Abstract
Classical eyeblink conditioning is used frequently to study the role of the cerebellum in associative learning. To understand the mechanisms involved in learning, the neural circuits that generate the eyeblink response should be identified. The goal of the present study was to examine cerebellar regions that are likely to control the human eyeblink response using event‐related functional magnetic resonance imaging (fMRI). In 14 healthy volunteers eyeblinks were evoked by unilateral air‐puff stimulation (total of 30 stimuli, inter‐trial interval 27–44 sec). With eyes closed throughout the experiment, eyeblinks were recorded using a video‐based system with infrared reflecting markers being attached to the upper eyelids. From each subject 500 scans were taken (TR = 2.2 sec, 22 slices per scan, slice thickness = 3 mm) using an echo planar imaging sequence (EPI). The statistical parametric maps of the experimental volume images were estimated with SPM99 specifying an appropriate event‐related design matrix. Two main regions of significant activation were found in the ipsilateral posterior lobe of the cerebellar hemisphere. In the more anterior region the maxima of activation were located in hemispheral lobules VI and Crus I, and in the more posterior region in hemispheral lobules VIIb, Crus II and VIIIa (nomenclature according to Schmahmann et al. [2000]: MRI Atlas of the Human Cerebellum). Although less pronounced, activity was found also in corresponding areas of the contralateral cerebellar hemisphere. These eyeblink‐related areas agree with trigeminal projection areas and blink reflex control areas shown in previous animal studies. Hum. Brain Mapping 17:100–115, 2002. © 2002 Wiley‐Liss, Inc.
INTRODUCTION
Reflex eyeblinking has been studied extensively in humans since it was first described as a reflex by Overend in 1896 [Esteban, 1999; Kimura, 1970; Kugelberg, 1952; Ongerboer de Visser, 1983a; Pellegrini et al., 1995; Shahani, 1970; Sibony and Evinger, 1998]. Research in clinical neurophysiology has been centered upon the anatomical organization of brainstem circuits involved in blink reflex control and the topographic value of blink reflex abnormalities for the evaluation of brainstem disorders [Aramideh et al., 1997; Hopf et al., 1991; Ongerboer de Visser, 1983a,b; Valls‐Sole et al., 1996].
Interest in the cerebellum in blink reflex control is coming from numerous animal and human lesion studies indicating that the cerebellum may be necessary for classical conditioning of eyeblink responses [Bracha et al., 1997; Daum et al., 1993; Thompson et al., 1997; Topka et al., 1993; Woodruff‐Pak et al., 1996; Yeo and Hesslow, 1998]. Interest in the role of the cerebellum in unconditioned eyeblink control is twofold. First, impaired eyeblink conditioning in cerebellar dysfunction has been related to impaired motor performance deficits, i.e., changes of unconditioned eyeblink responses [Harvey et al., 1993; Welsh and Harvey, 1989]. Results of previous studies of the effect of cerebellar damage on reflex blinking, however, are conflicting. After cortical lesions, amplitudes of eyeblink responses have been found to increase in rabbits [Gruart and Yeo, 1995; Yeo et al., 1985; Yeo and Hardiman, 1992] and to be unaffected in rats unless adaptive modifications of reflex blinks were required [Pelligrini and Evinger, 1997]. Findings after lesions of the cerebellar nuclei are also contradictory with decreased amplitudes being reported by some authors and unimpaired eyeblinks by others [Bloedel and Bracha, 1995; Kolb et al., 1997; Thompson and Krupa, 1994; Thompson et al., 1997; Welsh and Harvey, 1989, 1991]. Most human studies described preserved eyeblinks in patients with cerebellar disorders (Hacke et al., 1983; Daum et al., 1993; Topka et al., 1993; Woodruff‐Pak et al., 1996). There is one human case study comparing the affected and unaffected side in a patient with a unilateral, mainly cortical lesion, which showed a clear tendency of the unconditioned eyeblink amplitude to be larger on the affected side (Timmann et al., 1998).
There is some controversy about the relative roles of the cerebellar cortex and nuclei in eyeblink conditioning [Raymond et al., 1996; Thompson et al., 1997; Yeo and Hesslow, 1998]. The nucleus interpositus anterior and lobule H VI appear to be of particular importance. One possible reason for controversial results of lesion studies may be that the neural circuits that generate the unconditioned eyeblink response have not been identified exactly. For example, if areas controlling eyeblink are not confined to lobule H VI, variations in the extent of cerebellar cortical lesions could result in different effects.
There are only a few animal studies examining the sites in the cerebellar cortex that are likely to control unconditioned eyeblink responses [Hesslow, 1994; Pellegrini and Evinger, 1997]. Human studies demonstrating cerebellar areas involved in eyeblink control are lacking. So far, PET and fMRI studies in healthy human subjects examined cerebellar areas related to conditioned but not unconditioned eyeblinks [Blaxton et al., 1996; Logan and Grafton, 1995; Molchan et al., 1994; Ramnani et al., 2000; Schreurs et al., 1997].
The purpose of the present study was to investigate cerebellar areas that are activated during eliciting the blink reflex in healthy human subjects using functional MRI (fMRI).
Kugelberg in 1952 recognized two components in blink reflexes; an early ipsilateral reflex (R1) and a late bilateral reflex (R2), which is associated with clinically visible blinking. The difference of the R1 and R2 responses is attributed to differences in their central neural pathways [Esteban, 1999; Ongerboer de Visser, 1983a; Pellegrini et al., 1995; Sibony and Evinger, 1998]. In humans most authors assume that the R1 response is conducted through trigeminal pathways within the pons [Hopf et al., 1991; Kimura, 1970; Ongerboer de Visser, 1983a,b] and the R2 response through trigeminal pathways and adjacent reticular formation within the medulla oblongata before they reach the facial nuclei [Aramideh et al., 1997; Kimura and Lyon, 1972; Ongerboer de Visser and Kuypers, 1978; Valls‐Sole et al., 1996].
In the present study, blink reflexes mainly represent activity of the late R2 component and of the orbicularis oculi muscles. Eyeblinks were evoked by air‐puff stimulation to be in keeping with numerous eyeblink conditioning studies and to circumvent technical problems of electrical stimulation in the MR environment [Daum et al., 1993; Woodruff‐Pak et al., 1996; Yeo and Hesslow, 1998]. Air‐puff stimulation, however, is known to evoke R2 but not R1 responses of the reflex eyeblink [Kugelberg, 1952; Peshori et al., 2001; Shahani and Young, 1973]. Because repetitive stimulation results in habituation of the second component of the blink reflex [Gandiglio and Fra, 1967; Kugelberg, 1952; Rushworth, 1962; Shahani, 1970] an event‐related paradigm was applied with long interstimulus intervals.
Eyes were gently closed during the experiment to minimize effects of accompanying eye movements and spontaneous eyeblinks. Blink reflexes, therefore, represented mainly phasic activity in orbicularis oculi muscles (innervated by the facial nerve), but not additional transient relaxation with disappearance of tonic activity in the levator palpebrae muscles (innervated by the oculomotor nerve). Furthermore, in humans, unlike most vertebrates, an additional retractor bulbi muscle is absent, which causes a nictitating membrane to sweep the eyeball [Shahani and Young, 1973; Sibony and Evinger, 1998].
MATERIALS AND METHODS
Subjects
Fourteen healthy adult subjects participated. Their average age was 29.3 ± 5.4 (range 21–38) years. Seven subjects were female and seven male. Twelve were right‐handed. None of them had a history of neurological disease or showed neurological signs based upon neurological examination. The local ethical committee of the University of Essen approved the study. All subjects gave informed written consent.
Experimental design
Eyeblinks were evoked by an air‐puff lasting for 100 msec (4 bar at source) provided through a nozzle mounted on the head coil. The nozzle was set to direct the air‐puff to the periorbital region near the inner canthus of the left eye at a distance of approximately 10 mm. To minimize effects of habituation an event‐related fMRI‐paradigm (see below) was applied with a total of 30 air‐puffs being delivered pseudo‐randomly and with long inter‐trial intervals (range 27–44 seconds) during 500 consecutive MR scans. For each subject air‐puffs were delivered at the beginning of the 16th, 36th, 51st, 68th, 84th, and 96th MR scan, with the same order being repeated during the following 400 scans (116th, 136th, 151st, 168th, 184th, 196th, 216th, 236th, 251st, etc.).
Subjects were instructed to gently close their eyes throughout the experiment to minimize effects of accompanying eye movements and spontaneous eyeblinks. Kinematic measures of eyeblinks were taken to assure that subjects responded to an air‐puff with adequate squeezing of the eyes and to quantify the amount of possible habituation. Pilot data showed that although the eyes are closed air‐puff stimulation elicits a further compression of the eyelids that causes a marker attached to the upper outer quadrant of the eye to move toward the lower and inner quadrant of the eye (Fig. 1A). Two infrared reflecting markers were attached to the upper outer quadrant of each eyelid. For reference, one marker was attached to the root of each subject's nose and two markers were attached in a fixed distance (3 cm apart) to a mirror mounted on the head coil. A digital videocamera with an integrated infrared projector (Sony DCR‐TRV 11E) was placed outside the darkened MR scanning room in front of its window. Infrared light (conventionally used for night‐shoots) was emitted and reflected by the markers with the help of two mirrors (commonly used to enable subjects to look outside the scanner) attached to the head coil. Similarly, the reflected images of the infrared reflecting markers were videotaped by the use of the two mirrors (sample rate 25 Hz).
