Abstract
A functional magnetic resonance imaging (fMRI) study was conducted during which seven subjects carried out naturalistic tactile object recognition (TOR) of real objects. Activation maps, conjunctions across subjects, were compared between tasks involving TOR of common real objects, palpation of “nonsense” objects, and rest. The tactile tasks involved similar motor and sensory stimulation, allowing higher tactile recognition processes to be isolated. Compared to nonsense object palpation, the most prominent activation evoked by TOR was in secondary somatosensory areas in the parietal operculum (SII) and insula, confirming a modality‐specific path for TOR. Prominent activation was also present in medial and lateral secondary motor cortices, but not in primary motor areas, supporting the high level of sensory and motor integration characteristic of object recognition in the tactile modality. Activation in a lateral occipitotemporal area associated previously with visual object recognition may support cross‐modal collateral activation. Finally, activation in medial temporal and prefrontal areas may reflect a common final pathway of modality‐independent object recognition. This study suggests that TOR involves a complex network including parietal and insular somatosensory association cortices, as well as occipitotemporal visual areas, prefrontal, and medial temporal supramodal areas, and medial and lateral secondary motor cortices. It confirms the involvement of somatosensory association areas in the recognition component of TOR, and the existence of a ventrolateral somatosensory pathway for TOR in intact subjects. It challenges the results of previous studies that emphasize the role of visual cortex rather than somatosensory association cortices in higher‐level somatosensory cognition. Hum. Brain Mapping 21:236–246, 2004. © 2004 Wiley‐Liss, Inc.
Keywords: somatosensory, touch, haptic, tactile, fMRI, somatosensory cortex, second somatosensory cortex, human, object recognition
INTRODUCTION
People can recognize many common objects by touch in less than 2 sec [Klatzky et al.,1985]. Despite our proficiency, very little is known about the neural substrates underlying naturalistic tactile object recognition (TOR). Behavioral and neuroimaging studies have debated whether touch has a viable object recognition system independent from that of vision [e.g., Deibert et al.,1999; Klatzky et al.,1987]. In other words, to what extent does the somatosensory system recruit visual cortex, or share visual‐tactile processing streams, during TOR? Studies that have limited tactile exploration or that have used stimuli varying exclusively in contour or spatial extent tend to emphasize visual recruitment and the activation of primary visual areas [e.g., Sathian et al.,1997; Zangaladze et al.,1999]. In contrast, studies that have permitted naturalistic exploration and multidimensional stimuli that vary in texture and hardness as well as in shape and spatial extent have provided evidence that the somatosensory system has its own encoding processes and pathways that may or may not be shared with vision [Bonda et al.,1996; Klatzky et al.,1987; Servos et al., 1998]. This suggests that full activation of the neural substrates of TOR only occurs during naturalistic palpation of real, multidimensional objects in a recognition task.
A related point of debate is whether cortical areas carrying out high‐level somatosensory cognition can be differentiated from those areas involved in perceptual processing. Although human patient studies [Caselli,1991], human neuroimaging studies [e.g., Servos et al.,2001], and animal lesion studies [Friedman et al.,1986; Mishkin,1979] have documented that the primary somatosensory cortex (SI) is important for perceptual feature identification, it remains controversial whether somatosensory association areas, and specifically secondary somatosensory cortex (SII), are high‐level tactile object processing areas [Burton,1984; Corkin et al.,1970; Norrsell,1978; Penfield and Jasper,1954; Semmes,1965]. In humans, SII lies primarily in the upper bank of the Sylvian fissure, immediately posterior to the central sulcus [Burton et al.,1993; Kaas and Pons,1988; Luders et al.,1985; Moore et al., submitted; Penfield and Jasper,1954]. In primates, unilateral ablation of SII produces deficits in texture discrimination [Garcha and Ettlinger,1980]. Recently, primate studies have shown that SII is not a unitary area and have documented at least two separate areas sensitive to tactile stimuli: the parietal ventral area located rostrally and SII caudally [Krubitzer et al.,1995]. Further, areas surrounding SII also respond to somatosensory stimuli [Krubitzer et al.,1995; Robinson and Burton,1980]. In humans, much of our knowledge of SII comes from brain‐injured patients. Although studies of patients with SII lesions [Caselli,1991,1993] and rare cases of tactile agnosia [Platz,1996; Reed et al.,1996; Reed and Caselli,1994], demonstrated the involvement of SII and surrounding somatosensory association areas in TOR, they did not rule out the involvement of other, multimodal areas. It remains an open question whether tactile recognition can be associated with activation in SII or in other somatosensory association areas such as the insula.
We used functional magnetic resonance imaging (fMRI) to investigate the question of whether the somatosensory system has a specialized neural pathway for object recognition under conditions of naturalistic TOR performance: real, 3D objects with complex shapes are recognized and stereotypical hand movements are permitted. Identifying distinct cortical regions involved in TOR would provide support for theories postulating modality‐specific object recognition systems and help clarify the role of somatosensory association areas in somatosensory cognition. We compared TOR to Rest, expecting it to activate primary sensorimotor areas in addition to somatosensory association areas subserving TOR. To reveal cortical areas specifically involved in the recognition component, or nonsensorimotor components, of TOR, we compared the palpation of “nonsense” objects with TOR to subtract out motor and somatosensory activity.
SUBJECTS AND METHODS
Subjects
Seven right‐handed male subjects (mean age, 24.7 years; age range, 22–29 years) were paid to participate in this study. They had no history of neurologic or psychiatric disease, head injury, drug abuse, or serious medical problems of any kind, and had normal brain structure as established using an MRI scan. All subjects gave informed consent for a protocol approved by the Human Subjects Review Board of the University of Utah School of Medicine.
Stimuli
The stimuli consisted of 120 real objects and 16 “nonsense” objects. The real objects were easily named, nonmagnetic, hand‐sized items typically found around the house, in the office, the garage, and around sporting activities. These objects included both natural objects and artifacts, and were not plastic imitations of the real objects. As a result, the objects varied along multiple dimensions (e.g., texture, hardness, size, shape, and weight). The final set of objects was selected from a pilot experiment (n = 5) that confirmed that each object could be identified using the prescribed motor sequence (i.e., grasp, rub, rub) within 3 sec at an accuracy rate of 90% and above.
