Abstract
Despite the many studies highlighting the role of the amygdala in fear perception, few have examined differences between right and left amygdalar responses. Using functional magnetic resonance imaging (fMRI), we examined neural responses in three groups of healthy volunteers (n = 18) to alternating blocks of fearful and neutral faces. Initial observation of extracted time series of both amygdalae to these stimuli indicated more rapid decreases of right than left amygdalar responses to fearful faces, and increasing magnitudes of right amygdalar responses to neutral faces with time. We compared right and left responses statistically by modeling each time series with (1) a stationary fit model (assuming a constant magnitude of amygdalar response to consecutive blocks of fearful faces) and (2) an adaptive model (no assumptions). Areas of significant sustained nonstationarity (time series points with significantly greater adaptive than stationary model fits) were demonstrated for both amygdalae. There was more significant nonstationarity of right than left amygdalar responses to neutral, and left than right amygdalar responses to fearful faces. These findings indicate significant variability over time of both right and left amygdalar responses to fearful and neutral facial expressions and are the first demonstration of specific differences in time courses of right and left amygdalar responses to these stimuli. Hum. Brain Mapping 12:193–202, 2001. © 2001 Wiley‐Liss, Inc.
Keywords: amygdala, fear, laterality, time series, fMRI, face
INTRODUCTION
Several studies with animals [LeDoux, 1996], patients with amygdalar lesions [Adolphs et al., 1994, 1995; Calder et al., 1996; Young et al., 1995], and those employing functional neuroimaging techniques [Br‐eiter et al., 1996; Morris et al., 1996] have highlighted the predominant role of the amygdala in the perception of fear and fear conditioning [Bechara et al., 1995; LaBar et al., 1995]. These studies have often reported differences in the extent of activation of left and right amygdalae by fearful stimuli, but these differences have not been investigated systematically. Studies have demonstrated that activity in the left amygdala correlates significantly with perception of the intensity of fear depicted in standardised facial expressions [Morris et al., 1996] and with subjective ratings of aversive odorants [Zald et al., 1997]. Activation in the right amygdala has been reported in response to expressions depicting exaggerated intensities of fear [Phillips et al., 1997], to masked expressions of fear [Morris et al., 1998], to auditory presentations of fear [Phillips et al., 1998], and to aversive tastes [Zald et al., 1998]. Right amygdalar activity has also been correlated with memory for emotionally arousing film excerpts [Cahill et al., 1996]. Studies employing functional imaging techniques in the investigation of amygdalar responses during conditioned fear acquisition and extinction have reported bilateral amygdalar activation, with a bias toward the right amygdala [Furmark et al., 1997; LaBar et al., 1998; Buchel et al., 1998]. Methodological differences between these studies make the findings difficult to interpret with regard to the specific roles of right and left amygdalae in fear perception.
One possible reason for the discrepant findings is habituation of amygdalar responses to fearful stimuli [Breiter et al., 1996], which may result in difficulty in the detection of amygdalar signals to these stimuli. Differences may also exist in the extent of habituation to fear between right and left amygdalae. In order to investigate this further it is necessary to examine the nature of the time courses of right and left amygdalar responses to fearful stimuli. To our knowledge, there has been no such study to date.
We wished to first assess the variability of the time course of the amygdalar response to fear, and second to compare the extent of the variability of response of the right with that of the left amygdala. We extracted time series of amygdalar responses in three groups of healthy volunteers. In the first subject group, we examined the time series of amygdalar responses to prototypical (100%) facial expressions of fear. In the second group, we examined time series to differing intensities of fear (mildly or severely fearful facial expressions), and in the third group, we examined the effect of the task performed during presentation of expressions of fear (no task or a sex decision task). We then employed a novel technique of time series modeling in order to make statistical comparisons between the time series extracted from right and left amygdalae.
