Abstract
Both lesion and functional imaging studies have implicated sectors of high‐order association cortices of the left temporal lobe in the retrieval of words for objects belonging to varied conceptual categories. In particular, the cortices located in the left temporal pole have been associated with naming unique persons from faces. Because this neuroanatomical‐behavioral association might be related to either the specificity of the task (retrieving a name at unique level) or to the possible preferential processing of faces by anterior temporal cortices, we performed a PET imaging experiment to test the hypothesis that the effect is related to the specificity of the word retrieval task. Normal subjects were asked to name at unique level entities from two conceptual categories: famous landmarks and famous faces. In support of the hypothesis, naming entities in both categories was associated with increases in activity in the left temporal pole. No main effect of category (faces vs. landmarks/buildings) or interaction of task and category was found in the left temporal pole. Retrieving names for unique persons and for names for unique landmarks activate the same brain region. These findings are consistent with the notion that activity in the left temporal pole is linked to the level of specificity of word retrieval rather than the conceptual class to which the stimulus belongs. Hum. Brain Mapping 13:199–212, 2001. © 2001 Wiley‐Liss, Inc.
Keywords: left temporal pole, language, word retrieval, functional imaging, face processing, naming
INTRODUCTION
Evidence from both lesion and functional imaging studies has implicated relatively segregated sectors of inferotemporal (IT) and temporal polar (TP) cortex in the process of word retrieval for concrete entities belonging to different conceptual categories. For example, in studies of brain‐damaged subjects with stable unilateral cortical lesions, left TP was the most consistent site of cortical damage among subjects with defective retrieval of names for unique persons [H. Damasio et al., 1996; Papagno and Capitani, 1998; Fukatsu et al., 1999]. There is also evidence from PET imaging implicating relatively segregated sectors of left IT and TP cortices in the normal process of retrieval of words denoting concrete entities in different conceptual categories [H. Damasio et al., 1996]. The finding relevant to the study reported here was that unique‐level recognition and naming of persons from faces are associated with rCBF increases in left TP and a sector of the left middle temporal gyrus but not in left ventral or posterior IT, which were the sectors engaged when subjects recognized and named animals or tools. Because unique‐level recognition of known faces appears undisturbed by left temporal polar lesions (the effect being seen most consistently with damage to the right temporal pole [Tranel et al., 1997], it is plausible to assume that the finding of left temporal polar activation in the study mentioned above is related to the retrieval of the names of those unique persons.
Based on preliminary observations indicating that lesions in left TP can impair the retrieval of names at unique level for entities other than faces, we suspected that the anatomic effect was related to the specificity of the task. (In this manuscript, “unique entity” is to be understood to mean an entity normally processed at a conceptual level so specific that the entity is in a class with no other members.) However, as the effect was demonstrated by using faces of persons as stimuli, and because faces are special entities for a variety of reasons [Damasio et al., 1990], we considered the possibility that the effect might be explained by preferential processing of faces by the anterior temporal cortices. Our strategy was to employ another category of unique entities, famous landmarks, and predict that, if the effect was a result of the uniqueness of the items, the retrieval of names of unique landmarks would also produce the same effect at the left temporal pole.
MATERIALS AND METHODS
Subjects
Ten normal subjects participated in the present experiment (5 men, 5 women, aged 28 ± 8 years). All were native English speakers with 12 or more years of education. All were right‐handed (Geschwind‐Oldfield questionnaire +90 or higher) and had no left‐handed first‐degree relatives. None had a history of neurologic or psychiatric disease. Their informed consent was obtained in compliance with federal and institutional guidelines.
Experimental tasks
Subjects performed two lexical retrieval tasks and two control tasks during the injection and uptake of the radiotracer. In the two retrieval tasks, they were asked to (a) name famous persons (PN), presented as face photographs, at unique level (ISI 2.5 sec); or (b) name famous landmarks (LN), such as buildings and natural landscape features, also at unique level (ISI 2.5 sec). In the two baseline tasks, subjects were shown (a) upright and inverted unknown faces (po) and (b) upright and inverted unknown buildings (lo). In both cases they were required to say “up” or “down” (ISI 1.0 sec). The orientation decision baseline tasks were included for the following reasons: (1) The stimuli used belonged to the same conceptual category as the target stimuli, and therefore the tasks did not differ in the requirement for basic perceptual processing; (2) the stimuli were of unknown entities (faces, buildings), therefore avoiding unwanted recognition or naming at unique level. Such unintended name retrieval is also the reason why it is not possible, in normal subjects, to isolate unique recognition from unique naming. We realize that these two aspects cannot be distinguished in the present experiment. However, this limitation is not a problem for the present study, in which we hypothesized there would be no differences in the left temporal pole related to recognition or naming of two categories of entities at unique level.
The scanning session was divided into halves, with each task performed once, in random order in each half session.
Hypotheses
The study design was factorial for category (persons, landmarks/buildings) and task (naming, orientation decision). We hypothesized that cortices in the left temporal pole would be engaged when lexical retrieval was performed at unique level (i.e., entities were recognized and named at unique level), regardless of conceptual category. Thus we anticipated that the main effect of word retrieval at unique level (i.e. [PN+LN]‐[po+lo]) would include increased activity in left temporal polar cortices, and that there would not be a significant effect of category there. Further, we did not expect to find a significant interaction of task and category in the left temporal pole, i.e., the recognition and naming at unique level, relative to the orientation decision on entities recognized at basic object level, would evoke similar activity in the left temporal pole for persons presented as faces as for landmarks. Finally, we expected that the unique‐level recognition and naming of persons would engage the right temporal pole, consistent with the results of functional imaging and lesion studies discussed earlier that implicate this region in recognition at unique level [Damasio et al., 1996; Tranel et al., 1997). A priori, there is insufficient empirical basis for predicting right temporal polar activation for unique‐level recognition of landmarks.
Stimuli
Examples of our stimuli are shown in Figure 1. Face stimuli were black‐and‐white photographs with background details and telltale appendages deleted. Familiar faces for a given subject were selected during a pilot session 24–48 hours before PET by having the subjects view a collection of famous faces from the Iowa [Tranel et al., 1995] and Boston [Albert et al., 1979] Famous Faces tests, and a number of additional faces of contemporary famous actors, politicians, and sports figures. Subjects were not asked to name any of the persons, and no names were spoken by the investigators. Subjects were asked to indicate whether or not they recognized each person. The final stimulus set of famous faces for each subject was composed only of faces they had said they recognized.
Figure 1.
