Abstract
The main aim of this study was to investigate the differential processing of correct and incorrect equations to gain further insight into the neural processes involved in arithmetic reasoning. Electrophysiological studies in humans have demonstrated that processing incorrect arithmetic equations (e.g., 2 + 2 = 5) elicits a prominent event‐related potential (ERP) compared to processing correct equations (e.g., 2 + 2 = 4). In the present study, we investigated the neural substrates of this process using event‐related functional magnetic resonance imaging (fMRI). Subjects were presented with arithmetic equations and asked to indicate whether the solution displayed was correct or incorrect. We found greater activation to incorrect, compared to correct equations, in the left dorsolateral prefrontal cortex (DLPFC, BA 46) and the left ventrolateral prefrontal cortex (VLPFC, BA 47). Our results provide the first brain imaging evidence for differential processing of incorrect vs. correct equations. The prefrontal cortex activation observed in processing incorrect equations overlaps with brain areas known to be involved in working memory and interference processing. The DLPFC region differentially activated by incorrect equations was also involved in overall arithmetic processing, whereas the VLPFC was activated only during the differential processing of incorrect equations. Differential response to correct and incorrect arithmetic equations was not observed in parietal cortex regions such as the angular gyrus and intra‐parietal sulcus, which are known to play a specific role in performing arithmetic computations. The pattern of brain response observed is consistent with the hypothesis that processing incorrect equations involves detection of an incorrect answer and resolution of the interference between the internally computed and externally presented incorrect answer. More specifically, greater activation during processing of incorrect equations appears to reflect additional operations involved in maintaining the results in working memory, while subjects attempt to resolve the conflict and select a response. These findings allow us to further delineate and dissociate the contributions of prefrontal and parietal cortices to arithmetic reasoning. Hum. Brain Mapping 16:119–130, 2002. © 2002 Wiley‐Liss, Inc.
Keywords: arithmetic, N400, prefrontal cortex, angular gyrus, fMRI, interference, Stroop
INTRODUCTION
Arithmetic reasoning is a uniquely human skill that we utilize nearly everyday. Brain imaging studies have identified a distributed network involved in arithmetic reasoning including the lateral and ventral prefrontal cortex, and posterior parietal lobe, as well as subcortical regions including the caudate nucleus and cerebellum [Burbaud et al., 1995; Dehaene et al., 1999; Menon et al., 2000b]. Isolating the processes involved in arithmetic reasoning, and their neural bases, however, presents a considerable challenge. Investigations of the psychological and neural bases of arithmetic reasoning are frequently based on verification tasks in which subjects are presented with an equation of the form, “2 + 3 = 5”, and are asked to make a decision regarding whether the presented answer is correct or incorrect [Allen et al., 1997; Menon et al., 2000a, b; Rickard et al., 2000]. A key aspect of this type of arithmetic reasoning is the ability to distinguish between incorrect and correct arithmetic equations. In this study we examine differences in the way the brain processes incorrect and correct equations.
Analyses of brain imaging data from verification tasks have generally focused on processing both correct (e.g., 2 + 2 = 4) and incorrect (e.g., 2 + 2 = 5) equations. Typically, brain activation during the processing of these equations is averaged and compared to a control condition involving non‐arithmetic operations [Menon et al., 2000a,b; Rickard et al., 2000]. Although several common operations are involved in processing correct and incorrect equations, recent behavioral and electrophysiological evidence indicates that the brain processes these two types of arithmetic equations differently [Niedeggen and Rosler, 1999; Niedeggen et al., 1999; Zbrodoff and Logan, 2000]. The main aim of this study was to investigate the differential processing of correct and incorrect equations to gain further insight into the neural processes involved in arithmetic reasoning.
Research related to the processing of correct and incorrect arithmetic equations has been limited to a few studies. The strongest behavioral evidence for differential processing of correct and incorrect arithmetic equations comes from a recent study by Zbrodoff and Logan [2000] which found that it took participants, on average, 56 msec longer to produce the correct solution for equations displayed with incorrect, compared to correct, solutions. Zbrodoff and Logan have described this process as an arithmetic Stroop effect, in which the presentation of an incorrect answer interferes with the subject's ability to produce the correct answer. Two event‐related potential (ERP) studies from Neideggen et al. [1999] have provided more direct evidence that incorrect equations are processed differently from correct equations [Niedeggen and Rosler, 1999]. These studies have shown that during arithmetic verification tasks, processing of incorrect, compared to correct, equations evokes a large negative brain potential, peaking between 300 and 500 msec after presentation of the equation. This ERP component has been termed the arithmetic N400. The scalp topography of the arithmetic N400 is, however, fairly diffuse, making it difficult to identify specific brain areas involved in the differential processing of incorrect arithmetic equations.
In a previous study we used functional magnetic resonance imaging (fMRI) to examine which brain areas contribute uniquely to numeric computation [Menon et al., 2000b]. We used a classic arithmetic verification task involving simple addition and subtraction; task difficulty was manipulated by varying the number of operands (“3 + 5 = 8” or “3 + 5 − 1 = 6). Brain activation to incorrect and correct equations (e.g., ”3 + 5 = 8“ or ”2 + 3 = 6) was combined and analyzed together to examine activation resulting from general arithmetic processing. The results of this study showed that the contribution of the parietal and prefrontal cortices during general arithmetic processing tasks could be dissociated from each other. The angular gyrus, in particular, appears to be specifically involved in arithmetic computation independent of other processing demands [Menon et al., 2000b]. The prefrontal cortex, on the other hand, appears to be involved in supporting processes necessary for arithmetic reasoning, such as maintaining digits in working memory [Harmony et al., 1999; Kazui et al., 2000] and rapid processing of arithmetic stimuli [Menon et al., 2000b]. Accordingly, although lesions of the prefrontal cortex can result in poorer overall performance on arithmetic tasks [Fasotti et al., 1992; Lucchelli and De Renzi, 1993; Luria, 1966], lesions in the angular gyrus produce more profound and specific deficits in the ability to perform arithmetic computations [Kahn and Whitaker, 1991; Levin, 1993; Takayama et al., 1994]. Given that electrophysiological evidence suggests that the brain processes incorrect and correct equations differently, we used event‐related fMRI analyses [Burock et al., 1998; Dale, 1999; Menon et al., 1997b; Rosen et al., 1998] to examine differences in brain activation during the processing of these two types of equations.
