Abstract
Turner syndrome (TS), a genetic disorder characterized by the absence of an X chromosome in females, has been associated with cognitive and visuo‐spatial processing impairments. We utilized functional MRI (fMRI) to investigate the neural substrates that underlie observed deficits in executive functioning and visuo‐spatial processing. Eleven females with TS and 14 typically developing females (ages 7–20) underwent fMRI scanning while performing 1‐back and 2‐back versions of a standard visuo‐spatial working memory (WM) task. On both tasks, TS subjects performed worse than control subjects. Compared with controls, TS subjects showed increased activation in the left and right supramarginal gyrus (SMG) during the 1‐back task and decreased activation in these regions during the 2‐back task. In addition, decreased activation in the left and right dorsolateral prefrontal cortex (DLPFC) and caudate nucleus was observed during the 2‐back task in TS subjects. Activation differences localized to the SMG, in the inferior parietal lobe, may reflect deficits in visuo‐spatial encoding and WM storage mechanisms in TS. In addition, deficits in the DLPFC and caudate may be related to deficits in executive function during WM performance. Together these findings point to deficits in frontal‐striatal and frontal‐parietal circuits subserving multiple WM functions in TS. Hum. Brain Mapping 14:96–107, 2001. © 2001 Wiley‐Liss, Inc.
Keywords: neuroimaging, functional MRI, parietal cortex, prefrontal cortex, caudate, spatial cognition, X monosomy
INTRODUCTION
Turner syndrome, a genetic disorder characterized by partial or complete absence of one of the two X chromosomes in a phenotypic female, occurs in approximately 1 in 2,500 live births [Lippe, 1990]. Affected females share common physical characteristics including short stature, webbed neck, low set ears, shield chest, infertility, gonadal dysgenesis, and the absence of estrogen, progesterone and production of secondary sexual characteristics.
The neuropsychological profile of TS is notable for difficulties in visual memory and perception, mental manipulation of visuo‐spatial relationships among objects, and visual‐motor coordination, [Downey et al., 1989; Netley and Rovet, 1982; Romans et al., 1998]. In addition to visuo‐spatial impairments, previous behavioral research has also investigated increased impulsivity, decreased attention, and deficits in executive function in individuals with TS [Pennington et al., 1985; Romans et al., 1998; Waber, 1979]. Females with TS performed worse than controls on tests associated with frontal lobe function, including the Rey‐Osterreith complex figure (ROCF) [Reiss et al., 1995; Romans et al., 1998; Waber, 1979], and Wisconsin Card Sorting Test [Romans et al., 1998; Waber, 1979]. Despite these deficits, TS females possess relatively intact verbal skills. Reports of standardized cognitive tests indicate that average verbal IQ is in the normal range, whereas performance IQ is almost one standard deviation below the population mean [Garron, 1977; Rovet, 1993].
Figure 1.
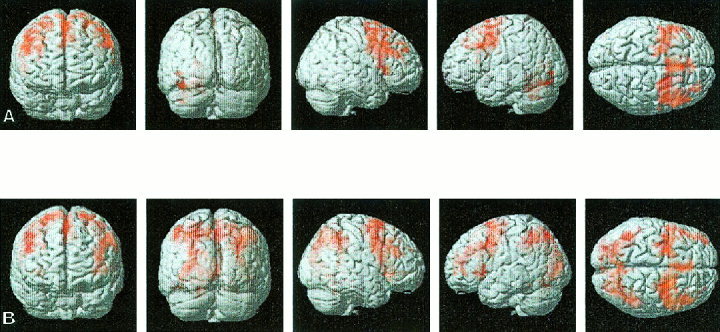
Surface rendering of group‐averaged brain activation during the 1‐ and 2‐back working memory tasks for control subjects. Significant clusters of activation were determined using the joint expected probability distribution of height and extent of Z scores, with height (Z > 1.67, P < 0.05) and extent threshold (P <0.05). (A) In the 1‐back task, significant activation was observed in the right inferior, middle and superior frontal gyrus, left middle and superior frontal gyrus, and left cerebellum. (B) In the 2‐back task, significant activation was observed in the left and right inferior, middle and superior frontal gyrus and premotor cortex, supramarginal gyrus, angular gyrus, superior parietal gyrus, intraparietal sulcus and left middle occipital gyrus.
Volumetric imaging studies have described neuroanatomical alterations in females with TS and have shown deficits in brain regions thought to be linked with visuo‐spatial processing. Murphy et al. [1993] demonstrated a volumetric reduction of the right posterior parietal/occipital regions. In a positron emission tomography (PET) study of five TS subjects, decreased glucose metabolism was observed in the parietal and occipital lobes bilaterally [Clark et al., 1990]. In a previous study from our laboratory, we used volumetric MRI and neurocognitive testing to address whether abnormalities in cognitive performance in TS reflected anomalies of brain development when compared with control subjects [Reiss et al., 1995]. We showed a proportional volume decrease primarily in the right parietal lobe but also a relative increase in the right occipital cortex. This study demonstrated both depressed performance on visuo‐spatial tasks such as the Judgment of Line Orientation (JLO), ROCF and for performance IQ measures, along with structural abnormalities in the parietal‐occipital regions [Reiss et al., 1995]. No study to date has reported metabolic or structural alterations in the frontal cortex of individuals with TS. The absence of such a finding is notable given the evidence of problems with attention and executive function tasks in TS females [Clark et al., 1990; Romans et al., 1998; Waber, 1979].
In this study, we used functional MRI (fMRI) to investigate executive function in TS using a visuo‐spatial working memory task. Working memory, defined as the ability to hold and manipulate information online in the brain, has been used to investigate basic operations underlying higher cognitive function. Imaging studies have utilized working memory tasks to elucidate underlying prefrontal as well as parietal cortex mechanisms [Baddeley and Hitch, 1974; Goldman‐Rakic, 1994; Smith and Jonides, 1999]. PET and fMRI studies demonstrated activity in the parietal lobes as well as the prefrontal cortex for both phonologically and visually‐encoded memory [Braver et al., 1997; Cohen et al., 1997; Elliott and Dolan, 1998; Jonides et al., 1993]. Recently, fMRI evidence from several studies in adults and children have demonstrated consistent activation of the left and right dorsolateral prefrontal cortex, and the parietal and occipital cortices during working memory operations [Belger et al., 1998; Carlson et al., 1998; Casey et al., 1998; Thomas et al., 1999].
