Abstract
The successful control of upper limb movements is an essential skill of the human motor system. Yet, the neural organization of bimanual actions remains an issue of debate. Their control can be directed from both hemispheres, or, coordinated motion might be organized from the dominant (left) hemisphere. In order to unravel the neural mechanisms of bimanual behavior, we analyzed the standard task‐related and directed coherence between EEG signals picked up over the primary sensorimotor cortices in right‐handed subjects during unimanual as well as bimanual in‐phase (symmetrical) and anti‐phase (asymmetrical) movements. The interhemispheric coherence in the β frequency band (>13–30 Hz) was increased in both unimanual and bimanual patterns, compared to rest. During unimanual actions, the drive in the β band from one primary sensorimotor cortex to the other was greater during movement of the contralateral as opposed to ipsilateral hand. In contrast, during bimanual actions, the drive from the dominant to the non‐dominant primary sensorimotor cortex prevailed, unless task constraints induced by an external perturbation resulted in a substantial uncoupling of the hand movements, when interhemispheric coherence would also drop. Together, these results suggest that the contralateral hemisphere predominantly organizes unimanual movements, whereas coupled bimanual movements are mainly controlled from the dominant hemisphere. The close association between changes in interhemispheric coupling and behavioral performance indicates that synchronization of neural activity in the β band is exploited for the control of goal‐directed movement. Hum. Brain Mapping 18:296–305, 2003. © 2003 Wiley‐Liss, Inc.
Keywords: EEG, task‐related coherence, functional coupling
INTRODUCTION
When performing bimanual patterns, there exists an innate tendency to simultaneity of motion. For rhythmical actions, this tendency translates itself into isofrequency coordination whereby the segments are moved according to an in‐phase or anti‐phase mode. Both activities are spontaneously adopted configurations, although the in‐phase mode represents the more archaic pattern of coordination [Kelso, 1984], as confirmed by functional imaging [Fink et al., 1999; Sadato et al., 1997] and transcranial magnetic stimulation [Serrien et al., 2002]. The neural organization of these bimanual patterns remains a controversial issue. Their control can be directed from both hemispheres, or, the coordinated motion might be organized from one hemisphere. In particular, the importance of the left hemisphere for the representation of skilled behavior has been emphasized [Haaland et al., 2000]. Lesion studies have shown that left hemisphere damage produces bilateral deficits on a variety of motor tasks whereas right hemisphere damage is more prone to produce contralateral deficits [Haaland and Harrington, 1994]. Conversely, it has been suggested that bimanual movements may be controlled by each contralateral motor cortex independently [Foltys et al., 2001; Garry and Franks, 2002]. Therefore, incongruent evidence exists concerning the control mechanisms that underlie coordinated movements of the upper limbs. In contrast to bimanual movements, the organization of unimanual movements has been more thoroughly evaluated. In particular, a variety of analysis techniques have shown that unimanual patterns involve a strong contralateral and a less prominent ipsilateral activation of the sensorimotor cortex, particularly on the left side [Chen et al., 1997; Kim et al., 1993; Kristeva et al., 1991; Pfurtscheller and Lopes da Silva, 1999].
Here we investigate whether bimanual in‐phase and anti‐phase movements are guided from both hemispheres or are controlled from one hemisphere. To this end, we determined the standard task‐related and directed coherence analysis of EEG between the primary sensorimotor cortices. Standard EEG coherence, which is defined as the normalized cross‐power spectrum of two signals recorded simultaneously at different sites on the scalp, reveals the spatio‐temporal correlation between a pair of recordings. It is interpreted as a measure of interregional functional communication according to frequency [Andres et al., 1999; Gerloff et al., 1998; Shaw et al., 1978]. Directed coherence is a different measure [Jing and Takigawa, 2000; Mima et al., 2001], and allows the determination of the direction of drive between coupled sites. It was used in the present study to ascertain whether any coupling might be due to a drive from left to right or right to left.
We demonstrate, first, that the results of the directed coherence analysis in unimanual movements are in accord with the existing literature that these patterns are predominantly organized from the contralateral hemisphere. Second, we provide evidence for an increased coupling compared to rest between the primary sensorimotor cortices during bimanual movements that largely consists of a drive from the dominant (left) to the non‐dominant (right) hemisphere. Third, through unilateral load perturbations, we show that the linkage between the sensorimotor areas drops when the task constraints lead to a partial uncoupling of the hand movements, as would be expected if interhemispheric coupling were to be functionally relevant.
SUBJECTS AND METHODS
Subjects (n = 6, age = 28 ± 8 years) who were right‐handed as determined by the Edinburgh handedness inventory [Oldfield, 1971] gave informed consent to participate in the study and were seated in front of a desk with two custom‐built manipulanda, which restricted motion of the hands around the wrist joint in the horizontal plane.
