Abstract
Acupuncture, an ancient therapeutic technique, is emerging as an important modality of complementary medicine in the United States. The use and efficacy of acupuncture treatment are not yet widely accepted in Western scientific and medical communities. Demonstration of regionally specific, quantifiable acupuncture effects on relevant structures of the human brain would facilitate acceptance and integration of this therapeutic modality into the practice of modern medicine. Research with animal models of acupuncture indicates that many of the beneficial effects may be mediated at the subcortical level in the brain. We used functional magnetic resonance imaging (fMRI) to investigate the effects of acupuncture in normal subjects and to provide a foundation for future studies on mechanisms of acupuncture action in therapeutic interventions. Acupuncture needle manipulation was performed at Large Intestine 4 (LI 4, Hegu) on the hand in 13 subjects [Stux, 1997]. Needle manipulation on either hand produced prominent decreases of fMRI signals in the nucleus accumbens, amygdala, hippocampus, parahippocampus, hypothalamus, ventral tegmental area, anterior cingulate gyrus (BA 24), caudate, putamen, temporal pole, and insula in all 11 subjects who experienced acupuncture sensation. In marked contrast, signal increases were observed primarily in the somatosensory cortex. The two subjects who experienced pain instead of acupuncture sensation exhibited signal increases instead of decreases in the anterior cingulate gyrus (BA 24), caudate, putamen, anterior thalamus, and posterior insula. Superficial tactile stimulation to the same area elicited signal increases in the somatosensory cortex as expected, but no signal decreases in the deep structures. These preliminary results suggest that acupuncture needle manipulation modulates the activity of the limbic system and subcortical structures. We hypothesize that modulation of subcortical structures may be an important mechanism by which acupuncture exerts its complex multisystem effects. Hum Brain Mapp 9:13–25, 2000. © 2000 Wiley‐Liss, Inc.
Keywords: neuroimaging, complementary medicine, pain, acupuncture
INTRODUCTION
Acupuncture, a traditional Chinese healing technique that can be traced back at least 2,500 years, is gaining popularity as an alternative and complementary therapeutic intervention in the Western world [Diehl et al., 1997; Eisenberg et al., 1998, 1993]. Acupuncture treatments for postoperative and chemotherapy‐induced nausea and vomiting and for postoperative dental pain are promising, and acupuncture can be a beneficial adjunct or alternative treatment for drug addiction, stroke rehabilitation, asthma, and chronic pain [NIH, 1998]. Further research is necessary to clarify the role of acupuncture for various health conditions and to elucidate the mechanisms by which acupuncture achieves its therapeutic effects. The purpose of this study is to obtain basic information on the effects of acupuncture stimulation in normal, healthy subjects to provide a foundation for future studies on disease states.
Clinical studies of adjunct acupuncture during surgery have shown that this treatment stabilizes vital functions, such as supporting blood pressure and heart rate, reducing emesis and enhancing immune function, that contribute to a faster postoperative recovery [Al‐Sadi et al., 1997; Bensoussan, 1991; Cao et al., 1997; Cheng et al., 1997; Du et al., 1998; Dundee et al., 1989]. Animal and clinical data indicate that although the therapeutic effects of peripherally applied acupuncture stimulation are mediated through multiple physiological systems, the initiation of these effects requires coordinate activation of multiple regions of the central nervous system [Han and Terenius, 1982; Nathan, 1978; Takagi, 1982]. For example, the analgesic effects of acupuncture are widely believed to be mediated through descending inhibitory pathways localized to the brainstem [Han and Terenius, 1982; Liu and Zhu, 1986; Melzack, 1989; Stux, 1997]. Moreover, it has been noted that elevation of pain tolerance threshold by acupuncture is even more crucial than elevation of pain perception threshold for efficacy of surgical analgesia [Cao et al., 1983]. This implies that the processing of affective behavior through limbic brain regions above the brainstem is important for the therapeutic efficacy of acupuncture.
We hypothesized that acupuncture needle manipulation would have profound effects on the activity of neurons in the brainstem, subcortical gray matter areas, and limbic brain regions. To test this hypothesis we employed functional magnetic resonance imaging (fMRI) during acupuncture at LI 4, an acupuncture point in the first dorsal interosseous space of the hand [Hui et al., 1997, 1998]. This noninvasive neuroimaging technique was chosen because it can detect the rapid changes in neural activity of discrete brain regions [Bandettini et al., 1992; Kwong et al., 1992; Ogawa et al., 1992] postulated to subserve acupuncture effects in human subjects. The acupuncture point LI 4 was selected because it is the most frequently used acupuncture point in Chinese acupuncture, especially for analgesia and sedation [Stux, 1997]. It is also used most widely for diverse disorders such as stress, agitation, depression, emesis, poststroke paralysis, facial palsy, epilepsy, and the common cold [Bensoussan, 1991; Edzard et al., 1998; Huang, 1996; Konefal et al., 1995; Naeser et al., 1992, 1994; Stux, 1997]. Superficial tactile stimulation was performed over the LI 4 region for comparison with acupuncture needle manipulation. Here, we report the effects of acupuncture needle manipulation on fMRI activity in the limbic system, subcortical structures, and somatosensory cortices.
MATERIALS AND METHODS
Subjects
The study was performed with informed consent on 13 right‐handed normal, healthy volunteer adults: five male and eight female, six Caucasian and seven Asiatic, ages 27–52. None had a history of psychiatric or neurological disorders or head trauma with loss of consciousness, or intake of tranquilizing drugs in the last 3 days. Four were naïve to acupuncture, six had some knowledge of acupuncture by way of cultural exposure or learning but had never received treatment, and three had received acupuncture therapy in the past for headache or back pain. No subject was in pain or distress at the time of the study. All subjects were briefed about the range of possible acupuncture sensations they might experience during needle manipulation before entering the magnet. There were no differences in the degree of acupuncture sensation experienced or in the fMRI results obtained due to differences in past acupuncture experience; thus the data were combined for analysis. The study was approved by the MGH Subcommittee on Human Studies.
Experimental protocol
Each subject was brought to the MGH NMR Center. The subject was settled into the scanner and instructed to close his or her eyes and relax throughout the imaging session. First, a scan was performed during intermittent tactile stimulation overlying the acupuncture point to be manipulated in the subsequent scan. Then, a scan was performed during intermittent acupuncture needle manipulation on the same hand. Three subjects remained in the scanner for a third scan during acupuncture needle manipulation on the contralateral hand.
