Abstract
Insight problem solving has been the topic of much investigation. It is believed widely that insight critically contains the process of breaking one's mental set. Recent functional magnetic resonance imaging (fMRI) research on puzzle solving showed that insight was associated with activities in anterior cingulate cortex (ACC) and other areas (Luo and Niki [2003]: Hippocampus 13:274–281). We proposed ACC might mediate processes of breaking one's mental set, given its well‐known role in cognitive conflict. In the present research, high‐density event‐related potentials (ERPs) were recorded to examine the electrophysiologic correlates of insight problem solving. One hundred twenty interesting Chinese riddles (half difficult and half easy) were adopted as materials. For each trial, subjects were either given an easy puzzle followed by a keyword that was consistent with the subject's initial thinking (“No‐aha answer”), or a difficult puzzle followed by a keyword that was consistent with an unusual interpretation, so that it broke the subject's initial mental set (“Aha answer”). Results from 14 subjects showed that Aha answers elicited a more negative ERP deflection than did No‐aha answers in the time window from 250–500 msec after onset of the answer. The ERP difference wave (Aha minus No‐aha answer) showed the maximum amplitude over the central site (Cz) with a peak latency of 380 msec (N380). Voltage and current density maps of the difference wave showed strong activity and current density in the frontocentral region. Dipole analysis localized the generator of the N380 in the ACC. N380 therefore probably reflects an “Aha!” effect, and the ACC generator may be involved in the breaking of mental set. Hum. Brain Mapp. 22:261–270, 2004. © 2004 Wiley‐Liss, Inc.
Keywords: insight problem solving, event‐related potentials, dipole source localization, anterior cingulate cortex
INTRODUCTION
Since Köhler [1925] observed that chimpanzees could resolve problems suddenly, the processes of insight in problem solving have been the subject of much investigation [e.g., Duncker, 1945; Kaplan and Simon, 1990; Knoblich et al., 1999; Lavric et al., 2000; Maier, 1931; Ormerod et al., 2002]. The term “insight” has been used to name the process by which a problem solver suddenly moves from a state of not knowing how to solve a problem to a state of knowing how to solve it. Usually, solving insight problems such as the candle problem, the two‐string problem, and the nine‐dot problem does not require specific cognitive skills; everyone is capable of finding the solution once the problem is thought about in the correct direction. Why, then, are insight problems so difficult to solve? Some researchers believe it is because of mental fixation on an inappropriate view of the problem content [Glucksberg, 1962; Isaak and Just, 1995; Smith and Blankenship, 1991; Weisberg and Alba, 1981]. For example, in the nine‐dot problem, subjects are asked to connect nine dots (in a three‐by‐three matrix‐like arrangement) with four connected straight lines without retracing and without lifting the pen off the paper until the end of the final line. The problem's difficulty is believed to arise from the unwarranted assumption that the lines must not extend outside the boundary of the square formed by the nine dots [Scheerer, 1963]. Only when participants break this assumption can they get to the solution of the problem. Insight happens at the instant when mental fixation is broken, which is sudden and characterized by the subjective “Aha!” experience. The occurrence of insight or Aha! experience means rethinking some basic assumptions about the problem content, which happens in a relatively sudden and unpredictable manner.
For more than half a century, insight in humans and animals has been investigated in behavioral studies [e.g., Epstein et al., 1984; Köhler, 1925; Knoblich et al., 1999; Lung and Dominowski, 1985; Ormerod et al., 2002; Scheerer, 1963; Weisberg and Alba, 1981]. However, the neural basis of insight remains unknown. Recently developed brain imaging techniques such as functional magnetic resonance imaging (fMRI), positron emission topography (PET), and event‐related potentials (ERPs) have made it possible for us to record precisely the brain activity associated with many high‐level cognitive processes. It remains difficult to investigate neural correlates of insight problem solving, however, given that they are sporadic, unpredictable, short‐lived moments of exceptional thinking. In our recent fMRI studies [Luo and Niki, 2003], we recorded the neural activity correlated with insight by providing a trigger (the solution) to catalyze the insightful riddle solving process. Our results showed that, relative to the resting state, insight riddle solving was associated with activities in wide cerebral areas that critically included anterior cingulate (ACC), prefrontal cortex (PFC), posterior parietal cortex, and medial temporal lobe. We proposed that ACC might mediate processes of breaking one's mental set, given its well‐known role in cognitive conflict [reviewed in Botvinick et al., 2001]. We believed that insight is a complex cognitive processing, including the cognitive conflict between correct and incorrect thought, and the meta‐cognitive process of error awareness when the error of one's own way of thinking is realized.
To investigate further the temporal course of brain processes underlying insight problem solving, we introduced the experimental paradigm of guessing riddles into an ERP study. We have all experienced the feeling of insight after being given the answer to a riddle we could not solve; thus, Aha! responses can be evoked in riddle tasks. In the present study, interesting Chinese riddles were presented to the participants (e.g., “The thing that is very old, but very valuable”). If they failed to resolve the riddle, participants reported they had a feeling of insight when they saw the correct answer (“antique”). The electrophysiologic correlates of insight were detected by recording the electroencephalogram (EEG) of the comprehension to the answer. Although recognizing a presented solution was not quite the same as finding a solution after having dwelled upon alternative unsuitable approaches, our procedure did induce a process containing the critical features of insight. Firstly, this process occurred after an impasse state in which: (1) the information to solve the problem was adequate; (2) the method to solve the problem was well within the competence of the problem solver; and (3) sufficient consideration was given to alternative approaches. Secondly, this procedure led to an Aha! reaction in which the impasse was broken suddenly and transformation from not knowing to knowing was attained rapidly. The experimental procedure therefore provided us with a good way to investigate the processes of insight or Aha! response in problem solving using brain‐imaging techniques.
