Abstract
In visual discrimination tasks, the relevant feature to discriminate is defined before stimulus presentation. In feature uncertainty tasks, a cue about the relevant feature is provided after stimulus offset. We used 15O‐butanol positron emission tomography (PET) in order to investigate brain activation during a feature uncertainty task. There was greater activity during the feature uncertainty task, compared with stimulus detection and discrimination of orientation and spatial frequency, in the lateral and medial prefrontal cortex, the cuneus, superior temporal and inferior parietal cortex, cortical motor areas, and the cerebellum. The most robust and consistent activation was observed in the dorsal anterior cingulate cortex (Brodmann area 32; x = 0 y = 16, z = 40). The insula, located near the claustrum (x = −38, y = 8, z = 4), was activated during the discrimination tasks compared with the feature uncertainty condition. These results suggest that the dorsal anterior cingulate cortex is important in feature uncertainty conditions, which include divided attention, expectancy under uncertainty, and cognitive monitoring. Hum. Brain Mapp. 21:26–33, 2004. © 2003 Wiley‐Liss, Inc.
Keywords: vision, feature uncertainty, discrimination, attention, anterior cingulate cortex, positron emission tomography
INTRODUCTION
In a previous study [Gulyás and Roland, 1995], we demonstrated the activation of an overlapping, widely distributed neuronal network during spatial frequency and orientation discrimination [see also Faillenot et al., 2001]. In these experiments, subjects were told the relevant feature to discriminate (spatial frequency or orientation) before stimulus presentation. In contrast to visual discrimination tasks, in feature uncertainty conditions subjects do not know the relevant feature to discriminate until after the offset of stimuli [Vogels et al., 1988; Wing and Allport, 1973]. In this task, subjects divide their attention among multiple visual features from which any can be relevant for discrimination. After stimulus offset, a cue is presented that signifies the relevant feature to discriminate.
Consider the paradigm shown in Figure 1. In the reference condition, the stimulus pair consists of a random dot pattern and a grating, and subjects are asked to indicate if the first member of the pair is the grating. In the orientation and spatial frequency discrimination conditions, only the relevant feature differs between the first and second grating. Subjects are asked to indicate if the first grating is closer to the vertical meridian (orientation discrimination) or to indicate if the spatial frequency of the first grating is higher than that of the second grating (spatial frequency discrimination). In the feature uncertainty condition, both features change and the color of the response signal indicates the relevant feature to discriminate. In discrimination tasks, the color of the response signal is irrelevant because the relevant feature is defined before stimulus onset.
Figure 1.
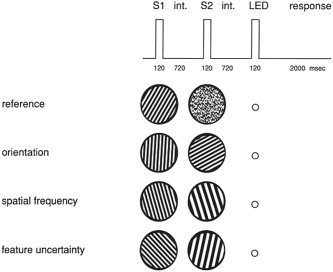
Visual discrimination and feature uncertainty tasks. S1, first stimulus; S2, second stimulus; int., interstimulus interval; LED, response signal.
Cortical fields in the dorsal and ventral visual stream and the prefrontal cortex are active during orientation and spatial frequency discrimination. Cortical areas in the occipito‐temporal and occipito‐parietal regions may be responsible for sensory representation, whereas prefrontal activation may reflect attentional and decision‐making processes [Faillenot et al., 2001; Gulyás and Roland, 1991, 1995; Vidnyánszky et al., 2000]. However, cerebral structures related to feature uncertainty tasks are unknown. It is possible that the visual cortex is especially active during feature uncertainty tasks because of the concurrent analysis of multiple visual features. Alternatively, increased activity may be observed in the prefrontal cortex and related structures because of the divided attention and expectancy components of the feature uncertainty task. To elucidate this issue, we measured regional cerebral blood flow (rCBF) with 15O‐butanol positron emission tomography (PET) during simple stimulus detection (reference condition), orientation and spatial frequency discrimination, and a feature uncertainty task.
SUBJECTS AND METHODS
Participants
Participants were eight male volunteers (age range 24–46 years) with no history of medical conditions that may affect brain functions. They were right‐handed as determined by the Edinburgh Handedness Inventory [Oldfield, 1971] and had normal or corrected‐to‐normal visual acuity. Electrooculography (EOG), electroencephalography (EEG), electromyography (EMG) (movements of the right thumb for responses), and arterial PaO2 and PaCO2 were registered during the experiment [Gulyás and Roland, 1995]. All subjects gave their written informed consent to participate in the study. The study was approved by the Ethical, Radiation Safety, and Magnetic Resonance Imaging Committees of the Karolinska Hospital.
