Abstract
The present study investigates human visual processing of simple two‐colour patterns using a delayed match to sample paradigm with positron emission tomography (PET). This study is unique in that we specifically designed the visual stimuli to be the same for both pattern and colour recognition with all patterns being abstract shapes not easily verbally coded composed of two‐colour combinations. We did this to explore those brain regions required for both colour and pattern processing and to separate those areas of activation required for one or the other. We found that both tasks activated similar occipital regions, the major difference being more extensive activation in pattern recognition. A right‐sided network that involved the inferior parietal lobule, the head of the caudate nucleus, and the pulvinar nucleus of the thalamus was common to both paradigms. Pattern recognition also activated the left temporal pole and right lateral orbital gyrus, whereas colour recognition activated the left fusiform gyrus and several right frontal regions. Hum. Brain Mapping 13:213–225, 2001. © 2001 Wiley‐Liss, Inc.
Keywords: PET, vision, visual attention, cortical colour processing, cortical pattern processing
INTRODUCTION
Over the past few decades there has been extensive research into the neuroanatomical substrates of information processing in both humans and primates particularly in vision. Visual processing, visual working memory, and recognition of colour and pattern are important but as yet incompletely understood functions of the visual system.
It is believed that the cortical processing of colour commences in the striate and extrastriate cortices with analysis of the wavelength composition of the image [Van Essen and Zeki, 1978; Howard et al., 1998; Walsh et al., 1992; Zeki and Marini, 1998]. These regions have extensive connections with V4 in primates [Zeki, 1978; Zeki and Shipp, 1989]. Area V4 performs higher level colour processing, in particular the comparison of wavelength differences with surrounding objects [Zeki and Marini, 1998; Zeki et al., 1991; Lueck et al., 1989]. Area V4 has known anatomical connections with the inferior temporal cortex [Dean, 1982]. In both humans and primates, neuroimaging studies demonstrate that colour processing extends from V4 into the inferotemporal cortex, but only in some cognitive tasks [Howard et al., 1998; Takechi et al., 1997; Zeki, 1993; Zeki and Marini, 1998]. Zeki and Marini [1998] have hypothesised that rather than V4 being the “colour centre,” there exists a multistaged cortical processing system for colour that results in the recruitment of various cortical regions stretching from V1 to the inferotemporal cortex, depending on the specific requirements of the task being undertaken. Certainly in macaques with V4 ablated, colour categorisation can still occur albeit with greater difficulty [Walsh et al., 1992]. Evidence from natural history studies of patients with circumscribed posterior cerebral infarcts demonstrates that colour discrimination can be affected independently of working memory for colour and of discrimination of colours, reinforcing the concept that there are multiple neural domains responsible for colour processing but that not all are required for any given task [Schoppig et al., 1999].
Two discrete though interconnected pathways are responsible for the processing of object information [Courtney et al., 1996; Haxby et al., 1992, 1994, 2000; Ungerleider et al., 1998; Ungerleider and Haxby, 1994; Ungerleider and Mishkin, 1982]. The dorsal stream is responsible for analysing spatial information and extends from the occipital cortex through the superior parietal regions into the dorsolateral prefrontal cortex. Information regarding form is processed in the occipitotemporal cortex extending through to the ventrolateral prefrontal cortex. The organisation of the occipitotemporal pathways has been explored predominantly using complex visual stimuli such as faces, the results of which suggested that the occipitotemporal cortex was made up of functionally discrete regions that responded to specific stimulus types [Clark et al., 1996; Courtney et al., 1996; Haxby et al., 1994, 1996; Sams et al., 1997; Sergent et al., 1992; Ungerleider et al., 1998]. More recently it has become apparent that although certain neural domains do respond preferentially to certain stimuli they still have significant activation to other stimuli, suggesting that human brain regions involved in pattern processing are recruited depending on the complexity of the stimulus and the requirements of the task being undertaken, in a similar manner to that proposed for colour processing [Haxby et al., 2000; Ishai et al., 1999; Nystrom et al., 2000].
The processing of simple two‐colour abstract patterns has received little attention to date. Those studies that have ventured into simple pattern processing have reported fewer activation areas and generally more posterior and medial fields in both occipital and temporal lobes than have studies of more complex patterns [Baker et al., 1996; Corbetta et al., 1991, 1993; Fink et al., 1997; Gulyas et al., 1994; Nobre et al., 1997].
The present study is unique in that we specifically designed the visual stimuli to be the same for both pattern and colour recognition, with all patterns being abstract shapes not easily verbally coded and composed of two‐colour combinations. The paradigm has been reported in detail previously, including psychophysical and event‐related potential data [Michie et al., 1999; Smith et al., 1998]. The paradigm enabled us to separate out the brain regions activated by simple pattern recognition from those activated by colour recognition. We hypothesised that colour processing would occur in the occipitotemporal cortices with contributions from parietal cortex, as suggested by the lesion work of Schoppig et al. [1999]. We also hypothesised that the processing of abstract patterns would also occur within the occipitotemporal cortices and have a different and more extensive distribution than colour.
METHODS
Subjects
Ten right‐handed male volunteers aged 18–35 were recruited. Subjects were not included if they were colour‐blind according to Ishihara's [1994] test or had a history of psychiatric disease, especially schizophrenia as defined by the DSM‐IV criteria (American Psychiatric Association, 1993]. Subjects all had normal or corrected‐to‐normal vision with normal colour vision as assessed by Ishihara's [1994] test. All subjects had a mean age of 25.4 (range 22–30, SD 3.13) and a mean full performance IQ of 118.0 (range 108.8–123.9, SD 4.78). Informed consent was obtained from each of the volunteers. The Royal Prince Alfred Hospital ethics committee approved the study.
Paradigm
The computerised visual attention battery (VAB) is a delayed matching to sample task that was specifically designed to separate the processing of colour and pattern information by using simple two‐colour geometric patterns that are not easily named.
Each task involved the serial presentation of a set of novel geometric patterns displayed in two‐colour combinations (Fig. 1). At the beginning of each task except the control task, an initial target stimulus was displayed for 3,000 ms and was followed by 80 stimuli, each displayed for 1,000 ms with an onset to onset interval of 3,650 ms. Each stimulus required a positive or negative response on a button press device. A 1,500‐ms response window starting at stimulus onset time was used to record reaction times. If no response was offered in this time an error was recorded. In each set there were 20 stimuli matching the target for a relevant feature that required a positive button press. These targets occurred pseudorandomly with 0–5 intervening nontarget stimuli. The remaining 60 filler stimuli required a negative button press and did not match the target for the relevant attributes. No feedback was given regarding the accuracy of responses. Incorrect responses to target or nontarget stimuli were counted as errors. All stimuli were identical in size (height 11.3 cm, width 20.2 cm) and luminance and were presented in the centre of the computer screen 50–70 cm from subjects' eyes.
