Abstract
Inhibitory control and performance monitoring are critical executive functions of the human brain. Lesion and imaging studies have shown that the inferior frontal cortex plays an important role in inhibition of inappropriate response. In contrast, specific brain areas involved in error processing and their relation to those implicated in inhibitory control processes are unknown. In this study, we used a random effects model to investigate error‐related brain activity associated with failure to inhibit response during a Go/NoGo task. Error‐related brain activation was observed in the rostral aspect of the right anterior cingulate (BA 24/32) and adjoining medial prefrontal cortex, the left and right insular cortex and adjoining frontal operculum (BA 47) and left precuneus/posterior cingulate (BA 7/31/29). Brain activation related to response inhibition and competition was observed bilaterally in the dorsolateral prefrontal cortex (BA 9/46), pars triangularis region of the inferior frontal cortex (BA 45/47), premotor cortex (BA 6), inferior parietal lobule (BA 39), lingual gyrus and the caudate, as well as in the right dorsal anterior cingulate cortex (BA 24). These findings provide evidence for a distributed error processing system in the human brain that overlaps partially, but not completely, with brain regions involved in response inhibition and competition. In particular, the rostal anterior cingulate and posterior cingulate/precuneus as well as the left and right anterior insular cortex were activated only during error processing, but not during response competition, inhibition, selection, or execution. Our results also suggest that the brain regions involved in the error processing system overlap with brain areas implicated in the formulation and execution of articulatory plans. Hum. Brain Mapping 12:131–143, 2001. © 2001 Wiley‐Liss, Inc.
Keywords: prefrontal cortex, cingulate cortex, fMRI, inhibition, Go/NoGo
INTRODUCTION
Cognitive theories of the human brain posit that executive task control and performance monitoring are critical functions of the prefrontal cortex (PFC) [Logan, 1985; Luria, 1966]. An important, but less well studied, aspect of prefrontal cortex function is performance monitoring, which includes error detection and error correction to enable improved task accuracy [Reason, 1990]. A number of indirect findings suggest that humans can monitor actions and detect and compensate for errors [Rabbitt 1966a, 1966b]. For example, subjects tend to slow down on trials subsequent to errors [Rabbitt, 1966b]. The strongest evidence for the existence of a neural system that implements error processing has come from the recent discovery of a component of event‐related potentials (ERPs). These studies have shown that when subjects make an error, an error‐related negativity (ERN) occurs 100 msec after response onset. The ERN is maximal at fronto‐central recording sites on the human scalp with a peak voltage of up to 10uV [Gehring et al., 1993; Falkenstein et al., 1995a].
Brain areas that contribute to error processing, particularly in relation to executive functions such as inhibitory control, remain poorly understood. Based on dipole modeling of the ERP, the anterior cingulate (AC) has been considered a putative source of the ERN [Dehaene et al., 1994]. However, given the inherent indeterminacy of dipole source localization, dipole sources cannot be used to make conclusive inferences about neural generators of the ERN [Miltner et al., 1997]. Two recent functional magnetic resonance imaging (fMRI) studies of error processing have been conducted using different paradigms. Carter et al. [1998] used a continuous performance task (AX‐CPT) and they reported that the dorsal AC was activated during error processing as well as during tasks with increased response competition. In exploratory analysis they also reported that the left and right dorsolateral prefrontal cortex were activated during error processing but not during response competition. Kiehl et al. [2000] used a Go/NoGo paradigm that is more closely related to electrophysiological studies of the ERN. They reported that the anterior rostral but not the dorsal AC, and the left but not the right dorsolateral prefrontal cortex were involved in error processing. Thus, the precise role of the AC in error processing and response inhibition is not known, and it is not clear whether the AC makes a unique contribution to error processing. Furthermore, the contribution of other brain areas, if any, to error processing is unknown.
Inhibitory control mechanisms are a critical component of the response selection processes that contribute to accurate performance [Roberts et al., 1998] and as such may or may not be related to error processing. Lesion and imaging studies have shown that the PFC plays a critical role in inhibition of perseverative behavior [Iversen and Mishkin, 1970], inhibition of distracting sensory information [Chao and Knight, 1998], and inhibition of inappropriate prepotent response tendencies in motor [Konishi et al., 1998; Sasaki and Gemba, 1986] and cognitive [Jonides et al., 1998] processes. These and other similar findings have led to the proposal that inhibitory control is a central function of the PFC [Fuster, 1997; Roberts et al., 1998]. Based on primate lesion studies, it was originally hypothesized that inhibitory control was a function localized to the ventral (orbitofrontal) PFC [Fuster, 1997; Rolls, 1996]. However, more recent lesion, neurophysiological, and brain imaging studies have implicated other regions of the PFC, including the dorsolateral prefrontal cortex (DLPFC) [Shimamura, 1995; Dias et al., 1997], the inferior frontal cortex [Konishi et al., 1998; Garavan et al., 1999] and the AC cortex [Taylor et al., 1997; Posner, 1998] in response inhibition. The role of these regions in error processing, if any, is poorly understood.
The Go/NoGo task provides a simple paradigm with which to investigate brain activation during operations such as error processing, as well as response inhibition and response competition. ERP studies have shown the presence of distinct components related to response inhibition and error‐related signals during the Go/NoGo [Roberts et al., 1994; Falkenstein et al., 1995b; Kiefer et al., 1998]. We used a Go/NoGo task in an fMRI study to investigate brain regions involved in error processing following failure to inhibit response. Previous imaging studies of the Go/NoGo task have focused exclusively on response inhibition and competition. Konishi et al. [1998] used an event‐related design with equiprobable Go and NoGo events and found activation in the right inferior frontal sulcus during correctly inhibited NoGo events. However, this study only used five subjects, sampled a limited area of the PFC, and did not involve the establishment of a prepotent response that the subjects had to inhibit. Two recent developmental fMRI studies of the Go/NoGo used a paradigm that involved buildup of a significant level of prepotent response, and reported diffuse activation of the PFC [Casey et al., 1997; Vaidya et al., 1998]. Limitations of these studies include the use of prespecified ROIs and the restricted brain area evaluated, often not including the entire PFC. Using an event‐related fMRI design, Garavan et al. [1999] found greater activation in the PFC, as well as the inferior parietal lobe, during response inhibition. To our knowledge, only one study has examined whole‐brain event‐related activity during error processing [Kiehl et al., 2000], and only one study has examined whole‐brain activation during response inhibition [Garavan et al., 1999].
