Abstract
Response selection, which involves choosing representations for appropriate motor behaviors given one's current situation, is a fundamental mental process central to a wide variety of human performance, yet the neural mechanisms underlying this mental process remain unclear. Research using nonhuman primates implicates ventral prefrontal and lateral premotor cortices in this process. In contrast, human neuroimaging research also highlights the role of dorsal prefrontal, anterior cingulate, and superior parietal cortices in response selection. This inconsistency may stem from the difficulty of isolating response selection within the constraints of cognitive subtraction methodology utilized in neuroimaging. We overcome this limitation by using an experimental procedure designed to selectively influence discrete mental processing stages and analyses that are less reliant on the assumptions of cognitive subtraction. We varied stimulus contrast to affect stimulus encoding and stimulus–response compatibility to affect response selection. Brain activation data suggest processing specific to response selection in superior parietal and dorsal prefrontal cortices, and not ventral prefrontal cortex. Anterior cingulate and lateral premotor cortices may also be involved in response selection, or these regions may mediate other response processes. Hum. Brain Mapping 17:193–201, 2002. © 2002 Wiley‐Liss, Inc.
Keywords: attention, prefrontal cortex, executive control, neuroimaging, functional magnetic resonance imaging, human performance
INTRODUCTION
To successfully interact with our environment, we must select appropriate behavioral responses given our current environmental situation and particular goals. The selection of appropriate responses is vital for the successful performance of a variety of tasks, from clinical and experimental ones like Wisconsin Card Sorting and Stroop to real‐world tasks like pressing the brake (or the accelerator, given one's particular situation, goals, and predilections) when the traffic light ahead turns yellow. Despite the ubiquity of this mental process, the neural mechanisms underlying it are poorly understood.
Research on nonhuman primates suggests that ventral prefrontal, lateral premotor, and primary motor cortical regions are involved in the selection of responses to arbitrary visual stimuli [Halsband and Passingham, 1982; Halsband and Passingham, 1985; Murray and Wise, 1997; Murray et al., 2000; Petrides, 1987; Petrides, 1982; Riehle et al., 1997]. Neuroimaging research on humans, however, implicates dorsal prefrontal and anterior cingulate as well as ventral prefrontal, lateral premotor, and superior parietal cortices in response selection processes [Dassonville et al., 2001; Deiber et al., 1991; Iacoboni et al., 1996; MacDonald et al., 2000; Meyer et al., 1998; Passingham et al., 2000; Pochon et al., 2001; Posner et al., 1988; Rowe et al., 2000; Taylor et al., 1994; Toni et al., 2001].
One reason for this inconsistency and confusion may be that, in neuroimaging experiments, it is difficult to localize activity related to response selection per se from other task‐related processes (e.g., stimulus encoding, movement production, or working memory. This difficulty arises from the complexity of the tasks often used in previous experiments and the frequent reliance of neuroimaging experiments on cognitive subtraction methodology, a method whose assumptions may fail [Friston et al., 1996; Sartori and Umilta, 2000a,b; Zarahn et al., 1997b].
We sought to identify the neural basis for response selection in humans during the performance of relatively basic perceptual‐motor tasks. We localize brain activity associated with specific mental processing stages by factorially manipulating experimental variables to affect the duration of distinct processes (i.e., stimulus encoding and response selection) within an experimental framework designed to isolate independent processing stages [Sternberg, 1969, 2001]. By manipulating the duration, rather than the occurrence, of particular mental processes across task conditions, we are less susceptible to artifacts of cognitive subtraction [Friston et al., 1996; Sartori and Umilta, 2000a,b; Zarahn et al., 1997b].
We selectively influence distinct mental processing stages by manipulating two experimental factors: stimulus contrast and stimulus–response (S–R) compatibility. Our stimulus contrast factor affects the duration of, and thus activity related to, stimulus encoding and not response selection [Sanders, 1980]. Our S–R compatibility factor affects the duration of, and thus activity related to, response selection and not stimulus encoding [Duncan, 1977; Kornblum et al., 1990]. We measure the effects of these variables both on participant reaction time and brain activity.
Sternberg [2001] suggested that each of these two dependent measures may provide evidence for separate psychological and neural modules related to stimulus encoding and response selection. Reaction times have been analyzed previously to show that stimulus encoding and response selection are psychologically distinct [Sanders, 1980; Sternberg, 1969, 2001]. Our brain activation data may provide evidence that these processes are neurally distinct as well. With it we may identify brain regions related to response selection (i.e., regions affected by S–R compatibility) and distinguish them from regions related to stimulus encoding.