Figure 1.
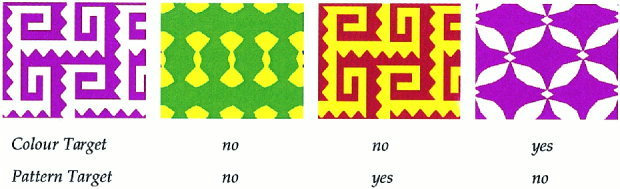
Stimulus set. An example stimulus set from the Visual Attention Battery. Each stimulus set consists of two pairs of similar patterns and a set of four two‐colour combinations. For each task, the subject had to press “yes” or “no” to indicate whether the stimulus matched the target stimulus. The VAB was completed on a 486DX Deltacom computer with a colour 4FG VGE monitor 50–70 cm from the subject's eyes. Confounding variables relating to task order were accounted for by counterbalancing task order across subjects using a Latin square.
Each stimulus set consisted of four geometric shapes presented in four two‐colour combinations. Within each set there were two pairs of stimuli. The intrapair similarity was judged greater than 50% and the interpair similarity was less than 25% in a pilot study [Smith et al., 1998]. Using a factorial manipulation of the four patterns and four colour combinations, 16 different stimuli were created for each stimulus set. There were 14 stimulus sets (i.e., 14 different versions of each task). Each overall paradigm had seven different task orders. The tasks, stimulus sets, and target orders were administered to participants using a Latin square design. Participants used different stimuli for each task and different participants used a different stimulus set for each task. These measures reduced the possibility of there being something inherent to either the pattern/colour combination or the stimulus order and ensured that stimuli were novel for each task.
There were seven different tasks completed during the paradigm (including the control task). The tasks reported in this paper are:
1. The control task (CON) had no target stimulus. Participants were required to alternatively press the positive and negative buttons whenever a stimulus appeared, irrespective of the colour or pattern of the stimulus. Participants were asked to respond as soon as possible after the presentation of each. Its aim was to allow for the control of simple motor output and noncognitive visual processing by direct subtraction of PET images.
2. The selective attention to colour (COL) task required participants to remember the colour combination of the target stimulus and to respond with a positive button press to all stimuli matching the target for colour (irrespective of the pattern) and pressing the negative button to all other stimuli.
3. The selective attention to pattern (PAT) task required participants to remember the pattern of the target stimulus and to respond with a positive button press to all stimuli matching the target for pattern (irrespective of the colour) and pressing the negative button to all other stimuli.
For each task there was a practice run of 30 stimuli completed prior to the commencement of the paradigm. The practice runs were administered in the set order of CON, COL, PAT and used a different stimulus set to the actual task. The practice runs were given to ensure that all subjects understood task instructions and had the ability to execute the requirements of all tasks.
During the pilot testing, the VAB was compared with standard neuropsychological tests of working memory and attention, including the Wisconsin Card Sorting test, the Stroop Colour‐Word test and Trails A and B. The results attested to the validity of the VAB as an assessment of specific attentional and working memory performance [Smith et al., 1998].
Data acquisition
PET scanning was performed using a Siemens‐CTI 951R ECAT scanner using the high sensitivity, septa‐out (3D) mode. Oxygen‐15 water was the radiotracer injected via an antecubital vein. To provide a constant rate of infusion, a standard hospital infusion device (IMED) was used. The 5 ml H2 15O bolus was infused over 50 sec. The individual's forearm‐brain blood transit time was determined. The target stimulus was shown immediately prior to tracer arrival in the brain. Data was acquired for 100 sec, during which the subjects matched the stimuli to the target. The task was completed prior to tracer washout to provide optimal signal to noise ratio [Cherry et al., 1993; Hurtig et al., 1994]. To prevent head movement during the experiment, all subjects were individually fitted with a thermoplastic facemask. An attenuation scan using a rotating germanium source was performed before and after the whole paradigm to allow correction for subject movement during the study. Each subject received a total of 5 mSv radiation for the entire paradigm.
Subjects completed each task once in the manner described above and were allowed to freely inspect each image with no control of visual fixation. EOG and EEG data was recorded. The resulting event related potential (ERP) data are reported elsewhere [Michie et al., 1999].
Data analysis
Analysis of the PET data was performed on a SunSparc workstation using SPM 95. Image reconstruction used the computerised algorithm of Townsend et al. [1991]. Image analysis was performed with ANALYZE [Robb et al., 1989]. All calculations and matrix manipulation were performed in MATLAB (Mathworks Inc.). Images from a single subject were realigned to that subject's first image to correct for any head movement during the scan. The scans were stereotactically scaled and linearly transformed to fit a standard model [Friston et al., 1991; Talairach and Tournoux, 1988]. Images were filtered using a Hanning filter at reconstruction (effective smoothing 8 mm at FWHM); a 10 mm (x dimension) × 10 mm (y dimension) × 12 mm (z dimension) filter at the stereotactic stage; and a Gaussian filter of 4 mm FWHM in x and y dimensions at the final stage of stereotactical comparison [Myers et al., 1996; Toga and Mazziotta, 1995]. Total brain activation acquired in each task was normalised to 50 mL/min/100 g using an ANCOVA correction [Friston et al., 1990]. MRI scans were performed separately on a GE Signa (1.5 Tesla) scanner.
The summed control scan was subtracted from each of the summed test conditions [Frackowiak et al., 1997]. Statistical analysis consisted of multiple t tests with the t statistic transformed into a normal distribution. A Bonferroni‐like adjustment was performed. Results were reported as significant if z score ≥ 3.1 with P < 0.05. To be considered significant, activation areas had to extend over at least three contiguous 2 × 2 × 2mm voxels. Activation areas were localised by comparison with a stereotactic map of the brain [Talairach and Tournoux, 1988] and plotted onto a standard MRI image for display as cortically rendered images using SPM 95.
RESULTS
Psychophysical performance
As can be seen from Table I, subjects performed both tasks with a similarly high degree of accuracy and reaction time.
Table I.
Psychophysical performance data for the two tasks and the control (CON)*
| Task | Correct response rate (%) | SD | Reaction time (ms) | SD |
|---|---|---|---|---|
| CON | 100 | 0 | 333 | 99.7 |
| COL | 98.6 | 1.49 | 544 | 90.7 |
| PAT | 98.9 | 1.44 | 544 | 59.1 |
There was no significant difference between percent correct or reaction time in the selective attention to colour task (COL) and the selective attention to pattern task (PAT).
