Abstract
Presenting various stimuli in an MRI scanner can be difficult due to the high magnetic field associated with the scanner. Mechanical vibration stimuli are difficult to deliver to subjects in the MRI environment because most vibration devices contain internal circuitry that can adversely interact with the high magnetic field. Piezoelectric ceramics can provide a solution to this problem since they do not require any internal circuitry to vibrate. Piezoceramics are nonmagnetic and they can be made to vibrate if supplied with an alternating current from a straight wire. We designed a piezoceramic vibrotactile stimulator that is safe and effective in functional MRI experiments. The stimulator was tested in an fMRI experiment at 35 and 150 Hz. The results yielded activation sites in the primary sensory cortex and Brodmann area 40 at both frequencies. Hum. Brain Mapping 10:140–145, 2000. © 2000 Wiley‐Liss, Inc.
Keywords: vibration, somatosensory cortex, piezoelectric, magnetic resonance imaging, brain mapping, safety
INTRODUCTION
Mechanical vibration stimuli have been used effectively to map the somatosensory cortex with positron emission tomography (PET) [Fox et al., 1987; Seitz et al., 1992]. The vibrators used for these studies were normally some type of commercial electromechanical motor that could generate very large amplitudes at various frequencies. These vibrators are not suitable for other brain mapping modalities such as electroencephalography (EEG), magnetoencephalography (MEG), and functional magnetic resonance imaging (fMRI) because the electric circuitry of the motors can interfere with the imaging apparatus. Different types of vibration stimuli have been tried with these brain mapping modalities, all with limitations. The most common method employed to activate the somatosensory cortex for all brain mapping modalities is to directly stimulate the skin with an electrical current [Disbrow et al., 1998; Forss et al., 1994; Hamalainen et al., 1990; Kaukoranta et al., 1986; Kurth et al., 1998; Rossini et al., 1996; Spiegel et al., 1999]. This method is limited in that it produces a nonspecific stimulation of the peripheral receptors and the area of stimulation is diffuse [Jagow et al., 1992]. Tactile stimuli are more natural than electrical stimuli and are therefore more desirable [Jousmaki et al., 1999]. Vibrotactile stimuli are not often used with fMRI due to the difficulty in finding a safe, effective stimulus that is compatible with a high magnetic field. Stimulation with air puffs, or vibrotactile devices powered by airpuffs, have been used with fMRI [Forss et al., 1994; Gelnar et al., 1998; Jousmaki et al., 1999; Rossini et al., 1996; Servos et al., 1998], as well as with EEG and MEG [Forss et al., 1994; Hashimoto et al., 1988; Rossini et al., 1996]. The primary drawback of the air puff stimulation techniques is that they tend to have a low maximum stimulation frequency. An electromagnetic device that utilizes the magnetic field of the MRI scanner (B 0) to produce a mechanical force is another type of vibrotactile stimulator that could be used with fMRI. Such a device can produce large displacements over a wide frequency range. The problem with electromagnetic devices is that they can interact with the changing magnetic fields of the MRI scanner (B 1) to produce artifacts in the image that mimic fMRI activation patterns.
An alternative to these methods is a vibrotactile stimulator using piezoceramics. Piezoelectric devices have been used previously with MEG [Hashimoto et al., 1999; Jagow et al., 1992; Ribary et al., 1992] and fMRI [Harrington et al., 1998]. A piezoelectric vibrotactile stimulator has been designed in our lab that is safe to use in an fMRI environment and does not produce any image artifacts. The stimulator has a large frequency range (1–300 Hz) which allows the user to optimize the stimulation for a specific peripheral receptor. The theory, design, and results of the fMRI experiments for this stimulator are presented in the next section.
Piezoceramic Theory
Piezoceramics are nonmagnetic devices that can produce displacements without internal circuits. These two properties of piezoceramics make them ideal for use in the fMRI environment because magnetic materials or devices with internal circuits cannot be used in or near the MRI scanner. The only requirement to achieve displacement of a piezoceramic is a small current supply.