SUBJECTS AND METHODS
Subjects
Ten healthy, right‐handed volunteers (four males, six females, ages 18–29) participated. All subjects were recruited from the University of Pennsylvania community and gave their informed consent. The data from one participant were not analyzed because he had an error rate above 15% overall and was among the slowest to respond in all four experimental conditions.
Behavioral procedure
Participants carried out two types of visual–manual choice‐reaction tasks. The stimulus display and responses were similar for both tasks. Only the fixation stimulus, the cue brightness, and the S–R pairings differed across task blocks. At the beginning of each trial, a fixation stimulus and four open circles, two to each side of fixation, appeared on a screen for 1,000 msec. The stimuli appeared white on a black background, were equidistant from each other, and the display subtended roughly 2.5‐degree visual angle. After a 1,000 msec foreperiod, the four circles filled in and remained on‐screen for an additional 2,000 msec. Three of the circles were distractors and turned dark gray (3.9 c/m2). The remaining circle turned either white (26.5 c/m2 for the high contrast condition) or light gray (9.1 c/m2 for the low contrast condition) on each trial.
A “+” fixation stimulus indicated the compatible task. On blocks of trials for this task, participants made an ordered manual button press to the location of the cue stimulus. That is, they pressed a button with their left middle, left index, right index, or right middle finger if the cue appeared on the far left, middle left, middle right, or far right position, respectively. An “x” fixation stimulus indicated the incompatible task. On blocks of trials for this task, participants made an unordered manual button press to the location of the cue stimulus. That is, they pressed a button with their left middle, left index, right index, or right middle finger if the cue appeared on the middle left, far right, far left, or middle right position, respectively.
The functional magnetic resonance imaging (fMRI) scanning session consisted of 12 fMRI runs for each participant. During each run, participants carried out blocks of trials for each of the four task conditions (i.e., high contrast compatible mapping, low contrast compatible mapping, high contrast incompatible mapping, and low contrast incompatible mapping). They carried out two blocks of the compatible mapping, one for each contrast condition; two blocks of the incompatible mapping, one for each contrast condition; and one 16‐sec fixation control block where they passively viewed a centrally presented “*”. Each experimental block lasted either 16 or 18 sec.1 These blocks were repeated three times during each fMRI run.
The order in which participants carried out the blocks was counterbalanced across subjects. The fixation control block always occurred between the low contrast compatible mapping block and the high contrast incompatible mapping blocks.
Participants practiced the four task conditions on a day prior to the scanning session. They carried out 14 blocks of 40 trials each. The first eight blocks consisted of two blocks in a row for each of the four task conditions in the following order: 1) high contrast compatible mapping, 2) low compatible, 3) high incompatible, and 4) low incompatible. The next six blocks consisted of one block for each compatible condition followed by two blocks for each of the incompatible conditions (high contrast before low). Participants then carried out four more blocks of trials, similar to those presented in the scanning session. Each of these consisted of 10 trials in a row of each task condition. The blocks were presented in the following order: 1) high compatible, 2) low compatible, 3) high incompatible, and 4) low incompatible, for each participant throughout the practice session.
fMRI procedure
Imaging was conducted on a 1.5 T SIGNA scanner (GE Medical Systems) equipped with a fast gradient system for echoplanar imaging. A standard radio frequency (RF) head coil was used with foam padding to restrict head motion comfortably. High‐resolution axial T1‐weighted anatomical images (21 axial slices) were obtained for each participant. A gradient echo, echoplanar sequence (TR = 2000 msec, TE = 50 msec, matrix size = 64 × 64, FOV = 24 cm) was used to acquire data sensitive to the blood oxygen level dependent signal. Each functional volume contained 21 contiguous 5‐mm axial slices. Each fMRI run lasted 4 min 30 sec (135 volumes/run) and was preceded by 20 sec of dummy gradient RF pulses to achieve a steady state of tissue magnetization.
fMRI data processing
Data processing and analysis were carried out with analysis routines written in Interactive Data Language (Boulder, CO). Data processing involved four steps. First, data were sync interpolated in time to correct for between‐slice timing differences in image acquisition [Aguirre et al., 1998]. Next, a slice‐wise motion compensation method removed spatially coherent signal changes using a partial correlation method for each slice in time [Zarahn et al., 1997a]. Additional head‐motion detection and correction was carried out using a six‐parameter, rigid‐body transformation algorithm [Friston et al., 1995]. Finally, the time series from each voxel was normalized by the mean signal value across the run to remove scaling difference across runs.
