Abstract
Brain imaging research has identified at least two regions in human extrastriate cortex responding selectively to faces. One of these is located in the mid‐fusiform gyrus (FFA), the other in the inferior occipital gyrus (IOG). We studied activation of these areas using fMRI in three individuals with severely impaired face recognition (one pure developmental and two childhood prosopagnosics). None of the subjects showed the normal pattern of higher fMRI activity to faces than to objects in the FFA and IOG or elsewhere. Moreover, in two of the patients, faces and objects produced similar activations in the regions corresponding to where the FFA and IOG are found in normal subjects. Our study casts light on the important role of FFA and IOG in the network of areas involved in face recognition, and indicates limits of brain plasticity. Hum. Brain Mapping 16:176–182, 2002. © 2002 Wiley‐Liss, Inc.
Keywords: prosopagnosia, face recognition, object recognition, brain plasticity, fMRI
INTRODUCTION
In the neurological syndrome of prosopagnosia, damage to ventral occipitotemporal regions (usually in the right hemisphere) leads to severe deficits in face recognition [Damasio et al., 1982, 1990]. In developmental prosopagnosia, selective deficits in face recognition [de Gelder and Rouw, 2001] exist throughout life in the absence of any known brain damage [Bentin et al., 1999; de Gelder and Rouw, 2000b]. Although prosopagnosia is often accompanied by impairments in some other recognition tasks [de Gelder et al., 1998], the deficit is relatively specific for faces, with essentially normal performance in the recognition of other classes of visual stimuli including animal faces [de Gelder, 1998; Gauthier et al., 1999; McNeil and Warrington, 1993].
A number of brain imaging studies carried out in the last decade have identified cortical regions that are activated when subjects view faces [Haxby et al., 1994; Sergent et al., 1992]. Several of these regions have been shown to respond in a highly selective fashion to face stimuli. A region in the mid‐fusiform gyrus known as the fusiform face area (FFA) produces at least twice the response in fMRI to face stimuli (including cartoon faces, cat faces, and inverted faces) as to a wide variety of control stimuli such as houses, hands, the backs of human heads, scrambled faces, and flowers [Chao et al., 1999; Kanwisher et al., 1997; McCarthy et al., 1997; Tong, 2000]. Another region in the inferior occipital gyrus called the IOG [Hoffman and Haxby, 2000] has been less well studied, but has been shown to respond more strongly to faces than to objects or houses in half or more of subjects scanned [Halgren et al., 1999; Haxby et al., 1999; Lerner et al., 2001; Levy et al., 2001; Puce et al., 1996].
In the present study, we scanned three prosopagnosic patients using fMRI. One of the patients (AV, M, 42 years) is a pure developmental prosopagnosic, whereas the two other suffered a closed head injury in childhood. Developmental prosopagnosia is a face recognition deficit occurring in the absence of an established neurological disease [McConachie, 1976]. Patient GA (M, 27 years) suffered from a head injury at 18 months, whereas Patient RP (M, 49 years) was injured at age 7 years. As is often the case in closed head injury, none of the patients had evident lesions on the MR scan. If the FFA or the IOG are the critical areas that produce prosopagnosia when damaged, absent, or undeveloped, then we should find no evidence for face‐selective response in either region in any of the three patients. Moreover, there might be an overlap between object and face activation in those areas. This would explain previous data indicating that abnormal inversion effect for faces are equally found for objects [de Gelder, 1998; de Gelder and Rouw, 2000a].
A further question addressed in the present study concerns cortical plasticity, and the consequences on cortical organization of a lesion acquired before face perception is fully developed [Farah et al., 2000; Le Grand et al., 2001]. Independently of the issue of localization and specificity of brain areas for face recognition, developmental prosopagnosia suggests that there are substantial limits on plasticity, and that the role of FFA and IOG in the network implementing face recognition may not be taken over by functionally or neuroanatomically related areas.
Detailed reports of results from AV and RP on a number of experimental tasks probing among other things the inversion effect for objects and faces, holistic processing, dependence on featural analysis and encoding in memory were reported previously [de Gelder and Rouw, 2000b; de Gelder and Rouw, 2001].