Both devices included a forearm rest and a hand plate that incorporated metal placements on either side to stabilize the hands in a posture such that the palmar surfaces faced each other when the devices were placed side by side in front of the subject. Two co‐axial potentiometers sensed the displacement of the hands. Servo motors were mounted underneath both devices and coupled to the shafts of the manipulanda. Subjects were instructed to produce cyclical flexion/extension movements to an auditory metronome. There were two bimanual conditions, i.e., in‐phase (both hands moved in a mirror mode) and anti‐phase (both hands moved in a parallel mode), performed with a cycle duration of 900 msec. One movement cycle was to be produced with every beat of the metronome. Visual feedback was provided. Trials with a unilateral load as well as without any load were executed. In the no load conditions, the motors were turned off whereas in the loaded conditions, a viscous load was used for which the applied torque was negatively proportional to hand velocity. Control conditions that included unimanual performances without loading were completed as well. Recordings of 90 sec were executed. Subjects were made familiar with the load requirements before the actual recording started so as to minimize modulations in the adopted movement amplitude. Rest (baseline) trials that involved passive listening to the metronome tone were also used. The order of the performance conditions—loading (no load, with viscous load), side of the imposed load (right, left)—as well as the movement patterns—in‐phase, anti‐phase, unimanual—were randomized.
Before the experiment started, silver‐silver chloride electrodes were fixed with collodion over the subject's scalp at C3/4 according to the International 10‐20 System, and referenced to linked ears. C3/4 are likely to overlie the primary sensorimotor areas [Homan et al., 1987]. The signals were amplified, band pass filtered (0.5–100 Hz) and digitized by a 1401 analogue to digital converter and sampled at a rate of 200 Hz (Spike 2, Cambridge Electronic Design, Cambridge, UK).
Analysis
Behavioral data
For the unimanual patterns, movement amplitude, movement time, and trajectory variability were measured in order to estimate motion stability. For the bimanual patterns, the measurements included movement amplitude, movement time, and the coefficient of determination, which denotes the square of the zero lag cross‐correlations of the trajectories (r2), and quantifies the co‐variation of the motions. We opted to use the coefficient of determination as a behavioral index of coordination due to its compatibility with the EEG estimate of coherence.
EEG data
The EEG measurement assessed functional coupling between the left and right primary sensorimotor areas in the frequency domain. We used EEG coherence, which refers to the complex analog of Pearson's correlation coefficient and represents the co‐variance between the recorded signals. It was analyzed using the discrete Fourier transform [Halliday et al., 1995] and evaluated in the α (8–13 Hz) and β (>13–30 Hz) frequency bands. Coherences were transformed using the inverse hyperbolic tangent, which resulted in values that were normally distributed as confirmed by the Shapiro‐Wilks W test. The α and β bands were chosen due to their distinct character and different roles in brain function [Salmelin et al., 1995]. Also, EEG activities in both frequency bands have been shown to be involved in earlier work on bimanual coordination [Andres et al., 1999].
There is considerable debate over which is the most appropriate way to avoid overestimated coherence values due to volume conduction and common references [Nunez, 2000]. We used linked earlobe electrodes as our reference. Although data obtained from such a recording can introduce a common signal to all other channels leading to inflated coherence estimates [Fein et al., 1988], this influence is only relevant if the activity recorded in the reference electrodes is significant [Rappelsberger, 1989]. We limited these effects by using a subtractive approach, with the assumption that movement‐related activity is not picked up by the reference electrodes. Therefore, in order to separate the task‐related from the background coherence, the values of the resting state were subtracted from those of the active state. This subtraction method also reduces between‐subject differences and any contribution from volume conduction of signals between electrodes. Coherence increments are expressed as positive values and can be interpreted as indicating greater interregional communication, whereas coherence decrements are depicted as negative values and denote a state of relative functional disconnection. Instances containing EEG artifacts were rejected while the number of evaluated epochs was kept the same for each condition. Eighty seconds of EEG data (62 non‐overlapping blocks of 256 data points each) were finally analyzed for each trial. In addition, logarithmic transformed EEG power was measured in the α and β bands at the individual electrodes (C3, C4). Task‐related power was calculated by subtracting the values of rest from those of the active state, and expressed power decreases in the case of negative scores. This measure was primarily used to ensure that changes in coherence were not due to modulations in non‐linearly related frequency components [Florian et al., 1998].
Coherence provides a measure of the linear functional coupling between two signals, but provides no directional information. To this end, a directed transfer function can be constructed to investigate any possible asymmetry in the flow of information between two regions [Jing and Takigawa, 2000; Kamiński and Blinowska, 1991]. One first finds the multivariate autoregressive (MAR) model that best describes the signals coming from the two (or more) regions of interest. Following the procedure detailed in Cassidy and Brown [2002], a Bayesian methodology was applied to estimate the parameters of the autoregressive model. This approach is desirable in that it provides full probabilistic distributions for all of the model parameters as well as a natural model order selection criterion, which is determined objectively based on the data supplied to the model. The Bayesian update equations for the MAR coefficients are similar in form to the standard maximum likelihood equations, but additionally incorporate a prior precision term α, and are given by:
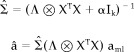 |
They are given by the normal distribution N (a|
 ,
,
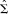 )Λ is the noise precision, X is a matrix containing the regressors, aml is the usual maximum likelihood solution for the coefficients, I
k is the identity matrix and k = pd2, where p is the model order and d is the number of channels, ⊗ denotes the Kronecker product. One can see that if the prior term is set to zero, these equations reduce to the usual solution. For further details, the reader is referred to Cassidy and Brown [2002]. An autoregressive model can be interpreted as a linear filter that is applied to a white noise sequence to produce the observed signals. With this interpretation, the transfer function that describes this mapping in the frequency domain is constructed as a complex function of the MAR coefficient matrices. The standard spectral matrix is proportional to the square of this transfer function and one usually calculates the normal coherence from this in the standard way. However, the directional content of the original transfer function is lost because of the squared operation. The directed transfer function is the simplest measure to be constructed from the original transfer function that is bounded between 0 and 1. There is no squaring, which means that this measure incorporates the directional content of the signals. In particular, it indicates the drive or dominant source from which the information flow is propagated. The directed transfer function for rest and movement sequences was therefore calculated in the α and β bands, so as to give estimates of information flow from C3 to C4 and C4 to C3 at the different frequencies. Directed coherences were transformed using the inverse hyperbolic tangent, and the values were normally distributed as confirmed by the Shapiro‐Wilks W test.