Statistical analyses were carried out using a modified general linear model [Worsley and Friston, 1995]. In this model, a design matrix including covariates for each task condition convolved with an idealized hemodynamic response function was created for each participant. Finally, frequencies below 0.01 and above 0.25 Hz were excluded from the data [Zarahn et al., 1997b].
Region of interest analysis of fMRI data
Regions of interest (ROIs) were created using the T1 anatomical images for each participant and reference to standard brain atlases [Duvernoy, 1991; Talairach and Tournoux, 1988]. The ROIs for dorsal prefrontal cortex included Brodmann areas (BA) 9 and 46 on the middle frontal gyrus; the ROIs for ventral prefrontal cortex included BA 44, 45, and 47 on the inferior frontal gyrus; and the ROIs for lateral premotor cortex included BA 6 ventral to the superior frontal sulcus.
Recent work has shown that anterior cingulate cortex is composed of psychologically distinct regions [Bush et al., 2000]. To test for the effect of our factors on the dorsal “cognitive” portion of anterior cingulate cortex, we applied a spherical ROI with a 15‐mm radius centered at stereotaxic coordinates (x = 4, y = 16, z = 36) for each participant. We chose this position so that these ROIs included the peak activations from previous studies implicating this region in response processing [Botvinick et al., 1999; Carter et al., 1998; MacDonald et al., 2000; Taylor et al., 1994]. The ROIs included regions of BA 24 and 32.
Finally, we applied two spherical ROIs with 5‐mm radii each within superior parietal cortex, one for the left hemisphere (x = 22, y = −64, z = 48) and another for the right hemisphere (x = −18, y = −52, z = 48). These ROIs were centered on the coordinates of peak activation reported by Iacoboni et al. [1996]. We used the peak activation from Iacoboni et al. [1996] because their study included a manipulation of S–R compatibility similar to the one used here.
We chose these brain regions for investigation because they have been implicated in response selection [Dassonville et al., 2001; Deiber et al., 1991; Halsband and Passingham, 1982, 1985; Iacoboni et al., 1996; MacDonald et al., 2000; Murray and Wise, 1997; Murray et al., 2000; Passingham, 1993; Passingham et al., 2000; Pochon et al., 2001; Posner et al., 1988; Rowe et al., 2000; Taylor et al., 1994; Toni et al., 2001]. One additional ROI was drawn for BA 18 and 19 in extrastriate cortex to investigate the effect of stimulus contrast on an early perceptual brain region.
Voxels that showed reliable activation increases in the four task conditions combined relative to the fixation control condition were identified within each ROI. For these comparisons, the degrees of freedom were computed based on the number of voxels in each ROI as were the error variances, which were the average of the error variances from each voxel in each ROI. False‐positive rates were controlled at α < 0.05 by Bonferroni correction for the number of voxels within each ROI. The average activity in these task‐active voxels were extracted for each of the four experimental conditions relative to the fixation control condition for each participant in each ROI. The extracted activation values were used in subsequent planned comparisons.
This analysis technique is appropriate given the cognitive model being tested. Our model assumes that stimulus encoding and response selection are involved at all levels of our independent variables and only the duration of these processes is affected by our factors. We therefore limited our analysis to voxels showing a reliable main effect of task.
RESULTS
Stimulus contrast selectively influenced activation in extrastriate cortex. More interestingly, S–R compatibility selectively influenced dorsal prefrontal and superior parietal cortices. Ventral prefrontal cortex showed no reliable effect of either experimental factor and anterior cingulate and lateral premotor cortices were affected by both stimulus contrast and S–R compatibility.
Behavioral results
Accuracy rates were above 95% in all conditions. Mean reaction times (RTs) for correct trials from the scanning session were analyzed using a within‐subjects analysis of variance (Fig. 1). Stimulus contrast reliably affected task RTs, with low contrast blocks taking longer than high contrast ones (F[1,8] = 158.41, P < 0. 0005). Stimulus–response compatibility also reliably affected task RTs, with incompatible mapping blocks taking longer than compatible ones (F[1,8] = 169.79, P < 0. 0005). The interaction between these factors was also reliable (F[1,8] = 12.13, P < 0.01).
Figure 1.
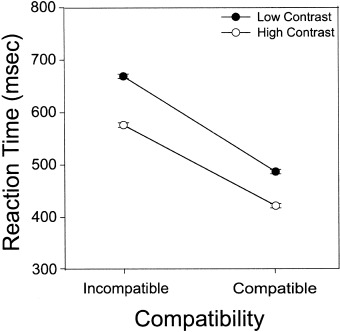
Mean reaction times and standard error bars for the effects of stimulus–response compatibility and stimulus contrast. Solid circles indicate low contrast; open circles indicate high contrast.