MATERIALS AND METHODS
Tasks
The low‐level vision tasks used were the Benton Visual Form Discrimination, and the Benton Line Orientation. Object recognition was tested with the Boston Naming test, the BORB [Riddoch and Humphreys, 1993] and a picture naming task [Snodgrass and Vanderward, 1980]. Face recognition was tested using the Warrington [1984] and the Benton tests [Benton and Van Allen, 1968].
Except for the modifications detailed below, the methods in this study are similar to those described previously [Tootell et al., 1997]. Informed written consent was obtained for each subject before the scanning session, and all procedures were approved by Massachusetts General Hospital Human Studies Protocol numbers #96‐7464. MR images of brain activity were collected from the patients and from two normal controls using a high‐field (3T) scanner, with echo‐planar imaging (gradient echo, echo time (TE) = 30 msec, repetition time (TR) 2 sec, flip angle 90°, FOV = 24 cm, 64 × 64 matrix). MR images were acquired using a custom‐built quadrature surface coil, shaped to fit to the posterior portion of the head. Sixteen contiguous 3–4 mm MR slices were obtained, with an in‐plane resolution of 3.1 × 3.1 mm, oriented approximately perpendicular to the calcarine fissure. A bite bar was used in most of the subjects to minimize head motion. High‐resolution T1‐weighted images spoiled gradient recall (SPGR) images were obtained for each subject to provide detailed anatomy (124 1.2 mm thick sagittal images, FOV = 24 cm). They were segmented, reconstructed, inflated and flattened [Dale et al., 1999; Fischl et al., 1999]. Data were analyzed in flattened cortical format, as described elsewhere [Hadjikhani et al., 1998; Tootell, 1997].
During the scanning session, AV and RP passively viewed stimuli of various kinds alternating with fixation epochs. Each scan lasted 5'36” and consisted of 16 16“ stimulus epochs arranged in blocks of four consecutive stimulus epochs (Faces, Objects, Houses, Scenes). A 16‐ sec fixation epoch was interleaved between each block. The order of the epoch type was counterbalanced over two versions of each experiment. During each epoch, 20 photographs of the same type were shown. Each photograph was presented for 300 msec followed by a blank interval of 500 msec. All stimuli consisted of grayscale photographs or photorealistic drawings 300 × 300 pixels in size. AV was run three times during two sessions, and RP was run twice in two sessions. These stimuli have been extensively used in previous work on normal viewers by Kanwisher et al. [1997].
GA and the normal controls were scanned using a slightly different protocol. Each scan lasted for 4'16”, and consisted of alternating 16‐sec epochs. Subjects were presented with grayscale pictures of faces and objects, and Fourier scrambled versions of these pictures. The stimuli all had a fixation point and were contained within a circle (480 pixels diameter) to control for retinotopic differences. The presentation order was faces vs. scrambled faces, objects vs. scrambled objects, and faces vs. objects. Each photograph was presented for 1,800 msec followed by a blank interval of 200 msec.
The subject's task in all experiment was to fixate the center of the visual stimulus throughout the period of scan acquisition.
Statistics
For periodic stimulus manipulations, (two‐condition comparisons), a Fourier analysis was done on the time series of each voxel. Significance values were computed by performing an F‐test on the ratio of the signal at the stimulus cycle frequency (8 cycles/scan) compared to the average power at all frequencies (4–64 cycles/scan), excluding the first and second harmonics and very low frequencies (1–3 cycles/scan) to remove baseline drift and head motion artifacts. Harmonic frequencies were excluded because any periodic signal that is not perfectly sinusoidal will be expressed by the sum of sine waves at its fundamental frequency and all of its harmonics. The phase of the signal at the stimulus frequency was used to distinguish between signal increases and decreases in the MR signal for two‐condition comparisons. Under the assumption of white (temporally uncorrelated) noise, the power at each frequency is an independent, identically distributed chi‐square random variable, and so the resulting ratio of signal power is F‐distributed. On this basis, the significance of the activation at each voxel was determined using an F‐statistic.
In the experiment using more than two conditions, the statistical significance maps were computed using linear regression analysis. The fMRI signal was modeled as a linear convolution of a hemodynamic impulse function with a 2‐sec interval introduced to account for hemodynamic delay [Dale and Buckner, 1997], with a neuronal activation function that was assumed to be constant during each epoch. The activation amplitude for each condition was estimated from the fMRI time course at each voxel, by fitting the fMRI signal model to the observed time course. The significance of the difference between the activation amplitudes of different conditions was computed using a standard t‐statistic.