The aims of our study were twofold: first, investigate which brain areas are involved in error processing over and above response inhibition and competition; and second, determine the extent to which this network overlaps with brain regions underlying response inhibition and competition on the one hand, and motor response execution on the other. We used a standard blocked design [Casey et al., 1997] in this study as a way to provide and maintain a high level of prepotent response. Randomly presenting an equal number of Go and NoGo stimuli would have eliminated buildup of a prepotent response. Further, altering the proportion of Go and NoGo stimuli would have invoked processes unrelated to either response inhibition or error processing [Menon et al., 1997; Miltner et al., 1997; Posner, 1998]. Weighted mean images time‐locked to correct and incorrect NoGo events and adjusted for the haemodynamic response function were computed using a random effects model [Holmes and Friston, 1998]. This event‐related approach provides not only greater specificity to events of interest but also greater generalizability to the normative population. Because errors were sparse and occurred randomly, activation to incorrect NoGo events could be statistically separated from ongoing task‐related activation. In the Kiehl et al. [2000] study of error processing, a fixed effects model was used. The fixed effects model provides inference only about the limited number of subjects studied. The random effects model used in the present study is more appropriate for establishing normative patterns of brain activation during error processing.
MATERIALS AND METHODS
Subjects
Fourteen healthy, right‐handed subjects (8 males and 6 females; ages 17–41 years; mean 23.6 ± 7.2) participated in the study after giving written informed consent.
Experimental design
The experiment consisted of a 30‐sec rest epoch, 12 alternating 26‐sec epochs of Go and Go/NoGo conditions, followed by a 30‐sec rest epoch. During the rest condition, subjects passively viewed a blank screen. During the experiment, subjects viewed a series of letters once every 2 sec and responded with a key press to every letter except the letter “X,” to which they were instructed to withhold response. All subjects responded using the forefinger of the right hand. In the Go (control) condition, subjects were presented a random sequence of letters other than the letter “X”. In the Go/NoGo (experimental) condition, subjects were presented with the letter “X” 50% of the time, thus requiring response to half the trials (Go trials) and response inhibition to the other half (NoGo trials). At the beginning of each epoch, a 2‐sec instruction warned the subject about the new task condition.
fMRI acquisition
Images were acquired on a 1.5T GE Signa scanner with Echospeed gradients using a custom‐built whole head coil that provides a 50% advantage in signal to noise ratio over that of the standard GE coil [Hayes and Mathias, 1996]. A custom‐built head holder was used to prevent head movement. Eighteen axial slices (6 mm thick, 1 mm skip) parallel to the anterior and posterior commissure covering the whole brain were imaged with a temporal resolution of 2 sec using a T2*‐weighted gradient echo spiral pulse sequence (TR = 2,000 msec, TE = 40 msec, flip angle = 89° and 1 interleave) [Glover and Lai, 1998]. The field of view was 240 mm and the effective inplane spatial resolution was 4.35 mm. To aid in localization of functional data, a high‐resolution T1‐weighted spoiled grass gradient recalled (SPGR) 3D MRI sequence with the following parameters was used: TR = 24 msec; TE = 5 msec; flip angle = 40°; 124 slices in sagittal plane; 256 × 192 matrix; acquired resolution = 1.5 × 0.9 × 1.2 mm. The images were reconstructed as a 124 × 256 × 256 matrix with a 1.5 × 0.9 × 0.9 mm spatial resolution.
The task was programmed using Psyscope [Cohen et al., 1993] on a Macintosh (Sunnyvale, CA) notebook computer. Initiation of scan and task was synchronized using a TTL pulse delivered to the scanner timing microprocessor board from a CMU Button Box microprocessor (http://www.psyscope.psy.cmu.edu) connected to the Macintosh. Letters were presented visually at the center of a screen using a custom‐built magnet compatible projection system (Resonance Technology, CA).
Image preprocessing
Images were reconstructed, by inverse Fourier transform, for each of the 120 time points into 64 × 64 × 18 image matrices (voxel size: 3.75 × 3.75 × 7 mm). fMRI data were preprocessed using SPM97 (http://www.fil.ion.bpmf.ac.uk/spm). Images were corrected for movement using least square minimization without higher‐order corrections for spin history, and normalized to the Montreal Neurological Institute (MNI) template provided with SPM. Images were then resampled every 2 mm using sinc interpolation.
Statistical analysis
Statistical analysis was performed on group data using a random effects model [Holmes and Friston, 1998] along with the theory of Gaussian random fields as implemented in SPM97. This method takes advantage of multivariate regression analysis and corrects for temporal and spatial autocorrelations in the fMRI data [Friston et al., 1995].
Confounding effects of fluctuations in global mean were removed by proportional scaling where, for each time point, each voxel was scaled by the global mean at that time point. Low frequency noise was removed with a high pass filter (0.5 cycles/min) applied to the fMRI time series at each voxel. A temporal smoothing function (4 mm Gaussian kernel corresponding to dispersion of 8 sec) was applied to the fMRI time series to enhance the temporal signal‐to‐noise ratio. Voxel‐wise t‐statistics were computed using the random effects model and normalized to Z scores to provide a statistical measure of activation independent of sample size. Finally, in order to determine the presence of significant clusters of activation, the joint expected probability distribution of height (Z > 1.67; P < 0.05) and extent (P < 0.05) threshold [Poline et al., 1997] was used in order to correct for spatial correlations in the data.
