Abstract
There is a large body of psychological and neuroimaging experiments that have interpreted their findings in favor of a functional equivalence between action generation, action simulation, action verbalization, and perception of action. On the basis of these data, the concept of shared motor representations has been proposed. Indeed several authors have argued that our capacity to understand other people's behavior and to attribute intention or beliefs to others is rooted in a neural, most likely distributed, execution/observation mechanism. Recent neuroimaging studies have explored the neural network engaged during motor execution, simulation, verbalization, and observation. The focus of this meta‐analysis is to evaluate in specific detail to what extent the activated foci elicited by these studies overlap. Hum. Brain Mapping 12:1–19, 2001. © 2001 Wiley‐Liss, Inc.
Keywords: activation studies, action generation, simulation, verbalization, observation, human
INTRODUCTION
Action is the means by which the self interacts or reacts with the external world. An action can be described as the final expression of several information‐processing stages: intention, programming, preparation, and execution. It is widely accepted that goal‐directed action is internally generated, and therefore its generation involves motor representation [Jeannerod, 1997].
There is a growing number of studies that have strengthened the idea that there is, to some degree, a functional equivalence between intending, simulating, observing, and performing an action (for a critical review, see Decety and Grèzes, 1999). On the basis of these data the concept of shared motor representations has been proposed [Gallese and Goldman, 1998]. A similar concept has been proposed by developmental psychologists to account for an innate system for coupling the perception and production of human acts [Meltzoff and Moore, 1997]. It has been argued that an action representation system is of considerable value to a social species in interpreting the actions and intentions of other members of the group [Annett, 1996; Meltzoff, 1999].
Furthermore, some authors have emphasized the possibility that language originates in manual gestures rather than in vocalization [Corballis, 1998; McNeilage, 1998]. According to Corballis [1992], the acquisition of the bipedy during the evolution of the early hominids has freed the hands and arms from primary involvement in locomotion and lent the upper body a new potential for expressive communication. Moreover, the concentration of areas specialized for language in the same hemisphere of the cerebral cortex as controlling the hand that is preferred for precise manipulative tasks may demonstrate the intimate connection between the two functions [Kimura and Archibald, 1974; Dawson et al., 1985]. Rizzolatti et al. [1996a] have proposed that Broca's area in the human left frontal cortex is homologous with an area in the monkey's ventral premotor cortex (F5 region according to Matelli et al.'s nomenclature [1985]) where neurons have been found to respond both to the production of visually guided actions and to the visual perception of the corresponding actions made by others. The neurological evidence does not clearly support a visuo‐manual over an audio‐oral evolutionary basis for language, but it might support a close relation between the two [Corballis, 1992].
The idea of a functional equivalence between representations involved in action execution, simulation, observation, and possibly even verbalization, offers a parsimonious explanation of the cognitive mechanisms that may be the basis for, or a precursor of, interpersonal mind‐reading. There are now quite a few neuroimaging studies that have explored the neural network underlying action execution, action simulation, action verb generation, and action observation. The goal of this meta‐analysis is to evaluate the equivalence at a structural level (i.e., anatomical) between these different cognitive states by means of a detailed meta‐analysis of neuroimaging activation studies. We believe that the structural level constrains the functional level. It is therefore important to bridge these two levels.
HUMAN FUNCTIONAL ANATOMY
Scope of the meta‐analysis
(1) Only studies performed with healthy volunteers were included; (2) We concentrated on cerebral blood flow studies measuring activity in the whole brain and included only one regions‐of‐interest (ROIs) study; (3) Our review is limited to those PET or fMRI studies that provide Talairach or MNI coordinates of activation foci. This choice was directed by the need for precise localization beyond global designation like “precentral gyrus”; (4) We focused on studies using the image subtraction method, which has until now been the standard method of cognitive PET studies; (5) The experimental conditions were marshaled into distinct categories (i.e., execution, simulation, observation, and verbalization). The selection of the experiments was based on the fact that they were close in terms of their target cognitive process. We have considered motor execution when tasks involved goal‐directed hand movements; mental simulation when hand movements were involved; action observation when hand movements were being watched; and verbalization when action verb generation associated with a manipulable object was involved.
Execution of action
There is a large body of experiments that were designed to evaluate rCBF changes related to movement execution such as grasping objects [Grafton et al., 1996a; Matsumura et al., 1996], complex manipulation of objects [Binkofski et al., 1999], joystick movements [Stephan et al., 1995; Jenkins et al., 1994], execution of sequential finger movements [Sadato et al., 1996; Catalan et al., 1998], or immediate imitation of hand movements [Krams et al., 1998]. Throughout these studies, rCBF increases were consistently detected in the primary motor cortex, premotor cortex, supplementary motor area, cingulate gyrus, cerebellum, and inferior and superior parietal lobes.
Mental simulation of action
Mental simulation of action, defined as mental rehearsal of a motor act without performing any overt movement, implies that the subject imagines himself performing a given action. In several PET studies, subjects were asked to imagine grasping a visually presented object [Decety et al., 1994; Grafton et al., 1996b; Grèzes and Decety, 2000], which correspond to externally guided tasks. Whereas, in some others, subjects were requested to imagine moving a joystick corresponding to an internally guided task [e.g., Stephan et al., 1995]. However, all these experiments referred to explicit motor imagery and are associated with rCBF increases in the dorsolateral prefrontal cortex, precentral gyrus, SMA, inferior parietal lobe, cingulate gyrus, subcortical nuclei, and cerebellum. These regions have also been detected during implicit motor imagery, which correspond to the covert access to motor representation. Parsons and Fox [1998] have demonstrated that the recognition of handedness of a visually presented hand depends on covert recruitment of sensorimotor processes, which are constrained by the neural structures controlling the side of the hand to be recognized. The study performed by Krams et al. [1998], concerning motor preparation, was included in this section, for the authors have considered that this very condition requires the imagination of movement.
The above‐mentioned cortical and subcortical areas found to be activated during motor imagery, namely the primary motor cortex, premotor cortex, SMA, anterior cingulate cortex, parietal lobule, and cerebellum, pertain to the neural network known to be involved during action execution.
Observation of action
In a first study, Bonda et al. [1996] measured cerebral metabolic activity in human subjects by PET during the perception of simulation of goal‐directed hand action and whole‐body movements with point light displays. In a second study, subjects were requested either to observe grasping movements of common objects performed by an experimenter, or to reach and grasp the same objects. The reference condition consisted on the observation of objects [Rizzolatti et al., 1996b]. A third related study compared the observation with the mental simulation of grasping movements [Grafton et al., 1996b]. Decety and colleagues have studied the observation of pantomimes of action, in which objects were only suggested. In a first study, subjects were asked to observe those pantomimes either to recognize or to imitate them later [Decety et al., 1997]. In a second study, subjects were asked to observe pantomimes either with no aim or with the aim to imitate them later. The reference condition consisted of the observation of stationary hand positions [Grèzes et al., 1998]. Finally, Perani et al. [2000] have studied the perception of grasping actions of geometrical objects made by real hand or by a different quality of 3D, virtual reality, hand reconstruction.
All above‐mentioned studies have reported rCBF increases during the perception of goal‐directed hand movements have been detected in the premotor cortex, middle temporal gyrus, inferior and middle frontal gyri, and parietal cortex in the left hemisphere.
Finally, when subjects were requested to observe in order to imitate later, activations were detected in the occipito‐parietal pathway extending to the premotor areas in both hemispheres. rCBF increases were also detected in the SMA, cingulate gyrus, and middle frontal gyrus. These regions are known to be involved during motor preparation and motor programming [Decety, 1996; Passingham, 1996].
Verbalization of action
Knowledge about the use of objects and tools can be based either on the retrieval of instructions of use from semantic memory or on a direct inference of function from their structure. Sensorimotor experience may have a crucial role in processing information to gain access to semantic knowledge for certain class of objects. For instance, the category of man‐made tools are strongly associated with actions they afford [Tucker and Ellis, 1998]. Neuropsychological observations have revealed dissociations between the impaired recognition of living things and the normal recognition of man‐made objects [Warrington and McCarthy, 1987; Silveri and Gainotti, 1988]. Moreover, neuroimaging studies by Martin et al. [1996] and Perani et al. [1999] demonstrated that the recognition of living and nonliving or man‐made objects rely on distinct neural regions. These results further suggest that man‐made items are partially identified by their functional significance, while animate items are identified by their physical attributes as already suggested by Rosch et al. [1976]. Martin et al. [1995, 1996] have shown that the attributes defining an object are represented close to the cortical regions that mediate its perception. The authors have suggested that object knowledge is stored as a distributed network, and that the location of these sites are not randomly distributed but rather mirror the organization of sensory and motor systems.
Several neuroimaging studies have explored the neural basis associated to silent naming of tools or to the generation of the verb associated with the use of an object, based on visual stimuli [Martin et al., 1995, 1996; Grafton et al., 1997; Perani et al., 1999; Grabowski et al., 1998] or on auditory stimuli [Warbuton et al., 1996]. Silent verbalization is consistently associated with increased activity located in the inferior frontal gyrus, corresponding to Broca's areas and in the middle and inferior temporal gyri. In addition, activations are found in the ventral premotor cortex and sometimes in the parietal lobule [Martin et al., 1995, 1996; Grafton et al., 1997; Grabowski et al., 1998]. The former result may be related to “pragmatic” processing, i.e. motor representations of how objects are used which are built in the dorsal stream and its connections to premotor areas. However, it may also be explained to be related to naming [Grafton et al., 1997].
Anatomical description
All the studies that are included in the meta‐analysis are presented in Table I. The relevant references are listed alphabetically and numbered consecutively according to each category. For sake of clarity, foci related to execution, simulation, observation, and verbalization are represented with four different colors.
Table I.