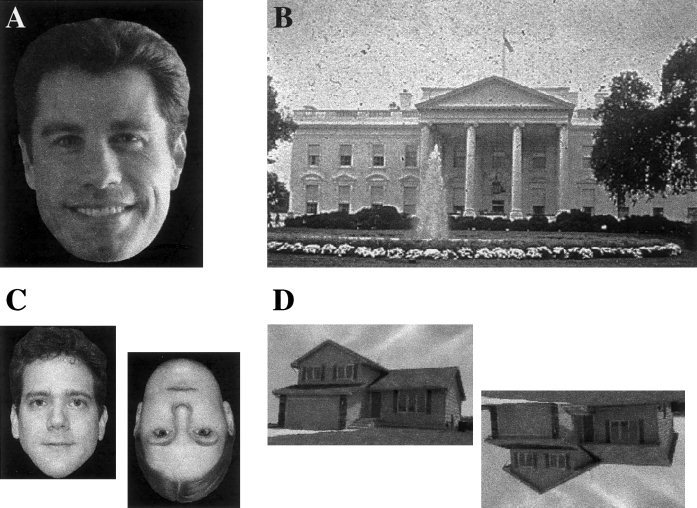
Examples of experimental stimuli. A. Photographs of faces of famous persons. B. Photographs of famous buildings and natural landmarks. C. Photographs of faces of unknown persons. D. Photographs of unknown buildings.
No attempt was made to control the ratio of male to female face stimuli for the person‐naming task. The ratio varied across subjects because of the procedure that customized the stimulus set. Fifteen stimuli were presented per injection. The mean number of males was 10.6 and the mean number of females was 4.4 (SD 1.8). Unfamiliar faces for the face baseline task were half male, half female, and half were inverted.
Famous landmark stimuli were photographs, including background details. Familiar landmark stimuli for the final stimulus set for a given subject were selected in a pilot session in a fashion analogous to that used for famous faces. Unfamiliar buildings for the building baseline task were scanned from real estate advertisements, and had background details deleted. Half were inverted.
Data acquisition
Positron emission tomography (PET) data were acquired with a General Electric 4096 Plus body tomograph, yielding 15 transaxial slices with a nominal interslice interval of 6.5 mm. For each injection, 50 mCi of [15O] water was administered as a bolus through a venous catheter. Arterial blood sampling was not performed. To improve the overlap in scanned volume across subjects, PET slice orientation was planned using PET‐Brainvox, as described previously [Grabowski et al., 1995; Damasio et al., 1996].
Subjects performed the tasks from 5 sec after injection until 40 sec after injection of [15O]water. The bolus of labeled water reached the brain 12–15 sec after injection [Hichwa et al., 1995]. Thus, subjects performed the requested task for 35 sec beginning 7–10 sec before bolus arrival. For the naming tasks, 15 stimuli were presented during this time. For the baseline tasks, 35 stimuli were presented. Subjects then viewed a fixation cross for an additional 60 sec after injection [Hurtig et al., 1994; Cherry et al., 1995]. The responses spoken by the subjects during each scan were audiotaped and later digitized. Latencies to voice onset were determined for each item, using custom software. Overall performance on each injection was indexed by the median latency to voice onset during the 30‐sec period beginning 5 sec before bolus arrival in the brain and ending 25 sec after bolus arrival in the brain.
Magnetic resonance (MR) images were obtained with a General Electric Signa scanner operating at 1.5 T, using the following protocol: SPGR 30, TR 24, TE 7, NEX 1, FOV 24 cm, matrix 256 × 192. We obtained 124 contiguous coronal slices with thickness 1.5–1.7 mm and interpixel distance 0.94 mm. The slice thickness was adjusted to the size of the brain so as to sample the entire brain, while avoiding wrap artifacts. Three individual 1NEX SPGR data sets were coregistered post hoc with Automated Image Registration (AIR 3.03) to produce a single data set of enhanced quality with pixel dimensions of 0.7 mm in‐plane and 1.5 mm between planes [Holmes et al., 1998].
MR and PET images were transferred to the Human Neuroanatomy and Neuroimaging Laboratory of the Division of Behavioral Neurology and Cognitive Neuroscience. All image processing was performed with Silicon Graphics Workstations (Silicon Graphics, Mountain View, CA) using Brainvox [H. Damasio and Frank, 1991; Frank et al., 1997].
MR images were reconstructed in three dimensions using Brainvox, prior to the PET scanning session. Extracerebral voxels were edited away manually. Talairach space was constructed directly for each subject via user‐identification of the anterior and posterior commissures and the midsagittal plane in Brainvox. An automated planar search routine defined the bounding box and a piecewise linear transformation was used [Frank et al., 1997], as defined in the Talairach atlas [Talairach and Tournoux, 1988]. After Talairach transformation, the MR data sets were warped (AIR 5th order nonlinear algorithm) to an atlas space constructed by averaging 50 normal Talairach‐transformed brains, rewarping each brain to the average, and finally averaging them again (analogous to the procedure described in Woods et al. [1999]). For simplicity, we will henceforth refer to this standard space as “Talairach space.”
Search volume
For this study, the search volume was defined as all stereotactic voxels that corresponded to the left or the right temporal pole in the averaged Talairach‐transformed MR data. Using Brainvox, we first constructed the long axis of the temporal lobe as a line between the junction of the lateral parieto‐occipital and lateral parieto‐temporal lines [Ono et al., 1990] and the temporal pole. The temporal pole was defined as all temporal lobe voxels anterior to a line drawn perpendicular to this long axis at the level of the anterior ascending ramus of the Sylvian fissure (see Fig. 2). The combined volume of the temporal poles was 21.3 cm3.
Figure 2.

Definition of the search volume. A. Upper left: The temporal pole was defined as the part of the temporal lobe anterior to a line (dotted yellow line) perpendicular to the long axis of the temporal lobe (red dotted line) at the level of the anterior ascending ramus of the Sylvian fissure. L and R ROIs were traced separately. Upper right: the three axial slices displayed in B. Lower left and right: 3D rendering of the average MR, with temporal poles, as defined for this study, painted red. B. Three Talairach axial sections, on which results are displayed in Figures 3 and 4. In the axial images, the right side of the image represents the left side of the brain.
PET data processing
Reconstructed images of the distribution of radioactive counts from each injection were coregistered with each other using Automated Image Registration (AIR 3.03, Roger Woods, UCLA). Three‐dimensional MR and the mean coregistered PET data were also coregistered using PET‐Brainvox and Automated Image Registration (AIR) [Woods et al., 1993]. PET data were Talairach‐transformed as described above. Because the search volume was proximate to the skeletal muscle in the temporal fossa, we took precautions to eliminate the possibility that the observed activity spilled in from this source. We used the coregistered MRI to mask away extracerebral voxels and then smoothed the data with an isotropic 16‐mm Gaussian kernel by Fourier transformation, complex multiplication, and reverse Fourier transformation. The final calculated image resolution was 18 × 18 × 18 mm FWHM. In a supplementary analysis a 6‐mm Gaussian kernel was used, with final calculated resolution 10 × 10 × 10 mm. PET data were analyzed with a pixelwise linear model that estimated coefficients for global flow (covariable) and task and block/subject effects (classification variables) [Friston et al., 1995; Grabowski et al., 1996].