Stimuli and Procedure
Stimuli were vertical luminance‐contrast gratings and random dot patterns (Fig. 1). The spatial frequency ranged between 0.42 and 4.6 cycles/degree in equal steps in a logarithmic scale. The orientation of the gratings covered 0–360 degrees in 15‐degree steps. Stimuli were matched for average luminance (13.5 cd/m2), geometrical complexity as determined by Euler numbers, internal contrast, and edge lengths [for details, see Gulyás and Roland, 1995].
During the experiment, subjects were placed in a supine position in the camera bed. Environmental noise was kept at a minimum. Stimuli were presented on a double‐polarized projection screen (Simda projectors). The viewing distance was 86 cm. The stimulus area covered a circular window. The diameter of the window was of 10 degrees of visual angle. The exposure time for both members of the stimulus pair (S1 and S2) was 120 msec. The inter‐stimulus interval was 720 msec. The second stimulus was followed by a 720‐msec interval, and then a response signal appeared on the screen for 100 msec. The LED‐generated response signal was a green or a red spot of 1 degree diameter. The LED colors were constant (red for spatial frequency and green for orientation). In the reference task, the color had no relevance for decision‐making. Finally, an interval of 2,000 msec was included, during which subjects responded by moving their right thumb (monitored by EMG) (Fig. 1). Before scanning, subjects were given a practice run to ensure that they were able to perform the task.
The experiment included four tasks. (1) In the reference detection task, the stimulus pair consisted of a grating and a random dot pattern. Subjects were asked to indicate if the first member of the pair was the grating. (2) In the orientation discrimination task, a pair of gratings with different orientations was presented. The spatial frequency was constant. Subjects were asked to indicate if the first grating was closer to the vertical meridian. (3) In the spatial frequency discrimination task, two gratings with different spatial frequency were presented. The orientation was constant. Subjects were asked to indicate if the spatial frequency of the first grating was higher than that of the second grating. (4) In the feature uncertainty task, both orientation and spatial frequency of S1 and S2 were different. If the response signal was green, subjects had to indicate in a similar way as they did in the orientation discrimination condition. If the signal was red, they had to use the response strategy of the spatial frequency condition. The order of tasks was randomized and counterbalanced across subjects. Subjects responded to each stimulus in a two‐alternative forced choice manner. The number of targets was matched across tasks. The time‐interval between the blocks was 20–25 min.
Brain Scanning
During the high‐resolution magnetic resonance imaging (MRI) and PET recordings, individually molded plastic head fixation helmets were used in order to hold the head in an identical position. For the PET measurements, a Scanditronix 2048‐15B camera was used. The 15O‐butanol tracer was given in a bolus injection before each task in the cubital vein [65 ± 5 mCi dissolved in 7 ml solution of physiological saline (90%) and ethanol (10%)]. Data were obtained for a total of 100 sec in 20 subsequent scans from which, following delay correction, the first 80 sec was included in the data analysis. The measurement of rCBF and stimulus presentation started simultaneously [for further methodological details, see Gulyás and Roland, 1995; Roland et al., 1993].
Data Analysis
The statistical parametric mapping software (SPM99, v. 1999, Welcome Department of Cognitive Neurology, London, UK) was used. The SPM software ran under a MATLAB package (Mathworks, Sherborn, MA) in an Octane‐2 Silicon Graphics workstation. T1‐weighted structural magnetic resonance images (Siemens) were coregistered to the PET images. Scans were realigned using the first scan for reference. Following realignment, images were stereotactically transformed to the Montreal Neurological Institute template space [Friston et al., 1995a], and then were smoothed with an isotropic Gaussian kernel of 5 mm FWHM. After spatial pre‐processing, statistical parametric maps were calculated, which were based on the general linear model and the theory of Gaussian fields [Friston et al., 1991, 1994]. Condition and subject effects were calculated at each voxel. Analysis of covariance (ANCOVA) was used, including global brain activity as covariate of no interest (fixed at 50 ml/dl/min) [Friston et al., 1995b]. Three contrasts were defined: (1) feature uncertainty vs. rest (FU > RE), (2) feature uncertainty vs. orientation discrimination (FU > OR), and (3) feature uncertainty vs. spatial frequency discrimination (FU > SF). The reverse of the three contrasts was also determined (RE > FU, OR > FU, SF > FU). For each contrast, the voxel values constituted a statistical parametric map of t‐statistics (SPM(t)). The SPM(t) data were transformed to the unit of normal distribution (SPM(Z)). The level of significance was set at P < 0.001 (uncorrected) and P < 0.05 (corrected according to the standard SPM procedure). The localization of brain areas was given using the Talairach–Tournoux coordinates [Talairach and Tournoux, 1988].
