Abstract
During the developmental stage, the brain undergoes anatomic, functional, and metabolic changes necessary to support the complex adaptive behavior of a mature individual. Estimation of developmental changes occurring in different regions of the brain would provide a means of relating various behavioral phenomena to maturation‐specific brain structures, thereby providing useful information on structure‐function relationships in both normal and disease states. We used multichannel near‐infrared spectroscopy (MNIRS), a new noninvasive imaging technique for revealing the course of neural activity in selected brain regions, to monitor the activities of the visual cortex as mirrored by hemodynamic responses in infants subjected to photostimulation during natural sleep. In the infants, oxyhemoglobin and total hemoglobin decreased and deoxyhemoglobin increased in the visual cortex with photostimulation. This pattern of responses was different from the response pattern in adults reported previously. The different patterns of responses to photostimulation in the visual cortices of infants and adults might reflect developmental and behavioral differences. It may reflect a different functional organization of the visual cortex in infants or ongoing retinal development. Our results demonstrated that regional hemodynamic change could be detected in a small area around the visual cortex. MNIRS offers considerable potential for research and noninvasive clinical applications. Hum. Brain Mapping 22:124–134, 2004. © 2004 Wiley‐Liss, Inc.
Keywords: developmental change, multichannel near‐infrared spectroscopy, hemodynamics, infants, visual cortex, photostimulation, optical imaging
INTRODUCTION
During the developmental stage, the brain undergoes anatomic, functional, and metabolic changes necessary to support the complex adaptive behavior of a mature normal individual. Positron emission tomography (PET) studies have shown that there are local changes in cerebral glucose utilization during infant development and that the changes match the process of initial overproduction and subsequent elimination of excessive neurons, synapses, and dendritic spines known to occur in the developing brain [Chugani and Phelps, 1986; Chugani et al., 1987]. Estimation of developmental changes occurring in different regions of the brain would provide a means of relating various behavioral phenomena to maturation‐specific brain structures, thereby providing useful information on structure‐function relationships in both normal and disease states. Visual functions in infants and young children have been studied extensively using electrophysiologic techniques [Placzek et al., 1985; Roy et al., 1995] and a behavioral technique [Atkinson, 1984] in clinical settings. Functional magnetic resonance imaging (fMRI) based on blood oxygenation level‐dependent (BOLD) contrast has been used widely for detection and localization of activation of cerebral functions in infants, including changes in patterns of visually induced cortical activation, during development. Born et al. [1996, 1998] reported that flickering light stimulation induced a signal decrease in the occipital region in infants, which is different from the signal increase in adults, and that the localization of the activation was age‐dependent. Furthermore, Yamada et al. [1997, 2000] reported an inverse response in infants that is related to a developmental process of white matter myelination.
Near‐infrared spectroscopy (NIRS) is a noninvasive method for detecting changes in the concentrations of oxyhemoglobin ([oxyHb]), deoxyhemoglobin ([deoxyHb]), and total hemoglobin ([totalHb]) at the bedside. In recent years, NIRS has been used to study functional activations of various areas of the brain. This is based on the assumption that an increase in [oxyHb] represents an increase in blood flow, which in turn reflects neural activation. Most studies using NIRS to assess cortical function have been carried out on adult subjects. In infants, NIRS has been used to assess the activities of the visual cortex induced by a checkerboard or flashlight simulation [Meek et al., 1998; Hoshi et al., 2000], activation of the frontal cortex by music stimulation [Sakatani et al., 1999], activation of the temporal cortex by acoustic stimulation [Zaramella et al., 2001] and activation of the olfactory cortex by odor stimulation [Bartocci et al., 2000].
Small multichannel near‐infrared spectroscopy (MNIRS), a technique that enables images of temporal changes in hemoglobin (Hb) concentration at any location in the brain to be obtained, has also become available recently. The results of measurements using MNIRS are shown as 2D images. We reported previously brain activities during a passive knee movement and distribution of cerebral blood flow (CBF), estimated by MNIRS, and the clinical usefulness of MNIRS for infants [Isobe et al., 2001; Kusaka et al., 2001a]. MNIRS can be carried out at the bedside and provides image of high resolution. We used MNIRS to estimate activities of the visual cortex as mirrored by hemodynamic responses in infants subjected to photostimulation during natural sleep, and we compared the activities to normal adult response patterns during non‐rapid eye movement (REM) sleep. Preliminary results of this study without imaging results have been published previously [Kusaka et al., 2003].
MATERIALS AND METHODS
Near‐Infrared Optical Imaging System
We used a near‐infrared optical imaging system with three laser diodes, 776, 804, and 828 nm, as light sources (OMM‐2000, Shimadzu Corp., Japan). The mean laser power at each light source was 2 mW. Each source fiber contained light fluxes of three wavelengths. We used eight sources and eight detectors. There were 8 such source fibers in the system, and the light fluxes were sent through the fibers to probe‐pads attached to the head of the infant. Diffusely reflected light fluxes from the infant were detected by another 8 fibers and transmitted to photomultipliers for final detection. Figure 1 shows the positions in which the 16 optical fibers were placed on the head of an infant and the 24 measurement positions. The resulting 24 source‐detector signals were used for time‐course graphics every 130 msec, and data were averaged for 1 sec [Kusaka et al., 2001b; Oda et al., 2001]. We defined [totalHb] as the sum of [oxyHb] and [deoxyHb] and measured the changes in concentrations of [oxyHb], [deoxyHb] and [totalHb] from prebaseline values.
Figure 1.
