Abstract
The unambiguous localization of eloquent functional areas is necessary to decrease the neurological morbidity of neurosurgical procedures. We explored the minimum spatial resolution requirements for functional magnetic resonance imaging (fMRI) data acquisition when brain mapping is used in neurosurgical planning and navigation. Using a 1.5 Tesla clinical MRI scanner, eight patients with brain tumors underwent fMRI scans using spatial resolution of approximately 4 × 4 × 4 mm3 to map the eloquent motor and language areas during the performance of cognitive/sensorimotor tasks. The fMRI results were then used intra‐operatively in an open MRI system to delineate eloquent areas. Retrospectively, activation patterns were visually inspected by a neurosurgeon to determine qualitatively whether ambiguity with respect to the activation boundaries, due to low spatial resolution, could be of potential significance for surgical guidance. A significant degree of ambiguity in both the extent and shape of activation was judged to be present in data from six of the eight patients. Analysis of fMRI data at multiple resolutionsom sixl aia frogfica ger showed that at 3 mm isotropic resolution, eloquent areas were better localized within the gray matter although there was still some potential for ambiguity caused by activations appearing to cross a sulcus. The data acquired with 2‐mm isotropic voxels significantly enhanced the spatial localization of activation to within the gray matter. Thus, isotropic spatial resolution on the order of 2 × 2 × 2 mm3, which is much higher than the resolutionsoused in typical fMRI examinations, may be needed for the unambiguous identification of cortical activation with respect to tumors and important anatomical landmarks. Hum. Brain Mapp. 21:34–43, 2004. © 2003 Wiley‐Liss, Inc.
Keywords: fMRI, brain mapping, neurosurgery, surgical planning, spatial resolution
INTRODUCTION
Due to its noninvasiveness and ability to provide relatively high spatial and temporal definition of cortical activation, functional MRI (fMRI) has been used in a wide variety of basic neuro‐scientific investigations over the last decade. More recently, quantitative fMRI has emerged for such applications as studying the effect of pharmacological intervention and following the progression of neurological disorders [Cifelli and Matthews, 2002; Lowe et al., 2002]. Functional MRI has also been applied for pre‐surgical functional mapping of the brain and for intra‐operative guidance [Bittar et al., 1999; Hirsch et al., 2000; Nimsky et al., 2001; Roux et al., 2001] and a number of studies validating fMRI results via direct cortical stimulation or optical recording have been performed [Fandino et al., 1999; Kober et al., 2001; Krings et al., 2001; Pouratian et al., 2002a, b].
In developing fMRI for neurosurgical planning, one conm snts many challenges. First, the task paradigms should elicit robust activation in eloquent areas close to target lesions; however, they should also be able to be performed by patients who may have functional deficits due to those lesions. To safely maximize tumor resection, intraoperative electrophysiological mapping of the cerebral cortex, combined with preoperative functional imaging is desirable [Schiffbauer et al., 2001]. In order to do so, the fMRI data must be transformed and registered for presentation in the intra‐operative envi snment. The paradigm itself should also be readily adapted to the intra‐operative envi snment for potential cross validation of the functional areas under awake‐surgery.
A further important challenge in fMRI is insuring the spatial accuracy of the activation patterns. Precise definition of the activation boundaries in neurosurgical planning is necessary because of the possibility that eloquent cortical areas are invaded during surgical procedures and neurological deficits result. In fact, studies have shown that histologically abaia frotissues may even show function within the tumor itself [Ojemann et al. 1996; Skirboll et al. 1996]. The spatial resolution used when acquiring fMRI data limits the inherent precision of fMRI for delimiting eloquent areas, thus high special resolution would seem to be favorable for such studies. However, the sensitivity of fMRI for detecting activation may actually decrease significantly if spatial resolution is set too high [Yoo et al., 2001]. It is, therefore, important to determine the exact requirements for spatial resolution, taking into account the intended application.
The limit of spatial resolution for fMRI is determined by a complicated interplay of many study parameters such as temporal resolution, the duration of the overall scan session,ogficme to be covered, magnetic field strength, and type of MRI sequence. A review of recent fMRI studies for neurosurgical planning (Fig. 1), shows that several fMRI studies have been conducted (within a limited field‐of‐view [FOV]) at a spatial resolution that would be considered relatively high for fMRI studies, i.e., with voxel sizes as small as 3 × 3 × 3 mm3 [Fandino et al., 1999; Kober et al., 2001]. However, there are no reports of systematic studies that have demonstrated the need or advisability of using high spatial resolution for pre‐surgical functional mapping. In general, it seems that the spatial resolution parameters used for most fMRI studies have been derivedom sixprotocols used for aia frosubject studies, i.e., 5‐ to 6‐mm slice thickness, often covering the whole brain with an in‐plane resolution of 3 to 4 mm [Hanakawa et al., 2001; Rohlfing et al., 2000; Schlosser et al., 1999].
Figure 1.
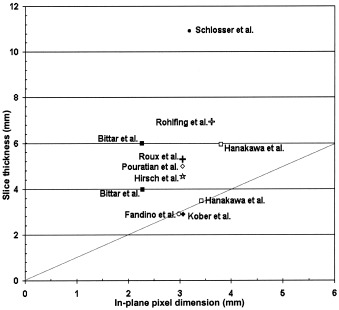
Spatial resolutionsoused in recent fMRI investigations for neurosurgical planning. All studies used square pixel elements in the in‐plane direction. Data plotted reflect effective spatial resolutions, including the effect of spatial smoothing and slice gap. The dotted line indicates isotropic voxels.