Piezoceramics are devices composed of special crystalline materials that are capable of producing an electric charge when subjected to a mechanical stress. This effect, known as the piezoelectric effect, produces a charge that is directly proportional to the applied force. The sign of the current (direction) depends on whether the force is compressive or tensile. The piezoelectric effect is also reversible; an applied voltage on a piezoelectric material will cause a mechanical strain.
The coupling between electrical and mechanical forces theoretically permits piezoceramics to work like other electromechanical devices such as electromechanical motors. The problem is that the displacements for piezoceramics are very small, thus limiting their applications. The magnitude of displacement for a piezoelectric material depends on the applied voltage and the piezoelectric constant (d). The mechanical strain (S) produced by and applied electric field (E) is simply [Jaffe, 1971]:
A high piezoelectric constant is required for materials intended to produce motion or vibration.
Vibrotactile Stimulator
The vibrotactile stimulator designed for this study consisted of a power source, insulated coaxial wire (Belden‐8216), and a piezoceramic wafer (Aura Ceramics Inc.) that was specially engineered for maximal displacement. The circular piezoceramic wafer was very thin (diameter 5.08 cm, thickness .035 cm) and it was reduced on one side, which made it very brittle (Fig. 1). The displacement of the wafer was 3.39 μm/V in the axial direction with an applied voltage limit of ± 150 V. The hand‐held, battery operated power source (12.7 × 10.0 × 7.5 cm, ∼225 g) contained a function generator chip that produced a square wave output. The power source was connected to the wafer by 6 m of insulated coaxial wire so that it could be operated from the MRI console room while the wafer was in the scanner. The design of the stimulator made it very easy to set up and operate.
Figure 1.
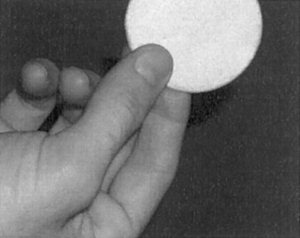
The subjects held the wafer between the tips of their thumb, middle finger, and index finger.
A problem with using piezoceramics for somatotopic brain mapping is that it takes a very large applied voltage to get a small mechanical displacement. It was necessary to use high voltage batteries and an operational amplifier (Apex Microtechnology Corporation) in order to supply the wafer with a large enough voltage differential to get a significant mechanical displacement. Feeding an alternating current to the wafer produced the mechanical vibration. A square wave signal was used to create an abrupt voltage change that caused more distinct mechanical displacements in the wafer. The vibration created with a square wave input was stronger than with a sine wave or a triangular wave. The frequency range of the input signal was 1–300 Hz with a maximum amplitude of ± 150 V. The piezoceramic vibrator made a humming noise that varied with the frequency and amplitude of the input signal. However, the noise was not very loud and it could not be heard while the MR scanner was in use.
Stimulator Safety and Compatibility
In designing any stimulus equipment for fMRI, it is necessary to address certain safety concerns involving any possible interactions between the high magnetic field of the scanner and the stimulus. Many of these concerns can be satisfied by keeping any objects containing magnetic material, materials of high magnetic susceptibility, or materials with electric circuits away from the bore of the magnet. For this reason, the power supply of the vibrotactile stimulator was kept in the MR console room at all times. The piezoelectric wafer and the coaxial wire connected to the wafer were near (or in) the scanner and hence needed to be checked for MR compatibility.
Materials that are magnetic, or have a high magnetic susceptibility, can easily get pulled in the bore of the magnet. These materials are considered to be incompatible with MRI and should not be near the scanner. Materials that are compatible with MRI can be divided in to two groups: compatibility of the first and second kind [Schenck, 1996]. Materials in the first group have a low magnetic susceptibility (10−5 <|χ−χwater|<10−2) and do not produce strong forces when they are in the presence of a strong magnetic field. However, the susceptibilities of these materials are large enough to create small changes in the static magnetic field of the scanner. If an object that has compatibility of the first kind is placed near the imaging plane, the inhomogoneities in the static filed caused by the object can produce distortions in the image. Materials in the second group have a susceptibility near that of human tissue (|χ−χwater| < 10−5). In the presence of a strong magnetic field, these materials do not produce a detectable force or cause image distortions [Schenck, 1996].
