Abstract
Despite its critical sociobiological importance, the brain processing of visual sexual stimuli has not been characterized precisely in human beings. We used Positron Emission Tomography (PET) to investigate responses of regional cerebral blood flow (rCBF) in nine healthy males presented with visual sexual stimuli of graded intensity. Statistical Parametric Mapping was used to locate brain regions whose activation was associated with the presentation of the sexual stimuli and was correlated with markers of sexual arousal. The claustrum, a region whose function had been unclear, displayed one of the highest activations. Additionally, activations were recorded in paralimbic areas (anterior cingulate gyrus, orbitofrontal cortex), in the striatum (head of caudate nucleus, putamen), and in the posterior hypothalamus. By contrast, decreased rCBF was observed in several temporal areas. Based on these results, we propose a model of the brain processes mediating the cognitive, emotional, motivational, and autonomic components of human male sexual arousal. Hum. Brain Mapping 11:162–177, 2000. © 2000 Wiley‐Liss, Inc.
Keywords: sex behavior; tomography, emission‐computed; erotica; motivation; emotions; basal ganglia; gyrus cinguli; prefrontal cortex; limbic system
INTRODUCTION
According to ethological studies of animal behavior, the main goal‐directed behaviors are searching for food, escaping predators, finding an ecological niche, and finding a sexual mate [Alcock, 1997]. Sexual behavior is a critical function for the survival of species. Copulatory behavior is the culminating point of a process that comprises several antecedent stages. At least in males, mating requires that the organism reaches a particular state of readiness, termed “sexual arousal” (SA), characterized by a series of adaptive physiological and behavioral changes. SA, in turn, may be initiated by external stimuli or may develop as a result of endogenous factors. The concept of sexual arousability, defined as the individual's propensity for sexual arousal given a source of external stimulation [Whalen, 1966], integrates the roles of external stimuli and endogenous factors.
According to recent sociobiological developments (reviewed in Rolls, 1999), the pressure of natural selection has led to the emergence of brain systems in which reward is associated with those sexual behaviors that increase fitness. Fitness is understood here as the aptitude to transmit one's genes to the next generation. Evolutionary pressure has thus selected the neurophysiological implementation of brain reward systems associated not only with copulation per se but also with SA and with sexual attraction to characteristics indicating a potential partner's fitness.
The central nervous system (CNS) plays a role at all successive stages of sexual behavior [Meisel and Sachs, 1994]. Regarding the stage of processing of external stimuli, it has been shown in various mammals, in particular in monkeys, that hormonally determined characteristics of females, such as odor [Baum et al., 1977] and visual signals [Bielert, 1982] promote sexual behavior in males. These characteristics are evaluated through the central processing of sensory information. In humans also, sexual attraction is based on various factors [Buss, 1989] and the significance of these external stimuli as sexual incentives has to be assessed for the sexual response to develop. Although it has been hypothesized that the brain is involved in the assessment of such factors and of their potential reward value [Rolls, 1999], these brain mechanisms have not been systematically studied.
The cognitive aspects involved in further stages of SA have been increasingly recognized in the literature. It has been proposed that human SA cannot be defined adequately without highlighting the critical role of cognitive labeling and subjective experience in determining the response to a given stimulus as sexual [Rosen and Beck, 1988b]. Likewise, the concept of central arousal [Bancroft, 1989] refers to CNS activation and attentional factors that underlie the psychological processing of sexual stimuli.
Finally, at least in humans, SA is also characterized by emotional responses and by motivational processes. The conscious perception of sexual desire belongs to the latter processes. In this sense, the study of the cerebral correlates of SA is a specific aspect of the broader field of research on the neural correlates of emotion and motivation [Davidson et al., 1999].
Knowledge on the brain regions involved in human sexual behavior is largely dependent on animal data [Bancroft, 1989; Herbert, 1996]. Numerous animal studies have considerably clarified the role played by subcortical structures in sexual behavior (septal nuclei, amygdala, and hypothalamic nuclei) [Meisel and Sachs, 1994]. However, human sexual behavior has species‐specific characteristics such as sexual imagery so that knowledge from animal research is not sufficient to understand these aspects of human sexuality. Therefore, studies on human subjects are needed to characterize the regions of the brain involved in these unique aspects of human sexual behavior.
In spite of the limited amount of research in this domain, some relevant data have been provided by different types of studies. First, electroencephalographic studies of healthy right‐handed volunteers presented with visual sexual stimuli demonstrated a pattern of right temporal activation and an increased right‐to‐left temporal activity asymmetry [Cohen et al., 1985]. Second, in partial epileptic seizures with bilateral genital sensations accompanied by fear or pleasure, the discharge was located in temporal regions and in limbic structures (amygdala, hippocampus, pararhinal region). Another type of seizures, where signs are essentially motor (e.g., patient grasping his genitals), has been related to a discharge arising in the anterior part of the cingulate cortex [Landré et al., 1993]. Third, studies of brain lesions have provided relevant findings. Neurosurgery has demonstrated the involvement of temporal and frontal lobes in the inhibitory control of human sexual behavior [Freeman, 1973; Terzian and Dalle Ore, 1955]. Moreover, in the sixties and seventies, neurosurgical unilateral lesions of hypothalamic nuclei were performed in West Germany in male volunteers with the purpose of suppressing deviant sexual behaviors. After these operations, several men showed a decreased sexual desire. However, the results of these studies are difficult to interpret, given the purely psychological impact of the neurosurgical procedure [Dieckmann et al., 1988]. Furthermore, in animals, unilateral lesions have been ineffective. The involvement of septal nuclei has also been implicated in the control of SA in human males [Gorman and Cummings, 1992]. Fourth, one Single Photon Emission Computed Tomography study, focusing on orgasm in right‐handed men, found an increased rCBF limited to the right prefrontal cortex [Tiihonen et al., 1994]. Fifth, a preliminary PET study [Stoléru et al., 1999] described a pattern of increased rCBF in temporooccipital regions, paralimbic areas and right caudate nucleus upon visual sexual stimulation. This preliminary study allowed us to develop and validate an experimental paradigm to study SA through PET. We demonstrated that it was possible to induce SA in human males in the experimental context of a PET study and then to identify regions controlling this emotional state in the intact human brain. Furthermore, this study allowed us to propose a neurobehavioral model of SA, comprising a perceptual‐cognitive, an emotional, a motivational, and a physiological component. Sixth, a recent fMRI study reported that SA was associated with a significant activation of the inferior temporal gyrus, the inferior frontal gyrus, and the pulvinar [Beauregard et al., 1998]. Finally, Rauch et al. [1999] used PET in a paradigm where subjects had to recall and imagine an event associated either with SA or with “competitive arousal.” They reported that the SA condition was characterized by a selective increase of rCBF in the brain stem and in the left claustrum.
Our preliminary study left several questions unanswered: (i) only young subjects (23–25 years old) selected on the basis of their high sexual arousability were included; (ii) the field of view of the scanner did not cover the whole brain; (iii) the observed temporooccipital activations might have been related to moving visual stimuli presented (films); (iv) in this mainly subtractive approach, only one level of sexual stimulation was contrasted with a neutral condition; therefore, the within‐subjects covariation between rCBF and increasing levels of SA elicited by different stimuli could not be investigated. However, in some regions, an across‐subjects correlation between rCBF and testosterone plasma level could be demonstrated in the stimulated condition; and (v) finally, as this study was mainly exploratory, its findings needed a confirmatory study.
Our objectives in the present study were therefore to use findings of the first study as working hypotheses to be tested; to increase the generalizability of the first study by including subjects with a larger range of age and sexual arousability; to map the neuroanatomical correlates of SA in the entire brain by using a whole‐brain PET scanner; to better interpret the temporooccipital activation by using still photographs as visual stimuli; and to identify regions where rCBF was linearly correlated with the magnitude of various sexual responses induced by sexual stimuli of graded intensity.
SUBJECTS AND METHODS
Subjects
Nine healthy right‐handed heterosexual men (mean age = 30.7; range = 21–39) were included in the study. The absence of physical disorders and of any pharmacological treatment was checked through a medical examination. The absence of psychopathological conditions was ascertained through a psychiatric interview and the administration of SCL‐90R [Derogatis, 1977]. Heterosexual orientation was confirmed using the ACSF questionnaire [Spira et al., 1993] and the Brief Sexual Function Questionnaire (BSFQ) [Reynolds et al., 1988]. The BSFQ was also used to screen out sexual dysfunctions. Candidates were not selected on the basis of a particularly high arousability in response to sexually explicit films. Information of subjects and protection of their intimacy were ensured following published guidelines [Rosen and Beck, 1988a]. The study was approved by the local ethics committee and written informed consent was obtained from all subjects.
Stimuli
The six experimental conditions were defined by the type of visual stimuli (video clips of different categories): (i) two emotionally neutral documentary films (N condition); (ii) two humorous films, inducing a pleasant but nonsexual emotion (H condition); video clips used in conditions (iii) to (v) were series of photographic sexual stimuli of graded intensity, i.e., two series of 18 nonsexually arousing (A), moderately arousing (B), and highly arousing (C) photographs representing women; and (vi) two sexually explicit films depicting heterosexual coitus (S). In an attempt to control for the perception of moving and interacting persons appearing in S films, N and H films also displayed moving and interacting persons. Similarly, in all categories of photographs, only one woman was displayed. Each clip was silent and lasted three minutes. Subjects were requested to watch the stimuli with no additional task. PET scanning was performed twice in each of these conditions.
