Abstract
Functional neuroanatomy of writing is relatively unknown compared to that of other linguistic processes. This study aimed at identifying brain regions crucial to the process of writing. Using functional magnetic resonance imaging (fMRI), brain hemodynamic activity was examined during three conditions that differentially engaged visual, linguistic, and/or motor functions: (1) writing names of pictures with the right index finger, (2) naming pictures silently, and (3) visually cued finger tapping. A writing minus naming comparison and a writing minus tapping comparison were performed, and brain regions commonly activated in these two contrasts were detected. Our main finding was that such common activation was observed in the anterior part of the left superior parietal lobule, the posterior part of the middle and superior frontal gyri, and the right cerebellum. The parietal and frontal regions were considered to subserve the process of writing as separated from that of naming and finger movements, which is consistent with the classical notion mainly proposed by studies of selective writing deficits called pure agraphia. The right cerebellar activation, on the other hand, was interpreted as the reflection of the execution of complex finger movements required for writing. Hum. Brain Mapping 13:34–42, 2001. © 2001 Wiley‐Liss, Inc.
Keywords: left superior parietal lobule, left premotor cortex, Exner's area, right cerebellum, agraphia
INTRODUCTION
Writing can be rated among the most useful communication tools because of its power to convey information over time and space. It is a late‐learned skill both phylogenetically and ontogenetically, and may therefore be relatively susceptible to injury [Friedland, 1990]. There have been many reports describing brain‐damaged patients who showed various types of writing deficits (i.e., agraphia) [for summary, see Hinkin and Cummings, 1996; Roeltgen, 1997]. Rare but interesting is a symptom termed pure agraphia, a writing impairment in the absence of other aphasic symptomatology. Since the first appearance of this symptom in the neuropsychological literature [Exner, 1881], two brain regions have been candidate cortical sites that can be called “the writing centers”: the posterior end of the left middle frontal gyrus (Exner's area) and the left superior parietal lobule [Vernea and Merory, 1975; Basso et al., 1978]. However, inconsistent evidence has been presented, reporting patients with pure agraphia following lesions in other sites [Rosati and De Bastiani, 1981; Yokota et al., 1990]. Functional neuroimaging techniques are of great use to supplement these confusing neuropsychological data. Sugishita et al. employed functional magnetic resonance imaging (fMRI) and investigated brain activity during mental writing [1996]. Activation was observed in left frontal, bilateral intraparietal, and cingulate regions. Among them the left intraparietal region was most extensively activated. Although this finding confirmed part of the previous lesion work, it remains to be ascertained whether the same regions are involved in the process of writing with actual hand movements. As for studies of positron emission tomography (PET), there have been several related studies [Seitz et al., 1997; Petrides et al., 1995], few of which directly examined the functional neuroanatomy of the process of writing. Thus, in both neuropsychological and neuroimaging literature, no sufficient explanation has been made regarding the existence and/or the location of the brain region responsible for writing.
The present study was designed to identify brain regions crucial to the process of writing. Using fMRI, brain hemodynamic responses were measured during three conditions which differentially engaged visual, linguistic, and/or motor processings: (1) writing names of pictures, (2) naming pictures silently, and (3) visually cued finger tapping. Comparisons were made among three conditions, and a conjunction between the results of these comparisons was performed as well. These analyses were expected to reveal candidate brain regions for “the writing centers.”
METHODS
Subjects
Subjects were 17 right‐handed men, ranging in age from 19 to 31 (mean 23.5). Edinburgh Handedness Inventory laterality quotients [Oldfield, 1971] ranged from 65 to 100 (mean 91.7), indicating strong right‐hand preference for all subjects. All of the subjects had normal vision without corrective lenses, or with contact lenses. None of the subjects reported any history of neurological or psychiatric diseases. The experimental procedures were approved by the Research Ethics Committee of the Faculty of Medicine, University of Tokyo, and written informed consent was obtained from all the subjects.