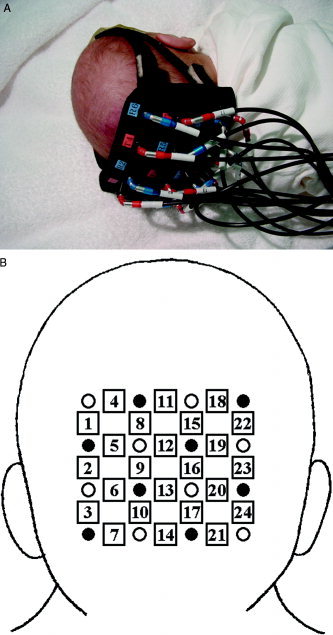
A: Measurement positions on the head of an infant. The probe was placed over the bilateral occipital region, with the center of probe at the inion. B: Measurement positions and coordinate system. Open circles, light incident positions; closed circles, detector positions; squares, measurement positions.
Subjects and Experimental Procedures
The subjects were five infants, age 29 to 111 days (postconceptional ages of 34–43 wk) and five healthy adult volunteers. All five infants had undergone clinical MRI examinations for a variety of pathologic conditions unrelated to visual function, which was normal in routine clinical examinations in all infants except for one infant who had been diagnosed as having periventricular leukomalacia with spastic diplegia at the age of 11 months. Informed consent was obtained in writing from the parents of the infants and from the adult volunteers, and the protocol was approved by the local ethics committee. Clinical data of the infants are shown in Table I.
Table I.
Summary of clinical data for infants
| Infant | Gestational age (week) | Birth weight (g) | Age (days) | Clinical diagnosis | Neurologic sign (age) |
|---|---|---|---|---|---|
| M.Y. | 28.0 | 1,010 | 111 | RDS, PDA | Normal (1 yr 2 mo) |
| M.K. | 28.0 | 896 | 111 | RDS, PDA | Normal (1 yr 2 mo) |
| I.K. | 29.4 | 1,035 | 78 | RDS | Normal (1 yr 1 mo) |
| K.M. | 30.6 | 1,518 | 29 | PVL | Spastic diplegia (11 mo) |
| U.A. | 33.1 | 1,704 | 34 | TTN | Normal (9 mo) |
RDS, respiratory distress syndrome; PDA, patent ductus arteriosus; PVL, periventricular leukomalacia; TTN, transient tachypnea of newborn.
A probe consisting of 16 optical fibers, 8 for transmission and 8 for detection, was placed over the bilateral occipital region, with the center of probe at the inion. The interoptode distances were 3 cm for adults and 2 cm for infants. Theoretical investigation of the photon path in a head model has indicated that the gray matter is part of the sampling volume even at interoptode distances as short as 2–2.5 cm [Okada et al., 1997]. We reported previously that an interoptode distance of 2 cm is sufficient for NIRS measurement of cerebral hemodynamics from the scalp in infants [Kusaka et al., 2001a]. The probe was covered with a dark cloth to prevent external light interference.
Measurements in the infants were carried out in the supine or prone position with the occipital region of the head touching the probe, and measurements in the adults were carried out in a comfortable sitting position or lateral position in a dark, quiet room. The adult subjects kept their eyes closed and were instructed to sleep throughout the examination. The subjects were exposed to stroboscopic white flashing light at a frequency of 8 Hz projected on the eyelids during the stimulation period (15 sec) and to non‐flashing light during the rest period (45 sec). The stimulation cycle was repeated 8–11 times. All infants were monitored by pulse oximetry during the examination. The infants were clinically in a state of quiet sleep during the study. The behavioral state of the infants, as defined clinically by Prechtl [1974] and Thoman [1990, 1995], was closely monitored at the bedside. In the adult subjects, the sleep stage was assessed by standard sleep‐laboratory polysomnographic techniques, including electroencephalography, submental electromyography, electrooculography and electrocardiography. A unipolar electroencephalogram was recorded. The sleep stage was estimated, and MNIRS measurement was carried out during the non‐REM sleep stage.
We corrected the baseline for consecutive analysis. The line connecting values of parameters obtained from 15 sec before the start of stimulation until 30 sec after the completion of stimulus presentation for each photostimulation was made the baseline. MNIRS measurements, even those carried out in a state of no stimulation, are usually contaminated by movement artifacts and spontaneous oscillation. Integration of data obtained from multiple stimulations is needed to eliminate these artifacts and spontaneous fluctuations in [oxyHb], [deoxyHb], and [totalHb] and to extract only data on responses to photostimulation. We therefore analyzed the means and standard deviations of changes in parameters calculated from 8–11 trials in which continuous stable measurements were possible.
Figure 2 shows the grand averages of concentration changes in each parameter as a function of time at all channels from 10 trials in an infant case. The patterns of changes in [oxyHb], [deoxyHb], and [totalHb] at all channels are similar. The fluctuations in both [oxyHb] and [deoxyHb] were greatest at channel 13. These results were the same in all infants. Because the interoptode distances were the same for all channels, it was thought that the sites of greatest activation in the brain would be the sites where measurements were carried out, i.e., the channel with the greatest signal change in [oxyHb]. The channel with the greatest change in [oxyHb] from the 24 source‐detector channels for each subject was selected for analysis of statistical significance in fluctuations of [oxyHb], [deoxyHb], and [totalHb] caused by photostimulation. Wilcoxon's signed rank test was used for statistical analysis of changes in parameters from 5 sec before the start of stimulation, and P values of less than 0.05 were considered significant. We selected a time of 5 sec before the start of stimulation for statistical analysis because Hb changes are thought to be minimal during the non‐stimulation period without the effect of previous photostimulation. In the activated area, the Mann‐Whitney U test was used for statistical analysis of differences between each of the three parameters of all tasks in the prestimulation period (from −15–0 sec before stimulation) and stimulation period (from 0–15 sec during stimulation) in all channels.