)Λ is the noise precision, X is a matrix containing the regressors, aml is the usual maximum likelihood solution for the coefficients, I
k is the identity matrix and k = pd2, where p is the model order and d is the number of channels, ⊗ denotes the Kronecker product. One can see that if the prior term is set to zero, these equations reduce to the usual solution. For further details, the reader is referred to Cassidy and Brown [2002]. An autoregressive model can be interpreted as a linear filter that is applied to a white noise sequence to produce the observed signals. With this interpretation, the transfer function that describes this mapping in the frequency domain is constructed as a complex function of the MAR coefficient matrices. The standard spectral matrix is proportional to the square of this transfer function and one usually calculates the normal coherence from this in the standard way. However, the directional content of the original transfer function is lost because of the squared operation. The directed transfer function is the simplest measure to be constructed from the original transfer function that is bounded between 0 and 1. There is no squaring, which means that this measure incorporates the directional content of the signals. In particular, it indicates the drive or dominant source from which the information flow is propagated. The directed transfer function for rest and movement sequences was therefore calculated in the α and β bands, so as to give estimates of information flow from C3 to C4 and C4 to C3 at the different frequencies. Directed coherences were transformed using the inverse hyperbolic tangent, and the values were normally distributed as confirmed by the Shapiro‐Wilks W test.
Statistics
Behavioral data
The trajectory variability, movement amplitude, and movement time of the unimanual patterns were analyzed by means of paired t‐tests with factor hands.
The coefficient of determination of the bimanual patterns was analyzed by a 2 × 3 (coordination mode × load) ANOVA. The first factor referred to the in‐phase and anti‐phase movements whereas the second factor represented the conditions: no load, left load, right load.
The movement amplitude and movement time were separately analyzed through 2 × 3 × 2 (coordination mode × load × hand) ANOVAs. The first factor corresponded to the coordination patterns, the second factor specified the conditions with and without loading, and the third factor represented the left and right hand.
EEG data
Interhemispheric task‐related coherence of the unimanual movements was estimated through a paired t‐test with factor hands. The directed coherence between the sensorimotor cortices was evaluated by a 2 × 2 (hand × direction) ANOVA. The first factor indicated both hands whereas the second factor referred to the directional flow of information, whether C3 to C4 or C4 to C3. Power was determined by individual analyses for the electrodes (C3, C4) by means of a paired t‐test with factor hands. Analyses were made per frequency band.
Interhemispheric task‐related coherence of the bimanual movements was analyzed by means of a 2 × 3 (coordination mode × load) ANOVA. The first factor included the coordination patterns whereas the second factor pointed to the load performances. The directed coherence between the sensorimotor cortices was established through a 2 × 3 × 2 (coordination mode × load × direction) ANOVA. The first factor represented the in‐phase and anti‐phase patterns, the second factor indicated the load conditions whereas the third factor referred to the direction of flow of information, whether C3 to C4 or C4 to C3. Power was evaluated by individual analyses for the electrodes (C3, C4) by means of a 2 × 3 (coordination mode × load) ANOVA. The first factor referred to the coordination patterns whereas the second factor indicated the load performances. Analyses were made per frequency band.
RESULTS
Unimanual Movements
For the α band, the standard task‐related and directed coherence between the sensorimotor regions did not change according to the hand moved (P > 0.05, for both). For the β band, the task‐related coherence between the sensorimotor areas was greater for left (non‐preferred) than right (preferred) hand motions [t(5) = 4.2, P < 0.01]. The mean scores were 0.10 and 0.04 for left and right hand movements, respectively. The directed coherence revealed a significant main effect of direction [F(1,5) = 71.7, P < 0.01]. The hand × direction interaction also reached significance [F(1,5) = 6.9, P < 0.05]. Figure 1, which includes the rest values for illustrative purposes, shows that the drive from the left sensorimotor cortex was greater when the right hand was moved than when the left hand was moved. The motion‐related status in the C3 to C4 drive compared to the rest state was 145% for right‐hand movements and 125% for left‐hand movements. Similarly, the drive from the right sensorimotor cortex was greater when the left hand was moved than when the right hand was moved. The motion‐related status in the C4 to C3 drive compared to the rest state was 148% for left‐hand movements and 83% for right‐hand movements. Overall, the strongest drive came from the left (dominant) hemisphere, although a bi‐directional asymmetry was already present at rest. Nevertheless, the drive of the contralateral hemisphere, whether left or right, was highest during unimanual movement as compared to rest, which indicates an augmented propagation due to motion processing.