List of neuroimaging studies included in the Meta‐analysis
| Execution (yellow) | ||
| 1 | Binkofski et al. (1999) | Complex manipulation of objects versus sphere manipulation |
| 2 | Catalan et al. (1998) | Sequential finger movements versus rest |
| 3 | Grafton et al. (1996a) | Grasping versus observation of targets |
| 4 | Jenkins et al. (1994) | Sequence of key‐presses versus rest |
| 5 | Krams et al. (1998) | Execution versus observation |
| 6 | Matsumura et al. (1996) | Grasping versus reaching |
| 7 | Sadato et al. (1996) | Sequential finger movements versus rest |
| 8 | Stephan et al. (1995) | Movement execution versus preparation |
| Simulation (green) | ||
| 1 | Decety et al. (1994) | Motor simulation of grasping objects versus observing of moving hands |
| 2 | Grafton et al. (1996b) | Motor simulation of grasping objects versus observing objects |
| 3 | Grèzes and Decety (2001) | Motor simulation of grasping objects versus observing non‐objects |
| 4 | Krams et al. (1998) | Motor preparation versus observation |
| 5 | Parsons et al. (1995) | Left‐right judgements of visually presented hands versus rest |
| 6 | Stephan et al. (1995) | Motor simulation versus motor preparation |
| Observation (blue) | ||
| 1 | Bonda et al. (1996) | Hand action to recognize versus body movements |
| 2 | Decety et al. (1997) | Meaningful hand actions to recognize versus meaningless actions |
| 3 | Grafton et al. (1996b) | Grasping observation versus observation of objects |
| 4 | Grèzes et al. (1998) | Meaningful hand actions to imitate versus meaningful actions |
| 5 | Grèzes et al. (1998) | Meaningful hand actions to imitate versus static hands |
| 6 | Grèzes et al. (1998) | Meaningful hand actions versus static hands |
| 7 | Perani et al. (2001) | Grasping observation versus observation of geometrical objects |
| 8 | Rizzolatti et al. (1996b) | Grasping observation versus observation of objects |
| Verbalization (red) | ||
| 1 | Grabowski et al. (1998) | Verb generation versus observation of unfamiliar faces |
| 2 | Grafton et al. (1997) | Verb generation versus object observation |
| 3 | Grèzes et Decety (2000) | Verb generation versus observation of non‐objects |
| 4 | Martin et al. (1995) | Verb generation versus objects naming |
| 5 | Martin et al. (1996) | Tools naming versus animals naming |
| 6 | Perani et al. (1999) | Non‐living entities discrimination versus meaningless shape or living entities |
| 7 | Tatsumi et al. (1999) | Verb generation versus rest |
| 8 | Warbuton et al. (1996) | Verb generation versus rest |
The activation foci are plotted on the left and right lateral and internal views of the MNI template (see Figs. 1 and 2), and on coronal sections (Figs. 3 and 4). The foci are numbered according to the reference list in Table I.
Figure 1.
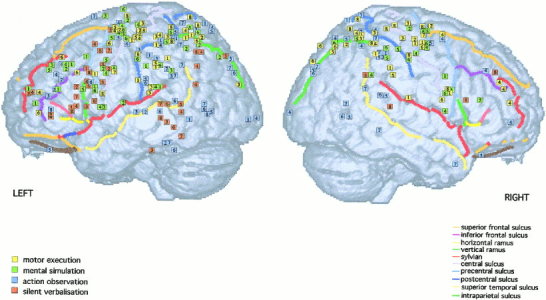
Activation foci plotted on left and right lateral surfaces of the MNI template.
Figure 2.
Activation foci plotted on the mid‐sagittal view of the MNI template.
Figure 3.
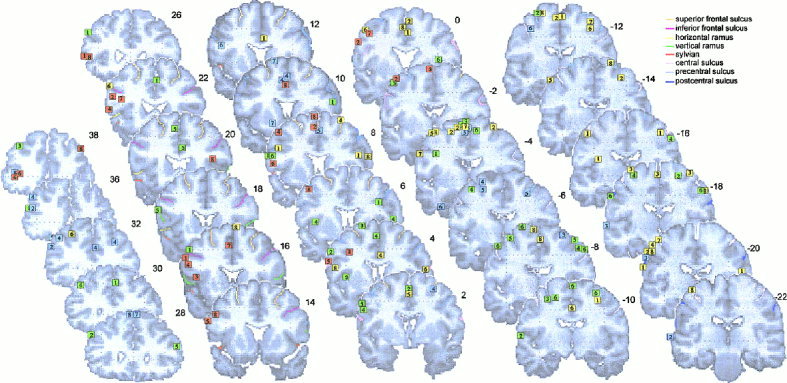
Activation foci plotted on coronal sections (38 ≥ y ≥ ‐22) from the MNI template. Numbers are millimeters (+, in front; ‐ behind) from the VCA line.
Figure 4.
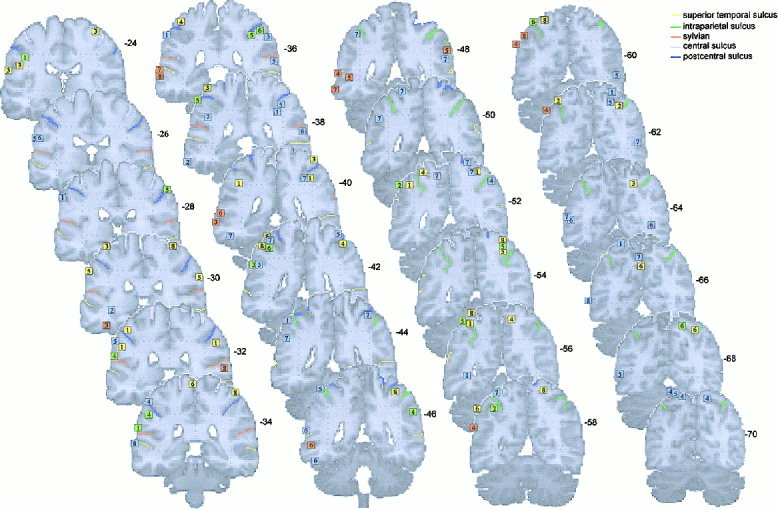
Activation foci plotted on coronal sections (‐24 ≥ y ≥ ‐70) from the MNI template. Numbers are millimeters (‐ behind) from the VCA line.
FRONTAL LOBE
Sensorimotor cortex activations
One group of activations is detected between –16 < y < ‐36, and between ‐40 < x < ‐60 in the left hemisphere with a symmetric one in the right hemisphere, at the level of hand representation in the primary motor cortex [e.g., Fink et al., 1997]. These groups are composed by activations found during motor execution (L: 1, 2, 3, 4, 5, 7, 8, R: 1, 2, 3, 8) and during one study on mental simulation (L: 4, R: 4). In addition, few foci were found to lie between the central and the postcentral sulci in the sensorimotor cortex, during motor execution (L: 1, 3, 4, R: 8).
Lateral premotor cortex activations
Dorsal premotor cortex
Two symmetric activation sites are detected during execution (L: 2, 4, 5, 6, 8; R: 1, 2, 3, 4, 6, 7, 8), simulation (L: 2, 3, 4, 5, 6; R: 2, 4, 5, 6), and observation (L: 4, 5, 6; R: 3, 4, 5) on the surface of the brain between –20 and 0 on y axis, –20 and –40 on x axis, and between 50 and 70 on the z axis. One activation was detected on the left hemisphere during verbalization (L: 2). Most of these foci are localized within the precentral sulcus or around it. We have considered that this area corresponds to the dorsal premotor area (PMd) in macaque brain. This area in human lies at roughly the level of the hand area of motor cortex and at the level of the superior frontal sulcus [Grafton et al., 1996a; Fink et al., 1997].
Ventral part of PMd cortex
A group of foci, below what is described as PMd, is detected in the left hemisphere between 20 and 50 on the z axis, between –10 and 5 on the y axis, and between –30 and –50 on the x axis. These activations were elicited by mental simulation (L: 1, 2, 4, 5), silent verbalization (L: 2, 3, 4, 5, 7, 8), and motor execution (L: 7). We considered this region corresponding to the ventral part of PMd.
Activation within the pars opercularis of the inferior grontal gyrus
It is difficult to precisely establish, because of considerable variability in both shape and location, whether an activation focus lies within the pars opercularis or whether it lies just anterior, in the pars triangularis, or just posterior in the premotor cortex [Amunts et al., 1999]. From the recent probabilistic map on the MNI brain constructed by Tomaiuolo et al. [1999], activated foci localized between y = 8 and y = 20 (corresponding to the occurrence frequencies of 25–50%) were considered to belong to the pars opercularis. These activations were elicited by silent verbalization (L: 1, 3, 4, 5, 6, 8), by mental simulation (L: 5), and by observation (L: 6).
Ventral opercular premotor cortex
The operculum is considered as the cortex lying in the upper bank of the Sylvian sulcus, lateral to the insula and anterior to the central sulcus [Fink et al., 1997]. Another group of foci, localized below the ventral part of PMd and slightly posterior to the inferior frontal area 44 in each hemisphere (between 2 < y < 8), is composed by activations elicited by mental simulation (L: 1, 3, 4, 6, R: 1, 4, 6) and during execution (L: 1, 8, R: 1, 6, 8).
The activations found in the left hemisphere by Binkofski et al. [1999] during complex manipulation at –52 8 28 (1), and by Stephan et al. [1995] at –53 3 14 (8) during joystick movements, are difficult to be precisely localized. However, on the coronal sections (see Figs. 3 and 4), these foci seem to lie within the precentral sulcus, which separates the inferior precentral gyrus from the inferior frontal gyrus. Two other activations found during mental simulation [Decety et al., 1994; Stephan et al., 1995] at y = 8 are also localized at the limit between the inferior frontal gyrus and the precentral sulcus, and have been described as lying in the opercular premotor cortex. These activations fall in the occurrence frequencies of 5–25% to be within the Broca area 44.