Each subject received superficial tactile stimulation to the first dorsal interosseous space at the acupuncture site for comparison with the effects of acupuncture needle manipulation. Tactile stimulation consisted of gently tapping the skin surface with the bent tip of a flexible wire at the rate of 120 times per min. Four subjects received tactile stimulation using the same timing paradigm as used for delivering acupuncture needle manipulation, described below (Fig. 1). Seven other subjects received tactile stimulation following timing paradigms already in use at the MGH‐NMR Center for mapping studies of somatosensory pathways [Moore et al., 1996]. Of these seven subjects, five received 30 sec of stimulation repeated four times with 30 sec rest intervals. Two subjects received 20 sec of stimulation repeated eight times separated by 20 sec. After 15 min rest, scanning was resumed and acupuncture needle manipulation was delivered to LI 4 on one hand (Fig. 1). In the three subjects who received acupuncture needle manipulation to both hands, the left hand was studied before the right, separated by an ∼15 min rest interval. The results of the superficial tactile stimulation were qualitatively similar regardless of which timing paradigm was used, and they are presented together.
Figure 1.
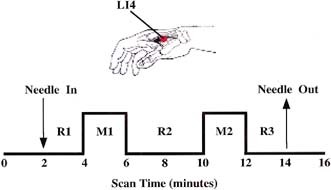
Experimental paradigm. Acupuncture needle manipulation was performed at LI 4 (arrow pointing to red dot) in the first interosseous muscle (gray shading). Scanning began 2 min prior to needle insertion. The needle was left at rest for 2 min before manipulation. Two epochs of needle manipulation (M1, M2) each lasting 2 min, were administered separated by a rest interval of 4 min. The needle was removed 2 min after M2. Scanning was continued for an additional 2 min. For statistical analyses, the mean signal intensity of the three periods of needle at rest (R1, R2, R3) served as the baseline for changes in signal intensity during needling. For the control experiments tactile stimulation was delivered to the skin surface overlying the acupuncture point with the tip of a flexible wire during M1 and M2. Total scanning time was 16 min.
Acupuncture was performed at acupuncture point LI 4 either on the left hand only (n = 6) or on the right hand only (n = 4), or sequentially on both hands (n = 3 with two sets of data from each subject). Of the 16 scans acquired during acupuncture, 12 sets were used to generate group average maps, five for the left hand and seven for the right hand. One scan could not be used for the group averaging due to inadvertent shortening of the rest period between epochs of needle manipulation, although the results were entirely consistent with the individuals included in the group average. Another two scans are discussed separately because the subjects experienced pain instead of the typical acupuncture sensation (deqi) during needle manipulation. One scan was discarded due to an error in the acupuncture stimulation parameters.
Intermittent acupuncture stimulation was delivered using a sterile disposable 38 gauge stainless steel acupuncture needle 0.18 mm in diameter (Fig. 1). The needle was inserted perpendicularly to the skin surface to a depth of ∼1.0 cm and allowed to stay in place for 2 min. Stimulation was then delivered by a balanced “tonifying and reducing” technique. The needle was rotated manually clockwise and counterclockwise for 2 min at a rate of 120 times per min, followed by 4 min of needle at rest and another 2 min of needle manipulation. The needle then remained in place for 2 min before removal. The procedure was performed by the same experienced and licensed acupuncturist (JL) on all subjects. Subjects were questioned as to the degree of deqi or pain that they experienced [Stux, 1997]. The goal was to generate acupuncture sensation with little or no pain.
Imaging
Scanning was performed with a 3‐axis gradient head coil in 1.5 Tesla GE Signa MRI System equipped for echo planar imaging [Kwong et al., 1992]. The head was cushioned with foam rubber pads to prevent motion. Ten coronal slices, each 6.5 mm thick with 0.5 mm gap, were placed parallel to the postcentral gyrus at 110 ± 5° to the AC‐PC line to encompass the major regions of interest. Thus, fMRI data was acquired for approximately the middle one‐third of the brain. High resolution structural maps were obtained by T1‐weighted echo‐planar recovery sequence for preliminary statistical mapping. A sagittal localizer scan (conventional T1‐weighted spoiled gradient‐echo sequence, 60 slices, 3 mm thick) was acquired for use as the structural scan for Talairach transformation [Talairach and Tournoux, 1988]. Functional MRI images were acquired by gradient echo T∗︁2‐weighted sequence with an in‐plane resolution of 3.1 mm, TE 50 msec and TR 4.8 sec. For acupuncture experiments, scanning began 2 min prior to needle insertion and continued for 2 min after its removal (Fig. 1). The experiments using a similar paradigm for acupuncture and tactile stimulation acquired 200 images in 16 min. The experiments employing unmatched paradigms for tactile stimulation used a shorter TR of 2.5 seconds; other imaging parameters were unchanged. A total of 110 images were acquired over 4.5 min for the 4 × 30 sec stimulation paradigm and 96 images over 4 min for the 6 × 20 sec stimulation paradigm.
Data analysis
Echo‐planar data were motion‐corrected using an algorithm adapted from Woods [Jiang et al., 1995; Woods et al., 1992]. The fMRI signal intensity during needle manipulation (50 time points) was compared with that of needle at rest (100 time points). The baseline was derived from the mean signal intensity before, between, and after the needle manipulations. The structural and functional scans were transformed into Talairach space and re‐sliced into 57 coronal slices with isotropic dimensions (x, y, z = 3.1 mm). Functional scans were fit to the structural scans by translation of exterior contours to correct for possible movement between acquisition of functional and structural images. The Talairach‐transformed data were normalized and then averaged across the subjects in each experimental group. Kolmogorov‐Smirnov (KS) statistical images using a 0.7 pixel Gaussian filter for smoothing were constructed from individual and averaged data [Purdon and Weisskoff, 1998]. We hypothesized a priori that both tactile stimulation and acupuncture manipulation would activate primary and secondary somatosensory cortex; therefore, we used a Bonferroni corrected threshold of P ≤ 10−3 for signal increases in these regions. Since we did not have a priori hypotheses for particular regions within the deep gray and limbic structures, the rest of the analysis was performed using a Bonferroni corrected threshold of P ≤ 10−5 for signal changes and a cluster constraint of a minimum of four adjacent voxels across two slices. Time‐course graphs were constructed for the putative activations identified on KS statistical maps to confirm that the change occurred during stimulation.
Neuroanatomical analysis
The anatomical localization of the functional data was determined both by Talairach co‐ordinates and inspection of individual maps by a neuroantomist (NM). Localization of the nucleus accumbens, amygdala, hippocampus, parahippocampus, ventral tegmentum, thalamus, cingulate gyrus, caudate putamen, and insula were defined according to the conventional methods of the MGH‐NMR Center for Morphometric Analysis [Caviness et al., 1996; Filipek et al., 1994; Rademacher et al., 1992; see also Breiter et al., 1997 for application of this method]. The SI was defined as the postcentral gyrus and SII as the parietal operculum.