To our knowledge, this is the first fMRI study to examine WM function in individuals with TS. We hypothesized that subjects with TS would show behavioral impairments during working memory performance and that these impairments would be accompanied by deficits in prefrontal and parietal cortex regions that are known to subserve critical operations underlying working memory.
MATERIALS AND METHODS
Subjects
All potential subjects were interviewed and screened by telephone for assessment of medical and psychiatric history. Documentation of X monosomy was obtained from the diagnosing physician or facility. Growth hormone and estrogen replacement status was determined. All TS subjects had received growth hormone and only three had started estrogen replacement therapy. Twelve right‐handed TS subjects and 14 right‐handed control subjects participated in the study after giving written informed consent. They received neurocognitive assessments and underwent fMRI scanning. Data from one of the TS subjects was not used because of excessive head movement (>5 mm). The remaining 11 right‐handed subjects with TS (ages 7–18 years; mean 12.6 years) and 14 control subjects (ages 7–20 years, mean 14.5 years) were used in the fMRI and neuropsychological analyses. The human subjects committee at Stanford University School of Medicine approved all protocols used in this study.
Neuropsychological Assessment
The Wechsler Adult Intelligence Scale‐III [Wechsler, 1991] was administered to participants over the age of 17, and the Wechsler Intelligence Scale for Children‐III [Wechsler, 1997] was given to participants between the ages 6–17 years. The Wechsler scales yield Verbal, Performance, and Full Scale IQ scores (u = 100, SD = 15) as well as scores on 11 subtests (u = 10, SD = 3). The Woodcock‐Johnson‐Spatial Relations (WCJ‐SR) test [Woodcock, 1989] (u = 50, SD = 10) was administered to all participants to assess spatial reasoning skills. The JLO test [Benton et al., 1994] was administered to assess spatial orientation processing. For the JLO, raw scores were reported.
1‐ Back and 2‐Back Working Memory Task Design
The 1‐back and 2‐back tasks consisted of rest, experimental (E) and control (C) epochs in the following order for each task: Rest‐E‐C‐E‐C‐E‐C‐Rest‐E‐C‐E‐C‐E‐C‐Rest. Thus, there were three rest epochs, six experimental epochs, and six control epochs in each task. The order of experiments was counter‐balanced across subjects. Each rest epoch was 30 sec long during which subjects passively viewed a blank screen. Control epochs began with a 4‐sec display of the instructions “Push for Center.” Experimental epochs began with a 4‐sec display of the instructions “Push for 1 Back” in the 1‐back task and “Push for 2 Back” in the 2‐back task. Each control and experimental epoch consisted of 16 stimuli presented for 500 msec each, with a 1,500 msec inter‐stimulus interval. The stimulus “O” was presented in one of nine distinct visuo‐spatial locations. In the 1‐back task, the subject was asked to respond if the stimulus was in the same location as the previous one. In the 2‐back task, the subject was asked to respond if the stimulus was in the same location two steps back. For the control condition, subjects were instructed to respond only when the stimulus appeared in the center.
Behavioral Data Analysis
The percent correct (PC) and reaction time (RT) for experimental and control events were computed separately for the 1‐back and 2‐back experiments. Percent correct refers to the percentage of stimulus trials in which the subject responded correctly, either with an appropriate button push or an appropriate inhibition. Percent correct and RTs were compared using an Analysis of Variance (ANOVA) with factors: Diagnosis, Task (1‐back, 2‐back). In a second analysis we used IQ as a covariate in an analysis of co‐variance (ANCOVA) to determine if behavioral differences were related to differences in IQ.
fMRI Acquisition
Images were acquired on a 1.5T GE Signa scanner with Echospeed gradients using a custom‐built whole head coil that provides a 50% advantage in signal to noise ratio over that of the standard GE coil. A custom‐built head holder was used to minimize head movement. Eighteen axial slices (6 mm thick, 1 mm skip) parallel to the anterior and posterior commissure covering the whole brain were imaged with a temporal resolution of 2 sec using a T2* weighted gradient echo spiral pulse sequence (TR = 2,000 msec, TE = 40 msec, flip angle = 89° and 1 interleave) [Glover and Lai, 1998]. The field of view was 240 mm and the effective in‐plane spatial resolution was 4.35 mm. To aid in localization of functional data, high resolution T1 weighted spoiled grass gradient recalled (SPGR) 3D MRI sequence with the following parameters was used: TR = 35 msec; TE = 6 msec; flip angle = 45°; 24 cm field of view; 124 slices in coronal plane; 256 × 192 matrix; acquired resolution = 1.5 × 0.9 × 1.2mm. The images were reconstructed as a 124 × 256 × 256 matrix with a 1.5 × 0.9 × 0.9 mm spatial resolution.
The working memory task was programmed using PsyScope [Cohen et al., 1993] on a Macintosh (Sunnyvale, CA) notebook computer. Initiation of scan and task was synchronized using a TTL pulse delivered to the scanner timing microprocessor board from a ‘CMU Button Box’ microprocessor (http://poppy. psy.cmu.edu/psyscope) connected to the Macintosh. Stimuli were presented visually at the center of a screen using a custom‐built magnet compatible projection system (Resonance Technology, CA).
Image Preprocessing
Images were reconstructed, by inverse Fourier transform, for each of the 120 time points into 64 × 64 × 18 image matrices (voxel size: 3.75 × 3.75 × 7 mm). FMRI data were pre‐processed using SPM99 (http://www.fil.ion.ucl.ac.uk/spm). Images were corrected for movement using least square minimization without higher‐order corrections for spin history, and normalized to stereotaxic Talairach coordinates [Talairach and Tournoux, 1988]. We used the standard SPM/MNI T2*weighted template image for normalizing fMRI images into a standard coordinate system. Images were then resampled every 2 mm using sinc interpolation and smoothed with a 4 mm Gaussian kernel to decrease spatial noise.
Statistical Analysis
Statistical analysis was performed on individual and group data using the general linear model and the theory of Gaussian random fields as implemented in SPM99. This method takes advantage of multivariate regression analysis and corrects for temporal and spatial autocorrelations in the fMRI data [Friston et al., 1995]. Activation foci were superposed on high‐resolution T1‐weighted images and their locations interpreted using known neuroanatomical landmarks.