PET results
Results of the mean selective attention to colour minus mean control task are displayed in Table II and Figure 2, and results of the mean selective attention to pattern minus mean control task in Table III and Figure 3. No statistically significant areas of activation were present in the mean selective attention to pattern minus mean selective attention to colour. The areas of activation are illustrated on a standardised atlas brain, but because they are cortical renders; not all activations are apparent if they extend into sulci, are superior to the cerebellum or are intracerebral. The tabulated data shows the Talairach coordinates, z scores and Brodmann's areas of the data.
Table II.
Results of the selective attention to colour minus control subtraction*
| Structure | z score | x | Y | z | BA |
|---|---|---|---|---|---|
| R middle frontal gyrus (inferior aspect) | 3.53 | 26 | 50 | 8 | 10/46 |
| R lateral orbital gyrus | 3.10 | 28 | 55 | −16 | 11 |
| R middle/superior frontal gyri | 3.50 | 40 | 50 | −4 | 10 |
| R inferior parietal lobule | 3.19 | 30 | −70 | 36 | 19 |
| L precuneus (deep medial aspect) | 3.34 | −16 | −54 | 40 | 7 |
| L cuneus | 3.99 | −14 | −98 | 4 | 18 |
| L middle occipital gyrus | 3.50 | −25 | −84 | 12 | 19 |
| L inferior occipital gyrus | 3.10 | −44 | −84 | −4 | 19 |
| R inferior occipital gyrus | 3.24 | 40 | −76 | −4 | 19 |
| L fusiform gyrus | 3.24 | −42 | −34 | −8 | 20/37 |
| R middle occipitotemporal sulcus | 3.71 | 34 | −74 | 20 | 19 |
| R posterior thalamus (pulvinar region) | 4.10 | 22 | −32 | 12 | |
| R head of caudate nucleus | 3.14 | 18 | 16 | 16 |
Results are expressed as coordinates in the space of the Talairach and Tournoux [1988] as well as anatomical location. The z score for each result is given. The Brodmann's area (BA) corresponding to each location is also given. Results are reported here if they are considered statistically significant (z score ≥ 3.1, P < 0.05 in three contiguous 2 × 2 × 2 mm voxels).
Figure 2.
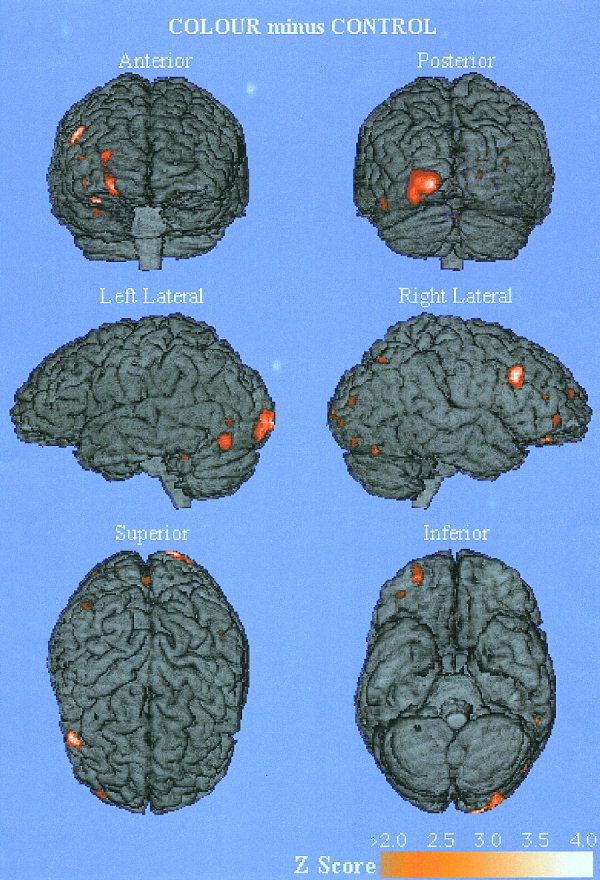
Cortical activation areas found in selective attention to colour minus control. The subtraction set of selective attention to colour minus control demonstrating all cortical regions with a z score greater than 2.0. The regions of activation are rendered onto a single brain. Talairach coordinates, anatomical locations, Brodmann's areas and z score are reported in Table II for statistically significant activation areas (z score ≥ 3.1, P < 0.05 in three contiguous 2 × 2 × 2 mm voxels).
Table III.
Results of the selective attention to pattern minus control subtraction*
| Structure | z score | x | Y | z | BA |
|---|---|---|---|---|---|
| R lateral orbital gyrus | 3.25 | 30 | 50 | −16 | 11 |
| R inferior parietal lobule | 3.29 | 26 | −76 | 36 | 19 |
| L middle temporal gyrus (pole) | 3.10 | −55 | 5 | −16 | 21 |
| L cuneus | 5.24 | −10 | −78 | 8 | 17 |
| R cuneus | 3.53 | 22 | −92 | 8 | 18 |
| L inferior occipital gyrus | 4.00 | −40 | −80 | −4 | 19 |
| R inferior occipital gyrus | 3.47 | 32 | −80 | −4 | 19 |
| R superior occipital gyrus | 3.50 | 32 | −80 | 28 | 19 |
| L middle/superior occipital gyri | 3.17 | −24 | −88 | 20 | 19 |
| R middle occipital gyrus | >3.5 | 40 | −84 | 16 | 19 |
| R middle occipitotemporal sulcus | 3.71 | 34 | −74 | 20 | 19 |
| R parahippocampal gyrus | >3.5 | 24 | −8 | −24 | 35 |
| R head of caudate nucleus | 3.45 | 20 | 12 | 16 | |
| R posterior thalamus (pulvinar region) | 3.09 | 26 | −34 | 12 | |
| Cerebellar vermis | >3.5 | 2 | −78 | −24 |
Results are expressed as coordinates in the space of the Talairach and Tournoux [1988] as well as anatomical location. The z score for each result is given. The Brodmann's area (BA) corresponding to each location is also given. Results are reported here if they are considered statistically significant (z score ≥ 3.1, P < 0.05 in three contiguous 2 × 2 × 2 mm voxels).
Figure 3.