Figure 1.
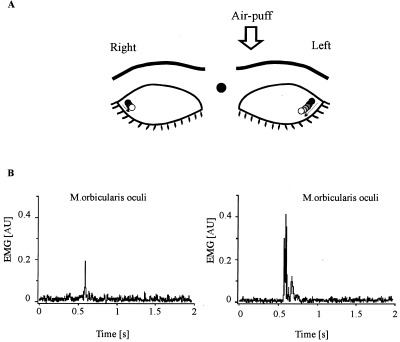
A: Experimental set‐up. Eyeblinks were evoked by an air‐puff directed to the left eye with eyes closed throughout the experiment. For kinematic recordings infrared reflecting markers were attached to the upper outer quadrant of each eye and, for comparison, one on the root of the nose and two markers (3 cm apart) to the mirror mounted on the head coil (not shown). The eyeblink (i.e., the further compression of the eyes) after the air‐puff is indicated by the color of the circles, with black circles indicating the rest position and open circles the maximum extent of the movement. B: Characteristic EMG recordings of air‐puff‐induced blink reflex in bilateral orbicularis oculi muscles in a healthy control subject obtained outside the MR scanner. R2 responses are present bilaterally. R1 responses are absent. Note larger ipsilateral (left eye) responses both in EMG and diagram of kinematic recordings.
A few air‐puff stimulations were presented before scanning to adjust the position of the nozzle and to familiarize subjects with the task (i.e., to control for startle‐like responses during scanning). Recording of eyeblink amplitudes was not significantly affected by head motion based on video data. In addition, individual plots of head motion correction parameters (see Image analysis) showed no local extremes at the time of the stimuli.
To quantify eyeblink amplitude the maximal air‐puff‐induced displacement of each eye marker was measured off‐line. To allow for comparison between subjects eyeblink amplitudes were normalized to maximally voluntary eye compression. At the beginning of each experiment, subjects were asked to maximally squeeze their eyes together starting from the status with their eyes gently closed. The mean marker displacement of three maximally voluntary eye compressions was set to 100% and each eyeblink amplitude expressed as a percentage of this value.
Although the kinematic measures of eyelid movements taken during the actual experiments could not distinguish between the occurrence of R1 and R2 responses, analysis of previous electromyographic recordings using a similar paradigm carried out outside the MRI‐scanner showed that air‐puff stimulation consistently evoked R2 but not R1 responses [Maschke et al., 2000]. Representative EMG examples are shown in Figure 1B.
Imaging
All fMRI scans were taken with a 1.5 T Siemens Sonata scanner (Dept. of Neuroradiology, University of Essen) with standard head coil. A multislice echo planar imaging sequence (EPI) was used to produce 22 continuous 3 mm thick axial slices covering the volume of the cerebellum and adjacent brainstem with TR = 2.2 sec, TE = 60 msec, flip angle = 90°, 64 × 64 matrix and voxel size = 3.59 × 3.59 × 3 mm3. An event‐related (fMRI) paradigm was used for the experiment. Event‐related fMRI permits the analysis of activity at the level of a single trial, thus conferring specific advantages in reflex studies. An electronic triggering signal was used to achieve synchronization between the time of initiation of the active event (air‐puff) and the MR acquisition. Each series consisted of 500 scans with a total duration of 18.3 min. Structural images were acquired for each subject using a T1 3D sequence with TR = 11.1 msec, TE = 4.3 msec, flip angle = 15°, 136 partitions, effective thickness = 1.2 mm and voxel size = 1 × 1 × 1.2 mm3.
Image analysis
The stereotactical transformations and statistical analysis were carried out on a Sun Sparc Ultra 80 computer with statistical parametric mapping software (Wellcome Department of Cognitive Neurology, London, UK), version SPM99, implemented in MATLAB (Mathworks, Sherborn, MA). The first five volumes in each subject were discarded to minimize the magnetization relaxation artifacts. All individual volumes were realigned after the six head‐movement parameters were estimated (3 translations and 3 rotations) from rigid body transformations that minimized the difference between each volume and the first. The functional and structural images were realigned and then spatially normalized [Friston et al., 1995] into the reference system of Talairach and Tournoux [1988], using a representative standard EPI template from the Montreal Neurological Institute (MNI) [Evans et al., 1994]. The functional images were subsampled to a voxel size of 2 × 2 × 2 mm3 and smoothed using an isotropic Gaussian kernel of 4 mm.
The statistical analysis was carried out for each time series after specifying the appropriate design matrix. The active event was modelled with a train of delta functions convolved with hemodynamic response function (HRF). One trial specific covariate was included in the design matrix introducing the effect of eyeblink habituation (i.e., decrease of reflex amplitude with time). Because blink reflex amplitudes were significantly larger on the stimulated left as compared to the right side (see Results), amplitude changes of the left eye were entered into statistical analysis.
The significance of effects was assessed using Z‐statistics for every voxel from the brain, and these sets of Z‐values were used to create statistical parametric maps (SPMs). A high‐pass filter was used to remove low‐frequency drifts and fluctuations of the signal [Friston et al., 1996], and proportional scaling was applied to remove global changes in the signal.
Data were analyzed for each subject individually and for all 14 subjects as a group. Group analysis is valuable for summarizing the activities across subjects and to increase the sensitivity of the analysis. Group effects were calculated using fixed and random effects models. For the latter model, contrast images, one for each single subject, were taken to the second level analysis and entered into the one sample t‐test model [Friston et al., 1999]. The statistical test of variance of these single contrast images from subject to subject consists of contributions from both the between and within subject components of variance and can be used to extend the inference to population whereas the fixed model analysis can make a conclusion valid only for the group studied.
In two subjects (Subject 2, Subject 7) only the first 200 MR scans were included in the statistical analysis, because kinematic analysis showed frequently missing eyeblinks during the last 300 scans (see Results). In all other subjects the total of the 500 functional volumes were taken for further estimation. The specified contrasts compared the active event condition with rest. For the group studies (both fixed and random model) thresholds of P < 0.001 (uncorrected) were adopted for analysis of cerebellar activations, and of P < 0.005 (uncorrected) for analysis of brainstem activations. For the analysis in single subjects a threshold of P < 0.005 for both the cerebellar and brainstem activations was used. “Area of significant activation” is used synonymous with “area of significant increase of BOLD effect (i.e., blood flow).” It is not well understood, within the cerebellum particularly, which neuronal activities correlate to these changes in blood flow. Animal data suggest that it is unlikely that the BOLD signal, or increase in blood flow in the cerebellum corresponds simply to an increase in firing of the Purkinje cells [Mathiesen et al., 1998].
Anatomical localization of MNI coordinates were defined based on the 3D MRI atlas of the human cerebellum in proportional stereotaxic space introduced by Schmahmann et al. [2000]. Each brain map of activation was superimposed and displayed onto the standard MNI brain anatomy to show the anatomic localization.
RESULTS
Kinematic data of blink reflexes
Kinematic measures of blink reflexes showed that each of the 30 air‐puff stimulations resulted in appropriate eyeblinks in all but two subjects (Subject 2, Subject 7) (Fig. 2A). Subjects 2 and 7 showed kinematic responses during the first 12 air‐puff stimuli, but failed to respond to seven and 13 of the last 18 stimuli, respectively. In these two subjects, only the first 200 MR‐scans (i.e., first 12 active events) were included in the fMRI‐analysis.
Figure 2.