Real objects were divided into three groups of 40 objects each, grouped by relative size to allow participants to consistently grasp the objects without dropping them. The object size manipulation was purely for handling ease and was not included as part of the experimental design. The group of small objects included objects typically held with the fingertips (e.g., Q‐tip, band‐aid, whistle, pacifier, cotton ball, garlic, die). The medium group included objects commonly held by several fingers (e.g., tennis ball, apple, measuring cup, baseball, hockey puck, orange). The large group included objects requiring the whole hand to manipulate them (e.g., book, hat, dish scrubber, carrot, football, mug). Participants received each object twice during the full course of the experiment.
The set of 16, 3‐D nonsense objects was constructed of balsa wood. They were hand‐sized (6 × 4.5 × 1.3 cm) unfamiliar complex shapes [see Reed et al.,1996, experiment 13]. Although each object primarily differed in shape, each piece had one to three pieces of fuzzy Velcro attached to its back to add texture and additional 3‐D contour. These items had no prior semantic associations. Pilot testing indicated that individual items were difficult to memorize, and therefore, they were relatively unlikely to produce repetition effects. When queried, subjects reported that they did not remember individual items during this task and did not know how many different items were in this set.
Procedure
Subjects participated in six 4‐min sessions of two types: TOR vs. rest and TOR vs. nonsense object palpation (NOP). The two types of sessions were presented in alternating order so that each was presented three times (e.g., TOR vs. rest, TOR vs. NOP, TOR vs. rest, TOR vs. NOP, TOR vs. rest, TOR vs. NOP). Each session consisted of five on–off 24‐sec block cycles (e.g., TOR, Rest, TOR, Rest, TOR, Rest, TOR, Rest, TOR, Rest). The experimental paradigm is illustrated in Figure 1.
Figure 1.

Diagram of experimental paradigm. Each subject participated in six 4‐min sessions. Three sessions used an on‐off block design for TOR and Rest tasks; three sessions used an on‐off block design for TOR and NOP tasks. Each block was 24‐sec long and included eight stimulus presentations.
For TOR on blocks, subjects lay in the scanner with their eyes closed and their right hand held flat with the palm facing upwards. The experimenter placed a real object in their right hand at a constant rate (every 3 sec). Subjects grasped the object, executed two rubbing movements in succession, and opened their hand. They were instructed to covertly recognize and name the object. No movement or response was required from the left hand. Subjects were told to keep it still in a relaxed position. Pilot testing demonstrated that the “grasp, rub, rub” motor sequence was a natural sequence of tactile exploration [see also Klatzky and Lederman,1992] and that the current set of real object stimuli could be recognized consistently and accurately using this motor sequence. Pilot testing also confirmed that the motor sequence was consistent across subjects and objects. Before scanning, subjects practiced using the motor sequence to recognize several objects that were not part of the experimental set. Experimenters ensured that subjects executed the motor sequence correctly before testing and then monitored subjects during testing.
For the Rest off blocks, subjects lay in the scanner with their eyes closed and their right hand held open with the palm facing upward. They were instructed to focus their attention on their hand. No stimuli were presented and no movement was required.
For TOR vs. NOP sessions, the TOR on blocks were the same as above. NOP off blocks were similar to the TOR blocks, except the stimuli were nonsense objects. The NOP task provided a simple, robust signal to subtract out activation that was not specific to the object recognition process, i.e., was due to purely motoric and sensory activity. Lying in the scanner with their eyes closed, subjects were handed a nonsense object every 3 sec, felt the objects using the grasp, rub, rub motor sequence (the same motor sequence used to feel real objects), and then opened their hand. They were instructed to focus on the motor sequence and what the objects felt like, but not to recognize them. Before scanning, subjects practiced feeling nonsense objects that were not part of the experimental set using the correct motor sequence and were monitored by the experimenters during testing to ensure that the motor sequence was correct and identical to the motor sequence used in TOR blocks.
In all sessions, hand movements were either monitored by videotape or by three separate experimenters inside the MR room. Each subject was observed during the entire experiment to ensure correct task performance. Analysis of videotaped data and experimenter reports documented that subjects adhered to the prescribed motor sequence with their right hand and did not move their left hand. Thus, any differences in motor movements between TOR and NOP tasks were subtle at best.
After scanning was completed, subjects were questioned regarding the stimuli they could and could not identify. Although there was no formal scoring of their responses during the experiment, all subjects reported that they could recognize approximately 90% of the real objects.
Functional Imaging
Data were acquired with a 1.5‐T GE Signa whole‐body MRI system (GE Medical Systems, Inc., Milwaukee, WI) equipped with a head volume coil. T1‐weighted anatomic images (7‐mm thickness with skip 0 mm, FOV = 26 cm, 256 × 256 pixels) and contiguous multislice T2*‐weighted echoplanar images (flip angle = 90 degrees, TE = 40 msec, 64 × 64 pixels, FOV = 26 cm, voxel size = 4.06 × 4.06 × 7 mm) were obtained. Volumes were obtained continually every 3 sec. Each volume comprised 15 slices (slice thickness of 7 mm). Six sessions of 4 min each were recorded. For each session, 88 volumes were acquired with eight “dummy” volumes acquired at the start of each session to allow for T1 equilibration effects. In total, 480 volumes of data were acquired for each subject.
Image Preprocessing
Image and statistical analyses were carried out using statistical parametric mapping (SPM) and the SPM99 software package (Wellcome Department of Cognitive Neurology, University College, London). All volumes were realigned to the first volume to correct for interscan movement and then resliced using a sinc interpolation in space [Friston et al.,1995a]. Each volume was normalized [Friston et al.,1995a] to a standard EPI template volume, based on the Montreal Neurological Institute (MNI) reference brain [Evans et al.,1993,1994], in the standardized space of Talairach and Tournoux [1988] using nonlinear basis functions. Finally, the data were smoothed with a Gaussian kernel of 8‐mm full width at half‐maximum to compensate for residual variability after spatial normalization. Smoothing also improved the applicability of the Gaussian random field theory used in the subsequent statistical inferences.
Two subjects demonstrated head movement of more than 3 mm in 3 of 42 sessions (7 subjects × 6 sessions). These data were discarded. The remaining data from these two subjects as well as the complete data sets from the other five subjects (39 sessions) were included in the subsequent analyses. In total, TOR vs. NOP sessions contained 1,440 images and TOR vs. Rest sessions contained 1,360 images.