RESULTS
Subjective effects
Subjects were questioned as to the type and intensity of sensation they experienced during the acupuncture scans. Deqi is a unique sensation of numbness, tingling, fullness, and dull ache that develops at the site of acupuncture and may spread some distance from the acupuncture point during needle manipulation [Stux, 1997]. The deqi sensation was experienced to some degree during needle manipulation by 11 of the 13 subjects in this study. In one person, the deqi sensation was felt only around the acupuncture point; in five others, the deqi sensation spread within the hand and in another five subjects the deqi sensation radiated up the arm. Despite efforts to avoid pain, two subjects experienced pain during needle manipulation instead of the characteristic acupuncture sensation; their data are briefly discussed in comparison with data from subjects who experienced deqi (see later section on pain during acupuncture needle manipulation).
Somatosensory cortex signal changes
Both acupuncture and tactile stimulation elicited signal increases in the somatosensory cortices (SSC). Images from a representative subject are shown in Figure 2. The increase in fMRI signal intensity in the secondary somatosensory cortex (SII) at the superior bank of the lateral fissure extended deep into the lateral sulcus. Compared to SII, signal increases in the primary somatosensory cortex (SI) on the postcentral gyrus were smaller and more variable in topography among the cohort for both acupuncture and tactile stimulation.
Figure 2.
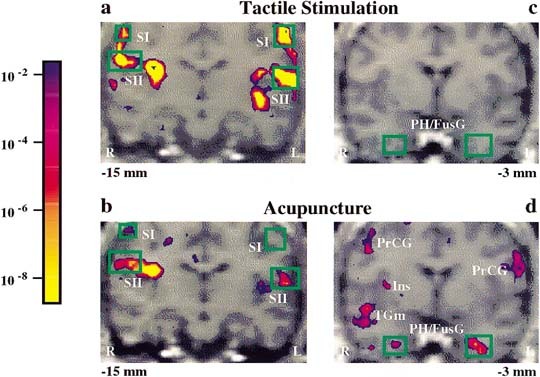
Bilateral fMRI signal increases in somatosensory cortices and signal decreases in deep structures: acupuncture needle manipulation of the left LI 4 versus tactile stimulation in a single subject. Pseudocolor KS statistical maps of signal increases (left column, a, b) and signal decreases (right column, c, d) overlaid on high‐resolution scans in gray scale at the indicated slice plane relative to the anterior commissure. Both tactile stimulation and acupuncture needle manipulation elicited signal increases in the primary and secondary somatosensory cortices (SI, SII), but the changes were more marked during tactile stimulation (a) than during acupuncture (b). Acupuncture needle manipulation elicited signal decreases in the parahippocampus/fusiform gyrus (PH/FusG) and insula (Ins) (d), whereas tactile stimulation did not (c). Also shown are acupuncture needle manipulation associated signal decreases in the middle temporal gyrus (TGm) and precentral gyrus (PrCG). Color bar shows significance, same color if signal increased or decreased.
In the group averaged data, bilateral signal increases (P ≤ 10−3) in SII were seen with acupuncture needle manipulation on either hand. SI increase was detected only in the contralateral hemisphere during acupuncture on the right hand. However, inspection of scans from individual subjects showed signal increases in SI in four of five acupuncture experiments performed on the left hand (2 bilateral, 1 contralateral, and 1 ipsilateral), and in six of seven acupuncture experiments performed on the right hand (3 bilateral, 2 contralateral, 1 ipsilateral). We attribute the discrepancy between group average and individual analysis regarding SI results to the interindividual variability in topography and the smaller size of the activated regions in the postcentral gyrus.
Acupuncture and tactile stimulation were delivered in exactly the same temporal sequence to the left hand in three subjects (Table I; see also Fig. 3). This quantitative comparison illustrates that the magnitude of fMRI activation in the primary afferent pathway by acupuncture needle manipulation is modest even compared to innocuous tactile stimulation delivered for a comparable period of time. Regions of interest (ROIs) representing SI and SII were used to compare signal increases during acupuncture and tactile stimulation. The volume of the four ROIs was determined by selecting all voxels activated by tactile stimulation above a threshold of P ≤ 10−5 for the right and left sides of SI and SII. The averaged group data are presented in Table I and Figure 3. Table I shows that the P values of the maximal voxel in SI and SII of both sides were more significant for tactile stimulation than acupuncture needle manipulation. Moreover, the magnitude of change was greater during tactile stimulation than during acupuncture in SI bilaterally (Student's t‐test comparing the two conditions, right side: P < 0.001 and left side: P < 0.0001) and in SII on the left side (P < 0.05). The extent of brain tissue showing signal increases was greater during tactile stimulation than acupuncture (Figs. 2 and 3, Table I). Qualitatively, the same result was found when an ROI determined by all voxels significantly activated during acupuncture was used for the percent change analysis.
Table I.
Signal increases in somatosensory cortex during acupuncture vs. tactile stimulation with identical paradigm (n = 3)
| Talairach coordinates | Activated volume (cc) | Tactile stimulation skin over left LI 4 area | Acupuncture left LI 4 | ||||
|---|---|---|---|---|---|---|---|
| R‐L | A‐P | S‐I | Signal change, % mean ± SD | P value max voxel | Signal change, % mean ± SD | P value max voxel | |
| Primary somatosensory cortex | |||||||
| 56 | −15 | 50 | 0.27 | 0.18 ± 0.12** | 2.7 × 10−6 | −0.03 ± 0.08 | 3.0 × 10−2 |
| −59 | −18 | 43 | 1.12 | 0.39 ± 0.15*** | 7.4 × 10−16 | 0.02 ± 0.12 | 8.9 × 10−1 |
| Secondary somatosensory cortex | |||||||
| 59 | −21 | 21 | 0.99 | 0.34 ± 0.14 | 2.8 × 10−12 | 0.19 ± 0.10 | 1.5 × 10−6 |
| −50 | −18 | 18 | 2.30 | 0.32 ± 0.14* | 3.8 × 10−17 | 0.11 ± 0.12 | 9.0 × 10−4 |
P < 0.05
P < 0.001
P < 0.0001.
Figure 3.