A within‐subjects procedure was used to model all the effects of interest for each subject. Individual subject models were identical across subjects (i.e., a balanced design was used). Confounding effects of fluctuations in global mean were removed by proportional scaling where, for each time point, each voxel was scaled by the global mean at that time point. Low frequency noise was removed with a high pass filter (0.5 cycles/min) applied to the fMRI time series at each voxel. A temporal smoothing function (Gaussian kernel corresponding to dispersion of 8 sec) was applied to the fMRI time series to enhance the temporal signal to noise ratio. The hemodynamic response was modeled with a gamma function [Friston et al., 1995]. We then defined the effects of interest for each subject with the relevant contrasts of the parameter estimates. Group analysis was performed using a random‐effects model that incorporated a two‐stage hierarchical procedure. This model estimates the error variance for each condition of interest across subjects, rather than across scans [Holmes and Friston, 1998] and therefore provides a stronger generalization to the population from which data are acquired. In the first stage, contrast images for each subject and each effect of interest were generated as described above. In the second stage, these contrast images were analyzed using a general linear model to determine voxel‐wise t‐statistics. One contrast image was generated per subject, per effect of interest. Finally, the t‐statistics were normalized to Z scores, and significant clusters of activation were determined using the joint expected probability distribution of height and extent of Z scores [Poline et al., 1997], with height (Z > 1.67; P < 0.05) and extent thresholds (P < 0.05). Contrast images were calculated using within subject design for the following conditions: (i) 1‐back, experimental‐control; (ii) 2‐back, experimental‐control; and (iii) 2‐back‐1‐back, experimental‐control. A one‐way t‐test with a two‐tailed probability threshold was then used to determine group activation for each effect. TS and control subjects were compared using an unpaired, two‐tailed t‐test. In a second analysis we used IQ as a covariate of no interest to determine if differences in activation in each of these conditions were related to differences in IQ.
RESULTS
Neuropsychological Assessment
The full‐scale IQ (FSIQ) scores of the TS subjects (range 56–135, mean = 100) were significantly lower (t = 2.28, P = 0.03) than the FSIQ of control subjects (range = 93–137, mean = 117) (Table I). The difference between the verbal IQ (VIQ) scores of the TS (range 59–142, mean = 107) and the control subjects (range 94–134, mean = 116) was not significant (Table I). The performance IQ (PIQ) scores of the Turner subjects (range = 62–120, mean = 92) were significantly lower (t = 3.91, P = 0.001) than the scores of the control subjects (range 93–132, mean = 114) (Table I). On the JLO task, TS subjects demonstrated decreased performance when compared with the control subjects (t = 2.63, P = 0.015) (Table I). On the WCJ‐SR task, decreased performance was observed in TS subjects when compared with controls (t = 3.30, P = 0.003) (Table I).
Table I.
Group means and standard deviations on selected psychological measures*
| Measures | Controls | Turner | df | T‐value | P |
|---|---|---|---|---|---|
| Age | 14.4 ± 4.2 | 12.5 ± 3.6 | 23 | 1.21 | 0.24 |
| Full scale IQ | 117 ± 13 | 100 ± 23 | 23 | 2.28 | 0.03 |
| Verbal IQ | 116 ± 13 | 107 ± 26 | 23 | 1.11 | 0.28 |
| Performance IQ | 114 ± 13 | 92 ± 16 | 23 | 3.91 | 0.001 |
| JLO | 25 ± 4 | 18 ± 9 | 23 | 2.63 | 0.015 |
| Woodcock‐Johnson | 63 ± 7 | 50 ± 12 | 23 | 3.30 | 0.003 |
For control group, n = 14; for Turner syndrome group, n = 11. All subjects were right‐handed. JLO, Judgment of Line Orientation.
Behavioral Performance
Behavioral performance on the 1‐back and 2‐back tasks was compared between these two groups. An ANOVA was performed with between‐group factors, Diagnosis (Control, TS). For PC, a significant main effect of diagnosis (df = 1,22, F = 4.06, P = 0.046) was observed with decreased accuracy in the TS subjects. The mean PC for TS subjects was 92 ± 5.6% on the 1‐back and 79 ± 15.1% on the 2‐back task. The mean PC for controls was 92 ± 5.9% on the 1‐back and 90 ± 7.5% on the 2‐back task. No interaction between diagnosis and task was seen and indicated that the TS group was not differentially impaired in the 2‐back task when compared with the 1‐back for this parameter of performance. After covarying for the effect of IQ, no main effect of diagnosis was observed for percent correct responses for either the 1 back or 2‐back‐task.
For RT, a main effect of diagnosis (df = 1,22, F = 5.4, P = 0.01) was observed with TS subjects showing significantly longer RTs. The mean RT was 890 ± 212 msecs on the 1‐back and 911 ± 258 msecs on the 2‐back for TS subjects. The mean RT for control subjects was 679 ± 131 msecs on the 1‐back and 695 ± 185 msecs on the 2‐back. No interaction between diagnosis and task was observed. This indicates that TS subjects also were not significantly different on the 2‐back task compared with the 1‐back task for this parameter of performance. After covarying for IQ, there was a main effect of diagnosis (df = 1,22, F = 4.06, P = 0.05) observed for RT.
Brain Activation
Whole brain analysis was performed on control and TS subjects during the 1‐back and 2‐back tasks. For each task, the experimental condition was contrasted with the control condition.
Control subjects
Significant activation was observed for the 1‐back task in the right inferior frontal gyrus (IFG) (BA 44/45), middle frontal gyrus (MFG) (BA 9/46), superior frontal gyrus (SFG) (BA 8) and premotor cortex (PMC) (BA 4/6), and in the left MFG (BA 9), SFG (BA 8) and PMC (BA 4/6) and the left cerebellum (Table II, Fig. 1A). In the 2‐back experiment, increased activation was observed in the left IFG (BA 44/45), MFG (BA 9), SFG (BA 8) and PMC (BA4/6). In addition, significant activation was observed in the left and right supramarginal gyrus (SMG) (BA 40), angular gyrus (ANG) (BA 39), superior parietal gyrus (SPG) (BA 7), intraparietal sulcus (IPS) (BA 7) and left middle occipital gyrus (MOG) (BA 19) (Table II, Fig. 1B).
