI data set to visualize the MEP map.
RESULTS
Subjects practiced the stringed instruments much more (170 ± 26 hr) during the latter half (especially the last month) of the 6‐month interscan period (Fig. 1). All participants passed the final audition for the annual concert. After the practice period, the left little finger LAFA movement became significantly faster (3.5 ± 0.6 Hz; P < 0.05, paired t‐test) than that before the practice (3.1 ± 0.4 Hz). This was not the case, however, for the right little finger movement (after/before, 3.5 ± 0.5/3.4 ± 0.4 Hz; P = 0.53) (Fig. 2). The improvement in left fingering speed seemed slightly lower in the novices (after/before, 3.5 ± 0.7/3.0 ± 0.4 Hz) than that in the previous players (after/before, 3.7 ± 0.3/2.9 ± 0.4 Hz); however, this did not reach statistical significance (P = 0.25, Mann–Whitney test). In a separate experiment using a computer keyboard, most subjects showed improved accuracy and increased speed of the left LAFA movement after the practice period (data not shown).
Figure 1.
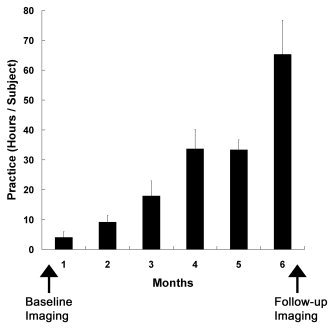
Mean (± SD) distribution of practice time over the 6‐month interimaging period.
Figure 2.
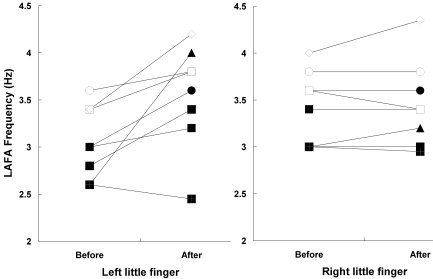
Lift‐abduction/falling‐adduction (LAFA) movement frequencies before and after the practice of playing stringed musical instruments.
Motor fMRI
Figure 3 depicts (in green) the areas activated during the LAFA movement before practice, shown as the pooled data of all 8 subjects, and served as a baseline activation map. The left little finger movement showed both contralateral and ipsilateral hemispheric activations. The bilaterality was not prominent for the right little finger, although the extent of contralateral activation in the sensorimotor cortex was larger than that of the left little finger movement. Areas in red denote the foci of activation, which after practice were significantly higher than was the baseline status. As shown in Figure 4, the higher post‐practice activations were observed at multiple brain regions including inferior parietal lobule (Brodmann area [BA] 39/40), premotor area (BA6, right PMv, and left middle frontal gyrus), left precuneus (BA 7), right anterior superior temporal gyrus (BA 38), and right posterior middle temporal gyrus (BA 37). In contrast, the right little finger LAFA movement showed only two minor areas of higher activation in the right middle temporal and inferior occipital gyrus. The coordinates of these activated regions in the Talairach and Tournoux space [Talairach and Tournoux, 1988] are listed in Table I.
Figure 3.
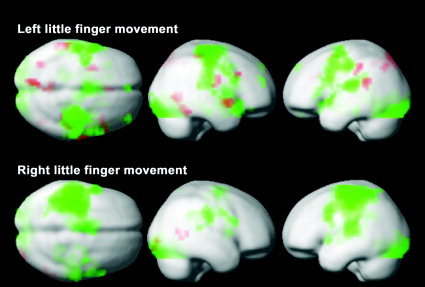
fMRI activation maps associated with the practice of playing stringed musical instruments. Activated regions by the LAFA finger movement, acquired before the practice, are displayed in green (pooled data of eight subjects). Bilateral hemispheric activation is more prominent for the movement of the left little finger (upper row). The extent of contralateral activation of the sensorimotor cortex was larger for the right finger movement (lower row). Activations significantly higher (P < 0.05, corrected) than baseline were observed for the left little finger in multiple regions (red) including inferior parietal lobule, premotor area, left precuneus, and temporal association cortices, 6 months after the practice of violin, viola or cello, Note that new activations for the right little finger movement are sparse.
Figure 4.
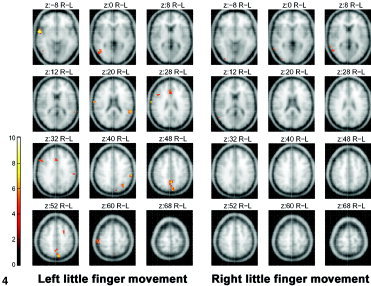
fMRI maps showing increased activation with the practice of stringed instruments, obtained by subtracting the activation areas of the LAFA movement before practice from activation areas acquired after practice. The activation maps on averaged brain MRI were thresholded at P < 0.05 (corrected for multiple comparisons) and a cluster size of 8 voxels.