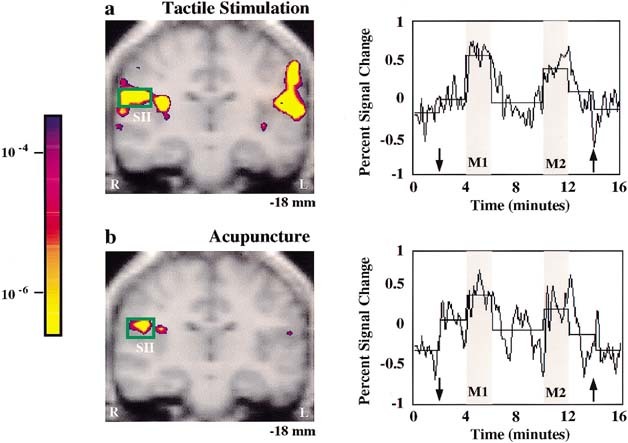
Signal increases in SII: tactile stimulation versus acupuncture needle manipulation at left LI 4 in the same subjects (n = 3). Group averaged data is presented in pseudocolor KS‐statistical maps of signal increases overlaid on gray scale high resolution scan with the time course of normalized signal intensity from the activated voxels as indicated by the green box. Note that SII signal increases occurred during both treatments, but the regional extent of brain activation was greater and the statistical significance more robust during tactile stimulation (a) than during acupuncture (b) see Table I. The time course of signal change correlated with the experimental paradigm. The horizontal line of each epoch represents the average signal intensity of that epoch.
Acupuncture associated signal decreases in deep structures
Multiple regions of signal decrease were observed in limbic, paralimbic, and subcortical gray structures during acupuncture needle manipulation of the right or left hand at LI 4, but not during tactile stimulation (Figs. 2 and 4; Table II). Table II reports the Talairach coordinates and P value associated with the maximally significant voxel for regions of signal decrease meeting a threshold criteria of P ≤ 10−5 in group averaged data. These regions included the nucleus accumbens, amygdala, hippocampus, parahippocampus, hypothalamus, ventral tegmental area, anterior cingulate (BA 24), caudate nucleus, putamen, anterior insula, and the temporal pole. Additionally, we observed signal decreases with P ≤ 10−4, but above our conservative threshold cutoff of P ≤ 10−5 in the basal forebrain, orbito‐frontal cortex, and posterior thalamus. The statistical maps and the time courses of signal change from the group averaged scans for several representative structures, including putamen, nucleus accumbens, amygdala, and hippocampus, are presented in Figure 4.
Figure 4.
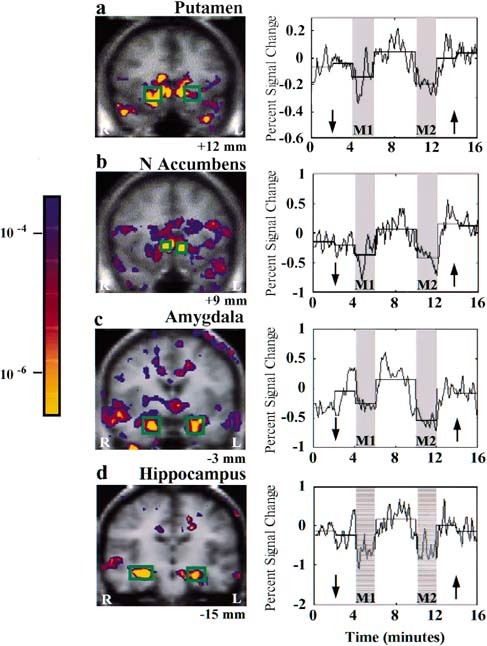
Signal decreases in deep structures during acupuncture needle manipulation: group averaged data. Left LI 4 stimulation (n = 5): putamen (a), nucleus accumbens (b). Right LI 4 stimulation (n = 7): amygdala (c), hippocampus (d). The onset and recovery of signal changes showed good temporal correlation with the experimental paradigm. There was no evidence of adaptation, but rather a suggestion of enhanced effect during M2 for the amygdala (c) and the hippocampus (d).
Table II.
Signal decreases in limbic and subcortical gray structures during acupuncture at LI 4
| Anatomic region | Left LI 4 (n = 5) | Right LI 4 (n = 7) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Talairach cor | P value KS Stat. | Signal change % (max voxel) | Proportion of individuals* | Talairach cor | P value KS Stat. | Signal change % (max voxel) | Proportion of indivduals | |||||
| R‐L | A‐P | S‐I | R‐L | A‐P | S‐I | |||||||
| N accumbens/ | 3 | 9 | −3 | 7.2 × 10−9 | −0.46 | 4/6 | 5/7 | |||||
| septi | −9 | 9 | 0 | 3.4 × 10−11 | −0.43 | 5/6 | −12 | 9 | 3 | 5 × 10−6 | −0.23 | 6/7 |
| Amygdala | 18 | −6 | −12 | 8.9 × 10−6 | −0.24 | 4/6 | 15 | −6 | −9 | 7.6 × 10−11 | −0.56 | 5/7 |
| −15 | −6 | −21 | 2.7 × 10−6 | −0.45 | 4/6 | −28 | −3 | −15 | 1.5 × 10−11 | −0.66 | 7/7 | |
| Hippocampus | 34 | −15 | −15 | 2.2 × 10−7 | −0.28 | 5/6 | 25 | −15 | 12 | 3.4 × 10−11 | −0.30 | 7/7 |
| −25 | −15 | −15 | 2.2 × 10−7 | −0.26 | 4/6 | −21 | −15 | −12 | 1.5 × 10−8 | −0.47 | 6/7 | |
| Parahippocampus | 25 | −21 | −15 | 2.7 × 10−5 | −0.18 | 5/6 | 31 | −12 | −25 | 5.9 × 10−8 | −0.32 | 7/7 |
| −28 | −21 | −21 | 7.9 × 10−5 | −0.2 | 5/6 | −28 | −9 | −25 | 1.5 × 10−6 | −0.35 | 6/7 | |
| Hypothalamus | 6 | 0 | −3 | −7.9 × 10−5 | −0.27 | 3/6 | 6 | −9 | −6 | −9.0 × 10−4 | −0.17 | 4/7 |
| −9 | 0 | −3 | −2.2 × 10−7 | −0.32 | 4/6 | −6 | −6 | −3 | −7.2 × 10−9 | −0.45 | 4/7 | |
| Ventral tegmental area | 12 | −21 | 0 | −4.3 × 10−7 | −0.17 | 3/6 | 9 | −18 | −12 | −1.2 × 10−7 | −0.22 | 4/7 |
| −9 | −12 | −3 | −7.2 × 10−9 | −0.24 | 5/7 | |||||||
| Ant. cingulate G | 6 | 3 | 34 | 7.2 × 10−9 | −0.29 | 5/6 | 12 | 0 | 37 | 2.7 × 10−6 | −0.18 | 4/7 |
| −3 | 3 | 37 | 2.7 × 10−5 | −0.2 | 5/6 | −6 | 0 | 31 | 3.5 × 10−9 | −0.15 | 5/7 | |
| Caudate N | 9 | 12 | 3 | 1.7 × 10−12 | −0.33 | 5/6 | 9 | 15 | 6 | 8.9 × 10−6 | −0.24 | 4/7 |
| −15 | 15 | 3 | 3.5 × 10−10 | −0.32 | 5/6 | −21 | 18 | 0 | 1.7 × 10−9 | −0.25 | 4/7 | |
| Putamen | 15 | 12 | −3 | 3.0 × 10−8 | −0.34 | 4/6 | 15 | 15 | 3 | 1.2 × 10−7 | −0.31 | 4/7 |
| −15 | 12 | 0 | 2.2 × 10−7 | −0.30 | 4/6 | |||||||
| Ant. insula | 31 | 18 | 0 | 3.5 × 10−9 | −0.25 | 4/6 | 28 | 15 | 6 | 5.0 × 10−6 | −0.19 | 4/7 |
| −31 | 12 | 3 | 4.3 × 10−7 | −0.17 | 4/6 | −25 | 18 | −3 | 1.2 × 10−7 | −0.21 | 4/7 | |
| Temporal pole | 40 | 15 | −12 | 3.5 × 10−9 | −0.32 | 5/6 | 50 | 12 | −21 | 3.6 × 10−10 | −0.48 | 5/7 |
| −50 | 6 | −18 | 7.6 × 10−11 | −0.26 | 4/6 | −34 | 15 | −21 | 3.5 × 10−9 | −0.62 | 6/7 | |
Subject with a shorter interval between needling could not be included in the group average, but was included in the individual data analysis.
