Abstract
Verbal fluency and confrontation naming, two tests of word retrieval, are of great utility in the field of cognitive neuroscience. However, in the context of functional magnetic resonance imaging (fMRI), movement artefact has necessitated the use of covert paradigms, which has limited clinical application. We developed two overt fMRI paradigms that allowed for performance measurement and hence were appropriate for use with patient groups. The paradigms incorporated a blocked‐design and compressed‐acquisition methodology where cues were presented and responses made in a “silent” period allowing for performance measurement. The slow response pace was specifically designed for older and potentially cognitively impaired participants. Verbal fluency was associated with activation in the middle frontal gyrus (Brodmann areas 46 and 9), anterior cingulate gyrus and inferior frontal gyrus (area 44 and 45). Confrontation naming activated areas of the temporo‐occipital cortices (areas 18, 19, and 37) and the inferior frontal gyrus. The two paradigms successfully activated regions involved in executive and word retrieval processes and overcame the potential artefacts resulting from overt speech during image acquisition, providing useful neuropsychological tools to investigate cognitive deficits in clinical populations. Hum. Brain Mapping 20:29–40, 2003. © 2003 Wiley‐Liss, Inc.
Keywords: fMRI, executive functions, word retrieval, speech, patient assessment
INTRODUCTION
Verbal fluency and confrontation naming are two tests of word retrieval frequently employed in clinical and experimental neuropsychology to assess language abilities. In confrontation naming the participant is presented with a series of pictures of objects (ranging from high frequency to rare object names) which they are required to name, e.g., Boston Naming Test, [Kaplan et al., 1983]; Graded Naming Test, [McKenna and Warrington, 1983]. This procedure has typically been employed to assess dysnomia, which is commonly found in cerebrovascular disorders and neurodegenerative conditions [Margolin et al., 1990]. Impairments on this test have been directly related to extent of temporal lobe pathology in Alzheimer's disease [Wilson et al., 1996]. In tests of verbal fluency the participant is required to say or write as many words as they can beginning with a given letter of the alphabet in a limited period of time (e.g., Controlled Oral Word Association [Benton and Hamsher, 1976]; Thurstone's Word Fluency Test [Thurstone and Thurstone, 1962]). This procedure also involves processes of word retrieval but is characterised by rapid intrinsic word generation, where responses are minimally specified by external cues or triggers [Frith et al., 1991a,b]. In terms of Baddeley's working memory model, [Baddeley, 1986, 1996] verbal fluency places heavy demands on the central executive with the initiation of effective retrieval strategies to organise thinking and aid generation [Estes, 1974; Laine, 1988; Troyer et al., 1997] and the continual switching between retrieval strategies [Troyer et al., 1997]. Hence, in addition to the processes of word retrieval, verbal fluency also strongly engages executive processes and has been employed to measure executive dysfunction in clinical populations [Baddeley and Wilson, 1988]. This task is particularly disrupted by lesions to the frontal lobes and is sensitive to disorders such as focal epilepsy, tumours, traumatic brain injury [Baldo and Shimamura, 1998] and neurodegenerative disease including frontal lobe dementia and amyotrophic lateral sclerosis [Abrahams et al., 2000].
Functional magnetic resonance imaging (fMRI) paradigms of verbal fluency [e.g., Paulesu et al., 1997; Pujol et al., 1996; Schlosser et al., 1998; Smith et al., 1996] and confrontation naming [Smith et al., 1996; Spitzer et al., 1998, 1995; Votaw et al., 1999] have been developed, but typically have been restricted to covert responses due to the potential artefacts resulting from overt speech during image acquisition. Two studies have employed an overt methodology in their investigation of verbal fluency [Phelps et al., 1997; Yetkin et al., 1995]. However, in the study by Phelps et al. [1997], much data needed to be discarded due to movement artefact. In addition Yetkin et al. [1995] showed that overt word generation produced greater artefactual activation than covert procedures which, they suggested, was related to the increased facial movements needed to perform the task during image acquisition. Movement artefacts are not solely produced by facial movements, but also by movement of the tongue and soft palate and of the air. To minimize such movements Small et al. [1996] used a bite plate during word reading in two subjects, whereas Barch et al. [1999] avoided scanning the plane of the mouth and throat during a Stroop paradigm. Such designs clearly limit the applicability of this procedure.
Performance measurement is essential for the investigation of patient groups who may be impaired on such cognitive tasks. Speech output cannot be monitored during covert procedures and overt designs have the additional problem of noise interference from rapidly switching MR gradients. An acquisition methodology that overcomes these problems allows for the stimulus presentation and response to occur in a “silent” period in between image acquisitions. Variations of this procedure have been described in auditory activation studies (clustered volume acquisition [Edmister et al., 1999]; sparse imaging [Elliott et al., 1999; Hall et al., 1999] and in a finger movement study (behaviour interleaved gradients technique [Eden et al., 1999]). The potential application of such procedures to clinical populations, however, has as yet been unrecognised. The aim of this study was to develop two overt word retrieval functional imaging tasks appropriate for use with clinical groups, using both auditory and visual presentation (verbal fluency and confrontation naming). A similar methodology (compressed acquisition) with more complex cognitive procedures than has been employed previously was used. Both procedures were developed to ensure that overt responses could be monitored effectively and could accommodate an older, potentially impaired, participant group. In addition, such investigations in healthy control subjects are a valuable and necessary first step before clinical application to determine the cerebral regions underlying these distinct cognitive processes.