Interacting effects of experimental factors on mean RTs are difficult to interpret. Assuming a strict serial stage model of processing, an interaction between factors suggests that they affect the duration of at least one processing stage in common [Sternberg, 1969]. Under situations in which processes are not temporally discrete, however, (e.g., cascade models), interactions between factors may arise even though they affect functionally distinct processes [McClelland, 1979].
The interaction between stimulus contrast and S–R compatibility found here is surprising because previous research has shown that these and similar factors produce additive effects on mean RTs [Sanders, 1980; Sternberg, 1969, 2001]. It may have one or more of the following implications: 1) the assumption of strict serial processing stages in this procedure failed in our participants; 2) stimulus contrast or S–R compatibility affected the duration of both stimulus encoding and response selection; or 3) stimulus contrast and S–R compatibility affected the duration of a third process in common.
Many previous reports have found evidence for serial and independent processing of stimulus encoding and response selection in a variety of situations [Sanders, 1980; Sternberg, 1969, 2001]. Our factors did not interact with each other during the practice session for these participants (F[1,8] = 0.67, P > 0.40), therefore the last option seems the most likely in our experiment. It seems unlikely that the MRI scanning environment (e.g., egocentric position, noise level) would fundamentally change the nature of these processes. Rather, more consistent with previous literature and our data is that in the MRI scanner, these factors may have affected a third process in common (e.g., general arousal or anxiety), a process not necessary in more standard testing situations.
It is important to note that the lack of independence of these factors does not prevent us from interpreting our brain activation data. Our data are interpretable as long as these factors are functionally distinct. The main effects of stimulus contrast and S–R compatibility on the mean RTs were quite large relative to their interaction, and both factors affected mean RTs at both levels of the other factor. Thus, localized effects of stimulus contrast and S–R compatibility on mean brain activation may still be found, and may be interpreted as evidence for neural processing related to each factor.
Brain activation results
Figure 2 shows the mean percent signal change across participants for each of the four task conditions relative to the fixation baseline in each of the six ROIs. As shown, stimulus contrast had a reliable effect on activation in the extrastriate ROI (BA 18, 19), with low contrast producing more activity than high contrast (F[1,8] = 6.92, P < 0.05). Stimulus–response compatibility, however, had no effect on extrastriate activation, (F[1,8] = 0.94, P > 0.35), and there was no reliable interaction (F[1,8] = 0.25, P > 0.60).
Figure 2.
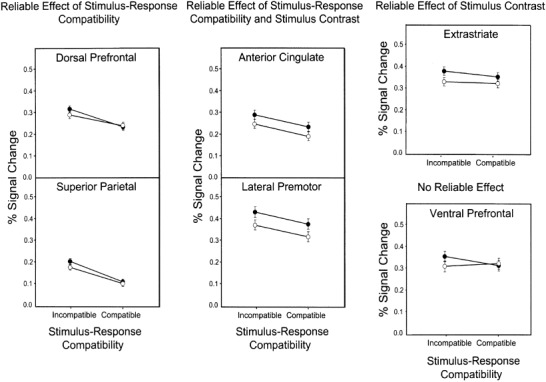
Signal change (mean percent and SE bars) for the effects of stimulus–response compatibility and stimulus contrast relative to the fixation baseline condition in six regions of interest: dorsal prefrontal, superior parietal, anterior cingulate, lateral premotor, extrastriate, and ventral prefrontal cortices. Open circles indicate high contrast; solid circles indicate low contrast.
A very different activity pattern appeared in dorsal prefrontal cortex. As shown in Figure 2, there was a reliable main effect of S–R compatibility, with incompatible trials producing more activation than the compatible ones (F[1,8] = 14.88, P < 0.01). There was no effect, however, of stimulus contrast (F[1,8] = 0.12, P > 0.70) and no interaction between these factors (F[1,8] = 1.06, P > 0.30).
Superior parietal cortex produced a similar pattern of activity. The S–R compatibility effect was reliable, (F[1,8] = 32.58, P < 0.01), but not the stimulus contrast effect, (F[1,8] = 2.06, P > 0.15), nor the interaction between these factors, (F[1,8] = 0.63, P > 0.45).