For group analysis, a random effects model was used to determine voxel‐wise t‐statistics contrasting specific conditions of interest. This model estimates the error variance for each condition of interest across subjects, rather than across scans [Holmes and Friston, 1998]. The random effects model provides better generalization to the subject population, albeit with some loss in power due to averaging in the time domain. This analysis proceeded in two steps. In the first step, adjusted images corresponding to the conditions/events of interest were determined. For each condition, a weighted average of the images was computed taking into account the haemodynamic response. In the second step, these condition‐specific images were contrasted in a general linear model to determine appropriate t‐statistics. The t‐statistics were normalized to Z scores to determine significant clusters of activation.
For each subject, mean images corresponding to correct NoGo events (“correct NoGo”) and false alarm events (“incorrect NoGo”) were computed for each subject. Brain activation during error processing was estimated using an event‐related contrast of “incorrect NoGo” and “correct NoGo” events. Missed Go events were not used in calculations of error processing in order to avoid possible confounds resulting from different types of errors (i.e., errors of commission vs. errors of omission).
In addition, mean images were derived for each of the three conditions: (1) Go (control), (2) Go/NoGo (experimental), and (3) rest. These images were contrasted (Go/NoGo versus Go) to determine brain activation during response inhibition and competition using a blocked design in order to compare this type of neural process with that of error processing. Brain activation related to motor response execution was investigated using a (Go versus Rest) contrast.
Neuroanatomical locations of activation were first determined using the standard Talairach atlas [Talairach and Tournoux, 1988] and then refined using the more detailed and thorough Duvernoy atlas [Duvernoy, 1999].
Behavioral data analysis
The reaction time (RT) and number of correct responses and misses to Go events were computed separately for the Go and Go/NoGo condition. The number of correctly withheld responses to the NoGo events, and the number of false alarm (FA) responses and their RTs, were computed. Percent correct and incorrect responses and RTs were compared using students' t‐test.
RESULTS
Behavioral
The average percentage correct for Go events was 98.51% in the control epoch and 98.02% in the experimental epoch. Six out of the 12 subjects had a total of 10 missed responses to Go events in the Go/NoGo condition. The average FA (incorrect responses to NoGo events) rate across subjects was 6.25% and there were a total of 54 errors across 12 subjects. Two subjects made no FA errors. Subjects made significantly more FAs than misses (t13 = 3.06; P < 0.01; two‐tailed paired t‐test).
RT for the correct Go trials was significantly faster in the Go condition (350 ± 61 msec) than in the Go/NoGo condition (449 ± 62 msec) (P = 7.84E‐08, t13 = ‐10.74; two‐tailed t‐test, paired by subject). The RT for FAs was 335 ± 48 msec, significantly faster than the RT for correct Go events in the Go/NoGo epochs (P = 1.78E‐06; t11 = ‐9.15; paired two‐tailed t‐test). For the 12 subjects who did commit FA errors, the RT for the first event following each FA (395 ± 61 msec) was significantly slower than the RT for FAs (P = 0.006; t10 = ‐3.46; paired two‐tailed t‐test).
Statistical analysis of distribution of events preceding and following incorrect and correct NoGo events
To prove that the error processing contrast (“incorrect NoGo” and “correct NoGo”) actually reflects error processing, it was important to ensure that the different events preceding and following correct and incorrect NoGo events were identically distributed. For the 12 out of 14 subjects (s) who made FA errors, psejk (the probability of event k following j seconds after event e) was computed and compared across subjects for events e = incorrect (I) and correct (C) NoGo events. No significant differences were found (P > 0.05) at any time point; that is, p. = p. . Thus, the distribution of stimulus events of interest preceding and following incorrect and correct NoGo events was statistically identical. This indicates that the contrast comparing incorrect and correct NoGo events reflects differences in brain activation between these events and not differences arising from overlap in haemodynamic response to events that precede or follow them.
Brain activation
Error processing (“Incorrect NoGo” versus “Correct NoGo” events)
When activation locked to incorrect and correct NoGo events was compared (N = 12; two subjects made no FA errors), four significant clusters of activation were observed in the anterior‐ventral aspect of the AC, the left and right insula and adjoining frontal operculum, and the precuneus and adjoining posterior cingulate (Table I, Fig. 1). Each cluster was significant after height (Z > 1.67; P < 0.05) and extent thresholding (P < 0.05).
Table I.