Table I.
Higher activation during the LAFA movement after the practice
| Contrast and region | BA | x | y | z | Voxels | Z |
|---|---|---|---|---|---|---|
| Right LAFA (post > pre) | ||||||
| R middle temporal gyrus | 21 | 63 | −46 | 6 | 11 | 6.46 |
| R inferior occipital gyrus | 18 | 28 | −94 | −5 | 12 | 6.44 |
| Left LAFA (post > pre) | ||||||
| R superior temporal gyrus | 38 | 55 | 4 | −7 | 26 | Inf |
| R postcentral gyrus | 43 | 67 | −15 | 15 | 10 | 7.78 |
| L precuneus | 7 | −4 | −52 | 43 | 45 | 7.13 |
| L superior temporal gyrus | 22 | −59 | −42 | 21 | 20 | 6.95 |
| R inferior temporal gyrus | 20 | 55 | −55 | −11 | 11 | 6.65 |
| L inferior parietal lobule | 40 | −63 | −29 | 38 | 14 | 6.63 |
| R middle temporal gyrus | 37 | 44 | −62 | 0 | 20 | 6.62 |
| R inferior parietal lobule | 40 | 48 | −32 | 53 | 18 | 6.37 |
| L inferior parietal lobule | 39 | −36 | −64 | 40 | 11 | 6.17 |
| L middle frontal gyrus | 6 | −28 | 3 | 51 | 8 | 5.80 |
| R ventral premotor cortex | 44 | 40 | 17 | 25 | 10 | 5.58 |
| Anterior cingulate | 32 | 0 | 28 | 24 | 10 | 5.41 |
Areas with higher activation during the lift‐abduction‐falling/adduction (LAFA) movement of the little finger after practice of stringed musical instruments than before practice are given as approximate Brodmann area (BA) and peak locations (x, y, z in mm) in the Talairach and Tournoux [ 1988] atlas with associated significance (Z value). Statistical threshold, (P < 0.05, corrected); spatial threshold, 8 contiguous significant voxels. See Frackowiak et al. [ 1997] for detailed descriptions of equations and assumptions involved in calculation of Z value and the probability associated with it.
R, right hemisphere; L, left hemisphere; voxels, number of activated voxels of the cluster; post, post‐practice; pre, pre‐practice; Inf, Z value > 8.
Figure 5 and Table II show brain areas with decreased activation after the practice period. They include left middle temporal gyrus, right cerebellar declive (vermis), middle and posterior cingulate gyrus (BA 23/24), right inferior frontal gyrus (BA 47), right basal ganglia, substantia nigra, and right medial prefrontal cortex (BA 9/10). Again, the right little finger LAFA movement showed only three minor areas of decreased activation.
Figure 5.
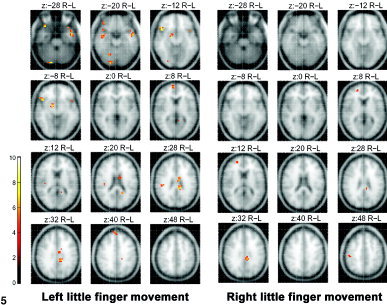
The fMRI maps showing decreased activation with the practice of stringed instruments, obtained by subtracting the activation areas of the LAFA movement after practice from activations acquired before practice. The activation maps on the averaged brain MRI were thresholded at P < 0.05 (corrected for multiple comparisons) and a cluster size of 8 voxels.
Table II.