The purpose of the present study was to investigate the neural basis of insight or Aha! response elicited during a guessing riddles task using high‐density (64 channel) ERP recordings. First, we wanted to know which ERP component is involved in insight in problem solving. Riddles were used to evoke insight or Aha! response in the present study. Semantic processes therefore were involved in problem solving. As an ERP component, the N400 has been used widely to measure different aspects of language comprehension. The N400, first described by Kutas and Hillyard [1980], was elicited in response to semantically deviant stimuli (e.g., “he spread the warm bread with sock” would produce an N400 to the word sock). It is considered to reflect neural activity associated with processes related to semantic or lexical access to word representations [Kutus and van Petten, 1994] or, alternatively, to processes integrating word representations with current context [Holcomb, 1993]. We examined whether N400 can be observed in the present study.
Second, the fMRI study by Luo and Niki [2003] found activation of ACC during insight. ACC was proposed to be involved in the resolution of cognitive conflict [Barch et al., 2000; Botvinick et al., 1999, 2001; MacDonald et al., 2000; van Veen and Carter, 2002a; van Veen et al., 2001]. This point of view is based partially on data from studies using ERPs. The ERP component N2 seems to increase in conditions in which response conflict was high and has been considered to be generated in ACC and reflect conflict detection [van Veen and Carter, 2002a]. Another conflict‐related ERP component, error negativity (Ne) or error‐related negativity (ERN), has been proposed also to be generated in ACC [Dehaene et al., 1994; Gehring and Willoughby, 2002; Gehring et al., 2000; Holroyd et al., 1998]. As a large negative‐going peak, the Ne/ERN was elicited after onset of the erroneous response and was taken as an electrophysiologic index of ACC activity [Botvinick et al., 2001; Carter et al., 1998; Falkenstein et al., 2000; Kiehl et al., 2000; Menon et al., 2001]. Because it is thought that the N2 and ERN/Ne are generated in ACC, we were also interested in whether N2 or ERN/Ne is obtained during insight. Moreover, the method of dipole source localization was used to determine the neural generator of the component involved in insight.
In summary, ERP recordings provide critical temporal information for analyzing the functional neuroanatomy of insight in problem solving. Bringing together the anatomic specificity of fMRI mapping and the time resolution of ERP recordings makes it possible to characterize the functional roles of specific brain areas in cognitive processes of insight. To the best of our knowledge, this work is the first ERP study to have investigated the electrophysiologic correlates of insight in problem solving.
SUBJECTS AND METHODS
Participants
As paid volunteers, 14 undergraduates and graduates (8 women, 6 men) aged 19–24 years (mean age, 22.2 years) from Peking University and Beijing Agricultural University participated in the experiment. All participants were healthy, right‐handed, and had normal or corrected to normal vision.
Stimuli
We selected 120 riddles that were evaluated as highly interesting and reasonable (matching with the solution to riddles) by a group of subjects who did not participate in the formal ERP experiment. About one‐half of the 120 riddles were very difficult and another one‐half was comparatively easy. It was not easy for participants to think the solutions to those difficult riddles so that the insight or Aha! response could be elicited when they knew the correct answer. For example, to the question “The thing that is very old, but very valuable,” the answer is “antique.” Easy riddles were also used as controls, because participants could easily think of solutions to these riddles and thus insight did not occur when participants found that the feedback answer was identical to one they had thought. For instance, to the question “Though they veil your eyes, you see clearer,” the answer is “glasses.” The things that the answers of the riddles indicated were common objects or phenomena. Participants were familiar with these things and were willing to do this task. The length of each question was within 20 Chinese characters, and each answer within three Chinese characters. The words that appeared in the questions and in the answers were high‐frequency words.
Procedure
The experimental paradigm was illustrated in Figure 1. At first the sentence was presented in the center of screen for 8 sec followed by a 2‐sec interval. Participants were instructed to try to think the solution to the riddle within the 10 sec. The standard answer or solution to the riddle was then presented in the center of the screen for 2 sec, followed by a 2‐sec interval. Participants were required to press the left or right key to indicate whether they understood the meaning of the riddle: (1) left key, the answer or solution participants thought of was identical to the revealed one (the standard answer); (2) right key, the answer they thought of was different from the standard one but they believed the standard was more reasonable, or did not think of the answer by themselves and believed the standard answer matched the riddle. The participants did not need to press any key if they could not understand the meaning of the standard answer, or did not think the answer matched the riddle. If subjects could understand the meaning of the answer, they were required to press the button as accurately and quickly as possible once the standard solution appeared. Only those trials in which the answer was judged as (1) or (2) mentioned above were included in the critical analysis. To familiarize the participants with the procedure and pace of this task, participants were trained with another set of similar materials in the same procedure before the formal ERP experiment.
Figure 1.
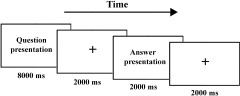
Illustration of the guessing riddle task.
ERP Recording
The EEG was recorded from 64 scalp sites using tin electrodes mounted in an elastic cap (NeuroScan Inc.), with the reference on the left and right mastoids. The vertical electrooculogram (EOG) was recorded with electrodes placed above and below the left eye. All interelectrode impedance was maintained below 5 kΩ. The EEG and EOG were amplified using a 0.1‐40 Hz bandpass and continuously sampled at 500 Hz/channel for off‐line analysis. Trials with EOG artifacts (mean EOG voltage exceeding ±100 μV) and those contaminated with artifacts due to amplifier clipping, bursts of electromyographic (EMG) activity, or peak‐to‐peak deflection exceeding ±100 μV were excluded from averaging. A 3‐D‐space FASTRAK digitizer was used to record the 3‐D coordinates of each electrode and of three fiducial landmarks (the left and right preauricular points and the nasion).