Subjects were presented with arithmetic equations involving simple addition or subtraction. Brain activation occurring during the presentation of correct equations (e.g., 5 + 3 = 8, or 5 + 3 − 1 = 7) was compared to that occurring during the presentation of incorrect equations (e.g., 5 + 2 = 8, or 5 + 2 − 1 = 5). Subjects were asked to indicate whether or not the solution displayed was correct. We hypothesized that processing incorrect, compared to correct, equations would involve the recruitment of additional cognitive resources and this would in turn result in greater activation in specific brain regions. Further, if the processing of incorrect equations involves increased calculation load, differential activation would primarily be observed in the angular gyrus of the parietal cortex [Menon et al., 2000b]. On the other hand, if processing incorrect equations was more closely related to resolving an interference between the presented and computed answers, or keeping digits in working memory, activation would primarily be observed in prefrontal cortex regions known to support these functions.
We also investigated the effect of increased level of arithmetic complexity on processing of incorrect, compared to correct, equations by comparing brain activation to 2‐ and 3‐ operand equations. The 3‐operand condition was more difficult due to the fact that subjects were required to manipulate an additional operand, and to switch between addition and subtraction within the problem (e.g., 5 + 2 − 1 = 5). We hypothesized that, if subjects engaged in recalculation of the equation during incorrect trials, greater activation of the parietal lobe should be seen for 3‐operand, compared to 2‐operand, incorrect equations. If, however, the processing of incorrect arithmetic solutions involves a more general process, such as detection of a discrepancy between correct and incorrect solutions and resolution of such an interference, we would not expect differential activation for the two types of equation in the parietal cortex. A major strength of this study is that a random effects model was used to analyze event‐related activation across 16 subjects. Using such a model ensured that only voxels consistently activated across subjects, rather than within individual subjects, would emerge as significant population activation [Holmes and Friston, 1998]. Findings from the present study provide new information on a key aspect of arithmetic reasoning and on the neural substrates of the arithmetic N400.
MATERIALS AND METHODS
Subjects
Sixteen healthy subjects (8 males and 8 females; ages 16–23) participated in this study after giving written informed consent, in accordance with the declaration of Helsinki and the Stanford Human Subjects Committee. Before the experiment, subjects were assessed using the Wechsler Adult Intelligence Scale (WAIS‐III) [Wechsler, 1991].
Experimental Design
A block fMRI experimental design (with event‐related data analyses, as described below), consisting of alternating experimental and control epochs was used in this study. In both epochs, numbers between 1 and 9 were presented visually for 5,250 msec, with an ISI of 750 msec. The experiment began with a 30‐sec rest epoch followed by six alternating 30‐sec epochs of “easy” (2‐operand equation) experimental trials and control trials. These six “easy” epochs were followed by a second 30‐sec rest epoch. After this rest epoch, subjects were presented with six alternating 30‐sec epochs of “difficult” experimental (3‐operand equation) and control trials. The experiment concluded with a 30‐sec rest epoch. During the rest condition, subjects passively viewed a blank screen. The easy epochs were presented before the difficult epochs for all subjects. The main reason for this aspect of the task design was that we wanted to use the same tasks in typically developing children as well as children and adults with difficulties in mathematical reasoning who might have become anxious and frustrated if they performed the difficult epochs first [Rivera et al., 2000, 2001]. Although the experimental design is slightly less than optimal for the present study, we do not believe that a significant confound from practice effects would be present because the 2‐ and 3‐operand computations were extremely simple for most subjects.
The “easy” experimental epochs consisted of five, 2‐operand addition or subtraction problems (randomly intermixed) with either a correct (e.g., 1 + 2 = 3) or an incorrect resultant (e.g., 5 − 2 = 4). The “difficult” experimental epochs consisted of five, 3‐operand addition and subtraction problems with either a correct (e.g., 6 − 3 + 5 = 8) or an incorrect resultant (e.g., 6 + 2 − 3 = 4). The 3‐operand equations each had one addition and one subtraction operation. Subjects were asked to respond with a button press only if the answer to the equation was correct. A total of fifteen different equations for each experimental condition (“easy” or “difficult”) were displayed, six correct and nine incorrect (Table I). The incorrect resultant trials were either one more than the correct answer (e.g., 5 + 3 = 9), or one less than the correct answer (e.g., 5 + 3 = 7) so that subjects would tend to perform more exact, rather than approximate, numerical calculations.
Table I.