Lower activation during the LAFA movement after the practice
| Contrast and region | BA | x | y | z | Voxels | Z |
|---|---|---|---|---|---|---|
| Right LAFA (pre > post) | ||||||
| L cingulate gyrus | 31 | −12 | −33 | 31 | 11 | 6.73 |
| R postcentral gyrus | 2 | 48 | −21 | 45 | 8 | 5.77 |
| R medial frontal gyrus | 10 | 20 | 47 | 9 | 8 | 5.54 |
| Left LAFA (pre > post) | ||||||
| L middle temporal gyrus | 21 | −51 | −1 | −20 | 51 | Inf |
| R cerebellum (declive) | — | 12 | −86 | −19 | 21 | Inf |
| — | 20 | −59 | −14 | 8 | 5.92 | |
| R inferior frontal gyrus | 47 | 44 | 19 | −11 | 27 | Inf |
| 47 | 32 | 26 | −25 | 17 | Inf | |
| R midbrain (substantia nigra) | ||||||
| R basal ganglia | — | 12 | −8 | −9 | 15 | 7.33 |
| L posterior cingulate | 23 | −16 | −30 | 28 | 27 | 7.04 |
| L middle cingulate | 24 | −12 | 5 | 22 | 26 | 6.70 |
| R superior frontal gyrus | 9 | 4 | 56 | 34 | 11 | 6.39 |
| R medial frontal gyrus | 10 | 4 | 59 | 4 | 9 | 5.97 |
| R temporal lobe | — | 40 | −5 | −16 | 11 | 6.02 |
| R postcentral gyrus | — | 40 | −22 | 27 | 17 | 6.02 |
Areas with lower activation during the lift‐abduction/falling‐adduction (LAFA) movement of the little finger after practice of stringed musical instruments than before practice are given as approximate Brodmann area (BA) and peak locations (x, y, z in mm) in the Talairach and Tournoux [ 1988] atlas with associated significance (Z value). Statistical threshold, (P < 0.05, corrected); spatial threshold, 8 contiguous significant voxels. See Frackowiak et al. [ 1997] for detailed descriptions of equations and assumptions involved in calculation of Z value and the probability associated with it.
R, right hemisphere; L, left hemisphere; voxels, number of activated voxels of the cluster; post, post‐practice; pre, pre‐practice; Inf, Z value > 8.
When the post‐practice fMRI activations for the left or right LAFA movement at maximum efforts were subtracted by those of slower speeds (left = 1.8 ± 0.8 Hz, right = 1.7 ± 0.8 Hz), as was expected from the previous report [Sadato et al., 1997], significant BOLD signal was observed primarily in the contralateral sensorimotor cortex and ipsilateral cerebellum (Table III). It is notable that these locations did not overlap with the new activation areas associated with the practice. Most areas active for maximal efforts (post‐practice LAFA with maximal effort − matching rest) were also activated during the slower movement (post‐practice LAFA with half effort − matching rest). In Figure 6, additional activations seemed to exist in the SMA after the left LAFA movement with maximal effort when compared to those carried out with half effort; however, subtraction analysis failed to reveal statistically significant differences (Table III).
Table III.
Higher activation during the LAFA movement with maximal efforts
| Contrast and region | BA | x | y | z | Voxels | Z |
|---|---|---|---|---|---|---|
| Right LAFA (fast > slow) | ||||||
| L postcentral gyrus | 1 | −44 | −21 | 53 | 149 | Inf |
| R declive (vermis) | — | 4 | −67 | −17 | 28 | 7.70 |
| Lt culmen (cerebellum) | — | −8 | −54 | −1 | 26 | 6.35 |
| Left LAFA (fast > slow) | ||||||
| L declive (cerebellum) | — | −4 | −67 | −17 | 148 | Inf |
| R precentral gyrus | 6 | 40 | −9 | 63 | 28 | Inf |
| L precentral gyrus | 4 | −55 | −6 | 37 | 14 | 7.67 |
| R/L cingulate gyrus | 24 | 0 | −2 | 37 | 50 | 6.81 |
| L postcentral gyrus | 40 | −59 | −18 | 19 | 9 | 6.74 |
| R hippocampal gyrus | 20 | 20 | −20 | −19 | 10 | 5.97 |
Areas with higher activation during the lift‐abduction/falling‐adduction (LAFA) movement of the little finger with maximal efforts (fast) than with half the maximum efforts (slow) are given as approximate Brodmann area (BA) and peak locations (x, y, z in mm) in the Talairach and Tournoux [ 1988] atlas with associated significance (Z value). Statistical threshold, (P < 0.05, corrected); spatial threshold, 8 contiguous significant voxels. See Frackowiak et al. [ 1997] for detailed descriptions of equations and assumptions involved in calculation of Z value and the probability associated with it. fMRI was carried out after practice of stringed musical instruments.
R, right hemisphere; L, left hemisphere; voxels, number of activated voxels of the cluster; post, post‐practice; pre, pre‐practice; Inf, Z value > 8.
Figure 6.
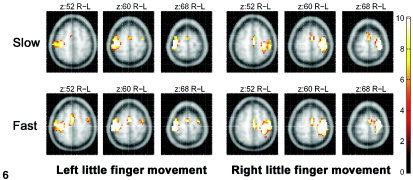
The fMRI activation areas for the LAFA movement with maximal effort (fast) and half the effort (slow), acquired after practice. The activation maps on the averaged brain MRI were thresholded at P < 0.05 (corrected for multiple comparisons) and a cluster size of 8 voxels.