Statistical Analysis
The data were analyzed using an across‐subject conjunction implemented in SPM99. The statistical analysis of each effect of interest had two stages. In the first stage, activation maps for each task‐pair contrast and each subject were calculated. In the second conjunction stage, areas of common activation for all subjects were identified for each effect of interest. Three effects of interest were studied separately in this manner: TOR vs. Rest, TOR vs. NOP, and NOP vs. TOR. Our analysis follows closely the method discussed in Friston et al. [1999] that formalizes the calculation of statistical thresholds corrected for a conjunction across a population of subjects.
Stage I analysis
The expected responses were modeled in an experimental design matrix by convolving a boxcar function with a standard hemodynamic response function (HRF). The temporal derivative of the expected hemodynamic response was also added as a regressor, allowing compensation for response‐delay variations. Low frequency artifacts, corresponding to aliased respiratory and cardiac effects and other slow variations in signal intensity, were removed by high‐pass filtering (>96‐sec period) the time series, and a low‐pass filter based on the HRF was used to remove transients. In addition, whole volume signal changes were removed by global scaling. Using the experimental design matrix and standard linear estimation, session‐specific t‐statistical maps (SPM[t]) pertaining to each effect of interest were calculated for each subject [Friston et al.,1995b].
Stage II analysis
An across‐subject conjunction was calculated by finding the minimal t‐value (at each voxel) across all subjects [Friston et al.,1999]. A P < 0.001 threshold corrected for family‐wise (type 1) error was applied. Areas of activation that survived the statistical threshold were characterized in terms of their peak heights (t‐value maxima) with their positions specified in coordinates (x, y, z) in stereotactic space defined by the MNI [Evans et al.,1993]. We reported cluster activations with a minimum extent of four voxels. The activation maps were then superimposed on high‐resolution MR scans of the standard MNI brain. Locations of peak activation were associated with their corresponding Brodmann areas using the MNI Space Utility (online at http://www.ihb.spb.ru/~pet_lab/) and by neuroanatomic analysis of the sulci and gyri. For reporting purposes, MNI‐space coordinates were translated into Talairach‐space coordinates using a nonlinear transformation function [Brett,1999].
To address the neuroanatomic prediction that SII was activated differentially during TOR, we conducted a region of interest (ROI) analysis using the MARSBAR procedure from the SPM99 toolbox [Brett et al.,2002]. The location of human SII coordinates was specified from an fMRI study [Moore et al., submitted] that defined human SII based on functional and neuroanatomic criteria. Left (LH) and right hemisphere (RH) SII regions of interest (ROIs) were specified as 6‐mm spheres with centers of activation positioned at (±53.3, −20.3, 22.4). ROI analyses were conducted for each individual subject for TOR vs. NOP and NOP vs. TOR contrasts.
RESULTS
To determine the basic pattern of activation for naturalistic TOR, we used the TOR vs. Rest contrast to reveal areas showing significantly increased blood oxygenation level‐dependent (BOLD) signal during conditions in which subjects were required to recognize real objects by touch compared signals during the control Rest condition. The Talairach‐space coordinates and sizes of activated clusters, their extent, and their significant t‐values (T7[607.5] = 1.41, P < 0.001 FWE corrected) are listed in Table I. Figure 2 shows clusters of activation as labeled color overlays on normalized anatomic MRI slices. As can be expected for stimulation and movement of the right hand, cortex adjacent to the central sulcus in the LH (i.e., contralateral to the hand used for palpation) was activated strongly in this contrast. This activation was most significant in the primary somatosensory and motor areas (Brodmann's areas [BA] 2–4), but extended into anterior parietal somatosensory association (BA 40) and premotor (BA 6) areas. Compared to the LH cluster, the RH cluster had less extensive activation and was located inferior and posterior to the left hemisphere cluster (BA 2/40). For both clusters, activation extended bilaterally into SII regions. An attention‐related network that included regions involved in covert object naming was activated that included the left inferior frontal gyrus/frontal eye fields (FEF; BA 6/9), right inferior frontal gyrus/prefrontal cortex (BA 9/45), and right premotor area (BA 6). In addition, regions in the ventral object recognition pathway were activated that included the left fusiform/inferior temporal gyrus (BA 19/37) and right inferior temporal gyrus (BA 20/37) or bilateral lateral occipital complexes (LOC). Last, mainly right (i.e., ipsilateral to the utilized hand) cerebellar and contralateral thalamic activation were observed. It is important to note that retinotopic visual cortex was not activated. The largest regions of selective activation thus occurred in modality‐specific somatosensory cortex.
Table I.
Brain areas activated during the TOR condition compared to the Rest condition
| Hemisphere, gyrus, area | BA | x | y | z | Voxels | t * |
|---|---|---|---|---|---|---|
| L prCG/poCG/IPL, SMI/SII | 2/3/4/6/40 | −42 | −20 | 56 | 1516 | 16.38 |
| R poCG/IPL, SI/SII | 2/40 | 59 | −21 | 45 | 491 | 8.69 |
| L IFG, premotor/prefrontal | 6/44 | −56 | 7 | 33 | 65 | 5.88 |
| R prCG/MFG, premotor | 6 | 39 | −3 | 58 | 139 | 7.70 |
| R IFG/MFG, prefrontal | 9/45 | 62 | 13 | 27 | 16 | 2.46 |
| R ITG/MTG, LOC | 37 | 57 | −56 | −7 | 19 | 3.26 |
| L ITG/MTG, LOC | 19/37 | −50 | −59 | −5 | 39 | 4.81 |
| L FG/cerebellum | 37 | −21 | −50 | −15 | 21 | 3.41 |
| R cerebellum | 18 | −53 | −18 | 220 | 9.27 | |
| L thalamus | −6 | −20 | 1 | 101 | 3.41 |
Talairach coordinates (x, y, z) and number of voxels given for activated clusters.
T7 (605.7) = 1.41, P < 0.001 (FWE corrected). Clusters restricted to a minimum of four voxels in extent.
TOR, tactile object recognition; BA, Brodmann's area; L and R, left and right; prCG/poCG, pre‐ and postcentral gyrus; IPL, inferior parietal lobule; SMI, primary sensorimotor cortex; SII, secondary somatosensory cortex; SI, primary somatosensory cortex; MFG, middle frontal gyrus; IFG, inferior frontal gyrus; ITG, inferior temporal gyrus; MTG, middle temporal gyrus; LOC, lateral occipital complex; FG, fusiform gyrus.
Figure 2.