Finally, two activations have been found in the insula during mental simulation (L: 3, 4), at the border with the pars opercularis of the inferior frontal gyrus, and with the opercular premotor cortex.
Activation within the pars triangularis of the inferior frontal gyrus
The area 45 is present at the free cortical surface of the triangularis part of the inferior frontal gyrus and is located rostral to the vertical ramus of the lateral fissure. The dorso‐rostral part of the pars triangularis is delimited by the inferior frontal sulcus [Amunts et al., 1999]. A group of activations found during silent verbalization (L: 1, 2, 4, 7, 8, R: 8) localized at 22 < y < 26 fall within area 45. One focus was detected in the right hemisphere during mental simulation (R: 5). All activations are localized in front of the vertical ramus (in green), above the horizontal ramus of the lateral fissure.
Activations along the inferior frontal sulcus and within the middle frontal gyrus
Rostral to Broca areas 45/44, between 30 < y < 60, and between 0 < z < 20, a group of activations elicited by silent speech (L: 1, 4, 6), observation (L: 3, 8, R: 4), mental simulation (L: 1), and motor execution (L: 4, 6, R: 4) is detected in the ventral part of the dorsolateral prefrontal cortex (Ba 9/46/10).
Another group, around 20 < y < 20, and 20 < z < 40, localized more dorsally to the first one, above the inferior frontal sulcus and below the superior frontal sulcus, lies within the middle frontal gyrus (Ba 9/46). It is composed by activations detected during mental simulation (L: 1, 2, 3, 4, 5), observation (L: 2, 4, R: 4), execution (L: 6, R: 4), and verbalization (R: 8).
PARIETAL LOBE
Inferior parietal lobule activations
This region is identified as the cortex lying below the inferior parietal sulcus and posterior to the postcentral sulcus. Within the inferior parietal lobule, in the supramarginal gyrus (area PF), activated foci were localized between –24 < y < ‐4. These foci are elicited by observation (L: 1, 3, 5, 6, 7, R: 1, 3, 4, 5, 7), mental simulation (L: 1, 2, 3, 4, 5, 6, R: 4, 5, 6), and by motor execution (L: 1, 3, 5, 8, R: 1, 5, 8). One focus was found during silent verbalization on the right hemisphere (R: 5). Within the angular gyrus (area PG: ‐56 < y < ‐66), activations were found during silent verbalization (L: 4, 8).
Intraparietal sulcus and superior parietal lobe (areas IPS and PE)
The superior parietal lobe is identified as the cortex lying above the inferior parietal sulcus and posterior to the postcentral sulcus. Activations within the IPS/SPL were detected during observation (L: 1, 4, 5, 7, R: 1, 4, 5, 7), motor execution (L: 1, 2, 3, 4, 6, 8, R: 1, 2, 3, 4, 5, 6, 8), and mental simulation (L: 2, 5, 6, R: 5, 6).
Precuneus and superior occipital gyrus
A group of activations is revealed within the superior occipital gyrus for observation (L: 3, 6, 7, R: 5, 6, 7), mental simulation (L: 2), execution (L: 3; R: 6), and verbalization (L: 3). In addition, activations in the precuneus were found during observation (L: 4, 6, 7, 8) and one during motor execution (L: 6).
TEMPORAL LOBE
Some scattered activations were observed in the middle temporal gyrus, between 0 < y < ‐55 during observation (L: 1, 2, 3, 6, 8, R: 7) and during silent verbalization (L: 3, 4, 5, 6, 7, 8). In the inferior temporal gyrus, activations were detected during observation (L: 2, 6, 7) and during silent verbalization (L: 3, 7).
Finally, around y = 70, activations were detected in the motion area MT/V5, only during observation (L: 5, 6, 7, 8, R: 5, 6, 7).
DISCUSSION
The main goal of this meta‐analysis was to explore to what extent there is an anatomical equivalence between various psychological states, which are supposed to be linked with action processing (i.e., execution, simulation, observation, and verbalization). Our analysis shows that there are common activation sites in favor of a functional equivalence between action execution, simulation, and observation across studies. There are also scattered foci that cannot account for a strict overlap between each of these processes. This is especially true for action verbalization. The discussion is organized region by region. Table II lists the percent of studies by categories that have reported activation foci in a specific cortical region.
Table II.
Percent of studies by categories that have reported activation in specific cortical area
| Cortical regions | Task | Left hemisphere | Right hemisphere |
|---|---|---|---|
| Primary motor cortex (M1) | Execution | 87.5% | 50% |
| Simulation | 16.6% | 16.6% | |
| Observation | 0 | 0 | |
| Verbalization | 0 | 0 | |
| Sensorimotor cortex (S1) | Execution | 37.5% | 12.5% |
| Simulation | 0 | 0 | |
| Observation | 0 | 0 | |
| Verbalization | 0 | 0 | |
| Dorsal part of the premotor cortex: dPMd | Execution | 50% | 87.5% |
| Simulation | 833% | 66.6% | |
| Observation | 37.5% | 37.5% | |
| Verbalization | 12.5% | 0 | |
| Ventral part of the dorsal premotor cortex: vPMd | Execution | 12.5% | 37.5% |
| Simulation | 66.6% | 50% | |
| Observation | 0 | 0 | |
| Verbalization | 75% | 0 | |
| Opercular premotor cortex | Execution | 25% | 37.5% |
| Simulation | 66.6% | 50% | |
| Observation | 0 | 0 | |
| Verbalization | 0 | 0 | |
| Broca area 44 | Execution | 0 | 0 |
| Simulation | 16.6% | 16.6% | |
| Observation | 12.5% | 0 | |
| Verbalization | 75% | 12.5% | |
| Broca area 45 | Execution | 0 | 0 |
| Simulation | 0 | 16.6% | |
| Observation | 0 | 0 | |
| Verbalization | 62.5% | 12.5% | |
| Ventral part of the dorsolateral prefrontal gyrus | Execution | 25% | 12.5% |
| Simulation | 16.6% | 0 | |
| Observation | 25% | 12.5% | |
| Verbalization | 37.5% | 0 | |
| Dorsal part of the dorsolateral prefrontal gyrus | Execution | 12.5% | 12.5% |
| Simulation | 83.3% | 0 | |
| Observation | 25% | 12.5% | |
| Verbalization | 0 | 12.5% | |
| Supramarginal gyrus | Execution | 50% | 50% |
| Simulation | 100% | 50% | |
| Observation | 75% | 50% | |
| Verbalization | 0 | 12.5% | |
| Angular gyrus | Execution | 12.5% | 12.5% |
| Simulation | 0 | 0 | |
| Observation | 0 | 0 | |
| Verbalization | 25% | 0 | |
| Superior parietal lobe | Execution | 50% | 87.5% |
| Simulation | 50% | 33.3% | |
| Observation | 50% | 50% | |
| Verbalization | 0 | 0 | |
| Superior occipital gyrus/Precuneus | Execution | 25% | 12.5% |
| Simulation | 16.6% | 0 | |
| Observation | 62.5% | 37.5% | |
| Verbalization | 12.5% | 0 | |
| Middle temporal gyrus/superior temporal gyrus | Execution | 0 | 0 |
| Simulation | 0 | 0 | |
| Observation | 62.5% | 37.5% | |
| Verbalization | 75% | 12.5% | |
| Inferior temporal gyrus | Execution | 0 | 0 |
| Simulation | 0 | 0 | |
| Observation | 37.5% | 0 | |
| Verbalization | 25% | 0 | |
| MT/V5 | Execution | 0 | 0 |
| Simulation | 0 | 0 | |
| Observation | 50% | 37.5% | |
| Verbalization | 0 | 0 |
Primary sensorimotor cortex
The controlateral sensorimotor cortex is involved only during motor execution (87.5% of the studies) and to a lesser extent the ipsilateral hemisphere (50% of the studies). There was a debate about the involvement of the primary motor and somatosensory areas during mental simulation. On one side, several PET studies have failed to show any significant involvement of the sensorimotor cortex [Decety et al., 1994; Stephan et al., 1995; Parsons et al., 1995; Grafton et al., 1996b]. In contrast, fMRI studies have reported activation of the sensorimotor cortex during mental simulation [Leonardo et al., 1995; Porro et al., 1996; Roth et al., 1996; Lotze et al., 1999]. However, these increases were significantly less during mental simulation as compared to motor execution. Interestingly, transcranial magnetic stimulations of the motor cortex have pointed in the same direction by demonstrating an increase of motor responses during mental simulation of movements [e.g., Abbruzzese et al., 1996]. This discrepancy may be explained by the difference in sensitivity of the neuroimaging techniques.
Supplementary motor area
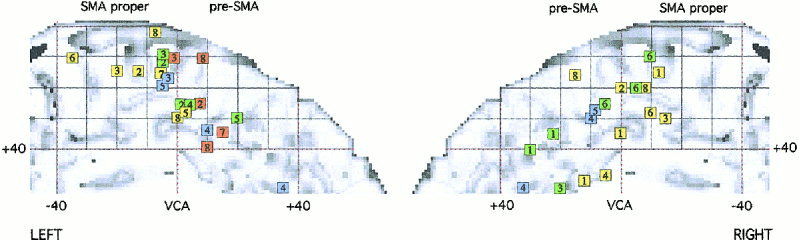
The data collected in the present review are coherent with the functional parcelization of the SMA and pre‐SMA that was proposed by Picard and Strick [1996]. The SMA proper sends direct projection to the primary motor cortex, whereas the pre‐SMA is connected with the prefrontal cortex [Dum and Strick, 1991]. The SMA and the pre‐SMA are associated with functional involvement in simple versus complex tasks, execution versus selection, automatic performance versus initial stage of skill acquisition, respectively. From our meta‐analysis, activation in the SMA proper is found during execution, simulation and observation, whereas the pre‐SMA is activated during mental simulation and observation with the aim to imitate. Within the SMA proper, Stephan et al. [1995] showed that the caudal part was mainly involved in motor execution, whereas the more rostral part, close to VCA line, was preferentially involved in mental simulation. In the present review, most of the activations in the SMA proper, found during execution, are caudal to those found during simulation and observation. This result is consistent with the notion that the SMA has both “higher” and “lower” motor functions [e.g., Wiesendanger and Wiesendanger, 1984].