Figure 1.
The directed coherence between the primary sensorimotor cortices in the β band when producing unimanual movements was strongest from the hemisphere directing the contralateral hand, with a smaller flow from the opposite hemisphere. Averaged transformed data (n = 6). The error bars denote the standard deviations from the means.
The distinction between the hemispheres is not likely to be explained by behavioral differences or EEG power changes. In particular, trajectory variability, movement amplitude, or movement time were not significantly different for the left (mean = 0.50, 58.7 degrees, 887 msec) and right (mean = 0.44, 61.2 degrees, 892 msec) hand patterns, P > 0.05 for all. Although the EEG power at C3 and C4 decreased due to movement, there were no significant differences according to which hand was moved (P > 0.05, for all). The mean scores for left and right hand movements were −0.06 and −0.03 (α), −0.06 and −0.05 (β) for C3; −0.08 and −0.05 (α), and −0.07 and −0.04 (β) for C4.
Bimanual Movements
Thus, the results of the directed coherence analysis of EEG recorded in unimanual movements were in accord with the evidence that these patterns are predominantly contralaterally organized [Chen et al., 1997; Kim et al., 1993; Kristeva et al., 1991; Pfurtscheller and Lopes da Silva, 1999]. Next we examined whether coupled bimanual movements are organized from both contralateral motor cortices or are controlled from the dominant hemisphere.
Behavioral data
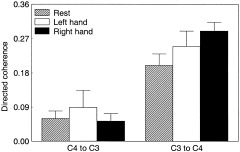
The analysis of the coefficient of determination demonstrated a significant main effect of coordination mode [F(1,5) = 45.5, P < 0.01] and load [F(2,10) = 11.4, P < 0.01]. The coordination mode × load interaction reached significance [F(2,10) = 4.9, P < 0.03] and is shown in Figure 2. It is illustrated that the in‐phase mode showed little modulation across the performance conditions whereas the anti‐phase mode deteriorated due to loading and this effect was most prominent when the load was added to the left side. The analysis of the movement amplitude did not reveal significant effects (P > 0.05). The mean values were 58.5 degrees, 59.8 degrees, and 61.2 degrees for the no load, left loaded, and right loaded conditions, respectively. Likewise, the analysis of the movement time did not show significant effects (P > 0.05). The mean values across conditions were 893 and 895 msec for left‐ and right‐hand movements, respectively.
Figure 2.
The behavioral output estimated by the coefficient of determination was modified as a function of loading (no load, left load, right load) and coordination mode (in‐phase, anti‐phase). The more successfully performed in‐phase pattern remained fairly stable across the loading conditions, whereas the anti‐phase pattern deteriorated due to loading and this effect was most prominent when the load was imposed on the left side. Averaged data (n = 6). The error bars denote the standard deviations from the means.
EEG data
For the α band, the task‐related coherence analysis of the primary sensorimotor areas revealed a significant main effect of load [F(2,10) = 4.1, P < 0.05]. In particular, loading resulted in a decreased coherence as revealed by the mean values that were 0.20, 0.08, and 0.09 for the no load, left loaded, and right loaded conditions, respectively. For the β band, the task‐related coherence analysis of the primary sensorimotor areas showed a significant coordination mode × load interaction [F(2,10) = 6.8, P < 0.01]. Figure 3A depicts that functional coupling was higher for anti‐phase than in‐phase movements in the no load condition whereas the reverse was observed when the load was imposed. The latter effect was more pronounced when the load was present on the left than on the right side. Figure 3B further depicts the lowered interhemispheric connectivity for the anti‐phase pattern due to loading, as substantiated by the coherence spectrum in a representative subject. Considering the task‐related coherence of unimanual vs. bimanual conditions without loading, it can be observed that functional coupling increases with task complexity. In particular, the values were 0.04 and 0.10 for right and left hand movements, whereas they were 0.16 and 0.19 for in‐phase and anti‐phase movements. These data illustrate the more complex nature of bimanual than unimanual actions, as well as the increased complexity within unimanual (left vs. right) and bimanual (anti‐phase vs. in‐phase) patterns.
Figure 3.
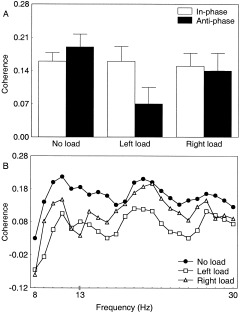
A: The functional coupling between the primary sensorimotor areas in the β band as measured by task‐related coherence drops as a function of loading. This was particularly evident for the anti‐phase pattern. Averaged transformed data (n = 6). The error bars denote the standard deviations from the means. B: The coherence spectrum of the interhemispheric connection for the anti‐phase mode further exemplifies the decrease due to loading. Individual transformed data.