Brain areas that showed significantly greater activation during error processing, response competition and inhibition, and response execution*
| Activated region | # of voxels | Z max | Peak location |
|---|---|---|---|
| Error Processing | |||
| Right Ant. Cing./Medial PFC (BA 24/32) | 869 | 3.55 | 10, 34, 22 |
| Left Insula/IFC (BA 47) | 618 | 4.09 | −44, −4, 2 |
| Right Insula/IFC (BA 47) | 575 | 3.87 | 40, 16, −2 |
| Precuneus/Post. Cing. (BA 7/31/29) | 643 | 3.91 | −8, −66, 36 |
| Response Inhibition | |||
| Left DLPFC (BA 9/46) | 614 | 4.18 | −34, 50, 32 |
| Right DLPFC (BA 9/46) | 1126 | 4.54 | 26, 46, 30 |
| Left IFC (BA 45/47) | 831 | 5.09 | −40, 30, 2 |
| Right IFC (BA 45/47) | 2095 | 4.93 | 40, 20, 2 |
| Left Premotor Cortex (BA 6) | 876 | 5.00 | −42, −8, 46 |
| Right Premotor Cortex (BA 6) | 493 | 4.51 | 50, −4, 42 |
| Right Anterior Cingulate (BA 24) | 3412 | 5.16 | 4, 16, 46 |
| Left Lingual Gyrus (BA 37) | 1063 | 3.77 | −18, −58, −6 |
| Right Lingual Gyrus (BA 37) | 502 | 3.12 | 20, −60, 8 |
| Right Inferior Parietal lobe (BA 39) | 1671 | 4.25 | 36, −56, 44 |
| Left Inferior Parietal lobe (BA 39) | 1433 | 3.50 | −22, −52, 46 |
| Left Caudate | 225 | 3.96 | −14, −6, 2 |
| Right Caudate | 468 | 4.11 | 16, −6, 12 |
| Response execution | |||
| Right Postcentral gyrus (BA 3) | 1188 | 5.17 | 38, −26, 56 |
| SMA (BA 6) | 994 | 5.05 | −8, 2, 56 |
| Left Lingual Gyrus (BA 37)* | 3523 | 5.02 | 44, −68, −20 |
| Right Lingual Gyrus (BA 37)* | 1335 | 4.58 | −46, −68, −16 |
| Left Superior/Inferior Parietal lobe (BA 7) | 843 | 4.41 | −34, −66, 56 |
| Left Motor and Premotor cortex (BA 4/6) | 1367 | 4.13 | −42, −4 46 |
| Right Premotor cortex (BA 4/6) | 991 | 3.64 | 50, 2, 38 |
Note: Cluster includes overlapping activation in the cerebellum and peristriate visual areas. Brain areas that showed significantly greater activation during error processing, response competition and inhibition and response execution. For each cluster, the activated region, number of voxels activated, maximum Z score and location of peak activation are shown.
Figure 1.
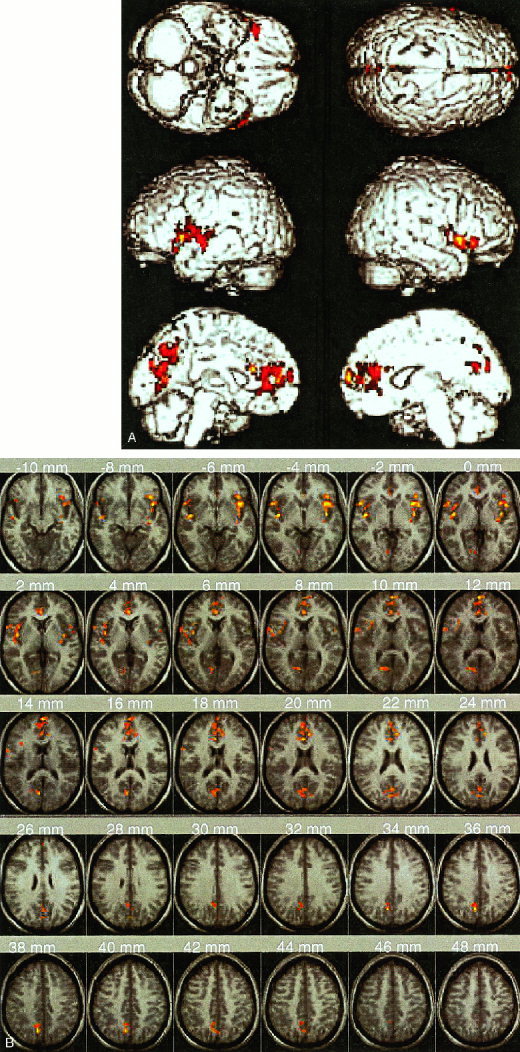
Brain areas showing significantly greater activation during error processing, compared to response inhibition and competition, include the left and right insula and adjoining inferior frontal cortex, right anterior cingulate, and left precuneus/posterior cingulate. (A) Surface rendered activation on a T1‐weighted single subject Montreal Neurological Institute (MNI) template provided with SPM. (B) Activation superposed on the average of all subjects' T1‐weighted structural MRI scans mapped into normalized MNI space. Axial slices from z = ‐10 to +48 mm are shown. Each cluster was significant after height (Z > 1.67; P < 0.05) and extent (P < 0.05) thresholding.
Although the number of errors that each subject made was small, our analysis used a random effects model, which estimates the statistical variance across subjects. Thus, even if individual signal levels were low, only brain regions consistently activated across subjects would emerge as significant population activation. We verified whether the observed results might have arisen from subjects who were outliers. The distribution of activation across subjects in all four brain regions where significant activation was observed during failed versus successful response inhibition is shown in Figure 2. No outliers were detected. In order to further verify that the error‐related activity in each of the four regions was normally distributed, we used the Shapiro‐Wilks's W test of normality [Shapiro et al., 1968]. If the W statistic is significant, then the hypothesis that the respective distribution is normal should be rejected. The results of the test were as follows: AC (W(24) = 0. 961356; P = 0.47), LIFC (W(24) = 0. 951242; P = 0.29), RIFC (W(24) = 0. 963180; P = 0.51), precuneus (W(24) = 0. 918060; P = 0.06). These results show that the activation in each brain region is normally distributed. Next, we did a power analysis to determine the effect size for the observed differences in each of these regions. The results of this power analysis are as follows: AC (Eta squared = 0.697), LIFC (Eta squared = 0.795), RIFC (Eta squared = 0.758), precuneus (Eta squared = 0.775). The large effect sizes in each case minimize the possibility of inaccuracy in the analysis.
Figure 2.

Scatter plot of activation levels in each of the four significant brain regions that showed greater activation during error processing compared to response inhibition and competition. Each point corresponds to the level of activation during incorrect and correct NoGo events for each of the 12 subjects.