There were no reliable effects on activation in ventral prefrontal cortex (contrast: F[1,8] = 0.50, P > 0.45; compatibility: F[1,8] = 0.49, P > 0.45; interaction: F[1,8] = 1.25, P > 0.25). Furthermore, S–R compatibility produced reliably less activity in ventral than dorsal prefrontal cortex (F[1,8] = 9.415, P < 0.05].
In anterior cingulate cortex, the incompatible condition produced reliably more activation than the compatible one (F[1,7] = 9.58, P < 0.05].2 There was also a reliable effect of stimulus contrast, with more activation in the low‐ than high‐contrast conditions (F[1,7] = 6.44, P < 0.05]. There was no reliable interaction between stimulus contrast and S–R compatibility (F[1,7] = 0.00, P > 0.95].
Finally, both factors affected activation in lateral premotor cortex. The incompatible mapping produced more activation than the compatible one (F[1,8] = 7.03, P < 0.05). The low contrast condition produced more activation than the high contrast one (F[1,8] = 15.04, P < 0.01). The interaction between these factors was not reliable, (F[1,8] = 0.00, P > 0.95).
There were no reliable hemispheric differences in any of the ROIs investigated (P > 0.05, in all cases).
DISCUSSION
We report a selective influence of our factors on activation in distinct brain regions. Stimulus contrast, but not S–R compatibility, affected brain activity in extrastriate cortex, implicating it in processes that encode task‐relevant stimuli. Conversely, S–R compatibility, but not stimulus contrast, affected activity in dorsal prefrontal and superior parietal cortices, implicating these regions in processes that select appropriate responses to environmental stimuli. Additionally, activation in ventral prefrontal cortex was not reliably affected by either factor, suggesting that this region may not be involved in either stimulus encoding or response selection. Finally, both factors affected brain activation in anterior cingulate and lateral premotor cortices. These regions may play a role in both stimulus encoding and response selection, or they may be involved in other processes affected by both factors.
Our finding that dorsal prefrontal cortex mediates response selection is consistent with two recent reports using fMRI implicating dorsal prefrontal cortex in response selection [Pochon et al., 2001; Rowe et al., 2000]. Rowe et al. [2000] found activation in dorsal prefrontal cortex when participants were cued to respond to a target location currently held in working memory. At cue onset, participants selected the appropriate response, thus activation in dorsal prefrontal cortex may reflect response selection. Dorsal prefrontal activation reported by Pochon et al. [2001] may also reflect response selection. Pochon et al. [2001] presented a sequence of locations on each trial. After a delay, participants identified either said whether another series of locations was identical (i.e., the matching condition) or reproduced the location order using the computer mouse (i.e., the reproduction condition). There were also control conditions where new sequences were presented at the time of response. The authors reported dorsal prefrontal activation, perhaps related to response selection, in the reproduction condition compared to the matching and the reproduction control conditions.
Neither Rowe et al. [2000] nor Pochon et al. [2001] designed their experiments to identify neural processing specific to response selection. Rather, both studies attempted to dissociate selection processes more generally from maintenance processes in working memory. Selection from working memory may be a psychologically distinct process from response selection. Our experiment was designed specifically to identify brain regions related to response selection per se, therefore we can be more confident than these previous studies that our activation in dorsal prefrontal cortex reflects response selection rather than some other selection process.
Our finding that superior parietal cortex is involved in response selection is also consistent with four recent neuroimaging studies. Like our current study, two studies manipulated spatial S–R compatibility and reported more superior parietal activation in the incompatible than the compatible mapping [Dassonville et al., 2001; Iacoboni et al., 1996]. Deiber et al. [1991] reported more activity in superior parietal cortex in a study in which participants made conditional joystick movements to the identity of a serially presented tone versus when they moved the joystick in the same direction regardless of the tone. Finally, Meyer et al. [1998] reported activation in this region in an experiment where participants made responses to the shape or color of a serially presented object. Based on the activation patterns produced when participants carried out blocks of one task or the other compared to blocks when they switched between the tasks, Meyer et al. [1998] concluded that brain activation in superior parietal cortex reflected response selection.
These studies did not report reliable activation in dorsal prefrontal cortex related to response selection, but this is not necessarily inconsistent with our results. Three of these studies did not include an ROI‐based analysis as we did here [Deiber et al., 1991; Iacoboni et al., 1996; Meyer et al., 1998]. Functional variability in between‐participant activation patterns and the conservative nature of analyses that correct for multiple comparisons across the entire brain may make an ROI analysis approach more sensitive to activation differences than the map‐wise activation approach used in these previous studies. Dassonville et al. [2001] analyzed their data with ROIs, but did not include one for dorsal prefrontal cortex specifically.