METHODS
Subjects and procedure
Three groups of right‐handed healthy volunteers viewed alternating 30s blocks of standardised neutral and fearful facial expressions, manipulated by computer software to demonstrate different intensities of emotion [Calder et al., 1997], in 5‐minute fMRI experiments. Informed consent was obtained from all subjects after the nature of the experimental procedure had been explained. The study was carried out in accordance with the code of ethics of the World Medical Association. Expressions of mild (25%) happiness were employed as the neutral stimuli, as described previously [Morris et al., 1996; Phillips et al., 1997] (Fig. 1). In one group, six subjects (four male and two female; mean age: 34 years; mean IQ estimate: 120) viewed expressions of 100% fear and mild happiness while deciding the sex of each face by pressing one of two buttons with the right thumb (implicit task). Seven subjects of a second group (two male and five female; mean age: 27 years; mean IQ estimate: 115) in two 5‐minute experiments viewed either expressions of 150% fear and mild happiness, or expressions of 75% fear and mild happiness, while performing the same task as the first group. In the third group, five subjects (all male; mean age: 25 years; mean IQ estimate: 120) viewed expressions of 100% fear and mild happiness in two 5‐minute experiments: in one experiment, subjects performed the sex decision task as above, and in the other, performed no task. The order of experiments was randomised over subjects in each of the latter two groups. After scanning, subjects identified the facial expressions presented in the experiments by choosing one expression out of the following: fear, anger, disgust, sadness, happiness, surprise. Mean accuracy ratings for identification of expressions of fear were 90% (range: 63%–100%) for Group 1; 66% (range: 25%–100%) for all fear expressions, 65% (range:0%–100%) for intense (caricatured) fear, and 38% (range: 0%–88%) for mild fear for Group 2; and 88% (range:63%–100%) for Group 3. Accuracies of recognition of the 75% and 150% morphed expressions of fear in Group 2 were lower than that for 100% fear in Groups 1 and 3. A possible reason for this is that 150% morphed expressions of fear are caricatures and therefore possibly less frightening than the 100% fear expressions, while 75% expressions are clearly milder versions of the 100% fearful expressions. Subjects were able to correctly identify the sex of the faces when requested.
Figure 1.
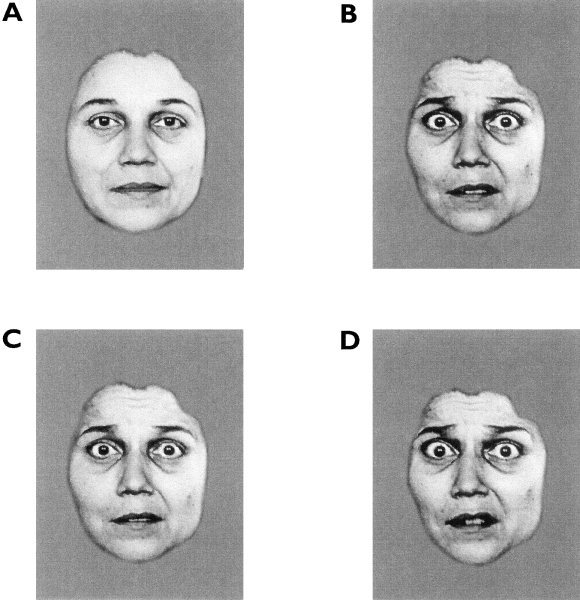
Examples of facial stimuli employed in the study. An expression of mild (25%) happiness is shown (A) and a prototypical (100%) expression of fear (B), in addition to expressions manipulated with morphing software to produce mild (75%; C) and intense (150%; D) fear.
Image acquisition and analysis
Gradient echo echoplanar images were acquired on a GE Signa 1.5T Neurovascular system (General Electric, Milwaukee, WI) at the Maudsley Hospital, London. 100 T2*‐weighted images depicting BOLD (blood oxygentation level dependent) contrast [Ogawa et al., 1990] were acquired over 5 min (for each task) at each of 14 near‐axial noncontiguous 5‐mm thick planes parallel to the intercommissural (AC‐PC) line: TE 40 msec, TR 3 sec, in‐plane resolution 5 mm, interslice gap 0.5 mm. This EPI data set provided complete coverage of the temporal lobes (including hippocampus and amygdala) and almost complete coverage of frontal, occipital, and parietal lobes [Simmons et al., 1999].