ERP Data Analysis and Statistics
EEG to answers was analyzed further and two types of items were defined in analysis: the Aha answer and the No‐aha answer. A solution or answer was classified as an Aha answer when participants could not solve the puzzle, and participants understood the ambiguous sentence when they saw the solution; or, when participants thought they could solve the puzzle, the solution participants thought about was different from the one revealed (the standard answer), and participants could understand the meaning of the standard solution and thought it was better than their own solution. A solution was classified as the No‐aha answer when participants solved the puzzle, and the solution participants thought about was the same as the standard solution.
The ERP waveforms were time‐locked to the onset of the standard answer. The averaged epoch for ERP was 1,000 msec including a 100‐msec pre‐answer baseline. The ERP waves under each condition were obtained after the ERP of the two types of items were overlapped and averaged respectively. For display of scalp topography and source localization, the difference wave was obtained by subtracting the averaged ERP of the No‐aha answer from the averaged ERP of the Aha answer.
The following 23 sites were chosen for statistical analysis: FPz, Fz, Cz, AF3, AF4, F1, F2, F5, F6, C3, C4, FT7, and FT8 (13 sites for anterior); Pz, Oz, P1, P2, P5, P6, O1, O2, TP7, and TP8 (10 sites for posterior). P1 was measured only from posterior sites in the 50–150‐msec time window. The visual N1 component may have separable anterior and posterior subcomponents. The anterior N1 and posterior N1 were thus measured separately in the 100–130‐ and 120–180‐msec time windows, respectively. Mean voltages in the time window of 250–500 msec (N380) and 500–800 msec (P300) were measured at both anterior and posterior electrodes.
Latencies and amplitudes (baseline to peak) of the early components (P1 and N1) and mean amplitudes in the time window of 250–500 msec (N380) and 500–800 msec (P300) were analyzed using two‐way repeated measures analysis of variance (ANOVA). The ANOVA factors were answer (two level: Aha, No‐aha), and electrode site (13 sites for anterior N1, 10 sites for posterior N1 and P1, and 23 sites for N380 and P300). For difference wave (Aha minus No‐aha), the latencies and amplitudes of N380 (in the time window from 250–500 msec) were measured at Fz, Cz, and Pz. The P values of all main and interaction effects were corrected using the Greenhouse‐Geisser method for repeated‐measures effects.
ERP Source Analysis
Source analysis was carried out on the Aha minus No‐aha difference wave, using Curry v4.5 software (a brain electrical source analysis software of Neurosoft, Inc.). Dipole source localization is quite sensitive to noise [Wang and Yang, 1995]. The grand average ERP was used to get the maximal signal–noise ratios for dipole modeling [Supek and Aine, 1993]. The mean values of these individual 3‐D coordinates of each electrode and of three fiducial landmarks (the left and right preauricular points and the nasion) were calculated over the 14 subjects and were fed into Curry. For the grand average data, coregistration of the ERP electrode reference frame with the MRI reference frame was accomplished with the average fiducial points in the ERP frame and the fiducial landmarks identified on the head MRI of one subject.
After the grand average ERP data, the averaged digitized electrodes and fiducial landmarks, the head MRI of one subject and the fiducial landmarks identified on it were fed into Curry, a computer algorithm was automatically carried out to calculate the best‐fit sphere encompassed by the array of electrode sites and to determine their spherical coordinates. The spherical coordinates for each site averaged across all subjects were used for ERP current density analysis and dipole source localization. In addition, spherical coordinates were related to the corresponding digitized fiducial landmarks and to fiducial landmarks identified on the head MRI of one subject.
Source reconstruction is an inverse problem and this problem does not have a unique solution. Two different classes of source models can be available, distributed and local sources. Distributed sources are found using the current density method, whereas local sources are computed by dipole fits. In the present study, we tentatively reconstructed the sources over the time range of 250–500 msec in a three‐shell head model using current density methods and dipole fits method. The low‐resolution electromagnetic tomography (LORETA) method was used in current density reconstruction. The moving dipole modeling was applied in dipole source analysis.
To estimate the position of the dipole source with respect to brain anatomy and fMRI activations, the dipole coordinates calculated from grand average ERP were projected onto the MRI of one subject [Rao, et al., 2003]. The line between the anterior (A) and the posterior (P) commissures was identified on the subject's MRI scan as the principal A‐P axis for the Talairach and Tournoux [1988] system, and the 3‐D coordinates of the dipole were determined on the MRI with respect to the Talairach space.
RESULTS
Behavioral Performance
In 120 riddles, mean trials for Aha answers were 56 (SE = 3) and 44 (SE = 3) for No‐aha answers. There were more than 31 trials for each type of event in each participant. Mean reaction times (RTs) were 2,179 msec for Aha answers (SE = 0.12) and 919 msec for No‐aha answers (SE = 0.07). RTs to Aha answers were longer than were RTs to No‐aha answers (t 13 = 12.78, P < 0.001). This indicates that participants responded quickly when the answer they thought about was identical to the standard one, but that they needed much more time to understand the meaning of the standard answer when it was not obtained in the riddle presentation phase or differed from the answer they thought of.