The directed coherence between the primary sensorimotor cortices in the α band revealed a significant main effect of load [F(1,5) = 4.2, P < 0.05]. The mean values were 0.06, 0.03, 0.00 for the no load, left and right loaded conditions, respectively. The directed coherence between the primary sensorimotor cortices in the β band demonstrated a significant main effect of direction [F(1,5) = 179.6, P < 0.01] and a significant load × direction interaction [F(2,10) = 4.1, P < 0.05]. The coordination mode × load × direction interaction also reached significance [F(2,10) = 4.3, P < 0.05]. Figure 4A shows that the directed coherence from the non‐dominant to the dominant hemisphere (C4 to C3) was small during all conditions, albeit slightly increased when the load was imposed on the left hand in the anti‐phase mode. Conversely, the directed coherence from the dominant to the non‐dominant hemisphere (C3 to C4) was strong, but degraded with loading, especially when the load was on the left side and movements were made in the anti‐phase mode. Once again, the rest values are shown for illustrative purposes and support previous MEG data that interhemispheric synchronization of β oscillations occurs in a resting condition [Nikouline et al., 2001]. Further, it can be observed that the drive from the non‐dominant hemisphere was reduced as compared to rest, which suggests a suppression of its activity. The average of the movement sequences for the C4 to C3 connection was 35% of the rest state. Conversely, the drive from the dominant hemisphere was increased during the performance conditions as compared to rest, which suggests an augmented propagation of EEG activity due to movement. In particular, the C3 to C4 drive was an average of 119% of the rest state. An exception was the anti‐phase mode during left sided loading for which a reduction and, therefore, suppression of activity was noted. In this particular situation, the C3 to C4 drive was 85% of the rest condition. Figure 4B further shows the asymmetry in directed coherence when performing the anti‐phase configuration, as revealed by the coherence spectrum in a representative subject.
Figure 4.
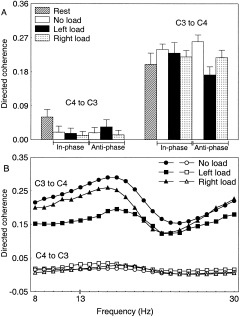
A: The directed coherence between the primary sensorimotor cortices in the β band when performing bimanual movements was small for the C4 to C3 connection (non‐dominant towards dominant hemisphere), whereas it was strong and deteriorated as a function of loading for the C3 to C4 connection (dominant towards non‐dominant hemisphere). Averaged transformed data (n = 6). The error bars denote the standard deviations from the means. B: The directed coherence spectrum of the C3 to C4 and C4 to C3 connections for the anti‐phase mode further illustrates the changes due to loading. Individual transformed data.
EEG power at C3 and C4 decreased due to movement but was not significantly different in the performance conditions for the α or β bands (P > 0.05, for all). Therefore, changes in EEG power could have contributed to increases in coherence with movement, but are not able to explain differences between coordination modes or variations due to loading. The mean scores for the no load and loaded conditions were −0.09 and −0.10 (α), −0.02 and −0.02 (β) for C3; −0.05 and −0.01 (α), −0.02 and −0.01 (β) for C4.
DISCUSSION
Bimanual movements performed according to an in‐phase or anti‐phase mode are spontaneously adopted configurations, and therefore represent basic coordination patterns. In the present study, we demonstrated that during unimanual actions, the drive in the β band from a given primary sensorimotor cortex to the other is greater during movement of the contralateral as opposed to ipsilateral hand. In contrast, during bimanual actions, the drive from the dominant (left) to the non‐dominant (right) primary sensorimotor cortex prevails, unless task constraints lead to a substantial uncoupling of coordinated behavior. Together, these results indicate that the direction of flow of activity between both hemispheres diverges for unimanual and bimanual activities. It suggests that interhemispheric transmission of information is selectively driven between the primary sensorimotor areas as a function of task requirements such that the contralateral hemisphere predominantly organizes unimanual movements, whereas the dominant hemisphere mainly controls bimanual movements.
Organization of Unimanual Movements
Even though the interhemispheric drive from the primary sensorimotor cortex was greater in unimanual movements executed by the contralateral hand there was an asymmetry of connectivity that reflected hand preference. The latter is a primary behavioral characteristic when performing manual activities. Using magnetic resonance morphometry, Amunts et al. [1996] estimated a larger hand motor area in the dominant than non‐dominant hemisphere, implicating a richer interconnectivity and increased dispersion of movement representations. This might enable an increased cortical encoding and result in a more refined motor skill repertoire of the preferred hand [Volkmann et al., 1998].
The present data underscore these earlier observations in several ways. First, at rest the drive from the dominant sensorimotor cortex far exceeded that from the homologous counterpart. This finding may relate to the fact that the non‐dominant (right) sensorimotor area has a strong tendency to shift to an idling state [Stančák and Pfurtscheller, 1996]. Second, unimanual left‐hand movements were characterized by a higher degree of interhemispheric coherence in the β band. Given that an association between interregional coupling and movement complexity exists [Gerloff et al., 1998], this result is compatible with left hand patterns being relatively more difficult to perform. The directed transfer function showed that this increased interhemispheric coupling with left‐hand movements involved not only an augmentation in the drive from the right to left sensorimotor cortex but also an increase in that from the left to right sensorimotor cortex, which is likely to reflect inhibitory mechanisms to suppress the homologous (preferred but non‐active) side. In contrast, only the drive from the left to the right sensorimotor cortex increased with right‐hand movements, suggesting that inhibitory processes of the homologous (non‐preferred but non‐active) side were limited. Thus, the interhemispheric drive from the dominant hemisphere augmented with both contralateral and ipsilateral movements, albeit more so with contralateral actions. These results emphasize a hemispheric asymmetry of ipsilateral activation during unimanual movements, and are in line with earlier work using functional imaging and transcranial magnetic stimulation [Kim et al., 1993; Ziemann and Hallett, 2001]. Lesion studies have similarly shown that left hemisphere damage produces bilateral deficits on a variety of motor tasks whereas right hemisphere damage is more prone to produce only contralateral deficits [Haaland and Harrington, 1994].