Figure 2.
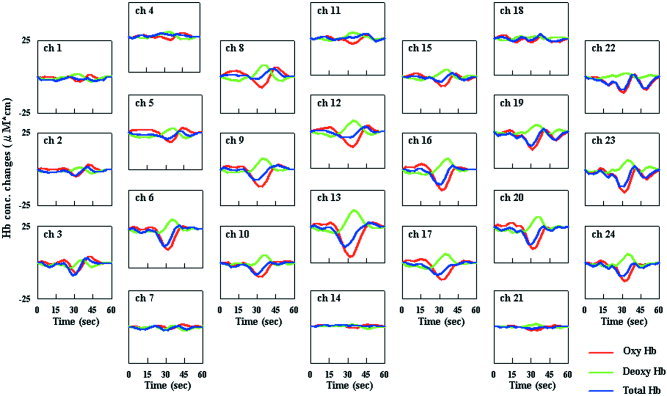
Grand averages of changes in [oxyHb], [deoxyHb], and [totalHb] as a function of time at all 24 channels from 10 trials in an infant case (MY). Photostimulation was induced for a period of 15 sec (from 15 to 30 sec).
RESULTS
Figure 3 shows topographical image patterns of grand averages of the time courses of [oxyHb], [deoxyHb] and [totalHb] during visual photostimulation in one infant and one adult volunteer. This figure shows changes in focal brain hemodynamics in the visual cortex of the occipital region, the changes in the infant's Hb apparently being different from those in the adult volunteer. In adults, there were rises in [oxyHb] and [totalHb] with photostimulation, whereas there were declines in [oxyHb] and [totalHb] and a rise in [deoxyHb] with brain activation in the infants. In the infants, [oxyHb] and [totalHb] gradually decreased and reached minimum plateaus approximately 17 and 13 sec after the onset of stimulus presentation and then returned to baseline levels. [deoxyHb] increased and reached maximum 20 sec after onset of stimulation and then returned to baseline level (Fig. 4).
Figure 3.
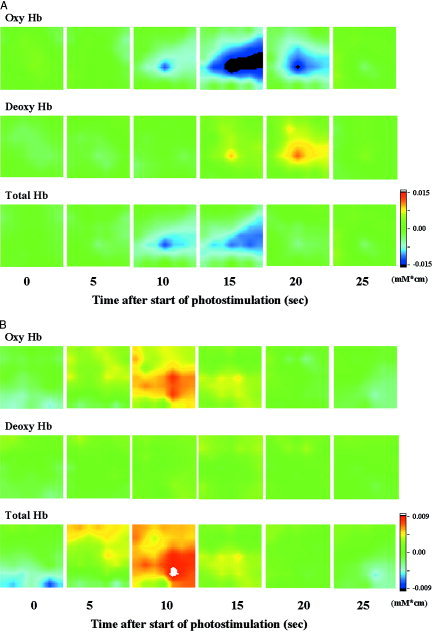
Dynamic 2D images of [oxyHb], [deoxyHb] and [totalHb] in an infant aged 111 days (43 wks postconception) (A) and an adult volunteer (B). Images were taken at 5‐sec intervals from 0–25 sec after the start of photostimulation. Photostimulation was induced for a period of 15 sec.
Figure 4.
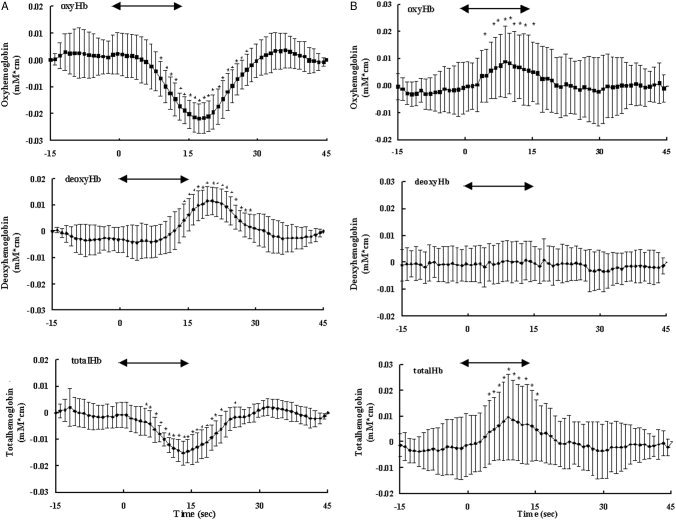
Grand averages of changes in [oxyHb] as a function of time in the same subjects as those for whom results are shown in Figure 3. From the 24 source‐detector signals, the signal with the greatest change in [oxyHb] in each subject was selected for statistical analysis. A: Average from 10 trials in an infant. B: Average from eight trials in an adult. The arrow indicates the 15‐sec period of visual flashlight stimulation. Changes in [oxyHb], [deoxyHb] and [totalHb] are given in mM · cm. The data were obtained every 130 msec and averaged for 1 sec. Error bars = SD of the mean. *Significance of mean concentration changes from 5 sec before the start of photostimulation (Wilcoxon's signed‐rank test; *P < 0.05).