Cortical activation areas found in selective attention to pattern minus control. The subtraction set of selective attention to pattern minus control demonstrating all cortical regions with a z score greater than 2.0. The regions of activation are rendered onto a single brain. Talairach coordinates, anatomical locations, Brodmann's areas and z score are reported in Table III for statistically significant activation areas (z score ≥ 3.1, P < 0.05 in three contiguous 2 × 2 × 2 mm voxels).
Colour task
In the selective attention to colour task, regions of activation were found predominantly in the occipitotemporal regions with activation of the left middle occipital (‐25, ‐84, 12) and fusiform gyri (‐42, ‐34, ‐8) as well as the left cuneus (‐14, ‐98, 4) and precuneus (‐16, ‐54, 40). Bilateral symmetrical activation areas were located in the inferior occipital gyri (‐44, ‐74, ‐4 and 40, ‐76, ‐4). An activation area was localised to the sulcus between the right middle temporal and occipital gyri (34, ‐74, 20). There were also several activation areas in the right occipital region that are apparent on Figure 2 that did not reach statistical significance but are symmetrically distributed with the left occipital activations. Subcortical activation areas were found in the right pulvinar nucleus of the thalamus (22, ‐32, 12) and in the right head of the caudate nucleus (18, 16, 16). An activation area was found in the right inferior parietal lobule (30, ‐70, 36), which localises to the sulcus posterior to the angular gyrus. Several foci of activation were found in the right frontal lobe—the inferior aspect of the middle frontal gyrus (26, 50, 8), the superior aspect of the middle frontal gyrus more anteriorly (40, 50, ‐4), and on the orbital surface of the frontal lobe in the lateral orbital gyrus (28, 55, ‐16).
Pattern task
In the selective attention to pattern task, regions of activation were found predominantly in the occipitotemporal regions, with activation of the bilateral superior (‐24, ‐88, 20 and 32, ‐80, 28), middle (‐24, ‐88, 20 and 40, ‐84, 16), and inferior (‐40, ‐80, ‐4 and 32, ‐80, ‐4) occipital gyri. Bilateral activation areas in the cuneus are present (‐10, ‐78, 8 and 22, ‐92, 8). A region in the sulcus between the right middle occipital and temporal gyri is also activated (34, ‐74, 20). An activation area was present near the pole of the left middle temporal gyrus (‐55, 5, ‐16) and the right parahippocampal gyrus also contained a statistically significant activation area (24, ‐8, ‐24). Subcortical activation areas were found in the right pulvinar nucleus of the thalamus (26, ‐34, 12) and in the right head of the caudate nucleus (20, 12, 16). An activation area was found in the right inferior parietal lobule (26, ‐76, 36) which localises to the sulcus posterior to the angular gyrus. The right lateral orbital gyrus contains one activation area that extends into the posterior orbital gyrus (30, 50, ‐16). A region of activation was found within the cerebellar vermis (2, ‐78, ‐24).
Task minus task subtraction
The selective attention to pattern minus selective attention to colour task subtraction did not reveal any statistically significant areas of activation.
DISCUSSION
The present study is a delayed match to sample paradigm using simple two‐colour pattern visual stimuli. The key aspect of the study design is that the stimuli are all abstract patterns made up of two‐colour combinations with any stimulus able to be used for any task. The study was designed in this manner so that differences and similarities between colour and pattern processing could be directly explored. A series of related cognitive processes are required for successful performance of the tasks including colour and pattern visual processing, visual working memory, visual recognition, and a two‐alternative forced choice (button press) response. The stimuli and motor responses were matched so that they would not be significantly represented in the subtraction images of control from task.
Selective attention to colour and the ventral pathway
Selective attention to colour resulted in predominantly left‐sided activation areas within the cuneus, precuneus, inferior, and middle occipital gyri as well as in the mid‐portion of the fusiform gyrus. Less extensive activation areas were present in the right inferior occipital gyrus and in the right middle occipitotemporal sulcus. These results are in keeping with the model of Zeki and Marini [1998], which proposes a multistaged cortical processing system for colour where various occipitotemporal cortical regions are recruited depending on the specific requirements of the task being undertaken. The processes and structures involved in modulating the cortical regions activated in each task have not yet been fully elucidated but have been attributed to prefrontal cortical regions. In the present study we found several right‐sided prefrontal activation areas that may be responsible for such modulation. Corbetta et al. [1991], using red or green stimuli, found extensive activation areas within the left lateral occipital cortex and the collateral sulcus. Imaging studies using the Farnsworth‐Munsell 100‐Hue test revealed extensive activation of occipitotemporal regions with passive colour viewing activating only the most posterior portions of the fusiform gyrus, whereas the anterior and middle portions of the fusiform gyrus were found with the active sorting of colours [Beauchamp et al., 1999]. Activation studies using Mondrian prints have demonstrated activation areas in bilateral lateral occipital cortex extending into the fusiform gyrus bilaterally [Howard et al., 1998; Zeki and Marini, 1998; Zeki et al., 1991]. Even though the activation found in Zeki's work was bilateral, left‐sided activation areas were more extensive. These studies used different complexities of stimuli and yet all resulted in similar early occipital activation areas with varying extension into temporal structures. Also there were essentially unilateral activation areas for the simplest discriminations [Corbetta et al., 1991], whereas more difficult discriminations demonstrated recruitment of bilateral activation areas, though left‐sided fields were generally more prominent [Beauchamp et al., 1999; Howard et al., 1998; Zeki and Marini, 1998; Zeki et al., 1991]. Increasing stimulus complexity also results in the recruitment of more anterior inferotemporal structures [Howard et al., 1998; Zeki and Marini, 1998]. Our paradigm, with its simple two‐colour patterns, has activated predominantly left‐sided more posterior occipitotemporal areas, which lends support to the model of Zeki and Marini [1998].
The present study did not result in an activation area in the V4 region but did show activation of a field just anterior to it. Cells in V4 respond to both colour and pattern information [Desimone et al., 1985; Corbetta et al., 1991]. However, both macaques and humans with lesions in the region of V4 can still categorise and discriminate colours as well as perform tasks of working memory using colour, suggesting that V4 is not essential for colour processing [Schoppig et al., 1999; Walsh et al., 1992]. From Zeki's work it would appear that V4 is not concerned with simple colour processing per se but rather may have a role in the investing of objects with colour, in colour constancy, or in another aspect of colour processing such as the relationship between colour and form. Our paradigm specifically required subjects to separate colour from pattern. Considering that V4 was not activated, it may be that V4 is more concerned with the relationship between colour and form rather than colour alone as suggested by Zeki's work.