Response inhibition and competition (Go/NoGo versus Go epochs)
When the Go/NoGo and Go epochs were compared (N = 14 subjects), significant activation was observed in the dorsal AC, left and right DLPFC, as well as in the pars triangularis region of the left and right IFC. Significant activation was also observed bilaterally in the premotor cortex, lingual gyrus, inferior parietal lobule (angular gyrus) and caudate (Table I, Fig. 3). Each cluster was significant after height (Z > 1.67; P < 0.05) and extent thresholding (P < 0.05).
Figure 3.

Brain areas showing significantly greater activation during response inhibition and competition include, bilaterally, the inferior frontal cortex, dorso‐lateral prefrontal cortex, premotor cortex, inferior parietal lobe, lingual gyrus, and caudate, in addition to the right dorsal anterior cingulate. (A) Surface rendered activation (B) Activation superposed on the mean of 14 individual T1‐weighted images in normalized space as in Figure 1. Each cluster was significant after height (Z > 1.67; P < 0.05) and extent (P < 0.05) thresholding.
Motor response execution (Go versus Rest epochs)
When the Go and Rest epochs were compared (N = 14 subjects), significant activation was observed in the left inferior frontal cortex, left posterior middle frontal gyrus, SMA (supplementary motor area), left and right premotor cortex, right postcentral gyrus, left superior and inferior parietal lobes, and the left and right lingual gyrus (extending into the cerebellum and peristriate visual areas) (Fig. 4). Each cluster was significant after height (Z > 1.67; P < 0.05) and extent thresholding (P < 0.05).
Figure 4.
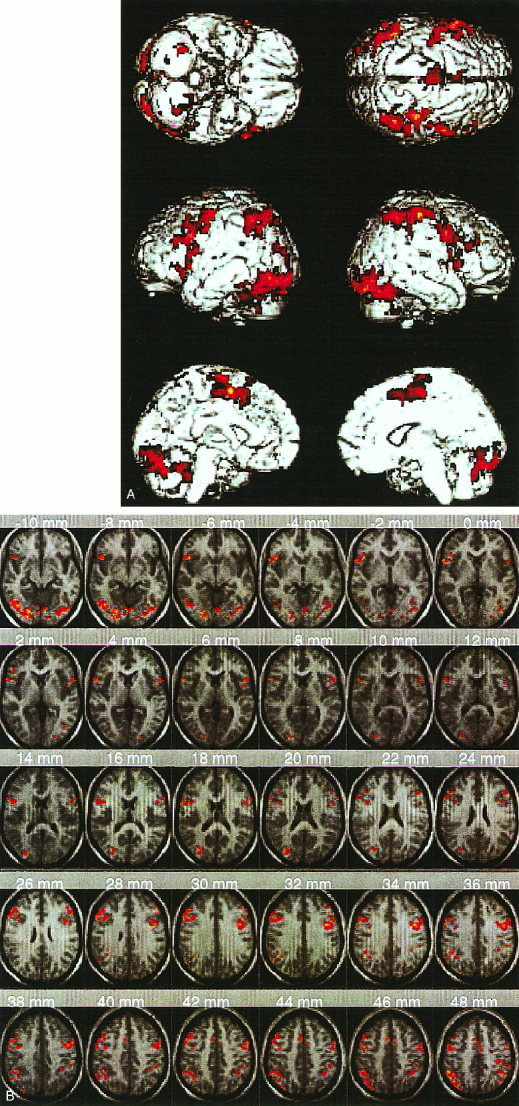
Brain areas showing significantly greater activation during response execution include left inferior frontal cortex, left posterior middle frontal gyrus, supplementary motor area, left and right premotor cortex, right postcentral gyrus, left superior and inferior parietal lobes, and the left and right lingual gyrus (extending into the cerebellum and peristriate visual areas). (A) Surface rendered activation (B) Activation superposed on the mean of 14 individual T1‐weighted images in normalized space as in Figure 1. Each cluster was significant after height (Z > 1.67; P < 0.05) and extent (P < 0.05) thresholding.
DISCUSSION
In the present study, we identified a network of brain areas involved in error processing by comparing activation during incorrect and correct NoGo events. This network consists of the left and right insular cortex and surrounding frontal operculum, rostro‐ventral AC and adjoining medial prefrontal cortex, and the posterior cingulate/precuneus. Our results suggest that error processing involves a more distributed network of brain regions than previously hypothesized in ERP studies [Dehaene et al., 1994; Falkenstein et al., 1995a; Gehring et al., 1995; Miltner et al., 1997b]. An exploratory analysis by Carter et al. [1998] suggested that lateral prefrontal cortex regions, including the left and right dorsolateral prefrontal cortex (BA 9/46), the left premotor cortex (BA 6), and right inferior frontal cortex (BA 44/45) were active during error processing. In contrast, Kiehl et al. [2000] have suggested that in addition to the AC, the left, but not the right, dorsolateral prefrontal cortex is involved in error processing. Our results indicate that a more inferior region in the lateral frontal/insular region cortex is involved in error processing. In addition, error processing also appears to involve regions in medial parietal cortex (precuneus and adjoining posterior cingulate) that have not been observed in previous studies.
Subjects performed the Go/NoGo task with a high level of accuracy (95%), indicating that the task was relatively easy and that stimuli were clearly distinguishable. The largest error‐related brain potentials are usually generated under conditions when subjects perform a task relatively well (at a level of approximately 90% or higher) [Gehring et al., 1993]. Although subjects performed the Go/NoGo task with a high level of accuracy, performance was not error free. Both false alarm responses to NoGo events and missed responses to Go events were committed, although subjects made more FAs than misses. RTs to incorrect NoGo events were significantly shorter than RTs to correct Go events in the experimental epoch, suggesting that these were not random errors or errors arising from factors such as fatigue. Instead, these errors appear to arise from fast guessing, as has been reported in studies of the ERN [Coles et al., 1995; Falkenstein et al., 1995a].