RESULTS
Before the present study, all three patients underwent extensive neuropsychological testing. Low level visual processing was tested with the Benton Visual Form discrimination, the Benton line orientation and selected subtests (line length, size, orientation, gap size) from the Birmingham Object Recognition Battery (BORB) [Riddoch and Humphreys, 1993]. Object recognition was tested with the Boston naming test, the Snodgrass and Vanderwart picture naming tests and selected subtests (overlapping shapes, minimal features, foreshortened views and object decision) from the BORB. Performance of all three patients on these tests fell within the normal range reported for these test batteries. Face recognition was tested with the Warrington face recognition test [Warrington, 1984] and the Benton face recognition test [Benton and Van Allen, 1968]. AV obtained a Warrington score of 34/50 (normal range is 38–50) and a Benton score of 34/54 (normal range is 41–54), GA received scores of 29/50 and 27/54 respectively and RP's performance was 32/50 and 31/54 on the same two tests.
It has been shown in many studies done with normal subjects that two regions respond more to faces than objects (Fig. 1, left). These regions are: 1) an area situated in the ventral temporal cortex, within the fusiform gyrus (FFA), which produces clear face‐specific activations in at least 80% of normal subjects [Halgren, 1999; Haxby, 1999; Kanwisher 1997; Lerner, 2001; Levy, 2001; Puce, 1996]; and 2) an area situated in the inferior occipital gyrus (IOG), between areas V8 [Hadjikhani, 1998], MT [Tootell et al., 1995] and LOC/LOP [Tootell and Hadjikhani, 2001], which produces face‐selective activations in at least half of normal subjects scanned [Halgren, 1999; Haxby, 1999; Kanwisher, 1997; Lerner, 2001; Levy, 2001; Puce, 1996]. The inverse comparison of objects vs. faces activated areas in the lateral occipital cortex (excluding the IOG area), the ventral temporal cortex and the posterior parietal cortex (see yellow activation, Fig. 1, left).
Figure 1.
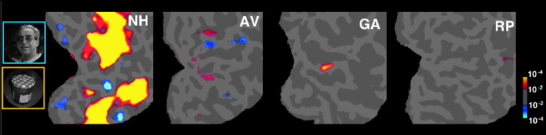
Regions in each subject showing a significantly greater response to faces than objects (in blue) or vice versa (in yellow) for one normal subject (NH) and the three prosopagnosic subjects. Note the FFA (lower blue activation spot) and the IOG (upper blue activation spot) in normal subject NH. Data are represented in a flattened representation of the occipital cortex in the right hemisphere [Hadjikhani, 1998; Sereno et al., 1995; Tootell, 1997]. Gyri appear in light gray whereas sulci are in darker gray.
We obtained very different results in our three prosopagnosic patients. None of the patients showed any face‐selective activation (i.e., a stronger response to faces than objects) anywhere in the ventral visual pathway, even at the very permissive statistical threshold of P < 10−2 (uncorrected). This finding is consistent with our hypothesis that intact FFA or IOG are an essential part of the network of brain areas involved in normal face recognition.
To see how the regions that are normally face‐selective respond to other stimuli, we next compared the response in each patient to faces and a control stimulus (scrambled objects for GA and the control patients; houses for Patients AV and RP). In AV, the developmental prosopagnosic, we found very similar activations for faces compared to houses as well as for objects compared to houses, in regions that appear to correspond to the FFA and the IOG (see Fig. 2C,H).
Figure 2.