Across‐subject conjunction activation for TOR vs. Rest. Areas of significant activation are overlaid on normalized brain slices based on the MNI brain. Activated regions include bilateral SM1, SII, LOC, and prefrontal regions, and are consistent with the TOR pathway proposed by Mishkin [1979]. Note absence of primary visual cortex activation.
The areas showing significantly increased BOLD signal during the TOR condition compared to the NOP condition revealed similar results without the primary somatosensory and motor components. The Talairach‐space coordinates of clusters activated, their extent, and their significant t‐values (T7[673] = 1.41, P < 0.001 FWE corrected) are listed in Table II. Figure 3 shows clusters of activation as labeled color overlays on normalized anatomic MRI slices. Regions associated with the TOR process included bilateral inferior parietal somatosensory association areas and SII (LH, BA 2/40; RH, BA 2/40). The activation in primary somatosensory and motor cortices observed when TOR was compared to Rest was absent, indicating that the NOP condition provided an effective control for sensorimotor activation. Regions associated with attentional processing included the left prefrontal cortex (BA 11/47, 46), left premotor/FEF (BA 6/9), and insula (BA 13). Regions associated with object recognition included the left LOC (BA 19/37). Last, activation was observed in bilateral basal ganglia and thalamic regions. Again, retinotopic visual cortex was not activated differentially.
Table II.
Brain areas activated during TOR conditions compared to NOP conditions
| Hemisphere, gyrus, area | BA | x | y | z | Voxels | t * |
|---|---|---|---|---|---|---|
| L poCG/IPL, SI/SII | 2/40 | −62 | −16 | 23 | 37 | 2.27 |
| L poCG/IPL, SI/SII | 2/40 | −65 | −25 | 29 | 4 | 1.59 |
| R poCG/IPL, SI/SII | 2/3/40 | 62 | −21 | 43 | 177 | 3.96 |
| L IFG, prefrontal | 11/47 | −36 | 34 | −12 | 6 | 1.74 |
| L IFG/MFG, prefrontal | 46 | −48 | 36 | 20 | 16 | 2.95 |
| L IFG/MFG/prCG, premotor | 6/9/44 | −39 | 7 | 27 | 66 | 2.42 |
| L ITG, LOC | 19/37 | −50 | −59 | −7 | 67 | 3.58 |
| L insula/basal ganglia | 13 | −36 | 0 | 0 | 106 | 2.71 |
| R basal ganglia | 24 | 0 | −8 | 24 | 1.77 | |
| LR thalamus | −3 | −23 | −1 | 35 | 1.97 |
Talairach coordinates (x, y, z) and number of voxels given for activated clusters.
T7 (673) = 1.41, P < 0.001 (FWE corrected). Clusters restricted to a minimum of four voxels in extent.
TOR, tactile object recognition; NOP, nonsense object palpation; BA, Brodmann's area; L and R, left and right; prCG/poCG, pre‐ and postcentral gyrus; IPL, inferior parietal lobule; SI, primary somatosensory cortex; SII, secondary somatosensory cortex; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; ITG, inferior temporal gyrus; LOC, lateral occipital complex.
Figure 3.
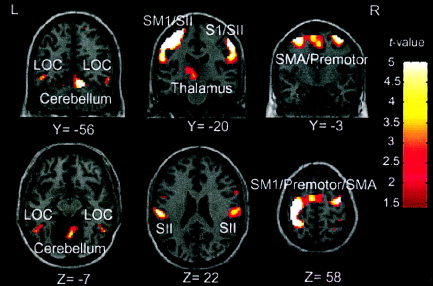
Across‐subject conjunction activation for TOR vs. NOP. Areas of significant activation are overlaid on normalized brain slices based on the MNI brain. In contrast to Figure 1, differential activation is seen in SII but not SM1 regions. Activated regions of SII, LOC, prefrontal, and SMA regions are associated with higher‐level TOR, motoric attention, and more general object recognition functions.
In contrast, areas showing significantly increased BOLD signal during the NOP condition compared to the TOR condition did not activate higher‐level somatosensory and object recognition regions. The coordinates of clusters activated, their extent, and their significant t‐values are listed in Table III. Activated regions included the right frontal pole (BA 10) and supplementary motor area (SMA; BA 8), precuneous (BA 7), and left supramarginal gyrus (SMG; BA 39). Similar precuneous activations have been found frequently for the control condition in other neuroimaging studies [Shulman et al.,1997]. Their significance is unknown, but they may partially reflect right hemisphere specialization for environmental monitoring [Mazziotta et al.,1983].
Table III.
Brain areas activated during the NOP condition compared to the TOR condition
| Hemisphere, gyrus, area | BA | x | y | z | Voxels | t * |
|---|---|---|---|---|---|---|
| R SFG, frontal pole | 10 | 36 | 61 | −6 | 15 | 2.49 |
| R SFG, SMA | 8 | 27 | 37 | 45 | 20 | 2.15 |
| R SMG, IPL | 39 | 53 | −60 | 28 | 7 | 1.73 |
| LR precuneus | 7 | −3 | −60 | 33 | 127 | 2.36 |
Talairach coordinates (x, y, z) and number of voxels given for activated clusters.
T7 (673) = 1.41, P < 0.001 (FWE corrected). Clusters restricted to a minimum of four voxels in extent. NOP, nonsense object palpation; TOR, tactile object recognition; BA, Brodmann's area; L and R, left and right; SFG, superior frontal gyrus; SMA, supplementary motor area; SMG, supramarginal gyrus; IPL, inferior parietal lobule.
ROI analyses for LH and RH SII were consistent with the locations of activation found in the conjunction group analyses. These results are listed in Table IV. For the TOR vs. NOP contrast, significant activation was found in LH SII for all seven subjects and in RH SII for five of seven subjects. In contrast, none of the subjects displayed significant activation in either LH or RH SII for the NOP vs. TOR contrast.
Table IV.