Two‐ and three‐operand arithmetic equations used in the study
| Two‐operand equations | Three‐operand equations |
|---|---|
| 6 − 2 = 4 | 0 + 0 + 0 = 0a |
| 2 + 2 = 3 | 3 − 1 + 5 = 8 |
| 3 + 3 = 6 | 2 + 2 + 1 = 5 |
| 4 + 4 = 9 | 3 − 3 + 2 = 3 |
| 5 + 5 = 9 | 5 − 5 + 4 = 5 |
| 2 + 1 = 2 | 7 − 5 + 6 = 8 |
| 3 + 2 = 6 | 9 − 9 + 8 = 9 |
| 1 + 7 = 8 | 3 − 2 − 0 = 0 |
| 5 + 4 = 9 | 4 + 3 − 1 = 7 |
| 1 + 8 = 8 | 1 − 1 + 0 = 0 |
| 3 + 1 = 4 | 5 − 3 + 0 = 2 |
| 4 + 2 = 5 | 7 − 5 + 2 = 5 |
| 5 + 3 = 9 | 9 − 7 + 4 = 7 |
| 4 + 3 = 6 | 0 + 8 − 5 = 3 |
| 4 + 1 = 5 | 6 + 3 − 8 = 2 |
a = A simple equation containing zeros was presented to alert subjects to the transition to 3‐operand equations.
The “easy” control epoch consisted of a string of five digits (e.g., 1, 4, 0, 3, 5), subjects were asked to respond with a button press if “0” was one of the numbers displayed in the string. The “difficult” control epoch consisted of a string of seven digits (e.g., 1, 4, 0, 2, 3, 6, 5); subjects were asked to respond with a button press if “0” was one of the numbers displayed in the string. A total of 15 different strings were displayed for each control condition (“easy” or “difficult”), 6 with “0” and 9 without “0”.
A single button press (rather than two different button presses for correct and incorrect trials) was used because we wanted to use the same tasks in low functioning children, adolescents and adults (60 < IQ < 80), some of whom had difficulty switching between responses. The number of responses was, however, counterbalanced between experimental and control epochs.
fMRI Acquisition
Images were acquired on a 1.5 T GE Signa scanner with EchoSpeed gradients using a custom‐built whole head coil that provides a 50% advantage in signal to noise ratio over that of the standard GE coil [Hayes and Mathias, 1996]. A custom‐built head holder was used to prevent head movement. Eighteen axial slices (6 mm thick, 1 mm skip) parallel to the anterior and posterior commissure covering the whole brain were imaged with a temporal resolution of 2 sec using a T2* weighted gradient echo spiral pulse sequence (TR = 2,000 msec, TE = 40 msec, flip angle = 89° and 1 interleave) [Glover and Lai, 1998]. The field of view was 240 mm and the effective in‐plane spatial resolution was 3.75 mm. To aid in localization of functional data, a high resolution T1 weighted spoiled grass gradient recalled (SPGR) 3D MRI sequence with the following parameters was used: TR = 24 msec; TE = 5 msec; flip angle = 40°; 24 cm field view, 124 slices in sagittal plane; 256 × 192 matrix; acquired resolution = 1.5 × 0.9 × 1.2 mm. The images were reconstructed as a 124 × 256 × 256 matrix with a 1.5 × 0.9 × 0.9 mm spatial resolution.
The task was programmed using Psyscope [Cohen et al., 1993] on a Macintosh computer. Initiation of scan and task was synchronized using TTL pulse delivery to the scanner timing microprocessor board form CMU Button Box microprocessor (http://poppy.psy.cmu.edu/psyscope) connected to the Macintosh. Stimuli were presented visually at the center of a screen using a custom‐built magnet compatible projection system (Resonance Technology, CA).
Image Preprocessing
Images were reconstructed, by inverse Fourier transform, for each of the 225 time points into 64 × 64 × 18 image matrices (voxel size: 3.75 × 3.75 × 7 mm). FMRI data were preprocessed using SPM (http://www.fil.ion.ucl.ac.uk/som). Images were corrected for movement using least square minimization without higher‐order corrections for spin history, and normalized to stereotaxic Talairach coordinates [Talairach and Tournoux, 1988]. Images were then resampled every 22 mm using sync interpolation and smoothed with a 4 mm Gaussian kernel to decrease spatial noise.
Statistical Analysis
Although a block fMRI design was used in the present study, fMRI data were analyzed using event‐related methods. The aim of the analysis was to determine brain activation to incorrect, compared to correct, equations. Statistical analysis was performed on individual and group data using the general linear model and the theory of Gaussian random fields as implemented in SPM99 [Friston et al., 1995]. This method takes advantage of multivariate regression analysis and corrects for temporal and spatial autocorrelations in the fMRI data [Worsley and Friston, 1995].
Because incorrect and correct equations occurred randomly with respect to each other, activation to these events could be statistically separated. To do this we first had to determine that brain activation to correct and incorrect equations were not statistically correlated. Expected waveforms for events corresponding to correct and incorrect equations were computed after convolution with the hemodynamic response function [Kruggel and von Cramon, 1999]. The correlation between these events was 0.05 for the 2‐operand condition and 0.10 for the 3‐operand condition, allowing us to independently assess brain activation to correct and incorrect equations [Clark et al., 1998].
A within‐subjects procedure was used to model all the effects of interest for each subject. Individual subject models were identical across subjects (i.e., a balanced design was used). Confounding effects of fluctuations in global mean were removed by proportional scaling where, for each time point, each voxel was scaled by the global mean at that time point. Low frequency noise was removed with a high pass filter (0.5 cycles/min) applied to the fMRI time series at each voxel. A temporal smoothing function (Gaussian kernel corresponding to dispersion of 8 sec) was applied to the fMRI time series to enhance the temporal signal to noise ratio. We then defined the effects of interest for each subject with the relevant contrasts of the parameter estimates.