ERPs
Early components
As shown in Figure 2, the anterior N120 and posterior P100 and N170 were elicited by both Aha and no‐aha conditions. There was no main effect of the answer (Aha/No‐aha) for the anterior N120, posterior P100, and N170 components. There was a significant electrode effect on the posterior P100 amplitude (F (9,117) = 6.16, P < 0.01), the anterior N120 amplitude (F (12,156) = 4.03, P < 0.01), the posterior N170 amplitude (F (9,117) = 5.98, P < 0.01), and posterior N170 latency (F (9,117) = 6.80, P < 0.001), but the answer by electrode site interaction was not significant. Because the Aha effect was not observed in these early ERP components, the characteristics of their scalp distributions were not analyzed further.
Figure 2.
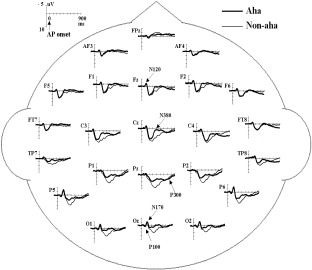
Grand average (n = 14) ERP to Aha and No‐aha answers at 23 electrode sites chosen for statistical analysis. Time = 0 msec corresponds to the onset of the answer presentation (AP onset). P100, N120, N170, N380, and P300 are indicated on the waveform plots.
N380
From ERP waveforms, we found Aha answers elicited a more negative ERP deflection than did No‐aha answers in the time interval between 250–500 msec and in the difference waves, the peak latency of the negative component was about 380 msec (N380) (see Fig. 2 and 3, left). Repeated measures ANOVA showed that the mean amplitude between 250–500 msec of the Aha answer was larger than that of No‐aha answer on negative orientation (F (1,13) = 42.83, P < 0.001). In addition, there was a main effect of electrode site (F (22,286) = 14.85, P < 0.001), and an answer by electrode site interaction (answer × electrode, F (22,286) = 8.43, P < 0.001). Hemisphere effect and interaction between answer and hemisphere did not reach significance. In the difference waves (Aha minus No‐aha answer), the peak amplitude and latency between 250–500 msec was measured at Fz, Cz, and Pz electrode sites and results showed that the maximal amplitude was at Cz (mean ± SE, −5.78 ± 0.76 μV) and the latency was about 380 msec (mean ± SE, 379 ± 5.06 msec). A voltage map (see Fig. 3, left) of the difference wave showed strong activity at the frontocentral region.
Figure 3.
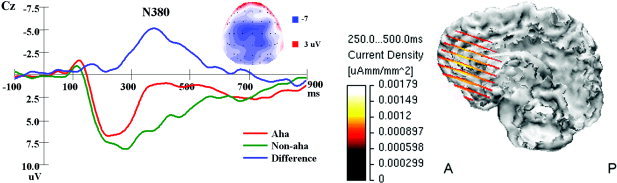
Left: Grand average (n = 14) ERP to Aha, No‐aha answers and the difference wave (Aha minus No‐aha) at Cz, and voltage map of the difference wave at 380 msec. Right: The averaged current density distribution of the difference wave (Aha minus No‐aha) in the time range of 250–500 msec reconstructed using the LORETA method, overlaid onto the cortex, and presented on a cut taken at the midsagittal plane.
P300
After the negative component, a small late positive component (LPC) or P300 was elicited by both Aha and No‐aha answers (see Fig. 2). We measured the mean amplitude of P300 in the time interval between 500–800 msec. Repeated measures ANOVA showed a significant electrode effect on the mean amplitude of P300 (F (22,286) = 8.61, P < 0.001). The maximal mean amplitude was measured at site Pz and it was significantly different from mean amplitudes measured at the central (Cz) and frontal (Fz) electrodes; however, neither the main effect of the answer (Aha/No‐aha) nor interaction of the answer and the electrode site was significant. In contrast to the negative component N380, the Aha effect was not observed on P300.
ERP Source Analysis: N380
The inverse source analysis based on a three‐shell spherical head model was carried out using Curry v. 4.5 on the grand average difference waves between the Aha answer and No‐aha answer to locate neural generators of N380. The current density distribution of the N380 was reconstructed in the time range 250–500 msec using the LORETA method. The reconstructed result overlaid onto the cortex of one subject showed strong current density in the medial frontal cortex (see Fig. 3, right). The dipole was fitted within the time interval of 250–500 msec without constraining orientation and location. A single dipole model located near the ACC was able to account for most of the variance in the observed data for N380 (location: x, y, z = 3.8, 23.0, 14.2; residual variance: 5.88%). Superimposing the dipole locations on a subject brain showed that the dipole location was close to the ACC (see Fig. 4).
Figure 4.
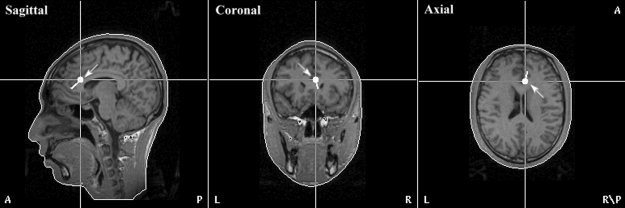
Dipole source localization for N380 was superimposed on the MR‐based head model of one subject. The dipole indicated by arrows was located in the anterior cingulate cortex.
DISCUSSION AND CONCLUSIONS
The present study was designed to examine the neural basis of the Aha! response by time course analysis of ERP. Since there is no significant difference in these early ERP components between Aha and No‐aha events, it may indicate that the visual processing occurring in the early stage is involved equally in both types of events. As higher‐level cognitive processes, insight in problem solving probably occurs later.