Extraction of time series
Motion‐corrected fMRI data for each subject [Bullmore et al., 1999] were registered in the standard space of Talairach and Tournoux [1988] and smoothed by a 2D Gaussian filter with full width half maximum = 11mm [Brammer et al., 1997]. A set of time series in both amygdalae was recovered from each subject. Each time series was the average of the time series at the voxel indexed by coordinates in standard space (±20, −8, −13) and the eight nearest neighbours in 2D (total regional volume = 0.57 cm3). These coordinates describe the center of the amygdala as defined by the Talairach atlas, and also are representative of the range of coordinates reported for the amygdala in previous studies [e.g., Morris et al., 1996, 1998; Phillips et al., 1997, 1998]. Each time series was corrected for slight temporal offsets due to the multislice acquisition protocol by linear interpolation prior to analysis of nonstationarity. We applied a median filter to all extracted time series in order to remove high‐frequency noise before making initial observations regarding the nature of right and left amygdalar responses.
RESULTS
Observation of time series extracted from all subjects indicated a trend for a maximal left amygdalar response to occur during presentations of blocks of fearful faces in each time series. The right amygdalar response to fearful facial expressions appeared to be greatest during earlier presentations of fearful faces, decreasing more rapidly than that of the left amygdala with subsequent presentations of these faces. Furthermore, the maximal right amygdalar response in many of the time series occurred during presentations of neutral rather than fearful facial expressions.
Examples of time series of right and left amygdalar responses, each smoothed with a median filter over five consecutive data points in order to remove high‐frequency noise, are shown for one subject from each group in Figure 2.
Figure 2.
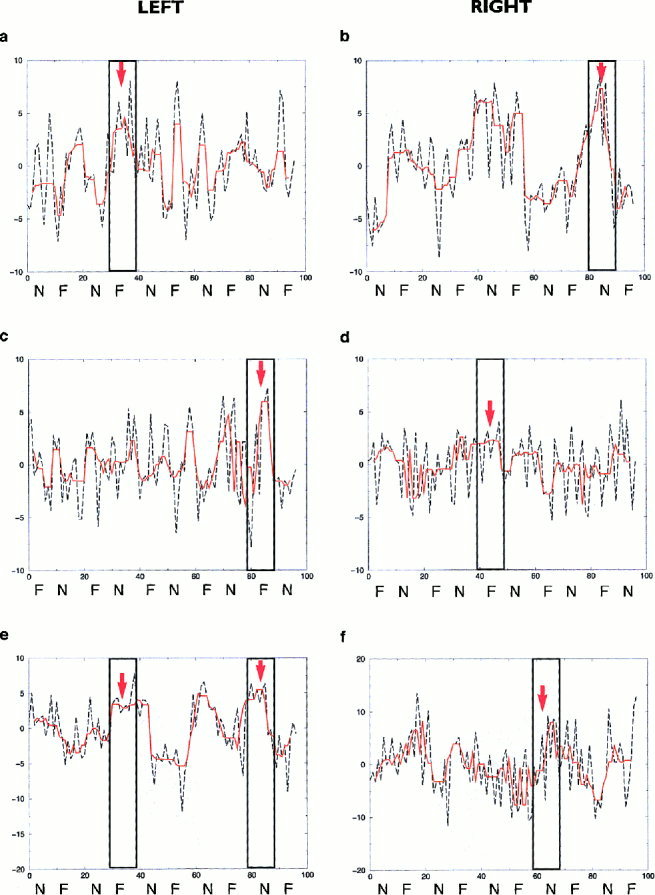
Examples of time series of left (a) and right (b) amygdalar responses of an illustrative subject in Group 1 during alternating presentation of 100% fearful and neutral facial expressions and performance of a sex decision task; left (c) and right (d) amygdalar responses of an illustrative subject in Group 2 during alternating presentation of 75% fearful and neutral facial expressions and performance of a sex decision task; and left (e) and right (f) amygdalar responses of an illustrative subject in Group 3 during alternating presentation of 100% fearful and neutral facial expressions and no task performance. Data were filtered (curve shown in orange) to suppress transient departures from stationarity which could be due to the presence of high‐frequency noise. The timing of presentation of stimuli (fear, F or neutral, N) is indicated for comparison with the individual time‐series. Labelling on the vertical axes on the time‐series plots refers to the change in signal or image intensity relative to the overall mean value (i.e, mean subtracted variation in signal in the amygdala). Labeling on the horizontal axes refer to the number of images acquired (20 images per 60‐sec period, and 100 images over each 5‐minute experiment). The maximal positive signal change in each amygdalar time series, occurring during presentation of either fearful or neutral faces, is indicated with an arrow.