Table II.
Brain regions that showed significant activation in the 1‐back and 2‐back tasks in control and Turner syndrome subjects*
| P value (COR) | Number of voxels | Z score (max) | Peak location Talaraich coordinates | |
|---|---|---|---|---|
| Control 1‐back | ||||
| Right inferior (BA 44/45), middle (BA 9/46) and superior frontal gyrus (BA 8) | <.001 | 3898 | 4.72 | 18, 6, 58 |
| Left middle (BA 9) and superior frontal gyrus (BA 8) | <.001 | 4063 | 4.26 | 42, 8, 40 |
| Right superior frontal gyrus (BA 8) and premotor cortex (BA4/6) | ||||
| Left cerebellum | <.001 | 1604 | 3.61 | −34, 52, −26 |
| Control 2‐back | ||||
| Left and right inferior (BA 44/45), middle (BA 9) and superior frontal gyrus (BA 8), premotor cortex (BA 4/6) | <.001 | 13878 | 4.9 | 36, 0, 42 |
| Left supramarginal gyrus (BA 40), superior parietal gyrus (BA 7), angular gyrus (BA 39), intraparietal sulcus (BA 7), middle occipital gyrus (BA18) | <.001 | 14124 | 4.63 | 42, −64, 56 |
| Right supramarginal gyrus (BA 40), superior parietal gyrus (BA 7), angular gyrus (BA 39), intraparietal sulcus (BA7) | ||||
| Turner 1‐back | ||||
| Left inferior semilunar lobule (Crus II) | .001 | 665 | 4.45 | −36, −56, −32 |
| Left supramarginal gyrus (BA 40) | <.001 | 1260 | 4.16 | −44, −58, 38 |
| Right inferior (BA 44) and middle frontal gyrus (BA 9), premotor cortex (BA 4/6) | <.001 | 3200 | 3.96 | 46, 8, 38 |
| Left inferior (BA 44) and middle frontal gyrus (BA 9), premotor cortex (BA 4/6), supramarginal gyrus (BA 40), angular gyrus (BA 7/39) | <.001 | 1524 | 3.63 | −32, 52, 36 |
| Right cuneus, superior occipital gyrus (BA 18/19) | <.001 | 1258 | 3.26 | 8, −90, 24 |
| Right supramarginal gyrus (BA 40), angular gyrus(BA 39) | <.001 | 758 | 3.18 | 46, −54, 34 |
| Turner 2‐back | ||||
| Right precuneus, angular gyrus (BA 7/39) | <.001 | 2403 | 3.66 | 8, −66, 60 |
| Right middle frontal gyrus (BA 9/10) | .001 | 749 | 3.35 | 30, −4, 52 |
| Right middle frontal gyrus (BA 8/9) | .012 | 536 | 3.72 | 24, 50, 42 |
| Left middle frontal gyrus (BA 9/10) | .018 | 504 | 3.93 | −36, 56, 26 |
For each significant cluster, region of activation, significance level, number of activated voxels, maximum Z score and location of peak in Talaraich coordinates are shown.
TS subjects
Significant activation in the 1‐back task was observed in the left and right IFG (BA 44), MFG (BA 9) and PMC (BA 4/6), SMG (BA 39), and IPS (BA 7). Activation was also observed in the right SPG (BA 39), cuneus, superior occipital gyrus (BA 18/19) and left cerebellum (Table II, Fig. 2A). Activation in the 2‐back task was seen in the left and right MFG (BA 9), right PMC (BA 4/6) SMG (BA 40) and SPG (BA 7/39) (Table II, Fig. 2B).
Figure 2.
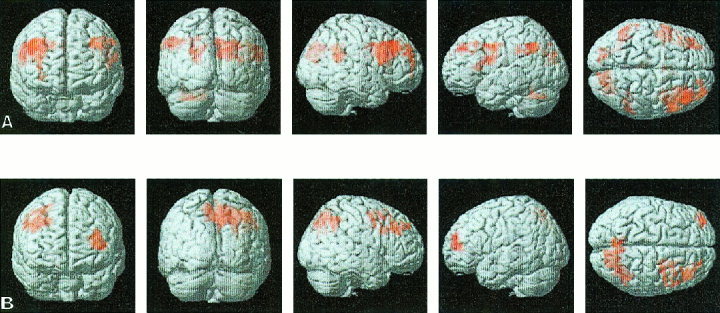
Surface rendering of group‐averaged brain activation during the 1 and 2‐back working memory task for Turner syndrome subjects. Analysis was similar to that in Figure 1. (A) In the 1‐back task, significant activation was observed in the left and right inferior and middle frontal gyrus, premotor cortex, supramarginal gyrus and angular gyrus and right cuneus and superior occipital gyrus (there was no activation in the top third of the brain during the 1‐back task). (B) In the 2‐back task, significant observation was observed in right middle frontal gyrus, right and left precuneus and angular gyrus. (The 2‐back task showed significant activation in these brain regions).
Control‐TS subjects
We then contrasted the control and TS subjects using unpaired t‐tests. In the 1‐back task, control subjects did not show greater activity than the TS group in any brain region. In the 2‐back task, the control group showed greater activation in the left and right IFG (BA 44), MFG (BA 9), SMG (BA 40) and head of the caudate. (Table III, Fig. 3A, Fig. 4). In the 2‐back minus 1‐back contrast, control subjects showed greater activation in the right IFG (BA 44), MFG (BA 9/46), PMC (BA 4/6), SMG (BA 40), and IPS (BA 7) (Table III, Fig. 5). In addition, small foci of activation were observed in the left MFG (BA 9) and left SMG (BA 40) in controls relative to TS subjects (Table III, Fig. 5).
Table III.