Activation in the other frontal regions investigated (i.e., ventral prefrontal, anterior cingulate, and lateral premotor cortices) showed either no reliable effect of either factor (ventral prefrontal cortex) or effects of both factors (anterior cingulate and lateral premotor cortices). The effect of S–R compatibility on brain activation in ventral prefrontal cortex was reliably different than the S–R compatibility effect in dorsal prefrontal cortex suggesting the involvement of dorsal, and not ventral prefrontal cortex in response selection.
Our interpretation of ventral prefrontal cortex differs from studies of response selection in nonhuman primates [Murray and Wise, 1997; Murray et al., 2000]. One possible explanation is that ventral prefrontal cortex is involved in response selection in nonhuman but not human primates. Unfortunately, the tasks used here have not been tested with nonhuman primates. When identical choice–reaction tasks have been tested on nonhuman and human primates, however, similar patterns of brain activation have been found [Nakahara et al., 2002]. Thus, the lack of inter‐species consistency described here is somewhat surprising.
Another possibility is that ventral prefrontal cortex is involved in the learning of arbitrary S–R mappings, but not the application of these mappings when well practiced. Consistent with this hypothesis, Murray and Wise [1997] found a greater deficit in the performance of novel S–R mappings than familiar ones for monkeys with lesions in ventral and orbital prefrontal cortices. Also consistent with this hypothesis is recent fMRI research on humans showing activity in ventral prefrontal cortex as participants learn a set of arbitrary S–R mappings, but not when participants perform a well‐practiced visual‐motor task, as they did in our current experiment [Toni et al., 2001].
Anterior cingulate and lateral premotor cortices were affected by both experimental factors. This suggests that these regions may be involved in the mental processing stages of stimulus encoding and response selection. Both regions have been implicated previously in response selection. For anterior cingulate cortex, Taylor et al. [1994] manipulated S–R compatibility in a task in which participants responded verbally to visually presented letters. On congruent blocks of trials, participants responded with the name of the letter presented. On incongruent blocks of trials, participants responded with the name of one of the other letters in the stimulus set; based on an arbitrary mapping rule. Using positron emission tomography, the authors reported greater activation in cingulate cortex for the incongruent than the congruent condition [Taylor et al., 1994]. More recently, using fMRI MacDonald et al. [2000] reported anterior cingulate cortex activation related to the performance of the incongruent version of the Stroop task (i.e., naming the color of the ink in which a color word is printed).
For lateral premotor cortex, Deiber et al. [1991] reported activation in this area when participants made random and sequential rather than fixed joystick movements to a serially presented tone; and Dassonville et al. [2001] showed that this region is affected by manipulations of spatial S–R compatibility.
Stimulus encoding has been shown to affect activity in anterior cingulate cortex [Barch et al., 1997]. It is surprising, however, that stimulus contrast would affect activity in lateral premotor cortex. However, neurons in motor cortex of nonhuman primates have been found to be sensitive to stimulus properties [Riehle et al., 1997]. If stimulus‐encoding neurons exist in primate motor cortex, it is conceivable for lateral premotor cortex to be involved in stimulus encoding as well.
Also consistent with our results is that activity in anterior cingulate and lateral premotor cortices reflects other mental processes affected by stimulus contrast and S–R compatibility, rather than stimulus encoding and response selection per se. Specifically, brain activation in these regions may be related to response activation, a process distinct from response selection [Hommel, 1998].
Anterior cingulate cortex, as suggested by previous research, may be involved in monitoring for response conflict [Botvinick et al., 1999; Carter et al., 1998; MacDonald et al., 2000]. Under this hypothesis, the difficult versions of both factors (i.e., low contrast and incompatible mapping) may increase response conflict because, with increased difficulty, more time elapses within each trial before a response is chosen. This leads to increased activation for, and thus increased competition between, potential responses.
Lateral premotor cortex may be affected in a similar manner. Perhaps in the presence of uncertainty about which response is correct, lateral premotor cortex activation reflects activation of all possible responses for the task. This suggestion is consistent with existing research using nonhuman primates [Fuster, 1995; Wise, 1985]. Nonhuman primates with lateral premotor lesions show impaired performance for tasks that require them to make a response based on a previously learned S–R mapping [Halsband and Passingham, 1982, 1985; Passingham, 1993; Petrides, 1982, 1987]. This may reflect a deficit in selecting the previously learned responses or a deficit in activating those responses. The nonhuman primate research conducted thus far does not differentiate between these processes.