There were sufficient FA NoGo events to enable us to investigate error‐related brain activation in direct contrast with brain activation to response inhibition. Brain activation to missed Go events could not be investigated because of the low frequency of these events (a total of 10 across 12 subjects) and we could not be sure that the subjects were aware of their error or if they had simply missed the stimulus altogether. As Scheffers et al. [1996] have pointed out, since FAs, unlike misses, cannot be corrected by executing another response during the same trial, FA activation reflects error processing itself rather than processes related to inhibition of error.
The statistical independence of incorrect and correct NoGo events allowed us to compute error‐related brain activation over and above the background activation due to response inhibition and competition (see Methods and also Lumer et al. [1998] for a similar analysis in a different context). The large effect size, lack of outliers, and normality of distribution of activation across subjects minimize inaccuracy in interpretation and meaning of results. Using a random effects model ensured that only brain regions consistently activated across subjects would emerge as significant population activation [Holmes and Friston, 1998]. Additionally, imaging the whole brain and not using prespecified regions of interest allowed us to compare and contrast error‐related activation with brain areas involved in response inhibition and competition. Further, our analysis used a larger number of subjects than previous fMRI studies, thus providing improved validity over results of previous studies.
Because incorrect NoGo events involved error as well as motor response processing, we compared the pattern of brain activation during error processing and motor response execution. The left and right insular cortex, rostro‐ventral AC and adjoining medial prefrontal cortex, and the posterior cingulate/precuneus were activated during error processing but not during motor response execution. Of all the brain regions activated during error processing, only the posterior inferior frontal cortex showed activation during response execution. Even in this region, activation was slightly more extensive during error processing. Therefore, the brain areas involved in error processing appear to be distinct from those involved in response execution.
The error processing network overlaps partly but not completely with the distributed network involved in response inhibition and competition. We found significant activation during response inhibition and competition in the right dorsal AC and bilaterally in the inferior and dorsolateral prefrontal cortex, premotor cortex, caudate, inferior parietal lobe and lingual gyrus. Note that the left and right insular cortex, rostro‐ventral AC and adjoining medial prefrontal cortex, and the posterior cingulate/precuneus which were activated during error processing, were not activated during response inhibition and competition.
Our results also suggest that a more ventral aspect of the IFC and the adjoining insula are specifically involved in error processing. The inferior frontal cortex (IFC) showed significant activation during both error processing and response inhibition. However, error‐related activation in the IFC was restricted to the most ventral (opercular) region of the IFC. Congruent with event‐related fMRI analysis of response inhibition during similar Go/NoGo tasks [Garavan et al., 1999; Konishi et al., 1998], the pars triangularis region of the IFC showed significant activation during response inhibition and competition. Additionally, both the left and right anterior insular cortex adjoining the IFC showed significant activation only during error processing. These observations suggest that the lateral prefrontal cortex areas involved in error processing are at least partially distinct from those involved in response inhibition and competition.
Further evidence for a specialized circuit involved in error processing comes from differences in the localization of activation in the medial prefrontal cortex and AC. During error processing, an anterior‐ventral region of the AC and the adjoining medial prefrontal cortex showed significant activation in our study. A more dorsal region of the AC showed greater activation during response inhibition and competition and the SMA was activated during response execution. The dorsal AC and the DLPFC did not show any detectable activation during our error‐processing comparison, although these regions showed significant activation during response inhibition and competition. However, these results do not preclude the possibility that the dorsal AC and the DLPFC may be involved in both error processing and response inhibition/competition. Such activation would only be detectable against a more low‐level background because error related activity in the present task is detected over and above activation due to response competition and inhibition. It should be noted both the Carter et al. [1998] and the Kiehl et al. [2000] studies have suggested a role for the left DLPFC in error processing.
These results provide further evidence that the rostro‐ventral region of the AC is distinctly involved in error processing and is consistent with findings from a recent fMRI study of error processing [Kiehl et al., 2000]. The ventro‐medial prefrontal cortex activated during error processing bordered the orbitofrontal cortex but was clearly superior to it. Note that the present study used a random effects model for data analysis and therefore provides better generalization to the population than the fixed effects model used in Kiehl et al. [2000]. Carter et al. [1998] reported activation of the dorsal AC during both response inhibition and error processing. They interpreted this finding to mean that AC activity was related to response competition that occurred during both conditions, rather than error processing per se. In agreement with this finding, the dorsal AC did not show greater activation during error processing, compared to response inhibition and competition. Our results suggest that the anterior‐ventral region of the AC and the medial PFC may make a unique contribution to error processing and converge on lesion studies which have suggested that the ventro‐medial prefrontal cortex is involved in self‐monitoring processes [Damasio, 1994]. Our results are also consistent with recent EEG studies [Bush et al., 2000; Luu et al., 2000] that argue for an emotive component in response to errors. The rostro‐ventral region of the AC activated during error processing in the present study has also been shown to be activated during the emotional Stroop task [Whalen et al., 1998]. Taken together, these results support recent findings of parcellation of the anterior cingulate into the cognition division, which is engaged in response selection, competition and inhibition and the affective subdivision that is engaged in modulation of internal and emotional responses [Devinsky et al., 1995].
Although dipole modeling has suggested that the dorsal AC is the main neural generator of the ERN, our results suggest that the anterior‐ventral region identified in the present study may be a more appropriate source. Further, it is possible that bilateral sources in the inferior frontal cortex identified in the present study may also contribute to the fronto‐central vertex ERN peak in the same way that the centro‐parietal vertex peak of the P300 ERP component arises from bilateral sources in the temporal‐parietal junction [Menon et al., 1997].