In the present study two colour pairs were compared and matched by subjects. Corbetta [1991] also used a simple task with subjects matching red or green stimuli, but Zeki [1991, 1998] used relatively complex Mondrian prints. Corbetta asked subjects to engage in active visual matching (as in the present study), whereas Zeki used passive tasks. Perhaps the results of Zeki's work reflect the general activation of colour‐sensitive regions to different types of information (complex Mondrian displays, normally and abnormally coloured objects), whereas the results of Corbetta's study and ours indicate modulated, task‐specific activation areas responsible for colour processing when it is being compared with a specific set of colours in working memory.
Selective attention to pattern and the ventral pathway
Selective attention to pattern resulted in activation areas extending from the bilateral occipital cortices to bilateral inferior temporal cortices. An activation area was also present in the right orbital frontal cortex. These results are in broad agreement with the literature. Several groups studying simple pattern processing have found activation areas in the posterior and lateral occipital cortex in studies examining selective attention to shape, especially in tasks where the other component of the stimulus is colour [Baker et al., 1996; Corbetta et al., 1991; Gulyas et al., 1994]. Activation areas are also reported in a variety of locations in the inferior temporal cortex, extending into inferolateral frontal cortex [Baker et al., 1996; Gulyas et al., 1994, 1998; Fink et al., 1997; Nobre et al., 1997; Paulesu et al., 1993].
As one might expect, our paradigm resulted in broad activation within BA19. Activation areas were also localised to the right parahippocampal gyrus and to the pole of the left middle temporal gyrus. In their studies of colour, form, and motion, Corbetta and colleagues [1991] reported bilateral activation areas in the parahippocampal gyri during matching to form but not during matching to colour. A right parahippocampal activation area was also reported by Courtney et al. [1996] in their comparison of working memory for faces and locations. They felt however that the activation was the result of deactivation in the location task rather than activation in the face task. Many other studies of human pattern processing have not reported parahippocampal activation [Baker et al., 1996; Fink et al., 1997; Gulyas et al., 1994, 1998; Heinze et al., 1994; Köhler et al., 1995; Nystrom et al., 2000]. The exact role of the parahippocampus in the present study is unclear. However the available evidence suggests that it has a role in memory and here it may be partly responsible for matching stimuli to the target.
The pole of the temporal lobe receives feedforward connections from temporal association cortex immediately posterior to it, with the inferior portions receiving visual input from the anterior portion of the inferotemporal cortex [Moran et al., 1987]. The temporal pole has connections with the orbitofrontal gyri as well as with the parahippocampal gyrus, both of which were activated in the present study [Moran et al., 1987]. Perhaps the activation area identified here is partly responsible for comparing the images in working memory with the target stimulus.
Studies of complex patterns (e.g., faces) result in activation areas extending into the fusiform gyrus and other inferior temporal structures in pattern processing [Haxby et al., 1991; Köhler et al., 1995; Sams et al., 1997; Sergent et al., 1992; Ishai et al., 1999]. Such fields have been less prominent but nonetheless present in studies requiring simple pattern processing [Corbetta et al., 1991; Gulyas et al., 1994, 1998]. Within the fusiform gyrus differences between lateral and medial regions have been described. The lateral fusiform gyrus responds most actively but not exclusively to faces [Chao et al., 1999; Clark et al., 1996; Haxby et al., 1996, 1999, 2000; Ishai et al., 1999; Sams et al., 1997]. Medial fusiform gyral activation areas have been demonstrated in studies examining less complex imagery [Chao et al., 1999; Clark et al., 1996; Haxby et al., 1996; Ishai et al., 1999]. Surprisingly the present study does not demonstrate any statistically significant activation within the fusiform gyrus. It may be that the power of the study is not high enough to demonstrate statistically significant activation above the control task, or that the fusiform gyrus was not required for the present paradigm either because of the simple nature of the stimuli or because of the task itself. Baker et al. [1996] presented one of the few pattern studies that did not report any statistically significant activation of the fusiform gyrus. Similar to the present study, their stimuli and tasks were relatively simple. Other studies using more complex nonface stimuli did result in fusiform gyral activation [Corbetta et al., 1991; Gulyas et al., 1998]. This along with evidence of the difference between medial and lateral fusiform gyrus suggests that pattern processing is organised in a processing‐specific manner.
A comparison of colour and pattern processing
From Figures 2 and 3 it appears that the occipital activation is different between the two paradigms. However, the apparent marked differences in extrastriate cortex activation between the tasks is not borne out in the task minus task comparison, suggesting that there is subthreshold activation of the right occipital cortex. Indeed it is apparent from the figures that in the colour task there are activation areas with a z score less than 3.1 in the same locations as statistically significant fields appear in the pattern task. This suggests that the same regions of cortex are activated by the same stimuli regardless of the feature being attended to. The more extensive activation seen in the pattern task implies that even at the earliest stage of cortical processing there is modulation of cortical areas. It is known that modulation in extrastriate cortices does occur in other aspects of visual processing such as directing visual attention to a single element in a cluttered visual field [Kastner et al., 1998].
Differences between the processing of colour and pattern emerge in the activation areas found in the temporal cortex. This difference implies a task‐specific role to the respective activation areas found in each of the tasks. Task‐specific activation areas are now being thought of not so much as being particular to faces or other objects but rather may fulfill some specific role that is required to complete either stimulus or task processing [Ishai et al., 1999; Haxby et al., 2000]. The prefrontal activation areas also demonstrate a marked difference between colour and pattern, possibly representing similar differences in working memory processes as have been found in previous studies [Corbetta et al., 1991; Courtney et al., 1996; Haxby et al., 1996, 2000; Ungerleider et al., 1998; Zeki and Marini, 1998; Fuster et al., 1985]. Our data is thus in concordance with recent work that suggests that once basic processing is completed, more complex comparisons to other objects and to items held in either working or long‐term memory occur in a task‐dependent manner by a variety of activation areas in the inferotemporal and prefrontal cortices [Haxby et al., 2000; Ishai et al., 1999; Nystrom et al., 2000].
Considering the apparent differences between the two paradigms in the task minus control subtractions, it was disappointing that the differences did not also emerge in the subtraction of the pattern and colour tasks. The absence of statistically significant activation areas in the pattern minus colour subtraction implies that there is subthreshold activation of the same extra‐occipital regions in the colour task as are demonstrated in the pattern task. One explanation lies with the contention of Haxby et al. [2000], that for each task similar fields are activated and it is in the relative strength of the activations that the clues to differences in processing lie. It is possible that our subject numbers (n = 10) were not sufficient to identify differences using SPM methodology and statistical thresholds.