Brain regions that showed significant activation differences between control and Turner syndrome subjects during the 1‐back and 2‐back tasks*
| P value (COR) | Number of voxels | Z score (max) | Peak location Talaraich coordinates | |
|---|---|---|---|---|
| Control minus Turner syndrome (1‐back) | ||||
| No foci of activation | ||||
| Control minus Turner syndrome (2‐back) | ||||
| Left and right caudate, inferior (BA 44) and middle frontal gyrus (BA 9) | <.001 | 2337 | 4.59 | − 18, 16, 4 |
| Left supramarginal gyrus (BA40) | <.001 | 1661 | 4.39 | − 44, − 44, 28 |
| Right supramarginal gyrus (BA40) | .003 | 756 | 4.17 | 32, − 42, 28 |
| Right inferior (BA 44) and middle frontal gyrus (BA 9) | <.001 | 2178 | 4.00 | 36, 10, 34 |
| Right middle frontal gyrus (BA 8/9) | .011 | 625 | 3.34 | 26, 34, 24 |
| Control minus Turner syndrome (2‐back minus 1‐back) | ||||
| Right inferior (BA 44) and middle frontal gyrus (BA 9/46); right premotor cortex (BA 4/6) | <.001 | 1990 | 4.13 | 46, − 12, 18 |
| Right supramarginal gyrus (BA 40) and intraparietal sulcus (BA 7) | <.001 | 676 | 3.92 | 30, − 44, 36 |
| Left supramarginal gyrus (BA 40) | .001 | 434 | 3.53 | − 44, − 44, 26 |
| Left middle frontal gyrus (BA 9) | .012 | 291 | 3.21 | − 36, 16, 28 |
| Turner syndrome minus control (1‐back) | ||||
| Left and right precuneus (BA 7) | <.001 | 2062 | 3.40 | − 4, − 48, 40 |
| Supramarginal gyrus (BA 40) | .039 | 401 | 3.32 | 44, − 54, 16 |
| Supramarginal gyrus (BA 40) | .031 | 418 | 3.25 | − 52, − 62, 34 |
| Turner syndrome minus control (2‐back) | ||||
| No foci of activation | ||||
| Turner syndrome minus control (2‐back minus 1‐back) | ||||
| No foci of activation |
For each significant cluster, region of activation, significance level and location of peak in Talaraich coordinates are shown.
Figure 3.
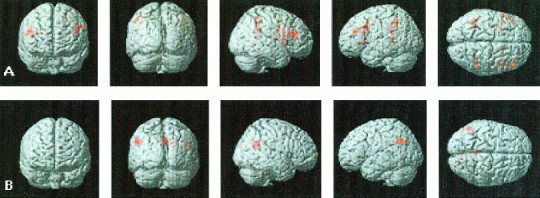
Direct comparison of brain activations in control and Turner syndrome (TS) subjects during the 1 and 2‐back working memory task. Unpaired t‐tests were used for comparisons. Analysis was similar to Figure 1. (A) During the 1‐back task, increased activation was observed in the left and right precuneus and supramarginal gyrus in TS subjects. (B) During the 2‐back task, decreased activation was observed in left and right inferior and middle frontal gyrus and left and right supramarginal gyrus in TS subjects.
Figure 4.
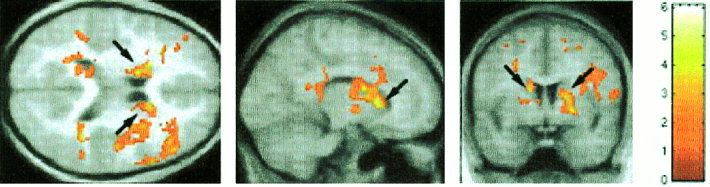
Direct comparison of brain activation in control and Turner syndrome (TS) subjects during the 2‐back task using unpaired t‐tests. Activation maps were superimposed on group‐averaged high‐resolution structural magnetic resonance images. Decreased activation was seen in TS subjects in the left and right middle frontal gyrus, supramarginal gyrus and caudate (arrows). Analysis was similar to that in Figure 1.
Figure 5.
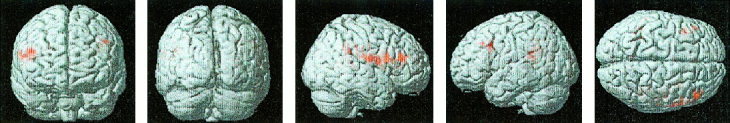
Surface rendering of brain activation during the 2‐back minus 1‐back working memory contrast for control subjects relative to Turner syndrome subjects. Analysis was similar to that in Figure 1. Control subjects show more activation in the left inferior frontal gyrus, middle frontal gyrus, premotor cortex, supramarginal gyrus, intraparietal sulcus and the right middle frontal gyrus and supramarginal gyrus.
TS‐Control subjects
In the 1‐back task, TS subjects showed greater activation than control subjects in the left and right SMG (BA 40) and precuneus (BA 7). During the 2‐back task, no brain region showed greater activation in TS subjects (Table III, Fig. 3B). In the 2‐back minus 1‐back contrast, no brain regions showed greater activation in TS subjects relative to controls.
Brain activation after covarying for IQ
In the 1‐back task, there were no differences between control and TS subjects. In the 2‐back task, compared with controls, TS subjects showed decreased activation in the left and right MFG (BA 9), PMC (BA 4/6), SMG (BA 40) and caudate (Table IV). TS subjects showed greater activation than controls in the left and right precuneus during the 1‐back and 2‐back tasks (Table IV).
Table IV.
Brain regions that showed activation differences between control and Turner syndrome subjects after using IQ as a covariate
| P value (COR) | Number of voxels | Z score (max) | Peak location Talaraich coordinates | |
|---|---|---|---|---|
| Control‐Turner syndrome 1‐back | ||||
| No foci of activation | ||||
| Turner syndrome‐control 1‐back | ||||
| Left and right precuneus (BA 7) | <.001 | 1576 | 3.37 | 4, − 76, 34 |
| Control‐Turner syndrome 2‐back | ||||
| Left supramarginal gyrus (BA 40) and precuneus (BA 7) | <.001 | 1629 | 4.52 | − 44, − 44, 28 |
| Left and right caudate, middle frontal gyrus (BA 9) | <.001 | 1673 | 4.22 | − 18, 16, 4 |
| Right supramarginal gyrus (BA 40) and precuneus (BA 7) | .001 | 875 | 3.79 | 44, − 40, 26 |
| Right middle frontal gyrus (BA 9/46) | <.001 | 2243 | 3.50 | 36, 10, 34 |
| Turner syndrome‐control 2‐back | ||||
| Left precuneus (BA 7) | .004 | 728 | 3.35 | 10, − 56, 28 |
* For each significant cluster, region of activation, significance level and location of peak in Talaraich coordinates are shown.