The left fingering triggered bilateral sensorimotor activation, whereas the right finger movement primarily recruited contralateral left sensorimotor areas (Figs. 3 and 6), which is in line with the previous report [Rao et al., 1993].
TMS
Figure 7 shows TMS motor maps before (green dots) and after (yellow dots) practice. For each subject, the specific loci at which stimulation produced an EMG response of the contralateral ADM were displayed on the corresponding 3‐D volumetric MR image. After practice, the motor map areas for the left ADM were similar or enlarged slightly. Moreover, new motor representational sites were observed in the premotor area (PMA) or supplementary motor area (SMA). In contrast, motor output maps for the right ADM were reduced significantly (P < 0.05, paired t‐test comparing differences in the number of TMS loci producing EMG responses before and after practice). After the practice period, the number of TMS sites for the right ADM (2.5 ± 0.7) were significantly less than were those for the left ADM (4.6 ± 0.7; P < 0.05, t‐test), which was not the case before practice (4.8 ± 0.6 and 4.4 ± 0.6, respectively).
Figure 7.
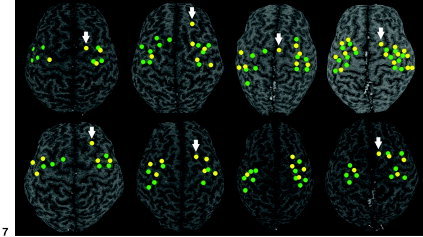
Motor maps acquired using TMS before (green) and after (yellow) practice of stringed musical instruments. Anatomic locations of each subject, where TMS produced an electromyographic response of the contralateral abductor digiti minimi (ADM), were displayed on the corresponding 3‐D volumetric MR image. Similar or slightly enlarged motor map areas are observed for the left ADM with new representation sites (arrows) in and around the PMA or SMA. Motor representations for the right ADM are reduced significantly.
The post‐practice motor thresholds to produce MEP responses in the left (76 ± 7% of maximum stimulator output) and right (79 ± 10%) ADMs were not significantly different from those of the pre‐practice runs (75 ± 9% and 76 ± 9%; P = 0.56 and P = 0.16, respectively, paired t‐test).
Sensory fMRI
The peak fMRI activation for sensory stimulation of the left little finger, which was newly generated in association with the practice of stringed instruments, was observed in the SMA (Fig. 8, Table IV).
Figure 8.
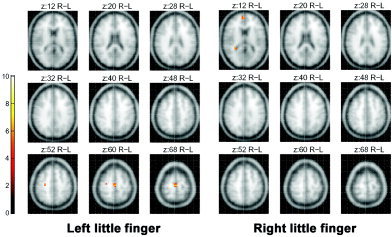
The fMRI maps obtained by subtracting the activation areas for the sensory stimulation on the little finger before practice of stringed musical instruments from those acquired after practice. Note that peak activation is observed in the posterior supplementary motor area. Images were thresholded at P < 0.05 (corrected for multiple comparisons).
Table IV.
Higher activation during the sensory stimulation after the practice
| Contrast and region | BA | x | y | z | Voxels | Z |
|---|---|---|---|---|---|---|
| Right little finger (post > pre) | ||||||
| R/L dorsal frontal gyrus | 10 | 0 | 55 | 8 | 25 | 5.99 |
| Left little finger (post > pre) | ||||||
| R/L medial frontal gyrus | 6 | 0 | −16 | 60 | 18 | Inf |
| R inferior occipital gyrus | 17 | 24 | −86 | −9 | 21 | 6.34 |
| R precentral gyrus | 4 | 24 | −13 | 52 | 8 | 6.17 |
Areas with higher activation during sensory stimulation of the little finger after practice of stringed musical instruments than before practice are given as approximate Brodmann area (BA) and peak locations (x, y, z in mm) in the Talairach and Tournoux [ 1988] atlas with associated significance (Z value). Statistical threshold, (P < 0.05, corrected); spatial threshold, 8 contiguous significant voxels. See Frackowiak et al. [ 1997] for detailed descriptions of equations and assumptions involved in calculation of Z value and the probability associated with it. fMRI was carried out after practice of stringed musical instruments.
R, right hemisphere; L, left hemisphere; voxels, number of activated voxels of the cluster; post, post‐practice; pre, pre‐practice; Inf, Z value > 8.