Organization of Bimanual Isofrequency Coordination
The directed transfer function revealed that the dominant hemisphere principally controlled coupling between the primary sensorimotor cortices in the β band during bimanual movements, increasing its drive with respect to the rest state. If anything, the drive from the non‐dominant side was suppressed compared to that recorded either at rest or during unimanual movements made with the left hand. Overall, these results highlight the responsibility of the dominant hemisphere for bimanual coordination, and are in agreement with imaging data [Jäncke et al., 1998; Viviani et al., 1998], lesion studies [Haaland and Harrington, 1994] and EEG recordings of the readiness potential [Cui et al., 2000]. They further indicate that bimanual patterns rely on interhemispheric connections as evidenced from specific deficits in callosal patients [Eliassen et al., 2000; Kennerly et al., 2002], which are more pronounced for anti‐phase than in‐phase movements [Serrien et al., 2001]. The previous implies that the nature of interhemispheric interactions is likely to be different for both coordination modes. In particular, the functional coupling can be considered to consist mainly of excitatory processes for in‐phase patterns, whereas that of anti‐phase patterns may additionally involve inhibitory processes for suppressing the more intrinsic tendency towards mirror movements. The increased degree of coherence during anti‐phase as compared to in‐phase actions under unloaded conditions may consequently be related to the greater need for information processing to maintain the coordination demands, which is in line with observations from functional imaging [Fink et al., 1999; Sadato et al., 1997] and repetitive transcranial magnetic stimulation [Serrien et al., 2002].
The bimanual data further indicated that performance was degraded when one limb was perturbed with an imposed load. The disturbing effect was more prominent for anti‐phase than in‐phase movements, which supports the additional processing requirements of the former as compared to the latter mode of coordination. These loading‐induced deteriorations in behavioral output were matched with a diminished communication between the primary sensorimotor areas in the β band, and accordingly suggest that interhemispheric coupling is of functional significance. The observed reduction in interhemispheric transfer is likely to have been induced either through alteration in the functional state of the sensorimotor system or through changes in the intensity requirements associated with task production. Previously, functional imaging has shown that sensory stimulation or awareness of the input [Deuchert et al., 2002; Hämäläinen et al., 2000] and force production or movement rate [Dettmers et al., 1995; Jäncke et al., 1998; Schubert et al., 1998] correlate with cortical activity. Any modulation in the functional state of the sensorimotor system may have involved the areas directly or indirectly, via other cortical regions such as the supplementary motor area (SMA). Previously we have shown that repetitive transcranial magnetic stimulation in the region of the SMA can modify the functional coupling between the primary sensorimotor cortices and accordingly modulate interlimb behavior [Serrien et al., 2002]. The latter implies that load‐induced changes in interhemispheric coherence, whether causal or consequential, could have been indirectly generated through SMA. Alternatively, the loading‐related deterioration in interhemispheric coherence might have been due to an increased coordinative effort. This seems unlikely because interregional information transfer rather tends to augment with task difficulty [Gerloff et al., 1998; Manganotti et al., 1998], which is underscored in the present study by the increased functional coupling in the anti‐phase as compared to the in‐phase mode when performing without any load. It also appears improbable that the bilateral coupling was attenuated due to non‐coherent rhythms, as EEG power did not change as a result of loading.
The fact that the uncoupling effect of loading reflected in coherence and behavioral performance was most powerful when the left hand was perturbed deserves comment. In this particular situation, the loading‐related information is relayed to the non‐dominant side and might thus not be appropriately transferred to the dominant side for assimilation into the motor plan due to the weak connectivity in this direction. Alternatively, the non‐dominant hemisphere might have exerted an inhibitory influence on the dominant hemisphere, in response to the afferent stimulation induced by loading. However, this seems less likely as the right motor cortex has a lower capacity to inhibit the left motor cortex than vice versa in right‐handers [Netz et al., 1995], also evidenced from behavioral data that the right hand experiences less mirror movements than the left hand in normal adults [Armatas et al., 1994]. That variation in interhemispheric coupling and associated behavioral performance was most closely related to β band activity, is in agreement with the hypothesis that modulations in this frequency range are particularly related to motor processes, including corticospinal output, whereas α band activity likely represents motor in combination with somatosensory processes [Salmelin et al., 1995]. Nevertheless, the EEG–EEG coherence in the β band in the present paradigm should not be considered equivalent to the cortex‐muscle coherence evident during isometric contractions, as the former increased, whereas the latter disappears during movement [Brown, 2000]. This in itself suggests that oscillatory activity in the β range may simultaneously serve different functions in the motor system.