We searched for increases in adjusted mean activity t‐statistic images generated for each planned contrast. Critical t values were calculated using Gaussian random field theory for t statistics [Worsley et al., 1992; Worsley, 1994]. The threshold t 65 value (P = 0.05) for the search volume (approximately 4 resels) was 3.09. The common intracerebral stereotactic volume was 1,237 cm3 (218 resels). The threshold for the post hoc whole‐brain search was 4.68. We also performed a supplementary analysis of the main effect of task at a finer spatial scale, using a Gaussian filter of 6 mm FWHM. For this analysis, we used a Bonferroni‐ and Gaussian field theory‐corrected critical t value (i.e., P = 0.025, 19 resels, t 65 = 4.06). We predicted that the search volume would contain voxels in the left TP with t values greater than the threshold dictated by random field theory for the main effect of task.
RESULTS
Imaging data: left temporal pole
When naming persons was contrasted directly with naming landmarks, no significant difference was found in the left TP (t 65max = +1.34; t 65min = −1.16) The observed difference in counts was <1% (range −4.4 counts to +6.6 counts). The main effect of retrieving names for unique entities (persons or landmarks), in comparison to the control tasks, identified a significant increase in activity in the left TP (t 65 = 6.17; see Fig. 3 and Table I). The center of mass of this region, within the search volume, was –37 +14 −20. However, this coordinate was not a local maximum because this region was confluent with another region of activation outside the search volume in the immediately adjacent frontal operculum. Therefore we also analyzed the data using a smaller spatial filter (6 mm FWHM) and confirmed the presence of a distinct and significant focus of activation in the left TP (t = 4.68, −41 +15 −19; see Fig. 4). The global‐adjusted mean activity at this coordinate in the four experimental conditions was as follows: naming persons from faces, 828 counts; naming landmarks, 827 counts; face orientation baseline, 812 counts; house orientation baseline, 801 counts. The local increase in activity was therefore about 2.5%. When naming persons was contrasted directly with the face orientation decision task, a significant increase in activity in the left TP was found (t 65 = 3.97). Likewise, when naming landmarks was contrasted directly with the house orientation decision task, a significant increase in the left TP (t 65 = 6.57) was also found.
Figure 3.
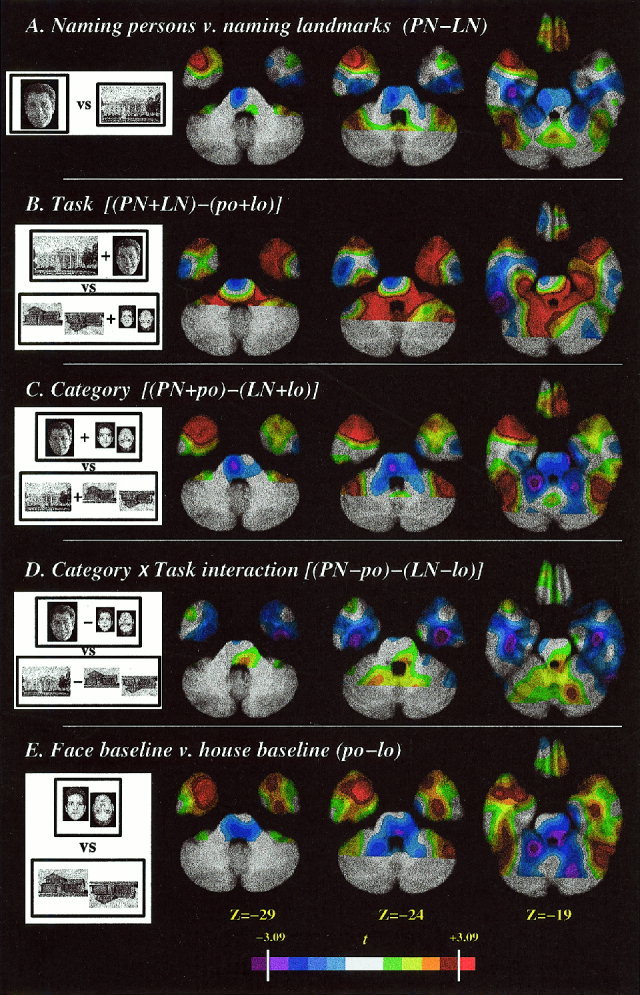
T statistic maps for planned contrasts, superimposed on axial slices of the averaged MRI scan. The temporal pole search volume is delineated by the white dotted line (see Fig. 2 for details). The color scale at the bottom is calibrated with the significance level (3.09) determined by Gaussian random field theory. A. Naming persons contrasted with naming landmarks. B. Main effect of task (naming landmarks or persons contrasted with the baseline tasks). C. Main effect of category (naming or baseline using faces contrasted with naming or baseline using landmarks/buildings). D. Task by category interaction. (The difference between person naming and face baseline contrasted with the difference between landmark naming and building baseline.) E. Face baseline contrasted with building baseline. In the axial images, the right side of the image represents the left side of the brain.
Table I.
Significant activation in the temporal polar search volumes
| Comparison | Tmax | X | Y | Z |
|---|---|---|---|---|
| A. Left temporal pole:a | ||||
| Naming persons vs. naming landmarks | 1.34* | |||
| Task: Naming persons or landmarks vs. baseline tasks | 6.17 | −37 | +14 | −20 |
| Naming persons vs. building baseline | 3.56 | −39 | +15 | −17 |
| Naming landmarks vs. face baseline | 4.46 | −33 | +11 | −24 |
| Category: tasks with faces vs. landmarks/buildings | 2.43 | |||
| Task by category interaction | 0.19** | |||
| Face baseline vs. building baseline | 3.06 | −28 | +10 | −34 |
| B. Right temporal pole:b | ||||
| Naming persons vs. naming landmarks | 4.22 | +40 | +11 | −25 |
| Task: Naming persons or landmarks vs. baseline tasks | 3.27 | +32 | +17 | −28 |
| 3.39 | +49 | +17 | −11 | |
| Naming persons vs. face baseline | 3.37 | +45 | +17 | −13 |
| Naming landmarks vs. building baseline | 2.98 | |||
| Category: tasks with faces vs. landmarks/buildings | 4.52 | +40 | +11 | −25 |
| Task by category interaction | 2.06*** | |||
| Face baseline vs. building baseline | 3.86 | +28 | +8 | −30 |
Significant foci in the left temporal pole are marked in bold font.
Significant foci in the right temporal pole are marked in bold font.
Tmin = −1.16; **Tmin = −2.83; ***Tmin = −1.75.
