Abstract
Learning is based on the remodeling of neural connections in the brain. The purpose of the present study was to examine the extent to which training‐induced improvements in tactile frequency discrimination in humans are correlated with an increase of cortical representations in the primary somatosensory cortex. Healthy male subjects (n = 16) were trained in a tactile frequency discrimination task of the left ring finger. During the first 15 days of training, there was a steep improvement in frequency discrimination, which generalized from the trained finger to its homologue on the opposite hand, and to a lesser extent, to the other fingers on both hands. During the following 15 days of training, there was only a minor improvement in tactile frequency discrimination. Retention of improved performance in frequency discrimination 30 days after training was demonstrated for all digits. Cortical finger representation in the primary somatosensory cortex, as measured by magnetic source imaging, did not change during training. Because of the generalized training effect and the lack of detectable increase in the cortical field evoked from the trained finger, we assume that skill improvement was mediated predominantly by regions outside the primary somatosensory cortex. Hum. Brain Mapping 18:260–271, 2003. © 2003 Wiley‐Liss, Inc.
Keywords: magnetoencephalogram, plasticity, primary somatosensory cortex, learning
INTRODUCTION
The topographic organization of somatosensory cortical zones reflects peripheral receptor density and the behavioral relevance of different body parts. However, peripheral afferents and cortical representation neurons do not match one‐to‐one. Rather, the terminal arborization of thalamocortical afferents provides a wide divergence of projections. [Darian‐Smith and Darian‐Smith, 1993] This divergence, together with the action of interneurons and tonic modulatory input, allows for the shaping of cortical response properties including sharpening of resolution and feature extraction [for a review, see Calford and Tweedale, 1990].
During the last two decades it has become obvious that what we measure as cortical body representation zones is not static but can reorganize to some extent [Jenkins et al., 1990; Kaas et al., 1979; Pons et al., 1991]. In the milestone work by Merzenich and colleagues [1983] somatotopic maps in owl monkeys 2 to 9 months after digit amputation were found to have shifted by 1 mm when reassessed by intracortical recordings. Using the same technology, Recanzone et al. [1992a] then reported that cortical representations increased in owl monkeys learning to discriminate different tactile frequencies. This work fostered the concept of representational plasticity. Cortical topographies came to be understood as modifiable physiological entities. Subsequently, it was shown that cortical reorganization need not be confined to distances determined by the length of cortical interneurons in the millimeter range but may also extend to several centimeters. Thus, Pons et al. [1991] demonstrated massive shifts in somatosensory lip representations in monkeys with longstanding arm amputation. Similarly, using magnetic source imaging, we found that in human subjects with arm amputation, the cortical somatosensory lip representation had moved up to several centimeters into the former arm representation [Elbert et al., 1994].
Considerable fascination came with the concept of representational plasticity because it seemed to provide a direct correlate of behavioral plasticity. It was then learned that this correlation may not be a trivial one. For example, although magnetic source imaging shows that the ipsilateral cortical lip zone in human arm amputees has shifted and expanded, there is no evidence to date that somesthesia of the ipsilateral lip in amputees is improved. Rather, somesthesia in these cases tends to become disorganized [Knecht et al., 1998]. Additionally, a number of indirect factors seem to affect cortical somatosensory representation. For example, modulation of cortical somatosensory maps has been observed during simple attentional focusing [Buchner et al., 2000]. Moreover, after amputation, shifts in cortical representations mostly seem to reflect phantom pain [Flor et al., 1995]. In some instances, the cortical reorganization can even be reversed by effective pain suppression [Birbaumer et al., 1997].
Despite these seemingly confounding observations, direct correlations between changes in behavior and cortical representational topography have been observed. In musicians who started playing the violin early in childhood, using magnetic source imaging Elbert et al. [1995] demonstrated an increased cortical representation of fingers of the left hand, which are most involved in string playing. However, the study by Elbert and colleagues [1995] also showed that expanded cortical representation is not a uniform phenomenon in highly proficient violin players. No marked increase in cortical representation was found in string players who started to practice after the age of 12 years [Elbert et al., 1995]. This raises the question whether increases in cortical representations, that are so marked as to be picked up by extracranial magnetoencephalography, constitute a necessary component of improved performance or are simply a by‐product of increased use over an extended length of time.
We followed up on this question. Using magnetic source imaging, we measured changes in somatosensory cortical finger representations in humans that were engaged in a frequency discrimination paradigm over a training period of one month. The role of the primary somatosensory (S1) cortex in such tasks has recently been reaffirmed by work from Romo and colleagues [1998, 2002]. Combining stimulation to the fingertip and to area 3b of S1 in monkeys, they demonstrated that the S1 cortex is critical for somatosensory flutter discrimination. Measurement of somatosensory cortical representations in our study was performed with the same magnetic source imaging device as in the study by Elbert and colleagues [1995].