The six types of visual stimuli mentioned above were selected out of a large series of clips by raters not otherwise involved in the experiment. These raters met the criteria used to include the experimental subjects in the study. To allow raters to assess the intensity of SA, humor, or of any other emotion generated by each stimulus of this series, they were provided with rating scales ranging from 1 to 9 and validated in the previous study [Stoléru et al., 1999]. The same scales were also used later with the experimental subjects of the present study to assess their subjective responses to the stimuli. Photographs were used in addition to films both to control for the cerebral processing of moving visual targets and to obtain a gradation in induced SA. The two sexually explicit films were rated as inducing marked SA but no negative emotion, such as disgust.
Procedure
Subjects lay on the camera bed, surrounded by a curtain isolating them from staff's view; head position was maintained by an individually molded face mask. Injection of radiolabeled water was timed so that PET data acquisition started 60 sec after the beginning of visual stimulation. PET scans were separated by 15‐min intervals. After the presentation of each of the stimuli, in order to allow subjects to regain a neutral emotional state, they were then presented with a 4‐min emotionally neutral clip, followed by a 3‐min rest period.
It has been shown in PET studies of obsessive‐compulsive disorders [Cottraux et al., 1996] that activations associated with the obsessive state were likely to be persistent and to bias data collected in control conditions. Therefore, in other studies on obsessive‐compulsive disorders, only the so‐called “off‐on” order has been used [Breiter et al., 1996; Rauch et al., 1994]. It is noteworthy that in these studies several of the stimuli used to induce the obsessive state were of a sexual nature. On these grounds, we reasoned that brain activations induced by sexual stimuli (B, C, and S) might be prolonged and might bias data collected in subsequent nonsexual conditions. If the order of presentation of stimuli had been counterbalanced and such a carry‐over effect had been demonstrated, only the data collected in the first conditions could have been compared, imposing across‐subjects comparisons and precluding within‐subjects analyzes [Hills and Armitage, 1979]. Therefore, we used the N‐H‐A‐B‐C‐S sequence for all subjects. Subjects were not informed of the order when the sessions started.
Measures
Positron Emission Tomography
PET measurements of rCBF were performed through a Siemens HR+ scanner (Knoxville, TN) generating 63 2.33 mm‐thick contiguous transaxial slices of the entire brain in tridimensional mode. Scans were performed over 60 sec after intravenous injection of 333 MBq of H2 15O. Attenuation‐corrected data were reconstructed into 63 image planes using a Hamming filter (cutoff frequency, 0.5 cycles per pixel) providing intrinsic axial resolution of 4.1 mm and transverse resolution of 4.4 mm at the center of the field of view.
Rating scales
After each scan, Likert‐type rating scales, ranging from 1 (extremely low) to 9 (extremely high), were presented to subjects to assess perceived sexual arousal (PSA) and humor. For instance, the question pertaining to SA was “During the presentation of the film [photographs], how great was the intensity of sexual arousal that you experienced?” These scales were validated in a previous study [Stoléru et al., 1999].
Penile plethysmography
While PET data were acquired, penile tumescence was measured by plethysmography (Parks Medical Electronics, Model 240A, Aloha, OR). Penile responses were recorded using the S.O.E.T. software (Sex Offender Evaluation Test, Parks Medical Electronics) providing a graph of the increase of penile circumference. As recommended in the literature [Chaplin et al., 1995], we converted the measure of tumescence (area under the curve) into z scores so that each subject's response had a mean of 0 and a standard deviation of 1.
Other hormonal and physiological measures
While PET data were acquired, heart rate and blood pressure were monitored using the Finapres® BP monitor (Ohmeda, BOC Inc., Englewood, CO). For each subject, the means of cardiovascular parameters were computed over each condition.
After each scan, 5 ml blood was drawn for later plasma testosterone radioimmunoassay [Forest et al., 1973].
Data analysis
The effect of experimental conditions on the measures of SA was tested with a repeated measures analysis of variance.
Image processing [Friston et al., 1995a] and statistical analysis [Friston et al., 1995b] were performed using Statistical Parametric Mapping (SPM96 software; Wellcome Department of Cognitive Neurology, London, UK). Individual data were realigned to correct for interscan head movements. All images were then normalized and transformed into the Montreal Neurological Institute standard stereotactic space that is provided in SPM96. Finally, data were smoothed with a 14.0 mm FWHM isotropic Gaussian kernel. The rCBF measurements were adjusted to a global mean of 50 ml dl−1 min−1.
In a first analytical approach, the effect of the Condition factor on rCBF was assessed with contrasts between the various conditions using the t‐statistic subsequently transformed into the Z statistic. To identify regions activated in the S condition, we searched for the regions where rCBF was significantly higher in the S condition than in both the N and H conditions and where the H‐N contrast showed no significant difference. The converse procedure was used to identify deactivated regions (rCBF lower in S than in both N and H, and no significant difference shown by the N‐H contrast). In addition, we contrasted conditions C and A on the one hand, and conditions B and A on the other. Conjunction analysis [Price and Friston, 1997] was then performed in order to identify brain activations that were both characteristically related to sexual arousal (i.e., S vs. N and S vs. H) and modality‐independent (i.e., films vs. photographs). Accordingly, we used conjunction analysis to identify those regions activated simultaneously in contrasts S‐N, S‐H, and C‐A (or B‐A). A masking procedure was performed in order to ensure that all the regions found with the conjunction analysis were also activated in the individual contrasts implicated. Conjunction analysis was also performed to identify deactivated regions.
It might be argued that emotions induced in the S and the H conditions were different, not only qualitatively but also quantitatively (e. g., level of emotional arousal induced by the S condition higher than emotional arousal induced by the H condition). Therefore, we performed an S‐H and H‐S contrast analysis where we used as a confounding variable the rating of perceived humor for the H condition and the rating of PSA for the S condition.
In a second analytical approach, we looked for the brain regions where rCBF was linearly correlated with specific markers of SA, i.e., the rating of PSA and the magnitude of penile tumescence. In this analysis, the conditions were not included in the model, i.e., the effect of conditions was not taken into account. The measures of markers of SA were simply used as covariates of interest and at each voxel the correlation between rCBF and the measures of the markers was tested.
However, there was some redundancy between this correlational approach and the previous subtractive analysis due to the relation between experimental conditions and measures of SA. Therefore, the above correlational analysis was then performed with the markers used again as covariates of interest but with the Condition factor included in the model and set to zero, thus partialing out the effect of conditions on rCBF and markers. In this way, we could assess that part of the correlation between rCBF and markers independent from the conditions; there was room for such a partial correlation as, within each experimental condition, there were 18 measures of rCBF and 18 measures of each of the markers (two measures per subject, i.e., the two replications for each condition, and nine subjects).
Finally, given the fixed order of presentation of stimuli, a supplementary analysis was performed: in order to take into account a potential time effect, i.e., a systematic change of rCBF as a function of time elapsed since beginning of session, the number (rank) of each scan was used as a confounding variable in the correlational analyzes.
We report regions activated/deactivated above the threshold of P < .001 (Z > 3.09), uncorrected for multiple comparisons. However, because of the arbitrary character of this threshold, we systematically mention regions where activation was statistically significant after correction.
RESULTS
Measures of sexual arousal
Subjects' rating of perceived sexual arousal (PSA) varied across conditions (P < .001). Highest ratings were obtained for the sexually explicit films (S) (Fig. 1a). Systolic and diastolic blood pressure (P < .01) and penile tumescence (P < .001) showed the same pattern of variation as PSA (Table I), but heart rate did not (P > .05). Plasma testosterone varied across conditions (P < .05) and was higher (P < .05) in the S condition than in the neutral conditions (N and A).
Figure 1.
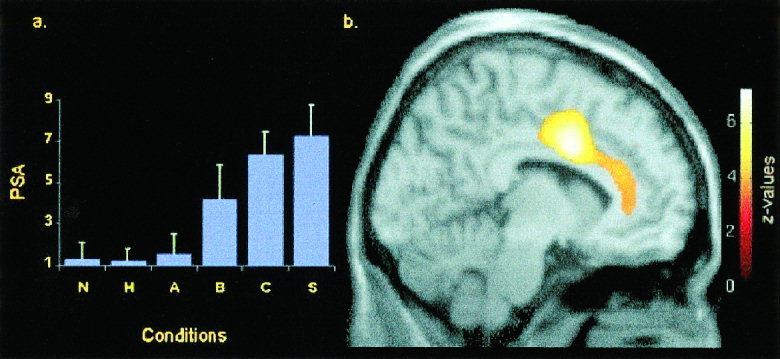
Perceived sexual arousal (PSA) and regional cerebral blood flow (rCBF) in left anterior cingulate gyrus. (a) Means and standard deviations of PSA in each condition. (b) Parasagittal section (4 mm left of midline) showing the positive correlation between rCBF in the left anterior cingulate gyrus (Brodmann area 24) and PSA. Height threshold: z = 3.71, P < 0. 0001, uncorrected. Anterior is to the right.