DISCUSSION
TS subjects showed decreased accuracy on both the 1‐back and 2‐back tasks. In addition, they had longer reaction times than the controls. The difference in performance between TS and control subjects, however, did not increase with the 2‐back compared with the 1‐back task. Thus, TS subjects showed overall impaired performance but did not perform worse with increasing working memory load. Working memory deficits were modest in comparison to the impairments in object location and spatial representation observed in the JLO and WCJ‐SR Task. The impaired performance in these two tasks provides further evidence for visuo‐spatial deficits in TS subjects. We next examined whether TS subjects showed significant differences in patterns of brain activation from control subjects during the working memory task.
For frontal regions, a direct comparison of TS and control subjects revealed no significant activation differences during the 1‐back task. Both TS and control subjects showed significant activation in the left and right MFG, SFG, PMC, the right IFG, and the left cerebellum. These results are consistent with previous neuroimaging studies of working memory demonstrating activation in the IFG (Talairach coordinates for Belger et al. [1998]: 42, 34, 12; −36, 31, 10; for Carlson et al. [1998]: −38, 34, −7), MFG (Talairach coordinates for Belger et al., [1998]: 30, 34, 37; −33, 33, 24), SFG (Talairach coordinates for Belger et al. [1998]: 21, 50, 35; for Carlson et al. [1998]: −8, −5, −55). Compared with control subjects, TS subjects, however, showed increased activation in the SMG and precuneus.
In contrast to the 1‐back group comparison, TS subjects showed significant activation deficits compared with controls in both frontal and parietal cortices during the 2‐back task. In the frontal cortex, controls showed increased activation in the left and right IFG, MFG, and SFG, whereas TS subjects showed less extensive activation in these regions. In the parietal lobe, controls showed increased left and right SMG, ANG, IPS and left MOG activation. Unlike the parietal findings in the 1‐back, TS subjects showed less robust activation levels in these regions than control subjects. In addition to the frontal‐parietal findings above, statistically significant activation differences between TS and control subjects were observed in the left and right caudate during the 2‐back task.
After the 1‐back and 2‐back analyses, we compared the activation differences between control and TS subjects in a 2‐back minus 1‐back contrast. The purpose of this contrast was to provide information about which brain regions were specifically modulated by working memory load. Our finding of a group by task interaction in the right IFG, MFG and SMG points to specific deficits in visuo‐spatial working memory in TS in these areas of the right hemisphere.
We then examined whether the working memory deficits observed in TS subjects were due in part to IQ differences between the two groups. TS subjects did not show significant impairment in accuracy after covarying out the effect of IQ; this finding is consistent with the observation of a correlation between IQ and performance during the spatial working memory task. Even after covarying out the effect of IQ, however, TS subjects demonstrated longer reaction times indicating that they process spatial working memory information less efficiently and rapidly relative to controls.
All differences in brain activation were preserved during the 2‐back task, including decreased activation in the DLPFC, caudate and inferior parietal cortex. These findings suggest that TS subjects show deficits in patterns of brain activation during the high‐load working memory task that are independent of deficits in IQ. Together with our behavioral findings, these results suggest that activation deficits may reflect differences in the efficiency and rapidity of visuo‐spatial information processing rather than performance ability.
Our findings of both behavioral deficits and functional activation differences in the prefrontal cortex in TS provide direct information regarding neural pathways underlying cognitive dysfunction in TS. The largest brain activation differences occurred in the DLPFC (Talairach coordinates for Smith et al. [1996]: 33, 44, 20; −35, 28, 29), a region of the prefrontal cortex that has been implicated in executive functions underlying visuo‐spatial working memory [Courtney et al., 1996; Elliott and Dolan, 1998; Jonides et al., 1993; Smith et al., 1996]. Our results suggest that DLPFC deficits occur in both hemispheres. This is consistent with recent fMRI studies indicating that both hemispheres, rather than just the right hemisphere, are involved in visuo‐spatial working memory processing [Belger et al., 1998; Carlson et al., 1998; McCarthy et al., 1994, 1996]. Further, several PET and fMRI studies have linked activation in the DLPFC with visuo‐spatial working memory and have shown a correlation between activation increases and increased memory load [Courtney et al., 1996; Klingberg et al., 1997]. A previous study using an n‐back task similar to the task in our study reported robust activation in the MFG only during the 2‐back task [Carlson et al., 1998]. Consistent with these reports, we observed activation differences in the DLPFC between the TS and control subjects only during conditions of increased memory load.
In addition to the DLPFC deficits, TS subjects also showed significant deficits in activation in the left and right caudate head. The DLPFC has strong projections to the caudate head as revealed by neuroanatomical path tracing techniques [Selemon and Goldman‐Rakic, 1988]. White matter tracts from the DLPFC originate in Brodman Areas 9/10 and project primarily to the head of the caudate [Alexander et al., 1986, 1990]. Neurobehavioral deficits associated with fronto‐striatal lesions have been well described in Huntington disease (HD), a genetic disorder affecting primarily the caudate and its projections from the prefrontal cortex. The cognitive deficits observed include impairments in shifting sets and organizational strategies for completing neuropsychological tasks [Cummings, 1993]. Overall deficits in working memory in HD may contribute the characteristic impairment in skill learning in these patients [Lawrence et al., 1998]. Together with planning and attentional set shifting, working memory forms the basis of executive function. This triad of impairments often present in TS indicates that individuals with TS may have abnormalities in fronto‐striatal circuitry subserving working memory operations. Older studies examining the effect of caudate lesions on behavior and electrical caudate stimulation in monkeys have suggested the involvement of the caudate nucleus in visuo‐spatial working memory [Battig et al., 1960; Dean and Davis, 1959]. More recently, fMRI has been used to characterize the involvement of the caudate in spatial working memory [Postle and D'Esposito, 1999]. These studies suggest that the caudate nucleus may play a role in the integration of spatially coded mnemonic information with motor preparation to guide behavior. Unlike the prefrontal cortex, however, the caudate does not seem to be involved in working memory maintenance. Rather, the caudate may be involved in support processes necessary for working memory such as set shifting, rapid sensory discrimination or coordinating sensorimotor activity [Postle and D'Esposito, 1999]. Moreover, as Rolls [1994] has suggested, frontal‐caudate pathways may mediate the cognitive flexibility necessary to switch attention during complex cognitive tasks such as reorienting and recognizing changes in visual patterned stimuli. We hypothesize that some or all of these operations may be deficient in TS. Our results showing activation deficits in the caudate are consistent with and extend the finding of decreased left caudate volume in TS [Murphy et al., 1993] and suggest that frontal‐striatal dysfunction may underlie deficits in visuo‐spatial working memory.