SUBJECTS AND METHODS
Subjects
Healthy male subjects (age: 21–34 years) were recruited through public advertisement. Twenty‐one male subjects gave written informed consent and participated in the study. All were strongly right‐handed, i.e., 100% by the Edinburgh Handedness Inventory [Oldfield, 1971]. Sixteen of the subjects served as a training group and the remaining five subjects served as a control group that received no training. The experimental procedures were in accordance with the Declaration of Helsinki and were approved by the Ethics Committee of the Medical Faculty of the University of Muenster.
Experimental design
Measurement of tactile frequency discrimination
Training was performed in a room with bright daylight. Subjects sat comfortably in a reclining chair, their forearms were placed palms downwards on an individually molded vacuum cushion. The tip of their finger contacted a convex‐shaped stimulator head of 2‐cm diameter with a hole in the center containing a flat‐tipped tactile probe of 1‐mm diameter. The tactile stimulator was a servo‐controlled mechanical transducer (Somedic, Sweden) set to a ramp displacement of 1‐msec duration and 100‐μm amplitude with a slope speed of 100 μm per msec. The contact force of the finger was not controlled. Subjects were allowed to place their fingertip onto the stimulator in a fashion they felt was most suitable for tactile perception. Because subjects made motor adjustments of the fingertip and very likely optimized fingertip placement during the training, we refer to the task as a procedural task rather than a purely perceptive one.
Stimulus presentation and data analysis were performed on a personal computer using custom‐tailored software. Each trial was initiated by a cueing tone (a beep on a personal computer) followed 200 msec later by a 20‐Hz burst of tactile stimuli with a 1‐sec duration. Five hundred milliseconds later, a second cueing tone occurred followed 200 msec later by a second burst of tactile stimuli of 1‐sec duration. The second burst was either identical with the first one (stimulus frequency of 20 Hz) or of a higher frequency. The difference in frequency between the first and the second bursts is referred to as “delta frequency” (ΔF). In 50% of 20 consecutive trials, the second burst frequency was higher than the first, alternating in a random manner (Fig. 1). Subjects were naïve to the experimental design. In a two‐alternative forced choice procedure, subjects were required to indicate after each trial whether they had perceived the burst frequency to be either “equal” or “different”. The difference in frequency between the first and second burst was adapted in a sequential two‐down/one‐up fashion to a performance level closely corresponding to 75% of correct responses (i.e., a performance of 0.75). The difference in frequency, i.e., the step size of delta F decreased from 2.0 to 1.0 to 0.5 and when necessary to 0.25 in some subjects. Interpolation was performed using a gamma function. For details, see Knecht et al. [1996b]. We controlled for response tendencies during the training and somethesia but did not find any significant changes. For this reason and to make our results accessible for readers who are not familiar with the Signal Detection Theory, neither a “d′” nor a “c” value were used for analysis [Green and Swets, 1996].
Figure 1.
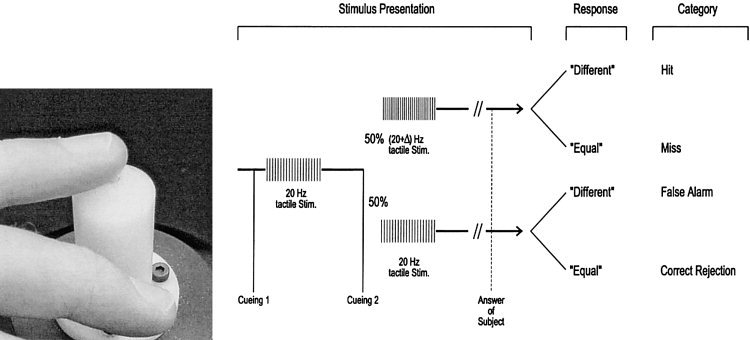
Course of the psychometric somatosensory testing and training procedure. Using a torque motor‐driving device (left), two bursts of tactile stimuli were presented after an auditory cue with the second burst differing from the first in 50% of trials (right). Subjects responded in a two‐alternative forced‐choice fashion with “equal” or “different.” (Modified from Knecht et al. [1996b]).
In order to exclude the possibility that subjects used information about the tactile frequency through the auditory modality, subjects were presented with continuous white noise at a comfortable level over headphones during the examination.
Training
Training was performed on 22 out of 30 days with breaks of maximally 2 days on weekends. The training consisted of repeated assessments of tactile frequency discrimination with feedback provided after each trial over 20 sequential trials. The delta frequency was adopted in two‐down/one‐up approximations of differing burst frequencies as used for the initial measurement. Subjects were requested to detect the smallest possible difference in tactile frequencies over at least six repeated runs that formed one block. A 75% performance (frequency discrimination threshold) could then be calculated. The procedure was repeated until 32 runs (= 640 trials) were completed. Each training session lasted between 60 and 90 min.
Testing of performance retention
Four weeks after the last training session, tactile frequency discrimination was reassessed. Subjects were retrained on three consecutive days in order to examine the retention and reactivation of tactile acuity.
Somesthesia
Assessments of tactile frequency discrimination of digits 2 to 5 of both hands were performed before training (baseline), after training sessions 11 (2 weeks) and 22 (4 weeks), 30 days after termination of training (first retraining, 4 weeks post), and again after 3 days of retraining (second retraining, retraining). The procedure used was the same as described before, except that no feedback was given.