Table I.
Physiological and emotional responses to experimental conditions†
| Condition | PSA* | Perceived humor* | Tumescence* | Heart rate**** | Diastolic pressure** | Systolic pressure** | Plasma testosterone*** |
|---|---|---|---|---|---|---|---|
| N | 1.3 (0.8) | 1.7 (1.1) | −0.87 | 64.2 (8.9) | 7.9 (1.0) | 15.1 (2.1) | 442.3 (104.2) |
| H | 1.2 (0.5) | 6.2 (1.2) | −0.75 | 63.3 (10.8) | 7.9 (1.0) | 14.9 (2.4) | 455.4 (117.2) |
| A | 1.6 (0.9) | 1.0 (0.0) | −0.55 | 63.6 (9.9) | 8.1 (0.8) | 15.2 (1.6) | 435.2 (103.1) |
| B | 4.2 (1.6) | 1.0 (0.0) | −0.12 | 62.4 (9.5) | 8.1 (0.8) | 15.4 (1.5) | 455.8 (112.9) |
| C | 6.4 (1.0) | 1.0 (0.0) | 0.94 | 65.6 (7.6) | 8.7 (1.1) | 16.4 (1.9) | 474.2 (125.2) |
| S | 7.3 (1.5) | 2.0 (1.8) | 1.35 | 69.0 (15.4) | 9.2 (1.1) | 17.0 (1.6) | 497.3 (136.9) |
Values are means (standard deviations). PSA: perceived sexual arousal. Scales for PSA and perceived humor: 1 to 9. Tumescence: z‐scores of penile tumescence. Diastolic/Systolic pressure: cm Hg. Testosterone: ng/dl.
ANOVA across conditions:
P < .001;
P < .01;
P < .05;
P = N.S.
Positron emission tomography
The results of the contrast and the correlational analyzes of rCBF (see Methods) are presented in Tables II to V and in Figures 1 and 2.
Table II.
Cerebral regions activated in sexual conditions†
| Brain region | BA | Hem | S‐N contrast | S‐N/S‐H/C‐A conjunction | S‐N/S‐H/B‐A conjunction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coordinates | Z scores | Coordinates | Z scores | Coordinates | Z scores | |||||||||
| x | y | z | x | y | z | x | y | z | ||||||
| Limbic/paralimbic cortex | ||||||||||||||
| Ant. cingulate, caudal | 24/32 | L | −4 | 6 | 36 | 6.00** | −6 | 6 | 34 | 8.72** | −4 | 6 | 32 | 8.00** |
| Ant. cingulate, rostral | 24 | L | 0 | 36 | 10 | 3.89‡ | −4 | 34 | 16 | 6.33** | −2 | 36 | 8 | 6.18** |
| Midcingulate | 24 | L | −16 | −8 | 40 | 5.03** | −16 | −8 | 40 | 5.82‡ | −8 | −4 | 36 | 5.65** |
| Gfd/cingulate | 9/32 | R | 20 | 40 | 20 | 4.43 | 26 | 40 | 20 | 5.21** | ||||
| Orbitofrontal cortex | 47/11 | R | 22 | 32 | −16 | 4.04 | 20 | 32 | −16 | 6.30** | ||||
| Subcortical structures | ||||||||||||||
| Claustrum | L | −28 | 4 | 12 | 6.07** | |||||||||
| Claustrum | R | 32 | 10 | −4 | 4.92** | 32 | 10 | −2 | 6.76** | 30 | 12 | −4 | 6.32** | |
| Caudate nucleus (head) | R | 16 | 14 | 12 | 4.51* | 14 | 14 | 14 | 5.56**, ‡ | 14 | 16 | 16 | 5.61** | |
| Putamen | L | −30 | 6 | −2 | 6.05** | −30 | 6 | 2 | 7.68* | |||||
| Putamen | R | 30 | 6 | −6 | 4.24‡ | 30 | 6 | −6 | 5.96** | |||||
| Thalamus (MD) | R | 4 | −20 | 6 | 3.03‡ | 4 | −20 | 6 | 4.48*, ‡ | 4 | −20 | 6 | 4.23 | |
| Thalamus (VPM) | R | 14 | −20 | 2 | 4.47 | 14 | −22 | 2 | 5.27** | |||||
| Thalamus (Pulvinar) | L | −28 | −22 | 8 | 3.93 | −26 | −20 | 10 | 4.92** | |||||
| All other | ||||||||||||||
| Precentral gyrus | 6 | L | −68 | 0 | 24 | 3.94 | −66 | 6 | 14 | 5.07** | ||||
| Central sulcus | 3 | L | −16 | −26 | 72 | 3.24 | ||||||||
| Parietal operculum | 40 | L | −72 | −24 | 22 | 3.83 | −70 | −24 | 26 | 6.08** | ||||
| Parietal lobules | 7/40 | R | 36 | −56 | 70 | 2.69‡ | 36 | −56 | 70 | 3.87 | ||||
Coordinates are defined in the MNI stereotactic space (Montreal Neurological Institute, Canada) and refer to the anterior commissure. x represents the lateral distance from midline (positive = right); y is the anteroposterior distance from the anterior commissure (positive = anterior); z represents the height relative to the intercommissural plane (positive = above).
P < .01, corrected for multiple comparisons;
P < .05, corrected;
no maximum activation at this location, but z‐score higher than 2.33.
BA: Brodmann area. Hem: hemisphere; R: right; L: left; Ant: anterior; Gfd: medial frontal gyrus; MD: mediodorsal; VPM: ventro‐postero‐medial.
Figure 2.
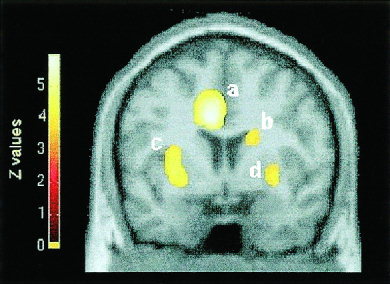
Coronal section demonstrating brain regions where rCBF was linearly correlated with levels of perceived sexual arousal. (a) Anterior cingulate gyrus; (b) head of caudate nucleus; (c) claustrum and (d) putamen. Section is located 4 mm rostral to anterior commissure. Height threshold: z = 4.40, P < 0. 00001, uncorrected. Right is to the right.
SPM contrast analysis
In the S‐N contrast, highly significant activations were found in the left anterior cingulate gyrus (ACG) in Brodmann area 24 (BA 24), with a caudal peak (just anterior to the vertical plane passing through the anterior commissure; y = 6) and a rostral peak just above the genu of the corpus callosum (y = 36). These two foci of activation were also found in the two conjunction analyses, which demonstrated activations induced both by films (S) and photographs (B and C). Other paralimbic areas were found activated in the left midcingulate, the right medial frontal gyrus, and right orbitofrontal cortex.
Activated subcortical structures were represented bilaterally by the claustrum, putamen, and thalami, and in the right hemisphere by the head of the caudate nucleus. Finally, other activations were found in cortical areas: left pre‐ and postcentral gyri, central sulcus, and right parietal lobules.
In the right orbitofrontal cortex (in the sulcus between the right inferior (BA 47) and medial (BA 11) frontal gyri), we observed a unique pattern of rCBF response, characterized by a maximum rCBF in the B condition (B = moderately arousing photographs). Moreover, in this region, rCBF in response to stimuli representing women (A, B, C, S) was much higher than in response to other stimuli (N, H) (Z = 7.46, P < .001, corrected; Fig. 3).
Figure 3.
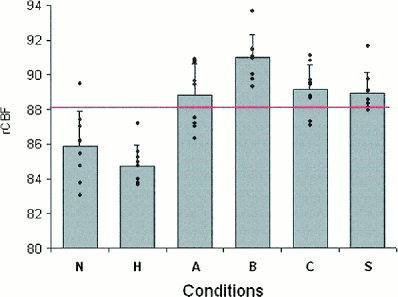
Regional cerebral blood flow (rCBF) in the right orbitofrontal gyrus in response to visual stimuli. Means, standard deviations and individuals values are in ml dl−1 min−1. rCBF is higher when presented stimuli contain women (conditions A, B, C, and S) than when women do not appear (conditions N and H). Red line indicates level of grand mean. Coordinates relative to anterior commissure: x = 22, y = 32, z = 16. See text for description of conditions.
The S‐N contrast revealed a significant activation of the left amygdala (−28; −2; −18; z = 3.41) and of the left (−52, −70, −6; z = 4.82) and right (52, −76, −6; z = 4.31) temporooccipital cortices. However, because these regions were also activated in the H condition (H‐N contrast; z = 3.12, z = 6.05, and z = 7.04, respectively), we did not find amygdalar and temporooccipital activations in the S‐H contrast (therefore, these regions do not appear in Table II).
The conjunction analysis between contrasts S‐N, S‐H. and C‐A revealed significant activations in all of the regions cited above, except for the right orbitofrontal cortex, the left central sulcus, the temporooccipital cortex, and amygdala. Conjunction analysis involving the B‐A contrast demonstrated an activation only in a subset of the above mentioned regions.