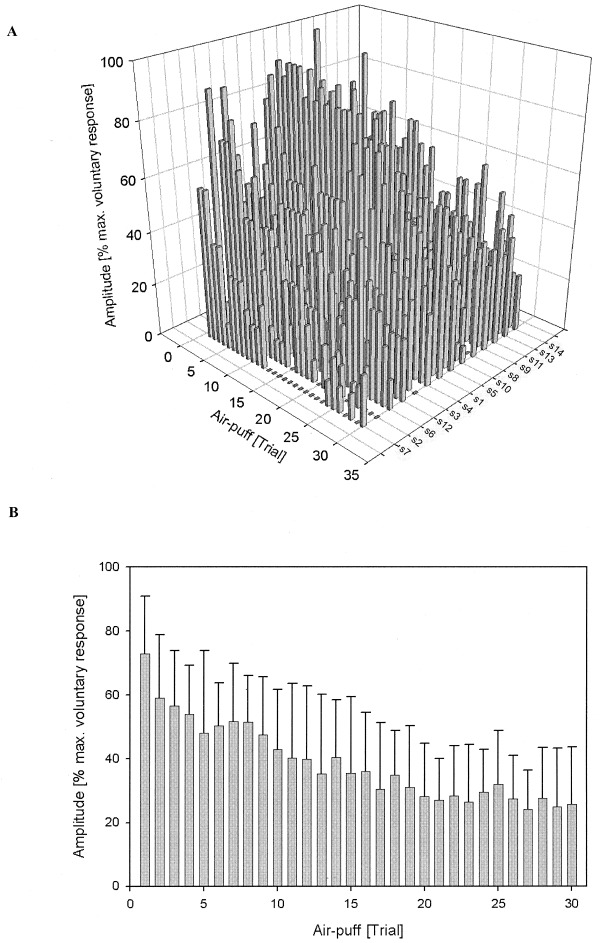
Kinematic data of blink reflex recordings during fMRI‐experiments. Normalized (% maximally voluntary eye closure) blink reflex amplitudes of the stimulated left eye are shown for all active events (total of 30 air‐puffs) in each individual subject (A) and for the group of 14 subjects (B). Note decrease of reflex amplitudes from the first to the last trial both in individual and group data (habituation).
Although special care was taken to minimize effects of habituation, reduction of blink reflex amplitude was present in all 14 subjects from the first to the 30th trial (Fig. 2A). Results are shown for the left eye because blink reflex amplitudes were significantly larger on the stimulated left side as compared to the contralateral right side (group mean of non‐normalized blink amplitude: left eye = 3.38 ± 1.84 mm, right eye = 2.16 ± 1.70 mm; P < 0.001, paired t‐test). The amount of amplitude reduction (first vs. last trial) varied between 33% and 100% (mean 63 ± 25%). Linear regression analysis showed a significant reduction in all subjects (all P‐values < 0.01). Similarly, one‐way ANOVA showed a significant trial effect on the group level (P < 0.001) (Fig. 2B). The amplitude of blink reflexes was considered covariate in the fMRI statistical analysis.
Imaging
Cerebellar activations
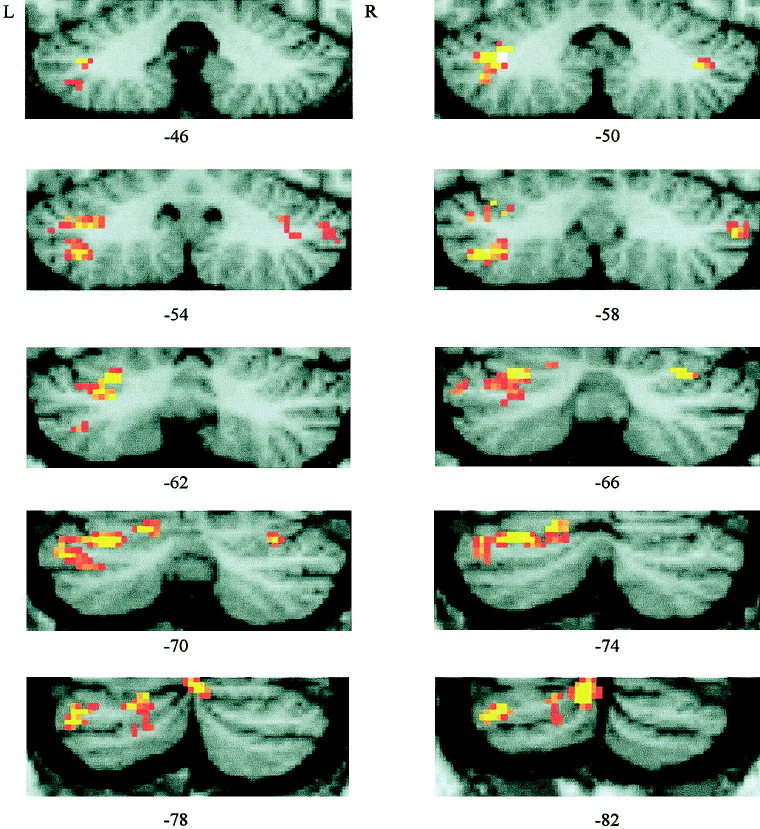
Table I summarizes the Z‐scores, MNI coordinates and cluster sizes for the cerebellar areas of significant increases of BOLD effect related to the air‐puff‐induced blink reflexes vs. rest for a fixed and random model group analysis as well as for each individual subject. There were robust activations in the first level group statistic (fixed model) localized in one big cluster (P < 0.001, cluster size = 4,022 voxels) with an absolute maximum in hemispheral lobule VI in the left posterior lobe (−36, −56, −34 mm). Activations were spread out to the anterior regions of the posterior lobe, with local maxima in hemispheral lobules VI and Crus I, and to a more posterior region of the posterior lobe, with local maxima in hemispheral lobules VIIb, Crus II, and VIIIa. Activations were more pronounced in the lateral hemispheres and on the left (ipsilateral) side, but extended to intermediate and medial cerebellar regions and to corresponding regions within the anterior and posterior parts of the contralateral right posterior cerebellar hemisphere. Coordinates and Z‐scores of the most significant local maxima in hemispheral lobules VI and Crus I, and lobules Crus II, VIIb and VIIIa (belonging to the same cluster) are given in Table I.
Table I.
Coordinates in MNI standard anatomical space for the peaks of cerebellar activation*
| Anterior region of posterior lobe: Lobules VI, Crus I | Posterior region of posterior lobe: Lobules Crus II, VIIb, VIIIa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left (ipsilateral) | Right (contralateral) | Left (ipsilateral) | Right (contralateral) | |||||||||
| x,y,z | Z | KE | x,y,z | Z | KE | x,y,z | Z | KE | x,y,z | Z | KE | |
| Group analysis | ||||||||||||
| Random model | −34,−50,−38a | 5.12 | 1058 | 30,−68,−24 | 4.59 | 39 | −38,−58,−46 | 4.79 | 1058 | |||
| −34,−82,−28 | 4.76 | 52,−66,−34 | 4.24 | 78 | −40,−52,−48 | 4.54 | ||||||
| −26,−66,−26 | 4.74 | 12,−86,−28 | 3.93 | 42 | ||||||||
| −30,−72,−26 | 4.60 | |||||||||||
| Fixed model | −36,−56,−34a | >7.77 | 4022 | 48,−72,−30 | 6.44 | 4022 | −34,−76,−54 | 7.36 | 4022 | 20,−76,−50 | 3.99 | 16 |
| −34,−66,−30 | >7.77 | 32,−66,−26 | 6.05 | −10,−86,−24 | 6.48 | 42,−56,−50 | 3.75 | 32 | ||||
| −30,−68,−28 | 7.55 | −40,−66,−46 | 4.88 | |||||||||
| −40,−74,−28 | 7.46 | |||||||||||
| Individual subjects | ||||||||||||
| 1 | −54,−62,−30 | 5.32 | 56 | 4,−76,−12 | 3.97 | 202 | −22,−70,−56 | 3.50 | 7 | |||
| 2 | −34,−54,−32b | 4.05 | 66 | 44,−76,−22 | 4.03 | 250 | −30,−70,−50 | 3.73 | 33 | |||
| 3 | −34,−66,−34b | 6.32 | 1859 | −20,−76,−50a | 6.53 | 1859 | ||||||
| 4 | −40,−72,−26 | 4.15 | 258 | 0,−72,−10 | 3.87 | 98 | −22,−64,−54b | 4.96 | 106 | 18,−68,−56 | 4.68 | 72 |
| −6,−80,−16a | 4.25 | 98 | ||||||||||
| 5 | −36,−48,−36 | 3.99 | 16 | 2,−76,−18 | 3.77 | 35 | −40,−70,−54 | 3.71 | 18 | |||
| 6 | −32,−64,−28b | 4.62 | 559 | 26,−68,−30 | 4.15 | 178 | −20,−76,−48 | 4.38 | 101 | |||
| 7 | −42,−80,−26 | 3.35 | 9 | |||||||||
| 8 | −12,−72,−22 | 3.39 | 12 | 40,−58,−36 | 3.17 | 8 | −44,−62,−54 | 2.97 | 6 | 54,−54,−42 | 3.92 | 46 |
| 9 | −40,−54,−32b | 5.08 | 475 | 36,−64,−24 | 4.21 | 246 | −34,−56,−54 | 4.95 | 409 | |||
| 10 | −36,−64,−22b | 3.67 | 80 | −34,−48,−44 | 3.67 | 113 | 36,−44,−52 | 3.67 | 11 | |||
| 11 | −34,−68,−26b | 5.83 | 1726 | 36,−54,−30 | 5.56 | 179 | ||||||
| 12 | −16,−72,−38a | 4.38 | 332 | 14,−74,−34 | 4.45 | 84 | −22,−78,−40 | 3.91 | 332 | |||
| 13 | 30,−58,−30 | 3.21 | 10 | −20,−62,−48 | 3.67 | 20 | 20,−66,−52 | 4.35 | 43 | |||
| 14 | −36,−62,−34b | 6.05 | 875 | 34,−60,−34 | 4.60 | 688 | −34,−58,−58 | 4.14 | 36 | |||
Coordinates in mm. Error probabilities for random model: P < 0.001; fixed model: P < 0.001; single subjects: P < 0.005.