MRI incompatibility of an object can be tested with a small permanent magnet [Schenck, 1996]. The piezoelectric wafer did not produce a force in the presence of the small magnet so it can be considered MRI compatible. The type of compatibility of the wafer, first or second kind, is not known because the exact susceptibility of the wafer is not known. This distinction is not important as long as the wafer is placed away from the imaging plane, so that it cannot cause any distortions in the image. In our studies, the subjects are holding the buzzer in their hands, which are kept near their hips. In this position, the wafer is far enough from the head coil that it will not cause any distortions in the images.
The interaction between the magnetic field of the scanner and the current in the coaxial wire of the vibrotactile stimulator will produce a small force according to Faraday's law. The use of shielded coaxial wire will help reduce this force as well as protect the subject from any possible heating of the wire. The force on the wire will be minimized if the wire is kept parallel to the bore of the magnet (there is no force when the wire is perfectly parallel), the wire is kept as far away from the scanner as possible, and the input current is kept low. Because the subject holds the buzzer in their hand while in the scanner, a portion of the wire will be inside the scanner. In our studies, the current in the wire ranged from .2 mA to .27 mA. To estimate the force on the wire, we assumed the wire made a 10° angle with B 0 and the wire had no shielding. In this case, the force on the wire in a 1.5T magnet would be .7 Newtons. This force is comparable to the weight of an object with a mass of approximately 71 g. In our studies, there was not a noticable increase in the temperature of the wire during the fMRI experiment.
MATERIALS AND METHODS
Functional Imaging Tests
All of the images were acquired with a 1.5T Siemens Vision scanner. The functional images were acquired with a gradient‐echo EPI sequence (TR 2.4 s, TE 64 ms, FOV 256 mm, flip angle 60°) covering the entire sensorimotor cortex with 17 contiguous axial slices (slice thickness 4 mm, in‐plane resolution 2 × 2 mm). This sequence was utilized to emphasize the blood oxygen level dependent (BOLD) contrast associated with neuronal activation. The two subjects were both right‐handed males, 22 and 24 years old. The stimulus was applied for five 19.2 second (eight sets of images) blocks alternated with a rest period of 36.0 seconds (15 sets of images). Two studies were performed, one with a vibration frequency of 35 Hz (± 270 μm displacement) and the other with a vibration frequency of 150 Hz (± 170 μm displacement). The displacement values were determined by the displacement/voltage value of the wafer given by the manufacturer. These values are slightly overestimated because they do not take in to account the reduction on displacement caused by the loading. The subjects held the buzzer between the tips of their thumb, right index finger, and middle finger (Fig. 1). Each subject practiced holding the wafer (outside the magnet) while the stimulus was turned on to make sure the stimulus was not painful.
RESULTS
The subjects did not report any negative effects other than a little surprise when the stimulator was initially turned on. This is likely due to the step function used by the generator to transition between off and on. A ramp function would likely produce a less startling effect for the subject and the next generation of the stimulator will likely have several options for this transition.
Active pixels were determined by correlating the fMRI time series with a boxcar reference waveform. The statistical map was achieved by setting the activation threshold at p < 1.0 × 10−5 (Bonferonni corrected). Significantly correlated voxels were detected for both subjects at each frequency. The majority of the activation was in the primary somatosensory cortex (S1, Brodmann areas 1, 2, and 3) and in Brodmann area 40 of the contralateral side (Figs. 2 and 3). There were some small clusters of activation located on the ipsilateral side of the parietal lobe for subject 1. There was a slight difference in activation patterns between the two different stimulus frequencies for each subject. For subject 1, there were stronger activations (p‐value and cluster size) and more detected clusters with the 35 Hz stimulus compared to the 150 Hz stimulus. Conversely, there was a greater number of active clusters detected with the 150 Hz stimulus for subject 2.
Figure 2.