Acupuncture needle manipulation related signal decreases were found bilaterally in the majority of structures regardless of which hand was stimulated. The bilateral regional activation was not necessarily equal on the left and right sides within a given structure. For example, the subject shown in Figure 2d had a bilateral signal decrease in response to acupuncture needle manipulation of left LI 4 in the parahippocampus/fusiform gyrus (PH/FusG) that was greater on the right side than left. In some cases the activation in a region was of comparable magnitude on both sides, but was not symmetrical with respect to the anterior to posterior localization. For example, in the group averaged data from the five subjects receiving acupuncture needle stimulation at the left LI 4, peak activation in the putamen was 12 mm anterior to the AC on the right (Fig. 4a) and 15 mm anterior to the AC on the left (not shown). There was no clear laterality pattern that could be attributed to the side of acupuncture needle manipulation (Table II). In the averaged scans, signal decreases of 0.15–0.66% were found in the nucleus accumbens, amygdala, hippocampus, parahippocampus, hypothalamus, ventral tegmental area, anterior cingulate gyrus (BA 24), caudate, putamen, anterior insula, and temporal pole during acupuncture needle manipulation (Fig. 4a–d and Table II).
The data from each individual were examined to ensure that the signal decreases noted in the group averaged data were representative of the majority of the cohort. The data from the subject in whom the rest period between needle manipulations was inadvertently shortened were included in determining the proportion of individuals who showed signal decreases in response to acupuncture (Table II). The individual data analysis strongly supports the group average results in the nucleus accumbens, amygdala, hippocampus, parahippocampus, and temporal pole with 10 or more of 13 studies demonstrating significant signal decreases. The hypothalamus, ventral tegmental area, anterior cingulate gyrus (BA 24), caudate, putamen, and anterior insula also reflected the majority response with signal decreases noted in 7–10 of the 13 studies. Due to the limits of the scanning volume, we could not assess the effects of acupuncture needle manipulation on the full extent of the cingulate gyrus. The cingulate could be reliably assessed only from the level of the AC to the level of the posterior commissure. The group averaged data of the nucleus accumbens during acupuncture needle manipulation on the right showed only a contralateral response; however, the data from individuals indicated that 5 of 7 studies had significant bilateral signal decreases. The discrepancy could be due to individual variability in signal change topography within the nucleus accumbens.
There was excellent correlation between the timing of the onset and offset of needle manipulation and the onset and offset of decreases in signal intensity in each of the brain regions listed in Table II for both the left and right hand (Fig. 4). The decrease in signal intensity occurred almost as soon as needle manipulation started and began to recover when needle manipulation stopped. The change in signal intensity during M2 did not show any adaptation, but rather was suggestive of an enhanced response in the amygdala, hippocampus and several other regions (Fig. 4). The mere presence of the needle did not cause the marked signal decreases in these deep structures; rather, we noted transient signal increases at the time of needle insertion in SSC and in some individuals in the amygdala and cingulate gyrus.
Pain during acupuncture needle manipulation
In the two subjects who experienced pain throughout acupuncture needle manipulation instead of deqi, marked differences in fMRI data were noted. Both of these subjects received manipulation of the left LI 4 acupuncture point. In contrast to the signal decreases observed in the other subjects described above, the acupuncture scan of these subjects showed a predominant pattern of signal increases in a number of regions including anterior cingulate gyrus (BA 24), putamen, anterior thalamus, and insula during needling. This network of activation is comparable to that reported in numerous functional neuroimaging studies of pain in human subjects [Becerra et al., 1999; Casey et al., 1994; Coghill et al., 1994; Craig et al., 1996; Davis et al., 1997; Jones et al., 1991; Talbot et al., 1991]. These two subjects showed signal increases in SI and SII in a manner similar to the rest of the cohort and similar to the results reported in the pain imaging studies.
DISCUSSION
This study demonstrates the consistent modulation of multiple bilateral cortical and subcortical limbic and paralimbic structures during acupuncture needling at LI 4 on either the right or left hand. The primary action was to decrease signal intensity in the nucleus accumbens, amygdala, hippocampus, parahippocampus, hypothalamus, ventral tegmental area, anterior cingulate gyrus (BA 24), caudate, putamen, temporal pole, and insula. In sharp contrast, signal increases were restricted to the SSC. The signal decreases were elicited by acupuncture needle stimulation in the majority of the cohort who experienced deqi and not in the two subjects who experienced pain during needle manipulation. Superficial tactile stimulation to the area elicited signal increases in the SSC as expected, but no signal decreases in the deep structures.
There are a number of limitations to this study. Acupuncture needle manipulation was performed with a matched imaging paradigm on all subjects. However, multiple paradigms, both matched and unmatched to the acupuncture paradigm, were performed for tactile stimulation in order to provide validation of our initial results. The fact that we got qualitatively similar results from all the variations provides strong support for our conclusions. Future studies need to confirm the results using a uniform study design in a larger cohort. Second, it is possible that the effects of acupuncture needle manipulation on regional brain activity could be influenced by a subject's expectation of what they would experience during the scan. Changes in attention and expectation can directly influence brain activity in the anterior cingulate [Davis et al., 1997; Murtha et al., 1996], one of the regions shown to have signal decreases during acupuncture needle manipulation. In this study, subjects had a range of prior experience with acupuncture from completely naïve to having successfully completed a therapeutic course in the past. We found no evidence for differences in the data from the subjects who were naïve and those with prior experience. However, future studies would benefit from the use of cohorts with no prior acupuncture experience and a systematic method for subject preparation that would minimize the variability in subject expectancy. Third, we did not systematically assess the degree of deqi experienced by each of the subjects. Our impression was that the most profound signal decreases in these deep brain structures were seen in the scans from subjects who experienced stronger deqi with spread of the sensation beyond the acupuncture site. However, since we did not attempt to quantify the magnitude of this sensation, we were not able to perform a correlation analysis of this effect on the fMRI signal changes. Future studies would benefit from the use of validated rating scales to determine the quality and spatial extent of the acupuncture sensation.