Task Design
Stimuli consisted of a red point and watercolor pictures of concrete objects. Items for the pictures were selected from commonly used words comprising one to three syllables from categories such as animals, plants, and buildings. The stimulus sequences were generated using a Macintosh computer and PsyScope software [Macwhinney et al., 1997] and were back‐projected on a translucent screen via a Panasonic liquid crystal projector TH‐L592J.
Three conditions were designed for fMRI scan periods: (1) writing names of pictures (written naming), (2) naming pictures silently, and (3) visually cued finger tapping. In the writing condition, subjects were presented with watercolor pictures one‐by‐one, and asked to write the names of the presented items in phonograms (Japanese kana) with their right index finger. Each stimulus was displayed on the screen for 4 sec with no interstimulus interval. Strictly speaking, this writing task is different from writing in the common usage of the word in that the former does not require a writing tool (e.g., a pen or a pencil), and therefore the subtle movements of the wrist and digits required for using these tools are not engaged in the task. However, patients with agraphia usually show difficulty in writing with their hand whether they use a pen or other writing tools, suggesting that underlying neural substrates are the same regardless of the writing tool. We considered that the same neural bases would also be engaged in writing with the right index finger. Another important reason for employing this task was its controllability. Because it involved the movements of only one finger, its motoric aspects were relatively easily controlled, as mentioned below. In the Naming condition, subjects were presented with pictures, one‐by‐one, and asked to name the presented items silently. Each stimulus was displayed on the screen for 2.5 sec with no interstimulus interval. The stimulus duration in the Naming condition was shorter than that in the Writing condition. The reason for this was to control for the difference between these two conditions in terms of time required to complete each task for each presented item. If we chose the same presentation rate for the two conditions, the Naming condition would be felt more sparsely than the Writing condition, and subjects might be engaged in other mental activities. We considered that equating subjective task density would be necessary to effectively cancel out naming processes from the task of writing. In the Tapping condition, subjects were presented with a red dot coming on and off, and asked to move their right index finger back and forth in the air while the red dot was on. The red dot was presented for 2 sec with a 2‐sec interstimulus interval. The duration and interval of the red point presentation was determined so that the Tapping condition would effectively control for finger movements involved in the Writing condition. Specifically, we measured in our preliminary experiments an average duration of finger movements required for writing each name of the pictures during the Writing condition, and the duration of the red point during the Tapping condition was set approximately equal to the average value. Then the interstimulus interval was set at 2 sec so that the frequency of the red dot would be equal to that of the pictures during the Writing condition (1 item/4 sec). Subjects were instructed to move their right index finger in the air with approximately the same speed and range as they did in the Writing condition. Padding was placed under the right wrist so that subjects could move their index finger in the air in the Writing and the Tapping conditions. The three conditions were alternated every 32 sec and repeated in a randomized order so that each condition would occur six times, namely, one experimental run comprised 18 epochs (576 sec) in total. To ensure that subjects would perform the tasks successfully, they were required prior to the fMRI scan to undergo a short practice session with different stimulus sets. During this practice session, each subject's finger movements for the Writing and the Tapping tasks were checked by examiners' eyes and corrected if necessary so that the finger movements for the two tasks would match in terms of timing, spatial range, and speed.
Image Acquisition
Imaging was performed on a 1.5 Tesla GE Signa LX system with a standard head coil. T2*‐weighted time‐series images depicting BOLD contrast [Ogawa et al., 1990] were acquired using a gradient‐echo EPI sequence (TR = 4,000 ms, TE = 50 ms, flip angle = 90°, FOV 24 × 24 cm, and voxel dimensions 3.75 × 3.75 × 8 mm). Eighteen axial contiguous 8‐mm‐thick slices covering the whole brain were collected. The first four volumes of fMRI time series were discarded to discount T1 saturation effects. Each epoch for one of the three conditions lasted for eight volumes, and each condition occurred six times intermittently. Thus, a total of 144 volumes were acquired for each subject. For anatomic localization and coregistration of images across subjects, high‐resolution T1‐weighted images of the entire brain were obtained using a SPGR sequence for contiguous 1.3‐mm‐thick axial slices.