TS subjects also showed significant activation differences bilaterally in the SMG of the inferior parietal lobe. A double dissociation in parietal activation was observed when TS subjects were directly compared with control subjects. That is, increased activation was observed in the 1‐back, whereas decreased activation was seen in the 2‐back task. These results show that the inferior parietal cortex activation observed in controls in the 2‐back task is similar to that observed in TS subjects during the 1‐back task. This suggests that TS subjects are unable to engage the parietal cortex adequately during conditions of higher working memory load.
In contrast to the observed activation deficits in the left and right SMG, no differences were observed in the superior parietal lobe, a region thought to be involved with spatial location processing [Owen et al., 1996]. These results extend previous findings of structural deficits in the parietal lobe [Murphy et al., 1993; Reiss et al., 1995]. Thus, although the TS subjects used in the present study were significantly deficient in spatial processing as measured by tasks such as the JLO and WCJ‐SR, the parietal cortex deficits observed during visuo‐spatial working memory processing do not seem to arise from dysfunction of the superior parietal cortex. Rather, the deficits seem to be related to deficits in spatial encoding and storage mechanisms that are involved in working memory, in which the SMG is known to play an important role [McCarthy et al., 1996; Smith et al., 1996]. Furthermore, our finding of both parietal and prefrontal cortex deficits in TS is consistent with neurophysiological findings of co‐activation in the parietal and prefrontal cortex during working memory [Friedman and Goldman‐Rakic, 1994; Mishkin et al., 1983; Ungerleider et al., 1998]. Therefore, it also is possible that deficits in integration of frontal and parietal circuits may underlie working memory deficits in TS.
Together these findings suggest that frontal‐parietal as well as the frontal‐striatal neural networks may be impaired in TS. Although early studies of neuropsychological function in TS postulated right hemisphere dysfunction related to spatial processing and relative sparing of left hemisphere processes such as verbal encoding [Money and Alexander, 1966; Silbert et al., 1977], the present study suggests that visuo‐spatial working memory deficits are bilateral. The extent to which the right hemisphere dysfunction predominates over left hemisphere dysfunction could not be assessed in the present study.
CONCLUSIONS
To our knowledge, this study is the first to use high‐resolution fMRI to examine cognitive brain function in individuals with TS. Behaviorally, we found that TS subjects had impaired performance during visuo‐spatial working memory. In conjunction, activation deficits were found bilaterally in the dorsolateral prefrontal, inferior parietal cortex and caudate. These results provide evidence for impairments in executive as well as the storage/retrieval operations underlying higher‐level cognition.
Future studies will focus on dissociating the various components of visuo‐spatial and executive dysfunction in TS and help provide valuable information about the role of × chromosome genes in the neurodevelopment of higher order cognitive function and brain structure. This information will ultimately serve to provide a framework from which the effectiveness of treatment studies can be assessed more accurately.
Acknowledgements
Support for this research was obtained from NIH grants HD3175 (NICHD/NIMH Human Brain Project) (A.L.R.), MH01142 (A.L.R.), MH50047 (A.L.R.) and M.I.N.D. Institute Grant K992247‐01. Additional funding was received from the Packard Foundation, and the Sinclair Foundation (A.L.R.) and the APA/Lilly Resident Research Award (M.F.H.).
REFERENCES
- Alexander GE, Crutcher MD, DeLong MR. 1990. Basal ganglia‐thalamocortical circuits: parallel substrates for motor, oculomotor, prefrontal and limbic functions. Prog Brain Res 85: 119–146. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. 1986. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357–381. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G. 1974. Working memory In: Bower GH, editor. The psychology of learning and motivation. New York: Academic Press; p 47–89. [Google Scholar]
- Battig K, Rosvold M, Mishkin M. 1960. Comparison of the effects of frontal and caudate lesions on delayed response and alternation in monkeys. J Comp Physiol Psychol 53: 400–404. [DOI] [PubMed] [Google Scholar]
- Belger A, Puce A, Krystal JH, Gore JC, Goldman‐Rakic P, McCarthy G. 1998. Dissociation of mnemonic and perceptual processes during spatial and nonspatial working memory using fMRI. Hum Brain Mapp 6: 14–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL, Sivan AB, Hamsher KD, Varney MR, Spreen O. 1994. Contributions to neuropsychological assessment: a clinical manual. New York: Oxford University Press. [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. 1997. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5: 49–62. [DOI] [PubMed] [Google Scholar]
- Carlson S, Martinkauppi S, Rama P, Salli E, Korvenoja A, Aronen HJ. 1998. Distribution of cortical activation during visuospatial n‐back tasks as revealed by functional magnetic resonance imaging. Cereb Cortex 8: 743–752. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, O'Craven K, Davidson RJ, Irwin W, Nelson CA, Noll DC, Hu X, Lowe MJ, Rosen BR, Truwitt CL, Turski PA. 1998. Reproducibility of fMRI results across four institutions using a spatial working memory task. Neuroimage 8: 249–261. [DOI] [PubMed] [Google Scholar]
- Clark C, Klonoff H, Hayden M. 1990. Regional cerebral glucose metabolism in Turner syndrome. Can J Neurol Sci 17: 140–144. [DOI] [PubMed]
- Cohen JD, MacWhinney B, Flatt M, Provost J. 1993. A new graphic interactive environment for designing psychology experiments. Behav Res Meth Instr Comp 25: 257–271. [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. 1997. Temporal dynamics of brain activation during a working memory task. Nature 386: 604–608. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. 1996. Object and spatial visual working memory activate separate neural systems in human cortex. Cereb Cortex 6: 39–49. [DOI] [PubMed] [Google Scholar]
- Cummings JL. 1993. Frontal‐subcortical circuits and human behavior. Arch Neurol 50: 873–880. [DOI] [PubMed] [Google Scholar]
- Dean WH, Davis GD. 1959. Behavior changes following caudate lesions in rhesus monkey. J Neurophysiol 22: 525–537. [DOI] [PubMed] [Google Scholar]