Regions deactivated in the S condition were principally located in the temporal lobes (Table III). They comprised the inferior (BA 20) and middle (BA 21 and 39) temporal gyri bilaterally, the right superior temporal gyrus (in the temporal pole, BA 38), the parahippocampal gyrus (BA 36) bilaterally, the left uncus and the left fusiform gyrus (BA 18).
Table III.
Cerebral regions deactivated in sexual conditions
| Brain region | BA | Hem | S‐N contrast | S‐N/S‐H/C‐A conjunction | S‐N/S‐H/B‐A conjunction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coordinates | Z scores | Coordinates | Z scores | Coordinates | Z scores | |||||||||
| x | y | z | x | y | z | x | y | z | ||||||
| Temporal lobes | ||||||||||||||
| Inferior temporal gyrus | 20 | L | −62 | −12 | −34 | 4.12 | −56 | −8 | −32 | 6.91** | −54 | −8 | −28 | 5.88** |
| Inferior temporal gyrus | 20 | R | 56 | −16 | −30 | 5.13** | 54 | −16 | −30 | 7.72** | ||||
| Middle temporal gyrus | 20 | L | −30 | −6 | −48 | 3.31 | −36 | −10 | −46 | 4.69* | ||||
| Middle temporal gyrus | 21 | L | −68 | −34 | −6 | 2.80 | −66 | −40 | 2 | 4.12 | ||||
| Middle temporal gyrus | 21 | R | 52 | −10 | −14 | 4.73* | 52 | −8 | −10 | 7.18** | ||||
| Middle temporal gyrus | 39 | L | −56 | −60 | 18 | 3.21 | −56 | −64 | 20 | 5.83** | ||||
| Middle temporal gyrus | 39 | R | 52 | −70 | 18 | 3.14 | 52 | −72 | 20 | 7.05** | ||||
| Superior temporal gyrus | 38 | R | 50 | 12 | −18 | 3.94 | 54 | 12 | −10 | 5.78** | ||||
| Superior temporal gyrus | 22 | L | −52 | −54 | 24 | 5.43** | ||||||||
| Parahippocampal gyrus | 36 | R | 34 | −28 | −12 | 3.45 | ||||||||
| Parahippocampal gyrus | 36 | L | −36 | −18 | −22 | 2.80 | −36 | −16 | −22 | 4.37 | ||||
| Fusiform gyrus | 18 | L | −16 | −96 | −12 | 6.20** | −16 | −94 | −6 | 7.16** | ||||
| Uncus | L | −32 | −8 | −48 | 3.22 | |||||||||
| All other | ||||||||||||||
| Middle occipital gyrus | 19 | L | −44 | −82 | 22 | 2.97 | −40 | −76 | 24 | 3.73 | ||||
| Orbital gyrus | 11 | R | 6 | 54 | −18 | 2.72 | 6 | 54 | −20 | 4.99** | ||||
| Posterior cingulate | 31 | L | −16 | −52 | 12 | 3.00 | −18 | −54 | 14 | 4.67* | ||||
| Precuneus | 7 | L | −4 | −72 | 66 | 3.31 | −6 | −54 | 50 | 4.71* | ||||
P < .01, corrected
P < .05, corrected
See legend of Table II for meaning of coordinates and abbreviations.
Additionally, deactivations were found in the left middle occipital gyrus, the left posterior cingulate, the left precuneus (BA 7), and the right orbital gyrus. All these regions, except the fusiform gyrus, the right parahippocampal gyrus, and the uncus, were also found deactivated in the conjunction analysis between contrasts N‐S, H‐S, and A‐C.
In the S‐H and H‐S contrast analysis where level of emotional ratings was used as a confounding variable, all the regions identified as activated or deactivated in the first contrast analysis were found again, except the sulcus between the right medial frontal gyrus and the cingulate gyrus (BA 9/32).
Covariation analysis
For nearly all regions found activated in the S‐N / S‐H / C‐A conjunction analysis (Table II), covariation analyzes showed: (i) a positive linear relationship between PSA and rCBF (except for the pulvinar); (ii) a positive linear relationship between penile tumescence and rCBF (except for the pulvinar and precentral gyrus) (Table IV).
Table IV.
Positive correlations between rCBF and markers of sexual arousal
| Brain region | BA | Hem | PSA | Penile tumescence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coordinates | Z scores | Coordinates | Z scores | |||||||
| x | y | z | x | y | z | |||||
| Limbic/paralimbic cortex | ||||||||||
| Ant. cingulate, caudal | 24 | L | −8 | 4 | 32 | 7.21* | −8 | 6 | 36 | 5.36* |
| Ant. cingulate, rostral | 24 | L | −4 | 34 | 10 | 4.29 | −2 | 34 | 8 | 3.64 |
| Ant. cingulate, rostral | 32 | L | −18 | 32 | 18 | 5.17* | −8 | 28 | 22 | 3.32 |
| Cingulate | L | −16 | −12 | 40 | 5.73*, † | −16 | −12 | 40 | 4.63** | |
| Sulcus cingulate/Gfd | 9/32 | R | 24 | 40 | 20 | 4.89† | 22 | 42 | 20 | 4.17 |
| Subcortical structures | ||||||||||
| Claustrum | L | −32 | 6 | 4 | 5.62*, † | −32 | 6 | 4 | 5.31* | |
| Claustrum | R | 30 | 10 | −2 | 6.01†, * | 30 | 10 | −2 | 4.14† | |
| Caudate nucleus (head) | R | 14 | 16 | 14 | 5.52* | 16 | 14 | 16 | 5.41* | |
| Caudate nucleus | R | 18 | 2 | 22 | 5.17* | 18 | 2 | 22 | 4.01† | |
| Putamen | L | −30 | 6 | 0 | 5.91* | −30 | 6 | 0 | 5.15*, † | |
| Putamen | L | −26 | −12 | 0 | 4.40 | −26 | −12 | 0 | 3.45† | |
| Putamen | R | 30 | 6 | −6 | 5.60*, † | 30 | 6 | −6 | 4.32 | |
| Thalamus (VPM) | R | 14 | −22 | 4 | 4.42 | 14 | −24 | 4 | 3.98 | |
| Thalamus (DM) | L/R | −2 | −24 | 14 | 4.40 | 0 | −20 | 4 | 3.83 | |
| All other | ||||||||||
| Sup. frontal gyrus | 6 | R | 20 | −12 | 62 | 5.23* | 20 | −14 | 64 | 4.06 |
| Precentral gyrus | 6 | L | −66 | 2 | 32 | 3.80 | ||||
| Central sulcus | 3/4 | L | −22 | −14 | 54 | 4.16† | −22 | −14 | 54 | 4.92* |
| Postcentral gyrus | 40/2 | L | −36 | −30 | 34 | 4.13 | −36 | −28 | 34 | 3.12 |
| Parietal operculum | 40 | L | −70 | −24 | 30 | 4.46 | −68 | −28 | 26 | 4.16 |
| Inf. parietal lobule | 7 | R | 36 | −60 | 60 | 4.73** | 36 | −58 | 62 | 3.71 |
| Fusiform gyrus | 19 | L | −48 | −74 | −16 | 3.43 | −38 | −80 | −14 | 3.42 |
| Fusiform gyrus | 18 | R | 50 | −82 | −6 | 3.70 | 46 | −70 | −12 | 4.32 |
| Precuneus | 7 | L | −18 | −46 | 54 | 2.97 | −22 | −48 | 54 | 3.32 |
| Transverse temporal gyrus | 41 | L | −30 | −26 | 10 | 4.70** | −30 | −24 | 10 | 4.32 |
| Cerebellum | L | −32 | −46 | −28 | 4.01 | −38 | −58 | −24 | 3.12 | |
| Cerebellum | L | −22 | −64 | −48 | 3.5 | −26 | −62 | −50 | 3.64 | |
| Cerebellar vermis | L | −4 | −72 | −26 | 4.00 | −2 | −72 | −22 | 3.89 | |
| Cerebellar vermis | L | −12 | −40 | −20 | 3.57 | −10 | −40 | −22 | 2.83 | |
| Cerebellar vermis declive | L | −38 | −80 | −14 | 3.42 | |||||
| Tuber of vermis | R | 60 | −64 | −24 | 2.80 | 54 | −62 | −26 | 3.70 | |
P < .01, corrected for multiple comparisons
P < .05, corrected
no maximum activation at this location, but z‐score higher than 2.33.
PSA: perceived sexual arousal; for other abbreviations and meaning of coordinates, see legend of Table II.
In addition, in some regions, while conjunction analysis did not demonstrate an activation, rCBF was positively correlated with PSA and/or penile tumescence. These regions were the right superior frontal gyrus, the left postcentral gyrus, the fusiform gyrus bilaterally, the left precuneus, the left transverse temporal gyrus, and several parts of the left half of the cerebellum.