Image Analysis
The data were analyzed using statistical parametric mapping (SPM) technique (using SPM96 from the Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab (Mathworks Inc., Sherborn, MA, USA). The analysis involved the following steps: to correct for head movement between scans, the functional images from each subject were realigned to the first image using rigid body transformation. A mean image was created using the realigned volumes. The high‐resolution T1‐weighted anatomical images were coregistered to this mean (T2*) images to ensure that the functional and anatomical images were spatially aligned. The anatomical images were then normalized into a standard space [Talairach and Tournoux, 1988] by matching to a standardized MNI template (Montreal Neurological Institute, Quebec, Canada), using both linear and nonlinear 3D transformations [Friston et al., 1995a]. The transformation parameters determined here were also applied to the functional images. Finally, these normalized images were smoothed with an 8‐mm (full width at half maximum) isotropic Gaussian kernel to accommodate intersubject differences in anatomy and to permit application of Gaussian random field theory to provide corrected statistical inference [Friston et al., 1995b]. A multisubject statistical analysis was performed employing a random effect model. Each subject's data set comprising 144 volumes was collapsed into three representative condition images, adjusted for global effects and with low frequency drifts removed via a high‐pass filter with a cutoff frequency of 0.47 cycles per min. The condition images from 17 subjects were then entered into a multisubject different‐condition model. Two contrasts and a conjunction between them were tested: (a) Writing vs. Naming and (b) Writing vs. Tapping. To confirm that the conjunction analysis successfully extracted brain areas commonly activated in the two contrasts, a mask analysis was also performed in which the contrast (a) masked with the result of the contrast (b) was tested. The assumption underlying our analysis is that the process of writing names of pictures can be divided into four components: (1) naming presented pictures by aid of visual and lexical processings, (2) retrieving the graphic images for the letters composing each name, (3) arousing the graphic motor images for the letters and planning the movement of the right index finger accordingly, (4) moving the finger actually. The contrast (a) was considered to represent the last three processes, while the contrast (b) was considered to represent the first three. Thus, the conjunction or mask analysis between these two contrasts was thought to reveal the brain regions involved in the intermediate processes (2) and (3). The difference between these two was not modeled in this analysis but is discussed in the Discussion section in relation to our results and previous lesion data. The SPMs{Z} for the two contrasts and their conjunction were generated and thresholded at a voxel‐wise P value of 0.001 and a cluster‐level significance level of P < 0.05 (corrected for multiple comparisons using the method described in Friston et al. [1995b]). The same significance level was applied to the SPM{Z} for the contrast (a) masked with the thresholded (P < 0.001, uncorrected) contrast (b).
RESULTS
Writing − Naming
The result of the Writing minus Naming comparison was summarized in Table I and Figure 1 (top row). Activation observed in the cerebral cortex showed strong left hemispheric lateralization. The most extensive activation was observed in a left parieto‐frontal cortical region including the superior parietal lobule, the superior and middle part of the precentral and postcentral gyri, and the posterior part of the superior and middle frontal gyri. Activation was also seen in the middle part of the left cingulate gyrus. The right supramarginal gyrus was the only cerebral region that showed activity on the right side. Subcortical activation, which was found in the thalamus and the caudate nucleus, also showed left dominance. In contrast, activation observed in the cerebellum was strongly right‐lateralized. A medial half of the cerebellum was extensively activated on the right side, while only a small superior part was activated on the left.
Table I.