Magnetoencephalography
Magnetoencephalographic measurements were obtained using a 37‐channel biomagnetometer equipped with first order gradiometers (Magnes I, BTI, San Diego, CA). Their spectral intrinsic noise was between 5 and 7 fT/Hz. Each detection coil measured 2 cm in diameter. The distance between the centers of two adjacent coils was 2.2 cm. The detection coils were arranged in a sensor array, which covered a circular concave area of 14.4 cm in diameter. Measurements were conducted in a magnetically shielded room (Vakuumschmelze Hanau, Germany).
A three‐dimensional digitizer unit (Polhemus 3space tracker) determined the spatial locations of the sensors in relation to the head. Prior to each measurement, patients laid comfortably on their back while reference points were registered with the digitization stylus. Based on this measurement, a head‐based coordinate system was defined. The origin was set at the midpoint of the medio‐lateral axis (y‐axis), which joined the center points of the entrance to the acoustic meati of the left and right ear (positive towards the left ear). The postero‐anterior axis (x‐axis) was oriented from the origin to the nasion (positive towards the nasion) and the inferior‐superior axis (z‐axis) was perpendicular to the x‐y plane (positive towards the vertex).
After localization of the reference points, subjects were moved to a lateral position with their head and body supported by a vacuum cushion. The sensor array was centered above C3 or C4 according to the international 10–20 system, contralateral to the stimulation site and as close as possible to the subjects' head. Prior to each 10‐min session, all accessible index points (the three forehead points and one ear point) were repeatedly localized in relation to the sensor. The glabrous skin of the second to fifth digits on both sides was stimulated in a randomized order by a tactile, pneumatic stimulator [Macmillan and Creelman, 1991]. At least 1,200 stimuli were delivered to each side with an interstimulus interval (ISI) of 500 ± 50 msec. The magnetic field in a frequency band between 0.01 to 200 Hz was sampled at a rate of 520.8 Hz.
The evoked magnetic responses were averaged, baseline corrected, and filtered with a low‐pass filter of 30 Hz (second‐order Butterworth). To exclude artifacts, stimulus‐related epochs were discarded if the difference between minimum and maximum exceeded 2pT. For each magnetic field distribution, a single equivalent current dipole (ECD) was fitted based on a spherical volume conductor. The maxima of the root mean square (RMS) and current dipole moment (Q) within an interval of 35–70 msec after stimulus onset were determined. The dipole fit results were rejected if the goodness‐of‐fit was less than 90%. The estimated dipole locations were specified in terms of their x, y, z coordinates.
The strength and location of current dipole moments for digits 2–5 of each hand were calculated.
Analysis of training data
The stimulation parameters and responses were recorded and used for on‐line calculation of the difference in tactile frequencies, which were distinguished with a 75% performance threshold (P = 0.75) on a given block. Changes in tactile frequency discrimination from the baseline assessment across the 22 training sessions, as well as across somesthesia measurements before, during, and after training were analyzed using an ANOVA with trend analysis on the repeated factors SESSION (baseline, training sessions 1–22) and BLOCK. Retention effects (from the last training session to the three re‐training sessions) were examined with the same model.
Because of the adaptive testing procedure of the training program, subjects were presented with a varying number of blocks during each training session. For data analysis, the thresholds of the first three blocks were averaged for each subject and each session.
Transfer assessments of tactile frequency discrimination
To examine training‐associated changes in the first and second neighbors of the trained digit as well as the corresponding four fingers of the opposite hand, an ANOVA was conducted with the repeated factors SIDE (left, right), DIGIT (d5, d4, d3, d2), and ASSESSMENT (trend analysis: baseline, training session 11, training session 22, first retraining session, and second retraining session). Greenhouse‐Geisser epsilon corrections to control for false‐positive results were used for within subject effects with two or more degrees of freedom [Keselman and Rogan, 1980]. Post‐hoc comparisons were analyzed using paired t‐tests with Bonferroni corrections.
The first MEG baseline measurement was excluded from the analysis because subjects first had to get accustomed to the procedure. The second and third baseline measurements were averaged for further analysis. Because not all subjects were available for the post‐training assessments, an ANOVA with n = 15 was conducted with the repeated factors SIDE (right, left), DIGIT (d5, d4, d3, d2), and ASSESSMENT (trend analysis: baseline, training day 11, training day 22). To examine the effects of retraining on cortical organization, an additional ANOVA was conducted with all subjects who were available for both retraining assessments (n = 12).
RESULTS
Training
Training led to a significant improvement of tactile acuity of the left ring finger. The initial improvement from baseline through training session 11 (first 2 weeks) was steeper than the improvement during the latter half of the training [Knecht et al. 2001].
We calculated the training‐induced threshold change, defined as 4 weeks post‐training minus baseline divided by baseline for each finger. We found a significant correlation for the trained finger (left ring finger) with the corresponding contralateral right ring finger (Pearson: r = 0.69, P < 0.005), the contralateral right middle finger (Pearson: r = 0.71, P < 0.005), and the ipsilateral left index finger (Pearson: r = 0.61, P < 0.05). No other correlations between the fingers were found.