DISCUSSION
After 6 months of practice with the stringed musical instruments, the fMRI showed new activation areas for left little finger LAFA movement when carried out with maximal effort. The extensive training of professional pianists was reported to yield greater performance efficiency, where a smaller number of neurons were recruited for the given movements [Jancke et al., 2000; Krings et al., 2000]. Due to the “maximal efforts without pacing” paradigm used in our study, new recruitment areas for the practice‐induced skillful fingering could have emerged. They were categorized into four distinctive regions: (1) left posterior parietal heteromodal cortices (BA 7 and 39/40; precuneus and inferior parietal lobule, respectively); (2) right parietal cortices (BA 43 and 40; postcentral gyrus and inferior parietal lobule, respectively); (3) premotor cortices (BA 6 and 44; left middle frontal gyrus and right ventral premotor cortex, respectively) and anterior cingulate gyrus (BA 32); and (4) temporal cortices including right anterior superior temporal gyrus (BA 38; temporopolar area), left posterior superior temporal gyrus (BA 22), and right posterior middle/inferior (BA 37/20) temporal gyrus. These activations were not attributable to the practice‐induced difference in the fingering speed, which is consistent with the previous study [Sadato et al., 1997]. For the right little finger, new activation areas were rarely observed. The TMS study showed new representation sites for the left little finger ADM in PMA, SMA, or both. Unexpectedly, motor representations for the right ADM were reduced significantly.
Among the new activation areas, the posterior parietal cortices including ipsilateral precuneus and left inferior parietal lobule that contains spatiotemporal representations of learned skilled movements, are known for multimodal interactions related to praxis, generation of motor plans, and spatial attention [Mesulam, 2000a]. The motor programs are translated subsequently into the appropriate motor output through mediation of the premotor cortex [Heilman et al., 1997], another brain region with new fMRI activation in the present study. The posterior parietal cortices are interconnected strongly with both cingulate gyrus and premotor cortex, indicating their roles for mediating the type of sensorimotor and cognitive integration that would be needed for spatial attention [Mesulam, 2000b]. The post‐practice functional coupling among these coactivated areas should contribute to the skillful performance of the stringed instruments. In addition to the left premotor area, our subjects showed activation in the right ventral premotor cortex, which is known to be a major source of input to the primary motor cortex and to have a role in transforming visual properties of 3‐D objects into hand shapes appropriate to interact with them [Rizzolatti et al., 1998]. It is notable that the subjects did not watch or touch the stringed instruments during the fMRI session. Although speculative, the recruitment of the PMv might contribute not only to the visual to hand shaping but also to the additional sensory (e.g., proprioception) to hand‐shaping transformative functions. In particular, a fluent transformation of 3‐D perception into hand shapes is essential for skillful playing of stringed instruments. As a reliable feedback loop, highly controlled interactive cross‐communication between sensory and motor systems seems crucial for the competence of string players. The well‐known brain connectivity also supports our findings. As pointed out previously, the fiber track connections between parietal and premotor areas, including the ventral premotor area, are regarded as responsible for sensorimotor transformations [Rizzolatti et al., 1998]. PMv neurons were also reported to encode the direction of wrist movement in space and were suggested to contribute to sensorimotor transformation between extrinsic and intrinsic coordinate frames [Kakei et al., 2001].
The rapid little finger LAFA, a necessary movement for improved performance in playing stringed instruments, was utilized for our fMRI paradigm. The LAFA movement is challenging and requires extensive training to master. To reflect any improved fingering ability after the practice, subjects were asked to exercise with maximum effort instead of paced movement. The participants, all of whom passed the final audition for the annual concert, showed a significant increase in the speed of the left little finger movement. The acquired skillful fingering of the adult amateur stringed instrument players may be explained by the new recruitment of additional neural resources in the posterior parietal cortices, the premotor areas, and the anterior cingulate gyrus. In support of our hypothesis, these areas were reported previously to be associated with precision grip, which involves fine control of direction and magnitude of fingertip forces [Ehrsson et al., 2000]. In that study, subjects were asked to use the tips of the thumb and index finger to grasp an instrumented handle for the precision grip task. For the power grip task, they clenched a cylindrical plastic tube with an overall force output much higher than that used in the precision grip task. The resulting fMRI representations acquired by subtracting the power grip activation from the precision grip areas corresponded well to the activation areas induced by the fingering practice in the present study.
