Abstract
Bimanual motor coordination is essential for piano playing. The functional neuronal substrate for high‐level bimanual performance achieved by professional pianists is unclear. We compared professional pianists to musically naïve controls while carrying out in‐phase (mirror) and anti‐phase (parallel) bimanual sequential finger movements during functional magnetic resonance imaging (fMRI). This task corresponds to bimanually playing scales practiced daily by pianists from the beginning of piano playing. Musicians and controls showed significantly different functional activation patterns. When comparing performance of parallel movements to rest, musically naïve controls showed stronger activations than did pianists within a network including anterior cingulate cortex, right dorsal premotor cortex, both cerebellar hemispheres, and right basal ganglia. The direct comparison of bimanual parallel to mirror movements between both groups revealed stronger signal increases in controls within mesial premotor cortex (SMA), bilateral cerebellar hemispheres and vermis, bilateral prefrontal cortex, left ventral premotor cortex, right anterior insula, and right basal ganglia. These findings suggest increased efficiency of cortical and subcortical systems for bimanual movement control in musicians. This may be fundamental to achieve high‐level motor skills allowing the musician to focus on artistic aspects of musical performance. Hum. Brain Mapping 22:206–215, 2004. © 2004 Wiley‐Liss, Inc.
Keywords: efficiency, fMRI, music, plasticity, sensorimotor
INTRODUCTION
Bimanual synchronized finger movements are a central element of piano playing. From the beginning of their training, playing scales bimanually is practiced daily by pianists. This exercise corresponds to sequential bimanual anti‐phase (parallel) movements that can experimentally be compared to less complex and more stable in‐phase (mirror) finger movements [Mechsner et al., 2001; Scholz and Kelso, 1990]. Several functional imaging studies have investigated the functional neuroanatomy underlying bimanual performance [Immisch et al., 2001; Jancke et al., 2000; Nair et al., 2003; Sadato et al., 1997; Stephan et al., 1999a, b]. Many studies emphasize the role in bimanual coordination of medial frontal motor areas (supplementary motor area [SMA] and cingulate motor cortex [CMA]) and of interhemispheric transcallosal communication [Andres et al., 1999; Kennerley et al., 2002; Serrien et al., 2001], as well as the role of dorsal premotor cortex (dPMC), prefrontal cortex, and cerebellum [Serrien and Wiesendanger, 2000; Tracy et al., 2001].
Studying professional musicians offers an ideal model to explore the effect of long‐term regular practice upon adaptive plasticity of the sensorimotor system. Structural studies revealed an increased size of the hand motor cortex [Amunts et al., 1997] and the corpus callosum [Schlaug et al., 1995a], and increased grey matter density within Broca's area in inferior frontal gyrus of professional musicians [Sluming et al., 2002]. An increased size of secondary auditory cortex was demonstrated in musicians as associated with the specialized skill of absolute pitch [Schlaug et al., 1995b; Zatorre et al., 1998]. Functional studies in musicians showed an enlargement of cortical finger representations [Elbert et al., 1995] and of auditory representation for tones [Pantev et al., 1998; Schneider et al., 2002]. In predisposed individuals, defective motor reorganization due to musical practice can lead to uncontrolled movements such as task‐induced dystonia with a smearing of representational zones [Elbert et al., 1998; Pujol et al., 2000]. Investigations of unimanual complex motor tasks demonstrated changes in motor activation patterns due to professional musical training [Hund‐Georgiadis and von Cramon, 1999; Krings et al., 2000]. An increased size of the corpus callosum [Schlaug et al., 1995a], reduced transcallosal inhibition [Ridding et al., 2000], and structural adaptation of the cerebellum [Schlaug, 2001] have been associated with elaborate bimanual abilities in musicians due to long‐term training.
Our present functional magnetic resonance imaging (fMRI) study aimed at investigating complex bimanual motor control in professional pianists compared to musically naïve controls. Our task comparing in‐phase (mirror) and more complex anti‐phase (parallel) bimanual finger movements has been established in various imaging studies in normal volunteers consistently showing stronger activations during parallel movements [Sadato et al., 1997; Stephan et al., 1999a, b]. Professional piano playing affords an extreme level of bimanual coordination. Years of bimanual training in pianists should lead to an adaptation of motor areas such that increased efficiency of this system is reflected in less extensive neuronal recruitment. Intense and prolonged motor activity has been shown to increase the number of synapses per neuron [Anderson et al., 1994; Black et al., 1990; Keller et al., 1992; Kleim et al., 1996]. Besides strengthening of existing synapses, it could be hypothesized that this renders the neuronal system more efficient [for review see Schlaug, 2001]. We therefore expected stronger activations in untrained controls compared to those in pianists during performance of complex parallel movements within a motor association network known to be involved in bimanual control, with a special focus on mesial premotor, cingulate, lateral premotor, and cerebellar activations.
SUBJECTS AND METHODS
Subjects
This study included 12 professional pianists (6 women, 6 men; mean age, 23.0 ± 2.2 years) and 12 musically naïve control subjects (6 women, 6 men; mean age, 25.0 ± 1.2 years). All subjects were strictly right‐handed according to the diagnostic criteria of the Edinburgh inventory [Oldfield, 1971]. Musical professionalism was defined as having at least a completed conservatory degree in piano playing. Mean history of piano practice was 15.9 ± 2.0 years. Musically naïve controls had to have never played a musical instrument and not received any musical teaching beyond normal school education. No subject had any history of neurologic disease. All subjects were naïve with regard to the goal of the study. All subjects gave written informed consent to the study in accordance with the guidelines from the Declaration of Helsinki. The study protocol was approved by the local Ethical Committee.