Figure 4.
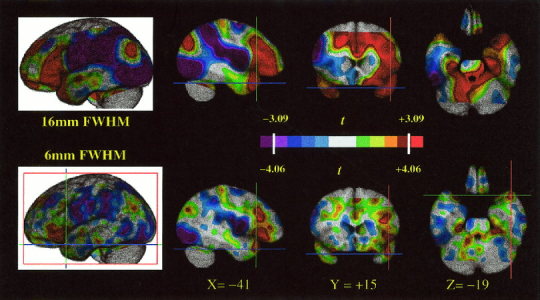
T statistic map for the main effect of task, with the data smoothed with a 16‐mm kernel (top tier) or a 6‐mm kernel (bottom tier). The color scale is calibrated with the significance levels (3.09, 4.06, respectively) determined by Gaussian random field theory. The coordinates of the temporal polar activation (−41 +15 −19) discussed in the text are indicated on orthogonal views. In the axial images, the right side of the image represents the left side of the brain.
There was no significant effect of category in the left TP (t 65max = 2.44; t 65min = −0.83). Thus, both naming persons and naming landmarks engaged the left TP, but neither category of stimuli engaged the left TP more than the other, results that support the hypothesis that the left TP is engaged by lexical retrieval at unique level, regardless of conceptual category. The interaction of task (naming at unique level vs. orientation judgment) and category (faces vs. landmarks/buildings) was not significant at any voxel.
Imaging data: right temporal pole
When naming persons was contrasted directly with naming landmarks, a significant difference was found in the right TP (t 65 = 4.54 +40 +11 −25). The main effect of task (retrieving names for unique persons or landmarks, in comparison to the control tasks) was associated with a significant increase (t 65 = 3.62) in activity in the right TP (+35 +10 −27). The global‐adjusted mean activity at this coordinate in the four experimental conditions was as follows: naming persons from faces, 855 counts; naming landmarks, 834 counts; face orientation baseline, 843 counts; house orientation baseline, 824 counts. There was also a main effect of category, showing significantly more engagement of the right TP when the stimuli were faces than when they were landmarks/buildings (t 65 = 4.64), and no significant task by category interaction effect.
In summary, the imaging results demonstrated (1) left temporal polar engagement during retrieval of words denoting unique entities for both unique faces and unique landmarks. Our previous result that left temporal polar cortex is engaged during the retrieval of names for persons was replicated; (2) right but not left temporal polar cortex engagement during the tasks involving face processing as opposed to house/landmark processing; and (3) no significant interaction of task and category in the left temporal pole.
Imaging data: exploratory analysis
A number of areas of significant activation were also found outside the search volume, using the more conservative statistical threshold appropriate for post hoc tests (see Table II and Fig. 4). Naming at unique level engaged the left inferior frontal gyrus, left superior mesial frontal regions, a region in the anterior left collateral sulcus, left retrosplenial region, and the right cerebellum. In addition to the right TP, a category effect (faces more than landmarks/buildings) was seen in the left and right lateral occipital and ventral occipito‐temporal (fusiform) regions. There was more activity in landmark/building tasks than face tasks in parahippocampal regions in both hemispheres. Significant category by task interaction was identified by post hoc tests in the right inferotemporal cortex (+54 −56 −10, reflecting greater difference between naming persons and face orientation than between naming landmarks and house orientation) and in the left lateral occipital lobe (−45 −82 +22, reflecting greater difference between naming landmarks and house orientation than between naming persons and face orientation).
Table II.
Significant activation outside the temporal polar search volumes found by post hoc, exploratory analysis
| Tmax | X | Y | Z | |
|---|---|---|---|---|
| A. Effect of task (naming unique entities vs. baseline tasks): | ||||
| L mesial frontal | 6.21 | −1 | +42 | +23 |
| L inferior frontal gyrus | 8.64 | −37 | +22 | +1 |
| L retrospenial region | 7.65 | +4 | −60 | +16 |
| L collateral sulcus | 6.73 | −20 | −27 | −21 |
| R cerebellum | 5.61 | +11 | −42 | −20 |
| L central cortex | 6.14 | −27 | −4 | +34 |
| L mesial frontal | 4.98 | −2 | +15 | +45 |
| R parietal | 4.94 | −46 | −73 | +29 |
| B. Effect of category (face tasks > place tasks): | ||||
| R temporal pole | 5.11 | +39 | +7 | −26 |
| L fusiform and lateral occipital | 5.12 | −44 | −75 | −19 |
| R fusiform and lateral occipital | 5.35 | +46 | −70 | −6 |
| C. Effect of category (place tasks > face tasks): | ||||
| R parahippocampal | 6.47 | +17 | −47 | −3 |
| L parahippocampal | 6.05 | −20 | −47 | −4 |
| R mesial occipital | 5.12 | +7 | −86 | +4 |
| R lateral occipital | 5.92 | +37 | −77 | +16 |
| L lateral occipital | 5.25 | −37 | −85 | +20 |
Performance data
The rates of nonresponses for naming persons and landmarks were 15.6% and 10.0%, respectively. The difference was not statistically significant. Latency data from six subjects were analyzed. (In the four remaining subjects, the stimulus time track was lost due to excessive electronic noise.) The median response latencies for naming persons and landmarks (sdv) were 1,556 msec (293) and 1,326 msec (117). The difference was not statistically significant (F1,5 = 2.98, P = n.s.). Median latency and error rate were highly correlated (r = 0.85). Performance success on the baseline tasks was nearly 100%, with response latencies for faces and buildings of 619 msec and 596 msec, respectively.
Effect of performance rate
The naming tasks were equivalently difficult for our subjects, based on the performance data. The stimuli for the control tasks were presented at a more rapid rate than the stimuli for the word retrieval tasks, in order to make the tasks of comparable difficulty [Damasio et al., 1996]. We have reported that this strategy tends to equate the tasks for syllable production and response latency, when integrated over the entire scan [Grabowski et al., 1998]. We considered the possibility that the difference in rate could nevertheless somehow account for the difference in activity in the temporal poles. However, we have now performed a number of PET studies using these and related tasks, and the activity generated at the left TP coordinate reported here appears specifically related to unique name retrieval tasks (Fig. 5).
Figure 5.