The combined TMS study revealed new representation sites in the PMA or SMA. MEPs during stimulation at or near those locations may have been due to increased sensitivity of the primary motor cortex to stimulation. Considering, however, no significant interval changes in motor thresholds between the time of pre‐ and post‐practices, a favorable explanation could be that the migration of the area responsive to TMS outside the usual boundaries may suggest activation of new synaptic connections [Rossini and Pauri, 2000]. Skill learning is thought to be followed by strengthening of horizontal cortical connections throughout many areas of the cerebral cortex as well as in the primary motor cortex [Rioult‐Pedotti et al., 1998]. We thus speculate that for the left little finger, transsynaptic projections between the SMA/PMA and the corresponding primary motor cortex were strengthened or newly generated as neural substrates for learning to play stringed instruments. The fact that focal TMS of the new foci in the SMA or PMA generated actual finger movements indicates the presence of the above‐mentioned connection with the peripheral motor system. A similar observation was made using TMS alone [Pascual‐Leone et al., 1995], but the combination of fMRI and TMS has not been tried.
Interestingly, we observed a significant reduction of the TMS‐responsive area for the right ADM. Higher numbers of motor representation sites for the left ADM than for the right ADM could be reflecting higher cortical excitability in the right motor cortex due to the unilateral left finger practice. Unilateral left finger movements could be associated with ipsilateral cerebral deactivation as well as the contralateral cerebral activation, possibly by transcallosal inhibition [Allison et al., 2000]. On the other hand, we could speculate that the shrinkage of the activatable motor areas partly resulted from continual relaxation of right fingers and their en‐bloc movements with proximal arm and shoulder muscles during the bowing motions of play. Subsequently, the finger representation could have been invaded by the representation of the proximal parts [Nudo et al., 1996]. String players specifically exercise the relaxation of their right fingers and practice the en‐bloc movement. For instance, they train by holding a pencil with only enough firmness to prevent dropping it.
Pianists often report that listening to a well‐trained piece of music can involuntarily trigger the respective finger movements, which suggests a possible coupling between music perception and motor activity in musically trained individuals [Haueisen and Knosche, 2001]. For reception and expression of music, the right auditory association cortex is thought to have an important role [Parsons, 2001]. When Parsons et al. [ 2001] contrasted brain activity during the piano performance of a musical piece by J.S. Bach with that during scales that required movements of nearly comparable frequency and complexity, they detected the primary difference in the temporal lobe [Parsons, 2001]. Although many activations were observed in the anterior secondary auditory association areas including BA 22, 21, and 20, the performance of the Bach piece activated superior, middle, and inferior temporal areas bilaterally, with more on the right than on the left [Parsons, 2001]. Likewise, in the present study, we may be able to understand the new emergence of the highly activated foci in similar temporal regions by correlating these areas to a variety of musical processing and production. The actual musical stimuli, however, were not incorporated in the study paradigm, and the production of sound is more related to the right‐handed bowing than to the left‐hand fingering. Another possibility is that scanner noise generated during fMRI activated auditory cortex and affected the results in some way. This hypothesis is very unlikely, however, because it cannot explain the fact that multiple areas in the temporal lobe were activated strongly during the left, not right, fingering. In the absence of the actual sound stimuli, nonverbal [Goldenberg et al., 1991], or musical imagery [Zatorre and Halpern, 1993], and auditory hallucinations [Shergill et al., 2000, 2001] were reported to be associated with a distinct set of brain areas, particularly the right temporal cortex. Although subjects in the current study were not asked to imagine musical pieces during the fMRI, we cannot exclude completely the possibility of the imagination processes. On the other hand, one may speculate that musical sound was generated spontaneously in the brain during the left fingering, because the movement had been associated extensively with the music production during the study period. It would have been interesting to observe whether right‐handed bowing, instead of fingering, could show similar or stronger activations in the right temporal cortex.
Musical processing (i.e., performance, perception, and comprehension) involves multiple brain systems including the motor, auditory, limbic, and executive systems. These processes thus require integration of the activities in all areas [Munte et al., 2003]. Motor and musical sciences are discovering a common field in which they can interact positively [Molinari et al., 2003], and future studies are expected to elucidate training‐induced transsynaptic connections between musical representation and motor areas. One intriguing finding in our study is that sensory stimulation of the left little finger induced new fMRI activation in the posterior SMA, not in the somatosensory cortex, unlike our expectation based on previous literature [Elbert et al., 1995]. The posterior SMA has a role for the initiating or executive activity of motor tasks [Lee et al., 1999]. As an anticipatory feed‐forward activity, the increased SMA activity after sensory stimulation might contribute to sensorimotor integration, which could optimize the interactive processes of “sensing‐the‐string” and “fingering‐the‐string” by coupling responsible brain areas; comprising an effective shortcut circuit to the execution stage, while presumably skipping the preparation stage that involves posterior SMA or PMA [Lee et al., 1999]. Subjects were instructed not to move their fingers during the sensory task and were monitored by the authors. The possibility remains, however, that imagining movements [Rao et al., 1993] or subtle reactive finger movements against the string was linked to SMA activation.