The number of trials for each subject, the time to peak (t max), the mean (SD) concentration changes during and after stimulation, and the levels of significance for these changes for each subject and parameter are shown in Tables II and III. In all infants, there were statistically significant decreases in [oxyHb] and increases in [deoxyHb]. In four of five infants, there were statistically significant decreases in [totalHb]. The average concentration changes during the respective peak responses were 13.1 ± 5.2 (SD) μM · cm for [oxyHb], 7.5 ± 4.2 μM · cm for [deoxyHb], and 7.0 ± 4.8 μM · cm for [totalHb]. The mean times corresponding to maximal changes were 15.6 ± 4.3 sec for [oxyHb], 16.9 ± 5.6 sec for [deoxyHb] and 13.1 ± 4.7 sec for [totalHb]. In four of five adults, there were statistically significant increases in [oxyHb] and [totalHb].
Table II.
Changes in [oxyHb], [deoxyHb], and [totalHb] in response to visual stimulation in infants
| Infant | n | OxyHb | DeoxyHb | TotalHb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t min (sec) | Mean (SD), mM · cm | P | t max (sec) | Mean (SD), mM · cm | P | t min (sec) | Mean (SD), mM · cm | P | ||
| M.Y. | 10 | 17.2 | −0. 0217 (0. 0055) | 0.005 | 20.4 | 0. 0011 (0. 0052) | 0.005 | 13.1 | −0. 0152 (0. 0046) | 0.005 |
| M.K. | 10 | 13.1 | −0. 0135 (0. 0048) | 0.005 | 14.1 | 0. 0122 (0. 0038) | 0.005 | 8.9 | −0. 0049 (0. 0020) | 0.005 |
| I.K. | 10 | 16.2 | −0. 0101 (0. 0062) | 0.009 | 17.2 | 0. 0067 (0. 0049) | 0.200 | 13.1 | −0. 0053 (0. 0030) | 0.047 |
| K.M. | 9 | 10.0 | −0. 0081 (0. 0055) | 0.038 | 12.0 | 0. 0078 (0. 0045) | 0. 0210 | 7.9 | −0. 0026 (0. 0022) | 0.051 |
| U.A. | 10 | 21.4 | −0. 0119 (0. 0134) | 0.005 | 25.1 | 0. 0099 (0. 0067) | 0. 0170 | 18.3 | −0. 0069 (0. 0110) | 0.005 |
Number of trials (n), time to bottom or peak (t min or t max), mean changes in [oxyHb], [deoxyHb], and [totalHb] (in mM · cm), and level of significance (Wilcoxon's signed‐rank test) for each subject.
Table III.
Changes in [oxyHb], [deoxyHb], and [totalHb] in response to visual stimulation in adults
| Adult | n | OxyHb | DeoxyHb | TotalHb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t max (sec) | Mean (SD), mM · cm | P | t min/max (sec) | Mean (SD), mM · cm | P | t max (sec) | Mean (SD), mM · cm | P | ||
| K.K. | 10 | 6.8 | 0. 0235 (0. 0157) | 0.013 | 21.4 | −0. 0037 (0. 0073) | 0.093 | 8.9 | 0. 0260 (0. 0160) | 0.013 |
| I.K. | 8 | 10.0 | 0. 0094 (0. 0062) | 0.017 | 21.4 | 0. 0002 (0. 0024) | 0.208 | 6.8 | 0. 0103 (0. 0060) | 0.012 |
| M.T. | 10 | 8.9 | 0. 0082 (0. 0091) | 0.075 | 13.1 | −0. 0023 (0. 0023) | 0.037 | 8.9 | 0. 0098 (0. 0124) | 0.092 |
| H.N. | 11 | 9.9 | 0. 0088 (0. 0146) | 0.026 | 14.1 | 0. 0019 (0. 0038) | 0.051 | 9.9 | 0. 0100 (0. 0177) | 0.012 |
| Y.E. | 10 | 26.6 | 0. 0103 (0. 0110) | 0.007 | 12.0 | 0. 0052 (0. 0073) | 0.241 | 20.3 | 0. 0106 (0. 0112) | 0.005 |
Number of trials (n), time to bottom or peak (t min or t max), mean changes in [oxyHb], [deoxyHb], and [totalHb] (in mM · cm), and level of significance (Wilcoxon's signed‐rank test) for each subject.
Figure 5 shows dynamic 2D images of [oxyHb], [deoxyHb] and [totalHb] in all cases. Images were taken at the time when the [oxyHb] signal reached a minimum or maximum value in the channel showing the signal with the greatest changes in [oxyHb] from among the 24 channels. In infants, the magnitude of decrease in [oxyHb] was greater than that of [totalHb]. In the adult volunteers, the magnitude of increase in [totalHb] was greater than that of [oxyHb].
Figure 5.