RESULTS
Behavioral and Physiological Measures
Performance and reaction time were as follows: RE: 100% (SD = 0), 609.3 msec (SD = 49.0); OR: 96.9% (SD = 4.6), 636.6 msec (SD = 56.8); SF: 97.4% (SD = 2.9), 633.6 msec (SD = 59.6); FU: 96.5% (SD = 4.9), 650.4 msec (SD = 44.7). There was no significant difference between test performances (Mann‐Whitney U test, P > 0.05). Log‐transformed reaction time data were entered into an analysis of variance (ANOVA). There was no significant main effect of test condition (P > 0.1). Table I shows the physiological parameters.
Table I.
Physiological parameters
| Parameters | Reference | Orientation | Spatial frequency | Feature uncertainty |
|---|---|---|---|---|
| gCBF | 54.5 ± 7.2 | 56.7 ± 9.9 | 57.1 ± 9.9 | 55.5 ± 6.9 |
| PaO2 | 12.3 ± 1.7 | 12.1 ± 1.3 | 12.5 ± 2.0 | 12.0 ± 1.7 |
| PaCO2 | 5.77 ± 0.5 | 5.77 ± 0.4 | 5.76 ± 0.4 | 5.72 ± 0.5 |
| EOG (Hz) | 0.46 ± 0.06 | 0.46 ± 0.04 | 0.45 ± 0.03 | 0.48 ± 0.03 |
| EEG (alpha blockade, %) | 6.8 ± 1.4 | 8.1 ± 2.4 | 4.7 ± 2.3 | 6.1 ± 1.0 |
Feature Uncertainty vs. Rest
Table II shows the brain areas that were active in the FU > RE contrast. At the cluster‐level, only two regions reached the level of statistical significance: the dorsal anterior cingulate cortex (dACC) (tentative BA 32) and the cuneus (tentative BA 18) (Fig. 2A). When data were corrected for multiple comparisons, only the dACC remained significant (t = 15.11, Z = 6.07, P < 0.05).
Table II.
Significant regional brain activity in the feature uncertainty vs. rest (FU > RE) contrast*
| Brain region | Coordinates (x, y, z) | Z‐score | t‐score |
|---|---|---|---|
| Anterior cingulate (BA 32) | 0, 16, 40 | 6.07 | 15.11 |
| Right cuneus (BA 18) | 4, −74, 20 | 4.41 | 6.94 |
| Right inferior frontal gyrus (BA 45) | 36, 48, 16 | 3.75 | 5.18 |
| Anterior cingulate (BA 32) | 0, 28, 32 | 3.75 | 5.17 |
| Right inferior parietal lobe (BA 40) | 58, −34, 26 | 3.60 | 4.85 |
| Right cerebellum | 38, −74, −32 | 3.52 | 4.68 |
| Right cerebellum | 36, −76, −26 | 3.31 | 4.26 |
Coordinates are given according to Talairach and Tornoux [ 1988]. BA: Brodmann area; P < 0.001, uncorrected.
Figure 2.
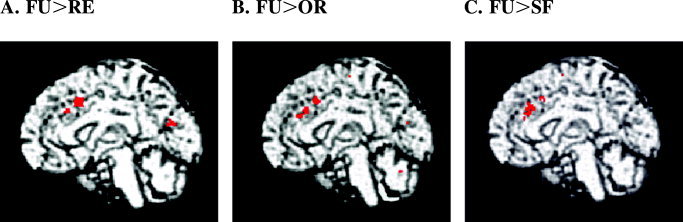
Brain activation in the feature uncertainty‐reference (FU > RE), feature uncertainty‐orientation discrimination (FU > OR), and feature uncertainty‐spatial frequency discrimination (FU > SF) contrasts (P < 0.001, uncorrected). The dorsal anterior cingulate cortex (Brodmann area 32; x = 0, y = 16, z = 40) was significantly activated in each contrast. In the FU > RE contrast, the cuneus was also activated (Brodmann area 18; x = 4, y = −74, z = 20).
Feature Uncertainty vs. Orientation Discrimination
Table III shows the significantly activated brain areas in the FU > OR contrast. When data were corrected for multiple comparisons, significant activity was found only in the dACC (tentative BA 32) (t = 10.78, Z = 5.38, P < 0.05) (Fig. 2B).