The post‐practice LAFA movement was associated not only with multiple neural recruitments but also with decreased activation in various brain areas. These included right cerebellar declive (vermis), left middle temporal gyrus, middle and posterior cingulate (BA 24/23), right medial prefrontal cortex (BA 9/10), inferior frontal gyrus (BA 47), basal ganglia, and substantia nigra. The anterior cingulate gyrus, which showed higher activation after the practice than before, is known to be involved in regulation of attention [D'Esposito et al., 1995] and monitoring of performance [Carter et al., 1998]. Areas such as middle and posterior cingulate, right inferior frontal gyrus, prefrontal cortex, and basal ganglia, however, are considered to be responsible for inhibition of responses [Aron et al., 2003; Booth et al., 2003]. We hypothesize that skillful repetitive fingering might have been coupled with the decreased inhibitory motor response, relaxed from some unnecessary constraints, along with the heightened level of attentive monitoring over possible inappropriate movements. Although the LAFA movement could be regarded as a relatively simple motor task, it would require attentional vigilance to maintain an efficient performance level if the fast fingering had to be sustained over a long time [Tracy et al., 2001].
There are several caveats to consider in our study. First, although motor training occurred in rich musical contexts, we focused mainly on measures of motor function. The contributions of the musical aspect of the training could have been made clearer by the addition of a control condition that involved motor practice alone without musical training. Second, although it is unlikely, the changes described might have resulted from merely scanning a subject twice. Carrying out the same fMRI/TMS studies in a control group may have resolved this problem. Third, further studies are warranted to explain the decreased activations in the left middle temporal gyrus and right posterior cerebellum after the practice.
Despite the limitations, our fMRI/TMS study demonstrates that practicing the playing of stringed musical instruments is linked with orchestrated reorganization of the sensorimotor and temporal association cortices, which contributes to skillful fingering and possibly to musical processing. The network plasticity is an example of the malleability of the adult brain circuits on which the lifelong ability to adapt to environmental needs and challenges is based [Pantev et al., 2001].
Acknowledgements
We thank the students from the Seoul National University Medical Orchestra, who participated in the study. We also thank Drs. D. Schellingerhout and K. Shah for proofreading the manuscript.
REFERENCES
- Allison JD, Meador KJ, Loring DW, Figueroa RE, Wright JC (2000): Functional MRI cerebral activation and deactivation during finger movement. Neurology 54: 135–142. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW (2003): Stop‐signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci 6: 115–116. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM (2003): Neural development of selective attention and response inhibition. Neuroimage 20: 737–751. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. (1995): The neural basis of the central executive system of working memory. Nature 378: 279–281. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H (2000): Cortical activity in precision‐ versus power‐grip tasks: an fMRI study. J Neurophysiol 83: 528–536. [DOI] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E (1995): Increased cortical representation of the fingers of the left hand in string players. Science 270: 305–307. [DOI] [PubMed] [Google Scholar]
- Frackowiak RS, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC (1997): Human brain function. San Diego: Academic Press. [Google Scholar]
- Goldenberg G, Podreka I, Steiner M, Franzen P, Deecke L (1991): Contributions of occipital land temporal brain regions visual and acoustic imagery—a SPECT study. Neuropsychologia 29: 695–702. [DOI] [PubMed] [Google Scholar]
- Haueisen J, Knosche TR (2001): Involuntary motor activity in pianists evoked by music perception. J Cogn Neurosci 13: 786–792. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Watson RT, Rothi LG (1997): Disorders of skilled movements: limb apraxia In: Reinberg TE, Farah MJ, editors. Behavioral neurology and neuropsychology. New York: McGraw‐Hill; p 227–235. [Google Scholar]
- Jancke L, Shah NJ, Peters M (2000): Cortical activations in primary and secondary motor areas for complex bimanual movements in professional pianists. Brain Res Cogn Brain Res 10: 177–183. [DOI] [PubMed] [Google Scholar]
- Kakei S, Hoffman DS, Strick PL (2001): Direction of action is represented in the ventral premotor cortex. Nat Neurosci 4: 1020–1025. [DOI] [PubMed] [Google Scholar]