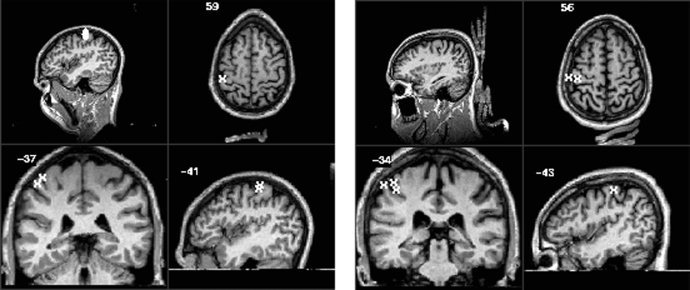
Activation overlays for the 35 Hz stimulus for subject 1 (left) and subject 2 (right). The Talairach 1988 coordinates (x,y,z) are −41 mm, −37 mm, 59 mm for subject 1 and −43 mm, −34 mm, 56 mm for subject 2 [Talairach, 1988; Lancaster, 1997].
Figure 3.
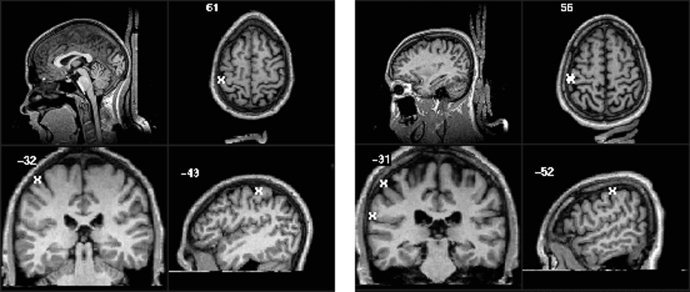
Activation overlays for the 150 Hz stimulus for subject 1 (left) and subject 2 (right). The Talairach 1988 coordinates (x,y,z) are −43 mm, −32 mm, 61 mm for subject 1 and −52 mm, −31 mm, 56 mm for subject 2 [Talairach, 1988; Lancaster, 1997].
DISCUSSION
The goal of this project was to build a vibrotactile device that was safe in an fMRI environment and could elicit a response in the somatosensory cortex. The current version of the piezoelectric buzzer is safe and clearly adequate for generating a BOLD signal in the somatosensory cortex. This piezoelectric‐buzzer stimulus can be used as a model for other piezoelectric devices to be used with fMRI. A different piezoceramic transducer could replace the wafer that we used as a transducer. Piezoelectric benders could possibly be used to get more deflection and to produce more of a brushing sensation. One of the drawbacks of the wafer used in this experiment was that it was difficult to stimulate one finger at a time. With piezoelectric benders, it is possible to set up a device to stimulate one finger at a time [Hashimoto et al., 1999].
Another drawback to the piezoelectric‐buzzer presented in this paper was that the perceived stimulus varied depending on how the subject held the buzzer between their fingers. The subjects were instructed to hold the wafer lightly between the tips of their thumb, middle finger, and index finger. When the subjects pressed too hard against the wafer, they reported some discomfort. One subject reported that it felt like the vibration was moving straight up their arm. When the subjects held the wafer lightly they reported only slight discomfort.
There are a few different methods for delivering mechanical vibration stimuli to a subject while in the MR scanner. Each one has its drawbacks and the best device may depend on the particular application. Air puff stimulation can be effective, but has a limited frequency range of vibration. Electromagnetic stimulators (solenoid) that use the magnetic field of the scanner are the easiest to manufacture and implement. However, if the solenoid interacts with the magnetic field in the head coil, then the artifacts produced by the interaction can confound the results. Piezoceramics do not interact with the magnetic field and they can be designed to work for a large range of frequencies. The primary drawback to piezoceramic vibration stimuli is the difficulty in generating large displacements from the piezoceramic and the necessity for specialized electronics. The stimulator we designed requires very large voltages (at least ± 50 V) to produce relatively small displacements (± 169.5 μm).