Task
The experimental task included the performance of two different kinds of externally paced bimanual finger tapping involving the index to little finger of both hands. In a first condition, subjects had to carry out parallel finger movements starting either with the index finger of the right and the little finger of the left hand or vice versa. The task then consisted in continuous, bidirectional finger movements sequentially involving the index, middle, ring, and little finger of one hand versus little, ring, middle, and index finger of the other hand. Movements had to be carried out as simultaneously as possible always involving one finger per hand at a time. The second condition demanded the performance of mirror‐like finger tapping. Differing from the first condition, the corresponding fingers of both hands (index–index, middle–middle, ring–ring, little–little) always had to be moved simultaneously in bidirectional, sequential order. A resting condition without finger movements served for control.
The movements were executed on in house‐built nonmagnetic button boxes with four buttons for each hand. Those boxes were placed beside the subjects' trunk so that they could be reached comfortably with the arms supported by cushions and the wrists and hands supported prone on the button boxes. The movements were triggered externally by a single computer generated pacing tone with a rate of 1/sec that was applied continuously during the entire acquisition period, i.e., during rest and activation phases. These tones were transmitted to the subjects via pneumatic earphones (EAR‐Link, 3A; Aearo Company, Indianapolis, IN). Subjects were instructed to always react to the tones, to avoid anticipation, and to press only one button per hand at a time. All subjects practiced the task over 15 min before scanning so that they could carry it out without difficulties. Task instructions were transmitted to the subjects by means of a computer image that was projected onto a transparent screen using a video beamer. This showed either the German word Ruhe, indicating rest periods, Parallel, for performance of parallel finger tapping, or Spiegel, signaling periods with mirror finger movements. The scanning room was darkened completely. Subjects viewed the screen using a mirror that was centered above their eyes.
Data Acquisition
FMRI measurements were carried out on a 1.5‐T Philips Gyroscan NT scanner (Hamburg, Germany) upgraded to Gyroscan Intera and equipped with a circular polarized birdcage head coil. A forehead restraining strip and various foam pads served for head fixation. The functional scans were acquired using a high‐speed gradient‐weighted echo planar imaging (EPI) sequence (TR = 3 sec, TE = 50 msec, flip angle = 90 degrees, field of view 230 mm, matrix 64 × 64 resulting in an in‐plane resolution of 3.59 mm). A volume of 30 continuous axial slices (single slice thickness of 5 mm) parallel to the AC‐PC line covering the whole brain was recorded during each scan. Additionally a whole‐brain 3D T1‐weighted image was acquired for each subject for high‐resolution anatomic reference. The audio system sound pressure level was optimized before each single scanning session so subjects could clearly hear the trigger tones during fMRI scanning.
We carried out two‐block design functional runs per subject (100 scans/run, single run duration 300 sec each). Each run comprised 10 epochs of 10 whole‐brain EPI scans (each epoch 30 sec). Four epochs of rest (R) and three epochs each of parallel (P) and mirror (M) movements were distributed over each run. Two fixed experimental runs were designed with mirrored epoch order.: R‐P‐M‐R‐M‐P‐R‐P‐M‐R and the reverse. The same two experimental runs were presented for all subjects, randomly alternating the order of runs.
The button boxes, the computer generating the pacing tones and video instructions, and the fMRI scanner were linked to a Lab View (National Instruments, Austin, TX)‐based electrophysiologic monitoring system. This allowed the precise online registration of task performance and of the timing of fMRI acquisition along with the experimental paradigm.
Data Analysis
Data were analyzed using SPM 99 software (online at http://www.fil.ion.ucl.ac.uk/spm) based on the general linear model [Friston et al., 1995]. Calculations were carried out on PCs running LINUX and Matlab v. 5.3 (Mathworks, Natick, MA). The first two scans of each run were omitted to allow equilibration of saturation effects resulting in 98 scans total per run. Data preprocessing included realignment of the images to the first image of the series and stereotactic normalization into a standard space (Montreal Neurological Institute [MNI]) approximating that of Talairach and Tournoux [ 1988]. To account for intra‐ and intergroup anatomic variability and given our interest in examining cortical and subcortical activations engaging rather large volumes of gray matter, smoothing was carried out with a Gaussian filter of 12 × 12 × 12 mm3.
A second level random‐effects approach was applied for statistical analysis. On the first level, an individual design matrix with two functional runs was built for each subject. Here two different conditions per run (parallel and mirror) were defined explicitly with the resting condition modeled implicitly as baseline. Data were convolved with a canonical hemodynamic response function. A high‐pass filter was applied for filtering low frequency noise (cut off, 180 sec). Four different contrast images were calculated per subject for determining activation differences between different conditions: parallel > rest, mirror > rest, parallel > mirror, mirror > parallel. The corresponding contrast images were entered into a second level (random effects) analysis for group comparison. The latter takes into account between‐subject variability and allows more generalized inferences from the data than does a fixed effects model [McGonigle et al., 2000; Strange et al., 1999]. Activation differences between professional pianists and musically naïve controls were calculated applying a two‐sample t‐test. Based on our a priori hypotheses that were derived from results of previous functional imaging studies on bimanual finger movements, a statistical threshold of P < 0.001 (10‐voxel extent threshold) was considered to show significant activation. To consolidate further the results for critical areas, a small volume correction (P svc < 0.05) was applied for anatomically defined a priori regions of interest, based upon a sphere of 12‐mm radius centered on coordinates derived from previous studies on bimanual motor control [Sadato et al., 1997; Tracy et al., 2001] and on functional neuroanatomy of mesial premotor areas [Picard and Strick, 1996]. Regions of interest were centered around stereotactic coordinates: x, y, z = 22, −10, 52 for right dPMC; x, y, z = 16, −20, 64 for caudal SMA [Sadato et al., 1997]; x, y, z = 0, 10, 58 for preSMA; x, y, z = 4, 26, 30 for anterior cingulate [Picard and Strick, 1996]; x, y, z = 24, −62, −22 for right cerebellar hemisphere; and x, y, z = −20, −58, −22 for left cerebellar hemisphere [Tracy et al., 2001]. For localization within the stereotactic space of Talairach [Talairach and Tournoux, 1988], the coordinates of activated voxels were transformed from MNI to Talairach coordinates using a routine provided by M. Brett (available online at http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html; updated 14 February 2002).