Modeling time series: stationary and adaptive model fitting
fMRI time series were modeled using the following approach. First, we employed a stationary fit model, which assumed an identical magnitude of amygdalar response to consecutive blocks of fearful faces. The “boxcar” alternating experimental design was convolved with three gamma density functions with means and variance equal to 4, 8, and 16 [Friston et al., 1998]. Least‐squares fitting was then used to compute the weighted combination of the three convolved functions that best approximated the observed time series. The use of three gamma density functions allows variability in the haemodynamic lag of the BOLD effect to be accommodated and good approximation of the shape of the response (including the so‐called “undershoot”). Although this fitting procedure allows flexible adaptation to the characteristics of the BOLD response, it assumes stationarity of the signal. In order to obtain an adaptive, or optimal (although more highly parameterised) fit to the time series—that is, that which made no assumptions about the magnitude of amygdalar response—the same fitting procedure was extended by using a separate set of convolved input functions for each “on” (fearful expressions) or “off” (neutral expressions) block. We reasoned that significant differences between these two model fits should be indicative of nonstationarity in the time series. In order to test the existence of such differences, we computed the sum of squares of differences between the two models across all time points. The sum of squares of differences was then repeatedly recomputed after randomly permuting the time series (in order to remove any systematic connection between the experimental design and the response) and refitting both sets of convolved input functions. The number of times that the sum of squares of differences between the fitted models was equal to or greater than that detected with the original time series data, divided by the total number of permutations, was the probability of the observed level of nonstationarity under the null hypothesis of no experimentally determined response. Similar comparisons of the sums of squares for the two models with their respective “randomised” equivalents were used to assess their individual probabilities under the null hypothesis of no experimentally determined response.
Before applying this methodology on time series whose nonstationarity characteristics were to be determined, the above methodology was applied to a time series expected to show good model fits to both the stationary and “adaptive” models but no significant nonstationarity (8 Hz visual stimulation in a 30 sec on and 30 sec off alternating block paradigm using goggles). The method was also tested on a mean time series drawn from 30 voxels in the lateral ventricles, which would be expected to exhibit no nonstationarity and a poor fit to both models. As predicted, in the visual cortex, both the stationary and adaptive model fits were significant (with a thousand randomisations, each had a probability under the null hypothesis of no experimentally determined response of P < 0.001). There was no significant nonstationarity in the time series (P = 0.87). In the ventricles, the fits of both stationary and adaptive models, and the nonstationarity of the time series were nonsignificant (P = 0.89, P = 0.54, and P = 0.81, respectively).
For all experimental conditions, the number of significant stationary and adaptive model fits and nonstationarity of responses of right and left amygdalar time series were determined using the above methodology, and compared with chi‐squared and Fisher exact tests.
We examined further the curves smoothed with a median filter (over five data points) indicating the areas of significant nonstationarity of right and left amygdalar time series. We wished to determine the nature of the stimuli (fearful or neutral expressions) in response to which the maximum sustained nonstationarity of response occurred for each amygdala. The total number of maxima of sustained nonstationarity occurring with presentation of fearful and neutral faces were then compared for right and left amygdalae with chi‐squared and Fisher exact tests.
The numbers of right and left amygdalar time series in each experimental condition for which there were significant (P < 0.05) stationary and adaptive model fits, and significant nonstationarity of response are shown in Table I. The nature of the stimuli (fearful or neutral facial expressions) to which the maximal nonstationarity of response occurred in each amygdalar time series is shown for all subjects in each experimental condition. Stationary and adaptive model fits, and curves representing areas in the time series at which significant nonstationarity of response occurred, are shown for the time series of illustrative subjects from Group 1 and Group 3 in Figures 3 and 4, respectively.
Table I.