Both the present study and an event‐related fMRI study [Garavan et al., 1999] found activation of the lateral parietal cortex during inhibitory control. Although it was involved in response inhibition and competition, the lateral parietal cortex did not show any error‐related activation. Electrophysiological studies of NoGo events [Kalaska and Crammond, 1995] have suggested that activity of the parietal cortex reflects stored potential motor responses to external inputs, while activity in the prefrontal cortex reflects the intended response. The lateral parietal cortex activation observed during response inhibition in the present study may therefore reflect PFC access to storage mechanisms, analogous to findings in working memory tasks [Smith and Jonides, 1997]. The lateral parietal cortex thus appears to be involved in response inhibition, but to have no role in error processing, unlike the lateral prefrontal cortex, which is involved in both response inhibition and error processing.
In contrast, the precuneus and adjoining posterior cingulate cortex in the medial parietal cortex, were activated only during error processing. Electrophysiological and lesion studies have suggested that this region is involved in evaluative functions such as monitoring behavior [Vogt et al., 1992]. Based on ERP studies, Badgaiyan and Posner [1998] have proposed that the posterior cingulate may be involved in processing feedback to errors. The precuneus is also transiently activated when external feedback shifts from “correct” to “incorrect” during tasks where subjects are required to alter stimulus‐response judgments [Nagahama et al., 1999], further supporting a role for this region in error monitoring.
While the medial prefrontal and parietal components of the error‐processing circuit appear to be involved in response evaluation and monitoring, evidence to date appears to indicate that the lateral prefrontal activation may partly reflect articulatory acknowledgement of error. Lesion studies have suggested that the left insula and adjoining inferior PFC play an important role in the execution of articulated plans [Dronkers, 1996; Donnan et al., 1997]. Furthermore, a recent PET study has suggested that the left insular cortex may play an even greater role in the formulation of articulatory plans than Broca's area [Wise et al., 1999]. The anterior insular cortex is also hypothesized to be involved in learning and acquisition of inhibitory avoidance behavior [Bermudez‐Rattoni et al., 1991] and stimulus predictability in the context of self‐generated actions [Blakemore et al., 1998], both processes that might be involved in initiation and regulation of future compensatory and remedial actions. Our findings of activation in these areas following FAs suggest a possible link between regions involved in articulation and regions involved in error processing.
These neurofunctional results are particularly suggestive of, and argue for, the existence of a neural system for error processing. Such a system could play a critical role in self‐monitoring processes [Frith, 1992]. Further research is needed to elucidate more precisely the neuroanatomical and neuropsychological substrates of such a system. The finding of consistent activity in specific brain regions during error processing may have significant implications for clinical research. In particular, such markers have potential utility in advancing our understanding of disorders in which deficits in self‐monitoring functions may play a prominent role, such as autism [Baron‐Cohen et al., 1997] and schizophrenia [Frith, 1992].
REFERENCES
- Badgaiyan RD, Posner MI (1998): Mapping the cingulate cortex in response selection and monitoring. Neuroimage 7: 255–260. [DOI] [PubMed] [Google Scholar]
- Baron‐Cohen S, Tooby J, Cosmides L (1997): Mindblindness: an essay in autism and theory of mind (learning, development and conceptual change). Cambridge, MA: MIT Press. [Google Scholar]
- Bermudez‐Rattoni F, Introini‐Collison IB, McGaugh JL (1991): Reversible inactivation of the insular cortex by tetrodotoxin produces retrograde and anterograde amnesia for inhibitory avoidance and spatial learning. Proc Natl Acad Sci USA 88: 5379–5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Rees G, Frith CD (1998): How do we predict the consequences of our actions? A functional imaging study. Neuropsychologia 36: 521–529. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD (1999): Conflict monitoring versus selection‐for‐action in anterior cingulate cortex. Nature 402: 179–181. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI (2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998): Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280: 747–749. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL (1997): A developmental functional MRI study of prefrontal activation during performance of a go‐no‐go task. J Cogn Neuro 9: 835–847. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT (1998): Contribution of human prefrontal cortex to delay performance. J Cogn Neurosci 10: 167–177. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J (1993): PsyScope: a new graphic interactive environment for designing psychology experiments. Behav Res Methods Instr Comp 25: 257–271. [Google Scholar]
- Coles MG, Scheffers MK, Fournier L (1995): Where did you go wrong? Errors, partial errors, and the nature of human information processing. Acta Psychol 90: 129–144. [DOI] [PubMed] [Google Scholar]
- Damasio AR (1994): Descartes' error. New York: Putnam. [Google Scholar]
- Dehaene S, Posner MI, Tucker DM (1994): Localization of a neural system for error detection and compensation. Psychol Sci 5: 303–305. [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA (1995): Contributions of anterior cingulate cortex to behaviour. Brain 118(Pt 1): 279–306. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC (1997): Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from “on‐line” processing. J Neurosci 17: 9285–9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnan GA, Darby DG, Saling MM (1997): Identification of brain region for coordinating speech articulation. Lancet 349: 221. [DOI] [PubMed] [Google Scholar]
- Dronkers NF (1996): A new brain region for coordinating speech articulation. Nature 384: 159–161. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM, Bourgouin P, Cabanis EA, Cattin F (1999): The human brain: surface, three‐dimensional sectional anatomy with MRI, and blood supply. New York: Springer‐Verlag. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J (1995a): Event‐related potential correlates of errors in reaction tasks In: Karmos G, Molnar M, Csepe V, Czigler I, Desmedt JE, editors. Perspectives of event‐related potentials research (EEG Suppl. 44). Amsterdam: Elsevier Science B.V. [PubMed] [Google Scholar]
- Falkenstein M, Koshlykova NA, Kiroj VN, Hoormann J, Hohnsbein J (1995b): Late ERP components in visual and auditory Go/Nogo tasks. Electroencephalogr Clin Neurophysiol 96: 36–43. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R (1995): Analysis of fMRI time‐series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Frith CD (1992): Cognitive neuropsychology of schizophrenia. San Diego, CA: LEA. [Google Scholar]
- Fuster J (1997): Prefrontal cortex. New York: Academic Press. [Google Scholar]
- Garavan H, Ross TJ, Stein EA (1999): Right hemispheric dominance of inhibitory control: an event‐related functional MRI study. Proc Natl Acad Sci USA 96: 8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Coles MG, Meyer DE, Donchin E (1995): A brain potential manifestation of error‐related processing. Electroencephalogr Clin Neurophysiol (Suppl) 44: 261–272. [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. (1993): A neural system for error detection and compensation. Psychol Sci 4: 385–390. [Google Scholar]
- Glover GH, Lai S (1998): Self‐navigated spiral fMRI: interleaved versus single‐shot. Magn Reson Med 39: 361–368. [DOI] [PubMed] [Google Scholar]
- Hayes C, Mathias C (1996): Improved brain coil for fMRI and high resolution imaging. Paper presented at the ISMRM 4th annual meeting, New York.