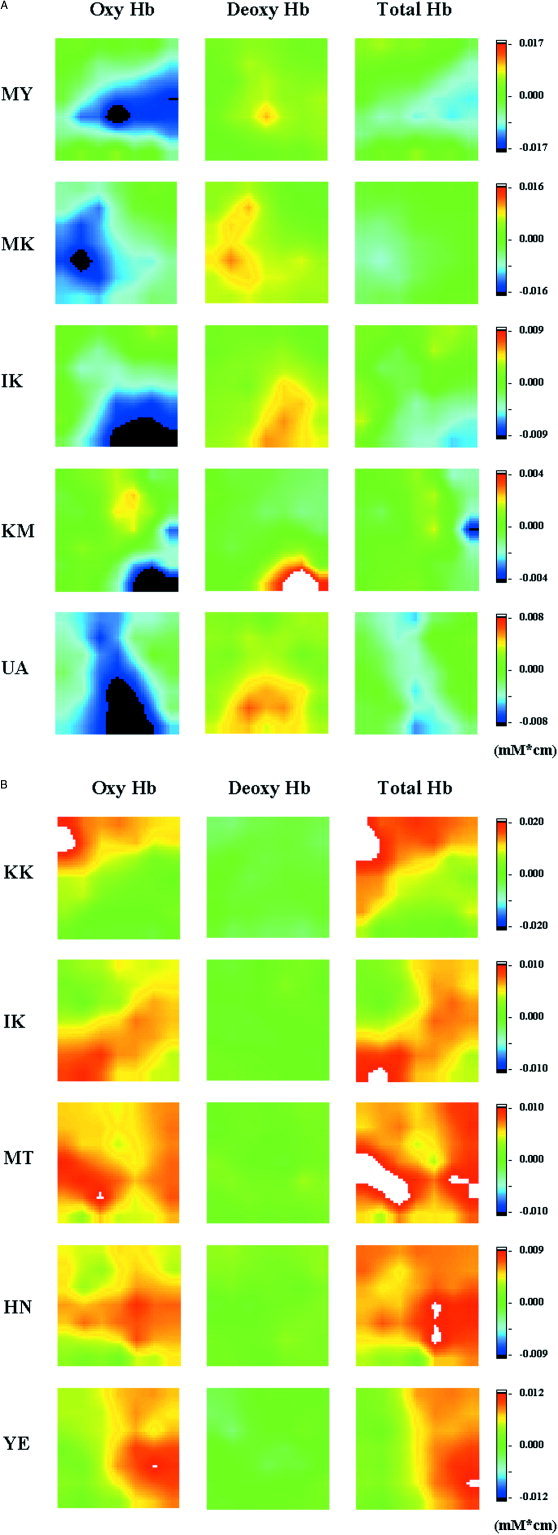
Dynamic 2D images of [oxyHb], [deoxyHb], and [totalHb] in all five infants (A) and five adult volunteers (B). Images were taken at the time when the [oxyHb] signal reached to minimum or maximum value in the channel showing the signal with the greatest changes in [oxyHb] from among the 24 channels.
Table IV summarizes the results of changes in parameters from prestimulation period to the stimulation period of all tasks in all channels. The results showed that, taking P < 0.01 as a significant level, [totalHb] increased mostly in the channels in which [oxyHb] increased in the adult volunteers; however, that was not the case in the infants.
Table IV.
Summary of individual P values in three parameters between the prestimulation period and stimulation period of all tasks in all channels
| Subjects | OxyHb | DeoxyHb | TotalHb | |||
|---|---|---|---|---|---|---|
| P < 0.01 | P < 0.001 | P < 0.01 | P < 0.001 | P < 0.01 | P < 0.001 | |
| Infants | ||||||
| M.Y. | 23 | 9, 12, 13, 15, 16–19, 22 | 5 | 1, 18, 22 | 16, 22–24 | 9, 12, 13, 15–17, 19, 20 |
| M.K. | 21 | 1–14, 16, 17, 19, 20, 23, 24 | 3, 11, 12, 14, 18, 20, 23, 24 | 1, 2, 4–7, 10, 13 | 14, 21 | 1–13, 15–17, 19, 20, 23, 24 |
| I.K. | 20 | 17, 18 | 1, 2, 4, 5, 22 | — | 8, 18 | 5, 9, 13, 14, 17, 20, 21, 24 |
| K.M. | 1, 4, 8, 15, 20 | 11, 12, 17, 21, 23, 24 | 7, 8, 17, 18 | 4, 5, 14, 20, 21 | 22 | 11, 12, 15, 16, 23, 24 |
| U.A. | 3, 11, 15, 19 | 1, 2, 4–6, 8, 18, 22, 24 | 1, 3 | 4, 8, 24 | 1, 5, 6, 22 | 2, 6, 15, 18, 24 |
| Adult | ||||||
| K.K. | — | 1–24 | 8, 18, 22 | 2, 3, 5–7, 9, 11, 12, 14–17, 19–21, 23, 24 | — | 1–24 |
| I.K. | — | 2, 3, 6–24 | 1, 2, 9 | 3–5, 7, 8, 10, 24 | 9 | 3, 6, 7, 10–24 |
| M.T. | 7, 21, 24 | 3 | 2, 7, 11 | 12 | 3, 21, 24 | — |
| H.N. | 3, 4, 5, 10, 11, 18, 22, 24 | 2, 6, 8, 9, 12, 13, 15–17, 19, 20, 23 | 1, 3, 13, 14, 17, 22 | 5, 7, 18 | 3, 8, 11, 13–15, 18, 20, 23 | 1, 2, 4–6, 9, 12, 16, 17, 19, 22 |
| Y.E. | 17 | 14, 21 | 22 | 18, 19 | — | 14, 17, 21 |
Individual P values in three parameters between the prestimulation period and stimulation period of all tasks in all channels using the Mann‐Whitney U test. Values are channel numbers.
DISCUSSION
We have demonstrated for the first time that patterns of changes in Hb detected by MNIRS in the visual cortices of infants induced by photostimulation is, surprisingly, different from that in adults.
Continuous‐wave NIRS has been used for noninvasive examination of brain functions, and results of studies using continuous‐wave NIRS have provided important information on the physiology of brain functions. Furthermore, recent developments in optical imaging technology have enabled information on localized brain hemodynamics to be obtained. We have presented here information on localization of brain functions in visual cortex in response to photostimulation that could not be obtained using a single channel method. The use of MNIRS enables selection of the site with the greatest activation and measurements of changes in surrounding areas, which had not been possible using other methods.