Left temporal polar activity (−41 +15 −19) across PET studies. This graph depicts PET activity, normalized to global activity of 1,000 counts, at the left temporal polar Talairach coordinate identified in Figure 4, across nine PET studies [including H. Damasio et al., 1996, 1999; Grabowski et al., 1998] including the one reported here, employing related tasks. Column 1: orientation decision baseline tasks with faces, ISI 1.0 sec, n = 6 studies; Column 2: orientation decision tasks using other concrete entities (unknown manmade objects, trees, or buildings), ISI 1.0 sec, n = 3. Column 3: orientation decision baseline tasks with faces, ISI 2.5 sec, n = 2; Column 4: orientation decision tasks using other concrete entities (unknown manmade objects, trees, or buildings), ISI 2.5 sec, n = 2; Column 5: retrieval of words for unique entities from this study and from Damasio et al. [1996], ISI 2.5 sec, n = 3. Column 6: Naming of manipulable manmade entities at basic object (n = 2) or subordinate level (n = 1), and verb generation (n = 1) for visually presented manipulable objects, ISI 2.5 sec. The normalized activity at this coordinate was greater in the three instances of unique level naming than it was in eight studies employing the face orientation control tasks (six with ISI 1 sec, two with ISI 2.5 sec); five studies employing orientation tasks with other classes of stimuli (buildings, trees, objects) at both 1 sec and 2.5 sec ISI; and four studies employing nonunique word production tasks using visual stimuli at an ISI of 2.5 sec (naming at basic object (2) and subordinate (1) level, and verb generation (1)). These data show that the activity at the left temporal polar coordinate in naming unique entities vs. baseline conditions is not simply a result of different ISIs for the retrieval and baseline tasks, as other retrieval tasks with the same ISI are not associated with relatively increased flow in the left temporal pole, and a reduced rate of performance of the baseline tasks is not associated with relatively increased flow in the left temporal pole.
DISCUSSION
We found significant activation of the left temporal polar region for both unique naming tasks (persons and landmarks) when compared to the baseline tasks, but no difference in left temporal polar activity between the two unique naming tasks, suggesting that the left temporal polar activity associated with both tasks relates to their common requirement to process entities at unique level.
Our intention was not to distinguish between recognition at unique level and name retrieval at unique level. The data we present do not allow such a distinction. However, they can be interpreted in light of dissociations demonstrated in brain‐damaged subjects. Lesions that lead to impaired retrieval of the names of persons affect primarily the left temporal cortex and its subcortical connections, while lesions that impair the recognition of those unique persons affect primarily the right anterior temporal and occipito‐temporal cortices, as demonstrated by lesion studies [Damasio et al., 1990a, 1990b; Ellis et al., 1989; Tranel et al., 1997] and by reports of “progressive prosopagnosia” associated with progressive atrophy of the right anterior temporal lobe [Tyrrell et al., 1990; Evans et al., 1995; Snowden, 1999]. Some authors [e.g., Bozeat et al., 2000; Mummery et al., 2000] have noted that “semantic dementia,” manifested by anomia and semantic memory loss, is associated with progressive atrophy of the left temporal lobe, which is usually conspicuous in the pole at the time of diagnosis. In addition, some stable, acquired lesions involving this area, usually a result of herpes simplex encephalitis, are also associated with semantic memory loss. However, in neither degenerative conditions nor HSV encephalitis can the damage, even when markedly asymmetric, be assumed to be unilateral, and the association of semantic knowledge with damage limited to the left temporal lobe is questionable. In fact, the salient and inaugural feature of “semantic dementia” resulting from left temporal lobar degeneration is usually anomia, with agnosia being a late feature. Interestingly, nondominant temporal lobar degeneration has been reported to invert this pattern, presenting with salient face and object agnosia instead of anomia [Tyrrell et al., 1990; Evans et al., 1995; Kazui et al., 1995; Snowden, 1999].
In the light of those lesion studies, we believe the best interpretation of the left temporal polar activity we observed in the target tasks is that it is related to naming at unique level. The right temporal polar activity we observed in the person naming task is most probably related to recognition of faces at unique level.
To our knowledge, there are no other reported functional imaging studies involving overt retrieval of the proper names of unique entities. In an abstract describing an fMRI study of normal subject, who covertly said names or occupations of known persons presented as faces, Tsukiura et al. [2000] showed that retrieving names, relative to retrieving occupations, was associated with activity in the left TP, with coordinates near ours. No other fMRI studies reporting engagement of either pole are available, possibly because magnetic susceptibility artifacts reduce and/or displace the available signal in this region [see Devlin et al., 2000].
A few studies have addressed the neural correlates of accessing unique level knowledge for concrete entities. Sergent et al. [ 1992, 1994] reported PET studies requiring decisions contingent on accessing conceptual knowledge pertaining to specific faces or printed names of specific persons. In the 1992 study, they reported activation of the temporal pole, bilaterally but more so on the right, when subjects categorized famous faces into superordinate categories (e.g., actor) compared to gender categorization of unfamiliar faces. The authors noted that the subjects reported being unable to prevent recalling the names of the persons, suggesting that some of the activity in left TP might be related to naming. The reported coordinates were within 10 millimeters (5 mm posterior and 7 mm inferior) of ours. In the 1994 study, the authors found left TP activation when subjects categorized famous names by occupation. Gorno‐Tempini et al. [1998] performed two PET experiments in which normal subjects performed a same/different judgment task on visually presented pairs of famous or nonfamous faces; and famous or nonfamous names. They found that the areas engaged when subjects viewed famous stimuli (faces or names) included the left anterior temporal and temporoparietal regions. In another study [Gorno‐Tempini et al., 2000] by the same investigators, reading or retrieving the name of famous persons presented as face photographs activated the same left anterolateral temporal region, relative to processing entities in five other categories: animals, objects, colors, body parts, and maps. There was no interaction of task (naming vs. reading) and category, but names were accessed in both naming and reading conditions.
As Gorno‐Tempini et al. [2000] acknowledged in their study, the distinction between the neural correlates of recognizing entities and naming them is extremely difficult to address with functional imaging in normal subjects because it is difficult to prevent naming during recognition tasks, particularly when the tasks call for recognition at unique level. There are several lines of evidence that processing of faces may “automatically” activate the anterior temporal structures associated with recognition and naming of unique entities, even when unique level processing is not required: (a) although not requiring recognition at unique level, our face orientation baseline task engaged the temporal poles, more so on the right, compared to the building orientation baseline task (Fig. 3); (b) although not requiring recognition at unique level, intentional encoding of unfamiliar faces [Kuskowski and Pardo, 1999] engaged the right temporal pole; (c) although not requiring naming, the face categorization task of Sergent et al. [1992] engaged the left TP. But when a briefer ISI was employed (2 sec vs. 3 sec), the temporal polar activation was diminished [Sergent et al., 1994]. Separation of the correlates of recognition and naming in functional imaging studies will call for designs that overcome such “automatic” processing. A possible exception in this regard is the PET study of Nakamura et al. [2000]. Normal subjects performed three discrimination tasks: deciding whether a presented face is that of a personally familiar person or an unfamiliar person, deciding whether an unfamiliar face faces left or right, and deciding whether a dot superimposed on a scrambled face is on the right or left of the screen. In an SPM conjunction analysis, the authors found that the right temporal pole was more active when classifying faces as familiar or unfamiliar than in either of the baseline tasks. The authors' suggestion that the right temporal pole participates in recognizing unique entities is consistent with the lesion and functional imaging studies reviewed above. Activation of the left temporal pole was not detected, possibly because the ISI was too brief to allow covert name retrieval (1 sec).