RESULTS
Behavioral Parameters
Reaction times were analyzed by calculating the times from the appearance of the auditory cue to the beginning of each single button press. A 2 × 2 × 2 repeated measurement analysis of variance (ANOVA) with hand and movement type as intra‐subject factors and group as between‐subject factor revealed no significant effect of the factors group, movement type, and hand onto reaction times. There was anticipation in 7 controls and 5 pianists. The measured reaction times were used to calculate two indices reflecting synchrony and regularity of bimanual motor performance: An average synchrony index (SI) reflecting the mean interval between right‐ and left‐hand button press onsets was determined for each single subject for anti‐phase and in‐phase movements separately. Here, a 2 × 2 repeated measurement ANOVA with movement type as intra‐subject factor and group as between‐subject factor revealed a significant effect of the factor group (F 1,22 = 12.49, P = 0.002) with musically naïve controls showing significantly higher SIs, i.e. lower synchrony, than was shown by pianists. No significant effect of movement type on SIs was observed for either group. Additionally, the individual variances of reaction times (VR) reflecting regularity of movement (high values mirroring low regularity) were calculated for right and left hand movements and both movement types separately. A 2 × 2 × 2 repeated measurement ANOVA revealed a significantly higher VR in musically naïve controls (F 1,22 = 10.6, P = 0.004) with no significant effect or interaction of hand and movement type in both groups. No significant differences concerning error rates were detected between both groups.
Functional Imaging Data
Performance of parallel and mirror bimanual movements compared to rest activated a well‐known motor network in both groups. Within‐group comparison of performance of anti‐phase (parallel) to in‐phase (mirror) movements in professional musicians showed no significant blood oxygen‐level‐dependent (BOLD) signal differences between both conditions. In contrast, musically naïve controls showed significantly stronger activations within a cortico‐subcortical network for performance of parallel compared to mirror bimanual movements: within right cerebellar hemisphere (anterior lobe) (P svc < 0.05); right middle frontal gyrus/prefrontal cortex (Brodmann's area [BA] 10, 46); left middle frontal gyrus/prefrontal cortex (BA 9) and precentral gyrus/ventral premotor cortex (vPMC) (BA 6); inferior parietal cortex (BA 40) bilaterally; right insular cortex (BA 13); bilateral occipital cortex/lingual gyrus (BA 19/17); left inferior temporal cortex/fusiform gyrus (BA 37); and right striatum (P < 0.001 uncorrected) (Table I).
Table I.
Areas with stronger activations in musically naive controls during performance of parallel compared to mirror movements
| Cluster | Area | BA | x, y, z | T‐level |
|---|---|---|---|---|
| 1 | R cerebellum, ant. hemisphere | — | 18, −57, −21 | 5.08a |
| 2 | L mid. frontal | 9 | −48, 10, 36 | 4.90 |
| L precentral, vPMC | 6 | −48, 4, 37 | 4.70 | |
| 3 | R mid. frontal | 10 | 40, 58, −5 | 5.14 |
| 4 | R mid. frontal | 46 | 50, 34, 19 | 4.36 |
| 5 | R inf. parietal | 40 | 44, −42, 59 | 7.79 |
| 6 | L postcentral, inf. parietal | 40 | −48, −32, 50 | 4.44 |
| 7 | R insula | 13 | 32, 19, −8 | 4.95 |
| 8 | R occipital, lingual gyrus | 19 | 6, −53, −6 | 6.28 |
| 9 | L occipital | 17 | −2, −95, −2 | 5.89 |
| 10 | L fusiform gyrus | 37 | −51, −63, −17 | 5.45 |
| 11 | R striatum | — | 26, 9, 18 | 5.86 |
Coordinates x, y, z express the position of the voxel with peak activation level (P < 0.001, uncorrected, extent threshold 10 voxels) within the cluster [in mm] relative to the anterior commissure [AC] in the stereotactic space [Talairach and Tournoux, 1988]. Coordinates: x, lateral distance from the midline (+ right, − left); y, anteroposterior distance from the AC (+ anterior, − posterior); z, height relative to the AC line (+ above, − below).
P < 0.05 small volume corrected for multiple spatial comparisons. BA, Brodmann area; L, left; R, right; ant., anterior; mid., middle; vPMC, ventral premotor cortex; inf., inferior.
Direct inter‐group comparison of activation induced by performance of parallel finger‐tapping movements relative to rest showed significantly greater BOLD signal increases in musically naïve controls than in pianists within cortical and subcortical motor association areas. Right dorsal premotor cortex (BA 6), anterior cingulate cortex (BA 32), both cerebellar hemispheres (anterior lobe) (P svc < 0.05), as well as right inferior temporal gyrus (BA 20), bilateral occipital cortex (BA 17, 19), and right striatum (P < 0.001 uncorrected) were activated more strongly in controls compared to pianists (Fig. 1; Table II). No significant activation differences at P < 0.001 uncorrected were detected between both groups when comparing performance of mirror movements versus rest. There was a trend at P < 0.01 uncorrected toward stronger signal increases due to mirror movements versus rest in controls compared to pianists within bilateral dPMC as well as left cerebellar hemisphere.