Subcortical structures
An activation area was present in the right pulvinar nucleus of the thalamus in both paradigms. Anatomical studies on the pulvinar nucleus of the thalamus have demonstrated reciprocal connections with the posterior parietal cortex, the orbital frontal cortex, and other prefrontal regions, as well as connections with the lateral geniculate nucleus and superior colliculus [Robinson et al., 1978]. Lesions of the pulvinar produce deficits of gaze in primates [Andersen, 1987; Ogren et al., 1984; Yin and Mountcastle, 1978], with unilateral lesions producing difficulty with visual search in the contralateral visual field [Ogren et al., 1984]. Primates with pulvinar lesions had particular difficulty discriminating complex patterns, suggesting a role for the pulvinar in visual search [Robinson and Peterson, 1992]. In human studies using PET, the pulvinar is activated during tasks requiring object discrimination, selective attention, and visual search [LaBerge and Buchsbaum, 1990; Corbetta et al., 1991, 1993; Nobre et al., 1997].
An activation area also was present in the head of the right caudate nucleus in both paradigms. Reciprocal connections between cortical regions and the caudate nucleus are well described [Goldman and Nausta, 1977; Yeterian and Van Hoesen, 1978]. Cells in the caudate nucleus are known from single‐unit studies in nonhuman primates to be involved in tasks requiring spatial working memory. The right caudate nucleus has been activated in PET studies testing human spatial working memory [Corbetta et al., 1993] and selective attention to colour [Corbetta et al., 1991]. It may also have a more general role as part of a network directing visual attention [Nobre et al., 1997]. The results of the present study support a general role for the caudate nucleus in visual attention because, like the right pulvinar nucleus of the thalamus, it was activated in both tasks.
The lateralisation of brain function seen in the present study has been commented on by several other authors, and the existence of a right‐sided attentional network has been proposed in visuospatial attention [Corbetta et al., 1993; Nobre et al., 1997]. That the present study examines form rather than spatial location suggests that this network of right‐sided structures is required for visual attention rather than a specific spatial function.
Cerebellum
We found activation of the cerebellar vermis with selective attention to pattern but not to colour. PET studies that have demonstrated activation of the cerebellum include those examining motor learning [Seitz et al., 1990], motor control [Fox et al., 1985], and tactile learning [Roland et al., 1987], as well as somatosensory discrimination [O'Sullivan et al., 1994]. It has been postulated that the cerebellum is involved in higher cognitive processing mediated through its reciprocal connections with the prefrontal cortex [Middleton and Strick, 1994]. Studies on patients who have had portions of their cerebellum removed for surgical treatment of carcinoma suggest that the cerebellum may have a role in changing the focus of attention from one component of a target to another [Akshoomoff and Courchesne, 1992, 1994]. In recent years it has been noted that the cerebellum has been activated in object identification tasks [Akshoomoff and Courchesne, 1992; Gulyas et al., 1998; Köhler et al., 1995; Raichle et al., 1994]. In the present study the activation is not merely caused by motor output because the same motor output was required in all tasks. Hence the cerebellar activation is likely to have arisen from the cognitive requirements of simple pattern processing/object identification.
CONCLUSION
The present study identified a series of activation areas in the occipital cortex that are required in processing basic stimulus attributes of colour and pattern. Task‐dependent processing occurs in the inferior temporal cortex with possible reference to a working memory store in the right prefrontal cortex. A right‐sided network involving the inferior parietal lobule, head of the caudate nucleus, and the pulvinar nucleus of the thalamus may have a role in visual search or has some other attentional role that is common to both paradigms. We also found evidence that the cerebellum contributes to pattern processing.
Acknowledgements
This research was made possible with the aid of the staff and resources of the National Medical Cyclotron and of the Department of Nuclear Medicine, Royal Prince Alfred Hospital, Sydney. N.A. Barrett was supported by the J. G. Hunter Fellowship from the Australian Medical Association and a BSc(Med)(Hons) Scholarship from the Faculty of Medicine, Sydney University. M.M. Large was supported by the Royal Australian and New Zealand College of Psychiatrists' Eli‐Lilly Fellowship. F. Karayanidis was supported by a Macquarie University Postdoctoral Research Fellowship.
REFERENCES
- Akshoomoff NA, Courchesne E (1992): A new role for the cerebellum in cognitive operations. Behav Neurosci 106: 731–738. [DOI] [PubMed] [Google Scholar]
- Akshoomoff NA, Courchesne E (1994): ERP evidence for a shifting attention deficit in patients with damage to the cerebellum. J Cogn Neurosci 6: 388–399. [DOI] [PubMed] [Google Scholar]
- Andersen RA (1987): Inferior parietal lobule function in spatial perception and visuomotor integration In: Mountastle V, Plum F, editors. Handbook of physiology, Sec. 1: the nervous system. Bethesda, MD: American Physiological Society; p 483–518. [Google Scholar]
- American Psychiatric Association (1993): Diagnostic and statistical manual of mental disorders. 4th ed. Washington, D.C.: American Psychiatric Association. [Google Scholar]
- Baker SC, Frith CD, Frackowiak RSJ, Dolan RJ (1996): Active representation of shape and spatial location in man. Cereb Cortex 6: 612–619. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Haxby JV, Jennings JE, DeYoe EA (1999): An fMRI version of the Farnsworth‐Munsell 100‐Hue test reveals multiple color‐selective areas in human ventral occipitotemporal cortex. Cereb Cortex 9: 257–263. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Goldberg ME, Robinson DL (1981): Behavioral enhancement of visual responses in monkey cerebral cortex. I: Modulation in posterior parietal cortex related to visual attention. J Neurophysiol 46: 755–772. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A, Haxby JV (1999): Are face‐responsive regions selective only for faces? Neuroreport 10: 2945–2950. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Miller EK, Duncan J, Desimone R (1993): A neural basis for visual search in inferior temporal cortex. Nature 363: 345–347. [DOI] [PubMed] [Google Scholar]
- Cherry S, Woods R, Mazziotta J (1993): Improved signal‐to‐noise activation studies by exploiting the dinetics of oxygen‐15‐labelled water. J Cereb Blood Flow Metab 13: S714. [Google Scholar]
