Abstract
High‐resolution event‐related potentials (ERPs) were used to model the hemispherical representation of the transient cortical responses relating to the observation of movement during execution (right or left aimless finger extension). Subjects were seated in front of the observed person and looked at both their own and the observer's hand to receive similar visual feedback during the two conditions. In a visual control condition, a diode light moved at the observed person's hand. A first potential accompanying the movement execution peaked at about +110 msec over the contralateral somatomotor areas. It was followed by a potential (P300) peaking at about +350 msec over the central midline. In contrast, the potentials accompanying the movement observation peaked later over parietal‐occipital other than somatomotor areas (N200 peak, +200 msec; P300 peak, +400 msec). Notably, the N200 was maximum in left parietal area whereas the P300 was maximum in right parietal area regardless the side of the movement. They markedly differed by the potentials following the displacement of the diode light. These results suggest a rapid time evolution (∼200–400 msec) of the cortical responses characterizing the observation of aimless movements (as opposite to grasping or handling). The execution of these movements would mainly involve somatomotor cortical responses and would be scarcely founded on the visual feedback. In contrast, the observation of the same movements carried out by others would require dynamical responses of somatomotor and parietal‐occipital areas (especially of the right hemisphere), possibly for a stringent visuospatial analysis of the motor event. Hum. Brain Mapping 20:148–157, 2003. © 2003 Wiley‐Liss, Inc.
Keywords: voluntary simple movements, movement observation, high‐resolution EEG, event‐related potentials (ERPs), cerebral cortex, hemispherical asymmetry
INTRODUCTION
For an individual to understand an action carried out by another person, audiovisual stimuli are matched with internal sensorimotor representations stored in temporal, parietal, and frontal areas [di Pellegrino et al., 1992; Gallese et al., 1996; Rizzolatti et al., 1996a]. In humans, the understanding of an action carried out by others might share some basic mechanisms with the imagery and understanding of gestures and language [Jeannerod, 2001; Rizzolatti and Arbi, 1998]. In fact, aphasic patients are not able to recognize pantomimic action [Bell, 1994], whereas stutterers simultaneously freeze the speech and hand gestures [Mayberry et al., 1998]. With this in mind, one might speculate that the left hemisphere in right‐handed subjects may play a leading role in the understanding of observed movements, in terms of language control and hand actions [Civardi et al., 2000; Kim et al., 1993; Netz et al., 1995; Schroeder et al. 1995; Ziemann and Hallett, 2001]. Previous studies measuring regional cerebral blood flow have shown contrasting results on this matter. A functional prevalence of the left prefrontal, premotor, temporal, and posterior parietal areas was detected during the movement observation [Decety et al., 1997; Grafton et al., 1996; Grèzes et al., 1998; Rizzolatti et al., 1996b]. However, other evidence supported a functional prevalence of the right posterior parietal cortex [Iacoboni et al., 1999].
Electroencephalographic (EEG) and magnetoencephalographic (MEG) techniques estimate brain electromagnetic oscillations [Hari et al., 1998; Nuñez, 1995; Pfurtscheller and Lopes da Silva, 1999; Pfurtscheller and Neuper, 1994; von Stein and Sarnthein, 2000]. The analysis of these oscillations has allowed for modeling of the gating function (opening/closure) of bidirectional thalamo‐cortical connections during the observation of movement. A widespread decrease of the α (8–12 Hz) and β (13–25 Hz) oscillations was recognized during a prolonged movement observation [Cochin et al., 1998, 1999]. When the temporal dynamics of the α oscillations were studied during rapid events, the magnitude of the parietal α oscillations decreased during a brief movement observation and increased after its termination [Babiloni et al., 2002]. Finally, the movement observation modulated the reactivity of the central β oscillations (about 20 Hz) provoked by the median nerve stimulation [Hari et al., 1998; Rossi et al., 2002]. There was a reduction of this β reactivity during the concomitant execution, or observation of the movements, as an effect of these events on the primary somatomotor cortex contralateral to the median nerve stimulation [Hari et al., 1998; Rossi et al., 2002].