Silent verbalization produces activations located in the pre‐SMA. Relatively simple speech tasks are associated with rCBF increases near or caudal to the VCA line, like simple repetition of words [Petersen et al., 1988, 1989] and production of overlearned verbal associations [Paus et al., 1993]. More complex tasks, like oral word production in new conditional associations [Paus et al., 1993], self‐ordered number generation [Petrides et al., 1993], and silent verb generation [Wise et al., 1991; Grafton et al., 1997; Warbuton et al., 1996; Tatsumi et al., 1999] are associated with activations in front of the VCA line. According to Wise et al. [1991], the act of retrieving words from memory automatically results in an “inner speech” process for which the SMA contains neural pathways concerned with it.
Lateral premotor cortex
There is no agreement on the subdivision within the premotor cortex in human. Anatomical studies in nonhuman primates distinguish in the lateral premotor cortex the dorsal part from the ventral part, termed PMd and PMv, respectively. These two regions are further subdivided into separate rostral and caudal sectors, with PMd consisting of areas F7 and F2, and PMv of areas F5 and F4 [Matelli et al., 1985]. In general, lateral premotor cortex has been found to be associated with planning, programming, initiation, guidance, and execution of simple and skilled motor tasks [Passingham, 1993]. Numerous studies in nonhuman primates suggest that the dorsal and the ventral regions of the premotor cortex participate in two independent neural networks, which may control different aspects of execution of action [Jackson and Husain, 1996]. The function of these dorsal and ventral circuits has been tied in the following different distinctions: movements planning as opposed to online control movement [Kurata, 1993, 1994]; movement executed in the visual as opposed to the somatic space [Rizzolatti et al., 1988; Graziano et al., 1994; Fox 1995]; and in the control of reaching as opposed to the control of grasping [Caminiti et al., 1996]. However, such dichotomies between PMd and PMv in monkeys probably turn out to be too simplistic in human.
Dorsal premotor cortex
The dorsal premotor cortex is mostly involved during action execution (L: 50%, R: 87%), simulation (L: 83%, R: 66%) in both hemispheres. Lotze et al. [1999] have recently reported that there is no difference in terms of level of activation in the premotor cortex, SMA, and cingulate gyrus between action simulation and action execution.
The results obtained during observation are less clear (L: 37%, R: 37%). Two activations (L: 4, 5, R: 4, 5) were detected during the observation of actions in order to imitate them later. This condition involved components of motor preparation, which is coherent with the implication of the premotor cortex [Passingham, 1993]. However, this area is also activated during two studies in which observation had no specific aim (L: 6, R: 5). The intention to act may be necessary in establishing a functional and an anatomical link between perception and action. However, there are good arguments in favor of a gradient of activation, at least in the precentral cortex, from observation, to simulation, and eventually execution. Some evidence comes from magnetoencephaloraphic (MEG) measurements that recorded rhythmic neuromagnetic oscillations of a frequency around 20 Hz as an indicator of precentral cortex activity. It has been shown that a rebound of the 20 Hz activity is abolished when subjects manipulate an object. Schnitzler et al. [1997] demonstrated that this rebound is significantly diminished during mental simulation. It corresponds to a suppression of 60% of that during real execution. More recently, the same group has shown that during observation of action, there is a significant suppression of 31–46% of that during real execution. Therefore, the effect is about 50% stronger during mental simulation as compared to observation [Hari et al., 1998]. In addition, Cochin et al. [1999] have demonstrated by electroencephamographic (EEG) recordings, similarities in the response of motor and frontal cortex during observation of human movements and execution. These results provide strong support to the idea of the existence a gradient of activity between these three tasks.
No studies dealing with verbalization, in our review, except that by Grafton et al. [1997], have detected an activation within the dorsal premotor cortex, corresponding to the hand/arm premotor cortex field.
Ventral premotor cortex
In the macaque brain, the inferior sector of Brodmann area 6, referred to as “ventral premotor cortex,” is constituted by two distinct areas: F4 and F5 [Matelli et al., 1985]. F5 is not homogeneous but formed by two major sectors [Matelli et al., 1996]. One is located on the posterior bank of the inferior arcuate sulcus (F5ab). The other is located in the cortical convexity immediately adjacent to the arcuate sulcus (F5c). Area F4 contains a representation of face, neck, and proximal arm movements, whereas F5 contains a distal movement representation of the hand and the mouth [Luppino et al., 1999]. The AIP‐F5ab circuit plays a crucial role in transforming the intrinsic properties of the object into the appropriate hand movements [Murata et al., 1997]. The description of object features, possibly in terms of their affordances, is carried out in the parietal area AIP and then transmitted to F5ab, where different types of grips are encoded. Finally, in F5c, mirror neurons show congruence between the observed and the executed action. Predominant inputs come from the parietal area PF where, according to Rizzolatti et al. [1998], neurons with mirror properties are certainly present. This PF‐F5c circuit, where an internal representation of action is evoked by an action made by others, is supposed to be involved in two functions: action imitation and action recognition.
There are controversies concerning the location of PMv in human brain, and three main hypotheses have been proposed. The first one is that the homology between monkey and human can be based on the location of the frontal eye field in the two species. The second possibility is that PMv lies more ventrally in opercular cortex. The third one is that Broca's area 44, in addition to control for oro‐facial movements, controls also hand/arm movements.
We will discuss each of these hypotheses and examine their respective explanatory power in the light of our meta‐analysis.
First hypothesis
In monkey, F5 is adjacent to the frontal eye field, with the two fields being located on the ventral and dorsal banks of the arcuate sulcus. Similarly in human, PMv would lie on the lateral surface, adjacent to the frontal eye field, located in the opposite bank of the precentral sulcus.
According to Rizzolatti et al. [1998], this middle region of human agranular frontal cortex, caudal to the middle frontal gyrus, is homologue of the arm field of monkey F4 as well as, inside the ascending branch of the inferior precentral sulcus, of F5ab. Winstein et al. [1997] have reported a focus of activity at z = 25 in the premotor cortex, during motor execution and have suggested that the ventral premotor cortex may be homologue of area F4 in monkeys. However, Preuss et al. [1996] have considered that the posterior medial frontal gyrus in human may correspond to the monkey dorsal cortex rather than the ventral premotor cortex. They have suggested that there are upper‐limb and orofacial movement representations, localized in the lateral surface of the premotor cortex, adjacent to the frontal eye‐field, and in between the superior and inferior frontal sulcus. This upper‐limb representation lies dorsally to the orofacial one.
In the present analysis, this region is found to mostly merge activations elicited by verbalization and mental simulation. Whereas the most dorsal part is related to activated foci elicited by execution, simulation, and observation of hand movements. Activations found in the ventral part of the premotor cortex might be explained in two ways: due to either verbal representation of the actions during mental simulation or to the mental imagery of the action during verbalization. The first explanation would be compatible with Preuss et al.'s view [1996] that the upper limb representation lies dorsally, whereas the orofacial representations lies ventrally as in monkeys. In human volunteers, Corina et al. [1999] elicited mouth and lips movements by stimulation of cortical sites in this region lying inferior to hand representation.
Many neuroimaging studies have detected rCBF increases in the ventral premotor cortex during various verbal tasks [Petersen et al., 1988; Price et al., 1996; Mummery et al., 1998; Paulesu et al., 1997; Tatsumi et al., 1999; Grabowski et al., 1998]. These activations lie between 20 to 42 on the z axis, and fall within the orofacial zone. Several authors have interpreted such activations within the premotor cortex in terms of prearticulatory phonological encoding [e.g., Demonet et al., 1992; Zatorre et al., 1992; Mummery et al., 1998]. Whereas, others have suggested that the formulation of an articulatory plan is a function of the left anterior insula and lateral premotor cortex and not of Broca's area, even when no verbal response is required [e.g., Petersen et al., 1988; Price et al., 1996; Wise et al., 1999].
Grabowski et al. [1998] have reported an activation within the premotor cortex during tools naming and verb generation, and considered it to be directly related to the conceptual content accessed during the performance of the task rather than the process of retrieval. Grabowski et al. [1998], Martin et al. [1995, 1995], and Grafton et al. [1997] have suggested that the premotor region in the vicinity of the junction of the inferior frontal and precentral sulcus is engaged by the generation of words denoting either actions or tools. This latter interpretation is in concordance with the second explanation, which considers that the activation of the ventral premotor cortex during silent generation can be due to the mental imagery of the action. However, the fact that other studies on semantic processing, which are not linked with tools or actions, have also found activated foci in the ventral premotor cortex, is in contradiction with this latter hypothesis.
Mental simulation tasks are associated with activations in both PMd and PMv. This raises the question whether these activations are due to two distinct hand representations as in monkey, or whether mental simulation engages an additional implicit verbal component. The same remark holds true for the activations localized in PMv found during motor execution.
It has been suggested that verbal encoding may participate in mental simulation of action [Decety and Ingvar, 1990]. In addition, it should be recalled that human voluntary actions are dominated by language in the sense that language often precedes action and forms a part of it. Motor programs can be seen as part of the meaning of verbal items that represent action [Engelkamp, 1986]. When mental simulation is compared to execution, activations are found within the caudal part of the inferior frontal gyrus [Broca area 44), in the ventral part of the dorsolateral prefrontal cortex (Ba 46/45), and within the middle temporal gyrus [Stephan et al., 1995]. The inferior frontal gyrus and the middle temporal gyrus are found in mostly all tasks involving silent verbalization.