Stereotaxic coordinates of significant activation foci revealed by the contrast Writing minus Naming*
| Brain region | Anatomical location (approximate BA) | x | y | z | Z‐score |
|---|---|---|---|---|---|
| Parietal | |||||
| Left | SPL (BA 7) | −18 | −42 | 64 | 5.02 |
| −12 | −52 | 64 | 3.19 | ||
| Right | SMG (BA 40) | 40 | −28 | 46 | 4.61 |
| Premotor | |||||
| Left | SFG (BA 6) | −20 | −4 | 58 | 7.21 |
| MFG (BA 6) | −28 | 0 | 46 | 6.68 | |
| Precentral | |||||
| Left | PreCG (BA 4, 6) | −34 | −8 | 50 | 7.22 |
| −34 | −18 | 52 | 6.40 | ||
| −26 | −14 | 66 | 6.15 | ||
| Postcentral | |||||
| Left | PostCG (BA 1, 2, 3) | −36 | −28 | 48 | 6.70 |
| Cingulate | |||||
| Left | CingG (BA 24, 31) | −8 | −4 | 44 | 5.33 |
| −6 | 4 | 34 | 5.32 | ||
| −10 | −16 | 42 | 4.21 | ||
| Basal ganglia | |||||
| Left | Thalamus | −14 | −16 | 4 | 6.25 |
| Caudate nucleus | −10 | 10 | −2 | 3.32 | |
| Cerebellum | |||||
| Left | −16 | −64 | −16 | 3.49 | |
| Right | 20 | −52 | −22 | 7.23 | |
| 6 | −64 | −16 | 7.14 | ||
| 6 | −56 | −12 | 6.84 | ||
| 24 | −66 | −50 | 6.26 |
Significance level was set at P < 0.05 (corrected for multiple comparisons).
Abbreviations: BA: probable Brodmann's area; SPL: superior parietal lobule; SMG: supramarginal gyrus; SFG: superior frontal gyrus; MFG: middle frontal gyrus; PreCG: precentral gyrus; PostCG: postcentral gyrus; CingG: cingulate gyrus.
Figure 1.
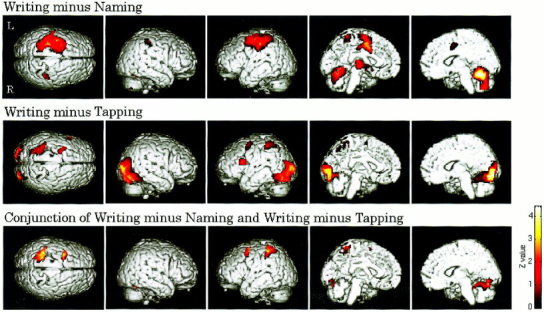
The activation maps revealed by the two contrasts and the conjunction between them. The top row shows the result of the Writing minus Naming comparison, the middle shows the result of the Writing minus Tapping, and the bottom shows the result of the conjunction between them. All the maps were thresholded at the significance level of P < 0.05 (corrected for multiple comparisons), and superimposed on the surface‐rendered T1 template brain. Z values for colored voxels are coded using the color bar at the lower right. Note that the activated regions in the bottom row correspond to the common activated areas of the top two rows.
Writing − Tapping
The result of the Writing minus Tapping comparison is shown in Table II and Figure 1 (middle row). The most extensive activation was observed in a bilateral occipital and occipitotemporal region. This activation showed no clear laterality and involved a large part of the middle and inferior occipital gyri on both hemispheres, spreading into the occipitotemporal cortex such as the lingual, fusiform, and inferior temporal gyri, and slightly extending into the superior part of the cerebellum. In contrast, parietal and frontal activation was restricted to the left hemisphere. Activation similar to that observed in the Writing − Naming comparison was seen in the left superior parietal lobule and the posterior part of the left middle and inferior frontal gyri, whereas the intermediate precentral and postcentral regions showed no activity. Notable was that the Broca area, the opercular part of the left inferior frontal gyrus, revealed activation, which was not observed in the Writing − Naming comparison.
Table II.