ROI analyses for SII
| Subject | Left SII (−53.3, −20.3, 22.4) | Right SII (53.3, −20.3, 22.4) | ||||||
|---|---|---|---|---|---|---|---|---|
| TOR vs. NOP | NOP vs. TOR | TOR vs. NOP | NOP vs. TOR | |||||
| t | P | t | P | t | P | t | P | |
| 1 | 4.64 | 0.0001 | −4.64 | 0.999 | 3.25 | 0.0001 | −3.25 | 0.999 |
| 2 | 2.67 | 0.004 | −2.67 | 0.996 | 2.82 | 0.0025 | −2.82 | 0.998 |
| 3 | 5.20 | 0.0001 | −5.20 | 1.00 | 4.24 | 0.0001 | −4.24 | 0.999 |
| 4 | 3.27 | 0.0001 | −3.27 | 0.999 | 1.11 | 0.128 | −1.14 | 0.872 |
| 5 | 2.02 | 0.02 | −2.02 | 0.978 | 2.56 | 0.005 | −2.56 | 0.995 |
| 6 | 1.77 | 0.04 | −1.77 | 0.961 | 1.14 | 0.128 | −1.14 | 0.872 |
| 7 | 5.01 | 0.0001 | −5.01 | 1.00 | 4.56 | 0.0001 | −4.56 | 0.999 |
ROI, region of interest; SII, secondary somatosensory cortex; TOR, tactile object recognition; NOP, nonsense object palpation.
DISCUSSION
Network for TOR Is Distinct From Primary Visual Cortex
Our results identify a network of cortical areas activated during TOR in intact individuals using naturalistic hand movements to recognize real, multidimensional objects. The network most prominently involves regions specific to the somatosensory system, but also includes regions that are part of the ventral visual object recognition pathway and motor attention systems. Furthermore, with the exception of primary sensorimotor cortex, the TOR‐specific areas are activated more by identification of real objects than by sensorimotor controls. No activation was found in primary visual areas (e.g., BA 17, 18, posterior 19) associated with retinotopic visual object perception and visual imagery. These results thus support a distinct stream of processing for TOR and provide evidence that somatosensory perceptual processes can be distinguished from higher‐level somatosensory cognition.
When activation due to primary motor and somatosensory stimulation was accounted for, primary somatosensory areas were no longer active differentially and three networks of activation were observed: a TOR network for higher‐level somatosensory processing, a somatomotor attention circuit, and a multimodal ventrotemporal object recognition stream. For the TOR network, significant activation was observed bilaterally in somatosensory association cortex including SII, insula, and inferior parietal or somatosensory association cortices. For the somatomotor attention circuit, activation was observed in contralateral prefrontal cortex, SMA, premotor cortex, FEF, and the basal ganglia. Last, for the ventral processing stream, contralateral activation was observed in the LOC and medial temporal lobe. These areas correspond well to anatomic and neurophysiological data regarding neural pathways for tactile processing. Based on his primate work, Mishkin [1979] proposed a ventrally directed pathway for tactile object processing in which information was processed serially from SI to SII to the insula to supramodal medial temporal lobe areas including the amygdala and hippocampal formation. Positron emission tomography (PET) studies of tactile memory for object shape [Bonda et al.,1996] and object discrimination based on object pattern [Ginsberg et al.,1987] support this ventrolateral pathway and its interconnections with frontal and parietal networks.
Activation of Somatosensory Association Cortex During TOR
The observation of significant bilateral activation in inferior somatosensory association areas when sensorimotor components were accounted for indicates their involvement in nonperceptual aspects of TOR. Our results are consistent with this activation of the ventrolateral somatosensory pathway, reflecting the more cognitive components of TOR. The ventrolateral pathway has been proposed as the key route for tactile object processing, especially in the integration of object features [Bonda et al.,1996; Ginsberg et al.,1987; Mishkin,1979]. Based on its connections with parietal and insular cortex and to parahippocampal cortex, Mishkin [1979] proposed that SII might be specialized for tactile learning. In monkeys, lesions to SII produce severe impairments in tactile discrimination [Mishkin,1979; Murray and Mishkin,1984]. In humans, lesions of SII and nearby somatosensory association areas produce tactile agnosia [Caselli,1991,1993; Reed et al.,1996; Reed and Caselli,1994]. Furthermore, the responses of SII cells suggest that SII may be involved in the ability to recognize the object as a whole, belonging to a category of similar objects, rather than the discrimination of component object features [Sinclair and Burton,1993]. In addition, SII has a role in active exploration. Connections between SII and motor cortex [Friedman et al.,1986] suggest the possibility that SII provides sensory feedback for the manipulatory movements necessary to obtain salient object information. It may also integrate sensory information gained from exploration to generate a coherent image of an object. This idea is supported by the co‐activations of SII and premotor areas. Finally, our observation of bilateral activation is consistent with both PET studies of active touch [O'Sullivan et al.,1994; Roland,1993; Roland et al.,1998] and with anatomic evidence [Jones and Powell,1970]. Overall, SII seems to be involved in the conscious detection of somatosensory stimuli [Moore et al., submitted].
Activation of Somatomotor Attention Circuit During TOR
Our results revealed an attention‐related network that was activated differentially by TOR. This network also included covert naming processes. It consisted of the contralateral inferior frontal gyrus (BA 11/47, 46), SMA, FEF, insula, and basal ganglia (BA 6/9, 13/34). When the activation evoked by palpation of nonsense objects was subtracted, TOR continued to activate motor association areas. A characteristic of object recognition in the tactile modality is that it requires an especially intense interaction between the sensory and motor systems. TOR evoked intense activation of somatomotor areas, especially contralateral to the palpating hand. These results correspond to previous neuroimaging studies of active tactile discrimination [Anton et al.,1996; Bonda et al.,1996; Ginsberg et al.,1987; Lin et al.,1996; O'Sullivan et al.,1994; Servos et al.,2001].
Although this somatomotor activation may reflect subtle differences between movements executed in TOR and NOP, movements were stereotyped and closely monitored to minimize such differences. Furthermore, differential activation between TOR and NOP was not observed in primary motor cortex or the cerebellum, areas that would be expected to be sensitive to subtle motoric differences. Thus, our results suggest that intentional tactile identification activates secondary motor areas. Previous neuroimaging studies have implicated the premotor and supplementary motor areas in intentional interactive movements that involve planning [e.g., Passingham,1996]. In primates, cells in somatomotor cortex often respond to somatosensory stimuli, and vice versa, corresponding to the intense anatomic interconnections and interactive physiologic activities of these structures [Jones and Powell,1970]. In addition, activation patterns are different from those found in fMRI studies investigating object‐related manipulation that have implicated Broca's area and infraparietal regions [Binkofski et al.,1999a,b]. Nonetheless, our results are consistent with these studies for activation of cortical regions involved in attention and conscious detection of objects, such as SII and SMA. The current data are consistent with the viewpoint that places the somatosensory and somatomotor cortices within a unified system.