When the effect of conditions was controlled for statistically (Condition factor included in the model), additional findings emerged: (i) the magnitude of penile tumescence was positively correlated with rCBF in the posterior part of the hypothalamic area (x = 0, y = −8, z = −6; Z = 3.18), the right midcingulate (6, −8, 34; Z = 3.17) and the right orbital gyrus (BA 11; 20, 36, −24; Z = 3.79); (ii) PSA was positively correlated with rCBF in the nucleus accumbens (−4, 6, −2; Z = 3.50) and in the middle frontal gyrus (BA 8; 30, 32, 36; Z = 3.27).
Regarding deactivations, in all the regions found in the N‐S/H‐S/A‐C conjunction analysis, rCBF was negatively correlated with markers of sexual arousal (PSA and penile tumescence), except for the middle occipital gyrus and the precuneus (Table V). Such negative correlations were found in additional regions, i.e., the calcarine sulcus and the fusiform gyrus, bilaterally, and in the right posterior cingulate.
Table V.
Negative correlations between rCBF and markers of sexual arousal
| Brain region | BA | Hem | PSA | Penile tumescence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coordinates | Z scores | Coordinates | Z scores | |||||||
| x | y | z | x | y | z | |||||
| Temporal lobes | ||||||||||
| Inf. temporal gyrus | 20 | L | −58 | −8 | −32 | 5.19* | −60 | −8 | −34 | 5.23* |
| Inf. temporal gyrus | 20 | L | −34 | −8 | −46 | 3.60 | −34 | −6 | −50 | 3.04 |
| Inf. temporal gyrus | 20 | L | −64 | −48 | −12 | 4.41 | −64 | −50 | −12 | 3.23 |
| Inf. temporal gyrus | 20 | R | 56 | −16 | −32 | 5.82* | 52 | −16 | −30 | 4.06 |
| Middle temporal gyrus | 21 | L | −66 | −36 | −2 | 4.92* | −68 | −34 | −2 | 3.63 |
| Middle temporal gyrus | 39 | L | −46 | −68 | 30 | 3.91 | −52 | −68 | 28 | 3.66 |
| Middle temporal gyrus | 39 | R | 52 | −70 | 22 | 4.69** | 54 | −74 | 24 | 4.18 |
| Middle temporal gyrus | 21 | R | 52 | −8 | −14 | 5.04* | 50 | −10 | −14 | 4.35 |
| Sup. temporal gyrus | 38 | L | −44 | 16 | −22 | 3.68 | −48 | 14 | −24 | 2.91 |
| Sup. temporal gyrus | 39 | L | −50 | −52 | 30 | 4.50** | −50 | −56 | 24 | 3.52 |
| Sup. temporal gyrus | 21 | R | 54 | 8 | −12 | 4.07 | 56 | 10 | −10 | 3.42 |
| Sup. temporal gyrus | 22 | R | 60 | −6 | 2 | 3.90 | 56 | −12 | 4 | 3.19 |
| Fusiform gyrus | 37 | L | −28 | −46 | −8 | 3.31 | −28 | −46 | −8 | 2.66† |
| Fusiform gyrus | 20 | R | 68 | −32 | −26 | 3.98 | 68 | −30 | −26 | 3.12 |
| Hippocampus | L | −32 | −20 | −18 | 3.49 | −34 | −22 | −20 | 2.72 | |
| Parahippocampal gyrus | 19 | L | −22 | −42 | −2 | 3.47 | −20 | −44 | 0 | 3.49 |
| All other | ||||||||||
| Calcarine sulcus | 17 | L | −14 | −92 | −6 | 4.70** | −16 | −98 | −12 | 3.36 |
| Calcarine sulcus | 17 | R | 12 | −100 | −4 | 4.24 | 10 | −100 | −12 | 3.28 |
| Post. cingulate | 30 | L | −16 | −54 | 14 | 3.24 | −18 | −54 | 14 | 3.61 |
| Post. cingulate | 30 | R | 14 | −52 | 14 | 3.75 | ||||
| Medial frontal gyrus | 11 | R/L | 2 | 56 | −20 | 3.77 | 2 | 54 | −18 | 2.64 |
P < .01, corrected
P < .05, corrected
no maximum activation at this location, but z‐score higher than 2.33. For meaning of abbreviations and of coordinates, see legend of Table II.
When the effect of conditions was controlled for statistically, we found that PSA was negatively correlated with rCBF in the left superior frontal gyrus (BA 9; −40, 48, 32; Z = 3.65) and in the left inferior frontal gyrus (BA 46; −52, 42, 12; Z = 3.49).
The analysis where the rank of each scan was used as a confounding variable revealed that the activity in the left ACG (BA 24; −6, −2, 44; Z = 3.52), the head of the right caudate nucleus (Z = 3.41), the right putamen (Z = 3.81), the dorsomedial nucleus of the left thalamus (Z = 3.95), the right orbital gyrus (BA 11, Z = 3.71), and the left middle temporal gyrus (BA 39, Z = 3.63) remained positively correlated with PSA (P < .001, uncorrected).
DISCUSSION
The present study confirms the results of our previous study [Stoléru et al., 1999]: using a sample of older subjects, a different set of stimuli, and a different PET scanner, we recorded again an increased rCBF in all the regions found activated in the sexual condition of the exploratory study. Moreover, the use of a whole‐brain PET scanner with a higher resolution demonstrated the activation of additional regions (claustra, putamens, thalami, hypothalamus, nucleus accumbens, central sulcus, parietal lobules). The present study confirmed that the temporooccipital activation found in the S‐N contrast of both studies was not specifically related to the processing of sexual stimuli: first, this activation was also found in the H‐N contrast analysis (between films), and second, it was not demonstrated by the C‐A contrast analysis (between photographs). Finally, this study identified regions where rCBF was positively or negatively correlated with the magnitude of sexual responses induced by sexual stimuli of graded intensity.
We have previously proposed [Stoléru et al., 1999] a neural/behavioral model of SA comprising four coordinated components, i.e., cognitive, emotional, motivational, and physiological (autonomic and endocrinological). Briefly, the cognitive component comprises a process of appraisal through which a stimulus is categorized as a sexual incentive and quantitatively evaluated as such. The emotional component includes the specific hedonic quality of SA, i.e., the pleasure associated with rising arousal and with the perception of specific bodily changes, such as penile tumescence. The motivational component comprises the processes that direct behavior to a sexual goal, including the perceived urge to express overt sexual behavior. The autonomic and endocrinological components include various responses (e. g., cardiovascular, respiratory, genital) leading the subject to a state of physiological readiness for sexual behavior. These four components are conceived as closely interrelated and coordinated. For instance, the emotional component is partly based on the perception of bodily changes generated by the autonomic component. Hereunder, we interpret the findings of the present experiment in the light of this model and use the new findings to further specify the model.
There is evidence that the activation of the right orbitofrontal cortex was correlated with the cognitive component of the proposed model. First, rCBF in this region was correlated with PSA. As indicated by Figure 3, this correlation was largely due to the increased rCBF in response to stimuli representing women (A, B, C, S vs. N, H). More specifically, the maximal rCBF response in this region was induced by the B condition. On debriefing, it is only for B photographs that subjects commented on the beauty of the presented women, reflecting the evaluation processes induced by this kind of stimuli. Because in the S condition rCBF in the right orbitofrontal cortex was as high as in the A and C conditions, this pattern of response cannot be explained by a slide vs. film effect. However, this pattern of response unique to the orbitofrontal cortex was a post hoc finding and needs to be replicated.
Second, as the right orbitofrontal cortex is important for the representation of rewards such as pleasant touch [Francis et al., 1999], activity in this region may be related to the representation of the pleasant bodily sensations induced by penile tumescence. This is consistent with the positive correlation found in the right orbitofrontal cortex between rCBF and penile tumescence. Finally, in the context of another motivated behavior, i.e., feeding, activity of neurons of the orbitofrontal cortex in response to the visual presentation of food was shown to reflect the incentive value of visual stimuli [Rolls, 1999], as it decreased sharply once monkeys were satiated.
Regarding the autonomic component of SA, the correlation found between the magnitude of penile tumescence and rCBF in the rostral portion of the anterior cingulate gyrus (ACG) is likely to reflect a causal relationship since electrical stimulation in this region elicits erection in monkeys [Dua and MacLean, 1964; Robinson and Mishkin, 1968]. In man also, lesion and electrical stimulation studies have demonstrated the role of the ACG (Brodmann area 24) in visceral responses and in the expression of affect [Devinsky et al., 1995]. The ACG is directly connected to autonomic nuclei in the medulla and to forebrain regions controlling autonomic functions, including the hypothalamus. In the present study, rCBF in the posterior part of the hypothalamic area was correlated with the magnitude of penile tumescence. In the rat, stimulation of the posterior hypothalamus induces copulatory behavior and bar pressing for the opportunity to copulate [Caggiula, 1970]. In freely moving monkeys, the stimulation of the dorsomedial hypothalamic nucleus elicits full erection and it is the only hypothalamic locus where radiostimulation leads not only to mounting and thrusting but also to ejaculation [Perachio et al., 1979]. The perception of tumescence is likely to be related to the activation in the most superior part of the left central sulcus (Brodmann area 3). This location corresponds closely to the primary somatosensory cortex receiving inputs from the external genitalia as shown by a recent study on cortical potentials evoked in human males by stimulation of the dorsal nerve of the penis [Bradley et al., 1998]. The activation in the left parietal operculum was located in the secondary somatosensory area (S II). In nonhuman primates, this area is the only somatosensory region that receives amygdalar projections [Amaral and Price, 1984]. This connection could form the anatomical basis of the emotional aspects of penile sensations. Regarding the emotional component of SA, one of the activated regions, the sulcus between BA 9 and BA 32, has been reported to be correlated with selective attention to subjective emotional responses [Lane et al., 1997b] and with the processing of affect‐related meanings [Teasdale et al., 1999]. Consistent with this interpretation, BA 9/32 was not found activated in the S‐H contrast analysis where level of emotional ratings was used as a confounding variable. This suggests that the activation of this region was related to the level of perceived emotion and not to the sexual quality of emotion.