Table III.
Significant regional brain activity in the feature uncertainty vs. orientation discrimination (FU > OR) contrast
| Brain region | Coordinates | Z‐score | t‐score | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Anterior cingulate (BA 32) | 0 | 16 | 40 | 5.38 | 10.78 |
| Left cerebellum | −2 | −68 | −26 | 4.28 | 6.54 |
| Right inferior frontal gyrus (BA 45) | 36 | 48 | 16 | 4.24 | 6.44 |
| Right cuneus (BA 18) | 4 | −74 | 20 | 4.10 | 6.04 |
| Anterior cingulate (BA 32) | 0 | 28 | 32 | 3.85 | 5.43 |
| Left middle frontal gyrus (BA 9) | −22 | 34 | 36 | 3.51 | 4.65 |
| Right cerebellum | 38 | −74 | −32 | 3.39 | 4.42 |
| Right precentral gyrus (BA 4) | 14 | −22 | 64 | 3.35 | 4.33 |
| Medial part of the right superior frontal gyrus (BA 6) | 6 | −4 | 64 | 3.32 | 4.28 |
* Coordinates are given according to Talairach and Tornoux [ 1988]. BA: Brodmann area; P < 0.001, uncorrected.
Feature Uncertainty Versus Spatial Frequency Discrimination
Table IV shows the significantly activated brain areas in the FU > SF contrast. Similarly to the FU > OR contrast, only the dACC (tentative BA 32) was active when data were corrected for multiple comparisons (t = 7.81, Z = 4.68, P < 0.05) (Fig. 2C).
Table IV.
Significant regional brain activity in the feature uncertainty vs. spatial frequency (FU > SF) discrimination contrast*
| Brain region | Coordinates | Z‐score | t‐score | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Anterior cingulate (BA 32) | 0 | 16 | 40 | 4.68 | 7.81 |
| Right superior temporal gyrus (BA 22) | 58 | −36 | 8 | 4.58 | 7.49 |
| Medial part of the right superior frontal gyrus (BA 9) | 4 | 34 | 32 | 4.36 | 6.79 |
| Right inferior frontal gyrus (BA 45) | 36 | 48 | 16 | 4.07 | 5.97 |
| Right middle frontal gyrus (BA 9) | 48 | 24 | 28 | 3.90 | 5.55 |
| Medial part of the right superior frontal gyrus (BA 6) | 4 | −2 | 62 | 3.81 | 5.32 |
| Right anterior cingulate (BA 32) | 6 | 36 | 20 | 3.80 | 5.31 |
| Right precentral gyrus (BA 4) | 42 | 0 | 44 | 3.30 | 4.24 |
Coordinates are given according to Talairach and Tornoux [ 1988]. BA: Brodmann area; P < 0.001, uncorrected.
Reverse Contrasts
In the RE > FU contrast, we found no supra‐threshold values. In the OR > FU comparison, the insula, near to the claustrum, was active (x = −38, y = 8, z = 4; t = 10.05, Z = 5.23, P < 0.05, corrected). The same area was observed in the SF > FU contrast (t = 8.26, Z = 4.80, P < 0.05, corrected).
DISCUSSION
Summary of Brain Activation
We found the dACC to be active in each contrast (FU > RE, FU > OR, FU > SF) (Fig. 3). This suggests that the dACC is the core of a complex network, which participates in divided attention and expectancy for the cue related to the selection of features and response strategies.
Beyond the dACC, several other areas are worthy of mention. The inferior lateral prefrontal cortex (tentative BA 45) was also active in each contrast. Extrastriatal visual areas (the cuneus located near to the middle occipital gyrus) were engaged in the FU > RE and FU > OR contrasts. Interestingly, a right‐sided (ipsilateral) activation of motor areas (tentative BA 4) was observed in the FU > OR and FU > SF contrasts but not in the FU > RE comparison. The most extensive prefrontal activity was observed in the FU > SF contrast, including the medial prefrontal cortex localized anterior to the dACC (Tables I, II, III). Finally, the insula, near the claustrum, was active in the SF > FU and OR > FU contrasts. In the following sections, we discuss the functional significance of these findings. Brain areas will be divided into four groups: (1) areas in the prefrontal cortex, (2) cerebellum and cortical motor areas, (3) visual cortex, (4) insula/claustrum.