We conclude that careful functional neuroimaging experimentation on humans using an appropriate experimental design implicates extrastriate cortex in processes that encode task‐relevant visual stimuli and superior parietal, dorsal prefrontal, but not ventral prefrontal cortices in processes that choose well‐learned responses to arbitrary visual stimuli. Anterior cingulate and lateral premotor cortices may be involved in these processes as well, or they may be involved in processes related to response monitoring and response activation.
We have shown that by using a relatively easy perceptual‐motor task and an experimental procedure sensitive to the effects of specific mental processes, we can localize activity related to relatively atomic processes like response selection. This study highlights the usefulness of manipulating experimental factors affecting the duration of specific mental processes within a task procedure to selectively influence brain regions associated with those processes. Further use of this technique will likely isolate other discrete mental processes required for successful performance in other task domains.
Acknowledgements
We thank D. Caggiano, P. Elston, K. Brodsky, and E. Diven for assistance with data collection or analysis, and E. Hazeltine, J. Jonides, D. Meyer, A. Osman, B. Postle, S. Sternberg, two anonymous reviewers, and the members of the D'Esposito laboratory for helpful discussions of this work.
Footnotes
If participants had not made a response for the previous trial by the end of a 16‐sec block, then the block was extended by another 2 sec so this response could be recorded. Participants carried out as many trials as possible within the time period. A duration‐dependent block length was used rather than a fixed number of trials to more equally equate the time participants spent on task for the easy and hard levels of both experimental factors [D'Esposito et al., 1997]. This manipulation had only a small effect on the number of trials per block, with all blocks having either eight or nine trials.
No reliable voxels were found in the map‐wise comparison for one participant.
REFERENCES
- Aguirre GK, Zarahn E, D'Esposito M (1998): The variability of human, BOLD hemodynamic responses. Neuroimage 8: 360–369. [DOI] [PubMed] [Google Scholar]
- Barch DA, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD (1997): Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia 35: 1373–1380. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nytrom LE, Fissell K, Carter CS, Cohen JD (1999): Conflict monitoring versus selection‐for‐action in anterior cingulate cortex. Nature 402: 179–181. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI (2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998): Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280: 747–749. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Lewis SM, Zhu XH, Ugurbil K, Kim SG, Ashe J (2001): The effect of stimulus–response compatibility on cortical motor activation. Neuroimage 13: 1–14. [DOI] [PubMed] [Google Scholar]
- Deiber M, Passingham RE, Colebatch J, Friston K, Nixon P, Frackowiak RSJ (1991): Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res 84: 393–402. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Zarahn E, Aguirre G, Shin R, Auerbach P, Detre J (1997): The effect of pacing stimuli on observed functional MRI activity. Neuroimage 6: 113–121. [DOI] [PubMed] [Google Scholar]
- Duncan J (1977): Response selection errors in spatial choice reaction tasks. Q J Exp Psychol 29: 415–423. [DOI] [PubMed] [Google Scholar]
- Duvernoy H (1991): The human brain surface, three‐dimensional sectional anatomy and MRI. New York: Springer‐Verlag Wien. [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J‐B, Heather JD, Frackowiak RSJ (1995): Spatial registration and normalization of images. Hum Brain Mapp 2: 165–189. [Google Scholar]
- Friston KJ, Price CJ, Fletcher P, Moore C, Frackowiak RSJ, Dolan RJ (1996): The trouble with cognitive subtraction. Neuroimage 4: 97–104. [DOI] [PubMed] [Google Scholar]
- Fuster JM (1995): Memory in the cerebral cortex. Cambridge: MIT Press. [Google Scholar]
- Halsband U, Passingham R (1982): The role of premotor and parietal cortex in the direction of action. Brain Res 240: 368–372. [DOI] [PubMed] [Google Scholar]
- Halsband U, Passingham RE (1985): Premotor cortex and the conditions for movement in monkeys (Macaca fascicularis). Behav Brain Res 18: 269–277. [DOI] [PubMed] [Google Scholar]
- Hommel B (1998): Automatic stimulus–response translation in dual‐task performance. J Exp Psychol Hum Percept Perform 24: 1368–1384. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Mazziotta JC (1996): Brain‐behavior relationships: evidence from practice effects in spatial stimulus–response compatibility. J Neurophysiol 76: 321–331. [DOI] [PubMed] [Google Scholar]