- Clark VP, Keil K, Maisog JM, Courtney S, Ungerleider LG, Haxby JV (1996): Functional magnetic resonance imaging of human visual cortex during face matching: a comparison with positron emission tomography. Neuroimage 4: 1–15. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin F, Dobmeyer S, Shulman G, Peterson S (1991): Selective and divided attention during visual discriminations of shape, colour and speed: functional anatomy by positron emission tomography. J Neurosci 11: 2383–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Miezin F, Shulman G, Petersen S (1993): A PET study of visuospatial attention. J Neurosci 13: 1202–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Kil K, Haxby JV (1996): Object and spatial visual working memory activate separate neural systems in human cortex. Cereb Cortex 6: 39–49. [DOI] [PubMed] [Google Scholar]
- Dean P (1982): Visual behaviour in monkeys with inferotemporal lesions In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behaviour. Cambridge, MA: MIT Press; p 597–628. [Google Scholar]
- Desimone R, Schein SJ, Moran J, Ungerleider LG (1985): Contour, color and shape analysis beyond the striate cortex. Vision Res 25: 441–452. [DOI] [PubMed] [Google Scholar]
- Fink GR, Dolan RJ, Halligan PW, Marshall JC, Frith CD (1997): Space‐based and object‐based visual attention: shared and specific neural domains. Brain 120: 2013–2028. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Thach WT (1985): Functional mapping of the human cerebellum with positron emission tomography. Proc Natl Acad Sci U S A 82: 7462–7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackowiak R (1994): Functional mapping of verbal memory and language. Trends Neurosci 17: 109–115. [DOI] [PubMed] [Google Scholar]
- Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC (1997): Human brain function. San Diego, CA: Academic Press. [Google Scholar]
- Friedman HR, Goldman‐Rakic PS (1994): Coactivation of prefrontal cortex and inferior parietal cortex in working memory tasks revealed by 2DG functional mapping in the rhesus monkey. J Neurosci 14: 1775–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Frith C, Liddle P, Dolan R, Lammertsma A, Frackowiak R (1990): The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab 10: 458–466. [DOI] [PubMed] [Google Scholar]
- Friston K, Frith C, Liddle P, Frackowiak R (1991): Plastic transformation of PET images. J comput assist tomogr 15: 634–639. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Bauer RH, Jervey JP (1985): Functional interactions between inferotemporal and prefrontal cortex in a cognitive task. Brain Res 330: 299–307. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Nausta WJH (1977): An intricately patterned prefronto‐caudate projection in the rhesus monkey. J Comput Neurol 171: 369–386. [DOI] [PubMed] [Google Scholar]
- Gulyas B, Cowey A, Heywood CA, Popplewell D, Roland PE (1998): Visual form discrimination from texture cues: a PET study. Hum Brain Mapp 6: 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas B, Heywood CA, Popplewell D, Cowey A, Roland PE (1994): Visual form discrimination from colour or motion cues: functional anatomy by positron emission tomography. Proc Natl Acad Sci U S A 91: 9965–9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J, Grady C, Ungerleider L, Horwitz B (1991): Mapping the functional neuroanatomy of the intact human brain with brain work imaging. Neuropsychologia 29: 539–555. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Horwitz B, Ungerleider L, Mishkin M, Carson RE (1992): Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proc Natl Acad Sci U S A 88: 1621–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL (1994): The functional organization of human extrastriate cortex: a PET‐rCBF study of selective attention to faces and locations. J Neurosci 14: 6336–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Horwitz B, Maisog JM, Rapoport SI, Grady CL (1996): Face encoding and recognition in the human brain. Proc Natl Acad Sci U S A 93: 922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Clark VP, Schouten JL, Hoffman EA, Martin A (1999): The effect of face inversion on activity in human neural systems for face and object perception. Neuron 22: 189–199. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Petit L, Ungerleider LG, Courtney SM (2000): Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. Neuroimage 11: 380–391. [DOI] [PubMed] [Google Scholar]
- Heinze HJ, Mangun GR, Burchert W, Hinrichs H, Scholz M, Munte TF, Gos A, Scherg M, Johannes S, Hundeshagen H, Gazzaniga MS, Hillyard SA (1994): Combined spatial and temporal imaging of brain activity during selective attention in humans. Nature 372: 543–546. [DOI] [PubMed] [Google Scholar]
- Howard RJ, Ffytche DH, Barnes J, McKeefry D, Ha Y, Woodruff PW, Bullmore ET, Simmons A, Williams SCR, David AS, Brammer M (1998): The functional anatomy of imagining and perceiving colour. Neuroreport 9: 1019–1023. [DOI] [PubMed] [Google Scholar]
- Hurtig R, Hichwa R, O'Leary D, Ponto L, Narayana S, Watkins G, Andreason N (1994): Effects of timing and duration of cognitive activation in [15O]water PET studies. J Cereb Blood Flow Metab 14: 423–430. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV (1999): Distributed representation of objects in the human ventral visual pathway. Proc Natl Acad Sci U S A 96: 9379–9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara S (1994): Ishihara's test for colour blindness. Tokyo: Kanehara. [Google Scholar]
- Jonides J, Smith E, Koeppe R, Awh E, Minoshima S, Mintun M (1993): Spatial working memory as revealed by PET. Nature 363: 623–625. [DOI] [PubMed] [Google Scholar]
- Kastner S, De Weerd P, Desimone R, Ungerleider LG (1998): Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science 282: 108–111. [DOI] [PubMed] [Google Scholar]
- Kastner S, De Weerd P, Ungerleider LG (2000): Texture segregation in the human visual cortex: a functional MRI study. J Neurophysiol 83: 2453–2457. [DOI] [PubMed] [Google Scholar]
- Köhler S, Kapur S, Moscovitch M, Winocur G, Houle S (1995): Dissociation of pathways for object and spatial vision: a PET study in humans. Neuroreport 6: 1865–1868. [DOI] [PubMed] [Google Scholar]
- LaBerge D, Buchsbaum M (1990): Positron emission tomographic measurements of pulvinar activity during an attention task. J Neurosci 10: 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueck CJ, Zeki S, Friston KJ, Deiber OJ, Cope P, Cunningham VJ, Lammertsma AA, Kennard C, Frackowiak RSJ (1989): The colour centre in the cerebral cortex of man. Nature 340: 386–389. [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG (1995): Discrete cortical regions associated with knowledge of color and knowledge of action. Science 270: 102–105. [DOI] [PubMed] [Google Scholar]