SUBJECTS AND METHODS
Participants
Eighteen right‐handed, healthy participants (14 men, 4 women), were recruited from advertisements placed in the local area and from voluntary organisations. Written informed consent was given by all participants in accordance with the declaration of Helsinki and the study was approved by the Bethlem and Maudsley NHS Trust/Institute of Psychiatry ethics committee. No participant had a history of neurological disorder, previous significant head injury, cerebrovascular disease, hypertension, or psychiatric disorder and none were taking psychoactive medication. The participants were between 39–76 years of age (mean age = 56.7 years, SD = 12.1), and the mean number of years of education was 13.9 (SD = 2.7). Intellectual ability was assessed using the National Adult Reading Test (NART) [Nelson and Willison, 1991] and the Raven's Standard Progressive Matrices (SPM) [Raven, 1958]. The mean NART predicted full scale IQ was 113.4 (SD = 8.9) and mean Raven's SPM score was 48.2 (SD = 9.3).
Image acquisition
Data were acquired using a 1.5 T GE Signa NV/i System (GE, Milwaukee, WI) at the Maudsley Hospital, London. Daily quality assurance was carried out to ensure high signal to ghost ratio, high signal to noise ratio and temporal stability using automated quality control procedures [Simmons et al., 1999]. A quadrature birdcage head coil was used for RF transmission and reception. One hundred T2*‐weighted echo planar images depicting BOLD contrast were acquired from 14 non‐contiguous planes parallel to the AC–PC plane (slice thickness = 7 mm, slice gap = 0.7 mm, TR = 6,000 msec, TE = 40 msec, θ = 90 degrees). A compressed pulse sequence was used where the data acquisition took place within the first 2 sec of each TR, with 4 sec during which the participant provided an overt response when there was no sound of the MR gradients. A high resolution inversion recovery echo planar image of the whole brain was also obtained (TE = 73 msec, TI = 180 msec, TR = 16,000 msec) for subsequent registration to the standard stereotactic space of Talairach and Tournoux [Talairach and Tournoux, 1998].
Experimental design
The two activation paradigms consisted of a periodic block design with alternating periods of baseline and experimental conditions for 60 sec each. Each condition was repeated five times across a 10‐min scanning schedule for each task. During the tasks the participant was presented with a stimulus cue every 6 sec and responded overtly with a single word in a 4‐sec quiet period. This was followed by 2 sec of compressed sequence acquisition.
Verbal fluency
The experimental condition consisted of letter‐based word generation in which the participant heard an auditory cue of a letter via headphones and responded overtly with a word beginning with that letter during the 4 sec period. On failure to generate an appropriate word the participant was required to say the word “PASS” during the quiet, 4 sec response time. A different letter (T, A, B, G, F) was presented for each 60‐sec block. To ensure that the participant correctly heard the cue, the first presentation of each block consisted of the letter followed by the corresponding word from the phonetic alphabet (e.g., “a for alpha”) during which they remained silent (Fig. 1). During the baseline condition the participant was cued by auditory presentation of the word “REST” which they were required to repeat in the 4‐sec period. The optimum rate of presentation of the letters in the experimental condition (one word every 6 sec) was established before the scanning investigation in a rigorous pilot study of paced verbal fluency in healthy controls (including older participants) and patients with degenerative disease. This slow rate of presentation was optimal for patients who performed poorly on standard tests of verbal fluency to perform the task successfully with few passes [Abrahams et al., 1996].
Figure 1.
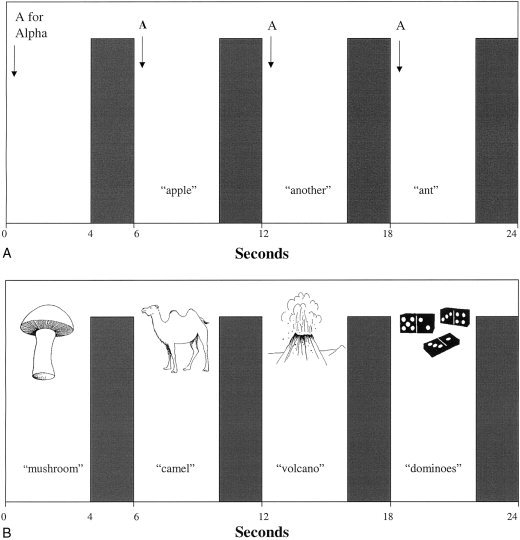
Schematic diagram of verbal fluency (above) and confrontation naming (below) paradigms. Participant is presented with stimulus cue and responds overtly during 4‐sec quiet period. This is followed by 2‐sec image acquisition.
Confrontation naming
In the experimental condition the participant was presented with a visual line drawing of an object for 4 sec and was required to say the correct name of the object during the response period (Fig. 1). Ten drawings were presented in each 60‐sec block. On failure to say an appropriate name the participant was required to say the word “PASS” during the 4‐sec response time. In the baseline condition the participant was presented with a meaningless fragmented picture and was required to say the word “REST”. The pictures were projected onto a screen located at the base of the scanning table. The participant viewed the screen via mirrors angled above their head in the scanner. The line drawings were selected from the Boston Naming Test [Kaplan et al., 1983] and were supplemented with pictures from the Snodgrass and Vanderwart [1980] series.
Immediately before the scanning session the participant underwent a practice verbal fluency and confrontation naming task using different stimuli, with simulated sound of the MR gradients. This familiarised them with the instructions and compressed acquisition scanning protocol and ensured that they were able to undertake the task with few passes and would only respond during the quiet periods.
Image analysis
Movement estimation and correction procedures as described by Friston et al. [1996] were first applied to the data. The data were then analysed by convolving the experimental design with two Poisson functions parameterising the haemodynamic delays of 4 and 8 sec [Friston et al., 1998]. The weighted sum of the two convolutions giving the best (least squares) fit to the time series at each voxel was computed and the sums of squares due to the fitted model and the residuals evaluated. The ratios of model/residual sum of squares were then evaluated for significance by comparison with a null distribution computed by repeating the fitting procedure ten times at each voxel after wavelet‐based random permutation of the time series. This non‐parametric procedure has been reliably validated for use with fMRI time series analyses [Bullmore et al., 2001]. Statistical testing at group level was carried out after transformation of the data into standard space [Brammer et al., 1997]. Median activation maps were computed at a voxel‐wise probability of a false activation of P < 0.005 and significant areas of in‐phase activation only are described below. Correlations between task performance and age and brain activation were calculated by fitting a linear regression model at each intracerebral voxel in standard space. Spatial statistics and permutation testing were used for inference [Bullmore et al., 2001; Sigmundsson et al., 2001].