Those results, in addition to the fact that this region was found to be involved both during verbalization (75% of the studies) and during mental simulation (66% of the studies) exclusively in the left hemisphere, support the view that mental simulation engages implicit verbal mediation.
Second hypothesis
PMv is homologue to the ventral opercular premotor cortex. In monkeys, movements of all body parts are represented throughout the insula with upper extremity represented more rostral than those of the lower extremity [Showers and Lauer, 1961]. Using PET, Fink et al. [1997] have shown that in human the insula and the opercular premotor area also contain motor maps organized somatotopically. However, much less is known about the functional and physiological significance of these different lateral premotor areas. Krams et al. [1998] have found an activated focus during preparation of action within Broca's area, which in fact, according to Tomaiuolo et al.'s [1999] probabilistic map of the MNI template, is localized posterior to the pars opercularis. These authors have argued that the ventral part of the premotor cortex may be concerned with the planning and selection of movements that required “standard” mapping of stimulus and response, or more precisely in the preparation of copied movements. Kurata [1994] has proposed that PMv is involved in the visual guidance of movement. However, Stephan et al. [1995] have found such activation both during execution and simulation of joystick movements while their subjects had their eyes closed.
The fact that this region has been revealed only during imagined and executed hand movements in both hemispheres lead us to suggest that it might be homologue to the ventral premotor cortex of monkeys where upper‐limb movement representations have been recorded.
Third hypothesis
PMv as homologue to Broca's area 44. The development of speech has provoked an enormous expansion of the mouth fields of areas homologous to F4 and F5, reflected by the increase of the lowest part of area 6 and even more by the areas 44 and 45. Human area 44 is agranular and belongs to the set of areas that form area 6, and it constitutes the rostral most part of inferior area 6 [Von Bonin and Bailey, 1947; Petrides and Pandya, 1994]. It has been suggested that area F5 is most likely the monkey homologue of area 44 in human [Petrides and Pandya, 1994; Passingham, 1993; Preuss et al., 1996]. The homology between 44 and F5 was drawn considering essentially that both areas are involved in larynbuccal movements.
Recent functional brain imaging studies have reported Broca's area activations during tasks outside the linguistic domain, namely during motor execution [e.g., Krams et al., 1998; Binkofski et al., 1999; Iacoboni et al., 1999], during perception of others actions [e.g., Grafton et al., 1996b; Rizzolatti et al., 1996b; Decety et al., 1997], and during mental simulation [e.g., Grafton et al., 1996b]. These results have led Rizzolatti and Arbib [1998] to argue that Broca's area function includes representational capacities related to action/recognition of oro‐facial and brachio‐manual based behaviors. Arbib and Rizzolatti [1996] have even proposed that the mirror zone (F5c) may have expanded in humans and would occupy the cortical convexity ventral to the inferior frontal sulcus, and that both areas 44 and 45 would originate from an area homologous to F5.
Our meta‐analysis shows that Broca's areas 44 and 45 are mainly involved during silent verbalization (75% of the studies for area 44, and 62.5% for area 45) There are other activated foci that have been labeled as lying in Broca's areas during nonverbal tasks, which may be subject to discussion or controversy. Parsons et al. [1995] have found one activated focus in area 44 (‐60 14 16) during implicit motor imagery. However, in a more recent article, this activation was not replicated [Parsons and Fox, 1998]. They have only found activations within the ventral premotor cortex (‐43 0 28) as in the first study (one activated foci at P = 0.01 was labeled Ba 44 with the following coordinates –46 –2 26, which appears to be within the premotor cortex in the MNI template). Binkofski et al. [1999] have reported activations in PMv during complex manipulation of object (‐56 4 28). This activation lies in the convexity, on the precentral sulcus. Additional activation has been found during complex manipulation with covert naming in the pars opercularis (‐60 12 8) and triangularis (‐40 32 16). Finally, one activation has been detected during observation of meaningful actions [Grèzes et al., 1998]. However, the fact that in this latter study this focus is not present during the observation in order to imitate is in contradiction with the Rizzolatti et al. [1996b] interpretation, i.e. the role of Broca area 44 in matching observation and execution in humans. Grèzes et al. [1998] interpreted this activation as reflecting silent speech processing.
Several neuroimaging studies have demonstrated that the left inferior frontal is involved in a variety of verbal tasks that require a phonological or semantic operation of words or nonwords, including the verbal working memory [Demonet et al., 1992; Zatorre et al., 1992; Hinke et al., 1993; Paulesu et al., 1993; Fiez et al., 1996a; Dolan et al., 1997] or word retrieving. Such processes are distinct from motor planning for speech articulation, which appears to be subserved by the precentral gyrus [Kuriki et al., 1999; Wise et al., 1999].
Altogether, these results indicate that silent generation involves cortical regions concerned with speech production and that Broca areas 44 and 45 are probably only engaged in language behavior. However, it is true that language dominates other cognitive processing and that it is difficult to clearly distinguish what belongs to what.
Middle frontal gyrus
The meta‐analysis shows that the dorsal part of the middle frontal gyrus is mainly concerned with mental simulation (83% of the studies). Prefrontal areas 9 and 46 in the dorsolateral prefrontal cortex are known to be involved in the self‐generated action when the decision and the selection is instigated by the subject [e.g., Frith et al., 1991] or on the basis of information held in working memory [Petrides et al., 1993]. Courtney et al. [1996] also suggested that the prefrontal cortex is involved in the general process of holding representations of stimulus information on line. This region has also been found during observation of action with an intended goal (e.g., to recognize or imitate later). The fact that both mental simulation and observation of action with a goal engage this region is coherent with Fuster's [1995] suggestion that the dorsal prefrontal cortex is important for prospective memory for what an individual plans to do next.
The ventrolateral prefrontal cortex was found to be involved in all target tasks of the meta‐analysis. Clinical observations in neurology [Lhermitte et al., 1986; Freedman et al., 1998] have demonstrated that the orbitofrontal and the ventral prefrontal cortex play a role in switching or reversing stimulus‐response associations. The engagement of those regions in inhibiting prepared motor programs is consistent with reports of ventral prefrontal activation during tasks using go/no‐go response choice [Kawashima et al., 1996; Konishi et al., 1998; Kiefer et al., 1998; Garavan et al., 1999] during the redirection of response based on a violation of stimulus contingencies [Nobre et al., 1999] and during tasks of motor selection and preparation requiring withholding of responses [Krams et al., 1998]. We suggest that action observation, simulation, and silent verbalization of action requires the suppression of behavioral output, which explains its involvement. However, the fact that two studies on motor execution involved this region may be in contradiction with this hypothesis. These activations may also be related to the attentional role of the dorsolateral prefrontal cortex [Passingham and Nixon, 1996; Rushworth et al., 1997a].
Parietal cortex
This region is known to play a critical role in linking sensation and action, to contribute to spatial representations and early sensorimotor transformations underlying action [e.g., Andersen et al., 1997; Goodale, 1997]. Caution is needed in defining homologous areas in the human and the monkey brain simply on the basis of the position of the intraparietal sulcus, and there has been considerable divergence between neuroanatomists. Moreover, monkey parietal parcelization based on both cytoarchitectony and functional data are in a more advanced stage than in human [Passingham, 1998].
Supramarginal gyrus (area PF)
In the present analysis, the supramarginal gyrus was found to be involved during execution (L: 50%, R: 50%), mental simulation (L: 100%, R: 50%), and observation (L: 75%, R: 50%). In human, inferior parietal lobe lesions are known to produce apraxia, an impairment of skilled movements, in absence of elementary sensory and motor deficits. Based on clinical observation of apraxic patients, it has been suggested that the parietal cortex may be a central node for storing, generating, or/and accessing motor representations (for a review, see Leiguarda and Marsden, 2000). Rushworth et al. [1997b] have shown that left parietal lesions, particularly including the supramarginal gyrus, are associated with impairments of motor attention. Moreover, they have demonstrated the left hemisphere dominance for movement selection, subserving by a distributed system composed by the dorsolateral frontal and parietal cortices [Rushworth et al., 1998].
Using functional brain imaging, the supramarginal gyrus has been involved when subjects decide when to make movements [Jueptner et al., 1996a] or learn new sequences of movements [Jenkins et al., 1994; Jueptner et al., 1996b]. Many neuroimaging studies have also pointed out the role of area 40 (PF) in motor preparation [e.g., Deiber et al., 1996; Krams et al., 1998].
It must be emphasized that in our analysis, only experiments that engaged fine fingers movements as complex manipulation of objects [Binkofski et al., 1999], grasping movements [Grafton et al., 1996; Matsumura et al., 1996], and immediate copying of finger movements [Krams et al., 1998] involved the inferior parietal lobe. This result may be related to a recent suggestion made by Binkofski [1999] of a possible homology between the supramarginal in human and the area AIP in monkey in which neurons discharge during object presentation visually guided hand shaping and hand‐object interactions. However, the fact that not all those studies have found activations within the ventral premotor cortex seems to be in contradiction with this hypothesis.
Intraparietal sulcus and the superior parietal lobule (areas IPS and PE)
The intraparietal sulcus and the superior parietal lobule were found to involved activations elicited by execution (L: 50%, R: 87%), mental simulation (L: 50%, R: 33%), and observation (L: 50%, R: 50%). There area several lines of evidence suggesting that Brodmann area 7 (PE) is involved in multimodal integration of external information and that it provides a sensory representation of extrapersonal space [Andersen et al., 1997]. PET studies have revealed that Ba 7 is related to motor selection with auditory cues as well as with visual cues, based on integration of spatial information [Deiber et al., 1991; Grafton et al., 1992], and that the dorsal parietal cortex and the precuneus respond to an increment of the spatial complexity of the task [Grafton et al., 1992]. Another issue related to Ba 7 (PE) is attention. Jenkins et al. [1994] found a bilateral activation within this region during complex sequential finger tapping auditory cued. They have interpreted these activations as reflecting spatial attention to fingers because these activations are prominent during learning new sequence as compared to well‐learned phase.