Absolute cluster maximum.
Coronar sections to actual y coordinates are shown in Figure 4.
Z, Z‐score; KE, cluster size.
Implementing of the second level statistic (i.e., random model analysis) did not significantly change the statistical results. At the same significance threshold (P < 0.001) one big cluster remained with an absolute maximum in hemispheral lobule VI (−34, −50, −38 mm) and additional local maxima in the anterior (lobules Crus I, VI) and posterior region (lobule VIIb) of the left posterior cerebellar hemisphere. Local maxima were also present in lobules VI and Crus I of the anterior part of the right posterior lobe. No significant maxima were found in the more posterior region of the right posterior lobe. The statistical group data from the random model are displayed on coronal sections of a typical canonical MNI brain in Figure 3.
Figure 3.
Cerebellar areas of blink reflex‐related activations: Group data (n = 14) are shown based on the random effects model (P < 0.001). SPM (t) maps are displayed on coronal sections of a typical canonical brain from the MNI. The y‐coordinate varies between −46 mm and −82 mm with a step of 4 mm. Laterality is indicated by L (left) and R (right).
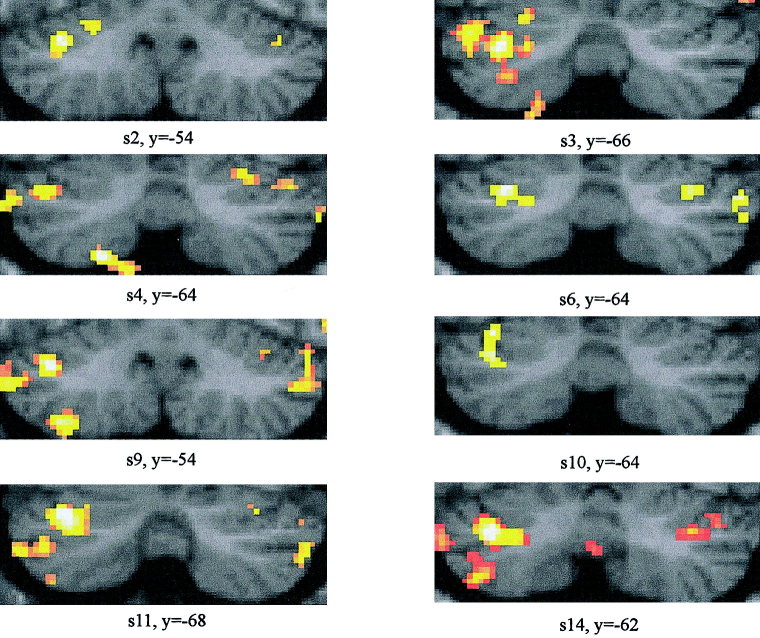
Group data were confirmed by findings in individual subjects. Figure 4 shows characteristic examples of eight single subjects with SPMs being displayed on coronal sections. The y coordinates shown in Figure 4 correspond to the y coordinates of the cluster with the highest Z‐score given in Table I (see Individual subjects). Individual findings were most consistent for activations within the anterior region of the posterior lobe. All but one (Subject 13) of the 14 subjects examined showed activation in hemispheral lobule VI and all but two in Crus I (Subject 7, Subject 13). In most cases activation was largest in more lateral regions of the cerebellar hemisphere, but, depending on the threshold used, extended frequently to intermediate and medial parts of the cerebellum. Individual findings were more variable within the more caudal regions of the posterior lobe. Although all but two subjects (Subject 7, Subject 11) showed activations in the caudal posterior lobe, local maxima of individual subjects were present to various extends in lobules Crus II, VIIb, or VIIIa. All but two (Subject 3, Subject 7) subjects showed bilateral activations (Table I).
Figure 4.
Cerebellar areas of blink reflex‐related activations: Data of eight single subjects are shown (P < 0.005). SPM (t) maps are displayed on coronal sections of a typical canonical brain from the MNI series. The y coordinates correspond to the cluster with the highest Z‐score in each subject (Table I). Laterality as in Figure 3.
No cerebellar activations were found within the anterior lobe (lobules I–V) on the group level (random and fixed models). Similarly, in single subjects no activations in the anterior lobe were found, with the exception of two subjects showing activations in lobule V (Subject 7: −26, −38, −28 mm, Z = 2.91; Subject 13: −10, −50, 20 mm, Z = 3.00). Group data (fixed model: −18, −56, −44 mm, Z = 3.68; 0, −54, −36 mm, Z = 3.42) and results in four individual subjects (Subject 3, Subject 7, Subject 12, Subject 14) showed additional activations in ipsilateral (left) lobule IX, primarily in vermal areas. For example, activation of vermal lobule IX is shown in single Subject 14 in Figure 4 (−2, −58, −42 mm, Z = 3.38).
Brainstem activations
Statistical results yielded also areas of significant increases of BOLD effect in the region of the brainstem based on group and single subject data. Table II summarizes MNI coordinates, Z‐scores and cluster sizes for the brainstem activations based on group (random and fixed model) and single subject analysis.
Table II.
Coordinates in MNI standard anatomical space for the peaks of brainstem activation
| Mesencephalon | Medulla oblongata upper part | |||||
|---|---|---|---|---|---|---|
| x,y,z | Z | KE | x,y,z | Z | KE | |
| Group data | ||||||
| Random model | 10,−22,−16 | 4.64 | 24 | |||
| Fixed model | 2,−14,−18a, b | 6.58 | 254 | 2,−28,−52b | 4.69 | 24 |
| −8,−12,−18 | 6.00 | 2,−38,−46b | 4.56 | 88 | ||
| Individual subjects | ||||||
| 1 | 8,−10,−18a | 4.68 | 119 | 8,−32,−52 | 3.63 | 51 |
| −14,22,−16 | 4.30 | |||||
| 2 | ||||||
| 3 | ||||||
| 4 | −8,−20,−14 | 3.91 | 44 | |||
| 5 | −6,−14,−16a | 3.82 | 37 | |||
| −4,−24,−12 | 3.78 | |||||
| 6 | 14,−20,−20 | 4.17 | 121 | |||
| 7 | ||||||
| 8 | 10,−18,−42 | 3.56 | 25 | |||
| 9 | −2,−18,−14 | 4.55 | 62 | |||
| 10 | −8,−36,−52 | 3.52 | 30 | |||
| 11 | −18,−24,−22 | 5.30 | 31 | −2,−38,−48 | 2.79 | 15 |
| 16,−28,−12 | 5.20 | 12 | ||||
| 12 | ||||||
| 13 | 2,−28,−52 | 3.21 | 16 | |||
| −2,−40,−50 | 3.13 | 11 | ||||
| 14 | −2,−20,−22 | 3.10 | 21 | |||
Error probabilities for random model: P < 0.005; fixed model: P < 0.005; single subjects: P < 0.005.
Absolute cluster maximum.
Fitted response plotted in Figure 6.
Z, Z‐score; KE, cluster size.
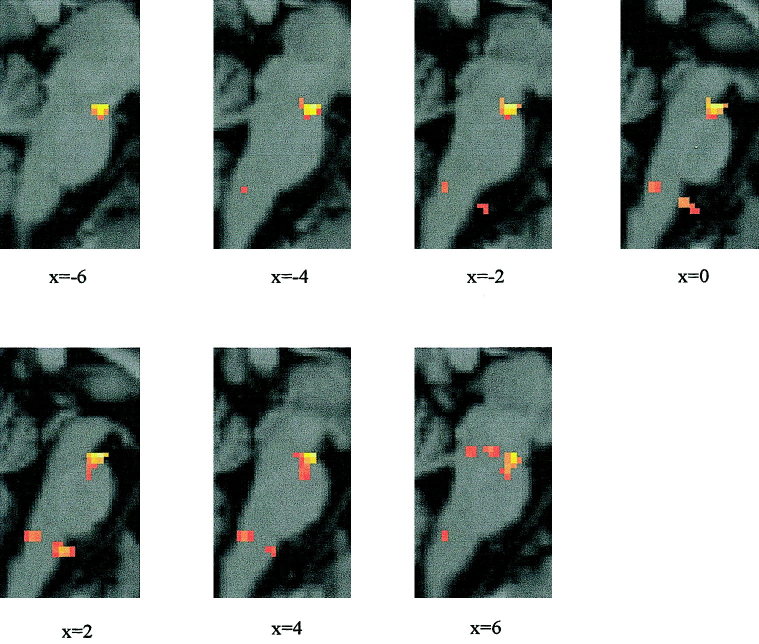
Group analysis (fixed model; P < 0.005) showed one area of activation at the level of the mesencephalon close to the upper pons (vertical z coordinate in the range of −12 mm to −20 mm) and two areas of activation at the level of the upper part of the medulla oblongata next to the lower pons (z ≈50 mm). Figure 5 shows the results of the group SPM (fixed model) for the brainstem region displayed on sagittal sections of a standard MNI brain. The most robust activation was found localized in one bigger cluster (cluster size = 254 voxels) with an absolute maximum at the level of the mesencephalon (4, 14, −18 mm). There was some spread‐out of activations to the upper and more posterior pontine region (local maximum 2, −28, −26 mm, Z = 3.79). In the upper medullary part two regions of activations were present, one located more anteriorly and one more posteriorly.