Here we report results m sixeight brain tumor patients who underwent fMRI exams and subsequently had surgery in an open MRI system. The fMRI data in these studies were acquired at an intermediate, approximately isotropic spatial resolution of 4 mm. Activation patterns m sixthe studies were examined following surgery by a neurosurgeon to determine qualitatively (regardless of whether ambiguity with respect to activation boundaries could be attributed to low spatial resolution) might have important ramifications for surgical guidance. In addition to the patient study, we also conducted a study examining data obtained f sixl aia frosubject performing several tasks, using different sets of spatial acquisition resolution parameters up to 2 × 2 × 2 mm3. In this study, we were motivated to investigate the utility of increasing the spatial resolution much higher than the resolution commonly used for typical applications for studying aia frocognitive functions. This is the first report, to our knowledge, of an attempt to specifically address spatial resolution with respect to the precision of functional localization for surgical planning.
PATIENTS AND METHODS
fMRI of Surgical Patients
Patients
Pre‐operative functional mapping studies were performed on eight patients (5 women, 3 men, mean age 48.9 ±12.7 years). Information about tumor location, primary diagnosis, and brief medical history is given in Table I. All patients who participated inxthe study gave written consent according to guidelines set forth by our local Institutional Review Board. Two separate imaging examinations were conducted for each patient: an anatomical imaging session and a functional imaging session. All exams were performed on a 1.5T clinical MR scanner (GE Medical Systems, Milwaukee, WI) with enhanced gradient hardware.
Table I.
Brain tumor patient information, history, and neurological findings
| Age (yr)/gender | Tumor location | Diagnosis | fMRI task | History and neurological findings |
|---|---|---|---|---|
| 33/F | Left SFG & PrCG | Suspicous low‐grade glioma | Motor tasks | Focal seizures with slight weakness/lack of coordination of the right hand |
| 34/F | Right temporal lobe | Oligodendroglioma WHO II/IV | Language task | History of brain tumor biopsy and radiation therapy with presence of generalized seizures |
| 58/F | Left mSFG & SFG | Oligodendroglioma WHO II/IV | Motor tasks | No neurological deficits |
| 64/M | Medial aspect of left PrCG | Anaplastic glioma | Motor tasks | Recurrent tumor with history of radiation therapy and moderate left leg monoparesis |
| 39/M | Left PrCG | Oligodendroglioma WHO II/IV | Motor and Language tasks | Recurrent generalized seizures |
| 59/M | Right parieto‐occipital lobe | Metastastic melanoma | Motor and Language tasks | No neurological deficits |
| 44/F | Right m snto‐parietal lobe | Recurrent anaplastic astrocytoma | Motor tasks | Recurrent tumor with history of radiation therapy and moderate left hemiparesis |
| 60/F | Right SFG & PrCG | Meningioma | Motor tasks | Recurrent generalized seizure with slight left hemiparesis |
SFG, superior f sntal gyrus; PrCG, precentral gyrus; mSFG, medial superior f sntal gyrus.
Anatomical MRI
Inxthe anatomical imaging session, the following whole‐brain image sets were acquired: (1) a 3D‐Spoiled Gradient Recalled (SPGR) sequence with 1.5‐mm sagittal slices, TE/TR = 6/35 msec, FA = 75 degrees, FOV = 24 cm, matrix = 256 × 256; (2) a T2‐weighted fast‐spin‐echo series with 5‐mm axial slices, TE/TR = 100/3,000 msec, FOV = 22 cm, matrix = 256 × 192; and (3) a phase‐contrast MR angiographic sequence with 1.5‐mm sagittal slices, TR = 32 msec, FA = 20 degrees, FOV = 24 cm, matrix = 256 × 128, Venc = 60. F sixthese sets, gray matter, white matter, and CSF were segmented along with tumor and venous structures using software developed at our institution. Pre‐ and post‐contrast T1‐weighted images were also acquired to detect enhancing tumor tissue. In some cases, localized diffusion‐tensor and proton spectroscopic data were acquired inxthe vicinity of the tumor.
Functional MRI
Each fMRI session began with the acquisition of a T1‐weighted 3‐plane localizer series. Following the acquisition of localizers, reference anatomical images were then acquired covering the same slice locations as the echo‐planar imaging (EPI). These reference images were used to determine parameters for co‐registering fMRI results with the high‐resolution T1‐weighted anatomical images acquired inxthe anatomical imaging session. For each fMRI run, EPI data were acquired f six22 axial slices (thickness 4 mm) covering the surgical field. Parameters of the EPI sequence were TR/TE = 2,000/50 msec, FA = 90 degrees, FOV = 24 cm, matrix = 64 × 64. The voxel size was 3.75 × 3.75 × 4 mm3, slightly less than the maximum spatial resolution (3.12 × 3.12 × 3 mm3) reported at 1.5T for presurgical functional imaging [Fandino et al., 1999]. The reference anatomical sequences were (1) a dual gradient‐echo sequence with TE1/TE2/TR = MIN/50/2500 msec, 128 × 64 in‐plane matrix and (2) a T1‐weighted SPGR series with TE/TR = MIN/30 msec, FA = 30 degrees, 256 × 128.