Between 250–500 msec after stimulus onset, Aha answers evoked a more negative ERP deflection than did No‐aha answers. For the difference wave, the peak latency was approximately 380 msec and was distributed broadly, but with a central focus. From the topographic map and current density map of the difference wave, we found strong activity and current density at frontal center. This implied the N380 might have a generator in medial PFC. Temporal‐spatial dipole source analysis showed a generator located near or in ACC. The N380 was actually the second negative component in the waveform, and it therefore may be an N2 component, similar to that found in other studies. The N2, observed by van Veen and Carter [2002a] in the Eriksen flanker task, is a negative wave with a latency of 340–380 msec after target stimuli onset. In the Eriksen flanker task, the N2 was enhanced to response‐incongruent stimuli because they involved high conflict. The frontocentral N2 was maximal at Cz and was generated by ACC. They believed that the ACC generator of the frontocentral N2 might reflect conflict detection. In addition, many other studies have suggested also that the ACC may be involved in conflict monitoring [Barch et al., 2000; Botvinick et al., 1999, 2001; MacDonald et al., 2000; van Veen et al., 2001]. The N380 in the present study had similar latency, frontocentral scalp distribution, and source localization of ACC with the N2 as was found in the ERP study using the Eriksen flanker task. After the question was presented in the present riddle‐guessing task, participants tried their best to answer it. No matter whether they could resolve the riddle, a mental set was formed; thus, when participants saw the standard answer, new and efficient ways of thinking were evoked and competed with the old mental sets. N380 therefore is probably an N2 component and embodies cognitive conflict in breaking a mental set.
N380 is similar with another ERP component named error negativity (Ne) or error‐related negativity (ERN) in some aspects. The Ne is a large negative‐going peak seen immediately after errors, first observed by Hohnsbein and colleagues [1989]. Gehring and coworkers [1993] observed the same phenomenon in error‐related processes and termed it ERN. Recent studies of ERPs provided further insights into this component. The ERN/Ne, elicited after the onset of the erroneous response, is maximal at frontocentral recording sites [Botvinick et al., 2001; Falkenstein et al., 2000; Gehring et al., 1993]. The ERN/Ne has been observed in many different tasks, such as a gambling task [Gehring and Willoughby, 2002], a guessing task [Ruchsow et al., 2002], the Eriksen flanker task [van Veen and Carter, 2002a], and a go/no‐go paradigm [Scheffers et al., 1996]. The ERN/Ne has been modeled repeatedly by a single dipole source, located in medial prefrontal areas, possibly in the ACC [Dehaene, et al., 1994; Gehring and Willoughby, 2002; Gehring et al., 2000; Holroyd et al., 1998]. Furthermore, studies using fMRI have also shown increased activation of the ACC during error trials relative to correct trials [Carter et al., 1998; Kiehl et al., 2000; Menon et al., 2001]. The ERN/Ne therefore is considered an electrophysiologic reflection of ACC functioning that mirrors response conflict. Although both N380 and ERN/Ne have similar frontocentral scalp distributions and ACC generators, the differences between them are obvious. ERN/Ne occurs immediately after error response, but N380 happens when an Aha response is evoked. Semantic processes are involved in the guessing riddle task and the latency of N380 therefore is longer than is ERN/Ne elicited in comparatively simple cognitive tasks, such as the Eriksen flanker paradigm, a gambling task, and a go/no‐go paradigm. The causes of these differences between N380 and ERN/Ne may lie with differences between the two tasks; however, similarity of ACC generators cannot be neglected. N380 is also probably related to cognitive conflict because the occurrence of insight in problem solving is based on resolving conflict and breaking the mental set. Moreover, both frontocentral N2 and ERN/Ne have been found to have the same ACC generator and to reflect conflict detection [van Veen and Carter, 2002a]. N380 may therefore be another form of ERN that involves semantic processes and relates to the cognitive conflict in breaking a mental set.
N380 also has a similar latency, scalp distribution, and neural generator, with the ERP component evoked by Stroop effect [Liotti et al., 2000]. In the Stroop color–word interference task, the stimuli were words describing colors, such as red and green, with the words presented in the congruent color (e.g., the word red in red ink) or in an incongruent color (e.g., the word red in green ink). Participants were instructed to decide the presentation color of the word. The ERP for incongruent and congruent color words diverged between 350–500 msec poststimulus, with the incongruent color word presenting a negative wave (peak at 410 msec) that was reduced in the ERP of the congruent color word. The negative component of the Stroop effect had an anterior‐medial focus of scalp distribution likely generated in ACC by dipole source analysis. ACC involvement in the Stroop color interference task was also delineated by cognitive neuroimaging studies using fMRI and PET [Carter et al., 1995; McKeown et al., 1998]. In the Stroop effect, the negative ERP component probably also reflects cognitive conflict because there is an obvious conflict between the meaning and the color of the word. N380, therefore, may be the same negative component observed in the Stroop effect mirroring cognitive conflict.