Figure 5.
Brainstem areas of blink reflex‐related activations: Group data (n = 14) are shown based on the fixed effects model (P < 0.005). SPM (t) maps are displayed on sagittal sections of a typical canonical MNI brain. The x coordinate varies between −6 mm and 6 mm with a step of 2 mm.
In Figure 6 plots of the individual fitted hemodynamic response functions (HRF) are shown for the voxels corresponding to the absolute maximum of the three clusters with significant fixed model statistical activation (Table II). These plots show that there was a common tendency for most of the individual subjects to react with positive HRF to the active event (compared to baseline) for areas in the mesencephalon (Fig. 6A) and upper medullary parts (Fig. 6B,C) despite some variance in the amplitude between subjects.
Figure 6.
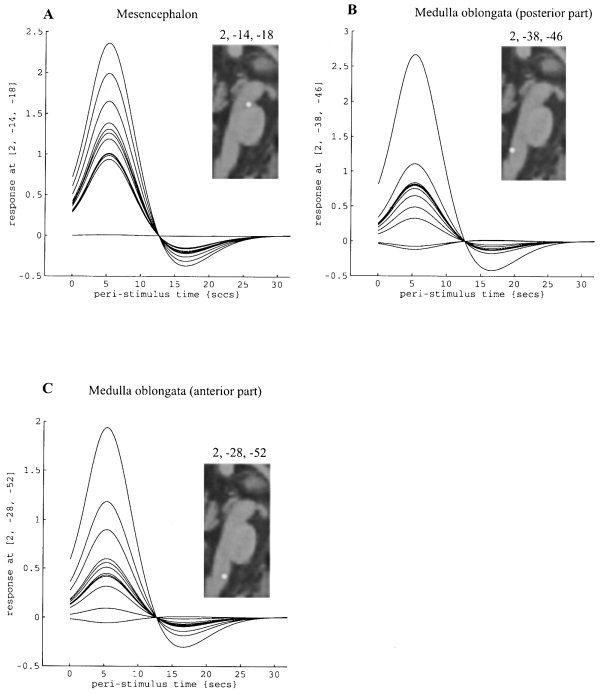
Fitted event‐related hemodynamic response functions for the absolute maxima voxel in each of the three main areas of blink reflex‐related brainstem activation shown in Figure 5 and Table II for each individual subject (n = 14): (A) within the mesencephalon, (B) within the more posterior part of the upper medulla oblongata, and (C) within the more anterior part of the upper medulla oblongata. Insets: schematic drawings of the locations of each of the three local maxima superimposed on a sagittal section of a typical canonical MNI brain.
Based on the statistically more conservative random effects model one region of significant brainstem activation was found corresponding to the region in the mesencephalon shown in Figure 5 and Figure 6A (10, −22, −16 mm; P < 0.005).
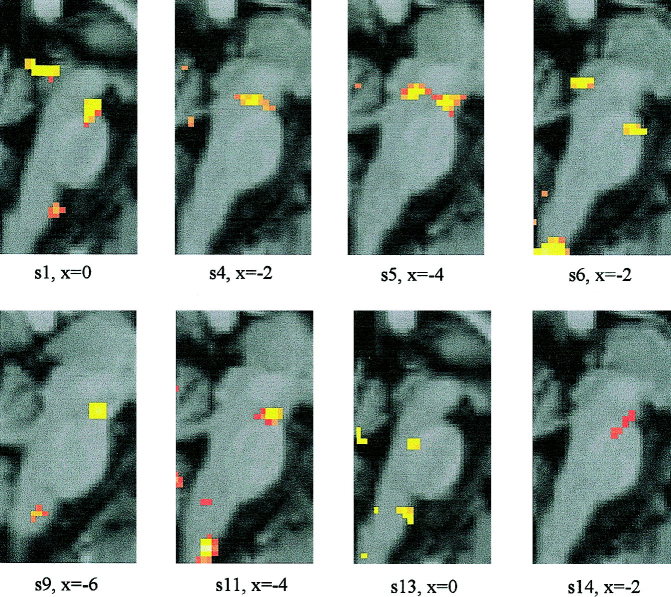
Results of single subjects show that there were consistent activations at P < 0.005 for eight of the subjects studied at the level of the mesencephalon (Fig. 7; Table II). Four subjects showed significant activations in the upper medullary region. In individual subjects, but not in the group data, additional activations were found within the region of the pons (Subject 13: −8, −36, −25 mm, Z = 3.5), lower medullary region (Subject 6: −10, −46, −62 mm, Z = 4.89; Subject 11: −6, −36, −62, Z = 5.24) and the superior colliculi (Subject 1: −2, −30, −4 mm, Z = 4.27; Subject 6: 4, −22, −16 mm, Z = 3.03). In Subject 6 the plot of HRF for the voxel corresponding to the absolute maximum of the cluster in the lower medullary region showed BOLD signal changes up to 6%. This unusual high BOLD signal change most likely reflected an artifact, e.g., due to swallowing movements correlating with the stimulus time, and was excluded from group statistical analysis. As the superior colliculi are related to eye movements, activations may represent eye movements accompanied with the blink reflex [Sibony and Evinger, 1998] or orientation of the eyes toward the acoustic stimulus evoked by the air‐puff (that may be heard by some of the subjects despite headphones and the noise of the scanner) [Baars, 1999].
Figure 7.
Brainstem areas of blink reflex‐related activations: Data of eight single subjects are shown (P < 0.005). SPM (t) maps are superimposed on sagittal sections of a typical canonical brain from the MNI series. The x coordinates were chosen to demonstrate most of potential activations in different parts of the brainstem in each subject. Regions do not necessarily correspond to coordinates of the local maxima within each cluster as shown in Table II.
DISCUSSION
Cerebellar activations
Two main areas of blink reflex related activity were found within the posterior lobe of the ipsilateral cerebellar hemisphere. As can be seen in Figure 3 (group data) and Figure 4 (findings in individual subjects) one region was located in the anterior part of the posterior lobe adjacent to the anterior lobe, and the other in more caudal parts of the posterior lobe. Blink reflex related activity was not limited to the ipsilateral cerebellum. Rather, although less pronounced, activity was also found in corresponding areas of the contralateral hemisphere. Our findings of two main areas of activation within each hemisphere agree with the existence of two body maps within the cerebellum coming from the early works of Adrian [1943], Snider and Stowell [1944], and confirmation in more recent fMRI‐studies in humans [Grodd et al., 2001; Nitschke et al., 1996; Rijntjes et al., 1999].
The present fMRI study does not allow a decision about which role the activated cerebellar areas play during evocation of the eyeblink. The cerebellar activation in response to an air‐puff may simply reflect trigeminal sensory inputs to the cerebellum from the stimulus or the blink response, cerebellar participation in trigeminal reflex blinks, or a combination of the two possibilities. There is evidence from animal studies supporting both possibilities. The cerebellum is a major target of both direct fibers and trigemino–olivo–cerebellar and trigemino–reticulo–cerebellar fibers originating in various parts of the sensory trigeminal nucleus. The present findings are essentially consistent with electrophysiological and histological studies showing that the main mossy and climbing fiber input from the face is to ipsilateral lobule H VI. Additional input to lobules H V and H VII and bilateral projections have also been described [Carpenter and Hanna, 1961; Cody and Richardson, 1979; Darian‐Smith and Phillips, 1964; Dunn and Matzke, 1968; Hesslow, 1994; Ikeda, 1979; Miles and Wiesendanger, 1975; Snider, 1943; Snider and Stowell, 1944; Somana et al., 1980; Stewart and King, 1963; van Ham and Yeo, 1992].
Although cerebellar areas with increased activity may simply represent areas receiving trigeminal input, it seems likely that these afferents are used for control of the eyeblink. Anatomical animal data suggest strongly that these cerebellar areas are connected with regions in the brainstem, which are known to be involved in the control of the eyeblink response. The red nucleus is known to receive afferents from the interposed nucleus and to project to the facial nuclei and premotor blink areas [Holstege et al., 1986; Morcuende et al., 2001; van Ham and Yeo, 1992]. The cerebello–rubral–olivary pathways provide a major route by which the cerebellum may modulate excitability of the eyeblink reflex pathways.