In conclusion, these results emphasize two related aspects of human motor function; hemispheric dominance and hand preference. The findings suggest a major role for the dominant sensorimotor cortex in organizing bimanual coordination whereas the interhemispheric drive from the non‐dominant sensorimotor cortex was suppressed. In addition, a close association was evident between cortical dynamics and behavioral states, suggesting that synchronized neural activity is exploited for the control of goal‐directed movement.
REFERENCES
- Andres GG, Mima T, Schulman AE, Dichgans J, Hallett M, Gerloff C (1999): Functional coupling of human cortical sensorimotor areas during bimanual skill acquisition. Brain 122: 855–870. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schlaug G, Schleicher A, Steinmetz H, Dabringhaus A, Roland PE, Zilles K (1996): Asymmetry in the human motor cortex and handedness. NeuroImage 4: 216–222. [DOI] [PubMed] [Google Scholar]
- Armatas CA, Summers JJ, Bradshaw JL (1994): Mirror movements in normal adult subjects. J Clin Neuropsychol 16: 405–413. [DOI] [PubMed] [Google Scholar]
- Brown P (2000): Cortical drives to human muscle: the Piper and related rhythms. Prog Neurobiol 60: 97–108. [DOI] [PubMed] [Google Scholar]
- Cassidy MJ, Brown P (2002): Hidden Markov based autoregressive analysis of stationary and non‐stationary electrophysiological signals for functional coupling studies. J Neurosci Methods 116: 35–53. [DOI] [PubMed] [Google Scholar]
- Chen R, Gerloff C, Hallett M, Cohen LG (1997): Involvement of the ipsilateral motor cortex in finger movements of different complexities. Ann Neurol 41: 247–254. [DOI] [PubMed] [Google Scholar]
- Cui RQ, Huter D, Egkher A, Lang W, Lindinger G, Deecke L (2000): High resolution DC‐EEG mapping of the Bereitschaftspotential preceding simple or complex bimanual sequential finger movement. Exp Brain Res 134: 49–57. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, Holmes A, Ridding MC, Brooks DJ, Frackowiak RS (1995): Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol 74: 802–815. [DOI] [PubMed] [Google Scholar]
- Deuchert M, Ruben J, Schwiemann J, Meyer R, Thees S, Krause T, Blankenburg F, Villringer K, Kurth R, Curio G, Villringer A (2002): Event‐related fMRI of the somatosensory system using electrical finger stimulation. Neuroreport 13: 365–369. [DOI] [PubMed] [Google Scholar]
- Eliassen JC, Baynes K, Gazzaniga MS (2000): Anterior and posterior callosal contributions to simultaneous bimanual movements of the hands and fingers. Brain 123: 2501–2511. [DOI] [PubMed] [Google Scholar]
- Fein G, Raz J, Brown FF, Merrin EL (1988): Common reference coherence data are confounded by power and phase effects. Electroencephalogr Clin Neurophysiol 69: 581–584. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Halligan PW, Frith CD, Driver J, Frackowiak RSJ, Dolan RJ (1999): The neural consequences of conflict between intention and the senses. Brain 122: 497–512. [DOI] [PubMed] [Google Scholar]
- Florian, G , Andrew C, Pfurtscheller G (1998): Do changes in coherence always reflect changes in functional coupling. Electroencephalogr Clin Neurophysiol 106: 87–91. [DOI] [PubMed] [Google Scholar]
- Foltys H, Sparing R, Boroojerdi B, Krings T, Meister IG, Mottaghy FM, Topper R (2001): Motor control in simple bimanual movements: a transcranial magnetic stimulation and reaction time study. Clin Neurophysiol 112: 265–274. [DOI] [PubMed] [Google Scholar]
- Garry MI, Franks IM (2002): Spatially precise bilateral arm movements are controlled by the contralateral hemisphere: Evidence from a lateralized visual stimulus parading. Exp Brain Res 142: 292–296. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Hadley J, Richard J, Uenischi N, Honda M, Hallett M (1998): Functional coupling and regional activation of human cortical motor areas during simple, self‐paced and metronome‐paced finger movements. Brain 121: 1513–1531. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL (1994): Limb‐sequencing deficits after left but not right hemisphere damage. Brain Cogn 24: 104–122. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, Knight RT (2000): Neural representations of skilled movement. Brain 123: 2306–2313. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF (1995): A framework for the analysis of mixed time series/point process data: theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 64: 237–278. [DOI] [PubMed] [Google Scholar]
- Hämäläinen H, Hiltunen J, Titievskaja I (2000): fMRI activations of SI and SII cortices during tactile stimulation depend on attention. Neuroreport 8: 1673–1676. [DOI] [PubMed] [Google Scholar]
- Homan RW, Herman J, Purdy P (1987): Cerebral location of international 10‐20 system electrode placement. Electroencephalogr Clin Neurophysiol 66: 376–382. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Peters M, Schlaug G, Posse S, Steinmetz H, Müller‐Gärtner H‐W (1998): Differential magnetic resonance signal change in human sensorimotor cortex to finger movements of different rate of the dominant and subdominant hand. Cogn Brain Res 6: 279–284. [DOI] [PubMed] [Google Scholar]