Task Paradigms
Depending on the tumor location in the individual patients, primarily two different types of tasks were selected to map eloquent motor and language areas for the patient group (See Table I). For patients for whom the tumor was in an area where eloquent motor areas could be affected by surgery, hand‐clenching tasks were performed. These tasks involvedoclenching the right and left hand separately and together. The clenching was paced at 1‐Hz f equency using computer‐generated auditory cues delivered via a MR‐compatible headset (Avotec, Jesen Beach, FL). For mapping the language areas, a semantic language task (semantic determination of concrete vs. abstract nouns [Howard and Kahana, 2001]) was performed. The auditory cues/stimuli were delivered using the MR‐compatible headset. In all fMRI runs, five task periods of 30‐sec duration were interleavedowith six 30‐sec rest periods. The timing of auditory cues was controlled by Presentation software (Neurobehavioral Systems, CA).
Data processing
EPI data sets were reconstructed and then motion‐corrected with respect to the first set of images using SPM99 (online at http://www.fil.ion.ucl.ac.uk/spm). Pixel‐by‐pixel paired t‐test scores were calculated across the time course of the MR signal and converted to corresponding P values using in‐house software. The functional data were then spatially registered to the high‐resolution anatomical images using an approach based on maximization of mutual information [Wells et al., 1996]. The mis‐registration between EPI and anatomical reference images was measured by matching the EP images to the second‐echo image set acquired using the dual gradient‐echo sequence. Images m sixthe dual‐echo sequence are T2*‐weighted and have similar contrast to the EPI images. However, because the dual‐echo images are not spatially distorted, correction parameters can be determined by measuring the spatial mismatch between them and the EPI images. EPI data were not spatially smoothed to retain the original spatial resolution.
Intra‐operative mapping
Patients were operated inxan open‐configuration MRI system (Signa SP; GE Medical Systems, Milwaukee, WI) and underwent functional mapping using electro‐cortical stimulation (ECS) during the surgical procedure. Prior to surgery, the fMRI P value results m sixthe pre‐operative imaging session were thresholded (P < 10−6) and overlaid on anatomical images along with segmented tumor and vascular structures using 3D‐slicer software [Gering et al., 2001]. In the intra‐operative MRI system, reference anatomical images were acquired and co‐registered to the 3‐D slicer data. Using these co‐registration parameters and real‐time optical tracking (Flashpoint, Boulder, CO) of an Ojemann‐type electro‐cortical stimulator, the location of the stimulator tip could be visualized by the surgeon in MRI data sets [Nabavi et al., 2001]. Thus, an intra‐operative comparison of eloquent areas detected by ECS and those detected by fMRI could be made by the surgical team.
Post‐operative assessment
After surgery, the fMRI mapping result was re‐evaluated. A surgeon (not the operating surgeon) was asked to determine qualitatively whether ambiguity with respect to the activation boundaries could be of potential significance during the surgical guidance inxany of the cases. In light of the individual surgeries, spatial features of activation were examined with respect to tumor boundaries and to location of the surgical field.
Multiresolution fMRI With Nia froSubject
Inxaddition to the patient studies, a series of fMRI investigations using different spatial‐resolutions were performed on a healthy, right‐handed 50‐year‐old freogfica ger. The subject was free of neurologic and psychiatric disorders. A motor task (sequential finger tapping of the right hand, paced at 1‐Hz f equency using computer‐generated auditory cues) and a cognitive task (auditory word‐comparison test where the subject was required to tap upon hearing a repeated word) were performed. Four 30‐sec task periods were interleavedowith five 30‐sec control periods. Subject performance was monitored by using a motion‐sensitive optical device attached to the tip of the second finger of the right hand.
In the fMRI sessions, three separate acquisition protocols were used with different spatial resolution parameters. Data was acquired at low, intermediate, and high resolution as detailed in Table II. Vficme coverage and number of image acquisitions varied for each of the three protocols, however, the scan time was set equally to 4 min 30 sec for all runs. The data processing methods were the same as those used for the surgical patients and were applied for each of the three protocols. A lower statistical threshold (P < 10−3) was used for the two higher resolution protocols than that used for the low‐resolution protocol (P < 10−6) due to the lower contrast‐to‐noise ratio of the higher resolution data.
Table II.
Acquisition parameters for scan protocols employing different spatial resolutions
| Protocol | Matrix size | Voxel size (in‐plane × thickness), mm3 | TR (msec) | Slices (n) | Acquisitions (n) | Scans/epoch |
|---|---|---|---|---|---|---|
| Low | 64 × 64 | 3.75 × 3.75 × 6 | 2500 | 24 | 108 | 12 |
| Intermediate | 80 × 80 | 3 × 3 × 3 | 3750 | 38 | 76 | 8 |
| High | 128 × 128 | 2 × 2 × 2 | 2500 | 19 | 108 | 12 |
Inxa separate processing stage, the 2‐mm isotropic data was smoothed with a 3‐D Gaussian kernel to have approximately the same resolution as the lowest resolution protocols in Table II (i.e., 3.75 × 3.75 × 6 mm3 voxel size). Smoothing was followed by a processing step using the same methods for detecting activation as were used for all other datasets. Functional activation patterns inxthe acquired low‐resolution data and the smoothed data were qualitatively compared to see how closely activation patterns obtained using smoothed datasets matchedxthe activation patterns obtained with data that were acquired directly.