REFERENCES
- Disbrow E. (1998): Somatosensory cortex: a comparison of the response to noxious thermal, mechanical, and electrical stimuli using functional magnetic resonance imaging. Hum Brain Mapp 6: 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forss N. (1994): Comparison of somatosensory evoked fields to airpuff and electric stimuli. Electroencephelalogr Clin Neurophysiol 92: 510–517. [DOI] [PubMed] [Google Scholar]
- Fox PT. (1987): Mapping human somatosensory cortex with positron emission tomography. J Neurosurg 67: 34–43. [DOI] [PubMed] [Google Scholar]
- Gelnar PA. (1998): Fingertip representation in the human somatosensory cortex: an fMRI study. NeuroImage 7: 261–283. [DOI] [PubMed] [Google Scholar]
- Hamalainen H. (1990): Human somatosensory evoked potentials to mechanical pulses and vibration: contributions of SI and SII somatosensory cortices to P50 and P100 components. Electroencephalogr Clin Neurophysiol 75: 13–21. [DOI] [PubMed] [Google Scholar]
- Harrington GS. (1998): Somatosensory Response to Vibrotactile Stimuli in fMRI. Fourth International Conference on Functional Mapping of the Human Brain, Montreal, Canada. p. 8.
- Hashimoto I. (1999): Are there discrete distal‐proximal representations of the index finger and palm in the human somatosensory cortex? A neuromagnetic study. Clin Neurophysiol 110: 430–437. [DOI] [PubMed] [Google Scholar]
- Hashimoto I. (1988): Somatosensory evoked potential correlates of psychophysical magnitude estimations for tactile air‐puff stimulation in man. Exp Brain Res 73: 459–469. [DOI] [PubMed] [Google Scholar]
- Jaffe B. (1971): Piezoelectric ceramics. London: Academic Press. [Google Scholar]
- Jagow R. (1992): A new sensory stimulator for the MEG environment: the Piezoundulative multifrequency apparatus (PUMA) In: Biomagnetism: clinical aspects. M. H. e. al. Amsterdam: Elsevier; p. 891–894. [Google Scholar]
- Jousmaki V. (1999): Somatosensory evoked fields to large‐area vibrotactile stimuli. Clin Neurophysiol 110: 905–909. [DOI] [PubMed] [Google Scholar]
- Kaukoranta E. (1986): Mixed and sensory nerve stimulations activate different cytoarchitectonic areas in the human primary somatosensory cortex SI. Exp Brain Res 63: 60–66. [DOI] [PubMed] [Google Scholar]
- Kurth R. (1998): fMRI assessment of somatotopy in human Brodmann area 3b by electrical finger stimulation. Neuroreport 9: 207–212. [DOI] [PubMed] [Google Scholar]
- Lancaster JL. (1997): The Talairach Daemon: A database server for Talairach atlas labels. NeuroImage 5(4): 633. [Google Scholar]
- Ribary U. (1992): The spatial and temporal organization of the 40Hz response in human brain: an MEG study In: Biomagnetism: clinical aspects. M. H. e. al. Amsterdam: Elsevier. [Google Scholar]
- Rossini PM. (1996): Topography and sources of electromagnetic cerebral responses to electrical and air‐puff stimulation of the hand. Electroencephalogr Clin Neurophysiol 100: 229–239. [DOI] [PubMed] [Google Scholar]
- Schenck J. (1996): The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med Phys 23: 815–850. [DOI] [PubMed] [Google Scholar]
- Seitz RJ. (1992): Vibratory stimulation increases and decreases the regional cerebral blood flow and oxidative metabolism: a positron emission tomography (PET) study. Acta Neurol Scand 86: 60–67. [DOI] [PubMed] [Google Scholar]
- Servos P. (1998): Somatotopy of the human arm using fMRI. Neuroreport 9: 605–609. [DOI] [PubMed] [Google Scholar]
- Spiegel J. (1999): Functional MRI of human primary somatosensory and motor cortex during median nerve stimulation. Clin Neurophysiol 110: 47–52. [DOI] [PubMed] [Google Scholar]
- Talairach J and Tournoux P. (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers. [Google Scholar]