Activation of Ventrotemporal Object Recognition Pathway During TOR
TOR activated contralaterally a lateral occipitotemporal area that seems to correspond to the visual object recognition area LOC. Activation was also maintained in medial temporal areas (fusiform and parahippocampal gyri) that may have multimodal roles in object recognition and memory [Bonda et al.,1996; Mishkin,1979]. Consistent activation was found contralaterally in the lateral occipitotemporal cortex (BA 37). This area is considered typically to be part of the visual association cortex, and lesions here are associated with visual agnosia for objects [Farah,1980]. The activated area is anterior to retinotopic cortex, and seems to correspond to an area labeled LOC that has been found in several previous studies to be activated by viewing objects, as compared to non‐object visual control stimuli [Halgren et al.,1999; Tootell et al.,1998]. It has been associated also with the detection of 3‐D shape. Although all objects used in this study were 3‐D, the real objects in this study varied more.
Visual imagery may have been used during TOR [Mellet et al.,1996], but the present pattern of activations does not correspond to general activation patterns produced by visual imagery [e.g., Chen et al.,1998; Klein et al.,2000; Thompson and Kosslyn,2000]. No activation was observed in the calcarine sulcus or other primary visual areas. Further evidence against a visual imagery hypothesis comes from a tactile discrimination study using magnetoencephalography [Reed et al.,2000]. LOC is activated only after prior processing by the somatosensory system.
One interpretation of LOC activation is that is that the ventrotemporal processing stream is multimodal, at least for vision and touch [Amedi et al.,2001,2002; James et al.,2002]. The object‐related LOC has been proposed to be one point of convergence for parallel pathways in specific multimodal brain regions that integrate information from multiple modalities [Amedi et al.,2002; Zangaladze et al.,1999; Zhou and Fuster,2000]. Amedi et al. [2001] found that LOC was activated by both visual and tactile inputs with a preference for objects over scrambled versions of the same objects or textures. Thus, LOC may be devoted mainly to visual object recognition but receives collateral transmodal activation during object recognition in non‐visual modalities.
Independence and Interaction in the TOR Pathway
Despite strong convergence of the present results with other tactile imaging and neurophysiological studies, it remains controversial whether higher‐level TOR occurs within the somatosensory system and the degree to which visual cortex is recruited during TOR. Theoretically, this has important implications for the argument that the somatosensory system has a viable, modality‐specific object recognition system. Some researchers have proposed that TOR is carried out by a visually based mechanism of object recognition and that tactile information is either converted to a visual image or stored in a temporary visual store [Bonda et al.,1996; Bushnell and Baxt,1999; Deibert et al.,1999; Easton et al.,1997; Kerst and Howard,1978; Klatzky and Lederman,1987; Reales and Ballesteros,1999]. According to this view, primary and secondary somatosensory areas are not involved in later stages of TOR. In a recent fMRI study, activation produced by active TOR of real objects was compared to texture discrimination for nonsense objects [Deibert et al.,1999]. Differential activation was found bilaterally in striate and extrastriate cortex, but not in somatosensory cortex. These findings are in direct contrast to our results showing activation in secondary somatosensory cortices but only secondary visual cortex activation. The discrepancies between these studies may be explained by the selection of control tasks. The lack of activation in somatosensory cortex in the study by Deibert et al. [1999] may be a consequence of their control task that required somatosensory texture evaluation. Given that texture is a salient cue for TOR, the control task may have subtracted out critical somatosensory contributions, leaving only residual visual areas.
Further, the extent of visual recruitment may be manipulated by the dimensions upon which stimuli vary. The tactile system processes information about substance properties (e.g., texture and hardness) more efficiently than information about structural properties (e.g., contour and size) [Klatzky et al.,1987]. Typically, studies demonstrating a role for visual cortex during tactile tasks involved the discrimination of shape and spatial characteristics, such as grating orientation [Sathian et al.,1997; Zangaladze et al.1999] and Braille [Sadato et al.,1996]. Zangaladze et al. [1999] found that transcranial magnetic stimulation (TMS) over the visual cortex interfered with tactile discrimination of grating orientation. Event‐related potential (ERP) recordings obtained during TMS revealed activation in the left parieto‐occipital cortex. Likewise, O'Sullivan et al. [1994] found bilateral activation in the angular gyrus during length discrimination but not during roughness discrimination. To create a representation of the object, these tasks required integration of contour and local spatial information over space and time. Under these circumstances, a tactile representation may be converted to a visual image and re‐perceived by visual processors. The above studies demonstrate the importance of stimulus and control task selection for interpreting the functional organization of somatosensory cortex.
CONCLUSION
Neural activation for naturalistic TOR is distinct from that produced by visual object recognition. Although some recruitment of visual regions related to object recognition was found, most activation was consistent with a separate stream of processing for TOR. Activation of the ventrolateral somatosensory pathway may be homologous to the ventrotemporal pathway that has been associated strongly with visual object recognition [Tootell et al.,1998; Ungerleider and Mishkin,1982]. Similarly, somatosensory cortical areas seem to have a functional hierarchy, with sensorimotor areas involved in more perceptual aspects of TOR and inferior parietal regions, including SII, involved in higher‐level somatosensory processing. Future research may be able to draw further parallels between the somatosensory and visual systems. As it has been important to clarify the roles of distinct regions of cortex in the visual system, it is important also to identify functional distinctions within somatosensory cortex.
Acknowledgements
We thank S. Baldwin, K. Cunningham, J. Jordin, N. Sevastyanenko, H. Buswell, J. Lee, and W. Stansbury for help in data collection and preparation. We also thank D. Post, W. Wariner, and the members of R. Normann's lab for help in data processing.
REFERENCES
- Amedi A, Malach R, Hendler T, Peled S, Zohary E (2001): Visuohaptic object‐related activation in the ventral visual pathway. Nat Neurosci 4: 324–330. [DOI] [PubMed] [Google Scholar]
- Amedi A, Jacobson G, Hendler T, Malach R, Zohary E (2002): Convergence of visual and tactile shape processing in the human lateral occipital complex. Cereb Cortex 12: 1202–1212. [DOI] [PubMed] [Google Scholar]
- Anton JL, Benali H, Guigon E, Di Paola M, Bittoun J, Jolivet O, Burnod Y (1996): Functional MR imaging of the human sensorimotor cortex during haptic discrimination. Neuroreport 7: 2849–2852. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund H (1999a): Fronto‐parietal circuit for object manipulation in man: evidence from an fMRI study. Eur J Neurosci 11: 3276–3286. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Stephan KM, Rizzolatti G, Seitz RJ, Freund HJ (1999b): A parieto‐premotor network for object manipulation: evidence from neuroimaging. Exp Brain Res 128: 210–213. [DOI] [PubMed] [Google Scholar]
- Bonda E, Petrides M, Evans A (1996): Neural systems for tactual memories. J Neurophysiol 75: 1730–1737. [DOI] [PubMed] [Google Scholar]
- Brett M (1999): The MNI brain and the Talairach atlas. Online at http://www.mrccbu.cam.ac.uk/Imaging/mnispace.html.