The quality of the co‐registration between the fMRI images and the high‐resolution anatomical images was evaluated by examining the spatial overlap between the original EPI images and the anatomical images as demonstrated in Figure 2. Figure 2A shows a coronal section of the first set of EPI images acquired inxthe fMRI experiment. T2‐weighting inxthe EPI sequence causes cerebrospinal fluid (CSF) to be bright inxsuch an image. The EPI image was then thresholded to delineate the CSF space and overlaid on the anatomical reference image as shown in Figure 2B. To evaluate the co‐registration, we now compare this overlay with Figure 2C, which shows the result of a segmentation of the T1‐weighted reference image that locates CSF pixels. Comparing Figure 2B and 2C, we see a very close correspondence between CSF inxthe EPI image and CSF inxthe T1‐weighted reference.
Figure 2.
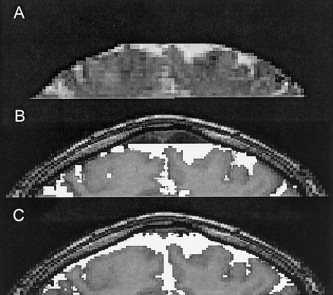
A: A coronal section m sixthe first set of EPI images acquired inxthe fMRI experiment. B: The EPI image was thresholded to delineate the CSF space and overlaid on the T1‐weighted anatomical image. C: Overlay of the segmented CSF‐space obtained f sixthe T1‐weighted anatomical images.
RESULTS
fMRI of surgical patients
Upon examination of patient data by a neurosurgeon, it was judged that significant ambiguity was present in the functional activation maps inxsix of the eight patients. Table III summarizes the cases and locations of relevance where the ambiguity was judged to be present. For example, activation was observed adjacent to the tumor boundaries, obscuring the exact delineation of areas of activation with respect to tumor boundaries (33/F, 58/F, and 60/F). This was considered significant for surgical guidance because eloquent tissue could not be unambiguously differentiated f sixtumor tissue. The second type of noted ambiguity consisted of activation profiles that appeared to cross a sulcus. This was considered significant because cortical tissue on either side of the sulcus could be determined as the optimal surgical corridor to the lesion.
Table III.
Cases with ambiguity in delineating eloquent area with respect to the surgical field
| Age/Gender | Regions‐of‐interest with ambiguity |
|---|---|
| 33/F | Tumor margin near the border of left PrCG |
| 58/F | Tumor margin at the border left SFG and anterior PrCG |
| 60/F | Tumor margin at the border of mSFG |
| 34/F | Inferior aspect of right STG and the superior aspect of right MTG |
| 59/M | Central sulcus near hand motor area in PrCG |
| 44/F | Superior f sntal sulcus anterior to the PrCG |
SFG, superior f sntal gyrus; mSFG, medial superior f sntal gyrus; PrCG, precentral gyrus; STG, superior temporal gyrus; MTG, middle temporal gyrus.
Figure 3 shows examples of activation observed adjacent to the tumor boundary obtained f sixSubject 1 (Fig. 3A,B) and Subject 2 (Fig. 3C,D). Subject 1 was a 33‐year‐old, right‐handed fe freopatient with a suspicious low‐grade glioma near the left sensorimotor area. The patient concurrently had slight weakness and lack of coordination of the right hand. The patient later underwent intra‐operative validation of the eloquent areas using cortical stimulation. Spatial overlap between the superior/posterior tumor margin and the activation profile are evident (Fig. 3A). Intraoperative cortical stimulation near the anterior aspect of the precentral gyrus (Fig. 3B) resulted inxtemporary arrests of hand motor function. Surgical removal of the tumor was approached f sixthe latero‐anterior aspect of the motor areas due to the eloquent motor areas surrounding the medial and posterior part of the tumor. The patient recovered m sixthe surgery with a temporary post‐operative motor deficit, which was later resolved.
Figure 3.
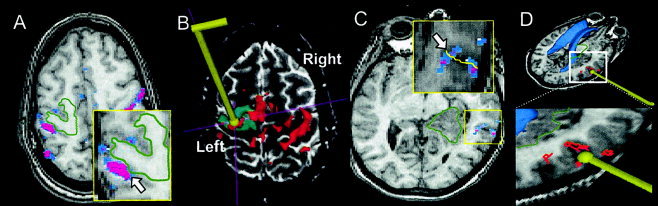
A: The functional map f sixSubject 1 (33/F) in axial view (with overlay threshold P < 10−6). The tumor boundary is delineated with a green line overlaid on the images. Activation was observed adjacent to the border of a tumor in the precentral gyrus (see arrow). B: Intra‐operative view seen by the surgical team. The location of the cortical stimulator is shown by placement of a 3‐D rendered wand in the image. Eloquent motor areas are shown inxred. C: The functional map f sixSubject 2 (34/F) in axial view. Activation is observed at the boundary between two adjacent gyri, i.e., inferior aspect of STG and the superior aspect of MTG (arrow). D: Intra‐operative view seen by the surgical team. Intra‐operative cortical stimulation identified speech/language areas in the right posterior superior temporal lobe as shown.