Stereotaxic coordinates of significant activation foci revealed by the contrast Writing minus Tapping*
| Brain region | Anatomical location (approximate BA) | x | y | z | Z‐score |
|---|---|---|---|---|---|
| Parietal | |||||
| Left | SPL (BA 7) | −26 | −56 | 40 | 5.93 |
| −28 | −46 | 58 | 4.55 | ||
| Premotor | |||||
| Left | SFG (BA 6) | −20 | −2 | 56 | 4.86 |
| MFG (BA 6) | −28 | 4 | 46 | 4.56 | |
| IFG (BA 44) | −44 | 12 | 22 | 5.36 | |
| Occipitotemporal | |||||
| Left | LG (BA 18) | −20 | −88 | −8 | 6.36 |
| FG (BA 18, 19) | −34 | −70 | −14 | 7.26 | |
| −34 | −54 | −16 | 7.17 | ||
| −24 | −82 | −14 | 6.46 | ||
| Right | FG (BA 18, 19) | 28 | −74 | −14 | 7.28 |
| ITG (BA 19, 37) | 50 | −62 | −10 | 5.51 | |
| 50 | −70 | 0 | 4.41 | ||
| Occipital | |||||
| Left | MOG (BA 18, 19) | −26 | −90 | 6 | 6.97 |
| −20 | −96 | 10 | 6.94 | ||
| IOG (BA 18, 19) | −34 | −80 | −6 | 6.53 | |
| Cuneus (BA 17, 18) | −12 | −98 | 2 | 7.09 | |
| −6 | −84 | 6 | 4.97 | ||
| Right | MOG (BA 18, 19) | 32 | −86 | 6 | 7.59 |
| IOG (BA 18, 19) | 28 | −94 | 8 | 6.79 | |
| 26 | −82 | −10 | 6.73 | ||
| 44 | −72 | −14 | 6.06 | ||
| 42 | −76 | −6 | 5.53 | ||
| Cerebellum | |||||
| Right | 34 | −50 | −22 | 7.01 | |
| 32 | −38 | −24 | 5.87 |
Significance level was set at P < 0.05 (corrected for multiple comparisons).
Abbreviations not listed in Table I are: IFG: inferior frontal gyrus; LG: lingual gyrus; FG: fusiform gyrus; ITG: inferior temporal gyrus; MOG: middle occipital gyrus; and IOG: inferior occipital gyrus.
Conjunction of Writing − Naming and Writing − Tapping
Table III and Figure 1 (bottom row) show the result of the conjunction analysis performed to detect brain regions commonly activated in the above two contrasts. Main cerebral activation was observed in two regions on the left hemisphere: the superior part of the left parietal lobe and the posterior part of the superior and middle frontal gyri. The parietal region involved the anterior part of the superior parietal lobule and slightly extended into the inferior parietal lobule, specifically, the superior margin of the supramarginal gyrus. The frontal region corresponded to the site called Exner's area. Similar to the result of the Writing minus Tapping comparison, there was no activation in the intermediate precentral and postcentral regions. A relatively weak activation focus was also found in the left lingual gyrus. Thus, the cerebral activation loci revealed by the conjunction was confined to the left cerebral hemisphere. In the cerebellum, on the other hand, activation was seen on the right side only. This activation was located in a superior middle and medial part of the right cerebellum, and the middle part was more strongly activated than the medial part. These cerebral and cerebellar activation patterns were similarly observed in the result of the mask analysis.
Table III.
Stereotaxic coordinates of significant activation foci revealed by the conjunction between Writing minus Naming and Writing minus Tapping*
| Brain region | Anatomical location (approximate BA) | x | y | z | Z‐score |
|---|---|---|---|---|---|
| Parietal | |||||
| Left | SPL (BA 7) | −32 | −38 | 56 | 5.71 |
| −28 | −46 | 58 | 5.61 | ||
| −22 | −42 | 64 | 5.00 | ||
| −14 | −52 | 64 | 3.54 | ||
| −14 | −40 | 68 | 3.10 | ||
| SMG (BA 40) | −42 | −32 | 46 | 4.48 | |
| Premotor | |||||
| Left | SFG (BA 6) | −22 | 0 | 64 | 4.96 |
| MFG (BA 6) | −28 | 4 | 48 | 6.14 | |
| −28 | 2 | 58 | 5.34 | ||
| Occipitotemporal | |||||
| Left | LG (BA 18) | −2 | −82 | −8 | 3.28 |
| Cerebellum | |||||
| Right | 26 | −52 | −20 | 7.10 | |
| 8 | −72 | −16 | 4.56 |
DISCUSSION
In this study, brain activation was examined during three conditions: writing names of pictures, naming pictures silently, and visually cued finger tapping. Our main analysis was the conjunction between the Writing minus Naming and the Writing minus Tapping comparisons. It revealed significant activation in the anterior part of the left superior parietal lobule, the posterior part of the middle and superior frontal gyri, and the right cerebellum, which indicated that these regions are commonly activated in the two contrasts. The following sections discuss specific roles of these brain regions in relation to previous neuropsychological and neuroimaging data, and also give an interpretation to other brain regions differentially activated in the two contrasts.