RESULTS
Task performance during scanning
In the verbal fluency paradigm the number of different words produced (excluding passes) was recorded as a measure of task performance during scanning. All participants were clearly capable of performing the word generation task successfully and on average produced 42.6 (SD = 1.8) different words from a total of 45 possible responses. In the confrontation naming task the number of correct names was recorded and participants produced on average 45.2 (SD = 3.8) correct responses from a possible maximum of 50. A series of non‐parametric correlations (using Spearman's rank order correlations) were conducted between measures of task performance on both scanning paradigms and participant characteristics of age and intellectual ability as assessed by the NART and Raven's SPM. A significant correlation was revealed between the number of correct names in the confrontation naming procedure and age only (r = −0.796, P < 0.001), with older participants displaying poorer performance. This finding is consistent with normative data on these tasks, in which verbal fluency performance displays little evidence of decline in participants up to 80 years old, whereas confrontation naming scores decline particularly in older age groups [Spreen and Strauss, 1998].
BOLD changes in verbal fluency
The cortical regions of significant activation during the verbal fluency task are presented in Figure 2 and details of corresponding regions are presented in Table I. The areas of significant activation during the experimental condition include extensive regions of the left middle frontal gyrus (Brodmann areas 46 and 9), inferior frontal gyrus (area 44 and 45), anterior cingulate gyrus (areas 32 and 24) and medial prefrontal cortex (area 6). In addition small areas of bilateral frontal activation are also displayed. Correlational analyses of BOLD changes and task performance (number of correct responses) and age showed no significant results.
Figure 2.
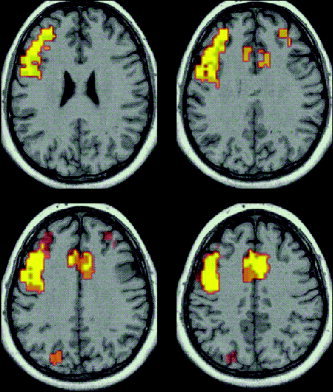
Generic brain activation map of verbal fluency. Areas of significant activation (P < 0.005) during word generation (highlighted). Axial slices parallel to the AC–PC plane are displayed for Talaraich z coordinates 22 mm, 27 mm (above) and 32 mm, 37 mm (below).
Table I.
Cortical regions of activation during overt verbal fluency
| Cerebral region (Brodmann Area) | Voxels (n) | Talairach coordinates (mm) | P < | ||
|---|---|---|---|---|---|
| x | y | z | |||
| L. Inferior frontal gyrus (44) | 60 | −46 | 0 | 26 | 0.0001 |
| L. Inferior frontal gyrus (44) | 53 | −46 | 7 | 31 | 0.0001 |
| L. Middle frontal gyrus (46) | 49 | −31 | 34 | 20 | 0.0001 |
| Anterior cingulate gyrus (24) | 43 | 4 | 7 | 37 | 0.0001 |
| L. Middle frontal gyrus (9) | 38 | −38 | 4 | 37 | 0.0001 |
| L. Anterior cingulate gyrus (24) | 31 | −4 | 0 | 42 | 0.0001 |
| R. Anterior cingulate gyrus (24) | 31 | 11 | 13 | 31 | 0.0001 |
| L. Medial frontal gyrus (6) | 30 | −4 | 0 | 48 | 0.0001 |
| L. Inferior frontal gyrus (45) | 28 | −42 | 26 | 15 | 0.0001 |
| L. Precentral gyrus (6) | 23 | −35 | −4 | 42 | 0.0001 |
| L. Precentral gyrus (4) | 16 | −35 | −17 | 48 | 0.0001 |
| Anterior thalamus (na) | 16 | −14 | −7 | 15 | 0.0001 |
| L. Insular cortex | 15 | −48 | 10 | 4 | 0.0001 |
| L. Inferior frontal gyrus (46) | 15 | −38 | 30 | 9 | 0.0001 |
| L. Anterior cingulate gyrus (32) | 14 | −7 | 23 | 26 | 0.0001 |
| L. Inferior frontal gyrus (47) | 10 | −42 | 17 | −2 | 0.0001 |
| L. Inferior frontal gyrus (47) | 10 | −38 | 20 | −7 | 0.0005 |
| R. Middle frontal gyrus (46) | 8 | 31 | 30 | 26 | 0.0001 |
| L. Inferior frontal gyrus (44) | 7 | −48 | 10 | 9 | 0.0001 |
| L. Lingular gyrus (18) | 7 | −4 | −70 | −7 | 0.0005 |
| L. Anterior thalamus | 6 | −14 | 0 | 9 | 0.001 |
| L. Cuneus (19) | 6 | −20 | −77 | 26 | 0.0001 |
BOLD changes in confrontation naming
The cortical regions of significant activation in confrontation naming are presented in Figure 3 with details of corresponding regions in Table II. The areas of significant in phase activation include the left inferior frontal gyrus (areas 44 and 45) and middle and inferior occipital gyri (areas 18 and 19) and inferior temporal gyrus (areas 19 and 37). Further analyses showed a significant positive correlation (P = 0.01) between BOLD changes and age in the anterior cingulate gyrus (area 32) and the middle frontal gyrus (area 8). In addition a significant positive correlation (P = 0.01) was revealed between BOLD changes and performance (number of correct responses) in the middle occipital gyrus (area 19) and the insula (Table III). Older participants produced greater activation in regions associated with attention and executive components of the task. A negative correlation emerged between age and task performance, however, indicating that despite greater activation in these regions older participants performed more poorly on the test.