Another limitation is the restricted brain volume imaged. The scanning parameters were selected to optimize signal detection in the central sulcus, the brainstem, and the deep gray structures. We did not determine the effects of acupuncture needle manipulation on the full extent of the cortex or the cerebellum. Cortical regions are thought to play a fairly modest role in the effects of acupuncture, e.g., acupuncture analgesia was not abolished in studies of decerebrate cats [Du and Chao, 1976; Kerr et al., 1978, and see discussion and references cited on pp. 253–255 in Gwei‐Djen and Needham, 1980]. However, we cannot rule out a role for these brain regions in mediating acupuncture effects. Furthermore, although we demonstrated the involvement of the anterior cingulate gyrus, only the portion of the cingulate caudal to the level of the AC was imaged. Future studies using whole brain imaging are necessary to determine the contribution of all brain regions to the effects of acupuncture needle manipulation.
The design of controlled experiments for acupuncture studies is fraught with difficulties, and few attempts have satisfied Western scientific standards [Richardson and Vincent, 1986; Vincent and Richardson, 1986]. One reason is the difficulty of finding a true “sham point” to use in comparison with the acupuncture point. According to traditional Chinese acupuncture, focal stimulation of a needle at relatively specific points with the evocation of the deqi sensation is important in generating therapeutic acupuncture effect. The specific location of acupuncture points along each meridian is derived from thousands of years of clinical experience. It is known that needling at points other than those indicated on acupuncture charts also can have therapeutic effects. For instance, Ah Shi and trigger points are focal tender points that are used in therapeutic acupuncture to treat chronic pain. An ideal “sham point” would be an area of skin located some distance from any known acupuncture point or trigger point. However, some of the physiological effects of acupuncture have been observed when such supposed “sham points” were stimulated [Margolin et al., 1993b]. This may be due to fact that the acupuncture “point” is actually an “area” overlying densely innervated muscle [Melzack, 1989].
We chose to use a number of controls including imaging during “needle at rest” and matched scans during tactile stimulation of the overlying area. Our data showed that neither “needle at rest” nor tactile stimulation produced the marked signal decrease in deep structures associated with needle manipulation. As can be seen from the time courses shown in Figures 2 and 4, insertion of the acupuncture needle caused at most transient signal changes (increases or decreases), never the sustained signal decreases observed during needle manipulation. The tactile stimulation paradigm used for the quantitative comparison was exactly matched to the acupuncture paradigm (2 epochs of 2 min stimulation separated by 4 min of rest and stimulus delivery 120 times per min) because the magnitude of the fMRI signal change is affected by these parameters. We tried another experimental design, needle manipulation within the subcutaneous tissue just beneath the corium (n = 2) (data not shown). This method did not yield significant signal decreases in the deep structures described for acupuncture at LI 4.
Our results indicate that insertion of the needle into the muscle and its manipulation are necessary to produce the deqi sensation and the prominent fMRI signal changes in the deep structures of the human brain. Additional evidence in support of this is provided by the results of an earlier preliminary study conducted in our center. They reported similar signal decreases in several of the limbic structures described above during acupuncture needle manipulation at acupuncture point Stomach 36 (ST 36, Zusanli) on the leg, but not during the control paradigm, pricking the skin over the ST 36 acupuncture point [Wu et al., 1997]. We did additional control scans (data not shown) in which several of the subjects were imaged for up to 8 min during needle insertion without manipulation. There were no accompanying signal decreases even during this prolonged needle insertion.
Although there are multiple interpretations for decreases in BOLD fMRI signal, we believe that the decreased signal during acupuncture needle manipulation reflects decreased cerebral blood flow (CBF) resulting from suppression of neuronal metabolic activity. The fact that we observed coordinate signal decreases, in the absence of significant signal increases, in a circuit of highly interconnected structures strongly supports this interpretation. Cognitive processes can result in decreased CBF as measured by BOLD fMRI signals [Buckner, 1998] or positron emission tomography [Shulman et al., 1997]. Further evidence to support this interpretation is provided by the results of BOLD and cerebral blood volume mapping methods during electrical stimulation to the forepaw in rats [Mandeville et al., 1998; Marota et al., 1996]. These investigators used a protocol that is similar to electroacupuncture at LI 4 in animal models with respect to the anatomical site and stimulation parameters (intensity and frequency). The demonstrated signal intensity increases in the SSC and found evidence for decreases in the striatum.
There are multiple pathways by which acupuncture stimulation could coordinately influence neuronal activity in cortical, subcortical limbic, and paralimbic structures. Acupuncture needle manipulation likely stimulates multiple peripheral sensory receptors. Activation of the SSC, as in the response to tactile stimulation of the overlying region, is most likely mediated by activation of the dorsal column medial lemniscal system. Modulation of activity in the deeper brain regions is more likely mediated by axons ascending in the spino‐thalamic, spino‐reticular, and spino‐mesencephalic tracts. Work in rodents, cats, and primates has documented that axons running in these bundles send collaterals to synapse directly on neurons in the dorsal thalamus, dorsal midbrain, medullary and pontine reticular formation, hypothalamus, amygdala, septum, and nucleus accumbens [see Willis, 1989; Willis and Westlund, 1997, and references therein]. In addition, reticular neurons are known to project both monosynaptically and polysynaptically to bilateral limbic and subcortical gray structures [Willis and Westlund, 1997]. Both direct spino‐amygdaloid and spino‐pontine‐amygdaloid pathways have been recently reported in rodents [Bernard et al., 1989, 1992; Burstein and Potrebic, 1993]. If such pathways exist in humans, they would provide relatively direct connections by which needle manipulation could influence the limbic system and other deep gray structures.
The widespread fMRI signal decreases in the limbic system are not surprising in view of the known therapeutic effects of acupuncture for disorders involving affective states such as anxiety, depression and substance abuse [Edzard et al., 1998; Margolin et al., 1993a]. Interestingly, two psychostimulant drugs, cocaine and nicotine, induced bilateral fMRI signal increases in many brain regions [Breiter et al., 1997; Stein et al., 1998], a subset of which, including the nucleus accumbens, putamen, hypothalamus, insula, and anterior cingulate gyrus, demonstrated signal decreases during acupuncture needle manipulation. The opposite effects of acupuncture needle manipulation and psychostimulant drugs on these limbic/paralimbic regions suggest that these brain regions could be involved in acupuncture treatments for drug craving and detoxification. We can further speculate that acupuncture analgesia for surgical procedures could work by decreasing neural activity in the thalamus, amygdala, and brainstem, structures that are known to modulate the conscious experience of pain [Becerra et al., 1999; Casey et al., 1994; Coghill et al., 1994; Craig et al., 1996; Davis et al., 1997; Jones et al., 1991; Talbot et al., 1991].