Left Parietal and Premotor Activation
The left superior parietal lobule and the posterior end of the middle frontal gyrus (Exner's area) have long been proposed as the centers for writing. Evidence for this view has mainly come from lesion studies describing cases of pure agraphia after damage to either of these two regions [Exner, 1881; Vernea and Merory, 1975; Basso et al., 1978]. Inconsistent data exist, however, which indicate that pure agraphia can be produced by lesions in other brain areas such as left posterior peri‐Sylvian [Rosati and De Bastiani, 1981], and left temporo‐parietal regions [Yokota et al., 1990]. In light of this confusing situation, Sugishita et al. performed an fMRI study of mental writing and showed the involvement of left premotor, bilateral intraparietal, and cingulate regions, among which the left intraparietal activation was most distinctive [1996]. This result can be interpreted as a piece of evidence supporting the classical notion. Yet, it remained to be determined whether these cortical sites are associated with the process of writing with actual hand movements and whether similar activation occurs when contrasting writing with other linguistic tasks (e.g., naming). Here, this study has employed a task design controlling these points and demonstrated that the two cerebral regions, the anterior part of the left superior parietal lobule and the posterior part of the left middle and superior frontal gyri, are engaged in the process of writing as compared to naming and finger tapping. This result successfully confirmed the classical notion that these two regions are important as the writing centers. The question remains, however, as to why damage to other cerebral regions results in the symptom of pure agraphia. Attention should be directed to the fact that patients with other apraxic, ataxic, or alexic symptoms have often been termed cases of pure agraphia [Auerbach and Alexander, 1981; Soma et al., 1989]. Even after these studies are left out, there still exists a considerable number of reports suggesting the crucial involvement of other sites such as left posterior peri‐Sylvian and left temporo‐parietal regions [Rosati and De Bastiani, 1981; Yokota et al., 1990]. One possible explanation is that the lesions were large enough to affect the superior part of the parietal lobe, as the reported lesion sites are adjacent to this part. The latter case was, in particular, a patient of thrombosis of the Labbe vein, and therefore it is possible that functions of extensive areas around were considerably disturbed. Individual variability is another possibility, as these cases are very rare compared to cases of pure agraphia with superior parietal lesions. Further examination of neuropsychological data will be needed to verify these possibilities.
What are the specific roles of the left parietal and premotor regions? A conventional model of the writing system holds that the left parietal region provides graphic images for letters while the left premotor region organizes graphic motor images [Brain, 1967]. The specific involvement of the parietal region in providing graphic images for letters has been relatively well supported by previous literature. In the Sugishita et al.'s [1996] study referred to earlier, the left parietal region was strongly activated during mental writing. Because their task required graphic visual imagery rather than motor imagery, the activation can be interpreted as the reflection mainly of graphic letter images. Several lesion studies have also provided consistent evidence. Patients with a left parietal lesion exhibit a disorder in recalling graphic images of letters with well‐formed morphology of their letters in writing and copying, indicating intact graphic motor engrams [Yaguchi et al., 1998; Miceli et al., 1985]. There is a contradicting report, which presented a patient with a left parietal lesion showing poorly formed letters in writing with intact ability to produce words with other methods such as typing [Alexander et al., 1992]. Yet, it is probable that their patient suffered other disorders in visuomotor coordination, as the patient exhibited impairments in copying letters and limb praxis. In addition, the possibility cannot be excluded that this seemingly intact graphic letter imagery was due to the participation of the right hemisphere. Thus, apart from such exceptional cases, the importance of the left parietal region in organizing graphic letter images has been generally accepted, and the parietal activation observed in our study can also be interpreted on the lines of this schema. In contrast, the specific involvement of the left premotor region in organizing graphic motor images for writing letters has not been well established. One patient with a left premotor lesion showed an impairment in writing words and sentences but no difficulty or ill‐formed morphology was observed in writing single letters and slavish copying. [Vernea and Merory, 1975]. Another patient with a left prefrontal infarction made phonological errors in writing but kept well‐formed morphology of his letters in writing, suggesting impaired retrieval of graphic letter images from given phonemes [Tohgi et al., 1995]. These cases imply that the left premotor region is not specifically involved in providing graphic motor images but is associated with other components of the writing system such as graphic letter imagery. Thus, the schema for the functional specificity of the left premotor region is yet to be refined.