- Kornblum S, Hasbroucq T, Osman A (1990): Dimensional overlap: cognitive basis for stimulus–response compatibility: a model and taxonomy. Psychol Rev 97: 253–270. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS (2000): Dissociating the role of dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- McClelland JL (1979): On the time relations of mental processes: an examination of systems of processes in cascade. Psychol Rev 86: 287–330. [Google Scholar]
- Meyer DE, Evans J, Lauber EJ, Gmeind L, Rubinstein J, Junck L, Koeppe RA (1998): The role of dorsolateral prefrontal cortex for executive cognitive processes in task switching. J Cogn Neurosci (Suppl): 106–106. [Google Scholar]
- Murray E, Wise S (1997): Role of orbitoventral prefrontal cortex in conditional motor learning. Abstr Soc Neurosci 23: 11. [Google Scholar]
- Murray EA, Bussey TJ, Wise SP (2000): Role of prefrontal cortex in a network for arbitrary visuomotor mapping. Exp Brain Res 133: 114–129. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Hayashi T, Konishi S, Miyashita Y (2002): Functional MRI of macaque monkeys performing a cognitive set‐shifting task. Science 295: 1532–1536. [DOI] [PubMed] [Google Scholar]
- Passingham RE (1993): The frontal lobes and voluntary action. Oxford: Oxford Psychology Series. [Google Scholar]
- Passingham RE, Toni I, Rushworth MF (2000): Specialization within the prefrontal cortex: the ventral prefrontal cortex and associative learning. Exp Brain Res 133: 103–113. [DOI] [PubMed] [Google Scholar]
- Petrides M (1987): Conditional learning and the primate frontal cortex In: Perecman E, editor. The frontal lobes revisited. New York: IRBN Press; pp 91–108. [Google Scholar]
- Petrides M (1982): Motor conditional associative‐learning after selective prefrontal lesions in the monkey. Behav Brain Res 5: 407–413. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Poline JB, Crozier S, Lehericy S, Pillon B, Deweer B, Le Bihan D, Dubois B (2001): The role of dorsolateral prefrontal cortex in the preparation of forthcoming actions: an fMRI study. Cereb Cortex 11: 260–266. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE, Fox PT, Raichle ME (1988): Localization of cognitive operations in the human brain. Science 240: 1627–1631. [DOI] [PubMed] [Google Scholar]
- Riehle A, Kornblum S, Requin J (1997): Neuronal correlates of sensorimotor association in stimulus–response compatibility. J Exp Psychol Hum Percep Perform 23: 1708–1726. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RSJ, Passingham RE (2000): The prefrontal cortex: response selection or maintenance within working memory? Science 288: 1656–1660. [DOI] [PubMed] [Google Scholar]
- Sanders A (1980): Stage analysis of reaction processes In: Stelmach GE, Requin J, editors. Tutorials of motor behavior. New York: North‐Holland; pp 331–354. [Google Scholar]
- Sartori G, Umilta C (2000a): The additive factor method in brain imaging. Brain Cogn 42: 68–71. [DOI] [PubMed] [Google Scholar]
- Sartori G, Umilta C (2000b): How to avoid the fallacies of cognitive subtraction in brain imaging Brain Lang 74: 191–212. [DOI] [PubMed] [Google Scholar]
- Sternberg S (1969): The discovery of processing stages: extensions of Donders' method. Acta Psychol (Amst) 30: 276–315. [Google Scholar]
- Sternberg S (2001): Separate modifiability, mental modules, and the use of pure and composite measures to reveal them. Acta Psychol (Amst) 106: 147–246. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Taylor, S, Kornblum S, Minoshima S, Oliver L, Koeppe R (1994): Changes in medial cortical blood flow with a stimulus–response compatibility task. Neuropsychologia 32: 249–255. [DOI] [PubMed] [Google Scholar]
- Toni I, Ramnani N, Joseph SO, Ashburner J, Passingham RE (2001): Learning arbitrary visuomotor associations: temporal dynamic of brain activity. Neuroimage 14: 1048–1057. [DOI] [PubMed] [Google Scholar]
- Wise SP (1985): The primate premotor cortex fifty years after Fulton. Behav Brain Res 18: 79–88. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ (1995): Analysis of fMRI time‐series revisited – again. Neuroimage 2: 173–182. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre GK, D'Esposito M (1997a): Empirical analyses of BOLD fMRI statistics I Spatially unsmoothed data collected under null‐hypothesis conditions. Neuroimage 5: 179–197. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre GK, D'Esposito M (1997b): A trial‐based experimental design for functional MRI Neuroimage 6: 122–138. [DOI] [PubMed] [Google Scholar]