Time series modelling parameters for right and left amygdalae
| Subject group | Right amygdala | Left amygdala | ||||||
|---|---|---|---|---|---|---|---|---|
| Stationary1 model fits | Adaptive2 model fits | Non‐stationarity3 of response | F:N4 | Stationary1 model fits | Adaptive2 model fits | Non‐stationarity3 of response | F:N4 | |
| (n = 6) 100% Fear Implicit task | 2/6 | 3/6 | 3/6 | 0:3 | 2/6 | 3/6 | 3/6 | 2:1 |
| (n = 7) 75% Fear Implicit task | 0/7 | 1/7 | 1/7 | 0:1 | 4/7 | 5/7 | 4/7 | 3:1 |
| 150% Fear Implicit task | 3/7 | 2/7 | 2/7 | 1:1 | 1/7 | 3/7 | 4/7 | 2:2 |
| (n = 5) 100% Fear No task | 1/5 | 3/5 | 2/5 | 0:2 | 2/5 | 3/5 | 2/5 | 1:1 |
| 100% Fear Implicit task | 0/5 | 2/5 | 3/5 | 1:2 | 3/5 | 1/5 | 1/5 | 0:1 |
| Total | 6/30* | 11/30* | 11/30* | 2:9** | 12/30* | 15/30* | 14/30* | 8:6** |
Proportions refer to the number of significant: 1. stationary model fits; 2. adaptive model fits; 3. non‐stationarity of response for right and left amygdalae for all subjects in each group. 4. Maximum significant non‐stationarity of response occurred either during presentation of fearful (F) or neutral (N) faces in time series. Ratios refer to the number of maxima occurring in response to fearful and neutral faces for the time series of each subject group.
Over all experimental conditions, there were no significant differences between right and left amygdalae time series in the number of significant model fits and number of areas of non‐stationarity of response.
P = 0.05 Chi‐squared = 3.90 (P = 0.06 Fisher exact test) comparing over all subjects for right versus left amygdalae the total number of areas of maximal significant non‐stationarity of response to fearful versus neutral faces.
Figure 3.
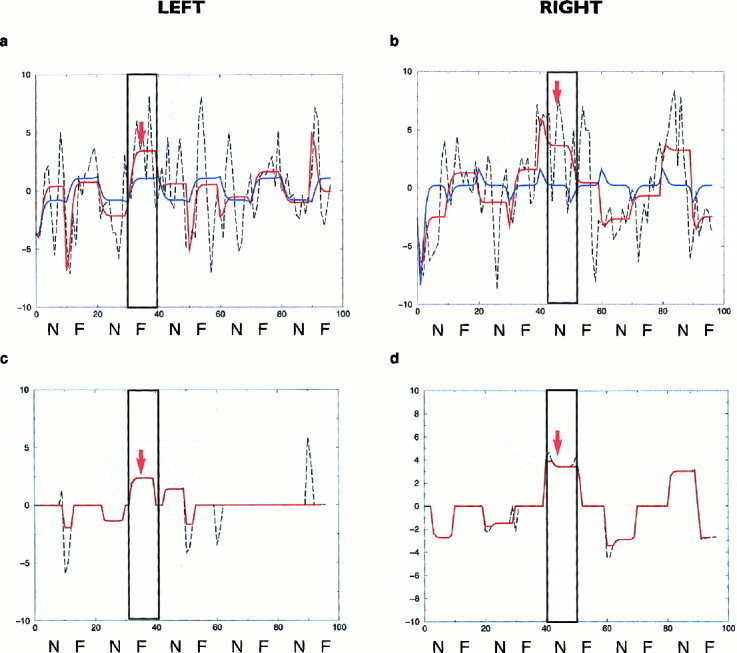
(a) stationary and adaptive model fits (blue and red curves, respectively) in an illustrative subject from Group 1 of the left amygdalar time series (P = 0.009 and P = 0.02: significance of the stationary and adaptive model fits, respectively); (b) stationary and adaptive model fits (colours as above) of the right amygdalar time series of the above subject (P = 0.002 and P = 0.03: significance of the stationary and adaptive model fits, respectively). Curves smoothed with a median filter (over five data points) are shown in red indicating time points of the left (c) and right (d) amygdalar time series where the fit to the adaptive model was significantly greater than that of the stationary model (i.e., areas of significant and near‐significant nonstationarity: P = 0.1 and P = 0.005: for the left and right amygdala, respectively). The labeling on both axes is as for Figure 2. The maximal modeled positive signal change and the area of maximal sustained nonstationarity of response in each amygdalar time series, occurring during presentation of either fearful or neutral faces, is indicated with an arrow.