Somesthesia
Transfer assessments of tactile frequency discrimination
An ANOVA with the repeated factors SIDE (2), DIGIT (4), ASSESSMENT (5) yielded a significant two‐way interaction of DIGIT * ASSESSMENT (quadratic trend: F(1, 15) = 7.69, P = 0.01), but no main effect or interaction involving side. For all four digits (pooled across side), a significant improvement was observed from baseline to the second post‐assessment, but the learning curves were steeper for digits 5 and 3 (main effects ASSESSMENT: quadratic trends: both Fs(1, 15) ≥ 148.11, P < 0.001) than for digits 4 and 2 (quadratic trends: both Fs(1, 15) ≥ 32.17, P ≤ 0.001). For all four digits, tactile acuity was significantly better after the 11th training session as compared to the baseline assessment (all ts(15) ≥ 6.31, P < 0.001), but the subsequent assessments were not significantly different from each other for any of the digits.
Furthermore, analyzing the main effects of DIGIT at each level of assessment showed significant differences between digits only for the baseline and two post‐training assessments (all Fs(3,45) ≥ 4.64, Ps ≤ 0.01). During the baseline and the two post‐training assessments, tactile acuity was significantly better for digits 2 and 3 as compared to digit 5 (all ts(15) ≥ 2.77, Ps < 0.001). In addition, during the second post‐training assessment (after 3 retraining sessions), digit 4 had a lower discrimination threshold than digit 5 (t (15) = 3.79, P = 0.002).
As shown in Figure 2, training effects were also reflected in somesthesia measurements before training, after the 11th and 22nd training sessions and after cessation of training (retrainings 1 and 2).
Figure 2.
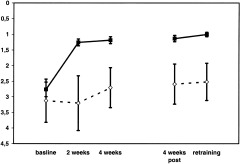
Training effect of left ring finger in the trained subjects vs. control subjects who did not receive tactile training. The No‐training‐group (dashed lines) did not improve significantly in tactile frequency discrimination. The training‐group (solid lines) improved significantly over the course of the training. Note the difference in standard errors, which are smaller in the Training‐ as compared to the No‐training‐group.
For the No‐training group, none of the interactions or main effects became significant and no effects of repeated testing were seen.
Performances on the tactile frequency discrimination training (baseline‐measurement, 11th, 22nd, 1st, and 2nd retraining sessions) and the respective somesthesia measurements (1 to 5), yielded a significant correlation for trainings 11 and 22 and retraining 1.
Magnetoencephalography
Dipole moment Q of the left and right ring finger for control subjects
Repeated measures ANOVA with the repeated factors SIDE (2) and assessment (baseline 1–3, 11th training day, 22nd training day) yielded no significant main effects or interactions. The dipolemoment Q of the left and right ring finger for the control group remained stable over all five time points during the study.
Repeated assessment of the dipolemoment Q from the left and right ring finger in the control group provided stable results over all 5 time points during the study (see Fig. 3).
Figure 3.
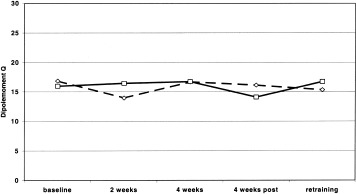
MEG assessment of dipolemoment Q of the left (dashed line) and right (solid line) ring finger in control group without training. Note that there is no habituation of Q over time.
Euclidean distance between D2 and D5
Repeated measures ANOVA with the factors SIDE (2) and ASSESSMENT (baseline 1–3, 2 weeks, 4 weeks, 4 weeks post, retraining) revealed a significant main effect for SIDE (linear Trend: F(1) = 7.579, P = 0.019). Post‐hoc analysis revealed a significantly smaller Euclidean Distance between the index and little finger for the left side (t (14) = 2.436, P = 0.029) (Fig. 4). We found no significant main effect for ASSESSMENT nor an interaction of SIDE * ASSESSMENT.
Figure 4.
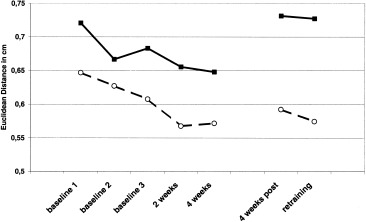
Changes in Euclidean distance between the index and little finger. The solid line shows the Euclidean distance between the index and little finger of the right hand; the dashed line shows the Euclidean distance between the index and little finger of the left hand.
Training‐related changes in dipole moment Q
Repeated measures ANOVA with the repeated factors SIDE (2), DIGIT (4), and ASSESSMENT (baseline, 11th training day, 22nd training day) yielded a significant interaction of all three factors (linear trend: F(1, 13) = 5.46, P = 0.04). To explain the 3‐way interaction, two‐way interactions of DIGIT * ASSESSMENT were examined separately for the left and the right hand. Neither the main effects of ASSESSMENT nor the interaction of ASSESSMENT * DIGIT were significant for either hand. There was, however a significant main effect of DIGIT. In a further step, two‐way interactions of SIDE * DIGIT were examined separately for each of the five assessments. Again, we found a significant main effect of DIGIT. In a third step, two‐way interactions of SIDE * ASSESSMENT were examined for each of the digits separately. A significant main effect of side for digit 5 (F(1, 13) = 5.74, P = 0.03) was found. Post hoc analysis showed that this effect was due to a significantly greater dipole moment Q of the left little finger as compared to the right one (t(13) = 2.53, P = .03).