Figure 3.
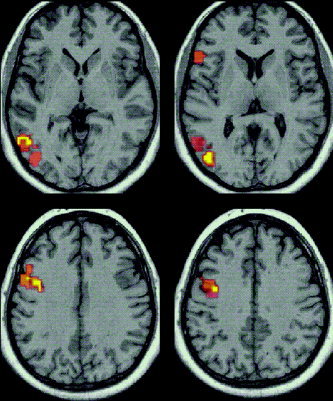
Generic brain activation map of confrontation naming. Areas of significant activation (P < 0.005) in object naming (highlighted). Contiguous axial slices parallel to the AC–PC plane are displayed for Talaraich z coordinates 4 mm, 9 mm (above) and 30 mm, 35 mm (below).
Table II.
Cortical regions of activation produced during overt confrontation naming
| Cerebral region (Brodmann Area) | Voxels (n) | Talairach coordinates (mm) | P < | ||
|---|---|---|---|---|---|
| x | y | z | |||
| L. Inferior frontal gyrus (44) | 17 | −46 | 10 | 26 | 0.0001 |
| L. Inferior temporal gyrus (19) | 12 | −46 | −60 | −2 | 0.0001 |
| L. Inferior frontal gyrus (44) | 11 | −48 | 4 | 20 | 0.0001 |
| L. Inferior frontal gyrus (44) | 10 | −35 | −7 | 31 | 0.0001 |
| L. Precentral gyrus (6) | 10 | −31 | −7 | 37 | 0.0001 |
| L. Precentral gyrus (6) | 8 | −35 | −7 | 42 | 0.0001 |
| L. Inferior temporal gyrus (37) | 8 | −42 | −56 | −7 | 0.0001 |
| R. Middle occipital gyrus (19) | 8 | 35 | −77 | 4 | 0.0001 |
| L. Inferior occipital gyrus (18) | 8 | −38 | −77 | −2 | 0.0001 |
| R. Inferior occipital gyrus (18) | 7 | 35 | −77 | −2 | 0.0001 |
| L. Inferior frontal gyrus (45) | 7 | −46 | 17 | 4 | 0.0001 |
| L. Middle occipital gyrus (19) | 6 | −35 | −77 | 4 | 0.0001 |
Table III.
Cortical regions of significant correlation during confrontation naming
| Cerebral region (Brodmann Area) | Voxels (n) | Talairach coordinates (mm) | ||
|---|---|---|---|---|
| x | y | z | ||
| Age | ||||
| Anterior cingulate gyrus (32) | 168 | 4 | 28 | 34 |
| Right middle frontal gyrus (8) | 104 | 28 | 24 | 43 |
| Task performance | ||||
| Left middle occipital gyrus (19) | 96 | −35 | −72 | −6 |
| Insula | 108 | −34 | −20 | −1 |
DISCUSSION
This study demonstrated two overt word retrieval fMRI activation paradigms with both auditory and visual stimulus presentation, using a blocked‐design compressed image acquisition methodology and slow paced response technique specifically designed for older and potentially cognitively impaired participants. Using this method this study overcame the potential artefacts resulting from overt speech during image acquisition. The paradigms successfully activated regions associated with word retrieval processes; verbal fluency activated regions of the prefrontal cortex corresponding to the executive demands of the task, whereas confrontation naming activated temporo‐occipital regions consistent with semantic processing of visual information.
The current paradigm was designed for an older and potentially cognitively impaired clinical population and hence the compressed block acquisition timing parameters provided 4 sec within which to make a verbal response, which with 2 sec of gradient noise gives a total TR of 6 sec. This longer TR was chosen after a pilot study in which patients with amyotrophic lateral sclerosis who are shown to be specifically impaired on verbal fluency [Abrahams et al., 2000] were able to conduct the task with a minimal number of passes. Alternative conventional block acquisition paradigms have typically used shorter TRs of 3 sec or less [e.g., Curtis et al., 1998; Paulesu et al., 1997; Smith et al., 1996; Votaw et al., 1999]. Increasing the TR to 6 sec increases the time within which longitudinal relaxation occurs and therefore increases the signal to noise ration (SNR). Increasing the TR also increases the total time of the experiment, however, which has the effect of reducing the SNR per unit time. Other authors have found little difference in practice between continuous and sparse blocked acquisitions [Edmister et al., 1999; Hall et al., 1999] due to the combination of these two effects.
In an attempt to overcome some of the previously described difficulties of overt responding in conventional uncompressed block design procedures [e.g., Yetkin et al., 1995] some studies have used event‐related fMRI to investigate speech production [Heim et al., 2002; Huang et al., 2001; Palmer et al., 2001]. These single trial designs involve an extended period after task performance during which the haemodynamic response is allowed to reach its peak and return to the baseline before another trial begins and an average haemodynamic response to the stimulus is obtained. This method can enable the dissociation of the haemodynamic response of interest from movement related signal changes that tend to occur during the actual performance of the task and have a sharper peak [Birn et al., 1999]. Huang et al. [2001] combined event‐related fMRI with motion reduction, detection and correction procedures to compare both overt and covert speech and noted that motion artefact was limited by using a training session before scanning. Palmer et al. [2001] also used an event related design to study overt and covert word stem completion and noted only minimal movement related artefact in the overt condition, which was primarily localised in the low brain region. Such event related designs (whether using a conventional or compressed acquisition sequence) can also be adapted to yield quiet periods within which a verbal response can be given. This includes silent event‐related (one‐time‐point) methods where a single set of images is acquired at approximately the peak of the haemodynamic response function and partially silent event‐related design as reviewed recently by Amaro et al. [2002]. In the present study we chose a block acquisition design for two reasons; firstly a blocked‐design is more appropriate for a letter‐based verbal fluency paradigm in which the cognitive activity of interest continues over an extended period and is not a discrete response to a stimulus presentation, secondly this design is more efficient in terms of SNR per unit time than an event‐related design. Other designs that use extended quiet periods (such as silent block designs where the silent gap is extended to 10 sec or more [Amaro et al., 2002]) would also be less efficient in terms of SNR per unit time. In terms of flexibility the compressed/sparse approach can be used for both block and event‐related designs. The analysis of these compressed block and event‐related designs is unchanged from conventional block and event‐related approaches. In this respect the approach is as flexible as other approaches but has clear practical application for clinical populations.