Our results provide a foundation for future functional brain imaging studies of the therapeutic effects of acupuncture. Many questions remain unanswered. For instance, acupuncture treatment is known to have both immediate and cumulative effects [Bensoussan, 1991; Stux, 1997]. How do these transient changes in fMRI signals that likely correspond to transient changes in regional brain activity lead to persistent health benefits? How do these results in healthy subjects generalize to patients with acupuncture responsive disorders?
Conclusions
This initial study provides evidence supporting a coordinated effect of acupuncture needle manipulation on a network of cortical and subcortical limbic and paralimbic structures in the human brain. Modulation of this neuronal network could constitute the initiating steps by which acupuncture regulates multiple physiological systems and achieves diverse therapeutic effects.
Acknowledgements
The authors thank Scott Fard and Todd Kramer for help with figure and table preparation, Sara Lazar for help with manuscript preparation, Barry Kosofsky for a critical review of manuscript and comments, and Paul Whalen, Jeffrey Bucci, I‐Wen Cheng, and Iris Chen for assistance with data analysis.
REFERENCES
- Al‐Sadi M, Newman B, Julious SA. 1997. Acupuncture in the prevention of postoperative nausea and vomiting. Anesthesia 52:658–661. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. 1992. Time course EPI during task activation. Magn Res Med 25:390–397. [DOI] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comie AR, Gonzales RG, Barsook D. 1999. Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn Reson Med 41:1044–1057. [DOI] [PubMed] [Google Scholar]
- Bensoussan A. 1991. The Vital Meridian: A Modern Exploration of Acupuncture. Melbourne: Churchill Livingstone. [Google Scholar]
- Bernard JF, Huang GF, Besson JM. 1992. Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol 68:551. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Peschanski M, Besson JM. 1989. A possible spino (trigemino)‐ponto‐amygdaloid pathway for pain. Neurosci Lett 100:83–88. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor H, Gastfriend DR, Riorden JP, Mathew RT, et al. 1997. Acute effects of cocaine on human brain activity and emotion. Neuron 19:591–611. [DOI] [PubMed] [Google Scholar]
- Buckner R. 1998. Functional anatomic study of episodic retrieval using fMRI: 1. Retrieval effort versus retrieval success. Neuroimage 7:151–162. [DOI] [PubMed] [Google Scholar]
- Burstein R, Potrebic S. 1993. Retrograde labeling of neurons in the spinal cord that project directly to the amygdala or the orbital cortex in the rat. J Comp Neurol 335:469–485. [DOI] [PubMed] [Google Scholar]
- Cao XD, Huang HN, Qu GL, Zhunag XL, Jiang CC, Wu GC. 1997. Acupuncture balanced anesthesia in China. Acupuncture Electro‐therapeutics Res Int J 22:60–61. [Google Scholar]
- Cao XD, Xu SF, Lu WX. 1983. Inhibition of the sympathetic nervous system by acupuncture. Acupuncture Electro‐therapeutics Res Int J 8:25–35. [DOI] [PubMed] [Google Scholar]
- Casey KL, Minoshima S, Berger KL, Kloeppe RA, Morrow TJ, Casey KL. 1994. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol 71:802–807. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Meyer J, Makris N, Kennedy DN. 1996. MRI‐based topographic parcellation of human neocortex: an anatomically specified method with estimate of reliability. J Cognitive Neurosci 8:566–587. [DOI] [PubMed] [Google Scholar]
- Cheng XD, WG C, HQZ Z, Cao XD. 1997. Effect of continuous electroacupuncture on induction of interleukin‐2 production of spleen lymphocytes from injured rats. Acupuncture Electrotherapeutics Res Int J 22:1–8. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH. 1994. Distributed processing of pain and vibration by the human brain. J Neurosci 14:4095–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, Reiman EM, Evans A, Bushnell MC. 1996. Functional imaging of an illusion of pain. Nature 384:258–260. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. 1997. Functional MRI of pain‐ and attention‐related activations in the human cingulate cortex. J Neurophysiol 77:3370–3380. [DOI] [PubMed] [Google Scholar]
- Diehl D, Kaplan G, Coulter I, Glik D, Hurwitz EL. 1997. Use of acupuncture by American physicians. J Alternative Complementary Med 1997:119–126. [DOI] [PubMed] [Google Scholar]
- Du HJ, Chao YF. 1976. Localization of central structures involved in descending inhibitory effect of acupuncture on viscero‐somatic reflex discharges. Scientia Sinica 19:137–148. [PubMed] [Google Scholar]
- Du LN, Wu GC, Cao XD. 1998. Modulation of orphanin FG or electroacupuncture on immune function of traumatic rats. Acupuncture Electro‐therapeutics Res Int J 23:117–124. [DOI] [PubMed] [Google Scholar]
- Dundee JW, Ghaly RG, Bill KM, Chestnut WN, Fitzpatrick KT, Lynas AG. 1989. Effect of stimulation of the P6 antiemetic point on postoperative nausea and vomiting. Br J Anaesth 63:612–618. [DOI] [PubMed] [Google Scholar]
- Edzard EE, Rand JI, Stevison C. 1998. Complementary therapies for depression. Arch Gen Psychiatry 55:1026–1032. [DOI] [PubMed] [Google Scholar]
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC. 1998. Trends in alternative medicine use in the United States, 1990–1997. JAMA 280:1569–1575. [DOI] [PubMed] [Google Scholar]
- Eisenberg DM, Kessler RC, Foster C. 1993. Unconventional medicine in the United States. N Engl J Med 328:246–252. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Verne S, Caviness J. 1994. The young adult human brain: an MRI‐based morphometric analysis. Cereb Cortex 4:344–360. [DOI] [PubMed] [Google Scholar]
- Gwei‐Djen L, Needham J. 1980. Celestial Lancets: A History and Rationale of Acupuncture and Moxa. Cambridge: Cambridge University Press. [Google Scholar]
- Han JS, Terenius L. 1982. Neurochemical basis of acupuncture analgesia. Ann Rev Pharmacol Toxicol 22:193–220. [DOI] [PubMed] [Google Scholar]
- Huang KC. 1996. Acupuncture in drug abuse, smoking, and alcoholism. Acupuncture 228–234. [Google Scholar]
- Hui KKS, Liu J, Chen AAJW, Wu MT, Rosen BR, Kwong KK. 1997. Effects of acupuncture on human limbic system and basal ganglia measured by fMRI. Neuroimage 5:S226. [Google Scholar]