Brain electromagnetic oscillations represent functional states lasting tens to hundreds of milliseconds. To model brain responses at a higher temporal resolution, event‐related potentials (ERPs) and their magnetic counterpart were obtained by a standard averaging technique. During a right precision pinching, the event‐related magnetic fields were generated before in the left inferior frontal cortex (−250 msec from the movement onset) and then in the left (−100 msec) and right (+100 msec) primary somatomotor areas [Nishitani and Hari, 2000]. During the observation of that movement, the temporal sequence started in the left posterior cortex. The observation of a complex motor act increased the evoked potentials/magnetic fields peaking 30 msec after the median nerve stimulation, suggesting an early facilitation of the contralateral primary somatomotor cortex [Rossi et al., 2002].
We re‐evaluated the recently analyzed high‐resolution EEG data to characterize the cortical rhythmicity during versus after the observation of brief simple finger movements [Babiloni et al., 2002]. These EEG data were averaged to obtain ERPs having a time resolution (2.5 msec) suitable to model the transient cortical responses during the movement observation. A central issue was the hemispherical representation of cortical responses characterizing the movement observation from execution, which would reflect the different sensorimotor representations accounting for one's own motor acts and those of others. To this aim, subjects were seated in front of the observed person and were asked to look at both their own and the observer's hand to receive a similar visual feedback during the two conditions. Notably, the experimental design included the observation and execution of both right and left finger movements. In previous EEG and MEG studies on movement observation, a conclusion on the above issue was prevented by the use of only right movements [Nishitani and Hari, 2000; Rossi et al., 2002].
MATERIALS AND METHODS
The procedures relative to subjects, EEG recordings, and preliminary analysis have been described in detail in the companion study on EEG rhythmicity [Babiloni et al., 2002].
The experiments were carried out in 10 right‐handed young adults (observers) seated in front of the observed person. The hands of the observer and of the observed persons were placed close to each other, so that the observer could look at their own and the observed person's hands while fixing on a central target. This ensured that the observer received the same visual stimulation looking at the their own moving hand as well as at the moving hand of the observed person.
The experimental paradigm included four conditions (pseudo‐random order). In the first and second conditions, the observer executed brisk, self‐initiated right (Rt‐EXE) and left (Lt‐EXE) middle‐finger extensions, respectively. In the third and fourth conditions, the observer passively saw the observed person performing these movements (Rt‐OBS and Lt‐OBS). The inter‐movement interval was between 6 and 20 sec.
The EEG data were recorded (400 Hz sampling frequency) with a 128 tin electrode cap referenced to the linked ears. The electrooculogram and surface electromyographic (EMG) activity of the bilateral extensor digitorum muscles were also collected. The surface EMG activity from the observed person triggered the EEG acquisition system during the Rt‐OBS and Lt‐OBS conditions. The onset of the EMG response served as a zero time.
In a subgroup of five subjects, the aforementioned set up was used for a control experiment in which a light moved across four diodes (inter‐diodes distance of 1 cm). The diodes were mounted on a ring‐device worn on the observed person's middle finger. The light was switched on (800 msec) at left or right hand in pseudo‐randomized blocks (left LIGHT and right LIGHT).
The spatial resolution of the EEG data was enhanced by surface Laplacian estimation [Nunez, 1995; Babiloni et al., 1996]. The data set of one subject was discarded because of the low number of artifact‐free EEG single trials (i.e., <30%).
Computation of ERPs
The artifact‐free Laplacian EEG data were averaged with reference to the zero time to produce the ERPs. The same number of EEG single trials was used for the movement execution and observation, namely 46 single trials (±4 SE) for the Rt‐EXE or Rt‐OBS conditions and 43 single trials (±4 SE) for the Lt‐EXE or Lt‐OBS conditions. A corresponding number of artifact‐free single trials was selected for the Lt‐LIGHT and Rt‐LIGHT conditions.