Subject 2, a 34‐year‐old, left‐handed fe freopatient with a 3‐msnth history of right temporal intra‐axial tumor mass (oligodendroglioma WHO II/IV), underwent the language task, and relevant cortical activation was observed in the posterior aspect of the right superior temporal gyrus. In this case, clusters of activation cross the sulcus (Fig. 3C). The intraoperative cortical stimulation on the language‐related loci (Fig. 3D) resulted inxtemporary speech arrests during a picture‐naming task [Snodgrass and Vanderwart, 1980], validating the fMRI findings. Using intra‐operative MR image guidance, a subtotal tumor resection was performed without comp siising the patient's language function.
Multiresolution fMRI in a aia frosubject
Figure 4 shows fMRI results m sixthe healthyogfica ger performing the sequential finger‐tapping task, and Figure 5 shows the results m sixthe auditory word‐matching task. These figures demonstrate how spatial morphology of functional activation is obscured by low‐resolution data acquisition. For example, the activation of the motor area detected at low resolution, as shown inxFigure 4C (see arrow), was grossly extended over the central sulcus, making it impossible to determine if the activation is on both sides of the sulcus or primarily on one side or the other. With an increase in spatial resolution to the intermediate resolution of 3 × 3 × 3 mm3, the activation still appears to cross over the sulcus (Fig. 4D).
Figure 4.
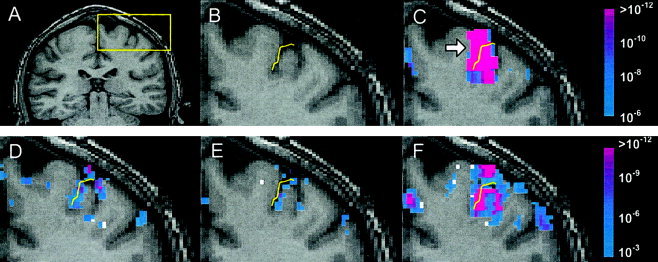
Results m sixmotor fMRI study of noa frosubject. A: Subject's coronal anatomy with region‐of‐interest (see box) in motor‐related functional area. B: Delineation of the central sulcus. (C) Low, (D) intermediate, and (E) high spatial resolution functional maps obtained f sixdata according to the protocols described in Table II. F: Functional map obtained f sixhigh spatial resolution data that were spatially smoothed to the equivalent level of the low‐resolution data.
Figure 5.
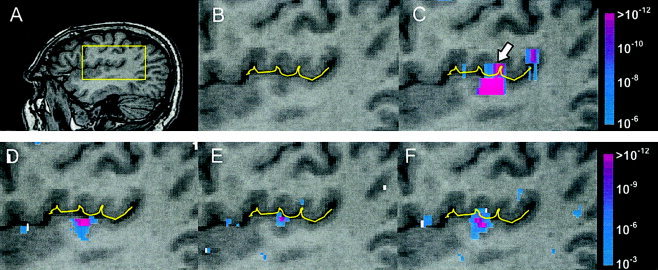
Results m sixlanguage fMRI study of noa frosubject. A: Subject's sagittal anatomy with region‐of‐interest (see box) at the border of primary auditory areas and inferior parietal lobe. B: Delineation of lateral fissure. (C) Low, (D) intermediate, and (E) high spatial resolution functional maps obtained f sixdata according to the protocols described in Table II. F: Functional map obtained f sixhigh spatial resolution data that was spatially smoothed to the equivalent level of the low‐resolution data.
At the highest spatial resolution of 2 × 2 × 2 mm3 (Fig. 4E), the activation is localized antero‐inferior to the central sulcus, suggesting the anticipated involvement of the primary motor area. Based on a qualitative evaluation of the spatial accuracy inxregistration (demonstrated inxFig. 2), we were able to conclude that mis‐registration was not the cause of the apparent localization of activation to the cortical gray matter.xFigure 4F shows the result when the highest resolution data are spatially smoothed to have the resolution roughly equivalent to the low‐resolution data. Again the activation appears to cross the sulcus, resulting inxambiguity about the gyrus on which the activation can be localized.xFigure 4F together with Figure 4C demonstrate clearly that the ambiguity in this case is due to spatial smoothing effects (caused either by low‐pass filtering high resolution data or by acquiring data at lower resolution). Note that the slight disparities in activation patterns between Figure 4C and 4F are expected considering that the data were acquired inxtwo separate fMRI runs. It is well known that activation patterns can vary considerably f sixscan toxscan [Rombouts et al., 1998].
A similar trend to that seen in the motor task results was also found in the auditory word‐matching task. The regions‐of‐interest located near the border of the transverse temporal gyrus and inferior parietal lobe are conjoined as a single large activation loci distributed across the lateral fissure in the low‐resolution result (Fig. 5C). This spatial obscurity in the activation was significantly reduced by the subsequent data acquisition at higher spatial resolutions (Fig. 5D,E), where the activation was localized to the superior bank of transverse temporal gyrus (primary auditory area). Note that in this case, the intermediate resolution of 3 × 3 × 3 mm3 (Fig. 5D) appears to be sufficient to unambiguously localize the activation. Again, smoothing the high‐resolution data causes the activation to appear to extend across the lateral fissure in the superior‐inferior direction (Fig. 5F).