Various stimuli were used in previous NIRS studies on visual brain function in adults, including picture observation [Villringer et al., 1993], flashing light at a frequency of 8 Hz [Kato et al., 1993], a multicolored moving dodecahedron [Wenzel et al., 1996], a green‐red checkerboard reversing at a frequency of 10 Hz [Heekeren et al., 1999], a checkerboard reversing at several frequencies [Takahashi et al., 2000], and a hemi‐field green‐white checkerboard reversing at a frequency of 8 Hz [Colier et al., 2001]. Results of those studies showed that the typical response of visual cortex activation in adults is an increase in [oxyHb] and a slight decrease in [deoxyHb]. Villringer and Chance [1997] described the significance of [oxyHb] and [deoxyHb] changes during neural activation. They reported that there is a decrease in [oxyHb] and corresponding increase in [deoxyHb] during a few seconds after stimulus onset in which oxygen is consumed, but such changes were not observed in the present study. The time resolution of our MNIRS (data were obtained every 130 msec and averaged for 1 sec) may have not ben sufficient for detection of the initial dip in Hb. Next, when regional CBF (rCBF) increases, there was a rise in [oxyHb] and often a decrease in [deoxyHb] due to washout. The relative contribution of these two effects determines whether [deoxyHb] increases or decreases, as was confirmed by the results of the present study.
Meek et al. [1998] used NIRS in a study in which ten infants aged 3 days to 14 weeks in an awake state viewed a checkerboard with a 5‐Hz pattern reversal and found that all infants showed an increase in [totalHb] in the occipital region with stimulation, nine of the infants showed an increase in [oxyHb], and nine showed an increase in [deoxyHb] related to the stimulus. These results are different to our results showing that [oxyHb] and [totalHb] decreased with photostimulation in infants. This discrepancy in results may be due to differences in sleeping stages and patterns of photostimulation.
Three groups have reported results of preliminary fMRI studies using sleeping or sedated infants. Martin et al. [1999] studied sedated neonates stimulated with red LED goggles at a frequency of 8 Hz. A negative BOLD signal indicating an increase in [deoxyHb] was observed in 5 of 18 infants between the age of 4 days and 4 months. Born et al. [1996, 1998] reported results from fMRI studies on sedated infants stimulated with 8‐Hz light flushes. An area of significant signal change was seen in the occipital region with a decrease in the BOLD signal. Yamada et al. [1997, 2000] reported that infants older than 8 weeks showed a signal decrease, whereas infants younger than 7 weeks showed a signal increase, which is related to progression of white matter myelination.
In this study, typical changes in infants with photostimulated brain activation were declines in [oxyHb] and [totalHb] and a rise in [deoxyHb], and the peak in [deoxyHb] increase was later than was the decrease in [oxyHb] in most of the infants. This is because, although photostimulation induces neural activation, compensatory rCBF decreases; thus, [oxyHb] and [totalHb] decrease. It is thought that [deoxyHb] increased due to the continued oxygen consumption in the area of neural activation. The reason for the decrease in [totalHb], indicating a decrease in rCBF in the visual cortex, is complicated. One possible reason is inaccuracy in the MNIRS measurements. There does not seem to be any problem with accuracy of measurements because we observed similar changes in Hb when measurements were carried out using another 24‐channel near‐infrared topography device (Hitachi Medico, Japan) in 10 normal infants in a state of natural sleep who had been subjected to stimulation similar to that used in the present study [Kusaka et al., 1999]. Another possible reason is a problem in the measurement area of MNIRS, which was measured from the surface of the scalp in the present study. It has been reported that spatial distributions of activation by photostimulation in infants and adults using fMRI measurements were different [Born et al., 1996, 1998]. In adults, the activation area is the whole length of the calcarine fissure. In contrast, in infants, activation area is restricted to the anterolateral part of the fissure. In infants, therefore, the decrease in rCBF in the measurement area may indicate that blood is being supplied to the activation area that is deeper from the scalp than that in adults. We considered the interoptode distance (20 mm) of MNIRS in the present study, however, to be sufficient for measurement of the activated area in infants. Because the differential pathlength factor in infants has been reported to be almost five [Duncan et al., 1995], the depth of the measurement position from the scalp surface in the present study was estimated to about 30 mm.
Indeed, Born et al. [2002a] reported a negative BOLD response in the primary visual cortex during photostimulation due to a decrease in rCBF in sedated children. Born et al. [2002b] also observed patterns of activation of the adult visual cortex using fMRI and PET. They reported that rCBF decreased during visual stimulation in a state of spontaneous sleep and that the decrease in rCBF was more rostro‐dorsal compared to the increase in rCBF along the calcarine sulcus found during visual stimulation in an awake state. They gave two possible explanations for the decrease in rCBF during visual stimulation in a state of sleep. One explanation is that the activation of primary sensory cortices that remain relatively active during sleep are suppressed by thalamic gating [Coenen et al., 1995; Steride et al., 1993]. Reduced rCBF during visual stimulation could be secondary to a direct neural inhibition of the visual cortex. Another possible explanation is that the rCBF during sleep remains elevated due to an energy consumption process during sleep that is not related to visual function. If the process is more energy consuming than the processing of the visual stimulus, neuronal activity and thus rCBF may decrease when the process is disrupted by a stimulus.