A random effects model [Holmes and Friston, 1998] was then used to determine which brain regions show greater activation during the processing of incorrect, compared to correct, equations for the 2‐ and 3‐operand conditions. Group analysis was performed using a two‐stage hierarchical procedure. In the first step, contrast images corresponding to the difference between brain responses to correct and incorrect equations were derived after adjusting for the hemodynamic response. In the second step, these contrast images were analyzed using a general linear model to determine voxel‐wise t‐statistics. A one‐way t‐test was then used to determine group activation for each condition of interest. Finally, the t‐statistics were normalized to Z scores, and significant clusters of activation were determined using the joint expected probability distribution of height and extent of Z scores [Poline et al., 1997], with height (Z > 2.33; P < 0.01) and extent threshold (P < 0.05).
Contrast images were calculated using a within‐subject design for the following analyses.
Contrast 1
The interaction between (i) processing incorrect vs. correct arithmetic equations and (ii) number of operands was examined using the following comparison: (3‐operand incorrect minus correct trials) minus (2‐operand incorrect minus correct trials).
Contrast 2
The main effect of processing incorrect vs. correct arithmetic equations was examined using the following comparison: (3‐operand incorrect minus correct trials) plus (2‐operand incorrect minus correct trials) minus control (no zero‐containing strings minus zero‐containing strings). Note that in this comparison, activation from control trials was subtracted out to eliminate the effect of response inhibition. The number of trials that required subjects to withhold response was identical during the arithmetic processing and the control conditions. Two additional analyses compared experimental and control epochs, irrespective of trial type.
Contrast 3
The main effect of arithmetic processing was examined with the following comparison: (3‐operand experimental epochs) plus (2‐operand experimental epochs) minus (all control epochs).
Contrast 4
The main effect of task difficulty during arithmetic processing was examined with the following comparison: (3‐operand experimental epochs) minus (2‐operand experimental epochs). Activation foci were superimposed on high‐resolution T1‐weighted images and their locations interpreted using known neuroanatomical landmarks [Duvernoy et al., 1999; Mai et al., 1997].
Behavioral Data Analysis
Mean percentage of correct responses and false alarms, and reaction time (RT) were computed and compared across the 2‐ and 3‐operand experimental and control conditions using a one‐way ANOVA.
RESULTS
Behavioral
Mean accuracy (percentage of correct responses) was 99.6% (SD = 1.1%), 98.8% (SD = 2.7%), and 96.7% (SD = 6.0%) for the control, and 2‐ and 3‐operand experimental trials respectively. For 2‐operand trials, the false alarm rate (responding “true” to an incorrect equation by making a button press) was 2.1% (SD = 4.5%) and the miss rate (responding “false” to a correct equation by withholding response) was 0%. For 3‐operand trials, the false alarm rate was 4.2% (SD = 6.9%) and the miss rate was 2.1% (SD = 5.7%). ANOVA of accuracy, false alarm rates, and misses revealed no significant differences between 2‐ and 3‐operand trials (P > 0.05).
Mean RT was 818.4 (SD = 144.0 msec) for the control trials, 1,233.8 msec (SD = 224.9 msec) for 2‐operand trials, 2,012.3 (SD = 327.3 msec) for 3‐operand trials, and. ANOVA on RTs revealed a significant main effect of condition (F[2,30] = 169.94; P < 0. 0000). RTs for 3‐operand trials were significantly longer than RTs for 2‐operand trials (t[15] = 7.84; P < 0.000).
Brain Activation
Contrast 1
No significant difference in brain activation was observed when incorrect versus correct trials in the 2‐operand condition were subtracted from those in the 3‐operand condition. We therefore examined the main effect of processing incorrect versus correct arithmetic equations by combining activation from the 2‐operand and 3‐operand conditions (Contrast 2 below).
Contrast 2
Significant brain activation to incorrect, compared to correct, arithmetic equations was observed in the left middle and inferior frontal gyri (Table II, Figs. 1 and 2).
Table II.
Brain areas that showed significant activation during the processing of incorrect, compared to correct, equations
| Brain area | Corrected P‐value | Number of voxels in cluster | Z max | Talairach coordinates |
|---|---|---|---|---|
| Left dorsolateral prefrontal cortex (BA 9/46) | 0.005 | 243 | 3.40 | −46, 22, 24 |
| Left ventrolateral prefrontal cortex (BA 47) | 0.042 | 168 | 3.57 | −46, 42, −6 |
Figure 1.
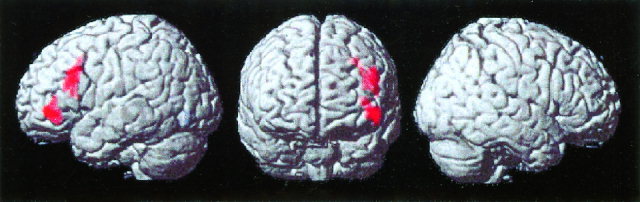
Surface rendering of brain areas that showed significant activation during the processing of incorrect, compared to correct, arithmetic equations. Activation was limited to two clusters in the left dorsolateral and ventrolateral prefrontal cortex (Brodmann areas 9/46 and 47). Results are from a random effects analysis of event‐related activation in 16 subjects; each activated cluster was significant after corrections for multiple spatial comparisons (P < 0.01).
Figure 2.