Figure 5a,b summarizes the main results of this study. An improvement of tactile frequency detection can be seen for all fingers tested. Learning thus generalized to all fingers and the training‐induced threshold changes remained stable for 4 weeks following training. The dipole moment Q did not differ across the sessions. Upon visual inspection, it appeared as though there was an increase in dipole moment from baseline during the first 2 weeks of training. Subsequent t‐tests with Bonferroni adjusted P levels did not reveal any significant results. A plot showing the combined dipolemoments for all fingers of the left/right hand showed that this increase was due to the increased dipolemoment of one single subject in the second session. Thus, in sum, there was no significant correlation of dipole moment or localization with somesthetic performance increase.
Figure 5.
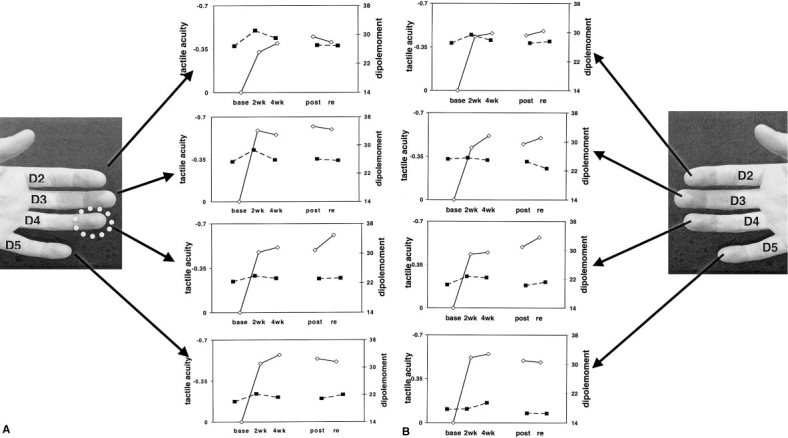
a: Left: The fingers of the left hand, which were assessed by tactile psychometry and MEG. The white circle marks the trained finger. Right: The mean changes in tactile acuity (solid lines) and cortical representation (dashed lines) over the course of the training. Note the steep improvement in frequency discrimination during the first 2 weeks of training, which is not mirrored by changes in dipole moment. Base = baseline assessment; 2wk = assessment after 2 weeks. b: Right: The fingers of the right hand, which were assessed by tactile psychometry and MEG. Left: The mean changes in tactile acuity (solid solid lines) and cortical representation (dashed lines) over the course of the training. Note the steep improvement in frequency discrimination during the first 2 weeks of training, which is not mirrored by changes in the dipolemoment. Base = baseline assessment; 2wk= assessment after 2 weeks of training, etc.
DISCUSSION
Three major findings were obtained in the present study: (1) Intensive training of tactile frequency discrimination in adult humans led to a persistent behavioral improvement. (2) Improvements in tactile acuity generalized to the digit homologue of the trained one, to neighboring digits (d3 left and d5 left) and, to a lesser extent, to the other digits of both hands. (3) No increase in cortical representation of the trained digit was detected.
Improvement in performance
Recanzone and coworkers [1992a, 1992b, 1992c, 1992d, 1992e] described results from intracortical microelectrode recordings in owl monkeys who had been trained in a tactile frequency discrimination task involving the comparison of a flutter‐frequency stimulus with a standard 20‐Hz stimulus similar to the one used in our study. They found a progressive improvement in discriminative abilities consistent with the concept of a strengthening of afferent excitatory synapses [Recanzone et al., 1992a]. The final tactile discrimination performance in our subjects was better than reported in a number of other human studies. It was also considerably better and more consistent across our subjects than reported by Recanzone et al. for owl monkeys (2–3‐Hz difference after training in monkeys vs. 1‐Hz difference after training in humans) [Goff, 1967; Mountcastle et al., 1990; Recanzone et al., 1992b]. The improvement in performance in our subjects was steep during the first 2 weeks. During the last 2 weeks of training, there was only a minor or no further improvement. However, this level of performance was maintained for another 4 weeks without training (Fig. 4a,b). These findings suggest a persistent alteration of neural networks, which allows retuning with very little repeated practice.