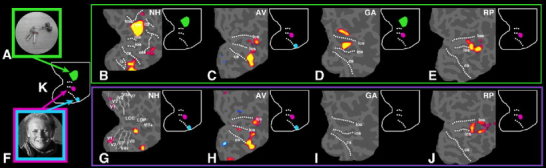
Activation obtained for objects (B–E) and faces (G–J) compared to control stimuli in the right hemisphere of a normal subject (B,G) and in three prosopagnosic patients (C–E,H–J). As in Figure 1, data are represented in a flattened representation of the occipital cortex, with gyri appearing in light gray whereas sulci are in darker gray. The dotted lines in (B) represent the major sulci: (tos, transverse occipital sulcus; ips, intraparietal sulcus; its, inferior temporal sulcus; ots, occipito‐temporal sulcus; los, lateral occipital sulcus; ios, inferior occipital sulcus; cs, collateral sulcus; ls, lingual sulcus). G: Shows the location of the different visual areas obtained by retinotopic mapping [Hadjikhani et al., 1998; Sereno, 1995; Tootell, 1997]. (A) Object and (F) face show an example of the stimuli used in the experiment, and (K) is a diagram of the activation obtained for objects (in green) compared to scrambled objects and faces compared to scrambled faces (in blue and magenta) in a normal representative subject. On the upper right part of each panel, a diagram indicates the areas activated in each subject in comparison with the normal subject (K). In normal subjects, the viewing of objects compared to scrambled objects (B) elicited activation principally in the lateral occipital cortex (LO, in green in the diagram), whereas faces compared to scrambled faces activated two areas: the anterior part of the collateral sulcus and fusiform gyrus (FFA, in blue) and the inferior occipital gyrus and sulcus (IOG, in magenta). In Patient AV, the developmental prosopagnosic, viewing both objects compared to houses (C) and faces compared to houses (H) activated the same region of cortex, in the anterior part of the collateral sulcus and the fusiform gyrus (FFA). Objects did not produce activation in the expected LO region, and faces failed to activate the IOG area. In Patient GA, injured at 18 months, faces failed to elicit any activation in the occipital cortex compared to scrambled faces (I) whereas objects compared to scrambled objects activated both the LO and the IOG area (D). Finally, Patient RP, injured at 7 years, showed similar activation to both faces compared to houses (J) and objects compared to houses (E), situated in the IOG region. No activation was seen in the FFA area. None of the prosopagnosic patients showed activation in both the FFA and the IOG to face presentation. Instead they showed activation in either one or the other, or none at all.
In Patient GA, who was injured at 18 months of age, presentation of faces (in comparison to scrambled faces) failed to activate any part of the brain. A comparison of objects vs. scrambled objects, however, did produce activation in the LO region and in the IOG (Fig. 2D,I).
In Patient RP, who sustained a head injury at age 7, faces vs. houses, and objects vs. houses, both activated the LO and the IOG region. In contrast, the comparison of faces vs. objects did not show any differential activation, suggesting that the same areas are involved (to a similar degree) in processing faces and objects (Fig. 2E,J and Table I).
Table I.
| Faces | Objects | |
|---|---|---|
| AV‐developmental | FFA | FFA |
| GA‐early affected | No activation | IOG and LO |
| RP‐affected later | IOG | IOG and LO |
| Control subjects | FFA and IOG | LO, inferior temporal cortex, posterior parietal cortex |
DISCUSSION
The two areas selectively activated for faces in our normal controls correspond to well known areas in the literature, namely the fusiform gyrus (FFA) and the inferior occipital gyrus (IOG) [Halgren, 1999; Haxby, 1999; Kanwisher, 1997; Lerner, 2001; Levy, 2001; Puce, 1996]. None of the three prosopagnosic patients we scanned showed any evidence of either of these face‐selective responses. At 1.5 T, 80% of normal subjects show face‐selective activation of the FFA [Kanwisher, 1997]. The lack of such activation in three prosopagnosic patients found here reflects a significant difference from the normal population, because the probability of finding three normal subjects without FFA by chance is P < 0.008 (i.e., 0.2 cubed). This lack of face‐selective activations occurred together with the fact that the same subjects showed a partly normal pattern of activation during object viewing. These findings are consistent with our hypothesis that the face‐selective responses in the FFA and IOG are important parts of the network that subserves the face recognition processes that are lost in prosopagnosia. The present study used a surface coil that covered the occipital cortex and the posterior part of the parietal and the temporal cortices. We cannot exclude from our data that more anterior areas in the brain that convey information about face identity [Allison et al., 1999; McCarthy et al., 1999; Puce et al., 1999] did respond normally to faces and objects.