Activation of regions in verbal fluency
The verbal fluency task selectively activated extensive regions of the prefrontal cortex, including the middle frontal gyrus (Brodmann areas 46 and 9), which closely resembles the pattern of activation found in positron emission tomography (PET) studies using overt word generation procedures [Friston et al., 1991; Frith et al., 1991a,b]. The activation of these prefrontal regions in the verbal fluency task is consistent with the demands on executive functioning involved when performing verbal fluency. In this task the responses were minimally specified by the external cue and hence the participant is required to initiate extensive searches for the appropriate words. The results from previous fMRI studies have been more variable in relation to activation of this region, however, and this may be related to the methodologies employed. Covert self‐paced techniques may less reliably produce activation of this region with only some studies reporting activation in the middle frontal gyrus [Gaillard et al., 2000; Pujol et al., 1996; Yetkin et al., 1995], whereas in others activation has not extended beyond the inferior frontal gyrus (area 45) [Paulesu et al., 1997; Rueckert et al., 1994; Smith et al., 1996]. In the study undertaken by Smith et al. [1996] both verbal fluency and confrontation naming were compared and it was reported that the two patterns of activation did not differ in terms of involvement of the prefrontal cortex and did not extend into area 46. Such self‐paced designs are likely to place fewer demands on executive resources in comparison to paced methodologies in which the participant has to respond on the presentation of a cue, as used in the current investigation. Covert verbal fluency studies using a paced technique have demonstrated activation of areas 46 and 9 [Brammer et al., 1997; Curtis et al., 1998]. In the study reported by Phelps et al. [1997], however, a paced overt verbal fluency procedure was conducted but activation of area 46 and 9 was not found. In their task a more rapid speed of word generation was employed (one word every 3 sec) relative to the current study (one word every 6 sec). Frith and Grasby [1995] suggest that these regions of the prefrontal cortex may only be involved when the subject has to reflect upon what response they are going to make. The slower pace of word generation employed here may have allowed the participants greater time to undertake more extensive searches for appropriate words and initiate more effective retrieval strategies. It is clear from the task performance that the participants were able to perform the task successfully with relatively few passes (on average 42.6 different words generated from a possible total of 45). This slow paced design may have allowed participants more time to reflect upon possible responses and engage in executive processes required for successful performance. The present results are consistent with those reported recently by Blank et al. [2002] who compared activation patterns of prepositional and nonprepositional speech (neither of which activated the more dorsolateral regions of the prefrontal cortex) with verbal fluency data that reliably activated the left inferior and middle frontal gyrus. They concluded that executive and working memory processes account for most of the activation observed in verbal fluency and language processes are restricted to the caudal and left inferior frontal gyrus. They suggest this limits the usefulness of verbal fluency in the study of recovery from aphasia. This implies, however, that verbal fluency activates regions of the prefrontal cortex not involved in everyday speech, which highlights its utility in investigating executive dysfunction in the absence of aphasia, as it is currently employed within clinical neuropsychology.
The verbal fluency paradigm also activated regions of the inferior frontal gyrus known to be involved with word retrieval, phonological processing and language production, namely Broca's area (area 44 and associated region of area 45). This is consistent with previous functional MRI studies [Paulesu et al., 1997; Phelps et al., 1997; Smith et al., 1996]. Although the task was designed to ensure that the speech output components of the experimental and baseline conditions were matched, the baseline conditions involved the repetition of the same word and hence there may have been greater variation in overt articulatory mechanisms during the experimental conditions, producing relatively increased activation of the inferior frontal gyrus. The experimental and baseline conditions in the current study may also have differed in the amount of subvocal speech undertaken by the participants, particularly in the verbal fluency task. Letter‐based fluency paradigms encourage the processing and manipulation of phonological information that is rehearsed through subvocalisation in verbal working memory [Baddeley, 1996]. Functional imaging studies have also associated the subvocal rehearsal system of working memory and the covert retrieval of words with activation in Broca's area [e.g., Hinke et al., 1993; Paulesu et al., 1997].
The current findings from the verbal fluency task also demonstrated bilateral BOLD changes in the prefrontal cortex, which is consistent with our previous findings using overt verbal fluency in PET [Abrahams et al., 1996]. Such bilateral activation has also been demonstrated using other tasks requiring the intrinsic generation of responses that are not fully determined by the immediate task and hence place heavy demand on executive resources. These include the internal generation of joystick movements [Deiber et al., 1991] and freely chosen finger movements [Frith et al., 1991a; Hyder et al., 1997]. The pattern of activation in verbal fluency also included the anterior cingulate gyrus (areas 32 and 24) that is consistent with both fMRI [Phelps et al., 1997] and PET activation studies [Friston et al., 1991; Frith et al., 1991a,b]. Frith and Grasby [1995] suggest that this region may be activated when a person reflects upon what they are attending to and it has been suggested that increased activation is related to the attentional demands of the task [Raichle et al., 1994].