Angular gyrus (PG)
Activations in this region were found only in two studies on silent verbalization. However, Price et al. [1996] detected a similar focus in Ba 39 specifically engaged for object naming and more recently for viewing objects [Moore and Price, 1999]. The importance of the left posterior parietal cortex for comprehending written and spoken language and semantic knowledge has been well established in neuropsychological studies since Dejerine [1892].
Temporo‐parietal junction
Finally, a group of activated foci located at the occipito‐temporal junction and another one located in the superior occipital gyrus (V3a) are mainly associated with observation of other's actions. The localization of the occipito‐temporal junction corresponds precisely to the coordinates of V5 given by Watson et al. [1993] from a PET study in human. These regions are known to be specifically engaged by visual motion [e.g., Zeki et al., 1991]. These activations are interpreted as reflecting hand movements analysis.
TEMPORAL LOBE
The cortical areas forming the ventral stream are known to compute visual attributes such as size, shape, and color and convey information on them to the inferotemporal cortex, which plays a central role in object recognition and categorization [Ungerleider and Mishkin, 1982]. Electrophysiological studies in monkeys have shown that neurons in the inferotemporal cortex respond to complex stimuli, such as faces or hand [e.g., Gross et al., 1985; Perrett et al., 1982, 1990], and are activated by specific object features [Tanaka et al., 1991; Kobatake and Tanaka, 1994]. Single‐cell recordings in monkeys, and neurophysiological and neuroimaging studies in humans, reveal that cerebral cortex in and near the superior temporal sulcus (STS) region is an important component of this perceptual system. In monkeys and humans, the STS region is activated by movements of the eyes, mouth, hands, and body, suggesting that it is involved in analysis of biological motion (for a recent review, see Allison et al., 2000).
From our analysis, the ventral pathway is engaged by silent verbalization (L: 75%) and observation (L: 62,5%). In humans, the posterior part of the middle temporal gyrus seems to be specifically linked with naming tools or verb generation [Martin et al., 1996; Damasio et al., 1996] and is considered to be a site for stored information about nonbiological object motion. This idea is supported by the proximity of this area to motion perception areas and by its selective activation when subjects generate action words [Wise et al., 1991; Martin et al., 1995; Fiez et al., 1996b], name and retrieve information about tools [Martin et al., 1996; Cappa et al., 1998; Mummery et al., 1998; Moore and Price 1999; Perani et al., 1999]. However, this region is also associated with the observation of hand actions, and therefore is related with the analysis of biological motion. Recently, Chao et al. [1999], from an fMRI study, suggested that there is a superior‐to‐inferior gradient from the posterior STS to the middle temporal gyrus that may be tuned to the features that distinguish biological motion from motion associated with the use of manipulable, man‐made objects.
Some neuroimaging studies on action observation did include real objects, while some others merely suggest them by pantomimes. The fact that observation of hand actions lead to more spread activations as compared to verbalization within the temporal lobe may be in favor of the involvement of the two processes. In addition, due to the complexity and the social importance of these stimuli, we cannot exclude the fact that subjects may have used different strategies for recognition.
CONCLUSIONS
An action may be described as the outcome of several information‐processing stages: intention, planning, preparation, and execution. It is widely accepted that the generation of a goal‐directed action involves a representational stage that is synonymous with mental representation [Jeannerod, 1997]. The concept of mental representation of action corresponds both to the mental content related to the goal and to the consequences of the given action and to the neural operations supposed to occur before an action begins. The existence of such system of mental representation accounts for many adaptive advantages, such as the ability to anticipate, to predict, to simulate, and even to understand others' actions and intentions, which are crucial for social interactions. Therefore, the cognitive and the neuronal structures underlying those functions should present some neurophysiological coherence. All the cognitive processes of interest in this review (i.e., execution, simulation, observation, and verbalization) are supposed to bear relationship to potential action and hence to the involvement of motor representation. Nonetheless, they may be dissimilar with regard to the nature of the component involved.
Of the four target processes, our meta‐analysis shows that there is a good overlap between action execution, simulation, and observation in the SMA, the dorsal premotor cortex, the supramarginal gyrus, and the superior parietal lobe. This makes sense in regard to their role for generating a motor plan appropriate to an intended goal. However, mental simulation is in addition associated with the ventral premotor cortex, which may be explained in terms of verbal mediation, whereas observation of action is associated with additional rCBF increases located in the temporal pathway, which is consistent with processing of the visual scene. Finally, both mental simulation and observation of actions with the intention to act engage the pre‐SMA and the dorsolateral frontal gyrus, associated with prospective memory for planned action.
The nonoverlapping areas between the three target processes may be the most interesting piece in this analysis. If one accepts the idea of shared representations for observation, simulation, or self‐production, one may wonder how we distinguish our own actions from the actions produced in the environment. This question remains to be elucidated.
An unanticipated finding from our meta‐analysis is the relative sparse overlap between regions elicited by silent verbalization and the other target processes. In addition to the fact that it is the sole process that is massively left‐hemispheric dominant as compared to simulation, execution, and observation, distinct and specific activations are found in Broca's areas, in the angular gyrus, and to a lesser extent in the temporal lobe. Silent verbalization of tools or action verb appears not to be associated with the same cortical regions that are involved in motor representation (as those engaged by simulation, execution, and observation of hand movement). Such a finding is not surprising if one accepts that language in its complexity is unique to humans, even though it has been hypothesized that there is a close evolutionary relationship between action and speech [Corballis, 1992; McNeilage, 1998].
Acknowledgements
We express our gratitude to Professors G. Rizzolatti and M. Matelli (University of Parma, Italy) for helpful comments on an earlier version of this article.
- X_mni
- 1.36 X_talairach + 0.91
- Y_mni
- 1.03 Y_talairach + 3.42
- Z_mni
- ‐0.06 Y_talairch+1.14 Z_talairach + 0.31.
REFERENCES
- Abbruzzese G, Trompetto C, Schieppati M (1996): The excitability of the human motor cortex increases during execution and mental imagination of sequential but not repetitive finger movements. Exp Brain Res 111: 465–472. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G (2000): Social perception from visual cues: role of the STS region. TICS 4: 267–278. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HBM, Zilles K (1999): Broca's region revisited: cytoarchtecture and intersubject variability. J Comp Neurol 412: 319–341. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Bradley DC, Xing J (1997): Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci 20: 303–330. [DOI] [PubMed] [Google Scholar]
- Annet J (1996): On knowing how to do things: a theory of motor imagery. Cogn Brain Res 3: 65–69. [DOI] [PubMed] [Google Scholar]
- Arbib MA, Rizzolatti G (1996): Neural expectations: a possible evolutionary path from manual skills to language. Commun Cogn 29: 393–424. [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund HJ (1999): A fronto‐parietal circuit for object manipulation in man: evidence from an fMRI‐study. Eur J Neurosci 11: 3276–3286. [DOI] [PubMed] [Google Scholar]
- Bonda E, Petrides M, Ostry D, Evans A (1996): Specific involvement of human parietal systems and the amygdala in the perception of biological motion. J Neurosci 16: 3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminiti R, Ferraina S, Johnson PB (1996): The sources of visual information to the primate frontal lobe: a novel role for the superior parietal lobule. Cereb Cortex 6: 319–328. [DOI] [PubMed] [Google Scholar]
- Cappa SF, Perani D, Schnur T, Tettamanti M, Fazio F (1998): The effects of semantic category and knowledge type on lexical‐semantic access: a PET study. Neuroimage 8: 350–359. [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M (1998): The functional neuroanatomy of simple and complex sequential finger movements: a PET study. Brain 121: 253–264. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A (1999): Attribute‐based neural substrates in temporal cortex for perceiving and knowing about objects. Nature Neurosci 2: 913–919. [DOI] [PubMed] [Google Scholar]
- Cochin S, Barthelemy C, Roux S, Martineau J (1999): Observation and execution of movement: similarities demonstrated by quantified electroencephalography. Eur J Neurosci 11: 1839–1842. [DOI] [PubMed] [Google Scholar]
- Corballis MC (1992): On the evolution of language and generativity. Cognition 44: 197–226. [DOI] [PubMed] [Google Scholar]
- Corballis MC (1998): Cerebral asymetry: monitoring on. TICS 2: 152–157. [DOI] [PubMed] [Google Scholar]
- Corina DP, McBurney SL, Dodrill C, Hinshaw K, Brinkley J, Ojemann G (1999): Functional roles of Broca's area and SMG: evidence from cortical stimulation mapping in a deaf singer. Neuroimage 10: 570–581. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV (1996): Object and spatial visual working memory activates separate neural systems in human cortex. Cereb Cortex 6: 39–49. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR (1996): A neural basis for lexical retrieval. Nature 380: 499–505. [DOI] [PubMed] [Google Scholar]
- Dawson G, Warrenburg S, Fuller P (1985): Left hemisphere specialization for facial and manual imitation. Psychophysiology 22: 237–243. [DOI] [PubMed] [Google Scholar]
- Decety J, Ingvar DH (1990): Brain structures participating in mental simulation of motor behavior: a neuropsychological interpretation. Acta Psychologica 73: 13–34. [DOI] [PubMed] [Google Scholar]