Generalization of improved performance
Improvement in frequency discrimination was not confined to the trained left ring finger. Similar observations have been made in animal and human experiments [Harris et al., 2001b; Nagarajan et al., 1998; Recanzone et al., 1992b]. Recanzone and coworkers observed some spread of learning to adjacent fingers in owl monkeys. However, thresholds of these digits were always higher than the ones of the trained digit [Recanzone et al., 1992b]. Nagarajan and colleagues [1998] performed a study involving somatosensory interval discrimination in humans and observed a generalization of the improvement not only to other digits but also to the auditory modality. In addition, generalization in humans may not be confined to purely temporal tasks. During training on grating discrimination in human subjects, a highly task‐specific widespread spatial generalization has been found [Sathian and Zangaladze, 1997]. Generalization in our study extended to all other digits tested (all but the thumbs). However, the generalization followed a specific pattern. The degree of improvement in the frequency discrimination ability of the trained left ring finger correlated with the improvement in its homologue finger, its neighboring fingers (left little and middle finger), and the right ring finger. This pattern of generalization resembles results from a recent study of tactile whisker learning in rats [Harris and Diamond, 2000]. Harris and Diamond [2000] found partial transfer of learning to neighboring whisker positions and to whisker positions on the other side of the snout, but only if the prosthetic whiskers were symmetrically opposite to the trained whiskers. They proposed that the underlying neuronal changes are distributed according to the topographic organization of the sensory cortical map. Furthermore, the transhemispheric spread of training effects in the study by Harris and Diamond [2000] and in our study, suggests that these changes involve bilateral cortical regions or regions receiving bilateral input.
No detectable increase in cortical representation
Recanzone et al. [1992e] reported that the training of temporal features of tactile stimuli alters the distributed spatial response properties of cortical neurons in the primary somatosensory cortex of owl monkeys. While these experiments showed increased cortical representations in the range of a few square millimeters, subsequent work on the effects of long‐term deafferentation demonstrated greater changes of cortical maps in adult monkeys [Pons et al., 1991]. Contrary to our study, Recanzone et al. [1992b, 1992c, 1992d, 1992e] used microelectrode measurements and this may explain the different results. Using magnetoencephalography before and after surgical separation of webbed fingers, Mogilner and coworkers, like others, demonstrated reorganization of the somatosensory cortex [Elbert et al., 1995; Flor et al., 1995; Knecht et al., 1996b, 1995; Mogilner et al., 1993; Sterr et al., 1998; Taub et al., 1995].
Recently, Pleger et al. [2001] reported SEP source changes consistent with short‐lived improvement of spatial tactile acuity. However, a study by Spengler et al. [1997], attempting to replicate the effects of transitory somatosensory training in owl monkey by using MEG in humans, obtained a paradoxical result. Instead of an increase in the evoked current dipoles strength, somatosensory training was associated with a decrease, indicating a decreased cortical finger representation. Unlike Spengler and coworkers, we found no paradoxical change in cortical finger representation.
The dipole strength of all four fingers of both hands remained stable across all training sessions and for another 4 weeks after training. In general, the MEG used by Spengler and colleagues [1997] as well as ours has a different sensitivity than intracortical recordings used in animal studies. It is estimated that a coherent neural activity extending over a cortical area of 40–400 mm2 is required for somatosensory‐evoked fields to be recorded from outside the scalp [Lu and Williamson, 1991]. It is unknown how many additional neurons need to be activated or how much stronger the coherence of activated neurons needs to be for increases in dipole strength to be detected by MEG.
We could not use the same stimuli used for the frequency discrimination training during MEG‐recordings because of electromagnetic interference. We used a torque motor for tactile training, and a pneumatically driven membrane for MEG. For technical reasons, only frequencies of 2 Hz were reached during the MEG measurements. However, we do not believe that this difference can explain a lack of representational increase. Elbert et al. [1995] have shown that an increase in finger representation can be detected in string players using pneumatic stimulation, although their expertise is in a different mode, i.e., string playing. In the present experiment, the magnetometer and mode of analysis were the same as the ones used by Elbert and colleagues [1995] and in a number of previous studies in which reorganization of the somatosensory cortex has been shown [Elbert et al., 1994, 1995; Flor et al., 1995; Knecht et al., 1995, 1996b]. Moreover, current dipole strengths were highly consistent across measurements indicating a high quality of recording. We believe that any training‐related changes of representation in the S1 cortex were considerably less pronounced than the one reported in string players [Elbert et al., 1995].
The age at the beginning of training as well as the amount of training may be among the reasons why an increase in cortical representation has been found in string players but not in our cohort. Such an increase was found in musicians who had started playing a string instrument before the age of 12, but less so in those who started later [Elbert et al., 1995]. All of our subjects were adults who were skilled users of their fingers and regularly engaged in tactile exploration. In adult humans, baseline performance on the frequency discrimination task is much better than in monkeys. Owl monkeys approached a compatible level of performance only after several weeks of intense training. Therefore, tactile performance in monkeys, and possibly also in children, has a wider margin for improvement. Additionally, the malleability of the primary somatosensory cortex in monkeys, and in children, may be greater than in human adults. One could speculate that such a greater malleability may also be associated with a greater generalization of training effects. This way, training in one submodality like high frequency oscillations in string playing would then also affect neural processing in another submodality like low‐frequency pneumatic stimulation during MEG.