On debriefing, subjects reported that in response to the presentation of the C photographs and even more of S clips, that they had felt the urge to act out their sexual desire. In particular, S clips induced the desire to perform the same actions as those depicted in the films. The activation of the caudal part of the left ACG may be related to this motivational component of SA, and specifically to the perceived urge to act. The activation of this region was highly correlated with the intensity of PSA (Fig. 1) and with the magnitude of penile tumescence. In keeping with this interpretation, the role of the caudal part of the ACG in motor function is known to be similar to the role of premotor and supplementary motor area cortices [Dum, 1993]. In the monkey, stimulation of the ACG elicits not only erections but also genital manipulation of a masturbatory character [Robinson and Mishkin, 1968]. In human partial seizures with sexual manifestations (such as patients initiating pelvic thrusting), the origin of discharge has been located in the ACG [Landré et al., 1993]. Our results are thus in agreement with the previous general conclusion that the caudal part of the ACG plays a crucial role in the initiation and the motivation of goal‐directed behaviors [Devinsky et al., 1995].
However, it should be noted that in a situation where an urge to act is not followed by actual behavior, there must be control mechanisms at least as powerful as the motivational processes. This is the case in a PET paradigm where overt behavior must be controlled. The caudal part of the ACG includes the response selection cortex [Devinsky et al., 1995], which is activated when a response of one kind has to be withheld in favor of another kind of response. In addition, the specific location of our peak activation in the caudal part of the ACG (x, y, z = −4, 6, 36) has also been found activated in a PET study of a simple GO/NO‐GO task (with no response selection involved) [Kawashima et al., 1996]. We therefore speculate that the pronounced activation of the caudal ACG may be the result of multiple conflicting inputs to this area: on the one hand, inputs of the GO type, correlated with the perceived urge to enact SA, and, on the other hand, inputs of the NO‐GO type, correlated with the perceived need to withhold any overt sexual behavior in the current circumstances. Both types of inputs would be associated with an increased rCBF, because it is the local synaptic activity that is energy consuming. Moreover, the characteristic perception of sexual tension might be related to the opposite nature of signals converging to this area.
In spite of the evidence for a role of the caudal ACG in the motivational component of SA, one may also ask whether its activation was related to SA per se or to other psychological phenomena elicited by sexual stimuli. For instance, the caudal part of the ACG has been implicated in numerous cognitive processes, such as selective attention, working memory, semantic and episodic memory retrieval [Cabeza and Nyberg, 1997]. Some of these cognitive processes may have participated in the processing of sexual stimuli without being specifically triggered by the sexual character of processed stimuli.
By contrast, the rostral part of the ACG is considered as the affective part of this structure [Whalen et al., 1998]. The most rostral of the cingulate activations that we recorded was located in BA 9 and BA 32, i.e., in the region identified as mediating subjective emotional responses [Lane et al., 1997a; Teasdale et al., 1999]. It is also in the rostral part of the ACG that the peak of activation was located in a study of sexual and competitive arousal [Rauch et al., 1999]. Finally, recent studies of motivational processes other than SA (i. e., thirst [Denton et al., 1999b] and hunger [Tataranni et al., 1999] reported an activation in the rostral ACG.
As regards this distinction between a caudal cognitive and a rostral affective divisions of the ACG, it should be noted, however, that both in our study and in Denton's [Denton et al., 1999b], a very large extent of the cingulate gyrus, covering both divisions, was found activated (with peaks ranging from y = −14 to y = 32 in our study and from y = −16 to +30 in Denton's). The involvement of the ACG in motivational processes is thus not confined to its rostral part. Furthermore, studies by Denton et al. [Denton et al., 1999b] and by Rauch et al. [Rauch et al., 1999] indicate that the activation of the ACG does not necessarily depend on the cognitive processing of ongoing external stimuli: in the former study, thirst was induced by IV infusion of hypertonic saline and in the latter study SA was based on recalling an event.
The pattern of connections of the ACG is consistent with this motivational function. It receives massive projections from the amygdala, which was activated by the presentation of the sexual stimuli. The amygdala receives from the activated temporooccipital cortex dense projections carrying highly processed visual information. In addition, the ACG is reciprocally connected with the orbitofrontal cortex. Finally, the ACG projects to the claustrum and the putamen.
The highly significant bilateral activation of the claustrum and of the putamen (Fig. 2) was one of the most striking findings. Bilaterally, rCBF in the claustrum and the putamen was positively correlated with PSA. There is now accumulating evidence of the involvement of the claustrum in motivational processes. One of the regions activated in the S–N contrast of our previous study [Stoléru et al., 1999], reported under the label right insula, extended over the claustrum. Rauch et al.'s [1999] study on sexual and competitive arousal reports a left claustral activation. The claustrum is one of the two only regions (along with the brain stem) where an activation was found in SA but not in competitive arousal. Moreover, both in a study of the neural correlates of thirst [Denton et al., 1999a] and in another PET study on hunger [Tataranni et al., 1999], a bilateral (thirst) or right (hunger) claustral activation was found. Additionally, there is further evidence for the role of the claustrum in emotional and motivational responses in rats [Hamamura et al., 1997] and in man [Reiman et al., 1989]. A recent study supports the view that the claustrum and the basolateral complex of the amygdala have a common embryological origin [Swanson and Petrovich, 1998]. The basolateral amygdala has been implicated in reward‐related processes [Everitt et al., 1991]. Specifically, the basolateral amygdala seems to interact with dopamine‐dependent processes in the ventral striatum in mediating the control by conditioned incentives over instrumental behavior, while being of relative little importance in the control of unconditioned consummatory responses elicited by primary incentives cues [Everitt, 1990]. Thus, it is possible that the human claustrum fulfills a function that in lower mammals is mediated by the basolateral amygdala.
Regarding the putamen, a study has suggested a possible relationship of its ventral part with the hedonic properties of the expected reward [Schultz et al., 1992]. The putamen is also a region where electrical stimulation evoked most often an erection and/or genital manipulation in Macaca mulatta [Robinson and Mishkin, 1968]. However, as argued below, another interpretation suggests that the putamen may also be involved in withholding the motor output of SA.
Another activated region, the nucleus accumbens, is likely to participate in the motivational component of SA. There is physiological evidence linking the nucleus accumbens to sexual appetitive behavior [Everitt, 1990]. It receives rich inputs from the basolateral region of the amygdala, the orbitofrontal cortex and the ACG. The output of the shell, limbic‐related, part of the nucleus accumbens is directed toward the ventral pallidum and thence to the mediodorsal nucleus of the thalamus, which projects to the prefrontal and cingulate cortices. The ventral striatum may thus be a route for limbic and paralimbic structures to influence output regions.
In the present paradigm, as no overt behavioral response was possible, it is the premotor aspects of responses, as well as responses of regions concerned with withholding behavior, which were investigated. A recent model of basal ganglia function in motivated behavior is helpful to interpret the observed correlation between PSA and rCBF in the head of right caudate nucleus [Rolls, 1999] (Fig. 2). According to this model, once the neurons in the orbitofrontal cortex have decoded the motivational significance of stimuli, it is essential that these reward‐related signals should not be interfaced directly with motor behavior. Instead, what is required is that the signals enter an arbitration mechanism, which takes into account the cost of obtaining reward. It has been proposed that the basal ganglia participate in this function [Rolls, 1999]. They receive inputs from numerous areas of the cerebral cortex, including the ACG, which is strongly connected with the caudate nucleus and with the putamen. Cortical inputs compete within the striatum for behavioral output, and the striatum maps each particular type of input to the appropriate behavioral output, implemented via the return basal ganglia connections to premotor/prefrontal cortex. This model is based on experimental findings in monkeys demonstrating the implication of the head of the caudate nucleus in the withholding of responses in a go/no‐go task [Dunnett and Iversen, 1981]. The model is also consistent with evidence from neuroimaging studies, i.e., (i) the activation of the putamen and/or the caudate nucleus in paradigms where the need for a motor response is conflicting with the need to withhold it [Pardo et al., 1990] and (ii) the activation of the head of the caudate nucleus upon volitional tic suppression in Tourette Syndrome [Peterson et al., 1998]. Finally, the model is supported by clinical evidence, such as hypersexuality in patients with lesions circumscribed to the head of the caudate nuclei [Richfield et al., 1987]. The above development strongly suggests that the initial model [Stoléru et al., 1999] should be complemented by an additional component consisting in the control of the motor expression of SA. This would be consistent with the dual model of the control of sexual behavior proposed by Bancroft [1999].