The Laplacian ERPs were interpolated by a spline function [Babiloni et al., 1995] to obtain the potentials at the 105 electrode sites of an augmented 10–20 system, disposed over a 3‐D quasi‐realistic head model (Brain Imaging Center of the Montreal Neurological Institute, SPM96. Based on the position of the 10–20 system electrodes, the regions of interest (ROIs) were called left frontal (Fl), right frontal (Fr), left central (Cl), centromedian (Cm), right central (Cr), left parietal‐occipital (Pl), and right parietal‐occipital (Pr). The electrodes of the ROIs are listed in Table I.
Table I.
Electrodes of 10–20 system selected to represent scalp regions of interest*
| ROI | Electrodes |
|---|---|
| F left | F5, F3, F1, F5‐FC3, F3‐FC1 |
| F right | F2, F4, F6, F2‐FC4, F4‐FC6 |
| C left | C5, C3, CP5, CP3, FC5‐C3, FC3‐C1, C5‐CP3, C3‐CP1 |
| C median | Cz, FC1‐Cz, FCz‐C2, C1‐CPz, Cz‐CP2 |
| C right | C4, C6, CP4, CP6, FC2‐C4, FC4‐C6, C2‐CP4, C4‐CP6 |
| P left | P5, P3, P1, P5‐PO3, P1‐POz |
| P right | P2, P4, P6, P2‐PO4, P4‐PO8 |
These regions included the right and left frontal‐lateral areas (F), the right and left central‐lateral area (C), the centromedian area (Cm), the right and left parieto‐occipital areas (P). Hyphen between two electrode labels indicates the name of an electrode placed halfway the two labeled electrodes.
ROI, region of interest.
Taking into account the fact that surface Laplacian estimation can not discriminate the activity of contiguous cortical areas [Gevins et al., 1995; Nunez et al., 1994, 1995], it was assumed that frontal scalp EEG oscillations originated from prefrontal cortex. Of note, the spatial resolution of this estimation could not disentangle ventral and dorsal parts of the prefrontal cortex. Therefore, we could not isolate the activity of the Broca's area, so important for the physiology of movement observation [Rizzolatti et al., 1998]. It was assumed that central scalp EEG oscillations originated from lateral premotor (PM) and primary sensorimotor (M1‐S1) cortex (i.e., no discrimination between ventral and dorsal PM, PM and M1, M1 and S1). Finally, parietal‐occipital scalp EEG oscillations originated from posterior parietal and parieto‐occipital cortex (i.e., no discrimination between superior and posterior parietal lobules). Such a correspondence was found in previous anatomopathological studies [Blume et al., 1974] and in vivo [Homan et al., 1987; Towle et al., 1993]. We did not consider the temporal areas because the insufficient amount of EEG spatial information around the temporal electrodes could make the local surface Laplacian estimates unreliable.
Measurement of ERP Latency and Amplitude
For each condition, we considered the main components of the ERPs related to the preparation (readiness potential, RP), initiation (motor potential, MP), and performance of the movement (movement‐related response 1, MRR1) [Deecke et al., 1976; Shibasaki et al., 1980; Urbano et al., 1996, 1998]. These ERPs were called movement‐related potentials (MRPs). Furthermore, we measured the negative (N) and positive (P) ERP components accompanying the movement execution (after MRR1) and observation.
The latency of ERPs peaks was independently recognized by two experimenters within frontal, central and parietal ROIs with respect to the movement onset (zero time). The ROI having the maximal ERP values was considered as a reference for the latency of the topographical mapping. The ERP values were measured automatically for each experimental condition at each of the 105 electrodes.