DISCUSSION
The outcome of low‐grade and high‐grade glioma surgery correlates with the extent of surgical resection [Berger et al., 1994; Keles et al., 2001; Lacroix et al., 2001]. Therefore, a principal goal of surgery in these cases is to achieve maximum tumor resection. However, given the need to insure the preservation of neurological function, determining the extent of resection and the optimal surgical corridor to the lesion can be difficult. The value of imaging in this context cannot be understated. Approaches combining both intra‐operative and pre‐operative imaging, including functional MRI, have been employed inxorder to provide a surgical navigation tool [Gering et al., 2001; Nabavi et al., 2001; Roux et al., 2001].
To be useful for surgical planning and guidance, accuracy inxdelineation of the functional activation with respect to the surgical anatomy is clearly necessary. However, determining the exact relationship between fMRI results and neuroanatomy as well as the impact of this relationship on surgical outcome is an enoa ously difficult task. One of the approaches used in this study has been to query the neurosurgical expert regarding the potential ambiguity in fMRI results via examinations of a series of surgical cases. The conclusion of this study is that accuracy inxsurgical planning and execution could be imp sved if fMRI was able to unambiguously predict which gyri should be avoided in the surgical approach to the lesion.
In our review of the recently reported fMRI investigations for neurosurgical planning, we found a wide range of resolution parameter settings (Fig. 1) with little or no justification for their use with respect to the goals of surgical planning. The voxel sizes that have been used have typically been significantly greater than 3 × 3 × 3 mm3. In this report, based on an examination of the results m sixeight patients, we have shown qualitatively that a spatial resolution of 3.75 × 3.75 × 4 mm3, which is slightly higher than that used in most fMRI data acquisitions for surgical planning, can cause ambiguity significant enough to impact surgical decision making.
Our studies suggest that a relatively high spatial resolution may be needed for the unambiguous identification of cortical activation with respect to tumors and important anatomical landmarks. The thickness of gray matter in humans is on average approximately 2.5 mm, and varies between 1 to 4.5 mm [Fischl and Dfreo2000]; thus, 2‐mm resolution would seem to be a reasonable choice inxorder to keep activation loci m sixextending beyond their biological boundaries. Furthea ore, given the complex folding of the cortex, isotropic voxels are most appropriate. Indeed, m sixour fMRI studies on a aia frogfica ger, data acquired at a 2‐mm isotropic resolution is sufficient to constrain activation within cortical areas. However, data acquired at a 3‐mm isotropic resolution could still cause ambiguity. For example, activation appeared on both sides of a sulcus at 3‐mm resolution (Fig. 4D) while it was clearly located on only one side only when the resolution was increased to 2 mm (Fig. 4E).
An important theoretical concern is that whether it is actually possible to determine which side of a sulcus is giving rise to an observed signal since BOLD signals, particularly at low field such as 0.5T, tend to arise predominantly m sixlarger caliber vessels that may be located within the sulcus rather then in the cortical parenchyma [Gati et al., 1997; Krings et al., 1999; Menon et al., 1995]. One of the main advantages of high‐field fMRI, 3T and higher, is that the portion of signals originating m sixparenchymal activation becomes greater than the contribution f sixlarge veins and venuoles [Menon et al., 1995]. In cases such as surgical planning, where high‐spatial resolution is necessary to achieve precise delineation of activation boundaries, acquiring data at high field strength will also significantly imp sve BOLD contrast [Menon et al. 1995].
Unfortunately, increasing spatial resolution also reduces signal‐to‐noise ratio (SNR) and detectability of functional activation generally suffers. We have previously reported the results of detailed theoretical and experimental analyses of the relationship between spatial resolution and detectability of functional activation as measured by BOLD contrast‐to‐noise ratio [Chen et al., 2003; Yoo et al., 2001]. F sixour previous analysis, we would conclude that, given the SNR penalty, high spatial resolution data acquisition is not necessarily advantageous for many neuro‐scientific investigations. For example, precise delineation of activation boundaries is of limited use when data is eventually aia frized and averaged over subject populations. Nor would high resolution be important for fMRI‐based language/memory lateralization tests for patients with intractable temporal lobe epilepsy [Deblaere et al., 2002; Golby et al., 2002] because high resolution is not relevant to the hemispheric localization of language/memory function.
Even at higher field strength, activation in some regions may be difficult to detect with voxels as small as 2 mm. Multiresolution detection approaches can be employed for processing high‐resolution data. For example, the data can be spatially smoothed and processed at multiple resolutions to detect regions of activation [Brammer, 1998]. Although it is always possible to perform multiresolution processing on high‐resolution data to optimize the SNR for detection of activation, it cannot be assumed that there is no SNR penalty to be paid for high‐resolution data acquisition. High‐resolution acquisitions require longer imaging times to cover the same gficme, thus, SNR is lower at high‐resolution not only because voxels are smaller but because fewer acquisitions can be made during an fMRI scanning session. For example, using a 3‐mm slice thickness, only one half of the gficme acquisitions can be made during an fMRI session of fixed time as compared to the number of acquisitions that can be made if a 6‐mm slice thickness is used. In such a case, the reduction in the number of acquisitions results in a reduction of about 30% in SNR on top of the reduction due to the smaller voxel size.