Figure 1.

Stronger activations in musically naïve controls compared to professional musicians during performance of parallel movements vs. rest. Clusters of significant activation (P < 0.001 uncorrected, extent threshold of 10 voxels) are overlaid onto consecutive axial slices of a T1‐weighted anatomic MRI that was normalized stereotactically into the MNI standard space (right side of image corresponds to right hemisphere). Positions of slices within this standard space are indicated (in mm) relative to the anterior commissure (+ above, − below).
Table II.
Areas with significantly stronger activations in musically naive controls compared to pianists during performance of parallel movements versus rest
| Cluster | Area | BA | x, y, z | T‐level |
|---|---|---|---|---|
| 1 | R mid. frontal gyrus, dPMC | 6 | 32, −3, 52 | 3.65a |
| 2 | Anterior cingulate | 32 | 10, 17, 25 | 3.79a |
| 3 | R cerebellum, ant. hemisphere | — | 22, −55, −17 | 4.93a |
| 4 | L cerebellum, ant. hemisphere | — | −24, −59, −24 | 3.77a |
| 5 | R inf. temporal gyrus | 20 | 55, −32, −15 | 4.44 |
| 6 | R occipital, lingual gyrus | 17 | 6, −90, −4 | 4.07 |
| L occipital, lingual gyrus | 19 | −2, −84, −8 | 3.71 | |
| 7 | R striatum | — | 28, 19, −3 | 3.74 |
Coordinates x, y, z express the position of the voxel with peak activation level (P < 0.001, uncorrected, extent threshold 10 voxels) within the cluster [in mm] relative to the anterior commissure [AC] in the stereotactic space [Talairach and Tournoux, 1988]. Coordinates: x, lateral distance from the midline (+ right, − left); y, anteroposterior distance from the AC (+ anterior, − posterior); z, height relative to the AC line (+ above, − below).
P < 0.05 small volume corrected for multiple spatial comparisons. BA, Brodmann area; L, left; R, right; mid., middle; ant., anterior; inf., inferior; dPMC, dorsal premotor cortex.
When comparing signal increases that were more pronounced for execution of parallel than for mirror‐like finger tapping between both groups, musically naïve controls again showed significantly stronger activations than did musicians within a variety of motor association areas. Rostral and caudal parts of mesial premotor cortex (pre‐SMA, SMA proper), bilateral cerebellar hemispheres reaching into bilateral dentate nucleus and cerebellar vermis (P svc < 0.05), left ventral premotor cortex (vPMC, BA 6), left prefrontal cortex, middle frontal gyrus (BA 9), right prefrontal cortex, middle/superior frontal gyrus (BA 10, 46), left inferior frontal/orbitofrontal cortex (BA 47), right insula (BA 13), left fusiform gyrus (BA 37), right inferior temporal gyrus (BA 20), bilateral occipital lobe (cuneus, lingual gyrus, BA 17/18, 19), and right striatum showed stronger signal increases in controls than in pianists for parallel versus mirror movements (Fig. 2, Table III). Inter‐group comparison revealed no areas showing significantly stronger activations for controls than for pianists during performance of mirror compared to parallel movements.
Figure 2.
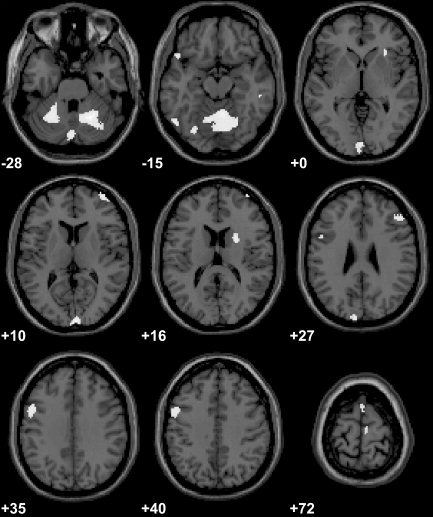
Brain regions showing significantly stronger activations in musically naïve controls than in professional pianists when comparing performance of parallel (anti‐phase) to mirror (in‐phase) finger movements (P < 0.001 uncorrected, extent threshold of 10 voxels). Clusters of activation are overlaid onto consecutive axial slices of a T1‐weighted anatomic MRI that was normalized stereotactically into the MNI standard space (right side of image corresponds to right hemisphere). Positions of the slices within this standard space are indicated (in mm) relative to the anterior commissure (+ above, − below).
Table III.