It has been argued that objects are represented in a distributed fashion across ventral temporal cortex with a relative degree of specialization for faces and objects [Chao, 1999; Haxby et al., 2000, 2001]. In line with this, we find similar extrastriate responses to faces and objects alike for subjects AV and RP, but no regions that respond selectively to faces. The presence of severe prosopagnosia in these subjects, however, indicates that non‐selective responses to faces in these subjects are apparently not sufficient to sustain normal face functions.
Another implication of the present work concerns the visual processes that apparently do not require face‐selectivity of the FFA or IOG. Our prosopagnosic subjects have no difficulty detecting faces in a noise pattern or deciding whether a stimulus is a face even from very brief exposures like 50 msec [de Gelder and Rouw, 2000b]. Their deficit is one of recognizing and discriminating between individual faces, not one of perceiving that the stimulus is a face. Thus if their behavioral deficits are indeed causally related to their lack of a normal FFA or IOG (as we suggest), the present data also indicate that these regions may be necessary for face recognition but not for face detection [de Gelder and Rouw, 2001].
Our data are relevant to plasticity of the cortical regions subserving face processing. Recovery from acquired prosopagnosia appears to be rare, suggesting constraints on the regions of cortex that can take over this task, at least in adulthood. Our results suggest that when early commitment of cortical regions for face recognition appears to be absent (as is typically the case in developmental prosopagnosia), functionally or anatomically related areas do not, at least in these patients, compensate for this and bootstrap the acquisition of normal face recognition behavior. The specialization observed in normal adults is not seen in AV and the area corresponding to the anatomical location of the FFA indistinctly processes faces and objects. Two recent studies suggest surprisingly early commitment of cortical regions for face recognition. In one, a patient developed prosopagnosia as a result of brain damage sustained at day one of age [Farah, 2000]. This case, however, had additional visual deficits in other visual tasks, making it somewhat difficult to draw conclusions about the development of face processing per se. In a striking recent finding, Le Grand et al. [2001] report that patients with congenital cataracts that were surgically corrected at a few months of age never developed normal face recognition as adults (despite normal performance on inverted faces). Our three cases of early‐onset prosopagnosia are consistent with this pattern, and further illustrate the apparent inability of other intact regions of the ventral visual pathway to take on face‐selective visual processing.
CONCLUSION
Our study shows that when areas subserving face recognition are damaged during development, face recognition does not get efficiently implemented and overlaps with other areas devoted to object recognition. Areas that are specialized for face recognition in normal adults can subserve object recognition in developmental prosopagnosia. Taken together, our results indicate some of the limits of cortical plasticity in the case of face perception.
Acknowledgements
We thank Dr. A. Dale and Dr. B. Fischl for the use of their software for analyzing and displaying results on flattened cortical surfaces, Dr. G. Ganis for helping in the preparation of the Fourier scrambled faces, Dr. N. Kanwisher for his critical comments on the paper, Dr. B. Rosen for all his support, and the patients for their patience and willingness to cooperate.
REFERENCES
- Allison T, Puce A, Spencer DD, McCarthy G (1999): Electrophysiological studies of human face perception. I: Potentials generated in occipitotemporal cortex by face and non‐face stimuli. Cereb Cortex 9: 415–430. [DOI] [PubMed] [Google Scholar]
- Bentin AL, Van Allen MW (1968): Selective visual streaming in face recognition: evidence from developmental prosopagnosia. Neuroreport 10: 823–827. [DOI] [PubMed] [Google Scholar]
- Benton AL, Van Allen MW (1968): Impairment in facial recognition in patients with cerebral disease. Trans Am Neurol Assoc 93: 38–42. [PubMed] [Google Scholar]
- Chao LL, Martin A, Haxby JV (1999): Are face‐responsive regions selective only for faces? Neuroreport 10: 2945–2950. [DOI] [PubMed] [Google Scholar]