The subjects in our study did improve with training of tactile frequency discrimination. This improvement could be related to reorganization outside the S1 cortex. Several subfunctions are involved in tactile frequency discrimination. The frequency discrimination is subserved by sensorimotor processing, by cued focusing of attention to the trained finger, by keeping an engram of the base frequency in store for comparison, and finally by activation of extended cortical areas allowing conscious stimulus perception [Harris and Diamond, 2001a; Knecht et al., 1996a; Macmillan and Creelman, 1991; Sörös et al., 2001].
The coexistence of topographically specific and unspecific learning effects indicate an involvement of brain regions, which are organized in a somatotopic manner as well as regions that are organized in a nonsomatotopic way. The specificity of the retraining effect and the somatotopy of generalization suggest that the somatotopically organized primary somatosensory cortex plays a role despite the lack of marked changes in cortical representation size. The transfer of learning to homologue sites would be compatible with an involvement of association cortices. An involvement of topographically specific and unspecific mechanisms is compatible with recent findings by Harris et al. [2001b], who reported evidence for a loose topographical framework of tactile working memory [Harris et al., 2001b]. However, recent data from Forss et al. [2001] suggest that neurons in S2 are poor at accurate timing and would, therefore, not be ideal candidates for learning of tactile frequency discrimination. We could not evaluate evoked responses in S2 because the measuring area of our magnetometer was focused on S1.
Recently the role of attentive modulation of primary and secondary somatosensory cortices has been stressed [Johansen‐Berg et al., 2000; Steinmetz et al., 2000]. Control of attentional shifts is known to be exerted by a lateralized parietal neural network (mostly of the right hemisphere) [Bäcker et al., 1999; Fink et al., 1997; Knecht et al., 1997]. The generalization of training effects indicates an involvement of these lateralized, attention‐mediating parietal regions.
The increased temporal tactile acuity in our study was a stable phenomenon even after termination of training. This suggests a persistent refinement of connections within existing neural assemblies involved in somatosensory processing. It seems reasonable to suspect that this refinement was achieved by an increase in synaptic transmission efficacy. So far, analyses of sensory maps may have helped to discover only the tip of the iceberg of learning‐induced sensory plasticity. Intracortical mapping of primary cortex in animals provides a model for functional neural reorganization. However, the results of the present study stress that the primary somatosensory cortex is not the only and probably not the major site of neural reorganization. Other areas and other integrated mechanisms need to be considered in future neuro‐imaging studies on learning related reorganization of the adult human brain.
Acknowledgements
We thank Erin Gibson for the helpful comments on the manuscript.
REFERENCES
- Bäcker M, et al (1999): Cortical tuning: a function of anticipated stimulus intensity. Neuroreport 5: 293–296. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, et al (1997): Effects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganization. J Neurosci 17: 5503–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner H, et al (2000): Differential effects of pain and spatial attention on digit representation in the human primary somatosensory cortex. Neuroreport 11: 1289–1293. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R (1990): Interhemispheric transfer of plasticity in the cerebral cortex. Science 249: 805–806. [DOI] [PubMed] [Google Scholar]
- Darian‐Smith C, Darian‐Smith I (1993): Thalamic projections to areas 3a, 3b, and 4 in the sensorimotor cortex of the mature and infant macaque monkey. J Comp Neurol 335: 173–199. [DOI] [PubMed] [Google Scholar]
- Elbert T, et al (1994): Extensive reorganization of the somatosensory cortex in adult humans after nervous system injury. Neuroreport 5: 2593–2597. [DOI] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E (1995): Increased cortical representation of fingers of the left hand in string players. Science 270: 305–307. [DOI] [PubMed] [Google Scholar]
- Fink GR, et al (1997): Neural mechanisms involved in the processing of global and local aspects of hierarchically organized visual stimuli. Brain 120: 1779–1791. [DOI] [PubMed] [Google Scholar]
- Flor H, et al (1995): Phantom limb pain as a perceptual correlate of massive cortical reorganization in upper extremity amputees. Nature 375: 482–484. [DOI] [PubMed] [Google Scholar]
- Forss N, Narici L, Hari R (2001): Sustained activation of the human SII cortices by stimulus trains. Neuroimage 13: 497–501. [DOI] [PubMed] [Google Scholar]
- Goff GD (1967): Differential discrimination of frequency of cutaneous mechanical vibration. J Exp Psychol 74: 294–299. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA (1966): Signal detection theory and psychophysics. New York: Wiley. [Google Scholar]
- Harris JA, Diamond ME (2000): Ipsilateral and contralateral transfer of tactile learning. Neuroreport 11: 263–266. [DOI] [PubMed] [Google Scholar]