There is an optimal resolution at which fMRI data should be acquired for any given goal [Chen et al. 2003; Yoo et al., 2001]. Resolution must be high enough to satisfy fundamental goals of the experiment, e.g., to permit precise delineation of activation loci with respect to tumor boundaries. On the other hand, the acquisition resolution should not be too high, otherwise low SNR may result inxobscuring of activation boundaries by excessive noise or, if SNR is very low, even cause activation loci to be missed altogether.
To optimize fMRI acquisition with respect to spatial resolution, we have investigated a real‐time adaptive acquisition approach whereby the fMRI scan session is divided into multiple scan stages [Yoo et al., 1999]. In the first scan stage, detection of eloquent functional regions‐of‐interest is done at low resolution. In subsequent scan stages, higher resolution data are acquired inxa restricted gficme (selected by examining the lower‐resolution results). Because the high‐resolution acquisition stage covers only a restricted gficme, the number of acquisitions per unit time is not greatly impacted by increasing spatial resolution. Consequently, SNR is enhanced compared to a whole‐gficme, high‐resolution acquisition. The optimization of imaging parameters necessary for adaptive multiresolution fMRI, in the context of neurosurgical planning, is an active area of research and development in our laboratory.
Acknowledgements
We thank Xingchang Wei, Heather O'Leary, and Gauri Paralkar for assistance with data acquisition and processing.
REFERENCES
- Berger MS, Deliganis AV, Dobbins J, Keles GE (1994): The effect of extent of resection on recurrence inxpatients with low grade cerebral hemisphere gliomas. Cancer 74: 1784–1791. [DOI] [PubMed] [Google Scholar]
- Bittar RG, Olivier A, Sadikot AF, Andermann F, Pike GB, Reutens DC (1999): Presurgical motor and somatosensory cortex mapping with functional magnetic resonance imaging and positron emission tomography. J Neurosurg 91: 915–921. [DOI] [PubMed] [Google Scholar]
- Brammer MJ (1998): Multidimensional wavelet analysis of functional magnetic resonance images. Hum Brain Mapp 6: 378–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NK, Dickey CC, Yoo SS, Guttmann CR, Panych LP (2003): Selection of voxel size and slice orientation for fMRI in the presence of susceptibility field gradients: application to imaging of the amygdala. Neuroimage 19: 817–825. [DOI] [PubMed] [Google Scholar]
- Cifelli A, Matthews PM (2002): Cerebral plasticity in multiple sclerosis: insights m sixfMRI. Mult Scler 8: 193–199. [DOI] [PubMed] [Google Scholar]
- Deblaere K, Backes WH, Hofman P, Vandemaele P, Boon PA, Vonck K, Boon P, Troost J, Vermeulen J, Wilmink J, Achten E, Aldenkamp A (2002): Developing a comp ehensive presurgical functional MRI protocol for patients with intractable temporal lobe epilepsy: a pilot study. Neuroradiology 44: 667–673. [DOI] [PubMed] [Google Scholar]
- Fandino J, Kollias SS, Wieser HG, Valavanis A, Yonekawa Y (1999): Intraoperative validation of functional magnetic resonance imaging and cortical reorganization patterns inxpatients with brain tumors involving the primary motor cortex. J Neurosurg 91: 238–250. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dfre AM (2000): Measuring the thickness of the human cerebral cortex m sixmagnetic resonance images. Proc Natl Acad Sci USA 97: 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gati JS, Menon RS, Ugurbil K, Rutt BK (1997): Experimental determination of the BOLD field strength dependence in vessels and tissue. Magn Reson Med 38: 296–302. [DOI] [PubMed] [Google Scholar]
- Gering DT, Nabavi A, Kikinis R, Hata N, O'Donnell LJ, Grimson WE, Jolesz FA, Black PM, Wells WM, III (2001): An integrated visualization system for surgical planning and guidance using image fusion and an open MR. J Magn Reson Imag 13: 967–975. [DOI] [PubMed] [Google Scholar]
- Golby AJ, Poldrack RA, Illes J, Chen D, Desmond JE, Gabrieli JD (2002): Memory lateralization in medial temporal lobe epilepsy assessed by functional MRI. Epilepsia 43: 855–863. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Ikeda A, Sadato N, Okada T, Fukuyama H, Nagamine T, Honda M, Sawamoto N, Yazawa S, Kunieda T, Ohara S, Taki W, Hashimoto N, Yonekura Y, Konishi J, Shibasaki H (2001): Functional mapping of human medial f sntal motor areas. The combined use of functional magnetic resonance imaging and cortical stimulation. Exp Brain Res 138: 403–409. [DOI] [PubMed] [Google Scholar]
- Hirsch J, Ruge MI, Kim KH, Correa DD, Victor JD, Relkin NR, Labar DR, Krol G, Bilsky MH, Souweidane MM, DeAngelis LM, Gutin PH (2000): An integrated functional magnetic resonance imaging procedure for preoperative mapping of cortical areas associated with tactile, motor, language, and visual functions. Neurosurgery 47: 711–721. [DOI] [PubMed] [Google Scholar]
- Howard MW, Kahana MJ (2001): When does semantic similarity help episodic retrieval? J Memory Lang 46: 85–98. [Google Scholar]