It is noteworthy that many of the classical neural models of writing proposed the importance of the left angular gyrus [Nielsen, 1946; Brain, 1967]. This is probably because lesions around this site often produce agraphia. However, patients with left angular lesions typically exhibit other linguistic disorders such as transcortical aphasia [Hinkin and Cummings, 1996; Crary and Heilman, 1988] and, moreover, lesions are often large enough to affect the superior part of the left parietal lobe or other subcortical areas [Crary and Heilman, 1988]. Therefore, it is conceivable that agraphic symptoms observed in such cases are secondary effects of other aphasic conditions or are caused by disturbed functions of the superior part of the left parietal lobe. In our study, parietal activation was observed in the left superior parietal lobule and superior margin of the left supramarginal gyrus, not in the left angular gyrus, after canceling out linguistic processes related to naming. Taken together with the lesion data, this result indicates that the superior part of the left parietal lobe is probably specific to the process of writing while the left angular gyrus is rather engaged in some aspects of speech language.
In this study, the visually cued finger tapping task was used to control for motoric aspects of the writing task. Both tasks involved movements of the right index finger with approximately the same timing, spatial range, and speed. However, because the writing task required more complex temporal and spatial control of the finger movements, some motoric components of writing may have possibly remained unsubtracted. Activation observed in the Writing minus Tapping comparison and in the conjunction analysis can therefore be partly associated with such differences in motoric aspects. In the light of this issue, we can not necessarily attribute the left parietal and premotor activation to the processes exclusive to writing. More neuroimaging data comparing writing with various control conditions will be necessary to fully understand the role of these regions.
Right Cerebellar Activation
Each hemisphere of the cerebellum is known to participate in motor functions of the ipsilateral side of the body. Our conjunction analysis between the Writing minus Naming and the Writing minus Tapping comparisons revealed activation in the superior part of the right cerebellum. As discussed above, the Writing condition inevitably included more complex movements than the Tapping condition. We consider that the right cerebellar activation revealed by the conjunction analysis represents the difference in the complexity of the finger movements, although the possibility exists that the region is associated with a certain linguistic function.
Differential Activation Between Writing − Naming and Writing − Tapping
The differences between the results of the two contrasts are also suggestive. Regions activated in the Writing minus Tapping comparison and not in the Writing minus Naming comparison can be thought to participate in the process of picture naming. Bilateral occipital, occipitotemporal, and the left inferior frontal regions showed this pattern of activation. Our interpretation is that the first two regions are associated with various visual processes required for the identification of pictures, and the last one, the region corresponding to the Broca area, is related to internal speech elicited by retrieving picture names. On the other hand, regions activated in the Writing minus Naming comparison and not in the Writing minus Tapping are interpreted as the areas involved in actual finger movements. The precentral and postcentral gyri and part of the basal ganglia were among those regions, which is consistent with previous knowledge on the neural substrates for finger movements.