Prefrontal Cortex
The exact functional organization of the cingulate cortex is unclear. Evidence from clinical research, animal models, and functional neuroimaging studies suggests that arousal, motor control, cognitive processes, and drive/emotion are integrated in the ACC [Allman et al., 2001; Bush et al., 2000; Devinsky et al., 1995; Paus, 2001]. The role of the dACC is emphasized in relation to voluntary actions/mental effort [Cohen et al., 1999; Paus et al., 1998], arousal and attention [Corbetta et al., 1991; Janer and Pardo, 1991; Koski and Petrides, 2002], response selection [Paus et al., 1993; Schumacher and D'Esposito, 2002; Turken and Swick, 1999], error detection [Dehaene et al., 1994; Gehring and Knight, 2000], conflict monitoring [Botvinick et al., 1999; Carter et al., 1998; Fallgatter et al., 2002; van Veen et al., 2001], reward and punishment [Bush et al., 2002; Gehring and Willoughby, 2002], and various aspects of social cognition [Calder et al., 2002; Frith, 2002; Hadland et al., 2003; Shallice, 2001].
There are at least four critical cognitive functions that are involved in the feature uncertainty task: (1) attention is divided between features, (2) features and response strategies are maintained on‐line in working memory, (3) the cue is anticipated under uncertainty, and (4) the appropriate response is selected according to the cue. These are essential components of decision‐making processes [Payne et al., 1992]. Consistent with our results, it has been demonstrated that dACC is activated during divided attention tasks [Corbetta et al., 1991]. The dACC also plays a critical role in anticipation and arousal in situations with uncertain outcomes [Critchley et al., 2001]. Pollmann et al. [ 2000] used a visual search task in which the feature of the target‐to‐be‐detected was uncertain (color or motion). These authors also found activation in the dACC during the uncertain condition, although the exact localization of this area was different from that in the present study.
The dACC and the lateral prefrontal cortex were activated together during the feature uncertainty task. It is possible that the lateral prefrontal cortex (tentative BA 45) is related to the maintenance of visual information in working memory [Glahn et al., 2002; Petrides, 1996; Postle and D'Esposito, 1999]. However, the exact functional significance of the dACC and lateral prefrontal activity is unclear. MacDonald et al. [ 2000] claimed that while the lateral prefrontal cortex participates in allocating and maintaining top‐down attentional control, the primary role of the dACC is conflict monitoring. An alternative hypothesis suggests that the dACC is responsible for the selection of motor responses, while decision‐making takes place in the lateral prefrontal cortex [Paus et al., 1993; Turken and Swick, 1999]. In the OR > RE and SF > RE comparisons, we found no significant activation in the dACC. Therefore, it is unlikely that this cortical area is related to the maintenance of visual information. In addition, response selection and basic decision‐making processes were also included in the discrimination tasks, which is against the possibility that activation under the feature uncertainty task is due to these processes.
Conflict monitoring is important when processing conflicts appear between simultaneously active and incompatible representations [Posner and DiGirolamo, 1998]. The feature uncertainty task includes a similar cognitive component because, in the pre‐cue phase, competitive features and related response strategies are processed. Similarly to previous studies emphasizing the significance of conflict monitoring [Carter et al., 1998; Dreher and Grafman, 2003; van Veen et al., 2001], the dACC was activated under the feature uncertainty task. It is important to emphasize that the four tasks had similar difficulties and performances revealed a ceiling effect. Therefore, the differential dACC responses can not be explained with task difficulty [Paus et al., 1998].
The feature uncertainty task is a complex procedure that can be solved by remembering both stimulus dimensions and associated responses (decisions) or by deciding after the cue. Another possibility is that subjects alternated between the two strategies. It is difficult to objectively determine and experimentally control the strategy. Verbal reports suggest that the majority of subjects decide only after the cue, and nobody seems to alternate between the two strategies.
Because of the limitation of our experimental design, it is not possible to conclude about the basic function of the dACC. In general, it is difficult to attribute a single cognitive function to the dACC. In a single cell study, Schall et al. [ 2002] found that neurons in the ACC did not control movement initiation but signaled the production of errors, the anticipation of reinforcement, and the presence of processing conflict. Rushworth et al. [ 2003] concluded that it is difficult to separate specific cognitive functions of the ACC from simple attentional effects. We have discussed the question of information maintenance, decision‐making/response selection, task difficulty, and switching between alternative strategies. These are not likely to specifically contribute to brain activation during feature uncertainty tasks. In contrast, divided attention, expectancy under uncertainty, and higher monitoring demands may be critical. However, it is also possible that the dACC activity reflects efforts by subjects to learn the task since they received no practice before scanning [Milham et al., 2003]. Further work is needed to test this hypothesis by training subjects in the feature uncertainty task before scanning.