In this study, we observed a decrease in rCBF in infants during natural sleep but not in adults during non‐REM sleep. A decrease in rCBF may be observed more easily in infants than in adults because of different functional organization of visual cortex in infants or ongoing retinal development. It was also confirmed in this study that [totalHb] increases in adults were mainly in the area in which [oxyHb] increases. This is thought to reflect sufficient blood supply to the area in which oxygen consumption increases due to neural activity. In infants, however, such a relationship was not found. This finding of a difference in areas in which rCBF changes is similar to the finding reported by Born et al. [2002b] of a difference in areas of adult brain in which rCBF increases and decreases. Further investigation is needed into responses to photostimulation in various stages of sleep and differences between responses to photostimulation of various intensities.
In conclusion, functional hemodynamic responses in infants during natural sleep can be estimated using MNIRS. Results of analysis of images obtained by MNIRS in this study confirmed that there are differences between hemodynamics accompanying neural activation in infants and adults in a sleep stage when they are subjected to photostimulation. The MNIRS signal response patterns in infants and adults are different. This difference may reflect differences in behavioral or developmental states. The use of MNIRS is expected to enable study of the physiology of the developing brain.
Acknowledgements
We thank Professor K. Hattori for his critical comments regarding statistical analysis.
REFERENCES
- Atkinson J (1984): Human visual development over the first 6 months of life. A review and a hypothesis. Hum Neurobiol 3: 61–74. [PubMed] [Google Scholar]
- Bartocci M, Winberg J, Ruggiero C, Bergqvist LL, Serra G, Lagercrantz H (2000): Activation of olfactory cortex in newborn infants after odor stimulation: a functional near‐infrared spectroscopy study. Pediatr Res 48: 18–23. [DOI] [PubMed] [Google Scholar]
- Born P, Rostrup E, Leth H, Peitersen B, Lou HC (1996): Change of visually induced cortical activation patterns during development. Lancet 347: 543. [DOI] [PubMed] [Google Scholar]
- Born P, Leth H, Miranda M, Rostrup E, Stensgaard A, Peitersen B, Larsson HB, Lou HC (1998): Visual activation in infants and young children studied by functional magnetic resonance imaging. Pediatr Res 44: 578–583. [DOI] [PubMed] [Google Scholar]
- Born AP, Rostrup E, Miranda MJ, Larsson HB, Lou HC (2002a): Visual cortex reactivity in sedated children examined with perfusion MRI (FAIR). Magn Reson Imaging 20: 199–205. [DOI] [PubMed] [Google Scholar]
- Born AP, Law I, Lund TE, Rostrup E, Hanson LG, Wildschiodtz G, Lou HC, Paulson OB (2002b): Cortical deactivation induced by visual stimulation in human slow‐wave sleep. Neuroimage 17: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME (1986): Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science 231: 840–843. [DOI] [PubMed] [Google Scholar]
- Chugani, HT , Phelps ME., Mazziotta JC (1987): Positron emission tomography study of human brain functional development. Ann Neurol 22: 487–497. [DOI] [PubMed] [Google Scholar]
- Coenen AM (1995): Neuronal activities underlying the electroencephalogram and evoked potentials of sleeping and waking: implications for information processing. Neurosci Biobehav Rev 19: 447–463. [DOI] [PubMed] [Google Scholar]
- Colier WN, Quaresima V, Wenzel R, van der Sluijs MC, Oeseburg B, Ferrari M, Villringer A (2001): Simultaneous near‐infrared spectroscopy monitoring of left and right occipital areas reveals contra‐lateral hemodynamic changes upon hemi‐field paradigm. Vision Res 41: 97–102. [DOI] [PubMed] [Google Scholar]
- Duncan A, Meek JH, Clemence M, Elwell CE, Tyszczuk L, Cope M, Delpy DT (1995): Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys Med Biol 40: 295–304. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Kohl M, Obrig H, Wenzel R, von Pannwitz W, Matcher SJ, Dirnagl U, Cooper CE, Villringer A (1999): Noninvasive assessment of changes in cytochrome‐c oxidase oxidation in human subjects during visual stimulation. J Cereb Blood Flow Metab 19: 592–603. [DOI] [PubMed] [Google Scholar]
- Hoshi Y, Kohri S, Matsumoto Y, Cho K, Matsuda T, Okajima S, Fujimoto S (2000): Hemodynamic responses to photic stimulation in neonates. Pediatr Neurol 23: 323–327. [DOI] [PubMed] [Google Scholar]
- Isobe K, Kusaka T, Nagano K, Okubo K, Yasuda S, Kondo M, Itoh S, Onishi S (2001): Functional imaging of the brain in sedated newborn infants using near infrared topography during passive knee movement. Neurosci Lett 299: 221–224. [DOI] [PubMed] [Google Scholar]
- Kato T, Kamei A, Takashima S, Ozaki T (1993): Human visual cortical function during photic stimulation monitoring by means of near‐infrared spectroscopy. J Cereb Blood Flow Metab 13: 516–520. [DOI] [PubMed] [Google Scholar]
- Kusaka T, Matsuura S, Fujikawa Y, Okubo K, Yasuda S, Nagano K, Suzuki T, Kondo M, Isobe K, Imai T, Itoh S, Ohnishi S (1999): [Usefulness of near‐infrared topography for monitoring of brain functions.] Acta Neonatol Jpn 35: 210. [Google Scholar]