- Holmes AP, Friston KJ (1998): Generalisability, random effects and population inference. Neuroimage 7: S754. [Google Scholar]
- Iversen SD, Mishkin M (1970): Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res 11: 376–386. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter‐Lorenz PA (1998): Inhibition in verbal working memory revealed by brain activation. Proc Natl Acad Sci USA 95: 8410–8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaska JF, Crammond DJ (1995): Deciding not to GO: neuronal correlates of response selection in a GO/NOGO task in primate premotor and parietal cortex. Cereb Cortex 5: 410–428. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Marzinzik F, Weisbrod M, Scherg M, Spitzer M (1998): The time course of brain activations during response inhibition: evidence from event‐related potentials in a go/no go task. Neuroreport 9: 765–770. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB (2000): Error processing and the rostral anterior cingulate: an event‐related fMRI study. Psychophysiology 37: 216–223. [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y (1998): No‐go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. Eur J Neurosci 10: 1209–1213. [DOI] [PubMed] [Google Scholar]
- Logan GD (1985): Executive control of thought and action. Acta Psychol 60: 193–210. [Google Scholar]
- Lumer ED, Friston KJ, Rees G (1998): Neural correlates of perceptual rivalry in the human brain. Science 280: 1930–1934. [DOI] [PubMed] [Google Scholar]
- Luria AR (1966): Higher cortical functions in man. New York: Basic Books. [Google Scholar]
- Luu P, Flaisch T, Tucker DM (2000): Medial frontal cortex in action monitoring. J Neurosci 20: 464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Ford JM, Lim KO, Glover GH, Pfefferbaum A (1997): Combined event‐related fMRI and EEG evidence for temporal‐parietal cortex activation during target detection. Neuroreport 8: 3029–3037. [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH (1997a): Event‐related brain potentials following incorrect feedback in a time‐estimation task: evidence for a “generic” neural system for error detection. J Cogn Neurosci 9: 788–798. [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Lemke U, Weiss T, Holroyd C, Scheffers MK, Coles MGH (1997b): The source of the magnetic equivalent of the error‐related negativity. Psychophysiology 34: S65. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Sawamoto N, Toma K, Nakamura K, Hanakawa T, Konishi J, Fukuyama H, Shibasaki H (1999): Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. Neuroimage 10: 193– 199. [DOI] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ (1997): Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage 5: 83–96. [DOI] [PubMed] [Google Scholar]
- Posner MI (1998): Executive attention: conflict, target detection and cognitive control. Cambridge, MA: MIT Press. [Google Scholar]
- Rabbitt PM (1966a): Error correction time without external error signals. Nature 212: 438. [DOI] [PubMed] [Google Scholar]
- Rabbitt PM (1966b): Errors and error correction in choice‐response tasks. J Exp Psychol 71: 264–272. [DOI] [PubMed] [Google Scholar]
- Reason J (1990): Human error. Cambridge: Cambridge University Press. [Google Scholar]
- Roberts AC, Robinns TW, Weiskrantz L. (Eds.) (1998): The prefrontal cortex: executive and cognitive functions. New York: Oxford University Press. [Google Scholar]
- Roberts LE, Rau H, Lutzenberger W, Birbaumer N (1994): Mapping P300 waves onto inhibition: Go/No‐Go discrimination. Electroencephalogr Clin Neurophysiol 92: 44–55. [DOI] [PubMed] [Google Scholar]
- Rolls ET (1996): The orbitofrontal cortex. New York: Oxford University Press. [Google Scholar]
- Sasaki K, Gemba H (1986): Electrical activity in the prefrontal cortex specific to no‐go reaction of conditioned hand movement with colour discrimination in the monkey. Exp Brain Res 64: 603–606. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MG, Bernstein P, Gehring WJ, Donchin E (1996): Event‐related brain potentials and error‐related processing: an analysis of incorrect responses to go and no‐go stimuli. Psychophysiology 33: 42–53. [DOI] [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB, Chen HJ (1968): A comparative study of various tests of normality. J Am Stat Assoc 63: 1343–1372. [Google Scholar]
- Shimamura AP (1995): Memory and frontal lobe function. Cambridge, MA: MIT Press. [Google Scholar]
- Smith EE, Jonides J (1997): Working memory: a view from neuroimaging. Cogn Psychol 33: 5–42. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain: 3‐dimensional proportional system: an approach to cerebral imaging (Rayport M, translator). New York: Thieme. [Google Scholar]
- Taylor SF, Kornblum S, Lauber EJ, Minoshima S, Koeppe RA (1997): Isolation of specific interference processing in the Stroop task: PET activation studies. Neuroimage 6: 81–92. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD (1998): Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA 95: 14494–14499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR (1992): Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex 2: 435–443. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL (1998): The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 44: 1219–1228. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Greene J, Buchel C, Scott SK (1999): Brain regions involved in articulation [see comments]. Lancet 353: 1057–1061. [DOI] [PubMed] [Google Scholar]