Furthermore, the local maxima of activation found in Schmahmann hemispheral lobules VI, Crus I and VIIb are also consistent with findings of animal lesion and stimulation studies examining cerebellar areas likely to be involved in blink reflex control. In his detailed study in cats, Hesslow [1994] found two eyeblink‐related areas located in the hemispheral part of Larsell lobule VI of the posterior lobe. A third area was located in the superior hemispheral part of Larsell lobule VII and a fourth in the superior paramedian lobe. It should be noted that the superior part of Larsell lobule H VII in cats corresponds to Schmahmann lobule Crus I in humans, and the superior part of the paramedian lobe corresponds to Schmahmann hemispheral lobule VIIb [Schmahmann et al., 2000; Voogd and Glickstein, 1998]. More recently, Pellegrini and Evinger [1997] described blink‐related Purkinje cells in Crus I of the ansiform lobule in rats, which corresponds to Schmahmann lobule Crus I in humans. Eyeblinks have also been observed after intracerebellar stimulation of the border between lobules V and VI in monkeys [Ron and Robinson, 1973].
In the present human study, maxima of activations were found predominantly within more lateral parts of the posterior hemisphere, whereas Hesslow [1994] described eyeblink control areas mainly in the intermediate parts of the cerebellum in cats. Findings may not be contradictory, because Hesslow [1994] discussed that for the two blink areas in H VI, the medial part was in the intermediate C3 zone, whereas the lateral area could either be in the hemispheral Y zone (initially named the D2 zone) or in the lateral part of the intermediate C3 zone. Given that the lateral parts of the cerebellar hemispheres are much more developed in humans compared to cats [Voogd and Glickstein, 1998], areas within the more lateral parts of corresponding lobuli may become more important in control of the eyeblink in humans.
In addition, the present fMRI study showed a weak activation in ipsilateral lobule IX (Larsell uvula and paraflocculus). Because of the limited accessibility of some cerebellar areas in his animal studies, Hesslow [1994] noted that it cannot be excluded that there are other areas in the cerebellar cortex that are directly or indirectly involved in eyeblink control. In fact, van Ham and Yeo [1992] found somatosensory trigeminal projections to lobule IX and Nagao et al. [1984] showed that eyeblink could be evoked from the rabbit flocculus. This area in the cerebellum, however, may be concerned with a different aspect of eyelid control. Eyelid movements may accompany reflex eye movements, which are known to be controlled by the flocculus and paraflocculus [Sibony and Evinger, 1998; Zee et al., 1981].
Discharge of deep cerebellar nuclei neurons related to eye blinks has been shown in cats [Gruart and Delgado‐Garcia, 1994]. Changes in BOLD response were not detected in the cerebellar nuclei in the present study, most likely because the BOLD signal is not as sensitive as recording with an electrode. Similarly, Ramnani et al. [2000] were unable to show changes in the BOLD contrast in the cerebellar nuclei in their fMRI study of eyeblink conditioning.
Blink reflex related regions overlap with cerebellar areas, i.e., hemispheral lobule VI, which has been shown to be of major importance in eyeblink conditioning in numerous animal studies [Yeo and Hesslow, 1998 for review] and a recent fMRI study in humans [Ramnani et al., 2000]. Although the present study does not address directly the issue of cerebellar involvement in eyeblink conditioning, results have some relevance for the interpretation of human eyeblink conditioning studies. First, the issue of impaired motor performance (i.e., impaired unconditioned eyeblinks) and its possible relation to deficits in eyeblink conditioning, needs to be reconsidered in cerebellar patients. The present results suggest that the human cerebellum is involved in the control of the eyeblink. The findings of unimpaired unconditioned eyeblink responses reported in all but one human lesion studies appear contradictory [Daum et al., 1993; Hacke et al., 1983; Topka et al., 1993; Woodruff‐Pak et al., 1996]. Previous human studies compared findings in groups of control and cerebellar subjects. Comparison of the affected and unaffected side in patients with unilateral lesions, however, is more likely to show small changes of amplitudes. This is supported by the finding of increased EMG amplitudes on the affected side compared to the unaffected side in a patient with an unilateral cerebellar lesion [Timmann et al., 1998]. Findings need to be confirmed in a larger group of patients with unilateral cerebellar lesions.
Second, assuming that the cerebellar areas involved in the control of the unconditioned blink reflex play a role in eyeblink conditioning, the cortical areas involved in conditioning of the eyeblink response may not be confined to hemispheral lobule VI in humans. This is supported by findings of previous PET and fMRI studies of eyeblink conditioning in healthy human subjects. Although in the PET studies no detailed description of the activated cerebellar lobuli is given, significant changes of blood flow are reported in more widespread areas of the cerebellar cortex bilaterally and in the cerebellar vermis [Blaxton et al., 1996; Logan and Grafton, 1995; Molchan et al., 1994; Schreurs et al., 1997]. In their fMRI study Ramnani et al. [2000] found learning related changes in ipsilateral cerebellar lobules H VI and Crus I. There are also animal lesion studies suggesting that ipsilateral cortical areas other than H VI and contralateral cortical areas play an additional role in eyeblink conditioning, although to a lesser extent than lobule H VI [Hardiman and Yeo, 1992]. In this case, eyeblink conditioning may not be completely abolished in cerebellar patients with lesions confined to hemispheral lobule VI because other cerebellar areas involved in eyeblink control (e.g., hemispheral lobules Crus I or VIIb; contralateral hemisphere) may step in. Future studies should address this question in patients with focal cerebellar lesions.
Brainstem activations
Because the main neuronal circuit of the blink reflex is located within the brainstem, brainstem activations would be a reasonable finding [Esteban, 1999; Sibony and Evinger, 1998]. In particular, activations of the main input nuclei (i.e., principle trigeminal nucleus within the pons and caudal spinal trigeminal nucleus within the medulla oblongata) and output nuclei (i.e., facial nuclei within the pons) are to be expected. In the brainstem, however, fMRI is complicated by cardiac‐related movement of the brainstem and liquid flow and the unfavorable anatomical characteristics of the vascular system [Backes and van Dyjk, 2002; Guimaraes et al., 1998; Liu et al., 2000].
Areas of activation were observed within the region of the lower mesencephalon and upper medulla oblongata, but not within the pons. Increased signal variability due to cardiac‐related, pulsatile brainstem motion may be one important reason why no activations were found within the pontine input and output nuclei of the blink reflex [Backes and van Dyjk, 2002; Guimaraes et al., 1998].
The area of most consistent activation at the level of the caudal mesencephalon most likely reflects MRI signal changes from brainstem veins (Fig. 6A). It overlapped best with the known location of one of the principal venous truncs of the mesencephalon (i.e., basal vein) [see Fig. 72 in Duvernoy, 1995]. Activation of the pontine principle trigeminal nucleus or the mesencephalic red nucleus, which is known to be involved in blink reflex control [Holstege et al., 1986; Morcuende et al., 2001; van Ham and Yeo, 1992], seems less likely. The principle trigeminal nucleus is localized more caudally within the pons, whereas the red nucleus is localized more cranially within the mesencephalon than the maximum of mesencephalic activation.
In the upper medullary region two areas were activated, one being located more anteriorly and the other more posteriorly (Fig. 6B,C). Although activation of medullary veins cannot be ruled out, the more anterior part appeared to overlap with the olivary nucleus, whereas parts of the medullary reticular formation and caudal spinal trigeminal nucleus are known to be located within the more posterior area. Although results were less consistent as compared to the region within the mesencephalon (significant group data present in fixed model only; only few single subjects showed a significant activation), results seemed to be valid because all but one of the subjects reacted with positive HRF. The caudal trigeminal nucleus and medullary reticular formation are thought to be involved in the control of the R2 component, whereas the inferior olive is likely to mediate cerebellar influences in blink reflex control. The first assumption is in good agreement with the hypothesis put forward by Ongerboer de Visser [1983a,b] that the R2 component in humans is conducted from the ipsilateral caudal spinal trigeminal nucleus through polysynaptic medullary pathways running both ipsilaterally and contralaterally to the stimulated side before making connections with the facial nuclei [Kimura and Lyon, 1972; Ongerboer de Visser and Kuypers, 1978; Valls‐Sole et al., 1996]. Similar findings have been reported in cats [Hiraoka and Shimamura, 1977; Holstege et al., 1986; Takada et al., 1984; Tamai et al., 1986]. Trigemino–cerebellar climbing fiber afferents are known to be conducted through the inferior olive [Hesslow, 1994; Miles and Wiesendanger, 1975; van Ham and Yeo, 1992] and models of eyeblink conditioning assume the unconditioned stimulus to be passed to the cerebellum via this pathway [Bracha and Bloedel, 1996; Thompson and Krupa, 1994].
Future fMRI studies preventing artifacts from cardiac‐related brainstem motions and excluding large MRI signal changes from brainstem veins need to readdress the question of blink reflex related areas within the brainstem. Techniques for synchronizing the MRI scans with heart rhythm and some post‐processing filtering for eliminating the pulsation effect have been suggested in the recent literature [Backes and van Dyjk, 2001; Guimaraes et al., 1998], as well as avoiding of the vascular artifacts by excluding all of the vascular voxels from the statistic calculation [Liu et al., 2000].