Areas with significantly stronger activations in musically naive controls compared to pianists during performance of parallel compared to mirror movements
| Cluster | Area | BA | x, y, z | T‐level |
|---|---|---|---|---|
| 1 | R mid. frontal, caudal SMA | 6 | 8, −12, 67 | 3.86a |
| 2 | R sup. frontal, pre‐SMA | 6 | 0, 13, 64 | 3.56a |
| 3 | R cerebellar hemisphere | — | 22, −59, −17 | 5.20a |
| L cerebellar hemisphere | — | −24, −54, −23 | 4.57a | |
| Cerebellar vermis | — | 8, −51, −8 | 5.00 | |
| 4 | L mid. frontal gyrus | 9 | −51, 6, 35 | 4.29 |
| L precentral, vPMC | 6 | −51, 2, 35 | 4.19 | |
| 5 | R mid. frontal gyrus | 46 | 48, 32, 21 | 4.05 |
| 6 | L inf. frontal | 47 | −57, 19, −6 | 3.75 |
| 7 | R insula | 13 | 30, 23, −3 | 3.86 |
| 8 | L fusiform gyrus | 37 | −51, −61, −14 | 4.68 |
| 9 | R inferior temporal gyrus | 20 | 55, −32, −15 | 4.36 |
| 10 | R occipital, lingual gyrus | 18 | 4, −94, 14 | 4.95 |
| L occipital, lingual gyrus | 17 | −2, −91, 1 | 4.34 | |
| 11 | L occipital, cuneus | 19 | −10, −92, 30 | 4.08 |
| 12 | R striatum | — | 24, 7, 14 | 4.33 |
Coordinates x, y, z express the position of the voxel with peak activation level (P < 0.001, uncorrected, extent threshold 10 voxels) within the cluster [in mm] relative to the anterior commissure [AC] in the stereotactic space [Talairach and Tournoux, 1988]. Coordinates: x, lateral distance from the midline (+ right, − left); y, anteroposterior distance from the AC (+ anterior, − posterior); z, height relative to the AC line (+ above, − below).
P < 0.05 small volume corrected for multiple spatial comparisons. BA, Brodmann area; L, left; R, right; mid., middle; SMA, supplementary motor area; sup., superior; vPMC: ventral premotor cortex; inf., inferior.
A 2 × 2 ANOVA for 4 subgroups including naïve controls or pianists with or without movement anticipation, respectively, revealed no significant overall or within‐group (controls, pianists) effect of anticipation upon activation patterns for contrasts of performance of parallel movements versus rest and versus mirror movements within any of the areas of interest.
DISCUSSION
Our findings show that professional musicians recruit an extensive motor network during complex bimanual movements to a lesser degree than do musically naïve controls. This comprises mesial premotor, rostral cingulate and right dorsal premotor cortex, bilateral cerebellar hemispheres, and additional activations within prefrontal cortex bilaterally, left ventral premotor cortex, inferior parietal cortex bilaterally, and right striatum. As this network was activated more strongly in musically naïve controls than in pianists during demanding anti‐phase finger movements, we infer that professional musical skills in pianists rely on an increased efficiency of the motor system for complex bimanual motor activity.
Our findings of stronger cortical and subcortical activations during performance of anti‐phase (parallel) compared to in‐phase (mirror) movements in musically naïve controls agree with previous work on bimanual motor control. The present bimanual motor task is well established and has been the subject of various functional imaging studies [Sadato et al., 1997; Stephan et al., 1999a, b]. They congruently demonstrated parallel movements to be more complex than mirror finger movements with stronger activations within a network of motor association areas including mesial premotor cortex, cingulate cortex, and right dorsal premotor cortex, similar to what we observed in our musically naïve controls. Likewise, increased activation in medial cerebellar areas has been attributed previously to control of bimanual movements [Tracy et al., 2001]. Neuronal recordings revealed contributions of SMA, cingulate motor areas, and dPMC in bimanual coordination [Kermadi et al., 2000; Tanji et al., 1988]. Lesion studies showed impaired bimanual coordination in patients with mesial premotor [Brinkman, 1984; Chan and Ross, 1988], lateral premotor [Freund and Hummelsheim, 1985; Halsband et al., 1993], and cerebellar pathologies [Ivry et al., 1988; Serrien and Wiesendanger, 2000].
In contrast to our study, many studies on bimanual motor control in healthy individuals focused on mesial and lateral premotor activations, as their analyses did not cover subcortical areas [Immisch et al., 2001; Jancke et al., 2000; Stephan et al., 1999a, b]. Other motor tasks were applied involving bimanual movements of the index finger [Immisch et al., 2001], nonsequential finger‐to‐thumb opposition movements [Nair et al., 2003], bimanual pronation–supination movements [Tracy et al., 2001], or hand tapping with different intermanual movement rates [Jancke et al., 2000]. Some investigated self‐paced movements [Jancke et al., 2000; Nair et al., 2003; Stephan et al., 1999b; Tracy et al., 2001] and most studies did not record kinematics during fMRI scanning. These methodologic differences could account for differences in our results from inter‐group comparison of untrained controls and highly trained pianists during performance of a complex, sequential, externally paced bimanual finger‐tapping task.
Intriguingly, professional musicians in our study revealed no significant differences in activation levels between parallel and mirror bimanual movements. Anti‐phase movements that are accepted to be more challenging for normal controls did not induce stronger signal increases in musicians than did less complex in‐phase movements. Accordingly, stronger signal increases were detected in musically naïve controls within the motor controlling network when comparing performance of parallel to mirror movements and to rest between both groups. We feel that this reflects a more efficient use of the motor control network in professional pianists compared to that in untrained controls. A less efficient bimanual control system in musically naïve controls implies that they had to invest more neuronal activity to achieve the same level of performance as that achieved by professional pianists. We suggest that this efficiency is the consequence of many years of motor training and experience, usually beginning in childhood, that is necessary to achieve highly overlearned levels of professional musical motor skills including an outstanding level of bimanual motor performance.