- Brett M, Anton JL, Valabregue R, Poline JB (2002): Region of interest analysis using an SPM toolbox [abstract]. Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan. Neuroimage 16: 497A. [Google Scholar]
- Burton H (1984): Second somatosensory cortex and related areas In: Peters A, Jones EG, editors. Cerebral cortex, Vol 5 Sensory‐motor areas and aspects of cortical connectivity. New York: Plenum Press; p 31–98. [Google Scholar]
- Burton H, Videen TO, Raichle ME (1993): Tactile‐vibration‐activated foci in insular and parietal‐opercular cortex studied with positron emission tomography mapping the second somatosensory area in humans. Somatosens Mot Res 10: 297–308. [DOI] [PubMed] [Google Scholar]
- Bushnell EW, Baxt C (1999): Children's haptic and cross‐modal recognition with familiar and unfamiliar objects. J Exp Psychol Hum Percept Perform 25: 1867–1881. [DOI] [PubMed] [Google Scholar]
- Caselli RJ (1991): Rediscovering tactile agnosia. Mayo Clin Proc 66: 129–142. [DOI] [PubMed] [Google Scholar]
- Caselli RJ (1993): Ventrolateral and dorsomedial somatosensory association cortex damage produces distinct somesthetic syndromes in humans. Neurology 43: 762–771. [DOI] [PubMed] [Google Scholar]
- Chen W, Toshinoir K, Xio‐Hong Z, Ogawa S, Tank DW, Ugurbil K (1998): Human primary visual cortex and lateral geniculate nucleus activation during visual imagery. Neuroreport 9: 3669–3674. [DOI] [PubMed] [Google Scholar]
- Corkin S, Milner B, Rasmussen T (1970): Somatosensory thresholds—contrasting effects of postcentral‐gyrus and posterior parietal‐lobe excisions. Arch Neurol 23: 41–58. [DOI] [PubMed] [Google Scholar]
- Deibert E, Kraut M, Kremen S, Hart J (1999): Neural pathways in tactile object recognition. Neurology 52: 1413–1417. [DOI] [PubMed] [Google Scholar]
- Easton RD, Srinivas K, Greene AJ (1997): Do vision and haptics share common representations? Implicit and explicit memory within and between modalities. J Exp Psychol Learn Mem Cogn 23: 153–163. [DOI] [PubMed] [Google Scholar]
- Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM (1993): 3‐D statistical neuroanatomical models from 305 MRI volumes. In: Proceedings IEEE‐Nuclear Science Symposium and Medical Imaging Conference. Piscataway, NJ: IEEE Inc. p 1813–1817. [Google Scholar]
- Evans AC, Kamber M, Collins DL, Macdonald D (1994): An MRI‐based probabilistic atlas of neuroanatomy In: Shorvon S, Fish D, Andermann F, Bydder GM, Stefan H, editors. Magnetic resonance scanning and epilepsy. NATO ASI Series A, Life Sciences, Vol 264 New York: Plenum Press; p 263–274. [Google Scholar]
- Farah MJ (1980): Visual agnosia. Cambridge, MA: MIT Press. [Google Scholar]
- Friedman DP, Murray EA, O'Neill JB, Mishkin M (1986): Cortical connections of the somatosensory fields of the lateral sulcus of macaques: evidence of a corticolimbic pathway for touch. J Comp Neurol 252: 323–347. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Poline JB, Frith CD, Heather JD, Frackowiak RS (1995a): Spatial registration and normalization of images. Hum Brain Mapp 2: 165–189. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS (1995b): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ (1999): Multisubject fMRI studies and conjunction analyses. Neuroimage 10: 385–396. [DOI] [PubMed] [Google Scholar]
- Garcha H, Ettlinger G (1980): Tactile discrimination learning in the monkey: the effects of unilateral or bilateral removals of the second somatosensory cortex (area SII). Cortex 16: 397–412. [DOI] [PubMed] [Google Scholar]
- Ginsburg MD, Yoshii F, Vibulsresth S, Chang JY, Duara R, Barker WW, Boothe TE (1987): Human task‐specific somatosensory activation. Neurology 37: 1301–1308. [DOI] [PubMed] [Google Scholar]
- Halgren E, Dale AM, Sereno MI, Tootell RB, Marinkovic K, Rosen BR (1999): Location of human face‐selective cortex with respect to retinotopic areas. Hum Brain Mapp 7: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TW, Humphrey GK, Gati JS, Servos P, Menon RS, Goodale MA (2002): Haptic study of three‐dimensional objects activates extrastriate visual areas. Neuropsychologia 40: 1706–1714. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TP (1970): An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain 93: 793–820. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Pons TP (1988): The somatosensory system of primates In: Steklis HP, Erwin J, editors. Comparative Primate Biology, vol. 4 New York: Alan R. Liss. [Google Scholar]
- Kerst SM, Howard JH Jr (1978): Memory psychophysics for visual area and length. Mem Cogn 6: 327–335. [DOI] [PubMed] [Google Scholar]
- Klatzky RL, Lederman SJ (1987): The intelligent hand In: Bower G, editor. The psychology of learning and motivation Vol 21 San Diego: Academic Press; p 121–151. [Google Scholar]
- Klatzky RL, Lederman S, Metzger V (1985): Identifying objects by touch: an “expert system.” Percept Psychophys 37: 299–302. [DOI] [PubMed] [Google Scholar]
- Klatzky RL, Lederman SJ, Reed CL (1987): There's more to touch than meets the eye: the salience of object attributes for haptics with and without vision. J Exp Psychol Gen 116: 356–369. [Google Scholar]
- Klatzky RL, Lederman SJ (1992): Stages of manual exploration in haptic object identification. Percept Psychophys 52: 661–670. [DOI] [PubMed] [Google Scholar]
- Klein I, Paradis AL, Poline JB, Kosslyn SM, Bihan DL (2000): Transient activity in the human calcarine cortex during visual‐mental imagery: an event‐related fMRI study. J Cogn Neurosci 12: 15–23. [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Clarey J, Tweedale R, Elston G, Calford M (1995): A redefinition of somatosensory areas in the lateral sulcus of macaque monkeys. J Neurosci 15: 3821–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Kuppusamy K, Haacke EM, Burton H (1996): Functional MRI in human somatosensory cortex activated by touching textured surfaces. J Magn Reson Imaging 6: 565–572. [DOI] [PubMed] [Google Scholar]