Producing a name for a visually presented concrete entity involves recognition of the entity and engagement of phonological and articulatory processes. Because the processes underlying concept retrieval and language implementation are supported by different neural systems, the process of word retrieval implies coordination of activity among separate neural regions. We interpret the role of the left TP in the context of a theoretical framework [Damasio, 1989; Damasio et al., 1990; Damasio and Damasio, 1994; H. Damasio et al., 1996; Tranel et al., 1997a, 1997b] in which word‐form production is dependent on three kinds of neural structures: (1) those structures which support conceptual knowledge (in early and high‐order sensory cortices of both hemispheres); (2) those structures which support the implementation of word‐forms in vocalization (in classical left perisylvian language areas); and (3) mediational structures, such as left TP, which are engaged by the structures in (1) to guide the implementation process executed in (2). The sensorimotor patterns on the basis of which word‐forms are explicitly represented in mind are triggered by relevant mediational circuits and occur in early sensory cortices (e.g., auditory) and motor structures. This architecture is not constituted by rigid “centers” but by neuron ensembles interconnected by bidirectional pathways. Moreover, the ensembles and pathways hypothesized to support a particular function, e.g., the retrieval of words for unique entities, are seen as “preferred systems” that support efficient performance, although other systems can support the performance in less efficient ways.
Based mainly on the study of nonhuman primates, Brodmann area (BA) 38 in the temporal pole has been identified as a multimodal association cortex that is reciprocally connected with visual, auditory, olfactory, other multimodal association cortices, entorhinal cortex, hippocampus, amygdala, and basal forebrain [see Arnold and Van Hoesen, 1994, for a review]. In anatomic terms, BA 38 receives multimodal feedforward sensory projections and reciprocates these projections. Given this convergence‐divergence anatomical arrangement, this region is well suited to function as an intermediary among posterior temporal‐occipital‐parietal cortices, which presumably support conceptual processing, and left perisylvian cortices, which presumably support phonological processing. In other words, the region is anatomically suited to participate in the coactivation of concepts and words.
Familiar face processing appears to be staged along the right and left occipito‐temporal axes. The recognition of faces as familiar appears to depend mostly on occipito‐temporal regions, especially in right hemisphere, while recognition at unique level appears to depend on right anterior temporal regions. A somewhat comparable anatomical staging might apply to the regions that support lexical retrieval along the left occipito‐temporal axis, with retrieval of words for unique entities requiring the integrity of the more rostral regions, including left TP.
Two additional points should be borne in mind. First, there may also be regions other than the temporal pole that mediate word retrieval for unique entities. We focused our anatomic search on the left temporal pole on the basis of findings in lesion studies. But the incompleteness of the word retrieval defect typically seen after left temporal polar lesions suggests other regions either normally participate or can compensate in the presence of damage to the left temporal pole. For example, although not included in our anatomic hypothesis, our prior study and the one reported here identified a region in the anterior superior temporal sulcus/middle temporal gyrus very close to the coordinates reported by Gorno‐Tempini et al. [1998, 2000]. If we had included this region in the search volume, this coordinate would have been significant. This observation emphasizes a similarity between our results and those of Gorno‐Tempini et al., though the latter did not find activation in the temporal pole, possibly because their tasks did not require word retrieval. Other lesion studies implicate the left anterior thalamus [Cohen et al., 1994, Moreaud et al., 1995; Semenza and Zettin, 1988; Luchelli and De Renzi, 1992]. Tracer studies in nonhuman primates have identified parts of the medial dorsal and pulvinar nuclei as connected to BA 38 [Trojanowski and Jacobson, 1974; Markowitsch et al., 1985; Gower, 1989]. It is possible that the lesions in those studies affected the interaction of anterior temporal cortex with thalamic nuclei. Second, though we do not find distinct left temporal polar sectors for retrieving names for both landmarks and persons, we can not exclude the possibility of functional segregation at a subcentimeter spatial scale, as the spatial resolution of our technique is about 1.5 cm [Grabowski et al., 1996]. Thus, it could be that the systems that support face processing are relatively independent of those supporting processing of houses and landmarks, within the temporal polar cortex.
Not surprisingly, outside of the left TP, we found that the tasks employing face stimuli engaged a different set of neural regions than the tasks employing landmarks or buildings as stimuli. Processing faces activated the right temporal pole and lateral occipital cortex bilaterally, and the fusiform (occipitotemporal) gyrus bilaterally. Processing landmarks and buildings activated early visual cortex, more so on the right, and the parahippocampal regions bilaterally (Table IIB, IIC). Engagement of the fusiform areas by faces has been a consistent finding in fMRI studies involving faces as stimuli [Allison et al., 1994; Kanwisher et al., 1997; Haxby et al., 1997]. This finding is consistent with results from lesion studies, because the region is consistently damaged in patients with associative prosopagnosia. Engagement of the parahippocampal regions by buildings and landmark stimuli replicates more recent reports that found this area to be active using fMRI during visual processing of houses, buildings, and pictures of the local environment [Epstein and Kanwisher, 1998; Aguirre et al., 1998], and with lesion work [Barrash et al., 2000]. The association of mesial occipital activity, more so on the right, with landmarks and buildings also is consistent with lesion studies, which have associated visual agnosia for landmarks with right medial occipital damage [see Aguirre et al., 1998].
In conclusion, retrieval of the proper names of persons and landmarks engages the left temporal pole to a comparable degree, supporting the hypothesis that the effect seen when naming persons is linked to the level of specificity of the retrieval, rather than to the special properties of face stimuli.