Activation of regions in confrontation naming
In the confrontation naming task increased activation was observed in left inferior temporal gyrus (Brodmann areas 19 and 37), and bilaterally in the middle and inferior occipital gyri (areas 19 and 18). The temporo‐occipital regions activated by this task have similarly been found in other functional imaging naming paradigms [Murtha et al., 1999; Smith et al., 1996; Zelkowicz et al., 1998] and have been suggested to be involved with semantic processing of the visual information. These regions form part of the occipito‐temporal ventral pathway involved in object recognition [Ungerleider and Mishkin, 1982]. Semantic processing of visual stimuli was not necessary in the baseline condition as the stimuli consisted of meaningless drawings.
Similar to the verbal fluency task the confrontation naming paradigm also activated regions of the inferior frontal gyrus namely Broca's area (area 44 and associated region of area 45). This is consistent with previous functional MRI studies of confrontation naming [Smith et al., 1996; Spitzer et al., 1998; Votaw et al., 1999]. It has been suggested that the inferior frontal gyrus is functionally heterogeneous, the more posterior region (including area 44) is thought to be associated with phonological and articulatory processes, whereas the more anterior region (including areas 45/47) with semantic processing [Gabrieli et al., 1998; Paulesu et al., 1997; Poldrack et al., 1999; Thompson‐Schill et al., 1997; Wagner et al., 2001]. Paulesu et al. [1997] noted that area 45 was selectively activated during semantic fluency but not during a letter based fluency procedure. Poldrack et al. [1999] demonstrated that the anterior/ventral portion (BA 47/45) was more active during semantic as compared to the phonological processing of words, whereas a more posterior/dorsal region (BA 44/45) was active in relation to both semantic and phonological processing. Previous studies have supported the hypothesis of left prefrontal cortex involvement in semantic processing and retrieval [Kapur et al., 1994; Nyberg et al., 1996; Petersen and Fiez, 1993], but there is now some debate as to which aspect of semantic processing is involved. Thompson‐Schill et al. [1997, 1998, 1999] suggested that it was not simply the retrieval of semantic information that was related to the left inferior frontal gyrus, but it was more specifically the selection of information among competing alternatives from semantic memory. Activation was associated with the selection demands of the tasks in which certain semantic features had to be selected and others ignored. However, the activated region was in the posterior inferior frontal gyrus (BA 44/45), an area that was thought to be more related to phonological processing. In contrast, Wagner et al. [2001] argued that the left inferior frontal cortex guides controlled semantic retrieval irrespective of whether retrieval requires selection against competing representations. Controlled retrieval is required when the association between the stimulus and response is weak and cannot be processed automatically. This hypothesis is supported by the demonstration of experiments in which activation of this region increases with semantic retrieval demands and the level of control required when the selection demands are held constant. Wagner et al. [2001] also noted that it was the anterior aspect (47/45) that was particularly associated with the controlled retrieval of semantic knowledge, which is more consistent with earlier observations. The concept of controlled retrieval has strong similarities with Norman and Shallice's supervisory attentional system associated with prefrontal lobe functions [1986]. Similarly it has also been suggested that the left inferior prefrontal cortex may serve as a semantic working memory system or semantic executive system, which accesses, maintains and manipulates semantic representations that are stored elsewhere [Gabrieli et al., 1998; Poldrack et al., 1999]. In relation to the present study, the activation of a semantic executive system may be particularly pertinent to verbal fluency in which although phonological processing is predominantly encouraged by the letter‐based design, semantic strategies for word generation can also be employed. Words may be retrieved on the basis of semantic relationships, which encourages the search of semantic memory. In confrontation naming, although word retrieval of high frequency names may involve more automatic processes, the naming of more difficult items (as found in the Boston Naming Test, employed in the current design) may involve a more extensive search of semantic memory and hence the involvement of a semantic executive system.
CONCLUSIONS
The overt verbal fluency and confrontation naming paradigms developed in this study successfully produced activation in cerebral regions corresponding to word retrieval processes and avoided the potential artefact resulting from overt speech during image acquisition. The slow paced design of these tasks is particularly appropriate for application to an older and potentially impaired population. These procedures are potentially applicable to the investigation of patients with acquired brain injury, such as resulting from cerebrovascular accident, or neurodegenerative disease such as amyotrophic lateral sclerosis or the initial stages of dementia of the frontal lobe type.
Acknowledgements
We thank the neuroimaging staff at the MRI Unit, Maudsley Hospital for their help in this study. We also thank V. Curtis and E. Amaro for their assistance with this study. Dr. S. Abrahams was funded by a Wellcome Fellowship during the period of this research.