- Decety J, Perani D, Jeannerod M, Bettinardi V, Tadary B, Woods R, Mazziotta JC, Fazio F (1994): Mapping motor representations with positron emission tomography. Nature 371: 600–602. [DOI] [PubMed] [Google Scholar]
- Decety J (1996): The neurophysiological basis of motor imagery. Behav Brain Res 77: 45–52. [DOI] [PubMed] [Google Scholar]
- Decety J, Grèzes J, Costes N, Perani D, Jeannerod M, Procyk E, Grassi F, Fazio F (1997): Brain activity during observation of actions. Influence of action content and subject's strategy. Brain 120: 1763–1777. [DOI] [PubMed] [Google Scholar]
- Decety J, Grèzes J (1999): Neural mechanisms subserving the perception of human actions. TICS 3: 172–178. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebach JG, Friston KJ, Nixon PD, Frackowiak RSJ (1991): Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res 84: 393–402. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Ibanez V, Sadato N, Hallett M (1996): Cerebral structures participating in motor preparation in humans: a positron emission tomography study. J Neurophysiol 75: 233–246. [DOI] [PubMed] [Google Scholar]
- Dejerine J (1892): Contribution à l'etude anatomoclinique et clinique des différentes variétés de cécités verbal. CR Hebdomadaire des Scéance et Mémories de la Société de Biologie 4: 61–90. [Google Scholar]
- Demonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JD, Wise R, Rascol A, Frackowiak RSJ (1992): The anatomy of phonological and semantic processing in normal subject. Brain 115: 1753–1768. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Fletcher PC (1997): Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature 388: 582–585. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL (1991): The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 11: 667–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelberg D, Galaburda AM (1984): Inferior parietal lobule: divergent architectonic asymetries of the human brain. Archiv Neurol 41: 843–852. [DOI] [PubMed] [Google Scholar]
- Engelkamp J (1986): Motor programs as part of the meaning of verbal items In: Kurcz I, Shugar GW, Danks JH, editors. Knowledge and language. Amsterdam: Elsevier. [Google Scholar]
- Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Petersen SE (1996a): A positron emission tomography study of the short‐term maintenance of verbal information. J Neurosci 16: 808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Raichle ME, Balota DA, Tallal P, Petersen SE (1996b): PET activation of posterior temporal regions during auditory word presentation and verb generation. Cereb Cortex 6: 1–10. [DOI] [PubMed] [Google Scholar]
- Fink GR, Frackowiak RSJ, Pietrzyk U, Passingham RE (1997): Multiple nonprimary motor areas in the human cortex. J Neurophysiol 77: 2164–2174. [DOI] [PubMed] [Google Scholar]
- Fox PT (1995): Broca's area: motor encoding in somatic space. Behav Brain Sci 18: 344–345. [Google Scholar]
- Freedman M, Black S, Ebert P, Binns M (1998): Orbitofrontal function, object alternation and perseveration. Cereb Cortex 8: 18–27. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston K, Liddle PF, Frackowiak RSJ (1991): Willed action and the prefrontal cortex in man: a study with PET. Proc R Soc Lond B 244: 241–246. [DOI] [PubMed] [Google Scholar]
- Fuster JM (1995): Memory in the cerebral cortex: an empirical approach to neural networks in the human and non‐human primate. Cambridge, MA: MIT Press. [Google Scholar]
- Gallese V, Goldman A (1998): Mirror neurons and the simulation theory of mind‐reading. TICS 2: 493–501. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA (1999): Right hemispheric dominance of inhibitory control: an event‐related functional MRI study. Proc Natl Acad Sci U S A 96: 8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale MA (1997): Visuomotor modules in the vertebrate brain. Can J Physiol Pharmacol 74: 390–400. [PubMed] [Google Scholar]
- Grabowski T, Damasio H, Damasio AR (1998): Premotor and prefrontal correlates of category‐related lexical retrieval. Neuroimage 7: 232–243. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Mazziotta JC, Woods RP, Phelps ME (1992): Human functional anatomy of visually guided finger movements. Brain 115: 565–587. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fagg AH, Woods RP, Arbib MA (1996a): Functional anatomy of pointing and grasping inhumans. Cereb Cortex 6: 226–237. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G (1996b): Localization of grasp representations in humans by positron emission tomography. Exp Brain Res 112: 103–111. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fadiga L, Arbib MA, Rizzolatti G (1997): Premotor cortex activation during observation and naming of familiar tools. Neuroimage 6: 231–236. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Yap GS, Gross CG (1994): Coding of visual space by premotor neurons. Science 266: 1054–1057. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Costes N, Decety J (1998): Top down effect of the strategy to imitate on the brain areas engaged in perception of biological motion: a PET investigation. Cogn Neuropsychol 15: 553–582. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Decety J (2001): Does visual object afford action? Evidence from a neuroimaging study. Neuropsychologia, in press. [DOI] [PubMed] [Google Scholar]
- Gross CG, Desimone R, Albright TD, Schwartz EL (1985): Inferior temporal cortex and pattern recognition In: Chagas C, Gattass R, Gross C, editors. Pattern recognition mechanisms. Berlin: Springer, p 179–201. [Google Scholar]
- Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G (1998): Activation of human primary motor cortex during action observation: a neuromagnetic study. Proc Natl Acad Sci U S A 95: 15061–15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinke RM, Hu X, Stillman AE, Kim SG, Merkle H, Salmi R, Ugurbil K (1993): Functional magnetic resonance imaging of Broca's area during internal speech. Neuroreport 4: 675–678. [DOI] [PubMed] [Google Scholar]
- Lacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G (1999): Cortical mechanisms of human imitation. Science 286: 2526–2528. [DOI] [PubMed] [Google Scholar]
- Jackson SR, Husain M (1996): Visuomotor functions of the lateral premotor cortex. Curr Opin Neurobiol 6: 788–795. [DOI] [PubMed] [Google Scholar]
- Jeannerod M (1997): The cognitive neuroscience of action. New York: Blackwell. [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RSJ, Passingham RE (1994): Motor sequence learning: a study with positron emission tomography. J Neurosci 14: 3775–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jueptner M, Frith CD, Brooks DJ, Frackowiak RSJ, Passingham RE (1996a): The anatomy of motor learning. II. Subcortical structures and learning by trial and error. J Neurophysiol 77: 1325–1337. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RSJ, Passingham RE (1996b): The anatomy of motor learning. I. The frontal cortex and attention to action. J Neurophysiol 77: 1313–1324. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Satoh K, Itoh H, Ono S, Furumoto S, Gotoh R, Koyama M, Yoshioka S, Takahashi T, Takahashi K, Yanagisawa T, Fukuda H (1996): Functional anatomy of GO/NO‐GO discrimination and response selection—a PET study in man. Brain Res 728: 79–89. [PubMed] [Google Scholar]
- Kiefer M, Marzinzik F, Weisbrod M, Sherg M, Spitzer M (1998): The time course of brain activations during response inhibition: evidence from event‐related potentials in a go/no go task. Neuroreport 9: 765–770. [DOI] [PubMed] [Google Scholar]
- Kimura D, Archibald Y (1974): Motor functions of the left hemisphere. Brain 97: 337–350. [DOI] [PubMed] [Google Scholar]
- Kobatake E, Tanaka K (1994): Neuronal selectivities to complex object features in the ventral visual pathway of the macaque cerebral cortex. J Neurophysiol 71: 856–867. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y (1998): No‐go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. Eur J Neurosci 10: 1209–1213. [DOI] [PubMed] [Google Scholar]
- Krams M, Rushworth MFS, Deiber MP, Frackowiak RSJ, Passingham RE (1998): The preparation, execution and suppression of copied movements in the human brain. Exp Brain Res 120: 386–398. [DOI] [PubMed] [Google Scholar]
- Kurata K (1993): Premotor cortex of monkeys‐set‐related and movement‐related activity reflecting amplitude and direction of wrist movements. J Neurophysiol 69: 187–200. [DOI] [PubMed] [Google Scholar]
- Kurata K (1994): Information processing for motor control in primate premotor cortex. Behav Brain Res 61: 35–142. [DOI] [PubMed] [Google Scholar]
- Kuriki S, Mori T, Hirata Y (1999): Motor planning center for speech articulation in the normal human brain. Neuroreport 10: 765–769. [DOI] [PubMed] [Google Scholar]
- Leiguarda RC, Marsden CD (2000): Limb apraxias: higher‐order disorders of sensorimotor integration. Brain 123: 823–879. [DOI] [PubMed] [Google Scholar]
- Leonardo M, Fieldman J, Sadato N, Campbell G, Ibanez V, Cohen L, Deiber MP, Jezzard P, Pons T, Turner R, Le Bihan D, Hallett M (1995): A functional magnetic resonance imaging study of cortical regions associated with motor task execution and motor ideation in humans. Hum Brain Mapp 3: 83–92. [Google Scholar]
- Lhermitte F (1986): Human autonomy and the frontal lobes. II. Patients behavior in complex and social situations. The “environmental dependency syndrom.” Ann Neurol 19: 335–343. [DOI] [PubMed] [Google Scholar]
- Lotze M, Montoya P, Erb M, Hulsmann E, Flor H, Klose U, Birbaumer N, Grodd W (1999): Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. J Cogn Neurosci 11: 491–501. [DOI] [PubMed] [Google Scholar]
- Luppino G, Murata A, Govoni P, Matelli M (1999): Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4). Exp Brain Res 128: 181–187. [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG (1995): Discrete cortical regions associated with knowledge of color and knowledge of action. Science 270: 102–105. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV (1996): Neural correlates of category‐specific knowledge. Nature 379: 649–652. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Rizzolatti G (1985): Patterns of cytochrome oxydase activity in the frontal agranular cortex of macaque monkey. Behav Brain Res 18: 125–137. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Govoni P, Geyer S (1996): Anatomical and functional subdivisions of inferior area 6 in macaque monkey. Soc Neurosci Abtr 22: 796.2. [Google Scholar]
- Matsumura M, Kawashima R, Naito E, Satoh K, Takahashi T, Yanagisawa T, Fukuda H (1996): Changes in rCBF during grasping in humans examined by PET. Neuroreport 7: 749–752. [DOI] [PubMed] [Google Scholar]