REFERENCES
- Aguirre GK, Zarahn E, D'Esposito M (1998a): An area within human ventral cortex sensitive to “building” stimuli: evidence and implications. Neuron 21: 373–383. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D'Esposito M (1998b): Neural components of topographical representation. Proc Nat Acad Sci U S A 95: 839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, Butters N, Levin JA (1979): Temporal gradients in the retrograde amnesia of patients with alcoholic Korsakoff's disease. Arch Neurol 36: 211–216. [DOI] [PubMed] [Google Scholar]
- Allison TG, McCarthy G, Nobre A, Puce A, Belger A (1994): Human extrastriate visual cortex and the perception of faces, words, numbers, and colors. Cereb Cortex 5: 544–554. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Van Hoesen, GW (1994): Neuropathologic changes of the temporal pole in Alzheimer's disease and Pick's disease. Arch Neurol 51: 145–150. [DOI] [PubMed] [Google Scholar]
- Barrash J, Damasio H, Adolphs R, Tranel D (2000): The neuroanatomical correlates of route learning impairment. Neuropsychologia 38: 820–836 [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR (2000): Non‐verbal semantic impairment in semantic dementia. Neuropsychologia 38: 1207–1215. [DOI] [PubMed] [Google Scholar]
- Burke DM, MacKay DG, Worthley JS, Wade E (1991): On the tip of the tongue: what causes word finding failures in young and older adults? J Mem Lang 30: 542–579. [Google Scholar]
- Cherry SR, Woods RP, Doshi NK, Bannerjee PK, Mazziotta JC (1995): Improved signal‐to‐noise in PET activation studies using switched paradigms. J Nucl Med 36: 307–314. [PubMed] [Google Scholar]
- Cohen L (1990): Why is it difficult to put names to faces? Brit J Psychol 81: 287–298. [Google Scholar]
- Cohen L, Bolgert F, Tinsit S, Chermann JF (1994): Anomia for proper names after left thalamic infarction. J Neurol Neurosurg Psych 57: 1283–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Damasio H, van Hoesen G (1982): Prosopagnosia: anatomic basis and behavioral mechanisms. Neurology 32: 331–341. [DOI] [PubMed] [Google Scholar]
- Damasio AR (1989): Time‐locked multiregional retroactivation: a systems level proposal for the neural substrates of recall and recognition. Cognition 33: 25–62. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H (1990): Face agnosia and the neural substrates of memory. Annu Rev Neurosci 13: 89–109. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H, Tranel D, Brandt JP (1990): Neural regionalization of knowledge access: preliminary evidence. Symposia on quantitative biology, Vol. 55 Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; p 1039–1047. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H (1992): Brain and language. Sci Am 267: 89–95. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H (1994): Cortical systems for retrieval of concrete knowledge: the convergence zone framework In: Koch C, Davis JL, editors. Large‐scale neuronal theories of the brain. Cambridge, MA: MIT Press; p 62–74. [Google Scholar]
- Damasio H, Frank R (1992): Three‐dimensional in vivo mapping of brain lesions in humans. Arch Neurol 49: 137–143. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR (1996): A neural basis for lexical retrieval. Nature 380: 499–505. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Ponto LLB, Hichwa RD, Damasio AR (1999): Neural correlates of retrieval of words for spatial relationships. Fifth International Conference on Functional Mapping of the Human Brain, Düsseldorf, Germany. Neuroimage 9: S918. [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Wilson J, Moss HE, Matthews PM, Tyler LK (2000): Susceptibility‐induced loss of signal: comparing PET and fMRI on a semantic task. Neuroimage 11: 589–600. [DOI] [PubMed] [Google Scholar]
- Ellis AW, Young AW, Critchley EMR (1989): Loss of memory for people following temporal lobe damage. Brain 112: 1469–1483. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N (1998): A cortical representation of the local visual environment. Nature 392: 599–601. [DOI] [PubMed] [Google Scholar]
- Evans JJ, Heggs AJ, Antoun N, Hodges JR (1995): Progressive prosopagnosia associated with selective right temporal atrophy: a new syndrome? Brain 118: 1–13. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Wilson KD, Drain HM, Tanaka JR (1995): The inverted face inversion effect in prosopagnosia: evidence for mandatory face‐specific perceptual mechanisms. Vis Res 35: 2089–2093. [DOI] [PubMed] [Google Scholar]
- Fery P, Vincent E, Bredart S (1995): Personal name anomia: a single case study. Cortex 31: 191–198. [DOI] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, Grabowski TJ (1997): Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage 5: 13–30. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J‐B, Frith CD, Frackowiak RSJ (1995): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Fukatsu R, Fujii T, Tsukiura BE, Yamadori A, Otsuki T (1999): Proper name anomia after left temporal lobectomy: a patient study. Neurology 52: 1096–1099. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Anderson AW, Tarr MJ, Skudlarski P, Gore JC (1997): Levels of categorization in visual recognition studied using functional magnetic resonance imaging. Curr Biol 7: 645–651. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Skudlarski P, Gore JC, Anderson AW. (2000): Expertise for cars and birds recruits brain areas involved in face recognition. Nat Neurosci 3: 191–197. [DOI] [PubMed] [Google Scholar]
- Gorno‐Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, Frackowiak RSJ (1998): The neural systems sustaining face and proper‐name processing. Brain 121: 2103–2118. [DOI] [PubMed] [Google Scholar]
- Gorno‐Tempini ML, Cipolotti L, Price CJ (2000): Category differences in brain activation studies: where do they come from? Proc R Soc Lond B Biol Sci 267: 1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower EC (1989): Efferent projections from limbic cortex of the temporal pole to the magnocellular medial dorsal nucleus in the rhesus monkey. J Comp Neurol 280: 343–358. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Frank R, Hichwa RD, Boles Ponto LL, Watkins GL (1995): A new technique for PET slice orientation and MRI‐PET coregistration. Hum Brain Mapp 2: 123–133. [Google Scholar]
- Grabowski TJ, Damasio H, Damasio AR (1998): Premotor and prefrontal correlates of category‐related lexical retrieval. Neuroimage 7: 232–243. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Frank RJ, Brown CK, Damasio H, Boles Ponto LL, Watkins GL, Hichwa RD (1996): Reliability of PET activation across statistical methods, subject groups, and sample sizes. Hum Brain Mapp 4: 23–46. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Brown CK (1998): Two aspects of task difficulty studied with PET. Neuroimage 7: S161. [Google Scholar]
- Grabowski TJ, Damasio H, Tranel D. (2000): Retrieving names of unique entities engages the left temporal pole. Neuroimage 11: S262. [Google Scholar]
- Harris DM, Kay J (1995): Selective impairment of the retrieval of people's names: a case of category specificity. Cortex 31: 575–582. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Martin A, Clark VP, Hoffman J, Schouten J, Ungerleider LG (1997): The processing of faces, inverted faces, and other objects in the ventral object vision pathway. Neuroimage 5: S4. [Google Scholar]