CONCLUSIONS
Two main cerebellar areas within the posterior lobe of the cerebellar hemisphere mainly on the ipsilateral side have been found to be activated during reflex eyeblinks in humans. These regions within hemispheral lobules VI, Crus I, and VIIb [nomenclature according to Schmahmann et al., 2000] agree with cerebellar trigeminal projection areas and blink reflex control areas shown in previous animal experiments.
Acknowledgements
We thank B. Brol and M. Erichsen for their help in analyzing the kinematic and EMG blink reflex data and editorial help.
REFERENCES
- Adrian ED (1943): Afferent areas in the cerebellum connected with the limbs. Brain 66: 289–315. [Google Scholar]
- Aramideh M, Ongerboer de Visser BW, Koelman JH, Majoie CB, Holstege G (1997): The late blink reflex response abnormality due to lesion of the lateral tegmental field. Brain 120: 1685–1692. [DOI] [PubMed] [Google Scholar]
- Baars BJ (1999): Attention vs. consciousness in the visual brain: differences in conception, phenomenology, behavior, neuroanatomy, and physiology. J Gen Psychol 126: 224–233. [DOI] [PubMed] [Google Scholar]
- Backes WH, van Dijk P (2002): Simultaneous sampling of event‐related BOLD responses in auditory cortex and brainstem. Magn Reson Med 47: 90–96. [DOI] [PubMed] [Google Scholar]
- Blaxton TA, Zeffiro TA, Gabrieli JD, Bookheimer SY, Carrillo MC, Theodore WH, Disterhoft JF (1996): Functional mapping of human learning: a positron emission tomography activation study of eyeblink conditioning. J Neurosci 12: 4032–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloedel JR, Bracha V (1995): On the cerebellum, cutaneomuscular reflexes, movement control and the elusive engrams of memory. Behav Brain Res 68: 1–44. [DOI] [PubMed] [Google Scholar]
- Bracha V, Bloedel JR (1996): The multiple‐pathway model of circuits subserving the classical conditioning of withdrawal reflexes In: Bloedel JR, Ebner TJ, Wise PS, editors. The acquisition of motor behavior in vertebrates. Cambridge, MA: MIT Press; p 175–204. [Google Scholar]
- Bracha V, Zhao L, Wunderlich DA, Morrissy SJ, Bloedel JR (1997): Patients with cerebellar lesions cannot acquire but are able to retain conditioned eyeblink reflexes. Brain 120: 1401–1413. [DOI] [PubMed] [Google Scholar]
- Carpenter MB, Hanna GR (1961): Fiber projections from the spinal trigeminal nucleus in the cat. J Comp Neurol 117: 117–132. [DOI] [PubMed] [Google Scholar]
- Cody FW, Richardson HC (1979): Mossy and climbing fibre mediated responses evoked in the cerebellar cortex of the cat by trigeminal afferent stimulation. J Physiol (Lond) 287: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian‐Smith I, Phillips G (1964): Secondary neurones within a trigemino–cerebellar projection to the anterior lobe of the cerebellum in the cat. J Physiol (Lond) 170: 53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum I, Schugens MM, Ackermann H, Lutzenberger W, Dichgans J, Birbaumer N (1993): Classical conditioning after cerebellar lesions in humans. Behav Neurosci 107: 748–756. [DOI] [PubMed] [Google Scholar]
- Dunn JD, Matzke HA (1968): Efferent fiber connections of the marmoset (Oedipomidas oedipus) trigeminal nucleus caudalis. J Comp Neurol 133: 429–437. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM (1995): The human brain stem and cerebellum. Surface, structure, vascularization, and three‐dimensional anatomy with MRI. Wien, New York: Springer Verlag; p 108–109. [Google Scholar]
- Esteban A (1999): A neurophysiological approach to brainstem reflexes. Blink reflex. Neurophysiol Clin 29: 7–38. [DOI] [PubMed] [Google Scholar]
- Evans AC, Kamber M, Collins DL, MacDonald D (1994): An MRI‐based probabilistic atlas of neuroanatomy In: Shorvon S, Fish D, Andermann F, Bydder GM, Stefan H, editors. Magnetic resonance scanning and epilepsy. New York: Plenum; p 263–274. [Google Scholar]
- Friston KJ, Ashburner J, Poline JB, Frith CD, Heather JD, Frackowiak RSJ (1995): Spatial registration and normalization of images. Hum Brain Mapp 2: 165–189. [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD (1996): Detecting activations in PET and fMRI: levels of inference and power. Neuroimage 4: 223–235. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ (1999): Multisubject fMRI studies and conjunction analyses. Neuroimage 10: 385–396. [DOI] [PubMed] [Google Scholar]
- Gandiglio G, Fra L (1967): Further observations on facial reflexes. J Neurol Sci 5: 273–285. [DOI] [PubMed] [Google Scholar]
- Grodd W, Hülsmann E, Lotze M, Wildgruber D, Erb M (2001): Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp 13: 55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruart A, Delgado‐Garcia JM (1994): Discharge of identified deep cerebellar nuclei neurons related to eye blinks in alert cat. Neurosci 61: 665–681. [DOI] [PubMed] [Google Scholar]
- Gruart A, Yeo CH (1995): Cerebellar cortex and eyeblink conditioning: bilateral regulation of conditioned responses. Exp Brain Res 104: 431–448. [DOI] [PubMed] [Google Scholar]
- Guimaraes AR, Melcher JR, Talavage TM, Baker JR, Ledden P, Rosen BR, Kiang NYS, Fullerton BC, Weisskoff RM (1998): Imaging subcortical auditory activity in humans. Hum Brain Mapp 6: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardiman MJ, Yeo CH (1992): The effect of kainic acid lesions of the cerebellar cortex and the conditioned nictitating membrane response in the rabbit. Eur J Neurosci 4: 966–980. [DOI] [PubMed] [Google Scholar]
- Hacke W, Schaff C, Zeumer H (1983): [Orbicularis oculi reflex in computerized tomography verified lesions of the posterior cranial fossa]. Fortschr Neurol Psychiatr 51: 313–324. [DOI] [PubMed] [Google Scholar]
- Harvey JA, Welsh JP, Yeo CH, Romano AG (1993): Recoverable and nonrecoverable deficits in conditioned responses after cortical cerebellar lesions. J Neurosci 13: 1624–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G (1994): Correspondence between climbing fibre input and motor output in eyeblink‐related areas in cat cerebellar cortex. J Physiol (Lond) 476: 229–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka M, Shimamura M (1977): Neural mechanisms of the corneal blinking reflex in cats. Brain Res 125: 265–275. [DOI] [PubMed] [Google Scholar]
- Holstege G, Tan J, van Ham JJ, Graveland GA (1986): Anatomical observations on the afferent projections to the retractor bulbi motoneuronal cell group and other pathways possibly related to the blink reflex in the cat. Brain Res 374: 321–334. [DOI] [PubMed] [Google Scholar]
- Hopf HC, Thomke F, Gutmann L (1991): Midbrain vs. pontine medial longitudinal fasciculus lesions: the utilization of masseter and blink reflexes. Muscle Nerve 14: 326–330. [DOI] [PubMed] [Google Scholar]
- Ikeda M (1979): Projections from the spinal and the principal sensory nuclei of the trigeminal nerve to the cerebellar cortex in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol 184: 567–585. [DOI] [PubMed] [Google Scholar]
- Kimura J (1970): Alteration of the orbicularis oculi reflex by pontine lesions. Study in multiple sclerosis. Arch Neurol 22: 156–161. [DOI] [PubMed] [Google Scholar]
- Kimura J, Lyon LW (1972): Orbicularis oculi reflex in the Wallenberg syndrome: alteration of the late reflex by lesions of the spinal tract and nucleus of the trigeminal nerve. J Neurol Neurosurg Psychiatry 35: 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb FP, Irwin KB, Bloedel JR, Bracha V (1997): Conditioned and unconditioned forelimb reflex systems in the cat: involvement of the intermediate cerebellum. Exp Brain Res 114: 255–270. [DOI] [PubMed] [Google Scholar]
- Kugelberg E (1952): Facial reflexes. Brain 75: 385–396. [DOI] [PubMed] [Google Scholar]
- Liu Y, Pu Y, Gao J‐H, Parsons LM, Xiong J, Lotti M, J. Bower JM, Fox PT (2000): The human red nucleus and lateral cerebellum in supporting roles for sensory information processing. Hum Brain Map 10: 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CG, Grafton ST (1995): Functional anatomy of human eyeblink conditioning determined with regional cerebral glucose metabolism and positron‐emission tomography. Proc Natl Acad Sci U S A 92: 7500–7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschke M, Erichsen M, Drepper J, Jentzen W, Nelles G, Müller SP, Kolb FP, Diener HC, Timmann D (2000): Representation of specific aversive reactions in the human cerebellum. Soc Neurosci Abstr 26: 457. [Google Scholar]
- Mathiesen C, Caesar K, Akgören N, Lauritzen M (1998): Modification of activity‐dependent increases of cerebral blood flow by excitatory synaptic activity and spikes in rat cerebellar cortex. J Physiol (Lond) 512: 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles TS, Wiesendanger M (1975): Climbing fiber inputs to cerebellar Purkinje cells from trigeminal cutaneous afferents and the SI face area of the cerebral cortex in the cat. J Physiol (Lond) 245: 425–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molchan SE, Sunderland T, McIntosh AR, Herscovitch O, Schreurs BG (1994): A functional anatomical study of associative learning in humans. Proc Natl Acad Sci U S A 91: 8122–8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcuende S, Ugolini G, Delgado‐Garcia JM (2001): Retrograde transneuronal tracing with rabies virus of neural centers controlling the movement of eyelid. Abstract presented at the meeting on “Neural control of movement,” Sevilla, Spain. Unpublished abstract.