Although the N380 has much in common with the above‐mentioned negative components, there remain differences in the conflict‐monitoring function of ACC, which may be the common generator of N380 and those components. As to the conflict‐monitoring theory of ACC functions, the levels of processing that are monitored by ACC remain debated [Botvinick et al., 2001]. Many recent studies about ACC functions have suggested that ACC activation occurs mainly at the response stage, i.e., ACC is associated with information‐processing conflict that occurs at the stimulus–response association level [van Veen and Carter, 2002b; van Veen et al., 2001]. Some studies, however, suggest that ACC activity can be elicited in situations without motor requirements. ACC activity has been observed in response to conflict induced by unexpected error feedback in the Wisconsin Card Sorting Task [Monchi et al., 2001], to conflict occurring at a conceptual level by having participants read stories that do not form an integrated narrative [Robertson et al., 2000], or to conflict induced when participants are instructed to inhibit sexual arousal while watching erotic films [Beauregard et al., 2001]. Depending on the task, ACC activation thus might not be limited to response conflict, but might respond to other sources of conflict as well. In the present study, insight occurring in problem solving based on the breaking of unwarranted mental impasse is not the same as the conflict that occurs at the response level (e.g., Stroop or Eriksen task). We hypothesize that ACC involvement is related to the information‐processing conflict occurring at the stimulus–response association level, but not at the level of response conflict. The function is to adjust the conflict between old and new cognitive modes at the instant of insight. This cognitive conflict has some similarity with response conflict, i.e., just like we have a strong tendency to name the color of a colored word according to its meaning, the old cognitive mode in insight is highly automatic. The old cognitive mode, which is highly automatic, thus interferes intensively with the process of the new effective cognitive mode at the moment when insight occurs. The interference is so intense that an ACC effect similar to the motor conflict is produced. The above hypothesis, which is in accordance with the present study, is also consistent with the current, more general understanding about ACC functions.
It is important to compare the Aha effect of N380 with N400 because N380 is in the time range of N400. N400 is a negative deflection in the ERP, peaking at approximately 400 msec and elicited by words presented in the absence of an appropriate sentence context [Kutas and Hillyard, 1980, 1982, 1983]. The N400 wave is associated with processing of semantic information that is incongruent with semantic expectancy [Gunter et al., 1994; McPherson and Holcomb, 1999; Neville et al., 1986; Salmon and Pratt, 2002]. Findings such as these suggest that the N400 may reflect the degree to which a word is expected within the current semantic context. N400 was therefore proposed to be underlying the process of semantic integration. In the present guessing riddle task, similar process could have also occurred, i.e., participants might have formed certain expatiations when they tried to solve the puzzle by themselves. The following answer, however, elicited a novel “script” that was different from their expatiations. The process of semantic integration thus might also be involved in Aha! effects and N380 might be just the N400. The neural generator of N380 in the present study, however, is different from that of N400 in previous studies. Many studies have demonstrated that a number of areas are active in N400 generation. ERP recording from intracranial electrodes have suggested that the N400 is recorded from medial temporal lobe [Elger et al., 1997; McCarthy et al., 1995; Smith et al., 1986], lateral temporal lobe [Elger et al., 1997], and various temporal, frontal, and parietal structures [Guillem et al., 1995]. Magnetoencephalography (MEG) studies have also suggested temporal lobe sites are involved in N400 generation [Simos et al., 1997]. Recently, an fMRI study has also found many cerebral regions are involved in the generation of the N400, including bilateral inferior frontal and inferiomedial temporal cortex, left lateral frontal cortex, and left posterior fusiform gyrus [Kiehl et al., 2002]. The generator of N380 was located in ACC in the present study using the method of dipole source localization; however, it should be stressed that dipole source analysis is an inverse problem because there is no unique solution. Due to inherent limitations of source localization, the brain areas implied by source localization are only tentative. The result of dipole source analysis therefore should be considered with caution, because N380 could embody complex brain processes accomplished by multiple areas and their interactions. It is somewhat risky to propose that there is only one generator to account for a high‐level cognitive process such as breaking a mental set. Regarding the involvement of brain regions in response to insight in problem solving, the current results only provide a model, rather than empirical data. Furthermore, in our recent event‐related fMRI study [Luo and Niki, 2003], we found other cerebral areas including PFC, posterior parietal cortex, and medial temporal lobe (MTL) were activated in insight riddle solving. N380 may therefore be N400, and other brain regions including the medial temporal lobe generator of N380 cannot be excluded.
In sum, the N380 probably reflects the breaking of a mental set in insight problem solving, and its possible generator ACC may be related to detection of conflict between old and new cognitive modes at the moment of insight. The N380 may also be the N400, and related to the activity of MTL. Although the results of the dipole source analysis tend to support the former (that N380 probably generates in ACC), the latter (that it may generate in other brain regions, especially MTL) cannot be excluded due to the inherent limitations of source localization. Further studies are needed to address these issues.
After the negative component P300, which was dominant at Pz, occurred in both Aha and No‐aha conditions. In contrast to N380, however, the Aha! effect was not observed on the late positive component between 500–800 msec. P300 is the most commonly researched component of the ERP waveform, and is elicited typically by oddball stimuli (i.e., those occurring only infrequently within a stream of visual or auditory stimuli). In general, the amplitude of the P300 is larger with stimuli that are more infrequent. On the other hand, the latency (which can vary widely between 300–800 msec) is thought to represent the relative duration of multiprocess stimulus evaluation/classification operations [Donchin and Coles, 1988]. One of the most prominent theories regarding the cognitive basis of the P300 is that it indexes on‐line updating of working memory [Donchin and Coles, 1988]. Some other studies suggest that P300 with latency in the range of 500–900 msec poststimulus (sometimes this component is called the LPC, P600, or P800) indexes recollective processes of a more elaborative nature, based on information stored in long‐term memory [Besson et al, 1992; Smith, 1993]. In the present study, P300 might reflect a confirmation of the subject's understanding of the riddles, which is based also on knowledge stored in long‐term memory, or might merely reflect the closure of the negativity.