- Hichwa RD, Ponto LB, Watkins GL (1995): Clinical blood flow measurement with [15O]water and positron emission tomography (PET) In: Emran AM, editor. Chemists' views of imaging centers. Symposium proceedings of the international symposium, “Chemists' View of Imaging Centers.” New York: Plenum. [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods RP, Evans AC, Toga AW (1998): Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 22: 324–333. [DOI] [PubMed] [Google Scholar]
- Hurtig RR, Hichwa RD, O'Leary DS, Boles Ponto LL, Narayana S, Watkins GL, Andreasen NC (1994): Effects of timing and duration of cognitive activation in [15O]water PET studies. J Cereb Blood Flow Metab 14: 423–430. [DOI] [PubMed] [Google Scholar]
- Indefry P, Levelt WJM (2000): The neural correlates of language production In: Gazzaniga M, editor. The cognitive neurosciences Cambridge, MA: MIT Press. [Google Scholar]
- Kanwisher N, McDermott J, Chun MM (1997): The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazui H, Tanabe H, Ikeda M, Hashimoto M, Yamada N (1995): A case of predominantly right temporal atrophy with disturbance of identifying familiar faces. No To Shinkei 47: 77–85. [PubMed] [Google Scholar]
- Kuskowski MA, Pardo JV (1999): The role of the fusiform gyrus in successful encoding of face stimuli. Neuroimage 9: 599–610. [DOI] [PubMed] [Google Scholar]
- Levelt WJM, Roelofs A, Meyer AS. (1999): A theory of lexical access in speech production. Behav Brain Sci 22: 1–38. [DOI] [PubMed] [Google Scholar]
- Luchelli F, De Renzi E (1992): Proper name anomia. Cortex 28: 221–230. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Emmans D, Irle E, Streicher M, Preilowski B (1985): Cortical and subcortical afferent connections of the primate's temporal pole: a study of rhesus monkeys, squirrel monkeys, and marmosets. J Comp Neurol 242: 425–458. [DOI] [PubMed] [Google Scholar]
- Martin A, Ungerleider LG, Haxby JV (2000): Category specificity and the brain: the sensory/motor model of semantic representations of objects In: Gazzaniga MS, editor. The new cognitive neurosciences. Cambridge, MA: MIT Press. [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV (1996): Neural correlates of category‐specific knowledge. Nature 379: 649–652. [DOI] [PubMed] [Google Scholar]
- McCarthy RA, Evans JJ, Hodges JR (1996): Topographic amnesia: spatial memory disorder, perceptual dysfunction, or category specific semantic memory impairment? J Neurol Neurosurg Psychiatry 60: 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milers M (2000): Naming famous faces and buildings. Cortex 36: 139–145. [PubMed] [Google Scholar]
- Moreaud O, Pellat J, Charnallet A, Carbonnel S, Brennen T (1995): Deficit de la production et de l'appentissage des noms propres apres lesion ischemique tubero‐thalamique gauche. Rev Neurol 151: 93–99. [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, Hodges JR (2000): A voxel‐based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol 47: 36–45. [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Sato N, Sugiura M, Kato T, Hatano K, Ito K, Fukuda H, Schorman T, Zilles K (2000): Functional delineation of the human occipito‐temporal areas related to face and scene processing: a PET study. Brain 123: 1903–1912. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathy CD (1990): Atlas of the cerebral sulci. New York: Thieme. [Google Scholar]
- Papagno C, Capitani E (1998): Proper name anomia: a case with sparing of the first‐letter knowledge. Neuropsychologia 36: 669–679. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJS, Frackowiak RSJ (1996): Demonstrating the implicit processing of visually presented words and pseudowords. Cereb Cortex 6: 62–70. [DOI] [PubMed] [Google Scholar]
- Ralph MAL, Graham KS, Ellis AW, Hodges JR (1998): Naming in semantic dementia—what matters? Neuropsychologia 36: 775–784. [DOI] [PubMed] [Google Scholar]
- Reinkemeier M, Markowitsch HJ, Rauch M, Kessler J (1997): Differential impairments in recalling people's names: a case study in search of neuroanatomical correlates. Neuropsychologia 35: 677–684. [DOI] [PubMed] [Google Scholar]
- Semenza C, Zettin M (1988): Generating proper names: a case of selective inability. Cogn Neuropsychol 5: 711–721. [Google Scholar]
- Semenza C, Zettin M (1989): Evidence from aphasia for the role of proper names as pure referring expressions. Nature 342: 678–679. [DOI] [PubMed] [Google Scholar]
- Sergent J, Ohta S, MacDonald B (1992): Functional neuroanatomy of face and object processing. Brain 115: 15–36. [DOI] [PubMed] [Google Scholar]
- Sergent J, Signoret J‐L (1992): Functional and anatomical decomposition of face processing: evidence from prosopagnosia and PET study of normal subjects. Philos Trans R Soc Lond Biol Sci 335: 55–62. [DOI] [PubMed] [Google Scholar]
- Sergent J, MacDonald B, Zuck E (1994): Structural and functional organization of knowledge about faces and proper names: a positron emission tomography study. Attention and performance XV. Cambridge, MA: MIT Press. [Google Scholar]
- Shallice T, Kartsounis LD (1993): Selective impairment of retrieving people's names: a category specific disorder? Cortex 29: 281–291. [DOI] [PubMed] [Google Scholar]
- Snowden, JS (1999): Semantic dysfunction in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord 10(Suppl): 33–36. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Tranel D, Damasio H, Damasio AR, Brandt JP (1995): Soc Neurosci Abst 21: 1497. [Google Scholar]
- Tranel D, Logan CG, Frank RJ, Damasio AR (1997): Explaining category‐related effects in the retrieval of conceptual and lexical knowledge for concrete entities: operationalization and analysis of factors. Neuropsychologia 35: 1329–1339. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR (1997): A neural basis for the retrieval of conceptual knowledge. Neuropsychologia 35: 1319–1327. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Jacobson S (1976): Areal and laminar distribution of some pulvinar cortical efferents in rhesus monkey. J Comp Neurol 169: 371–392. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Fujii T, Okuda J, Tabuchi M, Kurata K, Suzuki K, Umetsu A, Kawashima R, Yanagawa I, Nagasaka T, Ymadori A, Takahashi S, Fukuda H (2000): Contribution of the rostral part of the left temporal lobe to retrieving people's names: a functional MRI study. Neuroimage 11: S373. [Google Scholar]
- Tyrrell PJ, Warrington EK, Frackowiak RSJ, Rossor MN (1990): Progressive degeneration of the right temporal lobe studied with positron emission tomography. J Neurol Neurosurg Psychiatry 53: 1046–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Price CJ, Wise RJS, Josephs O, Frackowiak RSJ (1996): Functional anatomy of a common semantic system for words and pictures. Nature 383: 254–256. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR (1993): MRI‐PET registration with automated algorithm. J Comput Assist Tomogr 17: 536–546. [DOI] [PubMed] [Google Scholar]
- Woods RP, Daprett M, Sicotte NL, Toga AW, Mazziotta JC (1999): Creation and use of a Talairach‐compatible atlas for accurate, automated, nonlinear intersubject registration, and analysis of functional imaging data. Hum Brain Mapp 8: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley, KJ (1994): Local maxima and the expected Euler characteristic of excursion sets of c2, F, and t fields. Adv Appl Prob 26: 13–42. [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P (1992): A three‐dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12: 900–918. [DOI] [PubMed] [Google Scholar]