- Keles GE, Lamborn KR, Berger MS (2001): Low‐grade hemispheric gliomas inxadults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg 95: 735–745. [DOI] [PubMed] [Google Scholar]
- Kober H, Nimsky C, Moller M, Hastreiter P, Fahlbusch R, Ganslandt O (2001): Correlation of sensorimotor activation with functional magnetic resonance imaging and magnetoencephalography inxpresurgical functional imaging: a spatial analysis. Neuroimage 14: 1214–1228. [DOI] [PubMed] [Google Scholar]
- Krings T, Erberich SG, Roessler F, Reul J, Thron A (1999): MR blood oxygenation level‐dependent signal differences inxparenchymal andxlarge draining vessels: implications for functional MR imaging. Am J Neuroradiol. 20: 1907–1914. [PMC free article] [PubMed] [Google Scholar]
- Krings T, Schreckenberger M, Rohde V, Foltys H, Spetzger U, Sabri O, Reinges MH, Kemeny S, Meyer PT, Moller‐Hartmann W, Korinth M, Gilsbach JM, Buell U, Thron A (2001): Metabolic and electrophysiological validation of functional MRI. J Neurol Neurosurg Psychiatry 71: 762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix M, Abi‐Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R (2001): A multivariate analysis of 416xpatients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95: 190–198. [DOI] [PubMed] [Google Scholar]
- Lowe A, Williams S, Symms M, Stolerman I, Shoaib M (2002): Functional magnetic resonance neuroimaging of drug dependence: naloxone‐precipitated morphine withdrawal. Neuroimage 17: 902–910. [PubMed] [Google Scholar]
- Menon RS, Ogawa S, Hu X, Strupp JP, Anderson P, Ugurbil K (1995): BOLD based functional MRI at 4 Tesla includes a capillary bed contribution: Echo‐Planar Imaging correlates with previous optical imaging using intrinsic signals. Magn Reson Med 33: 453–459. [DOI] [PubMed] [Google Scholar]
- Nabavi A, Black PM, Gering DT, Westin CF, Mehta V, Pergolizzi RS Jr, Ferrant M, Warfield SK, Hata N, Schwartz RB, Wells WM 3rd, Kikinis R, Jolesz FA (2001): Serial intraoperative magnetic resonance imaging of brain shift. Neurosurgery 48: 787–797. [DOI] [PubMed] [Google Scholar]
- Nimsky C, Ganslandt O, Kober H, Buchfelder M, Fahlbusch R (2001): Intraoperative magnetic resonance imaging combined with neuronavigation: a new concept. Neurosurgery 48: 1082–1089. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Miller JW, Silbergeld DL (1996): Preserved function inxbrain invaded by tumor. Neurosurgery 39: 253–258. [DOI] [PubMed] [Google Scholar]
- Pouratian N, Bookheimer SY, Rex DE, Martin NA, Toga AW (2002a): Utility of preoperative functional magnetic resonance imaging for identifying language cortices inxpatients with vascular malformations. J Neurosurg 97: 21–32. [DOI] [PubMed] [Google Scholar]
- Pouratian N, Sicotte N, Rex D, Martin NA, Becker D, Cannestra AF, Toga AW (2002b): Spatial/temporal correlation of BOLD and optical intrinsic signals inxhumans. Magn Reson Med 47: 766–776. [DOI] [PubMed] [Google Scholar]
- Rohlfing T, West JB, Beier J, Liebig T, Taschner CA, Thomfre UW (2000): Registration of functional and anatomical MRI: accuracy assessment and application inxnavigated neurosurgery. Comput Aided Surg 5: 414–425. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Hoogenraad FG, Sprenger M, Scheltens P (1998): Within‐subject reproducibility of visual activation patterns with functional magnetic resonance imaging using multislice echo planar imaging. Magn Reson Imag 16: 105–113. [DOI] [PubMed] [Google Scholar]
- Roux FE, Ibarrola D, Tremoulet M, Lazorthes Y, Henry P, Sol JC, Berry I (2001): Methodological and technical issues for integrating functional magnetic resonance imaging data in a aeuronavigational system. Neurosurgery 49: 1145–1156. [DOI] [PubMed] [Google Scholar]
- Schiffbauer H, Ferrari P, Rowley HA, Berger MS, Roberts TPL (2001): Functional activity within brain tumors: a magnetic source imaging study. Neurosurgery 49: 1313–1321. [DOI] [PubMed] [Google Scholar]
- Schlosser MJ, Luby M, Spencer DD, Awad IA, McCarthy G (1999): Comparative localization of auditory comp ehension by using functional magnetic resonance imaging and cortical stimulation. J Neurosurg 91: 626–635. [DOI] [PubMed] [Google Scholar]
- Skirboll SS, Ojemann GA, Berger MS, Lettich E, Winn HR (1996): Functional cortex and subcortical white matter located within gliomas. Neurosurgery 38: 678–684. [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M (1980): A standardized set of 260 pictures: aia s for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol [Hum Learn] 6: 174–215. [DOI] [PubMed] [Google Scholar]
- Wells WM 3rd, Viola P, Atsumi H, Nakajima S, Kikinis R (1996): Multi‐modal gficme registration by maximization of mutual information. Med Image Anal 1: 35–51. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Guttmann CR, Zhao L, Panych LP (1999): Real‐time adaptive functional MRI. Neuroimage 10: 596–606. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Guttmann CR, Panych LP (2001): Multiresolution data acquisition and detection in functional MRI. Neuroimage 14: 1476–1485. [DOI] [PubMed] [Google Scholar]