- Harris JA, Harris IM, Diamond ME (2001a): The topography of tactile learning in humans. J Neurosci 21: 1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Harris IM, Diamond ME (2001b): The topography of tactile working memory. J Neurosci 21: 8262–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic‐Robles E (1990): Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol 63: 82–104. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H, Christensen V, Woolrich M, Matthews PM (2000): Attention to touch modulates activity in both primary and secondary somatosensory areas. Neuroreport 11: 1237–1241. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Nelson RJ, Sur M, Lin CS, Merzenich MM (1979): Multiple representations of the body within the primary somatosensory cortex of primates. Science 204: 521–523. [DOI] [PubMed] [Google Scholar]
- Keselman HJ, Rogan JC (1980): Repeated measures F tests and psychophysiological research: controlling the number of false positives. Psychophysiology 17: 499–503. [DOI] [PubMed] [Google Scholar]
- Knecht S,et al (1995): Cortical reorganization in human amputees and mislocalization of painful stimuli to the phantom limb. Neurosci Lett 201: 262–264. [DOI] [PubMed] [Google Scholar]
- Knecht S, et al (1996a): Persistent unihemispheric perceptual impairments in humans following focal seizures. Neurosci Lett 217: 66–68. [DOI] [PubMed] [Google Scholar]
- Knecht S, et al (1996b): Reorganization and perceptual changes after amputation. Brain 1213–1219. [DOI] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Bäcker M, Ringelstein E‐B, Henningsen H (1997): Regional cerebral blood flow increases during preparation for and processing of sensory stimuli. Exp Brain Res 116: 309–314. [DOI] [PubMed] [Google Scholar]
- Knecht S, et al (1998): Plasticity of plasticity? Changes in the pattern of perceptual correlates or reorganization after amputation. Brain 121: 717–724. [DOI] [PubMed] [Google Scholar]
- Knecht S, et al (2001): D‐amphetamine does not improve outcome of somatosensory training. Neurology 57: 2248–2252. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Williamson SJ (1991): Spatial extent of coherent sensory‐evoked cortical activity. Exp Brain Res 84: 411–416. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD (1991): Detection theory: a user's guide. Cambridge: Cambridge University Press. [Google Scholar]
- Merzenich MM, et al (1983): Topographic reorganization of somato‐sensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience 8: 33–35. [DOI] [PubMed] [Google Scholar]
- Mogilner A, et al (1993): Somatosensory cortical plasticity in adult humans revealed by magnetoencephalography. Proc Natl Acad Sci U S A 90: 3593–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB, Steinmetz MA, Romo R (1990): Frequency discrimination in the sense of flutter: psychophysical measurements correlated with postcentral events in behaving monkeys. J Neurosci 10: 3032–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan SS, Blake DT, Wright BA, Byl N, Merzenich MM (1998): Practice‐related improvements in somatosensory interval discrimination are temporally specific but generalize across skin location, hemisphere, and modality. J Neurosci 18: 1559–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Pleger B, et al (2001): Shifts in cortical representations predict human discrimination improvement. Proc Natl Acad Sci U S A 98: 12255–12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons TP, et al (1991): Massive cortical reorganization after sensory deafferentation in adult macaques. Science 252: 1857–1860. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Jenkins WM, Hradek GT, Merzenich MM (1992a): Progressive improvement in discriminative abilities in adult owl monkeys performing a tactile frequency discrimination task. J Neurophysiol 67: 1015–1030. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Dinse HR (1992b): Expansion of the cortical representation of a specific skin field in primary somatosensory cortex by intracortical microstimulation. Cereb Cortex 2: 181–196. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM (1992c): Frequency discrimination training engaging a restricted skin surface results in an emergence of a cutaneous response zone in cortical area 3a. J Neurophysiol 67: 1057–1070. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski HA, Dinse HA (1992d): Topographic reorganization of the hand represen tation in cortical area 3b of owl monkeys trained in a frequency‐discrimination task. J Neurophysiol 67: 1031–1056. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Schreiner, CE (1992e): Changes in the distributed temporal response properties of S1 cortical neurons reflect improvement in performance on a temporally based tactile discrimination task. J Neurophysiol 67: 1071–1091. [DOI] [PubMed] [Google Scholar]
- Romo R, Hernandez A, Zainos A, Salinas E (1998): Somatosensory discrimination based on cortical microstimulation. Nature 392: 387–390. [DOI] [PubMed] [Google Scholar]
- Romo R, et al (2002): Neural codes for perception. Rev Neurol 34: 364–371. [PubMed] [Google Scholar]
- Sathian K, Zangaladze A (1997): Tactile learning is task specific but transfers between fingers. Percept Psychophys 59: 119–128. [DOI] [PubMed] [Google Scholar]
- Sörös P, et al (2001): Functional reorganization of the human primary somatosensory cortex after acute pain demonstrated by magnetoencephalography. Neurosci Lett 298: 195–198. [DOI] [PubMed] [Google Scholar]
- Spengler F, et al (1997): Learning transfer and neuronal plasticity in humans trained in tactile discrimination. Neurosci Lett 232: 151–154. [DOI] [PubMed] [Google Scholar]
- Steinmetz PN, et al (2000): Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature 404: 187–190. [DOI] [PubMed] [Google Scholar]
- Sterr A, et al (1998): Changed perceptions in Braille readers [letter]. Nature 391: 134–135. [DOI] [PubMed] [Google Scholar]
- Taub E, Flor H, Knecht S, Elbert T (1995): The strong correlation between phantom limb pain and cortical reorganization: Causal considerations and general implications. J NIH Res 7: 49–50. [Google Scholar]