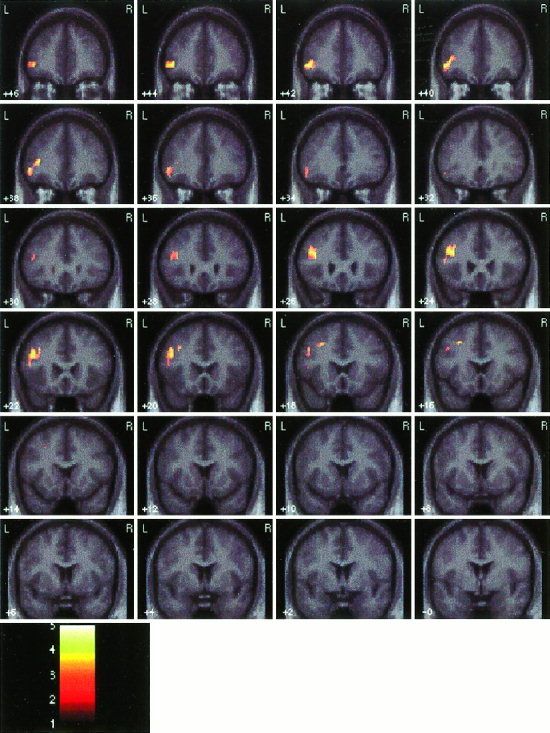
Coronal sections showing activation during the processing of incorrect, compared to correct, arithmetic equations. Other details as in Figure 1.
Contrast 3 Compared to Contrast 2
Of the several brain regions that were activated during arithmetic processing (irrespective of trial type), only the left middle frontal gyrus region showed overlapping activation during incorrect, compared to correct, arithmetic processing (Fig. 3).
Figure 3.
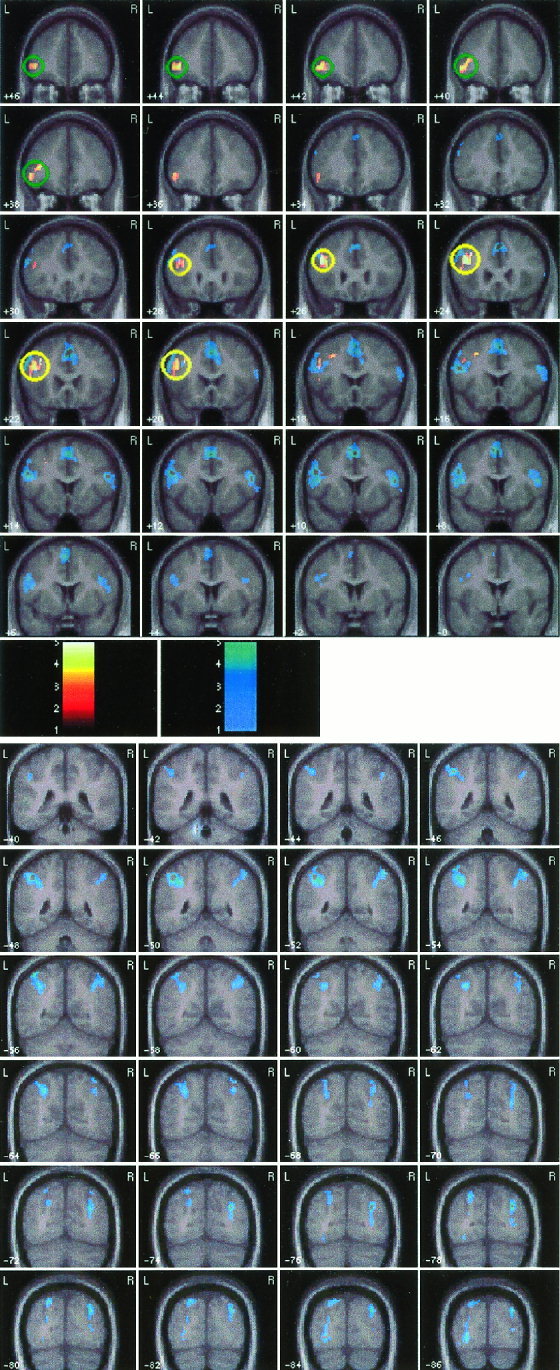
Overlap between brain areas activated during: 1) the processing of incorrect, compared to correct, arithmetic equations (shown in the yellow‐red‐black color scale), and 2) arithmetic processing (correct and incorrect equations compared to the control condition: shown in cyan). The left dorsolateral prefrontal cortex (DLPFC) in the middle frontal gyrus showed an overlap in activation in the two analyses (circled in yellow). The left ventro‐lateral prefrontal cortex (VLPFC) activation in the inferior frontal gyrus, on the other hand, was activated only during the differential processing of incorrect equations (circled in green).
Contrast 4
Although there were no differences between incorrect and correct trials for 3‐operand equations versus incorrect and correct trials for 2‐operand equations (Contrast 1), a significant difference in activation between processing of 3‐ and 2‐operand equations was found in the right angular gyrus/intra‐parietal sulcus (Fig. 4).
Figure 4.
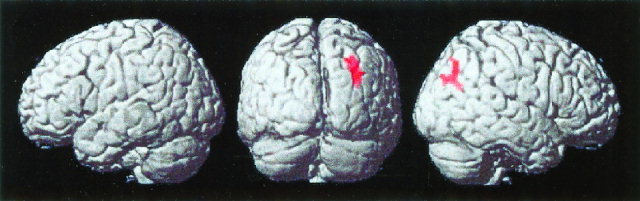
Surface rendering of brain areas that showed significant activation during the processing of 3‐operand, compared to 2‐operand, arithmetic equations, irrespective of trial type. Activation was limited to the right angular gyrus/intra‐parietal sulcus.