- Krings T, Buchbinder BR, Butler WE, Chiappa KH, Jiang HJ, Cosgrove GR, Rosen BR (1997): Functional magnetic resonance imaging and transcranial magnetic stimulation: complementary approaches in the evaluation of cortical motor function. Neurology 48: 1406–1416. [DOI] [PubMed] [Google Scholar]
- Krings T, Topper R, Foltys H, Erberich S, Sparing R, Willmes K, Thron A (2000): Cortical activation patterns during complex motor tasks in piano players and control subjects. A functional magnetic resonance imaging study. Neurosci Lett 278: 189–193. [DOI] [PubMed] [Google Scholar]
- Lee KM, Chang KH, Roh JK (1999): Subregions within the supplementary motor area activated at different stages of movement preparation and execution. Neuroimage 9: 117–123. [DOI] [PubMed] [Google Scholar]
- Mesulam MM (2000a): Behavioral neuroanatomy In: Mesulam MM, editor. Principles of behavioral and cognitive neurology. New York: Oxford University Press; p 39. [Google Scholar]
- Mesulam MM (2000b): Behavioral neuroanatomy In: Mesulam MM, editor. Principles of behavioral and cognitive neurology. New York: Oxford University Press; p 220. [Google Scholar]
- Mozart L (1948): A treatise on the fundamental principles of violin playing. London: Oxford University Press; (Translated by Edith Knocker). p 190. [Google Scholar]
- Munte TF, Nager W, Beiss T, Schroeder C, Altenmuller E (2003): Specialization of the specialized: Electrophysiological investigations in professional musicians. Ann N Y Acad Sci 999: 131–139. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, Milliken GW (1996): Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 272: 1791–1794. [DOI] [PubMed] [Google Scholar]
- Pantev C, Engelien A, Candia V, Elbert T (2001): Representational cortex in musicians. Plastic alterations in response to musical practice. Ann N Y Acad Sci 930: 300–314. [PubMed] [Google Scholar]
- Parsons LM (2001): Exploring the functional neuroanatomy of music performance, perception, and comprehension. Ann N Y Acad Sci 930: 211–231. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone A, Nguyet D, Cohen LG, Brasil‐Neto JP, Cammarota A, Hallett M (1995): Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74: 1037–1044. [DOI] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC (1997): Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci 17: 3178–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz A, Lisk LM, Morris GL, Mueller WM, Estkowski LD (1993): Functional magnetic resonance imaging of complex human movements. Neurology 43: 2311–2318. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP (2001): Cortical plasticity and music. Ann N Y Acad Sci 930: 330–336. [DOI] [PubMed] [Google Scholar]
- Rioult‐Pedotti MS, Friedman D, Hess G, Donoghue JP (1998): Strengthening of horizontal cortical connections following skill learning. Nat Neurosci 1: 230–234. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M (1998): The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol 106: 283–296. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al (1994): Non‐invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 79–92. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Pauri F (2000): Neuromagnetic integrated methods tracking human brain mechanisms of sensorimotor areas “plastic” reorganisation. Brain Res Brain Res Rev 33: 131–154. [DOI] [PubMed] [Google Scholar]
- Sadato N, Ibanez V, Campbell G, Deiber MP, Le Bihan D, Hallett M (1997): Frequency‐dependent changes of regional cerebral blood flow during finger movements: functional MRI compared to PET. J Cereb Blood Flow Metab 17: 670–679. [DOI] [PubMed] [Google Scholar]
- Schlaug G (2001): The brain of musicians. A model for functional and structural adaptation. Ann N Y Acad Sci 930: 281–299. [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH (1997): Neural correlates of motor memory consolidation. Science 277: 821–825. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Williams SCR, Murray RM, McGuire PK (2000): Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 57: 1033–1038. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Cameron LA, Brammer MJ, Williams SCR, Murray RM, McGuire PK (2001): Modality specific neural correlates of auditory and somatic hallucinations. J Neurol Neurosurg Psychiatry 71: 688–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Tracy JI, Faro SS, Mohammed F, Pinus A, Christensen H, Burkland D (2001): A comparison of “Early” and “Late” stage brain activation during brief practice of a simple motor task. Brain Res Cogn Brain Res 10: 303–316. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR (1993): MRI‐PET registration with automated algorithm. J Comput Assist Tomogr 17: 536–546. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Halpern AR (1993): Effect of unilateral temporal lobe excision on perception and imagery of songs. Neuropsychologia 31: 221–232. [DOI] [PubMed] [Google Scholar]