Electrophysiologic and neuroimaging studies investigating learning and training of various skills new to the subject showed sustained modulations and increases of cortical motor representations [Karni et al., 1995; Pascual‐Leone et al., 1995] as well as functional changes within different motor subsystems already due to learning over short periods [Grafton et al., 1995; Jenkins et al., 1994; Jueptner et al., 1997; Petersen et al., 1998; Raichle et al., 1994; Seitz and Roland, 1992; Shadmehr and Holcomb, 1997]. Long‐term training has been demonstrated to induce increases in structural and functional representations of cerebral areas considered especially developed in professional musicians [Amunts et al., 1997; Elbert et al., 1995; Sluming et al., 2002; Zatorre et al., 1998]. Activation levels of secondary motor association areas were lower in musicians compared to controls during unimanual complex motor tasks [Hund‐Georgiadis and von Cramon, 1999; Krings et al., 2000]. This agrees with our results, revealing that a highly developed motor control system is functionally less involved in musicians than in musically naïve controls during complex bimanual finger movements. This increased functional efficiency of an adapted motor control system together with experience‐driven structurally and functionally enlarged motor representations, as shown in professional musicians [Amunts et al., 1997], could be the basis for the highly specialized motor system in pianists leaving a higher number of degrees of freedom for extreme levels of bimanual motor performance.
The higher efficiency of motor association networks in musicians can be linked to different functional aspects of task‐computing that we suppose to be more strongly involved in untrained controls. We suggest the necessary amount of exact motor timing for synchronization of both hands to be higher in musically naïve controls and to decrease due to long‐term motor training in pianists. Supported by functional imaging data on motor timing [Jueptner et al., 1995; Kawashima et al., 2000; Penhune et al., 1998; Rao et al., 1997], this can especially explain higher cerebellar signal levels in our controls compared to pianists during the complex anti‐phase task [Ivry, 1997; Ivry et al., 1988].
A higher level of attention to action needed to carry out the demanding anti‐phase bimanual movements can explain further the stronger signal increases in musically naïve controls compared to professional pianists, within both anterior cingulate cortex and prefrontal cortex [Botvinick et al., 1999; Rowe et al., 2002; Smith and Jonides, 1999]. Additionally, stronger anterior cingulate and prefrontal activations could be attributed to higher demands for selection of the correct movement [Botvinick et al., 1999; Deiber et al., 1991] as well as for online monitoring of task performance and error detection [Carter et al., 1998; Cohen et al., 2000; Rowe et al., 2000]. In this context, stronger cerebellar activations in controls can be associated with monitoring and optimizing the outcome of movements using somatosensory feedback [Jueptner and Weiller, 1998].
Because of long‐term motor training, the increase in difficulty from mirror to parallel movements is reduced in pianists. The relative lack of bimanual motor training in musically naïve controls can be regarded as an increase in motor task complexity and is likely contributing to stronger activations of preSMA, dPMC, vPMC, and cerebellar structures in musically naïve controls in our study. These areas have been shown previously to be related to motor complexity [Boecker et al., 1998; Catalan et al., 1998; Harrington et al., 2000; Haslinger et al., 2002; Sadato et al., 1996; Tracy et al., 2001]. Compared to other bimanual motor studies in untrained controls these aspects of higher‐order motor control can contribute to stronger rostral premotor and prefrontal activation differences in controls opposed to pianists when contrasting parallel versus mirror movements.
Our findings show that the degree of functional recruitment of an extensive motor network differs in musicians and non‐musicians during complex bimanual movements. This suggests that long‐term musical motor training enables complex bimanual motor performance with a lower level of movement control, monitoring, selection, attention, and timing. Parallel to structural adaptations in musicians reported previously, we provide evidence of functional changes brought about by long‐term musical training. This could be the basis for an unequaled level of motor performance during professional piano playing, which is unreachable for musically untrained individuals. More than this, musicians can even focus their attention synchronously onto nonmotor, artistic aspects of musical performance.
Acknowledgements
We thank all subjects, especially the professional pianists, for participating in the study. Special thanks to M. Bauer (Hochschule für Musik und Theater, München) and Dr. T. Hitzlberger (Richard Strauss Konservatorium, München) for their support in recruiting the pianists.
REFERENCES
- Amunts K, Schlaug G, Jäncke L, Steinmetz H, Schleicher A, Dabringhaus A, Zilles K (1997): Motor cortex and hand motor skills: structural compliance in the human brain. Hum Brain Mapp 5: 206–215. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Li X, Alcantara AA, Isaacs KR, Black JE, Greenough WT (1994): Glial hypertrophy is associated with synaptogenesis following motor‐skill learning, but not with angiogenesis following exercise. Glia 11: 73–80. [DOI] [PubMed] [Google Scholar]
- Andres FG, Mima T, Schulman AE, Dichgans J, Hallett M, Gerloff C (1999): Functional coupling of human cortical sensorimotor areas during bimanual skill acquisition. Brain 122: 855–870. [DOI] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT (1990): Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA 87: 5568–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker H, Dagher A, Ceballos‐Baumann AO, Passingham RE, Samuel M, Friston KJ, Poline J, Dettmers C, Conrad B, Brooks DJ (1998): Role of the human rostral supplementary motor area and the basal ganglia in motor sequence control: investigations with H2 15O PET. J Neurophysiol 79: 1070–1080. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD (1999): Conflict monitoring versus selection‐for‐action in anterior cingulate cortex. Nature 402: 179–181. [DOI] [PubMed] [Google Scholar]