- Hui KKS, Liu J, Chen AJW, Makris N, Gollub RL, Kennedy DN, Moore CI, Rosen BR, Kwong KK. 1998. Acupuncture modulates the limbic system and subcortical gray structures of the human brain—direct evidence by fMRI. Neuroimage 7:S441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A, Kennedy DN, Baker JR, Weisskoff RM, Tootell RBH, Woods RP, Benson RR, Kwong KK, Brady TJ, Rosen BR, Belliveau JW. 1995. Motion detection and correction in functional MR imaging. Hum Brain Map 3:1–12. [Google Scholar]
- Jones AK, Brown DW, Friston KJ, Qi LY, Frackowizk RS. 1991. Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc Royal Soc London‐B 244:39–44. [DOI] [PubMed] [Google Scholar]
- Kerr FWL, Rosen PR, Nijensohn DE. 1978. Acupuncture reduces trigeminal evoked response in decerebrate cats. Experiment Neurol 61:84–95. [DOI] [PubMed] [Google Scholar]
- Konefal J, Duncan R, Clemence C. 1995. Comparison of three levels of auricular acupuncture in an outpatient substance abuse treatment program. Alternative Med J 2:8–17. [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng HM, et al. 1992. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA 89:5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu B. 1986. Relationship between electroacupuncture analgesia and descending pain inhibitory mechanism of nucleus raphe magnus. Pain 24:383–396. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJA, Kosofky BZ, Keltner JR, Weissleder R, Rosen BR, Weisskoff RM. 1998. Dynamic functional imaging of relative CBV during rat fore paw stimulation. Magn Res Med 39:619–624. [DOI] [PubMed] [Google Scholar]
- Margolin A, Avants SK, Chang P, Kosten TR. 1993a. Acupuncture for the treatment of cocaine dependence in methadone‐maintained patients. Am J Addictions 2:194–201. [Google Scholar]
- Margolin A, Chang P, Avants SK, Kosten TR. 1993b. Effects of sham and real auricular needling: implications for trials of acupuncture for cocaine addiction. Am J Chinese Med 21:103–111. [DOI] [PubMed] [Google Scholar]
- Marota J, Mandeville J, Kosofsky B, Keltner J, Berke J, LaPonte L, Weissleder R, Rosen B, Weisskoff R, Hyman S. 1996. Somatosensory stimulation mapping in rat brain. Soc Neurosci 22:19. [Google Scholar]
- Melzack R. 1989. Folk medicine and the sensory modulation of pain In: Wall PD, Melzack R, (eds): Textbook of Pain. Edinburgh: Churchill Livingstone, 897–905 [Google Scholar]
- Moore CI, Gehi A, Guimareas AR, Corkin S, Rosen BR, Stern CE. 1996. Somatotopic mapping of cortical areas SI and SII using fMRI. Neuroimage 3:S333. [Google Scholar]
- Murtha S, Chertkow H, Beauregard M, Dixon R, Evans A. 1996. Anticipation causes increased blood flow to the anterior cingulate cortex. Hum Brain Mapp 4:103–112. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Alexander MP, Stiassny D, Hobbs J, Bachmer D. 1992. Real versus sham acupuncture in the treatment of paralysis in acute stroke patients. A CT scan study. Neurologic Rehabil 6:163–173. [Google Scholar]
- Naeser MA, Alexander MP, Stiassny‐Eder D, Galler V, Bachman D. 1994. Acupuncture in the treatment of paralysis in chronic and acute stroke patients—improvement correlated with specific CT scan lesion sites. Acupuncture Electro‐Therapeutics Res Int J 19:227–249. [DOI] [PubMed] [Google Scholar]
- Nathan PW. 1978. Acupuncture anaesthesia. TRNS : 21–23. [Google Scholar]
- NIH Consensus Development Panel on Acupuncture . 1998. Acupuncture. JAMA 280:1518–1524. [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. 1992. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA 89:5951–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdon PL, Weisskoff RM. 1998. Effect of temporal autocorrelation due to physiological noise and stimulus paradigm on voxel‐level false‐positive rates in fMRI. Hum Brain Mapp 6:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher J, Galaburda AM, Kennedy DN, Filipek PA, Caviness VS. 1992. Human cerebral cortex: localization, parcellation and morphometry with magnetic resonance imaging. J Cognitive Neurosci 4:352–374. [DOI] [PubMed] [Google Scholar]
- Richardson PH, Vincent PA. 1986. Acupuncture for the treatment of pain: a review of evaluative research. Pain 24:15–40. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle M, Peterson SE. 1997. Common blood flow changes across visual tasks: II. decreases in cerebral cortex. J Cognitive Neurosci 9:648–663. [DOI] [PubMed] [Google Scholar]
- Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffman RG, Hawkins M, Rao SM, Bandettini PA, Bloom AS. 1998. Nicotine‐induced limbic‐cortical activation in the human brain: a functional MRI study. Am J Psychiatry 155:1009–1015. [DOI] [PubMed] [Google Scholar]
- Stux G, Pomeranz B. 1997. Basics of Acupuncture. Berlin: Springer‐Verlag. [Google Scholar]
- Takagi H. 1982. Critical review of pain relieving procedures including acupuncture: advances in pharmacology and therapeutics II. CNS pharmacology. Neuropeptides 1:79–92. [Google Scholar]
- Talairach J, Tournoux P. 1988. Co‐planar Stereotaxic Atlas of the Human Brain. 3‐D Proportional System: An Approach to Cerebral Imaging. New York: Thieme. [Google Scholar]
- Talbot JD, Marret S, Evans AC, Meyer E, Bushnell MC, Duncan GH. 1991. Multiple representations of pain in human cerebral cortex. Science 251:1355–1358. [DOI] [PubMed] [Google Scholar]
- Vincent CA, Richardson PH. 1986. The evaluation of therapeutic acupuncture: concepts and models. Pain 24:1–13. [DOI] [PubMed] [Google Scholar]
- Willis WD. 1989. The origin and destination of pathways involved in pain transmission In: Wall PD. and Melzack R. (eds): Textbook of Pain. Edinburgh: Churchill Livingstone, 112–127. [Google Scholar]
- Willis WD, Westlund KN. 1997. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clinical Neurophysiol 14:2–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. 1992. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomog 16:620–33. [DOI] [PubMed] [Google Scholar]
- Wu MT, Xiong J, Yang PC, Hsieh JC, Tsai G, Rosen BR, Kwong KK. 1997. Acupuncture modulating the limbic brain detected by functional MR imaging. Hum Brain Mapp 5:S15. [Google Scholar]