Figure 4.
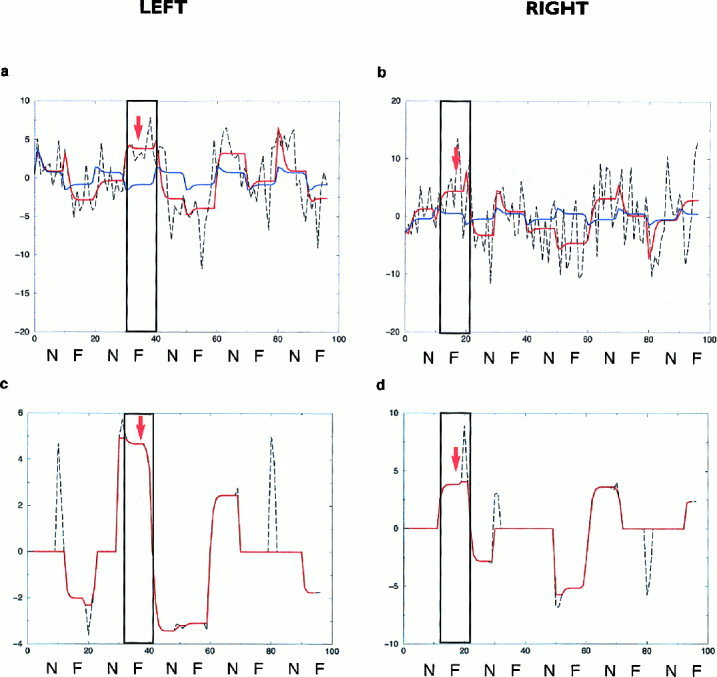
(a) stationary and adaptive model fits (blue and red curves, respectively) in an illustrative subject from Group 3 of the left amygdalar time series (P = 0.001 and P = 0.07: significance of the stationary and adaptive model fits, respectively); (b) stationary and adaptive model fits (colours as above) of the right amygdalar time series of the above subject (P = 0.03 and P = 0.6: significance of the stationary and adaptive model fits, respectively). Curves smoothed with a median filter (over five data points) are shown in red indicating time points of the left (c) and right (d) amygdalar time series where the fit to the adaptive model was significantly greater than that of the stationary model (i.e., areas of significant nonstationarity: P = 0.001 and P = 0.02: for the right and left amygdala, respectively). The labeling on both axes is as for Figure 2. The positioning of the arrows is as for Figure 3.
There was no significant difference over all experimental conditions in the number of significant model fits and nonstationarity of response in right compared with left amygdalar time series. There were, however, more areas of maximal significant nonstationarity of right amygdalar responses to presentation of neutral than fearful faces but similar numbers of areas of maximal significant nonstationarity of left amygdalar responses to presentation of fearful and neutral faces: for the eleven right amygdalar time series in which there were areas of significant nonstationarity of response, maximal nonstationarity occurred in response to neutral faces on nine occasions, and for the fourteen left amygdalar time series in which there was significant nonstationarity of response, maximal nonstationarity occurred in response to fearful faces on eight occasions. This difference in right and left amygdalar responses to fearful and neutral faces was significant: chi‐squared = 3.90; P = 0.05; Fisher exact test: P = 0.06. Further examination of these differences in right and left amygdalar responses within individual experimental conditions and different subject groups was not performed in view of the relatively small number of areas of significant sustained nonstationarity of amygdalar response in the time series of subjects within each group.
DISCUSSION
There has been little previous investigation of the laterality of the amygdalar response to fear. In this study, we aimed to assess the variability over time of the amygdalar response to fearful stimuli and examine the differences between right and left amygdalae in the extent of this variability by extracting time series of right and left amygdalar BOLD responses to alternating blocks of fearful and neutral expressions. Our initial observations of these time series suggested an earlier but more rapidly decreasing response of the right than the left amygdala to fearful faces and a maximal response of the right amygdala during presentations of neutral, and not fearful, facial expressions. We therefore employed a novel technique to model individual amygdalar time series in order to determine the extent of nonstationarity of right and left amygdalar responses, i.e., areas in the time series at which the amygdalar response differed significantly from that predicted by assuming a similar magnitude of response to each presentation of fearful stimuli over the course of the experiment. This procedure enabled us to make statistical comparisons between the extent of significant nonstationarity of right and left amygdalar responses to the above stimuli and also to determine in response to which block of stimulus presentation (fearful or neutral faces) maximal nonstationarity of right and left amygdalar responses occurred.