The present study is the first one using ERP to investigate electrophysiologic correlates of insight in problem solving, and the results suggest that the ERP component N380 is probably an electrophysiologic reflection of ACC functioning that mirrors the cognitive conflict in the breaking of mental set. The experimental design of the present research, however, should be improved further. In the present study, the Aha event includes those that can elicit strong Aha responses and those that can only evoke weak Aha responses. Future studies will be needed to separate the two types of events to observe whether there is any difference in ERP recordings between strong and weak Aha responses. Moreover, further studies should be done using both ERPs and fMRI to determine the role of the ACC in high cognitive functioning, and whether there are brain areas other than ACC that are involved in insight.
Acknowledgements
We thank Dr. P. Steven for his editorial assistance. We also thank X. Wei, X.J. Qu, C. Liu, and other members of the Key Laboratory of Mental Health, Chinese Academy of Sciences, for collecting riddles.
REFERENCES
- Barch DM, Braver TS, Sabb FW, Noll DC (2000): Anterior cingulate and the monitoring of response conflict: evidence from an fMRI study of overt word generation. J Cogn Neurosci 12: 298–309. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Le'vesque J, Bourgouin P (2001): Neural correlates of conscious self‐regulation of emotion. J Neurosci 21: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, Kutas M, van Petten C (1992): An event‐related potential (ERP) analysis of semantic congruity and repetition effects in sentences. J Cogn Neurosci 4: 132–149. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD (2001): Conflict monitoring and cognitive control. Psychol Rev 108: 624–652. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Nystrom LE, Fissell K, Carter CS, Cohen JD (1999): Conflict monitoring versus selection‐for‐action in anterior cingulate cortex. Nature 402: 179–181. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll DC, Cohen JD (1998): Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280: 747–749. [DOI] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Cohen JD (1995): Interference and facilitation effects during selective attention: an H2O‐15O PET study of Stroop task performance. Neuroimage 2: 264–272. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM (1994): Localization of a neural system for error detection and compensation. Psychol Sci 5: 303–305. [Google Scholar]
- Donchin E, Coles MGH (1988): Is the P300 component a manifestation of context updating? Behav Brain Sci 11: 355–372. [Google Scholar]
- Duncker K (1945): On problem solving. Psychol Monogr 58: 1–113. [Google Scholar]
- Elger CE, Grunwald T, Lehnertz K, Kutas M, Helmstaedter C, Brockhaus A, van Roost D, Heinze HJ (1997): Human temporal lobe potentials in verbal learning and memory processes. Neuropsychologia 35: 657–667. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kirshnit CE, Lanza RP, Rubin LC (1984): “Insight” in the pigeon: antecedents and determinants of an intelligent performance. Nature 308: 61–62. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J (2000): ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol 51: 87–107. [DOI] [PubMed] [Google Scholar]
- Gehring W, Goss B, Coles MGH, Meyer DE, Donchin E (1993): A neural system for error detection and compensation. Psychol Sci 4: 385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG (2000): Action‐monitoring dysfunction in obsessive‐compulsive disorder. Psychol Sci 11: 1–6. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR (2002): The medial frontal cortex and the rapid processing of monetary gains and losses. Science 295: 2279–2282. [DOI] [PubMed] [Google Scholar]
- Glucksberg S (1962): The influence of strength of drive on functional fixedness and perceptual recognition. J Exp Psychol 63: 36–41. [DOI] [PubMed] [Google Scholar]
- Guillem F, N'Kaoua B, Rougier A, Claverie B (1995): Intracranial topography of event‐related potentials (N400/P600) elicited during a continuous recognition memory task. Psychophysiology 32: 382–392. [DOI] [PubMed] [Google Scholar]
- Gunter TC, Jackson JL, Kutas M, Mulder G, Buijink BM (1994): Focusing on the N400: an exploration of selective attention during reading. Psychophysiology 31: 347–358. [DOI] [PubMed] [Google Scholar]
- Hohnsbein J, Falkenstein M, Hoormann J (1989): Error processing in visual and auditory choice reaction time tasks. J Psychophysiol 3: 32. [Google Scholar]
- Holcomb PJ (1993): Semantic priming and stimulus degradation: implications for the role of the N400 in language processing. Psychophysiology 30: 47–61. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Dien J, Coles MG (1998): Error‐related scalp potentials elicited by hand and foot movements: evidence for an output‐independent error‐processing system in humans. Neurosci Lett 242: 65–68. [DOI] [PubMed] [Google Scholar]
- Isaak MI, Just MA (1995): Constraints on thinking in insight and invention In: Sternberg RJ, Davidson JE, editors. The nature of insight. Cambridge, MA: Bradford Books/MIT Press; p 281–325. [Google Scholar]
- Kaplan CA, Simon HA (1990): In search of insight. Cogn Psychol 22: 273–419.2376113 [Google Scholar]
- Kiehl KA, Laurens KR, Liddle PF (2002): Reading anomalous sentences: an event‐related fMRI study of semantic processing. Neuroimage 17: 842–850. [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB (2000): Error processing and the rostral anterior cingulate: an event‐related fMRI study. Psychophysiology 33: 282–294. [PubMed] [Google Scholar]
- Knoblich G, Ohlsson S, Haider H, Rhenius D (1999): Constraint relaxation and chunk decomposition in insight problem solving. J Exp Psychol Learn Mem Cogn 25: 1534–1556. [Google Scholar]
- Köhler W (1925): The mentality of apes. London: Routledge and Kegan Paul. [Google Scholar]