- Brinkman C (1984): Supplementary motor area of the monkey's cerebral cortex: short‐ and long‐term deficits after unilateral ablation and the effects of subsequent callosal section. J Neurosci 4: 918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998): Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280: 747–749. [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M (1998): The functional neuroanatomy of simple and complex sequential finger movements: a PET study. Brain 121: 253–264. [DOI] [PubMed] [Google Scholar]
- Chan JL, Ross ED (1988): Left‐handed mirror writing following right anterior cerebral artery infarction: evidence for nonmirror transformation of motor programs by right supplementary motor area. Neurology 38: 59–63. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Botvinick M, Carter CS (2000): Anterior cingulate and prefrontal cortex: who's in control? Nat Neurosci 3: 421–423. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS (1991): Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res 84: 393–402. [DOI] [PubMed] [Google Scholar]
- Elbert T, Candia V, Altenmuller E, Rau H, Sterr A, Rockstroh B, Pantev C, Taub E (1998): Alteration of digital representations in somatosensory cortex in focal hand dystonia. Neuroreport 9: 3571–3575. [DOI] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E (1995): Increased cortical representation of the fingers of the left hand in string players. Science 270: 305–307. [DOI] [PubMed] [Google Scholar]
- Freund HJ, Hummelsheim H (1985): Lesions of premotor cortex in man. Brain 108: 697–733. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J‐B, Frith CD, Frackowiak RSJ (1995): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Grafton ST, Hazeltine E, Ivry R (1995): Functional mapping of sequence learning in normal humans. J Cogn Neurosci 7: 497–510. [DOI] [PubMed] [Google Scholar]
- Halsband U, Ito N, Tanji J, Freund HJ (1993): The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain 116: 243–266. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Rao SM, Haaland KY, Bobholz JA, Mayer AR, Binderx JR, Cox RW (2000): Specialized neural systems underlying representations of sequential movements. J Cogn Neurosci 12: 56–77. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Weilke F, Ceballos‐Baumann AO, Bartenstein P, Grafin von Einsiedel H, Schwaiger M, Conrad B, Boecker H (2002): The role of lateral premotor‐cerebellar‐parietal circuits in motor sequence control: a parametric fMRI study. Brain Res Cogn Brain Res 13: 159–168. [DOI] [PubMed] [Google Scholar]
- Hund‐Georgiadis M, von Cramon DY (1999): Motor‐learning‐related changes in piano players and non‐musicians revealed by functional magnetic‐resonance signals. Exp Brain Res 125: 417–425. [DOI] [PubMed] [Google Scholar]
- Immisch I, Waldvogel D, van Gelderen P, Hallett M (2001): The role of the medial wall and its anatomical variations for bimanual antiphase and in‐phase movements. Neuroimage 14: 674–684. [DOI] [PubMed] [Google Scholar]
- Ivry R (1997): Cerebellar timing systems. Int Rev Neurobiol 41: 555–573. [PubMed] [Google Scholar]
- Ivry RB, Keele SW, Diener HC (1988): Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Exp Brain Res 73: 167–180. [DOI] [PubMed] [Google Scholar]
- Jancke L, Peters M, Himmelbach M, Nosselt T, Shah J, Steinmetz H (2000): fMRI study of bimanual coordination. Neuropsychologia 38: 164–174. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE (1994): Motor sequence learning: a study with positron emission tomography. J Neurosci 14: 3775–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jueptner M, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE (1997): Anatomy of motor learning. II. Subcortical structures and learning by trial and error. J Neurophysiol 77: 1325–1337. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Rijntjes M, Weiller C, Faiss JH, Timmann D, Mueller SP, Diener HC (1995): Localization of a cerebellar timing process using PET. Neurology 45: 1540–1545. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Weiller C (1998): A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain 121: 1437–1449. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG (1995): Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377: 155–158. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Okuda J, Umetsu A, Sugiura M, Inoue K, Suzuki K, Tabuchi M, Tsukiura T, Narayan SL, Nagasaka T and others (2000): Human cerebellum plays an important role in memory‐timed finger movement: an fMRI study. J Neurophysiol 83: 1079–1087. [DOI] [PubMed] [Google Scholar]
- Keller A, Arissian K, Asanuma H (1992): Synaptic proliferation in the motor cortex of adult cats after long‐term thalamic stimulation. J Neurophysiol 68: 295–308. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Diedrichsen J, Hazeltine E, Semjen A, Ivry RB (2002): Callosotomy patients exhibit temporal uncoupling during continuous bimanual movements. Nat Neurosci 5: 376–381. [DOI] [PubMed] [Google Scholar]
- Kermadi I, Liu Y, Rouiller EM (2000): Do bimanual motor actions involve the dorsal premotor (PMd), cingulate (CMA) and posterior parietal (PPC) cortices? Comparison with primary and supplementary motor cortical areas. Somatosens Mot Res 17: 255–271. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT (1996): Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci 16: 4529–4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krings T, Topper R, Foltys H, Erberich S, Sparing R, Willmes K, Thron A (2000): Cortical activation patterns during complex motor tasks in piano players and control subjects. A functional magnetic resonance imaging study. Neurosci Lett 278: 189–193. [DOI] [PubMed] [Google Scholar]
- McGonigle DJ, Howseman AM, Athwal BS, Friston KJ, Frackowiak RS, Holmes AP (2000): Variability in fMRI: An examination of intersession differences. Neuroimage 11: 708–734. [DOI] [PubMed] [Google Scholar]
- Mechsner F, Kerzel D, Knoblich G, Prinz W (2001): Perceptual basis of bimanual coordination. Nature 414: 69–73. [DOI] [PubMed] [Google Scholar]