DISCUSSION
This is the first brain imaging study to investigate brain areas involved in the differential processing of incorrect and correct arithmetic equations. Using even‐related fMRI analyses we found that processing incorrect equations resulted in significantly greater activation than processing of correct equations in particular brain regions. This result confirms findings from electrophysiological studies, which have found that processing incorrect, compared to correct, equations results in larger electrophysiological signals [Niedeggen et al., 1999]. Together, these studies provide new information about the spatial and temporal characteristics underlying the processing of incorrect arithmetic equations.
Increased activation during processing of the incorrect equations was restricted to the left lateral prefrontal cortex, a region that several studies have implicated in arithmetic processing [Burbaud et al., 1995; Fasotti et al., 1992; Luria, 1966; Menon et al., 2000b; Rueckert et al., 1996]. Activation foci were localized to middle and inferior frontal gyri (Figs. 1 and 2), primarily encompassing the posterior dorsolateral prefrontal cortex (DLPFC; Brodmann Area 9/46) and the ventrolateral prefrontal cortex (VLPFC; Brodmann Area 47). It is unlikely that the prefrontal cortex activation was due to the motor component of response inhibition because the number of trials with response inhibition demands was balanced across the experimental and control conditions. Rather, the activation appears to be related to cognitive interference processing as discussed below.
There are a number of cognitive processes that might be invoked specifically by processing of incorrect, compared to correct, arithmetic equations. When presented with an equation, subjects must presumably produce an answer through mental calculation, and compare the result with the answer presented on the screen. Cognitive operations related to detecting the incorrect solution, and resolving the resultant interference between the calculated and displayed results, would then be evoked. Zbrodoff and Logan [2000] have examined the psychological processes underlying production during arithmetic processing tasks. Using a number of different manipulations, they provide convincing evidence that when a false answer is presented, it interferes with production of solutions to simple arithmetic equations (“the arithmetic Stroop effect”). Thus, interference detection and resolution should represent key operations involved in the differential processing of incorrect arithmetic equations during arithmetic verification tasks. We would therefore expect that activation observed during incorrect, compared to correct, arithmetic processing would overlap with brain areas that have previously been reported to be activated during proactive interference and conflict resolution. It is possible that subjects engage in a process of recalculating the answer for further verification when they realize that a discrepancy exists between the calculated answer and the one displayed. In this case, additional neural activity related to the recalculation process itself might be initiated. It is also possible that subjects made decisions about the veracity of equations based on violation of parity rules. For example, the sum of even numbers should be even. Research on parity has shown that participants are able to more easily identify the solution as incorrect when a proposed solution to an equation violates the rules of parity. Furthermore, most participants are unaware of having used parity rules, suggesting that they used these rules in an automatic fashion, as a form of tacit knowledge or skill [Krueger and Hallford, 1984]. The important point here is that even if subjects detected an incorrect equation without any real calculation, an interference process similar to the one discussed above would still be evoked.
The pattern of brain response observed in this study is consistent with the hypothesis that processing incorrect equations involves detection of an incorrect answer and resolution of the interference between the internally computed and externally presented incorrect answer. Sub‐regions of the DLPFC and the VLPFC cortex, that are differentially activated during the processing of incorrect equations, have been shown to be very generally involved in interference resolution [Jonides et al., 1998; Menon et al., 2001].
Recent imaging studies have suggested that although the VLPFC is involved more in the organization of information in working memory, interference resolution and selective retrieval, the DLPFC is more involved in maintenance and manipulation of information held in working memory [Owen, 1997; Rowe et al., 2000]. It is likely that activations in these regions are closely related, because operations involving real‐time interference resolution would also require obligatory access to the verbal rehearsal as well as the storage components of working memory. In other words, greater activation during processing of incorrect equations appears to reflect additional operations involved in maintaining the results in working memory, while subjects attempt to resolve the conflict and select a response. Thus, it is not surprising that the DLPFC and VLPFC regions activated in the present study are consistently activated during verbal working memory tasks and furthermore, show greater activation when interference processes related to working memory task performance must be resolved [D'Esposito et al., 1999; Jonides et al., 1998]. Furthermore, the DLPFC region differentially activated by incorrect equations was also involved in overall arithmetic processing (Fig. 3). On the other hand, the VLPFC was activated only during the differential processing of incorrect equations. These results provide evidence that the contribution of the DLPFC and VLPFC regions during the processing of incorrect equations can be dissociated.
No activation was observed in the anterior cingulate cortex (ACC), a brain region thought to be involved in response selection, inhibition and competition [Carter et al., 2000; Menon et al., 2001]. The differential role of the DLPFC and ACC in interference processing remains a topic of debate in the literature [Cohen et al., 2000]. One recent study has suggested that the DLPFC is more involved in implementation of cognitive control and the anterior cingulate cortex is more involved in performance monitoring [MacDonald et al., 2000]. In the present study, performance monitoring as well as other operations such as response inhibition and response competition were balanced between the experimental and control conditions. These factors may underlie lack of ACC activation specifically related to the processing of incorrect arithmetic equations.