REFERENCES
- Abrahams S, Goldstein LH, Kew JJ, Brooks DJ, Lloyd CM, Frith CD, Leigh PN (1996): Frontal lobe dysfunction in amyotrophic lateral sclerosis. A PET study. Brain 119: 2105–2120. [DOI] [PubMed] [Google Scholar]
- Abrahams S, Leigh PN, Harvey A, Vythelingum GN, Grise D, Goldstein LH (2000): Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia 38: 734–747. [DOI] [PubMed] [Google Scholar]
- Amaro E Jr, William SC, Shergill SS, Fu CHY, MacSweeney M, Picchoini MM, Brammer MJ, McGuire PK (2002): Acoustic noise and functional magnetic resonance imaging current strategies and future prospects. J Magn Reson 16: 497–510. [DOI] [PubMed] [Google Scholar]
- Baddeley AD (1986): Working memory. London: Oxford University Press. [Google Scholar]
- Baddeley AD (1996): Exploring the central executive. Q J Exp Psychol 49A: 5–28. [Google Scholar]
- Baddeley AD, Wilson BA (1988): Frontal amnesia and the dysexecutive syndrome. Brain Cognit 7: 212–230. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP (1998): Letter and category fluency in patients with frontal lobe lesions. Neuropsychology 12: 259–267. [DOI] [PubMed] [Google Scholar]
- Barch DM, Sabb FW, Carter CS, Bravewr TS, Noll DC, Cohen JD (1999): Overt verbal responding during fMRI scanning: empirical investigations of problems and potential solutions. Neuroimage 10: 642–657. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher KS (1976): Multilingual aphasia examination. Iowa City: University of Iowa Press. [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Shaker R (1999): Event‐related fMRI of tasks involving brief motion. Hum Brain Mapp 7: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank SC, Scott SK, Murphy K, Warburton E, Wise RJS (2002): Speech production: Wernicke, Broca and beyond. Brain 125: 1829–1838. [DOI] [PubMed] [Google Scholar]
- Brammer MJ, Bullmore ET, Simmons A, Williams SC, Grasby PM, Howard RJ, Woodruff PW, Rabe‐Hesketh S (1997): Generic brain activation mapping in functional magnetic resonance imaging: a nonparametric approach. J Magn Reson 15: 763–770. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Long C, Suckling J, Fadili J, Calvert G, Zelaya F, Carpenter TA, Brammer M (2001): Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum Brain Mapp 12: 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis VA, Bullmore ET, Brammer MJ, Wright IC, Williams SC, Morris RG, Sharma TS, Murray RM, McGuire PK (1998): Attenuated frontal activation during a verbal fluency task in patients with schizophrenia. Am J Psychiatry 155: 1056–1063. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS (1991): Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res 84: 393–402. [DOI] [PubMed] [Google Scholar]
- Eden GF, Joseph JE, Brown HE, Brown CP, Zeffiro TA (1999): Utilizing hemodynamic delay and dispersion to detect fMRI signal change without auditory interference: the behavior interleaved gradients technique. Magn Reson Med 41: 13–20. [DOI] [PubMed] [Google Scholar]
- Edmister WB, Talavage TM, Ledden PJ, Weisskoff RM (1999): Improved auditory cortex imaging using clustered volume acquisitions. Hum Brain Mapp 7: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Bowtell RW, Morris PG (1999): The effect of scanner sound in visual, motor, and auditory functional MRI. Magn Reson Med 41: 1230–1235. [DOI] [PubMed] [Google Scholar]
- Estes WK. 1974. Learning theory and intelligence. Am Psychol 29: 740–749. [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS (1991): Investigating a network model of word generation with positron emission tomography. Proc R Soc Lond B Biol Sci 244: 101–106. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Josephs O, Rees G, Turner R (1998): Nonlinear event‐related responses in fMRI. Magn Reson Med 39: 41–52. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R (1996): Movement‐related effects in fMRI time‐series. Magn Reson Med 35: 346–355. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston K, Liddle PF, Frackowiak RSJ (1991a): Willed action and the prefrontal cortex in man: a study with PET. Proc R Soc Lond B Biol Sci 244: 241–246. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston TKJ, Liddle PF, Frackowiak RSJ (1991b): A PET study of word finding. Neuropsychologia 29: 1137–1148. [DOI] [PubMed] [Google Scholar]
- Frith CD, Grasby PM (1995): rCBF studies of prefrontal function and their relevance to psychosis In: Boller F, Graffman J, editors. Handbook of neuropsychology Vol 10 Amsterdam: Elsevier; p 383–403. [Google Scholar]
- Gabrieli JD, Poldrack RA, Desmond JE (1998): The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci U S A 95: 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, Hertz‐Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH (2000): Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology 54: 180–185. [DOI] [PubMed] [Google Scholar]
- Hall DA, Haggard MP, Akeroyd MA, Palmer AR, Summerfield AQ, Elliott MR, Gurney EM, Bowtell RW (1999): “Sparse” temporal sampling in auditory fMRI. Hum Brain Mapp 7: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S, Opitz B, Friederici AD (2002): Broca's area in the human brain is involved in the selection of grammatical gender for language production: evidence from event‐related functional magnetic resonance imaging. Neurosci Lett 328: 101–104. [DOI] [PubMed] [Google Scholar]
- Hinke RM, Hu X, Stillman AE, Kim SG, Merkle H, Salmi R, Ugurbil K (1993): Functional magnetic resonance imaging of Broca's area during internal speech. Neuroreport 4: 675–678. [DOI] [PubMed] [Google Scholar]
- Huang J, Carr TH, Cao Y (2001): Comparing cortical activations for silent and overt speech using event‐related fMRI. Hum Brain Mapp 15: 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Phelps EA, Wiggins CJ, Labar KS, Blamire AM, Shulman RG (1997): “Willed action”: a functional MRI study of the human prefrontal cortex during a sensorimotor task. Proc Natl Acad Sci U S A 94: 6989–6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S (1983): The Boston naming test. Philadelphia: Lea and Febiger. [Google Scholar]
- Kapur S, Rose R, Liddle PF, Zipursky RB, Brown GM, Stuss D, Houle S, Tulving E (1994): The role of the left prefrontal cortex in verbal processing: semantic processing or willed action? Neuroreport 5: 2193–2196. [DOI] [PubMed] [Google Scholar]
- Laine M (1988): Correlates of word fluency performance In: Koivuselka‐Sallinen P, Sarajarvi L, editors. Studies in languages Vol 12 Joensuu, Finland: University of Joensuu, Faculty of Arts. [Google Scholar]
- Margolin DI, Pate DS, Friedrich FJ, Elia E (1990): Dysnomia in dementia and in stroke patients: different underlying cognitive deficits. J Clin Exp Neuropsychol 12: 597–612. [DOI] [PubMed] [Google Scholar]