Almost all the regions where rCBF decreased in the S condition (and where rCBF was negatively correlated with PSA and penile tumescence) belonged to the temporal lobes. It is well known that the removal of temporal lobes is followed by dramatic hypersexuality [Klüver and Bucy, 1939]. Together, these facts suggest that the alleviated inhibition from temporal lobes allows for the development of SA. In other words, these temporal regions could exert a tonic inhibition on the development of SA, while the role of the basal ganglia would be to withhold the behavioral expression of an already current state of SA. These deactivated temporal regions were clearly distinct from the temporooccipital regions which were found activated, in our previous study [Stoléru et al., 1999] and in the present experiment, both in the S‐N and in the S‐H contrast analysis.
Finally, our results support the notion that SA is a composite psychophysiological state correlated with the activation/deactivation of several brain regions. Among those regions, a majority, when considered individually, have been associated with other emotional states. For instance, the ACG and the claustrum have been activated in several affective states, including negatively valenced emotions [Benkelfat et al., 1995; Dougherty et al., 1999]. Then, what is specific of the neuroanatomical correlates of SA? This specificity may be related to: (i) a distinctive pattern of activated/deactivated areas and/or (ii) the activation/deactivation of discrete areas within the broad regions demonstrated by PET, e.g., the part of the rostral ACG reported to control erection in animals and the part of the somatosensory cortex related to the perception of penile tumescence.
Regarding the limitations of the study, it should first be noted that sexual stimuli activated simultaneously several components of SA and their neural substrates. Results, therefore, do not allow us to demonstrate directly the involvement of a particular brain region in a specific component of SA. Interpretations presented above should be considered as working hypotheses to be tested in future studies targeted, if possible, on specific components of SA.
Second, in the circumplex model of emotion [Russell, 1980], the dimensions of emotion are valence (pleasure vs. displeasure) and degree of arousal (low to high). In our study, the degree of arousal, irrespective of its valence, may have varied across conditions. For instance, emotional arousal may have been higher in condition S than in condition H. Although rCBF in several brain regions was correlated with specific markers of SA, this finding may still reflect that SA requires or induces a general emotional arousal. However, when controlling statistically for the level of perceived emotion in the analysis of the S–H contrast, we found that activations/deactivations remained associated with SA. This suggests that the correlation between activated/deactivated regions and SA was not due to a greater level of general emotional arousal induced by S films. Nevertheless, instead of controlling this parameter statistically, it would be preferable in future studies to use stimuli generating the same level of emotional arousal.
Third, in connection with the response inhibition needed in SA, it should be acknowledged that humor did not generate a motivational state comparable to the one induced by sexual conditions. In future studies, one strategy to overcome this limitation could be to use control conditions generating motivational states such as anger or greed for savory foods.
Fourth, for reasons mentioned above, we did not use a counterbalanced order for the presentation of the various stimuli. Counterbalancing is classically used to distribute various carry‐over effects so that they will not be confounded with the different experimental treatments [Yaremko et al., 1982] nor with a time effect. However, there is evidence that neither a time effect nor carry‐over effects affected the findings: (i) most of the activations were found in the analysis where the rank of condition was used as a confounding covariate; (ii) the H condition is very unlikely to have had a carry‐over effect on the subsequent conditions, as the rating of humor was the minimum possible rating (= 1 on a 1 to 9 scale) in the three subsequent conditions. Even if such carry‐over effect occurred, in the regions reported as activated in the S condition, both “Sexual – Neutral” and “Sexual – Humor” contrasts yielded a significant increase of rCBF.
CONCLUSION
In conclusion, we have shown that visually evoked SA was predominantly correlated with an activation in limbic and paralimbic cortex and in subcortical structures, along with a deactivation in several parts of the temporal cortex. Thus, for an experience to be perceived and labeled as “sexual arousal” by healthy males, it appears that concurrent functional changes are needed in multiple brain regions. This probably explains why the experimental stimulation of single regions of the brain generally failed to evoke SA [Penfield and Jasper, 1954]. Finally, these results, relevant to healthy subjects, should help to better identify, and potentially to better treat, the functional changes characterizing pathological alterations of sexual desire [Stoléru et al., 1998].
Acknowledgements
We thank V. Berthier (CERMEP), C. Vighi (CERMEP), M. Lionnet (CERMEP), L. Veyre (CERMEP), F. Bonnefoi (CERMEP), M.P. Monneret (INSERM 329), J.P. Fauvel (Hospices Civils de Lyon), B. De Gayffier (Université Paris XIII), and O. Nargues (Hospices Civils de Lyon) for their technical assistance.
This work was performed at the Centre d'Exploration et de Recherche Médicale par Emission de Positons, Lyon, France.
REFERENCES
- Alcock J (1997): Animal behavior. Sunderland, MA: Sinauer Associates, Inc; 625p. [Google Scholar]
- Amaral DG, Price JL (1984): Amygdalo‐cortical projections in the monkey (Macaca fascicularis). J Comp Neurol 230: 465–496. [DOI] [PubMed] [Google Scholar]
- Bancroft J (1989): Human sexuality and its problems. London: Churchill Livingstone; 748p. [Google Scholar]
- Bancroft J (1999): Central inhibition of sexual response in the male: a theoretical perspective. Neurosci Biobehav Rev 23: 763–784. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Everitt BJ, Herbert J, Keverne EB (1977): Hormonal basis of proceptivity and receptivity in female primates. Arch Sex Behav 6: 173–192. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Karama S, Leroux JM, Lecours AR, Beaudoin G, Bourgouin P (1998): The functional neuroanatomy of amusement, disgust and sexual arousal. Paper presented at the 4th International Conference on Functional Mapping of the Human Brain. Montreal, Quebec, Canada.
- Benkelfat C, Bradwejn J, Meyer E, Ellenbogen M, Milot S, Gjedde A, Evans A (1995): Functional neuroanatomy of CCK4‐induced anxiety in normal healthy volunteers. Am J Psychiatry 152: 1180–1184. [DOI] [PubMed] [Google Scholar]
- Bielert C (1982): Experimental examinations of baboon (Papio ursinus) sex stimuli In: Snowdown CT, Brown CH, Petersen MR, editors. Primate communication. London: Cambridge University Press, p 373–395. [Google Scholar]
- Bradley WE, Farrell DF, Ojemann GA (1998): Human cerebrocortical potentials evoked by stimulation of the dorsal nerve of the penis. Somatosens Mot Res 15: 118–127. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, Kendrick AD, Davis TL, Jiang A, Cohen MS, Stern CE, Belliveau JW, Baer L, O'Sullivan RL, Savage CR, Jenike MA, Rosen BR (1996): Functional magnetic resonance imaging of symptom provocation in obsessive‐compulsive disorder. Arch Gen Psychiatry 53: 595–606. [DOI] [PubMed] [Google Scholar]
- Buss DM (1989): Sex differences in human mate preferences: evolutionary hypotheses tested in 37 cultures. Behav Brain Sci 12: 1–14. [Google Scholar]
- Cabeza R, Nyberg L (1997): Imaging cognition: An empirical review of PET studies with normal subjects. J Cogn Neurosci 9: 1–26. [DOI] [PubMed] [Google Scholar]
- Caggiula AR (1970): Analysis of the copulation‐reward properties of posterior hypothalamic stimulation in male rats. J Comp Physiol Psychol 70: 399–412. [DOI] [PubMed] [Google Scholar]
- Chaplin TC, Rice ME, Harris GT (1995): Salient victim suffering and the sexual responses of child molesters. J Consult Clin Psychol 63: 249–255. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Rosen RC, Goldstein L (1985): EEG hemispheric asymmetry during sexual arousal: psychophysiological patterns in responsive, unresponsive, and dysfunctional men. J Abnorm Psychol 94: 580–590. [DOI] [PubMed] [Google Scholar]
- Cottraux J, Gérard D, Cinotti L, Froment JC, Deiber MP, Le Bars D, Galy G, Millet P, Labbé C, Lavenne F, Bouvard M, Mauguière F (1996): A controlled positron emission tomography study of obsessive and neutral auditory stimulation in obsessive‐compulsive disorder with checking rituals. Psychiatry Res 60: 101–112. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Abercrombie H, Nitschke JB, Putnam K. 1999. Regional brain function, emotion and disorders of emotion. Curr Opin Neurobiol 9: 228–234. [DOI] [PubMed] [Google Scholar]