We demonstrated significant stationary model fits of left and right amygdalar time series to alternating fearful and neutral facial expressions, but also demonstrated significant adaptive model fits and nonstationarity of both amygdalar responses to these stimuli. These data indicate a variability of both right and left amygdalar responses to fearful stimuli over the course of an experiment. These findings suggest that when there is a significant signal change in the amygdala in response to fear, this signal change is variable over time, and the assumption of a constant amplitude of response of the amygdala to fear is clearly an oversimplification.
Our initial observations had suggested a further interesting finding: the presence of a maximal right amygdalar response to presentations of neutral faces. Modeling time series allowed us to demonstrate a significant difference between right and left amygdalae in the number of areas of maximum significant nonstationarity of response to presentation of fearful faces compared with that for neutral faces. Although maximal nonstationarity of the left amygdalar response occurred to presentation of both fearful and neutral facial expressions over the course of an experiment: to fearful expressions on eight and to neutral expressions on six out of fourteen occasions of significant nonstationarity of response, maximal nonstationarity of the right amygdalar response occurred primarily to presentation of neutral expressions: on nine out of eleven occasions of significant nonstationarity of response. Furthermore, for the second subject group, to whom were presented 75% and 150% morphed expressions of fear rather than 100% fear, there were similar differences in right and left amygdalar responses: five out of eight occasions of significant nonstationarity occurring in response to presentation of fearful expressions in the left amygdalae; two out of three in response to neutral facial expressions in the right amygdalae.
The demonstration on many occasions of maximal nonstationarity of both right and left amygdalar responses (i.e., a larger signal change than predicted by the stationary model) to presentation of neutral rather than fearful stimuli suggests a relative decrease in the response to fearful stimuli. A possible explanation for this is habituation of both right and left amygdalar responses to fear over time [Breiter et al., 1996]. This is, however, an insufficient explanation for the demonstration in right compared to left amygdalae of significantly more occasions of maximal nonstationarity of response to neutral stimuli. An alternative explanation is that neutral faces may become conditioned stimuli when associated with presentation of fearful faces over the course of an experiment, to the right but not the left amygdala. Although studies examining amygdalar responses in conditioned fear paradigms have reported a bias toward the right amygdala [Furmark et al., 1997; LaBar et al., 1998; Buchel et al., 1998], no study to our knowledge has examined the extent of conditioning of either amygdalar response, which may occur to neutral faces presented in association with fearful faces. In the current study, we did not aim specifically to examine right and left amygdalar responses during a fear‐conditioning paradigm, but merely to examine differences in the variability of right and left amygdalar responses over time. Our findings indicate that examination of the responses of right and left amygdalae during fear conditioning paradigms should be the subject of future studies.
The use of block design experiments allowed us to examine the variability of amygdalar responses between blocks of presentation of fearful and neutral facial expressions and over the course of an experiment. Future studies could also examine the variability of amygdalar responses to individual stimuli over the course of an experiment with the employment of more sophisticated (e.g., event‐related) experimental designs.
We examined and modeled time series extracted from right and left amygdalae during presentation of different intensities of fearful facial expression and when subjects were requested to perform different tasks during the experiment. The findings from this study do not indicate that either of these two variables had a major effect on the differences observed in the responses of right and left amygdalae to alternating fearful and neutral facial expressions. The most striking finding over all experimental conditions was the demonstration of maximal nonstationarity of the right but not the left amygdalar response to neutral faces.
Using a novel method of time series modeling, we have been able to demonstrate the presence of specific differences in the variability over time of right and left amygdalar responses to repeated presentations of fearful and neutral facial expressions. While the findings are consistent with habituation of both right and left amygdalar responses to fearful expressions during the experiments, they also suggest as a hypothesis for future direct experimental testing that neutral expressions may become conditioned stimuli when associated with fearful expressions, for right but not left amygdalae. To our knowledge, this is the first demonstration of specific differences in the time courses of right and left amygdalar responses to fearful and neutral facial expressions, and it merits further study.