- Mennemeier MS, Chatterjee A, Watson RT, Wertman E, Carter LP, Heilman KM (1994): Contributions of the parietal and frontal lobes to sustained attention and habituation. Neuropsychologia 32: 703–716. [DOI] [PubMed] [Google Scholar]
- Michie PT, Karayanidis F, Smith GL, Barrett NA, Large MM, O'Sullivan BT, Kavanagh DJ (1999): An exploration of varieties of visual attention: ERP findings. J Cogn Res 7: 419–450. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (1994): Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science 266: 458–461. [DOI] [PubMed] [Google Scholar]
- Moran MA, Mufson EJ, Mesulam MM (1987): Neural inputs into the temporopolar cortex of the rhesus monkey. J Comput Neurol 256: 88–103. [DOI] [PubMed] [Google Scholar]
- Myers R, Cunningham V, Bailey D, Jones T (1996): Quantification of brain function using PET. London: Academic Press. [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Mesalum MM, Frackowiak RSJ, Frith CD (1997): Functional localization of the system for visuospatial attention using positron emission tomography. Brain 120: 515–533. [DOI] [PubMed] [Google Scholar]
- Nystrom LE, Braver TS, Sabb FW, Delgado MR, Noll DC, Cohen JD (2000): Working memory for letter, shapes and locations: fMRI evidence against stimulus‐based regional organization in human prefrontal cortex. Neuroimage 11: 424–446. [DOI] [PubMed] [Google Scholar]
- O'Sullivan BT, Roland PE, Kawashima R (1994): A PET study of somatosensory discrimination in man—microgeometry versus macrogeometry. Eur J Neurosci 6: 137–148. [DOI] [PubMed] [Google Scholar]
- Ogren MP, Mateer CA, Wyler AR (1984): Alterations in visually related eye movements and following left pulvinar damage in man. Neuropsychologia 188: 179–200. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith C, Frackowiak R (1993): The neuronal correlates of the verbal component of working memory. Nature 362: 342–345. [DOI] [PubMed] [Google Scholar]
- Raichle M, Fiez J, Videen T, Macleod A, Pardo J, Fox P, Peterson S (1994): Practice‐related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex 4: 8–26. [DOI] [PubMed] [Google Scholar]
- Robb RA, Hanson DP, Karwoski RA, Larson AG, Workman EL, Stacy MC (1989): ANALYZE: a comprehensive, operator‐interactive software package for multidimensional medical image display and analysis. Comput Med Imaging Graph 13: 433–454. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Goldberg ME, Stanton GB (1978): Parietal association cortex in the primate: sensory mechanisms and behavioral modulations. J Neurophysiol 41: 910–932. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Peterson SE (1992): The pulvinar and visual salience. Trends Neurosci 15: 127–132. [DOI] [PubMed] [Google Scholar]
- Roland P, Friberg L (1985): Localization of cortical areas activated by thinking. J Neurophysiol 53: 1219–1243. [DOI] [PubMed] [Google Scholar]
- Roland PE, Eriksson L, Stone‐Elander S, Widén L (1987): Does mental activity change the oxidative metabolism of the brain? J Neurosci 7: 2373–2389. [PMC free article] [PubMed] [Google Scholar]
- Sams M, Hietanen JK, Hari R, Ilmoniemi RJ, Lounasmaa OV (1997): Face‐specific responses from the human inferior occipito‐temporal cortex. Neuroscience 77: 49–55. [DOI] [PubMed] [Google Scholar]
- Schoppig A, Clarke S, Walsh V, Assal G, Meuli R, Cowey A (1999): Short‐term memory for colour following posterior hemispheric lesions in man. Neuroreport 10: 1379–1384. [DOI] [PubMed] [Google Scholar]
- Seitz R, Bohm C, Greitz T, Roland P, Eriksson L, Blomqvist G, Rosenqvist G, Nordell B (1990): Accuracy and precision of the computerized brain atlas programme for localization and quantification in positron emission tomography. J Cereb Blood Flow Metab 10: 443–457. [DOI] [PubMed] [Google Scholar]
- Sergent J, Ohta S, MacDonald B (1992): Functional neuroanatomy of face and object processing. Brain 115: 15–36. [DOI] [PubMed] [Google Scholar]
- Smith GL, Large MM, Kavanagh DJ, Karayanidis F, Barrett NA, Michie PT, O'Sullivan BT (1998): Further evidence for a deficit in switching attention in schizophrenia. J Abnorm Psychol 107: 390–398. [DOI] [PubMed] [Google Scholar]
- Takechi H, Onoe H, Shizuno H, Yoshikawa E, Sadato N, Tsukada H, Watanabe Y (1997): Mapping of cortical areas involved in color vision in non‐human primates. Neurosci Lett 230: 17–20. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. Paris: Thieme. [Google Scholar]
- Toga AW, Mazziotta JC (1995): Brain mapping: the methods. San Diego, CA: Academic Press. [Google Scholar]
- Townsend D, Geissbuhler A, Defrise M, Hoffman E, Spinks T, Bailey D, Gilardi M, Jones T (1991): Fully three‐dimensional reconstruction for a PET camera with retractable septa. IEEE Trans Med Imag 10: 505–512. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M (1982): Two cortical visual systems In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behaviour. Cambridge, MA: MIT Press; p 549–586. [Google Scholar]
- Ungerleider LG, Haxby JV (1994): 'What' and 'where' in the human brain. Curr Opin Neurobiol 4: 157–165. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Courtney SM, Haxby JV (1998): A neural system for human visual working memory. Proc Natl Acad Sci U S A 95: 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Zeki SM (1978): The topographic organisation of rhesus monkey prestriate cortex. J Physiol 277: 193–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh V, Kulikowski JJ, Butler SR, Carden D (1992): The effects of lesions of area V4 on the visual abilities of macaques: colour categorization. Behav Brain Res 52: 81–89. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Bachevalier J, Ungerleider LG (1994): Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cereb Cortex 4: 470–483. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Van Hoesen GW (1978): Cortico‐striate projections in the rhesus monkey: the organization of certain corticocaudate connections. Brain Res 139: 43–63. [DOI] [PubMed] [Google Scholar]
- Yin TCT, Mountcastle VB (1978): Mechanisms of neural integration in the parietal lobe for visual attention. Fed Proc 37: 2251–2257. [PubMed] [Google Scholar]
- Zeki SM (1978): The cortical projections of foveal striate cortex in the rhesus monkey. J Physiol 277: 227–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki SM, Shipp S (1989): Modular connections between areas V2 and V4 of macaque monkey visual cortex. Eur J Neurosci 1: 494–506. [DOI] [PubMed] [Google Scholar]
- Zeki S, Watson JDG, Lueck CJ, Friston KJ, Kennard C, Frackowiak RSJ (1991): A direct demonstration of functional specialization in human visual cortex. J Neurosci 11: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S (1993): A vision of the brain. Oxford: Blackwell. [Google Scholar]
- Zeki S, Marini L (1998): Three cortical stages of colour processing in the human brain. Brain 121: 1669–1685. [DOI] [PubMed] [Google Scholar]