- Kusaka T, Isobe K, Nagano K, Okubo K, Yasuda S, Kondo M, Itoh S, Onishi S (2001a): Estimation of regional cerebral blood flow distribution in infants by near‐infrared topography using indocyanine green. Neuroimage 13: 944–952. [DOI] [PubMed] [Google Scholar]
- Kusaka T, Isobe K, Nagano K, Okubo K, Yasuda S, Kawada K, Itoh S, Onishi S, Oda I, Wada Y, Konishi I, Tsunazawa T (2001b): Estimation of regional cerebral blood flow distribution in infants by multichannel near‐infrared spectroscopy with indocyanine green In: Chance B, Alfano RR, Tromberg B, Tamura M, Sevick‐Muraca EM, editors. Optical tomography and spectroscopy of tissue. IV Proceedings of SPIE, Vol. 4250 Online at http://spie.org. [Google Scholar]
- Kusaka T, Okubo K, Nagano K, Yasuda S, Kawada K, Imai T, Isobe K, Itoh S (2003): Activation of the visual cortex in newborn infants under natural sleep using multichannel near‐infrared spectroscopy. Adv Exp Med Biol 510: 255–259. [DOI] [PubMed] [Google Scholar]
- Martin E, Joeri P, Loenneker T, Ekatodramis D, Vitacco D, Hennig J, Marcar VL (1999): Visual processing in infants and children studied using functional MRI. Pediatr Res 46: 135–140. [DOI] [PubMed] [Google Scholar]
- Meek JH, Firbank M, Elwell CE, Atkinson J, Braddick O, Wyatt JS (1998): Regional hemodynamic responses to visual stimulation in awake infants. Pediatr Res 43: 840–843. [DOI] [PubMed] [Google Scholar]
- Oda I, Wada Y, Takeuchi S, Oikawa Y, Sakauchi N, Ito Y, Konishi I, Tsunazawa Y, Kusaka T, Isobe K, Itoh S, Onishi S (2001): Near infrared optical imager for cerebral blood flow and oxygenation detection In: Chance B, Alfano RR, Tromberg B, Tamura M, Sevick‐Muraca EM, editors. Optical tomography and spectroscopy of tissue. IV Proceedings of SPIE, Vol. 4250 Online at http://spie.org. [Google Scholar]
- Okada E, Firbank M, Schweiger M, Arridge SR, Cope M, Delpy DT (1997): Theoretical and experimental investigations of near infrared light propagation in a model of the adult head. Appl Opt 36: 21–31. [DOI] [PubMed] [Google Scholar]
- Placzek M, Mushin J, Dubowitz LM (1985): Maturation of the visual evoked response and its correlation with visual acuity in preterm infants. Dev Med Child Neurol 27: 448–454. [DOI] [PubMed] [Google Scholar]
- Prechtl HF (1974): The behavioral states of the newborn infants (a review). Brain Res 76: 185–212. [DOI] [PubMed] [Google Scholar]
- Roy MS, Barsoum‐Homsy M, Orquin J, Benoit J (1995): Maturation of binocular pattern visual evoked potentials in normal full‐term and preterm infants from 1 to 6 months of age. Pediatr Res 37: 140–144. [DOI] [PubMed] [Google Scholar]
- Sakatani K, Chen S, Lichty W, Zuo H, Wang YP (1999): Cerebral blood oxygenation changes induced by auditory stimulation in newborn infants measured by near infrared spectroscopy. Early Hum Dev 55: 229–236. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ (1993): Thalamocortical oscillations in the sleeping and aroused brain. Science 262: 679–685. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Ogata S, Atsumi Y, Yamamoto R, Shiotsuka S, Maki A, Yamashita Y, Yamamoto T, Koizumi H, Hirasawa H, Igawa M (2000): Activation of the visual cortex imaged by 24‐channel near‐infrared spectroscopy. J Biomed Opt 5: 93–96. [DOI] [PubMed] [Google Scholar]
- Thoman EB (1990): Sleeping and walking states in infants: a functional perspective. Neurosci Biobehav Rev 14: 93–107. [DOI] [PubMed] [Google Scholar]
- Thoman EB (1995): Monitoring of sleep in neonates and young children In: Ferber R, Kryger MH, editors. Principle and practice of sleep medicine in the child. Philadelphia, PA: W.B. Saunders; p 55–63. [Google Scholar]
- Villringer A, Planck J, Hock C, Schleinkofer L, Dirnagl U (1993): Near infrared spectroscopy (NIRS): a new tool to study hemodynamic changes during activation of brain function in human adults. Neurosci Lett 154: 101–104. [DOI] [PubMed] [Google Scholar]
- Villringer A, Chance B (1997): Non‐invasive optical spectroscopy and imaging of human brain function. Trends Neurosci 20: 435–442. [DOI] [PubMed] [Google Scholar]
- Wenzel R, Obrig H, Ruben J, Villringer K, Thiel A, Bernarding J, Dirnagl U, Villringer A (1996): Cerebral blood oxygenation changes induced by visual stimulation in humans. J Biomed Opt 1: 399–404. [DOI] [PubMed] [Google Scholar]
- Yamada H, Sadato N, Konishi Y, Kimura K, Tanaka M, Yonekura Y, Ishii Y (1997): A rapid brain metabolic change in infants detected by fMRI. Neuroreport 8: 3775–3778. [DOI] [PubMed] [Google Scholar]
- Yamada H, Sadato N, Konishi Y, Muramoto S, Kimura K, Tanaka M, Yonekura Y, Ishii Y, Itoh H (2000): A milestone for normal development of the infantile brain detected by functional MRI. Neurology 55: 218–223. [DOI] [PubMed] [Google Scholar]
- Zaramella P, Freato F, Amigoni A, Salvadori S, Marangoni P, Suppjei A, Schiavo B, Chiandetti L (2001): Brain auditory activation measured by near‐infrared spectroscopy (NIRS) in neonates. Pediatr Res 49: 213–219. [DOI] [PubMed] [Google Scholar]