- McKenna P, Warrington EK (1983): The graded naming test. Oxford: NFER‐Nelson. [Google Scholar]
- Murtha S, Chertkow H, Beauregard M, Evans A (1999): The neural substrate of picture naming. J Cognit Neurosci 11: 399–423. [DOI] [PubMed] [Google Scholar]
- Nelson HE, Willison JR (1991): Restandardisation of the NART against the WAIS‐R. Windsor: NFER‐Nelson. [Google Scholar]
- Norman DA, Shallice T (1986): Attention to action: willed and automatic control of behaviour In: Schwartz GE, Shapiro D, editors. Consciousness and self‐regulation Vol 4 New York: Plenum Press. [Google Scholar]
- Nyberg L, Cabeza R, Tulving E (1996): PET studies of encoding and retrieval: the HERA model. Psychon Bull Rev 3: 135–148. [DOI] [PubMed] [Google Scholar]
- Palmer ED, Rosen HJ, Ojemann JG, Buckner RL, Kelley WM, Petersen SE (2001): An event‐related fMRI study of overt and covert word stem completion. Neuroimage 14: 182–193. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Goldacre B, Scifo P, Cappa SF, Gilardi MC, Castiglioni I, Perani D, Fazio F (1997): Functional heterogeneity of left inferior frontal cortex as revealed by fMRI. Neuroreport 8: 2011–2017. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fiez JA (1993): The processing of single words studied with positron emission tomography. Ann Rev Neurosci 16: 509–530. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Hyder F, Blamire AM, Shulman RG (1997): FMRI of the prefrontal cortex during overt verbal fluency. Neuroreport 8: 561–565. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD (1999): Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage 10: 15–35. [DOI] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Deus J, Kulisevsky J, Marti‐Vilalta JL, Garcia C, Junque C, Capdevila A (1996): Frontal lobe activation during word generation studied by functional MRI. Acta Neurol Scand 93: 403–410. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod AM, Pardo JV, Fox PT, Petersen SE (1994): Practice‐related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex 4: 8–26. [DOI] [PubMed] [Google Scholar]
- Raven JC (1958): Guide to standard progressive matrices. New York: Psychological Corporation. [Google Scholar]
- Rueckert L, Appollonio I, Grafman J, Jezzard P, Johnson R Jr, Le Bihan D, Turner R (1994): Magnetic resonance imaging functional activation of left frontal cortex during covert word production. J Neuroimaging 4: 67–70. [DOI] [PubMed] [Google Scholar]
- Schlosser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, Dewey SL, Brodie JD (1998): Functional magnetic resonance imaging of human brain activity in a verbal fluency task. J Neurol Neurosurg Psychiatr 64: 492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmundsson T, Suckling J, Maier M, Williams SCR, Bullmore ET, Greenwood KE, Fukuda R, Ron MA, Toone BK (2001): Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry 158: 234–243. [DOI] [PubMed] [Google Scholar]
- Simmons A, Moore E, Williams SC (1999): Quality control for functional magnetic resonance imaging using automated data analysis and Shewhart charting. Magn Reson Med 41: 1274–1278. [DOI] [PubMed] [Google Scholar]
- Small SL, Noll DC, Perfetti CA, Hlustik P, Wellington R, Schneider W (1996): Localizing the lexicon for reading aloud: Replication of a PET study using fMRI. Neuroreport 7: 961–965. [DOI] [PubMed] [Google Scholar]
- Smith CD, Andersen AH, Chen Q, Blonder LX, Kirsch JE, Avison MJ (1996): Cortical activation in confrontation naming. Neuroreport 7: 781–785. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M (1980): A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learn 6: 174–215. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Kischka U, Guckel F, Bellemann ME, Kammer T, Seyyedi S, Weisbrod M, Schwartz A, Brix G (1998): Functional magnetic resonance imaging of category‐specific cortical activation: evidence for semantic maps. Cogn Brain Res 6: 309–319. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Kwong KK, Kennedy W, Rosen BR, Belliveau JW (1995): Category‐specific brain activation in fMRI during picture naming. Neuroreport 6: 2109–2112. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E (1998): A compendium of neuropsychological tests. Oxford: Oxford University Press. [Google Scholar]
- Talairach J, Tournoux P (1998): Co‐planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers. [Google Scholar]
- Thompson SL, D'Esposito M, Kan IP (1999): Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron 23: 513–522. [DOI] [PubMed] [Google Scholar]
- Thompson‐Schill SL, Esposito MD, Aguirre GK, Farah MJ (1997): Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci U S A 94: 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson‐Schill SL, Swick D, Farah MJ, D'Esposito M, Kan IP (1998): Verb generation in patients with focal frontal lesions A neuropsychological test of neuroimaging findings. Proc Natl Acad Sci U S A 95: 15855–15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurstone LL, Thurstone TG (1962): Primary mental abilities. Chicago: Science Research Associates. [PubMed] [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G (1997): Clustering and switching as two components of verbal fluency: evidence from younger and older healthy adults. Neuropsychology 11: 138–146. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M (1982): Two cortical pathways In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behaviour. Cambridge: MIT Press; p 549–586. [Google Scholar]
- Votaw JR, Faber TL, Popp CA, Henry TR, Trudeau JD, Woodard JL, Mao H, Hoffman JM, Song AW (1999): A confrontational naming task produces congruent increases and decreases in PET and fMRI. Neuroimage 10: 347–356. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare‐Blagoev EJ, Clark J, Poldrack RA (2001): Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron 31: 329–338. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Sullivan M, deToledo‐Morrell L, Stebbins GT, Bennett DA, Morrell F (1996): Association of memory and cognition in Alzheimer's disease with volumetric estimates of temporal lobe structures. Neuropsychology 10: 459–463. [Google Scholar]
- Yetkin FZ, Hammeke TA, Swanson SJ, Morris GL, Mueller WM, McAuliffe TL, Haughton VM (1995): A comparison of functional MR activation patterns during silent and audible language tasks. Am J Neuroradiol 16: 1087–1092. [PMC free article] [PubMed] [Google Scholar]
- Zelkowicz BJ, Herbster AN, Nebes RD, Mintun MA, Becker JT (1998): An examination of regional cerebral blood flow during object naming tasks. J Int Neuropsychol Soc 4: 160–166. [DOI] [PubMed] [Google Scholar]