Cerebellum and Motor Areas
In the FU > RE and FU > OR contrasts, the cerebellum was activated. In the FU > OR and FU > SF contrasts, activation was observed in the cortical motor areas (tentative BA 4 and BA 6). Cerebellar activation was repeatedly found when subjects performed tasks that required selective and divided attention, anticipation, and working memory [Cabeza and Nyberg, 2000; Middleton and Strick, 1998; but see Nixon and Passingham, 1999]. However, during the feature uncertainty task, expectancy may result in the imagery of motor responses, which may lead to the activation of cortical motor areas and the cerebellum [Decety et al., 1994; Naito et al., 2002; Roth et al., 1996]. It is notable, however, that the BA 6 also receives an intensive visual input [Okano, 1992], which may contribute to its role in our present paradigm. Interestingly, activity in these areas is common during higher‐level cognitive tasks [Cabeza and Nyberg, 2000].
Visual Areas
In the feature uncertainty task, more features are processed simultaneously (spatial frequency and orientation) than in discrimination tasks (spatial frequency or orientation), which may result in enhanced activity in the visual cortex. Indeed, the cuneus was activated in the FU > RE contrast in which the reference task was perceptually simple. Herath et al. [ 2001] found the cuneus to be activated during the recognition of visual patterns. The anteromedial cuneus is believed to interact with the primary visual cortex and may modify information transfer to higher‐order extrastriate cortices [Vanni et al., 2001]. It is notable that stimulus pairs differed in both spatial frequency and orientation only in the feature uncertainty condition. However, it is unlikely that dACC activation in each contrast is due to this fact.
In the FU > SF contrast, extensive activations were detected in the prefrontal cortex. Is spatial frequency processing special? In our previous study, we found that the volume of activated cortical fields during spatial frequency discrimination was two and a half times larger than that during orientation discrimination [Gulyás and Roland, 1995]. These findings are consistent with recent theories of natural image perception that emphasize the importance of multi‐resolution filtering in early stages of visual processing. The early filtering process may be mediated by specific frequency‐tuned channels [Brady, 1997; and see also Dragoi et al., 2002].
The superior temporal cortex (tentative BA 22) was exclusively activated in the FU > SF contrast. This area was also identified in a visual search task during an uncertain condition [Pollmann et al., 2000]. The superior temporal cortex receives both object‐based and space‐based visual information, and in the right hemisphere it is believed to be related to spatial awareness and exploration [Karnath, 2001].
Insula/Claustrum
The insula, located near the claustrum, was activated in the SF > FU and OR > FU contrasts. This suggests that this area is engaged when visual processing is focused on a single feature (discrimination), but not when multiple features and associated response strategies must be simultaneously processed (feature uncertainty). There is some evidence that the insula receives information from multiple sensory modalities and may play a role in attentional selection [Augustine, 1996; Hadjikhani and Roland, 1998; Lewis et al., 2000].
Conclusions
We found the dACC to be active during the feature uncertainty task as compared with simple discrimination tasks. This area is important in higher cognitive processes [Allman et al, 2001; Bush et al., 2000; Devinsky et al., 1995; Paus, 2001] and seems to coordinate the processing of competing features and response‐sets. Divided attention, anticipation under uncertainty, and cognitive monitoring may be essential components of the feature uncertainty task. The insula/claustrum was activated during the discrimination tasks as compared with feature uncertainty, suggesting its role in single feature processing during discrimination.
Acknowledgements
Szabolcs Kéri was supported by a post doctoral stipend from the Wenner‐Gren Foundation, Stockholm, Sweden. We express our gratitude to Dr. Rufin Vogels for his suggestions and advice.
This work was done at the Department of Neuroscience, Karolinska Institute.