- Denton D, Shade R, Zamarippa F, Egan G, Blair‐West J, McKinley M, Fox P (1999a): Correlation of regional cerebral blood flow and change of plasma sodium concentration during genesis and satiation of thirst. Proc Natl Acad Sci U S A 96: 2532–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton D, Shade R, Zamarippa F, Egan G, Blair‐West J, McKinley M, Lancaster J, Fox P (1999b): Neuroimaging of genesis and satiation of thirst and an interoceptor‐driven theory of origins of primary consciousness. Proc Natl Acad Sci U S A 96: 5304–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR (1977): The SCL‐90R Manual. I: Scoring, Administration and Procedures for the SCL‐90R. Baltimore, MD: Clinical Psychometrics. [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA (1995): Contributions of anterior cingulate cortex to behaviour. Brain 118 (Pt 1): 279–306. [DOI] [PubMed] [Google Scholar]
- Dieckmann G, Schneider‐Jonietz B, Schneider H (1988): Psychiatric and neuropsychological findings after stereotactic hypothalamotomy, in cases of extreme sexual aggressivity. Acta Neurochir(Wien) Suppl 44: 163–166. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Shin LM, Alpert NM, Pitman RK, Orr SP, Lasko M, Macklin ML, Fischman AJ, Rauch SL (1999): Anger in healthy men: a PET study using script‐driven imagery. Biol Psychiatry 46: 466–472. [DOI] [PubMed] [Google Scholar]
- Dua S, MacLean PD (1964): Localisation for penile erection in medial frontal lobe. Am J Physiol 207: 1425–1434. [DOI] [PubMed] [Google Scholar]
- Dum RP (1993): Cingulate motor areas In: Vogt BA, Gabriel M, editors. Neurobiology of the cingulate cortex and limbic thalamus. Boston: Birkhäuser, p 415–441. [Google Scholar]
- Dunnett SB, Iversen SD (1981): Learning impairments following selective kainic acid‐induced lesions within the neostriatum of rats. Behav Brain Res 2: 189–209. [DOI] [PubMed] [Google Scholar]
- Everitt BJ (1990): Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev 14: 217–232. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O'Brien A, Robbins TW (1991): The basolateral amygdala‐ventral striatal system and conditioned place preference: further evidence of limbic‐striatal interactions underlying reward‐related processes. Neuroscience 42: 1–18. [DOI] [PubMed] [Google Scholar]
- Forest MG, Cathiard AM, Bertrand JA (1973): Total and unbound testosterone levels in the newborn and in normal and hypogonadal children: use of a sensitive radioimmunoassay for testosterone. J Clin Endocrinol Metab 36: 1132–1142. [DOI] [PubMed] [Google Scholar]
- Francis S, Rolls ET, Bowtell R, McGlone F, O'Doherty J, Browning A, Clare S, Smith E (1999): The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport 10: 453–459. [DOI] [PubMed] [Google Scholar]
- Freeman W (1973): Sexual behavior and fertility after frontal lobotomy. Biol Psychiatry 6: 97–104. [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ (1995a): Spatial registration and normalisation of images. Hum Brain Mapp 2: 165–189. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ (1995b): Statistical parameter maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Gorman DG, Cummings JL (1992): Hypersexuality following septal injury. Arch Neurol 49: 308–310. [DOI] [PubMed] [Google Scholar]
- Hamamura T, Ichimaru Y, Fibiger HC (1997): Amphetamine sensitization enhances regional c‐fos expression produced by conditioned fear. Neuroscience 76: 1097–1103. [DOI] [PubMed] [Google Scholar]
- Herbert J (1996): Sexuality, stress and the chemical architecture of the brain. Annu Rev Sex Res 7: 1–43. [Google Scholar]
- Hills M, Armitage P (1979): The two‐period cross‐over clinical trial. Br J Clin Pharmacol 8: 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima R, Satoh K, Itoh H, Ono S, Furumoto S, Gotoh R, Koyama M, Yoshioka S, Takahashi T, Takahashi K, Yanagisawa T, Fukuda H (1996): Functional anatomy of GO/NO‐GO discrimination and response selection‐a PET study in man. Brain Res 728: 79–89. [PubMed] [Google Scholar]
- Klüver H, Bucy PC (1939): Preliminary analysis of functions of the temporal lobes in monkeys. Arch Neurol Psych 42: 979–1000. [DOI] [PubMed] [Google Scholar]
- Landré E, Ghossoub M, Chassoux F, Broglin D, Devaux B, Turak B, Bancaud J (1993): Sensations génitales paroxystiques bilatérales d'origine temporo‐sylvienne dans l'épilepsie partielle (à propos de cinq observations). Epilepsies 5: 205–213. [Google Scholar]
- Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ (1997a): Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry 154: 926–933. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE (1997b): Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia 35: 1437–1444. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Sachs BD (1994): The physiology of male sexual behavior In: Knobil E, Neill JD, editors. The physiology of reproduction Vol. 2 New York: Raven Press, p 3–105. [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME (1990): The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci U S A 87: 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Jasper H (1954): Epilepsy and the functional anatomy of the human brain. Boston: Little, Brown; 896p. [Google Scholar]
- Perachio AA, Marr LD, Alexander M (1979): Sexual behavior in male rhesus monkeys elicited by electrical stimulation of preoptic and hypothalamic areas. Brain Res 177: 127–144. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, Leckman JF, Gore JC (1998): A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry 55: 326–333. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ (1997): Cognitive conjunction: a new approach to brain activation experiments. Neuroimage 5: 261–270. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, Fischman AJ (1994): Regional cerebral blood flow measured during symptom provocation in obsessive‐compulsive disorder using oxygen 15‐labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry 51: 62–70. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Dougherty DD, Alpert NM, Orr SP, Lasko M, Macklin ML, Fischman AJ, Pitman RK (1999): Neural activation during sexual and competitive arousal in healthy men. Psychiatry Res 91: 1–10. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Raichle ME, Robins E, Mintun MA, Fusselman MJ, Fox PT, Price JL, Hackman KA (1989): Neuroanatomical correlates of a lactate‐induced anxiety attack. Arch Gen Psychiatry 46: 493–500. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, Frank E, Thase ME, Houck PR, Jennings JR, Howell JR, Lilienfeld SO, Kupfer DJ (1988): Assessment of sexual function in depressed, impotent, and healthy men: factor analysis of a Brief Sexual Function Questionnaire for men. Psychiatry Res 24: 231–250. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Twyman R, Berent S (1987): Neurological syndrome following bilateral damage to the head of the caudate nuclei. Ann Neurol 22: 768–771. [DOI] [PubMed] [Google Scholar]
- Robinson BW, Mishkin M (1968): Penile erection evoked from forebrain structures in Macaca mulatta. Arch Neurol 19: 184–198. [DOI] [PubMed] [Google Scholar]
- Rolls ET. 1999. The brain and emotion. New York: Oxford University Press; 367p. [Google Scholar]
- Rosen RC, Beck JG (1988a): Concerns involving human subjects in sexual psychophysiology In: Rosen RC, Beck JG, editors. Patterns of sexual arousal. Psychophysiological processes and clinical applications. New York: Guilford, p 345–355. [Google Scholar]
- Rosen RC, Beck JG (1988b): Patterns of sexual response In: Rosen RC, Beck JG, editors. Patterns of sexual arousal. Psychophysiological processes and clinical applications. New York: Guilford, p 23–52. [Google Scholar]
- Russell JA (1980): A circumplex model of affect. J Pers Soc Psychol 39: 1161–1178. [Google Scholar]
- Schultz W, Apicella P, Scarnati E, Ljungberg T (1992): Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci 12: 4595–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira A, Bajos N, ACSF (1993): Les comportements sexuels en France. Paris: La Documentation Francaise. [Google Scholar]
- Stoléru S, Gregoire MC, Gerard D, Decety J, Lafarge E, Cinotti L, Lavenne F, Le Bars D, Vernet‐Maury E, Rada H, Collet C, Mazoyer B, Forest MG, Magnin F, Spira A, Comar D (1999): Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav 28: 1–21. [DOI] [PubMed] [Google Scholar]
- Stoléru S, Redouté J, Grégoire MC, Lavenne F, Le Bars D, Cinotti L, Spira A, Pujol JF (1998): Cerebral correlates of hypoactive sexual desire disorder in men. Paper presented at the 24th annual meeting of the International Academy of Sex Research. Sirmione, Italy.
- Swanson LW, Petrovich GD (1998): What is the amygdala? Trends Neurosci 21: 323–331. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E (1999): Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci U S A 96: 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD, Howard RJ, Cox SG, Ha Y, Brammer MJ, Williams SC, Checkley SA (1999): Functional MRI study of the cognitive generation of affect. Am J Psychiatry 156: 209–215. [DOI] [PubMed] [Google Scholar]
- Terzian J, Dalle Ore G (1955): Syndrome of Kluver and Bucy reproduced in man by bilateral removal of temporal lobes. Neurology 5: 373–380. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuikka J, Kupila J, Partanen K, Vainio P, Airaksinen J, Eronen M, Hallikainen T, Paanila J, Kinnunen I (1994): Increase in cerebral blood flow of right prefrontal cortex in man during orgasm. Neurosci Lett 170: 241–243. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL (1998): The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 44: 1219–1228. [DOI] [PubMed] [Google Scholar]
- Whalen RE (1966): Sexual motivation. Psychol Rev 73: 151–163. [DOI] [PubMed] [Google Scholar]
- Yaremko RM, Harari H, Harrison RC, Lynn E (1982): Reference handbook of research and statistical methods in psychology. New York: Harper and Row. [Google Scholar]