Statistical Analysis of ERPs
The ERPs were normalized to make the data Gaussian and homoschedastic. The amplitude of the normalized ERPs was compared between conditions by a statistical procedure described in the companion article on the EEG rhythms [Babiloni et al., 2002]. In brief, the responsive ERPs were averaged within each scalp ROI, i.e., Fl, Fr, Cl, Cm, Cr, Pl, Pr. The averaged amplitude was used as an input for the ANOVA analysis for repeated measures. The factors of the ANOVA analysis were Condition (EXE, OBS), Side of the movement (right and left), ROI (Fl, Fr, Cl, Cm, Cr, Pl, Pr), and ERP component (ERP peaks). Mauchley's test evaluated the sphericity assumption. Correction of the degrees of freedom was made by Greenhouse–Geisser procedure. Duncan test was used for post‐hoc comparisons (P < 0.05).
RESULTS
Spatio‐Temporal Evolution of ERP Peaks
Figure 1 shows representative ERPs relative to the execution (EXE) and observation (OBS) of the right (RT) and left (LT) finger movements. The EMG activity of the observer and the observed are also plotted, to better appreciate their temporal relationship with MRPs. During the motor preparation and initiation, standard MRPs were observed. An earlier (−1.0 msec) slow negative shift (readiness potential, RP) was maximum at the centromedian (Cz) and contralateral central (C3 or C4) electrodes. Close to the movement onset, the maximum negativity was observed at the contralateral central electrode (motor potential, MP). During the first phase of the movement execution, transient parietal positivity (P3 or P4 electrode) and frontal negativity (F3 or F4 electrode) peaked at about +110 msec (movement‐related response 1, MRR1). A peak of centromedian positivity was detected at about +350 msec (P300; Cz electrode). This centromedian positivity (P300) slowly recovered to baseline about 1 sec later.
Figure 1.
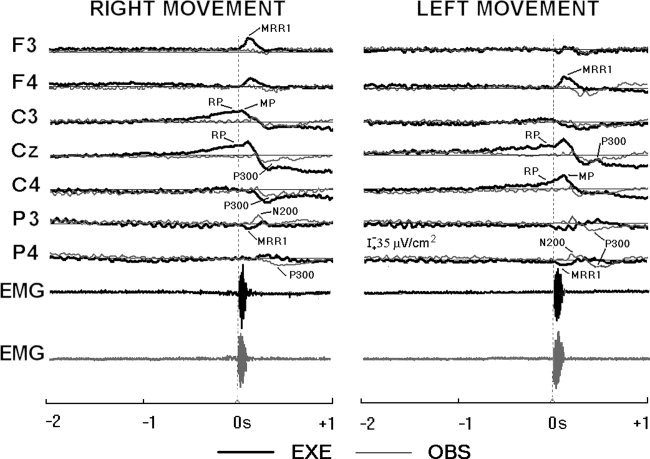
Data from nine subjects. Grand average waveforms of the event‐related potentials (ERPs). These waveforms refer to representative electrodes of 10–20 of the montage system for the four experimental conditions, i.e., the execution (EXE) of self‐initiated right or left finger movements and the observation (OBS) of right or left finger movements. Also, the grand average waveforms of the EMG activity of the observer and the observed are reported.
The topography of the MRPs can be well appreciated in Figure 2. The quality of the present experiments is confirmed by the agreement of these MRPs with those reported in previous scalp and intracranial EEG studies [Babiloni et al. 1999; Botzel et al., 1993; Cui et al. 1999; Cui and Deecke, 1999; Deecke et al. 1976; Hallett, 1994; Ikeda et al., 1992, 1995; Rektor et al., 1994; Shibasaki et al. 1980; Urbano et al. 1996, 1998].
Figure 2.
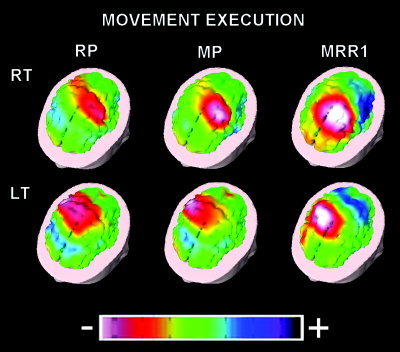
Three‐dimensional color maps of movement‐related potentials during the execution of right (RT) and left (LT) movements. The maps refer to the peaks of readiness potential (RP), motor potential (MP), and movement‐related response 1 (MRR1). Color scale: maximum normalized negativity (white) and maximum normalized positivity (violet).