- McNeilage PF (1998): The frame/content theory of evolution of speech production. Behav Brain Sci 21: 499–511. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK (1997): Explaining facial imitation: a theoretical model. Early Dev Parenting 6: 179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN (1999): Origins of theory of mind, cognition and communication. J Commun Disord 32: 251–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CJ, Price CJ (1999): Three distinct ventral occipitotemporal regions for reading and object naming. Neuroimage 10: 181–192. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, Price CJ (1998): Functional neuroanatomy of the semantic system: divisible by what? J Cogn Neurosci 10: 766–777. [DOI] [PubMed] [Google Scholar]
- Murata A, Fadiga L, Fogassi L, Gallese V, Rao V, Rizzolatti G (1997): Object representation in the ventral premotor cortex (area F5) of the monkey. J Neurophysiol 78: 2226–2230. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Coull JT, Frith CD, Mesulam MM (1999): Orbitofrontal cortex is activated during breaches of expectation in tasks of visual attention. Nat Neurosci 2: 11105–11612. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Fox PT, Downs JH, Glass T, Hirsch TB, Martin CC, Jerabek PA, Lancaster JL (1995): Use of implicit motor imagery for visual shape discrimination as revealed by PET. Nature 375: 54–58. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Fox PT (1998): The neural basis of implicit movements used in recognizing hand shape. Cogn Neuropsychol 15: 583–615. [Google Scholar]
- Passingham RE (1993): The frontal lobes and voluntary action. Oxford Psychology series 21 Oxford: Oxford University Press. [Google Scholar]
- Passingham RE (1996): Functional specialization of the supplementary motor area in monkeys and humans In: Lüders HO, editor. Advances in neurology, supplementary sensorimotor area Vol. 70 Philadelphia: Lippincott‐Raven, p 105–116. [PubMed] [Google Scholar]
- Passingham RE, Nixon PD (1996): Lesions of the cerebellar nuclei do not impair the acquisition of a visual‐motor conditional learning task. Soc Neurosci Abstr 22: 116.1. [Google Scholar]
- Passingham RE (1998): The specializations of the human neocortex In: Milner AD, editor. Comparative neuropsychology. Oxford: Oxford University Press, p 271–298. [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS (1993): The neural correlates of the verbal component of working memory. Nature 362: 342–345. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Goldacre B, Scifo P, Cappa SF, Gilardi MC, Castiglioni I, Perani D, Fazio F (1997): Functional heterogeneity of left inferior frontal cortex as revealed by fMRI. Neuroreport 8: 2011–2016. [DOI] [PubMed] [Google Scholar]
- Paus T, Petrides M, Evans AC, Meyer E (1993): Role of human anterior cingulate cortex in the control of oculomotor, manual and speech responses: a positron emission tomography study. J Neurophysiol 70: 453–469. [DOI] [PubMed] [Google Scholar]
- Perani D, Schnur T, Tettamanti M, Gorno‐Tempini M, Cappa SF, Fazio F (1999): Word and picture matching: a PET study of semantic category effects. Neuropsychologia 37: 293 Ablex Publishing Corporation 306. [DOI] [PubMed] [Google Scholar]
- Perani D, Fazio F, Borghese A, Tettamanti M, Ferrari S, Lomonaco F, Gilardi MC, Decety J (2001): How the brain works for watching at real and virtual (hand) actions. Neuroimage, in press. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Rolls ET, Caan W (1982): Visual neurons responsive to faces in the monkey temporal cortex. Exp Brain Res 47: 329–342. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Mistlin AJ, Harries MH, Chitty AJ (1990): Understanding the visual appearance and consequence of actions In: Goodale MA, editor. Vision and action. Norwood, NJ: Ablex Publishing Corp, p 163–180. [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME (1988): Positron emission tomographic studies of the cortical anatomy of single word processing. Nature 331: 585–589. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME (1989): Positron emission tomographic studies of the processing of single words. J Cogn Neurosci 1: 153–170. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Meyer E, Evans AC (1993): Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci U S A 90: 878–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya D (1994): Comparative architectonic analysis of the human and macaque frontal cortex In: Boller F, Grafman J, editors. Handbook of neuropsychology Vol. 9 Amsterdam: Elsevier, p 17–58. [Google Scholar]
- Picard N, Strick PI (1996): Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 6: 342–353. [DOI] [PubMed] [Google Scholar]
- Porro CA, Francescato MP, Cettolo V, Diamond ME, Baraldi P, Zuiani C, Bazzocchi M, di Prampero PE (1996): Primary motor and sensory cortex activation during motor performance and motor imagery: a functional magnetic resonance imaging study. J Neurosci 16: 7688–7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM, Stepniewska I, Kaas JH (1996): Movement representation in the dorsal and ventral premotor areas of owl monkeys: a microstimulation study. J Comp Neurol 371: 649–676. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Frackowiak RSJ, Friston K (1996): The neural regions sustaining object recognition and naming. Proc R Soc Lond B 263: 1501–1507. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M (1988): Functional organisation of inferior area 6 in the macaque monkey. I. Somatotopy and the control of proximal movements. Exp Brain Res 71: 491–507. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L (1996a): Premotor cortex and the recognition of motor actions. Cogn Brain Res 3: 131–141. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Perani D, Fazio F (1996b): Localization of cortical areas responsive to the observation of hand grasping movements in humans: a PET study. Exp Brain Res 111: 246–252. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA (1998): Language without our grasp. TINS 21: 188–194. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M (1998): The organization of the cortical motor system: new concepts. Electroencephal Clinical Neurophys 106: 283–296. [DOI] [PubMed] [Google Scholar]
- Rosch E, Mervis CB, Gray WD, Johnsen DM, Boyes‐Braem P (1976): Basic objects in natural categories. Cogn Psychol 8: 382–440. [Google Scholar]
- Roth M, Decety J, Raybaudi M, Massarelli R, Delon‐Martin C, Sege‐barth C, Morand S, Gemignani A, Decorps M, Jeannerod M (1996): Possible involvement of primary motor cortex in mentally simulated movement: a functional magnetic resonance imaging study. Neuroreport 7: 1280–1284. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Nixon PD, Eacott MJ, Passingham RE (1997a): Ventral prefrontal cortex is not essential for working memory. J Neurosci 17: 4829–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MFS, Nixon PD, Renowden S, Wade DT, Passingham RE (1997b): The left parietal and motor attention. Neuropsychologia 35: 1261–1273. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Nixon PD, Wade DT, Renowden S, Passingham RE (1998): The left hemisphere and the selection of learned actions. Neuropsychologia 36: 11–24. [DOI] [PubMed] [Google Scholar]
- Sadato N, Campbell G, Ibanez V, Deiber MP, Hallett M (1996): Complexity affects regional cerebral blood flow change during sequential finger movements. J Neurosci 16: 2693–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler A, Salenius S, Salmelin R, Jousmaki V, Hari R (1997): Involvement of primary motor cortex in motor imagery: a neuromagnetic study. Neuroimage 6: 201–208. [DOI] [PubMed] [Google Scholar]
- Showers MJC, Lauer EW (1961): Somatovisceral motor patterns in the insula. J Comp Neurol 117: 107–115. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Gainotti G (1988): Interaction between vision and language in category‐specific semantic impairment. Cogn Neuropsychol 5: 677–709. [Google Scholar]
- Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos‐Baumann AO, Frith CD, Frackowiak RSJ. (1995): Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol 73: 373–386. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. Stuttgart: Thieme. [Google Scholar]
- Tanaka K, Saito HA, Fukada Y, Moriya M (1991): Coding visual images of objects in the inferotemporal cortex of the macaque monkey. J Neurophysiol 66: 170–189. [DOI] [PubMed] [Google Scholar]
- Tatsumi IF, Fushimi T, Sadato N, Kawashima R, Yokoyama E, Kanno I, Senda M (1999): Verb generation in japanese—a multicenter PET activation study. Neuroimage 9: 154–164. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F, MacDonald JD, Caramanos Z, Posner G, Chiavaras M, Evans AC, Petrides M (1999): Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: an in vivo MRI analysis. Eur J Neurosci 11: 3033–3046. [DOI] [PubMed] [Google Scholar]
- Tucker M, Ellis R (1998): On the relations between seen objects and components of potential actions. J Exp Psychol Hum Percept Perform 24: 830–846. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M (1982): Two cortical visual systems In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behaviour. Cambridge, MA: MIT Press, p 549–586. [Google Scholar]
- Von Bonin G, Bailey P (1947): The neocortex of macaca mulatta. Urbana: University of Illinois Press. [Google Scholar]
- Warbuton E, Wise RJS, Price CJ, Weiller C, Hadar U, Ramsay S, Frackowiak RSJ (1996): Noun and verb retrieval by normal subjects, studies with PET. Brain 119: 159–179. [DOI] [PubMed] [Google Scholar]
- Warrington EK, McCarthy RA (1987): Categories of knowledge: further fractionations and an attempted integration. Brain 110: 1273–1296. [DOI] [PubMed] [Google Scholar]
- Watson JDG, Myers R, Frackowiak RSJ, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki (1993): Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex 3: 79–94. [DOI] [PubMed] [Google Scholar]
- Wiesendanger M, Wiesendanger R (1984): The suplementary motor cortex in the light of recent investigations. Exp Brain Res Suppl 9: 382–392. [Google Scholar]
- Winstein CJ, Grafton ST, Pohl PS (1997): Motor task difficulty and brain activity: investigation of goal‐directed reciprocal aiming using positron emission tomography. J Neurophysiol 77: 1581–1594. [DOI] [PubMed] [Google Scholar]
- Wise R, Chollet F, Hadar U, Friston K, Hoffner E, Frackowiak R (1991): Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain 114: 1803–1817. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Greene J, Buchel C, Scott SK (1999): Brain regions involved in articulation. Lancet 353: 1057–1061. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E, Gjedde A (1992): Lateralization of phonetic and pitch discrimination in speech processing. Science 256: 846–849. [DOI] [PubMed] [Google Scholar]
- Zeki S, Watson JDG, Lueck CJ, Friston KJ, Kennard C, Frackowiak RSJ (1991): A direct demonstration of functional specialization in human visual cortex. J Neurosci 11: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]