In contrast to the lateral prefrontal cortex, the parietal cortex was not differentially activated by incorrect, compared to correct, trials. Previous imaging studies have implicated both the prefrontal and parietal cortices in arithmetic processing [Burbaud et al., 1995; Dehaene et al., 1999; Menon et al., 2000a] and we have recently proposed that the prefrontal cortex may be more involved in retrieval of arithmetic facts, working memory, and other support processes, whereas the posterior parietal cortex may be more involved in the calculation process per se [Menon et al., 2000a]. In the present study, the parietal cortex was activated by both 3‐operand and 2‐operand trials (Fig. 3B); furthermore, the angular gyrus showed greater activation during 3‐operand, compared to 2‐operand, trials (Fig. 4) [see also Menon et al., 2000b]. The lack of differential activation to incorrect trials in the parietal cortex, and particularly in the angular gyrus, suggests that subjects were not recalculating the result to verify whether the solution displayed on the screen was incorrect. If subjects were recomputing the result, we would have expected an increase in activation in the angular gyrus and other parietal lobe regions that are critically involved in arithmetic computations during 3‐operand equations, which require more computation than 2‐operand equations. It is important to note that participants in this study performed both the 2‐ and 3‐operand tasks with a high level of accuracy. In the more difficult 3‐operand task, the average accuracy was 98.7% and half of the subjects responded correctly to 100% of the trials. It is possible that if task performance was less automatized and subjects performed these arithmetic tasks poorly, they might recompute the resultant during verification thereby engaging other brain areas including the angular gyrus in the parietal lobe.
We propose that the regions of DLPFC and VLPFC activated in the present study may contribute significantly to the scalp‐recorded arithmetic N400 effect [Niedeggen and Rosler, 1999]. It is nevertheless possible that there are sources not uncovered by the present study. The scalp N400 elicited during arithmetic tasks used by Neideggen et al. [1999] had a diffuse topography over the centro‐frontal as well as parietal electrode locations. It is important to note that the ERP studies have used more difficult multiplication tasks, compared to simple addition and subtraction tasks used in the present study. ERPs elicited in posterior scalp regions during the more difficult multiplication tasks may in fact reflect brain activation related to recomputation of the answer. To date there have been no ERP studies of the arithmetic N400 using the simple and relatively well automatized arithmetic equations used in the present study. So far no source analyses of the arithmetic N400 during either addition/subtraction or multiplication tasks have been done; however, it is unlikely given the inherent indeterminacy of dipole source localization, that dipole analysis could be used exclusively to make conclusive inferences about neural generators of the arithmetic N400. To resolve these issues it will be necessary to make use of identical event‐related fMRI and ERP paradigms in a within‐subject design [Menon et al., 1997a]. Intracranial recordings will also be needed to enhance and verify findings from non‐invasive brain imaging techniques. Together, these methods have the potential to probe the neural processes involved in arithmetic reasoning more precisely, and also to investigate how the human brain makes judgments and decisions in the context of formal rule based reasoning on a more general level.
In summary, our results indicate that the left lateral prefrontal cortex plays a key role in processing incorrect, as opposed to correct, arithmetic equations. Our results suggest that processing incorrect arithmetic equations reflects resolution of an interference effect, rather than a process of recomputation and reanalysis of the equations. These results provide further insight into the neural processes involved in arithmetic reasoning by helping to delineate and disassociate the contributions of the prefrontal and parietal cortices. Future studies will examine whether incorrect statements in other domains activate similar brain regions.
Acknowledgements
We would like to thank two anonymous reviewers for their helpful comments.
REFERENCES
- Allen PA, Smith AF, Jerge KA, Vires‐Collins H (1997): Age differences in mental multiplication: evidence for peripheral but not central decrements. J Gerontol B Psychol Sci Soc Sci 52: 81–90. [DOI] [PubMed] [Google Scholar]
- Burbaud P, Degreze P, Lafon P, Franconi JM, Bouligand B, Bioulac B, Caille JM, Allard M (1995): Lateralization of prefrontal activation during internal mental calculation: a functional magnetic resonance imaging study. J Neurophysiol 74: 2194–2200. [DOI] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM (1998): Randomized event‐related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport 9: 3735–3739. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD (2000): Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA 97: 1944–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Maisog JM, Haxby JV (1998): fMRI study of face perception and memory using random stimulus sequences. J Neurophysiol 79: 3257–3265. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Botvinick M, Carter CS (2000): Anterior cingulate and prefrontal cortex: who's in control? Nat Neurosci 3: 421–423. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J (1993): PsyScope: an interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behav Res Methods Instrum Comput 25: 257–271. [Google Scholar]
- Dale AM (1999): Optimal experimental design for event‐related fMRI. Hum Brain Mapp 8: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S (1999): Sources of mathematical thinking: behavioral and brain‐imaging evidence. Science 284: 970–974. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Jonides J, Smith EE (1999): The neural substrate and temporal dynamics of interference effects in working memory as revealed by event‐related functional MRI. Proc Natl Acad Sci USA 96: 7514–7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM, Bourgouin P, Cabanis EA, Cattin F (1999): The human brain: functional anatomy, vascularization and serial sections with MRI. New York: Springer. [Google Scholar]
- Fasotti L, Eling PA, Bremer JJ (1992): The internal representation of arithmetical word problem sentences: frontal and posterior‐injured patients compared. Brain Cogn 20: 245–263. [DOI] [PubMed] [Google Scholar]
- Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R (1995): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Map 2: 189–210. [Google Scholar]
- Glover GH, Lai S (1998): Self‐navigated spiral fMRI: interleaved versus single‐shot. Magn Reson Med 39: 361–368. [DOI] [PubMed] [Google Scholar]
- Harmony T, Fernandez T, Silva J, Bosch J, Valdes P, Fernandez‐Bouzas A, Galan L, Aubert E, Rodriguez D (1999): Do specific EEG frequencies indicate different processes during mental calculation? Neurosci Lett 266: 25–28. [DOI] [PubMed] [Google Scholar]
- Hayes C, Mathias C (1996): Improved brain coil for fMRI and high resolution imaging. Paper presented at the ISMRM 4th annual meeting, New York.