REFERENCES
- Alexander MP, Fischer RS, Friedman R (1992): Lesion localization in apractic agraphia. Arch Neurol 49: 246–251. [DOI] [PubMed] [Google Scholar]
- Auerbach SH, Alexander MP (1981): Pure agraphia and unilateral optic ataxia associated with a left superior parietal lobule lesion. J Neurol Neurosurg Psychiatry 44: 430–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso A, Taborelli A, Vignolo LA (1978): Dissociated disorders of speaking and writing in aphasia. J Neurol Neurosurg Psychiatry 41: 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain L (1961): Speech disorders: aphasia, apraxia and agnosia. London: Butterworths. [Google Scholar]
- Crary MA, Heilman KM (1988): Letter imagery deficits in a case of pure apraxic agraphia. Brain Lang 34: 147–156. [DOI] [PubMed] [Google Scholar]
- Exner S (1881): Untersuchungen über die Lokalisation der Funktionen in der Grosshirnrinde des Menschen. Vienna: Wilhelm Braumuller. [Google Scholar]
- Friedland J (1990): Development and breakdown of written language. J Comm Dis 23: 171–186. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J‐B, Heather JD, Fracko‐wiak RSJ (1995a): Spatial registration and normalization of images. Hum Brain Mapp 2: 1–25. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J‐P, Frith CD, Fracko‐wiak RSJ (1995b): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Hinkin CH, Cummings JL (1996): Agraphia In: Beaumont JG, Kenealy PM, Rogers MJC. The Blackwell dictionary of neuropsychology. Cambridge, MA: Blackwell Publishers; P 21–31. [Google Scholar]
- Macwhinney B, Cohen J, Provost J (1997): The PsyScope experiment‐building system. Spat Vis 11: 99–101. [DOI] [PubMed] [Google Scholar]
- Miceli G, Silveri MC, Caramazza A (1985): Cognitive analysis of a case of pure dysgraphia. Brain Lang 25: 187–212. [DOI] [PubMed] [Google Scholar]
- Nielsen JN (1946): Agnosia, apraxia, aphasia: their value in cerebral localization. New York: Poal B. Hoeber. [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW (1990): Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 87: 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC (1995): Functional activation of the human ventrolateral frontal cortex during mnemonic retrieval of verbal information. Proc Natl Acad Sci USA 92: 5803–5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeltgen DP (1997): Agraphia In: Feinberg TE, Farah MJ. Behavioral neurology and neuropsychology. New York: McGraw‐Hill; p 209–217. [Google Scholar]
- Rosati G, De Bastiani P (1979): Pure agraphia: a discrete form of aphasia. J Neurol Neurosurg Psychiatry 42: 266–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz RJ, Canavan AG, Yaguez L, Herzog H, Tellmann L, Knorr U, Huang Y, Homberg V (1997): Representations of graphomotor trajectories in the human parietal cortex: evidence for controlled processing and automatic performance. Eur J Neurosci 9: 378–389. [DOI] [PubMed] [Google Scholar]
- Soma Y, Sugishita M, Kitamura K, Maruyama S, Imanaga H (1989): Lexical agraphia in the Japanese language. Pure agraphia for Kanji due to left posteroinferior temporal lesions. Brain 112: 1549–1561. [DOI] [PubMed] [Google Scholar]
- Sugishita M, Takayama Y, Shiono T, Yoshikawa K, Takahashi Y (1996): Functional magnetic resonance imaging (fMRI) during mental writing with phonograms. Neuroreport 7: 1917–1921. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain: 3‐dimensional proportional system: an approach to cerebral imaging. Stuttgart: Thieme. [Google Scholar]
- Tohgi H, Saitoh K, Takahashi S, Takahashi H, Utsugisawa K, Yo‐nezawa H, Hatano K, Sasaki T (1995): Agraphia and acalculia after a left prefrontal (F1, F2) infarction. J Neurol Neurosurg Psychiatry 58: 629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernea JJ, Merory J (1975): Frontal agraphia, (including a case report). Proc Aust Assoc Neurol 12: 93–99. [PubMed] [Google Scholar]
- Yaguchi H, Bando M, Kubo T, Ohi M, Suzuki K (1998): [A case of pure agraphia due to left parietal lobe infarction]. Rinsho Shinkeigaku—Clin Neurol 38: 499–505. [PubMed] [Google Scholar]
- Yokota T, Ishiai S, Furukawa T, Tsukagoshi H (1990): Pure agraphia of kanji due to thrombosis of the Labbe vein. J Neurol Neurosurg Psychiatry 53: 335–338. [DOI] [PMC free article] [PubMed] [Google Scholar]