REFERENCES
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P (2001): The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann NY Acad Sci 935: 107–117. [PubMed] [Google Scholar]
- Augustine JR (1996): Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 22: 229–244. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD (1999): Conflict monitoring versus selection‐for‐action in anterior cingulate cortex. Nature 402: 179–181. [DOI] [PubMed] [Google Scholar]
- Brady N (1997): Spatial scale interactions and image statistics. Perception 26: 1089–1100. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI (2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR (2002): Dorsal anterior cingulate cortex: a role in reward‐based decision making. Proc Natl Acad Sci USA 99: 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L (2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Lawrence AD, Keane J, Scott SK, Owen AM, Christoffels I, Young AW (2002): Reading the mind from eye gaze. Neuropsychologia 40: 1129–1138. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998): Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280: 747–749. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Kaplan RF, Zuffante P, Moser DJ, Jenkins MA, Salloway S, Wilkinson H (1999): Alteration of intention and self‐initiated action associated with bilateral anterior cingulotomy. J Neuropsychiat Clin Neurosci 11: 444–453. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE (1991): Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci 11: 2383–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ (2001): Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron 29: 537–545. [DOI] [PubMed] [Google Scholar]
- Decety J, Perani D, Jeannerod M, Bettinardi V, Tadary B, Woods R, Mazziotta JC, Fazio F (1994): Mapping motor representations with positron emission tomography. Nature 371: 600–602. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM (1994): Localization of a neural system for error detection and compensation. Psychol Sci 5: 303–305. [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA (1995): Contributions of anterior cingulate cortex to behaviour. Brain 118: 279–306. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Grafman J (2003): Dissociating the roles of the rostral anterior cingulate and the lateral prefrontal cortices in performing two tasks simultaneously or successively. Cereb Cortex 13: 329–339. [DOI] [PubMed] [Google Scholar]
- Dragoi V, Sharma J, Miller EK, Sur M (2002): Dynamics of neuronal sensitivity in visual cortex and local feature discrimination. Nat Neurosci 5: 883–891. [DOI] [PubMed] [Google Scholar]
- Faillenot I, Sunaert S, Van Hecke P, Orban GA (2001): Orientation discrimination of objects and gratings compared: an fMRI study. Eur J Neurosci 13: 585–596. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Bartsch AJ, Herrmann MJ (2002): Electrophysiological measurements of anterior cingulate function. J Neural Transm 109: 977–988. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RSJ (1991): Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab 11: 690–699. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziota JC, Evans AC (1994): Assessing the significance of focal activation using their spatial extent. Hum Brain Mapp 1: 214–220. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Poline JB, Frith CD, Heather JD, Frackowiak RSJ (1995a): Spatial realignment and normalization of images. Hum Brain Mapp 3: 165–189. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD (1995b): Statistical parametric maps in human functional imaging: a general linear approach. Hum Brain Mapp 3: 189–210. [Google Scholar]
- Frith C (2002): Attention to action and awareness of other minds. Conscious Cogn 11: 481–487. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT (2000): Prefrontal‐cingulate interaction in action monitoring. Nat Neurosci 3: 516–520. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR (2002): The medial frontal cortex and rapid processing of monetary gains and losses. Science 295: 2279–2282. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Kim J, Cohen MS, Poutanen VP, Therman S, Bava S, Van Erp TG, Manninen M, Huttunen M, Lonnqvist J, Standertskjold‐Nordenstam CG, Cannon TD (2002): Maintenance and manipulation in spatial working memory: dissociations in the prefrontal cortex. Neuroimage 17: 201–213. [DOI] [PubMed] [Google Scholar]
- Gulyás B, Roland PE (1991): Cortical fields participating in form and colour discrimination in the human brain. Neuroreport 2: 585–588. [DOI] [PubMed] [Google Scholar]
- Gulyás B, Roland PE (1995): Cortical fields participating in spatial frequency and orientation discrimination: functional anatomy by positron emission tomography. Hum Brain Mapp 3: 133–152. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Roland PE (1998): Cross‐modal transfer of information between tactile and the visual representations in the human brain: a positron emission tomography study. J Neurosci 18: 1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland KA, Rushworth MF, Gaffan D, Passingham RE (2003): The effect of cingulate lesions on social behaviour and emotion. Neuropsychologia 41: 919–931. [DOI] [PubMed] [Google Scholar]
- Herath P, Kinomura S, Roland PE (2001): Visual recognition: evidence for two distinctive mechanisms from a PET study. Hum Brain Mapp 12: 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janer KW, Pardo JV (1991): Deficits in selective attention following bilateral anterior cingulotomy. J Cogn Neurosci 3: 71–86. [DOI] [PubMed] [Google Scholar]