- Kutas M, Hillyard SA (1980): Reading senseless sentences: brain potentials reflect semantic incongruity. Science 207: 203–205. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA (1982): The lateral distribution of event‐related potentials during sentence processing. Neuropsychologia 20: 579–590. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA (1983): Event‐related brain potentials to grammatical errors and semantic anomalies. Mem Cogn 11: 539–550. [DOI] [PubMed] [Google Scholar]
- Kutas M, van Petten CK (1994): Psycholinguistics electrified In: Gernsbacher MA, editor. Handbook of psycholinguistics. San Diego: Academic Press; p 83–143. [Google Scholar]
- Lavric A, Forstimeier S, Rippon G (2000): Differences in working memory involvement in analytical and creative task: an ERP study. Neuroreport 11: 1613–1618. [DOI] [PubMed] [Google Scholar]
- Liotti M, Woldorff MG, Perez R 3rd, Mayberg HS (2000): An ERP study of the temporal course of the Stroop color‐word interference effect. Neuropsychologia 38: 701–711. [DOI] [PubMed] [Google Scholar]
- Lung CT, Dominowski RL (1985): Effects of strategy instructions and practice on nine‐dot problem solving. J Exp Psychol Learn Mem Cogn 11: 804–811. [Google Scholar]
- Luo J, Niki K (2003): Function of hippocampus in “insight” of problem solving. Hippocampus 13: 274–281. [DOI] [PubMed] [Google Scholar]
- MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS (2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- Maier NR (1931): Reasoning in humans: II. The solution of a problem and its appearance in consciousness. J Comp Psychol 12: 181–194. [Google Scholar]
- McCarthy G, Nobre AC, Bentin S, Spencer DD (1995): Language‐related field potentials in the anterior‐medial temporal lobe. I. Intracranial distribution and neural generators. J Neurosci 15: 1080–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown MJ, Jung TP, Makeig S, Brown G, Kindermann SS, et al. (1998): Spatially independent activity patterns in functional MRI data during the Stroop color‐naming task. Proc Natl Acad Sci USA 95: 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson WB, Holcomb PJ (1999): An electrophysiological investigation of semantic priming with pictures of real objects. Psychophysiology 36: 53–65. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL (2001): Error‐related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp 12: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A (2001): Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event‐related functional magnetic resonance imaging. J Neurosci 21: 7733–7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville HJ, Kutus M, Chesney G, Schmidt AL (1986): Event‐related brain potentials during initial encoding and recognition memory of congruous and incongruous words. J Mem Lang 25: 75–92. [Google Scholar]
- Ormerod TC, MacGregor JN, Chronicle EP (2002): Dynamics and constraints in insight problem solving. J Exp Psychol 28: 791–799. [DOI] [PubMed] [Google Scholar]
- Rao H, Zhou T, Zhuo Y, Fan S, Chen L (2003): Spatiotemporal activation of the two visual pathways in form discrimination and spatial location: a brain mapping study. Hum Brain Map 18: 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DA, Gernsbacher MA, Guidotti SJ, Robertson RR, Irwin W, Mock BJ, et al. (2000): Functional neuroanatomy of the cognitive process of mapping during discourse comprehension. Psychol Sci 11: 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchsow M, Grothe J, Spitzer M, Kiefer M (2002): Human anterior cingulate cortex is activated by negative feedback: evidence from event‐related potentials in a guessing task. Neurosci Lett 325: 203–206. [DOI] [PubMed] [Google Scholar]
- Salmon N, Pratt H (2002): A comparison of sentence‐ and discourse‐level semantic processing: an ERP study. Brain Lang 83: 367–383. [DOI] [PubMed] [Google Scholar]
- Scheerer M (1963): Problem solving. Sci Am 208: 118–128. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MGH, Bernstein P, Gehring WJ, Donchin E (1996): Event‐related brain potentials and error‐related processing: an analysis of incorrect responses to go and no‐go stimuli. Psychophysiology 33: 42–53. [DOI] [PubMed] [Google Scholar]
- Simos PG, Basile LF, Papanicolaou AC (1997): Source localization of the N400 response in a sentence‐reading paradigm using evoked magnetic fields and magnetic resonance imaging. Brain Res 762: 29–39. [DOI] [PubMed] [Google Scholar]
- Smith ME (1993): Neurophysiological manifestations of recollective experience during recognition memory judgments. J Cogn Neurosci 5: 1–13. [DOI] [PubMed] [Google Scholar]
- Smith SM, Blankenship SE (1991): Incubation and the persistence of fixation in problem solving. Am J Psychol 104: 61–87. [PubMed] [Google Scholar]
- Smith ME, Stapleton JM, Halgren E (1986): Human medial temporal lobe potentials evoked in memory and language tasks. Electroencephalogr Clin Neurophysiol 63: 145–159. [DOI] [PubMed] [Google Scholar]
- Supek S, Aine CJ (1993): Simulation studies of multiple dipole neuromagnetic source localization: model order and limits of source resolution. IEEE Trans Biomed Eng 40: 529–540. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- van Veen V, Carter CS (2002a): The timing of action‐monitoring processes in the anterior cingulate cortex. J Cogn Neurosci 14: 593–602. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS (2002b): The anterior cingulate as a conflict monitor: fMRI and ERP studies. Phyiol Behav 77: 477–482. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS (2001): Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage 14: 1302–1308. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yang F (1995): Dynamic extraction of visual evoked potentials through spatial analysis and dipole localization. IEEE Trans Biomed Eng 42: 762–768. [DOI] [PubMed] [Google Scholar]
- Weisberg RW, Alba JW (1981): An examination of the alleged role of “fixation” in the solution of several “insight” problems. J Exp Psychol Gen 110: 169–192. [Google Scholar]