- Lüders H, Lesser RP, Dinner DS, Hahn JF, Salanga V, Morris HH (1985): The second sensory area in humans, evoked potentials and electrical stimulation studies. Ann Neurol 17: 177–184. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Phelps ME, Halgren E (1983): Local cerebral glucose metabolic response to audiovisual stimulation and deprivation: studies in human subjects with positron CT. Hum Neurobiol 2: 11–23. [PubMed] [Google Scholar]
- Mellet E, Tzourio N, Crivello F, Joliot M, Denis M, Mazoyer B (1996): Functional anatomy of spatial mental imagery generated from verbal instructions. J Neurosci 16: 6504–6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M (1979): Analogous neural models for tactual and visual learning. Neuropsychologia 17: 139–150. [DOI] [PubMed] [Google Scholar]
- Moore CI, Crosier E, Greve DN, Savoy R, Merzenich MM, Dale AM (2002): Cortical correlates of vibrotactile detection in humans. San Francisco: Cognitive Neuroscience Society. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M (1984): Relative contributions of SII and area 5 to tactile discrimination in monkeys. Behav Brain Res 11: 67–83. [DOI] [PubMed] [Google Scholar]
- Norrsell U (1978): Sensory defects caused by lesions of the first (SI) and second (SII) somatosensory areas of the dog. Exp Brain Res 32: 181–195. [DOI] [PubMed] [Google Scholar]
- O'Sullivan BT, Roland PE, Kawashima R (1994): A PET study of somatosensory discrimination in man: microgeometry versus macrogeometry. Eur J Neurosci 6: 137–148. [DOI] [PubMed] [Google Scholar]
- Passingham RE (1996): Functional specialization of the supplementary motor area in monkeys and humans. Adv Neurol 70: 105–116. [PubMed] [Google Scholar]
- Penfield W, Jasper H (1954): Epilepsy and the functional anatomy of the human brain. Boston, MA: Little, Brown and Co. [Google Scholar]
- Platz T (1996): Tactile agnosia. Casuistic evidence and theoretical remarks on modality‐specific meaning representations and sensorimotor integration. Brain 119: 1565–1574. [DOI] [PubMed] [Google Scholar]
- Reales JM, Ballesteros S (1999): Implicit and explicit memory for visual and haptic objects: Cross‐modal priming depends on structural descriptions. J Exp Psychol Learn Mem Cogn 25: 644–663. [Google Scholar]
- Reed CL, Caselli RJ (1994): The nature of tactile agnosia: a case study. Neuropsychologia 32: 527–539. [DOI] [PubMed] [Google Scholar]
- Reed CL, Caselli RJ, Farah MJ (1996): Tactile agnosia. Underlying impairment and implications for normal tactile object recognition. Brain 119: 875–888. [DOI] [PubMed] [Google Scholar]
- Reed CL, Dale AM, Dhond RP, Post D, Paulson K, Halgren E (2000): Activation of ventrolateral somatosensory cortex for tactile pattern discrimination using MEG. Neuroimage 11: S688. [Google Scholar]
- Robinson CJ, Burton H (1980): Organization of somatosensory receptive fields in cortical areas 7b, retroinsula, postauditory and granular insula of M. fascicularis. J Comp Neurol 192: 69–92. [DOI] [PubMed] [Google Scholar]
- Roland PE (1993): Brain activation. New York: John Wiley and Sons. [Google Scholar]
- Roland PE, O'Sullivan B, Kawashima R (1998): Shape and roughness activate different somatosensory areas in the human brain. Proc Natl Acad Sci USA 95: 3295–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadato N, Pascual‐Leone A, Grafman J, Ibanez V, Deiber MP, Dold G, Hallett M (1996): Activation of the primary visual cortex by Braille reading in blind subjects. Nature 380: 526–528. [DOI] [PubMed] [Google Scholar]
- Sathian K, Zangaladze A, Hoffman JM, Grafton ST (1997): Feeling with the mind's eye. Neuroreport 8: 3877–3881. [DOI] [PubMed] [Google Scholar]
- Semmes J (1965): A non‐tactual factor in astereognosis. Neuropsychologia 3: 295–315. [Google Scholar]
- Servos P, Lederman S, Wilson D, Gati J (2001): fMRI‐derived cortical maps for shape, roughness, and hardness. Soc Neurosci Abstr:24. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE (1997): Common blood flow changes across visual tasks. II: Decreases in cerebral cortex. J Cogn Neurosci 9: 648–663. [DOI] [PubMed] [Google Scholar]
- Sinclair RJ, Burton H (1993): Neuronal activity in the second somatosensory cortex of monkeys (Macaca mulatta) during active touch of gratings. J Neurophysiol 70: 331–350. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Thompson WL, Kosslyn SM (2000): Neural systems activated during visual mental imagery: a review and meta‐analysis In: Toga AW, Mazziotta JC, editors. Brain mapping. II: The systems. San Diego: Academic Press; p 535–560. [Google Scholar]
- Tootell RB, Hadjikhani NK, Mendola JD, Marrett S, Dale AM (1998): From retinotopy to recognition: fMRI in human visual cortex. Trends Cogn Sci 2: 174–183. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin, M (1982): Two cortical visual systems In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. Cambridge, MA: MIT Press; p 549–586. [Google Scholar]
- Zangaladze A, Epstein CM, Grafton ST, Sathian K (1999): Involvement of visual cortex in tactile discrimination of orientation. Nature 401: 587–590. [DOI] [PubMed] [Google Scholar]
- Zhou YD, Fuster JM (2000): Visuo‐tactile cross‐modal associations in cortical somatosensory cells. Proc Natl Acad Sci USA 97: 9777–9782. [DOI] [PMC free article] [PubMed] [Google Scholar]