- Nair DG, Purcott KL, Fuchs A, Steinberg F, Kelso JA (2003): Cortical and cerebellar activity of the human brain during imagined and executed unimanual and bimanual action sequences: a functional MRI study. Brain Res Cogn Brain Res 15: 250–260. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Pantev C, Oostenveld R, Engelien A, Ross B, Roberts LE, Hoke M (1998): Increased auditory cortical representation in musicians. Nature 392: 811–814. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone A, Nguyet D, Cohen LG, Brasil‐Neto JP, Cammarota A, Hallett M (1995): Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74: 1037–1045. [DOI] [PubMed] [Google Scholar]
- Penhune VB, Zattore RJ, Evans AC (1998): Cerebellar contributions to motor timing: a PET study of auditory and visual rhythm reproduction. J Cogn Neurosci 10: 752–765. [DOI] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME (1998): The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci USA 95: 853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL (1996): Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 6: 342–353. [DOI] [PubMed] [Google Scholar]
- Pujol J, Roset‐Llobet J, Rosines‐Cubells D, Deus J, Narberhaus B, Valls‐Sole J, Capdevila A, Pascual‐Leone A (2000): Brain cortical activation during guitar‐induced hand dystonia studied by functional MRI. Neuroimage 12: 257–267. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod AM, Pardo JV, Fox PT, Petersen SE (1994): Practice‐related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex 4: 8–26. [DOI] [PubMed] [Google Scholar]
- Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR (1997): Distributed neural systems underlying the timing of movements. J Neurosci 17: 5528–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Brouwer B, Nordstrom MA (2000): Reduced interhemispheric inhibition in musicians. Exp Brain Res 133: 249–253. [DOI] [PubMed] [Google Scholar]
- Rowe J, Friston K, Frackowiak R, Passingham R (2002): Attention to action: specific modulation of corticocortical interactions in humans. Neuroimage 17: 988–998. [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE (2000): The prefrontal cortex: response selection or maintenance within working memory? Science 288: 1656–1660. [DOI] [PubMed] [Google Scholar]
- Sadato N, Campbell G, Ibanez V, Deiber M, Hallett M (1996): Complexity affects regional cerebral blood flow change during sequential finger movements. J Neurosci 16: 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadato N, Yonekura Y, Waki A, Yamada H, Ishii Y (1997): Role of the supplementary motor area and the right premotor cortex in the coordination of bimanual finger movements. J Neurosci 17: 9667–9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G (2001): The brain of musicians. A model for functional and structural adaptation. Ann N Y Acad Sci 930: 281–299. [PubMed] [Google Scholar]
- Schlaug G, Jancke L, Huang Y, Staiger JF, Steinmetz H (1995a): Increased corpus callosum size in musicians. Neuropsychologia 33: 1047–1055. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Jancke L, Huang Y, Steinmetz H (1995b): In vivo evidence of structural brain asymmetry in musicians. Science 267: 699–701. [DOI] [PubMed] [Google Scholar]
- Schneider P, Scherg M, Dosch HG, Specht HJ, Gutschalk A, Rupp A (2002): Morphology of Heschl's gyrus reflects enhanced activation in the auditory cortex of musicians. Nat Neurosci 5: 688–694. [DOI] [PubMed] [Google Scholar]
- Scholz JP, Kelso JA (1990): Intentional switching between patterns of “bimanual” coordination depends on the intrinsic dynamics of the pattern. J Mot Behav 22: 98–124. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Roland PE (1992): Learning of sequential finger movements in man: a combined kinematic and positron emission tomography (PET) study. Eur J Neurosci 4: 154–165. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Nirkko AC, Wiesendanger M (2001): Role of the corpus callosum in bimanual coordination: a comparison of patients with congenital and acquired callosal damage. Eur J Neurosci 14: 1897–1905. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Wiesendanger M (2000): Temporal control of a bimanual task in patients with cerebellar dysfunction. Neuropsychologia 38: 558–565. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH (1997): Neural correlates of motor memory consolidation. Science 277: 821–825. [DOI] [PubMed] [Google Scholar]
- Sluming V, Barrick T, Howard M, Cezayirli E, Mayes A, Roberts N (2002): Voxel‐based morphometry reveals increased gray matter density in Broca's area in male symphony orchestra musicians. Neuroimage 17: 1613–1622. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J (1999): Storage and executive processes in the frontal lobes. Science 283: 1657–1661. [DOI] [PubMed] [Google Scholar]
- Stephan KM, Binkofski F, Halsband U, Dohle C, Wunderlich G, Schnitzler A, Tass P, Posse S, Herzog H, Sturm V and others (1999a): The role of ventral medial wall motor areas in bimanual co‐ordination. A combined lesion and activation study. Brain 122: 351–368. [DOI] [PubMed] [Google Scholar]
- Stephan KM, Binkofski F, Posse S, Seitz RJ, Freund HJ (1999b): Cerebral midline structures in bimanual coordination. Exp Brain Res 128: 243–249. [DOI] [PubMed] [Google Scholar]
- Strange BA, Portas CM, Dolan RJ, Holmes AP, Friston KJ (1999): Random effects analyses for event‐related fMRI. Neuroimage 9: 36. [Google Scholar]
- Talairach J, Tournoux PA (1988): Co‐Planar stereotactic atlas of the human brain. Stuttgart: Thieme. [Google Scholar]
- Tanji J, Okano K, Sato KC (1988): Neuronal activity in cortical motor areas related to ipsilateral, contralateral, and bilateral digit movements of the monkey. J Neurophysiol 60: 325–343. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Faro SS, Mohammed FB, Pinus AB, Madi SM, Laskas JW (2001): Cerebellar mediation of the complexity of bimanual compared to unimanual movements. Neurology 57: 1862–1869. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Perry DW, Beckett CA, Westbury CF, Evans AC (1998): Functional anatomy of musical processing in listeners with absolute pitch and relative pitch. Proc Natl Acad Sci USA 95: 3172–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]