- Dale AM, Buckner RL (1997): Selective averaging of individual trials using fMRI. Hum Brain Mapp 5: 329–340. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis I: segmentation and surface reconstruction. Neuroimage 9: 179–194. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H, Van Hoesen GW (1982): Prosopagnosia: anatomic basis and behavioral mechanisms. Neurology 32: 331–341. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H (1990): Face agnosia and the neural substrates of memory. Annu Rev Neurosci 13: 89–109. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Bachoud‐Levi AC, Degos JD (1998): Inversion superiority in visual agnosia may be common to a variety of orientation polarized objects besides faces. Vision Res 38: 2855–2861. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Rouw R (2000a): Structural encoding precludes recognition of parts in prosopagnosia. Cognitive Neuropsychology 17: 89–102. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Rouw R (2000b): Configural face processes in acquired and developmental prosopagnosia: evidence for two separate face systems? Neuroreport 11: 3145–3150. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Rouw R (2001): Beyond localization: a dynamic dual route account of face recognition. Acta Psychol 107: 183–207. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Rabinowitz C, Quinn GE, Liu GT (2000): Early commitment of neural substrates for face recognition J Cogn Neurosci 17: 117–124. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM (1999): Cortical surface‐based analysis. II: inflation, flattening, and a surface‐based coordinate system. Neuroimage 9: 195–207. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Behrman M, Tarr MJ (1999): Can face recognition really be dissociated from object recognition. J Cogn Neurosci 11: 349–370. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Liu AK, Dale AM, Cavangh P, Tootell RBH (1998): Retinotopy and color selectivity in human visual cortical area V8. Nat Neurosci 1: 235–241. [DOI] [PubMed] [Google Scholar]
- Halgren E, Dale AM, Sereno MI, Tootell RB, Marinkovic K, Rosen BR (1999): Location of human face‐selective cortex with respect to retinotopic areas. Hum Brain Mapp 7: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P (2001): Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293: 2425–2430. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI (2000): the distributed human neural system for face perception. Trends Cogn Sci 4: 223–233. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Horowitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL (1994): The functional organization of human extrastriate cortex: a PET‐rCBF study of selective attention to faces and locations. J Neurosci 14: 6336–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Clark VP, Schouten JL, Hoffman EA, Matin A (1999): the effect of face inversion on activity inhuman neural systems for and object perception. Neuron 22: 189–199. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Haxby JV (2000): Distinct representations of eye gave and identity in the distributed human neural system for face perception. Nat Neurosci 3: 80–84. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM (1997): The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand R, Mondloch CJ, Maurer D, Brent HP (2001): Early visual experience and face processing. Nature 410: 890. [DOI] [PubMed] [Google Scholar]
- Lerner Y, Hendler T, Ben‐Nashat D, Harel M, Malach R (2001): A hierarchical axis of object processing stages in the human visual cortex. Cereb Cortex 11: 287–297. [DOI] [PubMed] [Google Scholar]
- Levy I, Hasson U, Avidan G, Hendler T, Malach R (2001): Center‐periphery organization of human object areas. Nat Neurosci 4: 533–539. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Belger A, Allison T (1999): Electrophysiological studies of human face perception. II: Response properties of face‐specific potentials generated in occipitotemporal cortex. Cereb Cortex 9: 431–444. [DOI] [PubMed] [Google Scholar]
- McConachie HR (1976): Developmental prosopagnosia. A single case report. Cortex 12: 76–82. [DOI] [PubMed] [Google Scholar]
- McNeil JE, Warrington RK (1993): Prosopagnosia: a face‐specific disorder. Q J Exp Psychol A 46: 1–10. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Asagari M, Gore JC, McCarthy G (1996): Differential sensitivity of human visual cortex to faces, letter strings and textures: a functional magnetic resonance imaging study. J Neurosci 16: 5205–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddoch MJ, Humphreys GW (1993): Birmingham object recognition battery. Hove: Psychology Press. [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB (1995): Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging [see comments]. Science 268: 889–893. [DOI] [PubMed] [Google Scholar]
- Sergent J, Ohta S, MacDonald B (1992): Functional neuroanatomy of face and object processing. A positron emission tomography study. Brain 115: 15–36. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderward M (1980): A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol 6: 174–215. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Hadjikhani N (2001): Where is ‘dorsal v4’ in human visual cortex? Retinotopic, topographic, and functional evidence. Cereb Cortex 11: 298–311. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Mendola JD, Hadjikhani NK, Ledden PJ, Liu AK, Reppas JB, Sereno MI, Dale AM (1997): Functional analysis of V3A and related areas in human visual cortex. J Neurosci 17: 7060–7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Rosen BR, Belliveau JW (1995): Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci 15: 3215–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK (1984): Recognition memory test. Nelson, Windsor: NFER. [Google Scholar]