- Downey J, Ehrhardt AA, Gruen R, Bell JJ, Morishima A. 1989. Psychopathology and social functioning in women with Turner syndrome. J Nerv Ment Dis 177: 191–201. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ. 1998. Neural response during preference and memory judgments for subliminally presented stimuli: a functional neuroimaging study. J Neurosci 18: 4697–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HR, Goldman‐Rakic PS. 1994. Coactivation of prefrontal cortex and inferior parietal cortex in working memory tasks revealed by 2DG functional mapping in the rhesus monkey. J Neurosci 14: 2775–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiack RS, Turner R. 1995. Analysis of fMRI time‐series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Garron DC. 1977. Intelligence among persons with Turner's syndrome. Behav Genet 7: 105–127. [DOI] [PubMed] [Google Scholar]
- Glover GH, Lai S. 1998. Self‐navigated spiral fMRI: interleaved versus single‐shot. Magn Reson Med 39: 361–368. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic PS. 1994. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci 6: 348–357. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. 1998. Generalizability, random effects, and population inference. Neuroimage 7:S754. [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA. 1993. Spatial working memory in humans as revealed by PET [see comments]. Nature 363: 623–625. [DOI] [PubMed] [Google Scholar]
- Klingberg T, O'Sullivan BT, Roland PE. 1997. Bilateral activation of fronto‐parietal networks by incrementing demand in a working memory task. Cereb Cortex 7: 465–471. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Hodges JR, Rosser AE, Kershaw A, Constant C, Rubinsztein DC, Robbins TW, Sahakian BJ. 1998. Evidence for specific cognitive deficits in preclinical Huntington disease. Brain 121: 1329–1341. [DOI] [PubMed] [Google Scholar]
- Lippe B. 1990. Primary ovarian failure In: Kaplan SA, editor. Clinical pediatrics. Philadelphia: WB Saunders; p. 325–366 [Google Scholar]
- McCarthy G, Blamire AM, Puce A, Nobre AC, Bloch G, Hyder F, Goldman‐Rakic P, Shulman RG. 1994. Functional magnetic resonance imaging of human prefrontal cortex activation during a spatial working memory task. Proc Natl Acad Sci USA 91: 8690–8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, Goldman‐Rakic P. 1996. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cereb Cortex 6: 600–611. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA. 1983. Object vision and spatial vision: two cortical pathways. Trends Neurosci 6: 414–417. [Google Scholar]
- Money J, Alexander D. 1966. Turner syndrome: further demonstration of the presence of specific cognitional deficiencies. J Med Genet 3: 47–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DG, DeCarli CD, Daly E, Haxby JV, Allen G, White BJ, McIntosh AR, Powell CM, Horwitz B, Rapoport SI. 1993. X chromosome effects on female brain: a magnetic resonance imaging study of Turner syndrome. Lancet 342: 1197–1200. [DOI] [PubMed] [Google Scholar]
- Netley C, Rovet J. 1982. Atypical hemispheric lateralization in Turner syndrome subjects. Cortex 18: 377–384. [DOI] [PubMed] [Google Scholar]
- Owen AM, Milner B, Petrides M, Evans AC. 1996. Memory for object features versus memory for object location: a positron‐emission tomography study of encoding and retrieval processes. Proc Natl Acad Sci USA 93: 9212–9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington BF, Heaton RK, Karzmark P, Pendleton MG, Lehman R, Shucard DW. 1985. The neuropsychological phenotype in Turner syndrome. Cortex 21: 391–404. [DOI] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ. 1997. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage 5: 83–96. [DOI] [PubMed] [Google Scholar]
- Postle BR, D'Esposito M. 1999. Dissociation of human caudate nucleus activity in spatial and nonspatial working memory: an event‐related fMRI study. Brain Res Cogn Brain Res 8: 107–115. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Mazzocco MM, Greenlaw R, Freund LS, Ross JL. 1995. Neurodevelopmental effects of × monosomy: a volumetric imaging study. Ann Neurol 38: 731–738. [DOI] [PubMed] [Google Scholar]
- Rolls ET. 1994. Neurophysiology and cognitive functions of the striatum. Rev Neurol (Paris) 150: 648–660. [PubMed] [Google Scholar]
- Romans SM, Stefanatos G, Roeltgen DP, Kushner H, Ross JL. 1998. Transition to young adulthood in Ullrich‐Turner syndrome: neurodevelopmental changes. Am J Med Genet 79: 140–147. [PubMed] [Google Scholar]
- Rovet JF. 1993. The psychoeducational characteristics of children with Turner syndrome. J Learn Disabil 26: 333–341. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman‐Rakic PS. 1988. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci 8: 4049–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert A, Wolff PH, Lilienthal J. 1977. Spatial and temporal processing in patients with Turner syndrome. Behav Genet 7: 11–21. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. 1999. Storage and executive processes in the frontal lobes. Science 283: 1657–1661. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA. 1996. Dissociating verbal and spatial working memory using PET. Cereb Cortex 6: 11–20. [DOI] [PubMed] [Google Scholar]
- Talairach T, Tournoux P. 1988. Co‐planar stereotaxic atlas of the human brain: a 3‐dimensional proportional system, an approach to cerebral imaging. New York: Thieme Medical Publishers. [Google Scholar]
- Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, Birmaher V, Casey BJ. 1999. A developmental functional MRI study of spatial working memory. Neuroimage 10: 327–338. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Courtney SM, Haxby JV. 1998. A neural system for human visual working memory. Proc Natl Acad Sci USA 95: 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waber DP. 1979. Neuropsychological aspects of Turner syndrome. Dev Med Child Neurol 21: 58–70. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1991. Wechsler Intelligence Scale for Children‐Third Edition San Antonio: The Psychological Corporation (Harcourt Brace and Company). [Google Scholar]
- Wechsler D. 1997. Wechsler Adult Intelligence Scale‐Third Edition San Antonio: The Psychological Corporation (Harcourt Brace and Company). [Google Scholar]
- Woodcock R, NM. 1989. Woodcock‐Johnson tests of cognitive ability: standard and supplemental batteries. San Antonio: DLM Teaching Resources. [Google Scholar]