- Nagao S, Ito M, Karachot L (1984): Sites in the rabbit flocculus specifically related to eye blinking and neck muscle contraction. Neurosci Res 1: 149–152. [DOI] [PubMed] [Google Scholar]
- Nitschke MF, Kleinschmidt A, Wessel K, Frahm J (1996): Somatotopic motor representation in the human anterior cerebellum. A high‐resolution functional MRI study. Brain 119: 1023–1029. [DOI] [PubMed] [Google Scholar]
- Ongerboer de Visser BW, Kuypers HG (1978): Late blink reflex changes in lateral medullary lesions. An electrophysiological and neuro‐anatomical study of Wallenberg's syndrome. Brain 101: 285–294. [DOI] [PubMed] [Google Scholar]
- Ongerboer de Visser BW (1983a): Anatomical and functional organization of reflexes involving the trigeminal system in man: jaw reflex, blink reflex, corneal reflex, and exteroceptive suppression In: Desmedt JE, editor. Advances in neurology. New York: Raven Press; p 727–738. [PubMed] [Google Scholar]
- Ongerboer de Visser BW (1983b): Comparative study of corneal and blink reflex latencies in patients with segmental or with cerebral lesions In: Desmedt JE, editor. Advances in neurology. New York: Raven Press; p 757–772. [PubMed] [Google Scholar]
- Overend W (1896): Preliminary note on a new cranial reflex. Lancet 1: 619. [Google Scholar]
- Pellegrini JJ, Horn AK, Evinger C (1995): The trigeminally evoked blink reflex. I. Neuronal circuits. Exp Brain Res 107: 166–180. [DOI] [PubMed] [Google Scholar]
- Pellegrini JJ, Evinger C (1997): Role of the cerebellum in adaptive modification of reflex blinks. Learn Mem 3: 77–87. [DOI] [PubMed] [Google Scholar]
- Peshori KR, Schicatano EJ, Gopalaswamy R, Sahay E, Evinger C (2001): Aging of the trigeminal blink system. Exp Brain Res 136: 351–363. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Toni I, Josephs O, Ashburner J, Passingham RE (2000): Learning‐ and expectation‐related changes in the human brain during motor learning. J Neurophysiol 84: 3026–3035. [DOI] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG, Mauk MD (1996): The cerebellum: a neuronal learning machine? Science 272: 1126–1131. [DOI] [PubMed] [Google Scholar]
- Rijntjes M, Buechel C, Kiebel S, Weiller C (1999): Multiple somatotopic representations in the human cerebellum. Neuroreport 10: 3653–3658. [DOI] [PubMed] [Google Scholar]
- Ron S, Robinson DA (1973): Eye movements evoked by cerebellar stimulation in the alert monkey. J Neurophysiol 36: 1004–1022. [DOI] [PubMed] [Google Scholar]
- Rushworth G (1962): Observations on the blink reflexes. J Neurol Neurosurg Psychiatry 25: 93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Dojon J, Toga AW, Petrides M, Evans AC (2000): MRI atlas of the human cerebellum. San Diego: Academic Press. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, McIntosh AR, Bahro M, Herscovitch P, Sunderland T, Molchan SE (1997): Lateralization and behavioral correlation of changes in regional cerebral blood flow with classical conditioning of the human eyeblink response. J Neurophysiol 77: 2153–2163. [DOI] [PubMed] [Google Scholar]
- Shahani B (1970): The human blink reflex. J Neurol Neurosurg Psychiatry 33: 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahani BT, Young RR (1973): Blink reflex in orbicularis oculi In: Desmedt JE, editor. New developments in electromyography and clinical neurophysiology. Basel: S; Karger. p 641–648. [Google Scholar]
- Sibony PA, Evinger C (1998): Anatomy and physiology of normal and abnormal eyelid position and movement In: Miller NR, editor. Walsh and Hoyt's clinical neuroophthalmology. 5th Ed. Baltimore: Williams and Wilkins; p 1509–1592. [Google Scholar]
- Snider RS (1943): A fifth cranial nerve projections to the cerebellum. Fed Proc Am Soc Exp Biol 2: 46. [Google Scholar]
- Snider RS, Stowell A (1944): Receiving areas of the tactile, auditory, and visual systems in the cerebellum. J Neurophysiol 7: 331–357. [Google Scholar]
- Somana R, Kotchabhakdi N, Walberg F (1980): Cerebellar afferents from the trigeminal sensory nuclei in the cat. Exp Brain Res 38: 57–64. [DOI] [PubMed] [Google Scholar]
- Stewart WA, King RB (1963): Fiber projections from the nucleus caudalis of the spinal trigeminal nucleus. J Comp Neurol 121: 271–286. [DOI] [PubMed] [Google Scholar]
- Takada M, Itoh K, Yasui Y, Mitani A, Nomura S, Mizuno N (1984): Distribution of premotor neurons for orbicularis oculi motoneurons in the cat, with particular reference to possible pathways for blink reflex. Neurosci Lett 50: 251–255. [DOI] [PubMed] [Google Scholar]
- Talaraich J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Georg Thieme Verlag. [Google Scholar]
- Tamai Y, Iwamoto M, Tsujimoto T (1986): Pathway of the blink reflex in the brainstem of the cat: interneurons between the trigeminal nuclei and the facial nucleus. Brain Res 380: 19–25. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Krupa DJ (1994): Organization of memory traces in the mammalian brain. Annu Rev Neurosci 17: 519–549. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Bao S, Chen L, Cipriano BD, Grethe JS, Kim JJ, Thompson JK, Tracy JA, Weninger MS, Krupa DJ (1997): Associative learning. Int Rev Neurobiol 41: 151–189. [DOI] [PubMed] [Google Scholar]
- Timmann D, Baier C, Diener HC, Kolb FP (1998): Impaired acquisition of limb flexion reflex and eyeblink classical conditioning in a cerebellar patient. Neurocase 4: 207–217. [Google Scholar]
- Topka H, Valls‐Sole J, Massaquoi SG, Hallett M (1993): Deficit in classical conditioning in patients with cerebellar degeneration. Brain 116: 961–969. [DOI] [PubMed] [Google Scholar]
- Valls‐Sole J, Vila N, Obach V, Alvarez R, Gonzalez LE, Chamorro A (1996): Brain stem reflexes in patients with Wallenberg's syndrome: correlation with clinical and magnetic resonance imaging (MRI) findings. Muscle Nerve 19: 1093–1099. [DOI] [PubMed] [Google Scholar]
- van Ham JJ, Yeo CH (1992): Somatosensory trigeminal projections to the inferior olive, cerebellum and other precerebellar nuclei in rabbits. Eur J Neurosci 4: 302–317. [DOI] [PubMed] [Google Scholar]
- Voogd J, Glickstein M (1998): The anatomy of the cerebellum. Trends Neurosci 21: 370–375. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Harvey JA (1989): Cerebellar lesions and the nictitating membrane reflex: performance deficits of the conditioned and unconditioned response. J Neurosci 9: 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JP, Harvey JA (1991): Pavlovian conditioning in the rabbit during inactivation of the interpositus nucleus. J Physiol (Lond) 444: 459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff‐Pak DS, Papka M, Ivry RB (1996): Cerebellar involvement in eyeblink classical conditioning in humans. Neuropsychology 10: 443–458. [Google Scholar]
- Yeo CH, Hardiman MJ, Glickstein M (1985): Classical conditioning of the nictitating membrane response of the rabbit. II. Lesions of the cerebellar cortex. Exp Brain Res 60: 99–113. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ (1992): Cerebellar cortex and eyeblink conditioning: a reexamination. Exp Brain Res 88: 623–638. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hesslow G (1998): Cerebellum and conditioned reflexes. Trends Cognit Sci 2: 322–330. [DOI] [PubMed] [Google Scholar]
- Zee DS, Yamazaki A, Butler PH, Gucer G (1981): Effects of ablation of flocculus and paraflocculus on eye movements in primate. J Neurophysiol 46: 878–899. [DOI] [PubMed] [Google Scholar]