- Karnath H‐O (2001): New insights into the functions of the superior temporal cortex. Nat Rev Neurosci 2: 568–576. [DOI] [PubMed] [Google Scholar]
- Koski L, Petrides M (2002): Distractibility after unilateral resections from the frontal and anterior cingulated cortex in humans. Neuropsychologia 40: 1059–1072. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Beauchamp MS, DeYoe E (2000): A comparison of visual and auditory motion processing in human cerebral cortex. Cereb Cortex 10: 873–888. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS (2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulated cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (1998): Cerebellar output: motor and cognitive channels. Trends Cogn Sci 2: 348–354. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus ED, Cohen NJ (2003): Practice‐related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage 18: 483–493. [DOI] [PubMed] [Google Scholar]
- Naito E, Kochiyama T, Kitada R, Nakamura S, Matsumura M, Yonekura Y, Sadato N (2002): Internally simulated movement sensations during motor imagery activate cortical motor areas and the cerebellum. J Neurosci 22: 3683–3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon PD, Passingham RE (1999): The cerebellum and cognition: cerebellar lesions do not impair spatial working memory or visual associative learning in monkeys. Eur J Neurosci 11: 4070–4080. [DOI] [PubMed] [Google Scholar]
- Okano K (1992): Temporal priority of premotor cortex over nearby areas in receiving visual cues in primates. Neuroreport 3: 389–392. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness, the Edinburgh Inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Paus T (2001): Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2: 417–424. [DOI] [PubMed] [Google Scholar]
- Paus T, Petrides M, Evans AC, Meyer E (1993): Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J Neurophysiol 70: 453–469. [DOI] [PubMed] [Google Scholar]
- Paus T, Koski L, Caramanos Z, Westbury C (1998): Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. Neuroreport 9: R37–47. [DOI] [PubMed] [Google Scholar]
- Payne JW, Bettman JR, Johnson EJ (1992): Behavioral decision research: a constructive processing perspective. Annu Rev Psychol 43: 87–131. [Google Scholar]
- Petrides M (1996): Specialized systems for the processing of mnemonic information within the primate frontal cortex. Philos Trans R Soc Lond B Biol Sci 351: 1455–1461. [DOI] [PubMed] [Google Scholar]
- Pollmann S, Weidner R, Muller HJ, von Cramon DY (2000): A fronto‐posterior network involved in visual dimension changes. J Cogn Neurosci 12: 480–494. [DOI] [PubMed] [Google Scholar]
- Posner MI, DiGirolamo GJ (1998): Executive attention: conflict, target detection and cognitive control In: Parasuraman R, editor. The attentive brain. Cambridge: MIT Press; p 401–423. [Google Scholar]
- Postle BR, D'Esposito M (1999): “What”‐then‐“Where” in visual working memory: an event‐related fMRI study. J Cogn Neurosci 11: 585–597. [DOI] [PubMed] [Google Scholar]
- Roth M, Decety J, Raybaudi M, Massarelli R, Delon‐Martin C, Segebarth C, Morand S, Gemignani A, Decorps M, Jeannerod M (1996): Possible involvement of primary motor cortex in mentally simulated movement: a functional magnetic resonance imaging study. Neuroreport 7: 1280–1284. [DOI] [PubMed] [Google Scholar]
- Roland PE, Levin B, Kawashima R, Akerman S (1993): Three‐dimensional analysis of clustered voxels in 15O‐butanol brain activation images. Hum Brain Mapp 1: 3–19. [Google Scholar]
- Rushworth MF, Hadland KA, Gaffan D, Passingham RE (2003): The effect of cingulate cortex lesions on task switching and working memory. J Cogn Neurosci 15: 338–353. [DOI] [PubMed] [Google Scholar]
- Schall JD, Stuphorn V, Brown JW (2002): Monitoring and control of action by the frontal lobes. Neuron 36: 309–322. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, D'Esposito M (2002): Neural implementation of response selection in humans as revealed by localized effects of stimulus‐response compatibility on brain activation. Hum Brain Mapp 17: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T (2001): “Theory of mind” and the prefrontal cortex. Brain 124: 247–248. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. Stuttgart: Thieme Verlag. [Google Scholar]
- Turken AU, Swick D (1999): Response selection in the human anterior cingulate cortex. Nat Naurosci 2: 920–924. [DOI] [PubMed] [Google Scholar]
- Vanni S, Tanskanen T, Seppa M, Uutela K, Hari R (2001): Coinciding early activation of the human primary visual cortex and anteromedial cuneus. Proc Natl Acad Sci USA 98: 2776–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS (2001): Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage 14: 1302–1308. [DOI] [PubMed] [Google Scholar]
- Vidnyánszky Z, Gulyás B, Roland PE (2000): Visual exploration of form and position with identical stimuli: functional anatomy with PET. Hum Brain Mapp 11: 104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels R, Eeckhout H, Orban GA (1988): The effect of feature uncertainty on spatial discriminations. Perception 17: 565–577. [DOI] [PubMed] [Google Scholar]
- Wing A, Allport DA (1973): Multidimensional encoding of visual form. Percept Psychophys 12: 474–476. [Google Scholar]