- Jing H, Takigawa M (2000): Observation of EEG coherence after repetitive transcranial magnetic stimulation. Clin Neurophysiol 111: 1620–1631. [DOI] [PubMed] [Google Scholar]
- Kamiński MJ, Blinowska KJ (1991): A new method of the description of information flow in the brain structures. Biol Cybern 65: 203–210. [DOI] [PubMed] [Google Scholar]
- Kelso JAS (1984): Phase transitions and critical behavior in human bimanual coordination: Am J Physiol Regul Integrativ Comparat Physiol 15: R1000–R1004. [DOI] [PubMed] [Google Scholar]
- Kennerly SW, Diedrichsen J, Hazeltine E, Semjen A, Ivry RB (2002): Callosotomy patients exhibit temporal uncoupling during continuous bimanual movements. Nat Neurosci 5: 376–381. [DOI] [PubMed] [Google Scholar]
- Kim S‐G, Ashe J, Hendrich K, Ellermann JM, Merkle H, Uǧurbil K, Georgopoulos AP (1993): Functional magnetic resonance imaging of motor cortex: Hemispheric asymmetry and handedness. Science 26: 615–617. [DOI] [PubMed] [Google Scholar]
- Kristeva R, Cheyne D, Deecke L (1991): Neuromagnetic fields accompanying unilateral and bilateral voluntary movements: topography and analysis of cortical sources. Electroencephalogr Clin Neurophysiol 81: 284–298. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Gerloff C, Toro C, Katsuta H, Sadato N, Zhuang P, Leocani L, Hallett M (1998): Task‐related coherence and task‐related spectral power changes during sequential finger movements. Electroencephalogr Clin Neurophysiol 109: 50–62. [DOI] [PubMed] [Google Scholar]
- Mima T, Matsuoka T, Hallett M (2001): Information flow from the sensorimotor cortex to muscle in humans. Clin Neurophysiol 112: 122–126. [DOI] [PubMed] [Google Scholar]
- Netz J, Ziemann U, Hömberg V (1995): Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res 104: 527–533. [DOI] [PubMed] [Google Scholar]
- Nikouline VV, Linkenkaer‐Hansen K, Huttunen J, Ilmoniemi RJ (2001): Interhemispheric phase synchrony and amplitude correlation of spontaneous beta oscillations in human subjects: A magnetoencephalographic study. Neuroreport 12: 2487–2491. [DOI] [PubMed] [Google Scholar]
- Nunez PL (2000): Toward a quantitative description of large‐scale neocortical dynamic function and EEG. Behav Brain Sci 23: 371–437. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness. The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH (1999): Event‐related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110: 1842–1857. [DOI] [PubMed] [Google Scholar]
- Rappelsberger P (1989): The reference problem and mapping of coherence: a simulation study. Brain Topogr 2: 63–72. [DOI] [PubMed] [Google Scholar]
- Sadato N, Yonekura Y, Waki A, Yamada H, Ishii Y (1997): Role of the supplementary motor area and the right premotor cortex in the coordination of bimanual finger movements. J Neurosci 17: 9667–9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmelin R, Hämäläinen M, Kajola M, Hari R (1995): Functional segregation of movement‐related rhythmic activity in the human brain. Neuroimage 2: 237–243. [DOI] [PubMed] [Google Scholar]
- Schubert T, von Cramon DY, Niendorf T, Pollmann S, Bublak P (1998): Cortical areas and the control of self‐determined finger movements: an fMRI study. Neuroreport 9: 3171–3176. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Nirkko AC, Wiesendanger M (2001): Role of the corpus callosum in bimanual coordination: A comparison of patients with congenital and acquired callosal pathology. Eur J Neurosci 14: 1897–1905. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Strens LHA, Oliviero A, Brown P (2002): Repetitive transcranial magnetic stimulation of the supplementary motor area (SMA) degrades bimanual movement control in humans. Neurosci Lett 328: 89–92. [DOI] [PubMed] [Google Scholar]
- Shaw JC, O'Connor KP, Ongley OC (1978): EEG coherence as a measure of cerebral functional organization In: Brazire MAR, Petsche H, editors. Architectonics of the cerebral cortex. New York: Raven Press; p 245 – 256. [Google Scholar]
- Stančák A Jr, Pfurtscheller G (1996): Event‐related desynchronisation of central beta‐rhythms during brisk and slow self‐paced finger movements of dominant and nondominant hand. Brain Res Cogn Brain Res 4: 171–183. [DOI] [PubMed] [Google Scholar]
- Viviani P, Perani D, Grassi F, Bettinardi V, Fazio F (1998): Hemispheric asymmetries and bimanual asynchrony in left‐ and right‐handers. Exp Brain Res 120: 531–536. [DOI] [PubMed] [Google Scholar]
- Volkmann J, Schnitzler A, Witte OW, Freund H (1998): Handedness and asymmetry of hand representation in human motor cortex. J Neurophysiol 79: 2149–2154. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Hallett M (2001): Hemispheric asymmetry of ipsilateral motor cortex activation during unimanual motor tasks: further evidence for motor dominance. Clin Neurophysiol 112: 107–113. [DOI] [PubMed] [Google Scholar]