REFERENCES
- Adolphs R, Tranel D, Damasio A, Damasio H (1994): Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372: 669–672. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A (1995): Fear and the human amgydala. J Neurosci 15: 5879–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR (1995): Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 269: 1115–1118. [DOI] [PubMed] [Google Scholar]
- Brammer M, Bullmore E, Simmons A, Williams S, Grasby P, Howard R, Woodruff P, Rabe‐Hesketh S (1997): Generic brain activation mapping in functional magnetic resonance imaging: a non parametric approach. Magn Reson Imaging 15: 763–770. [DOI] [PubMed] [Google Scholar]
- Breiter H, Etcoff N, Whalen P, Kennedy W, Rauch S, Strauss M, Hyman S, Rosen B (1996): Response and habituation of the human amygdala during visual processing of facial expressions. Neuron 17: 875–887. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ (1998): Brain systems mediating aversive conditioning: an event‐related fMRI study. Neuron 20: 947–957. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Brammer M, Williams S, Rabe‐Hesketh S, Janot N, David A.S, Mellers J, Howard R, Sham P (1996): Statistical methods of estimation and inference for functional MR image analysis. Magn Reson Med 35: 261–277. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Brammer M, Rabe‐Hesketh S, Curtis V, Morris R, Williams S, Sharma T, McGuire P (1999): Methods for the diagnosis and treatment of stimulus correlated motion in generic brain activation studies using fMRI. Hum Brain Mapp 7: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL (1996): Amygdala activity at encoding correlated with long‐term, free recall of emotional information. Proc Natl Acad Sci USA 93: 8016–8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder AJ, Young AW, Rowland D, Perrett DI, Hodges J, Etcoff N (1996): Facial emotion recognition after bilateral amygdala damage: differential severe impairment of fear. Cognit Neuropsychol 13: 699–745. [Google Scholar]
- Calder AJ, Young A, Rowland D, Perrett D (1997): Computer‐enhanced emotion in facial expressions. Proc R Soc Lond B 264: 919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen W (1976): Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Friston KJ, Josephs O, Rees G, Turner R (1998): Nonlinear event‐related responses in fMRI. Magn Res Med 39: 41–52. [DOI] [PubMed] [Google Scholar]
- Furmark T, Fischer H, Wik G, Larsson M, Fredrikson M (1997): The amygdala and individual differences in human fear conditioning. Neuroreport 8: 3957–3960. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA (1998): Human amygdala activation during conditioned fear acquisition and extinction: a mixed‐trial fMRI study. Neuron 20: 937–945. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA (1995): Impaired fear conditioning following unilateral temporal lobectomy in humans. J Neurosci 15: 6846–6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J (1996): The emotional brain. New York: Simon & Schuster. [Google Scholar]
- Morris J, Frith C, Perret D, Rowland D, Young A, Calder A, Dolan R (1996): A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383: 812–815. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ (1998): Conscious and unconscious emotional learning in the human amygdala. Nature 393: 467–470. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW (1990): Brain magnetic resonance imaging with contrast dependent blood oxygenation. Proc Natl Acad Sci USA 87: 8868–8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Young A, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Rowland D, Perrett DI, Williams SCR, Gray JA, David AS (1997): A specific neural substrate for perceiving facial expressions of disgust. Nature 389: 495–498. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, Williams SCR, Bullmore ET, Brammer MJ, Gray JA (1998): Neural responses to facial and vocal expressions of fear and disgust. Proc R Soc Lond B 265: 1809–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A, Moore E, Williams SCR (1999): Quality control for functional magnetic resonance imaging using automated data analysis and Shewhart charting. Magn Reson Med 41: 1274–1278. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotactic atlas of the human brain. Stuttgart: Thieme. [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike M (1998): Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Lee JT, Fluegel KW, Pardo JV (1998): Aversive gustatory stimulation activates limbic circuits in humans. Brain 121: 1143–1154. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV (1997): Emotion, olfaction and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci USA 94: 4119–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]