- Holmes AP, Friston KJ (1998): Generalizability, random effects, and population inference. Neuroimage 7: S754. [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter‐Lorenz PA (1998): Inhibition in verbal working memory revealed by brain activation. Proc Natl Acad Sci USA 95: 8410–8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn HJ, Whitaker HA (1991): Acalculia: an historical review of localization. Brain Cogn 17: 102–115. [DOI] [PubMed] [Google Scholar]
- Kazui H, Kitagaki H, Mori E (2000): Cortical activation during retrieval of arithmetical facts and actual calculation: a functional magnetic resonance imaging study [In Process Citation]. Psychiatry Clin Neurosci 54: 479–485. [DOI] [PubMed] [Google Scholar]
- Krueger LE, Hallford EW (1984): Why 2 + 2 = 5 looks so wrong: on the odd–even rule in sum verification. Mem Cognit 12: 171–180. [DOI] [PubMed] [Google Scholar]
- Kruggel F, von Cramon DY (1999): Temporal properties of the hemodynamic response in functional MRI. Hum Brain Mapp 8: 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Goldstein FC, Speirs PA (1993): Acalculia In: Heilmann KM, Valenstein E. (eds.). Clinical neuropsychology. New York: Oxford University Press; p 91–122. [Google Scholar]
- Lucchelli F, De Renzi E (1993): Primary dyscalculia after a medial frontal lesion of the left hemisphere. J Neurol Neurosurg Psychiatry 56: 304–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria AR (1966): The higher cortical functions in man. New York: Basic Books. [Google Scholar]
- MacDonald AW III, Cohen JD, Stenger VA, Carter CS (2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- Mai JK, Assheur J, Paxinos G (1997): Atlas of the human brain. London: Academic Press. [Google Scholar]
- Menon V, Adleman N, White CD, Glover GH, Reiss AL (2001): Error‐related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp 12: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Ford JM, Lim KO, Glover GH, Pfefferbaum A (1997a): Combined event‐related fMRI and EEG evidence for temporal‐parietal cortex activation during target detection. Neuroreport 8: 3029–3037. [DOI] [PubMed] [Google Scholar]
- Menon V, Lim KO, Anderson JH, Pfefferbaum A (1997b): A head coil bite bar for reducing movement related artifacts during functional MRI scanning. Behav Res Meth Inst Comp 29: 589–594. [Google Scholar]
- Menon V, Rivera SM, White CD, Eliez S, Glover GH, Reiss AL (2000a): Functional optimization of arithmetic processing in perfect performers. Cogn Brain Res 9: 343–345. [DOI] [PubMed] [Google Scholar]
- Menon V, Rivera SM, White CD, Glover GH, Reiss AL (2000b): Dissociating prefrontal and parietal cortex activation during arithmetic processing. Neuroimage 12: 357–365. [DOI] [PubMed] [Google Scholar]
- Niedeggen M, Rosler F (1999): N400 effects reflect activation spread during retrieval of arithmetic facts. Psychol Sci 10: 271–276. [Google Scholar]
- Niedeggen M, Rosler F, Jost K (1999): Processing of incongruous mental calculation problems: evidence for an arithmetic N400 effect. Psychophysiology 36: 307–324. [DOI] [PubMed] [Google Scholar]
- Owen AM (1997): The functional organization of working memory processes within human lateral frontal cortex: the contribution of functional neuroimaging. Eur J Neurosci 9: 1329–1339. [DOI] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ (1997): Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage 5: 83–96. [DOI] [PubMed] [Google Scholar]
- Rickard TC, Romero SG, Basso G, Wharton C, Flitman S, Grafman J (2000): The calculating brain: an fMRI study. Neuropsychologia 38: 325–335. [DOI] [PubMed] [Google Scholar]
- Rivera SM, Menon V, White CD, Glaser B, Reiss AL (2000): Functional brain activation during arithmetic processing in females with Fragile X syndrome. J Cognit Neurosci (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera SM, Margolin L, Menon V, Reiss AL (2001): A developmental fMRI study of arithmetic processing. Monogr Soc Res Child Dev (in press). [Google Scholar]
- Rosen BR, Buckner RL, Dale AM (1998): Event‐related functional MRI: past, present, and future. Proc Natl Acad Sci USA 95: 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE (2000): The prefrontal cortex: response selection or maintenance within working memory? Science 288: 1656–1660. [DOI] [PubMed] [Google Scholar]
- Rueckert L, Lange N, Partiot A, Appollonio I, Litvan I, Le Bihan D, Grafman J (1996): Visualizing cortical activation during mental calculation with functional MRI. Neuroimage 3: 97–103. [DOI] [PubMed] [Google Scholar]
- Takayama Y, Sugishita M, Akiguchi I, Kimura J (1994): Isolated acalculia due to left parietal lesion. Arch Neurol 51: 286–291. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain: 3‐dimensional proportional system: an approach to cerebral imaging (Rayport M, translator). New York: Thieme Medical Publishers. [Google Scholar]
- Wechsler D (1997): Manual for Wechsler adult intelligence scale‐III (WAIS‐III). San Antonio: The Psychological Corporation; and Harcourt Brace Jovanovich, Inc. [Google Scholar]
- Worsley KJ, Friston KJ (1995): Analysis of fMRI time‐series revisited—again. Neuroimage 2: 173–181. [DOI] [PubMed] [Google Scholar]
- Zbrodoff NJ, Logan GD (2000): When it hurts to be misled: a Stroop‐like effect in a simple addition production task. Mem Cognit 28: 1–7. [DOI] [PubMed] [Google Scholar]