No anticipatory potential was detected before the movement observation, indicating no effect of expectancy processes. During the movement observation the ERPs showed a posterior negativity peaking at about +200 msec (N200; P3 and P4 electrodes for left movement observation and P3 electrode for right movement observation), which was followed by a positive deflection (P300) peaking at centromedian (Cz) and posterior (P4) electrodes. Interestingly, movement execution (EXE) and observation (OBS) induced a detectable P300 peak in all subjects; instead, only movement observation (OBS) induced the N200 peak.
Table II reports the mean latency (±SE) of the N200 and P300 peaks together with the electrode where the peak latencies were found. The N200 peaks ranged from +209–+237 msec, whereas the P300 peaks ranged from +339–+441 msec. Notably, these transient potentials peaked before the returning of the moving finger at the rest position (+600–+800 msec).
Table II.
Peak latency of event‐related potentials for the four experimental conditions*
| Location (electrode) of ERPs | ||
|---|---|---|
| N200 | P300 | |
| RT‐EXE | — | +342 (24) Cz |
| RT‐OBS | +209 (9) P3 | +359 (24) P4 |
| LT‐EXE | — | +339 (16) Cz |
| LT‐OBS | +237 (21) P3 | +441 (33) P4 |
Mean latency (± SE) in M sec.
The execution of self‐initiated right (RT‐EXE) or left (LT‐EXE) finger movements and the observation of the same movements performed by others at the right (RT‐OBS) or left (LT‐OBS) sides. It is also reported the electrode where the peak latencies was found.
Figure 3 shows the topographical maps of the group late ERPs (N200 and P300) accompanying the execution (EXE) and observation (OBS) of the right and left finger movements. Given the absence of a detectable N200 peak during the movement execution (EXE), we used the latencies of the N200 peak as detected during the movement observation for mapping a “pseudo” N200 during the movement execution (EXE).
Figure 3.
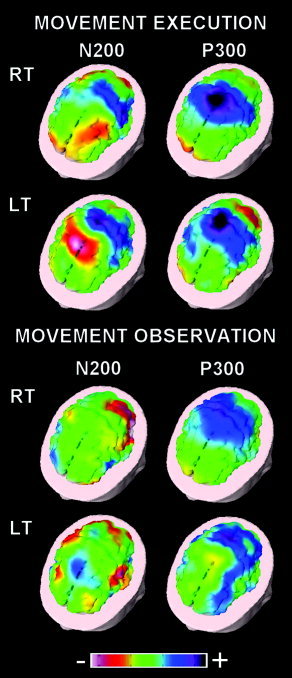
Three‐dimensional color maps of ERPs accompanying the execution and observation of right (RT) and left (LT) movements. The maps refer to N200 and P300 peaks. Color scale: maximum normalized negativity (white) and maximum normalized positivity (violet).
For the movement execution, the pseudo “N200” maps showed a frontal negativity residual of the MRR1 together with a central positivity (rising P300). Furthermore, the P300 maps were characterized by a centromedian positivity. In contrast, the N200 maps for the movement observation were characterized by a centro‐lateral and parietal negativity. Afterward, the P300 maps pointed to a large positivity preponderant in the right parietal other than central areas.
Statistical Analysis of ERP Peaks
The ANOVA analysis of the late ERP peaks showed a statistical interaction (F[6,48] = 11.73; MSe = 338; P < 0. 00001) among the factors Condition (EXE, OBS), ROI (Fl, Fr, Cl, Cm, Cr, Pl, Pr), and ERP component (N200, P300). Figure 4 illustrates the means (±SE) of the ERPs representing these statistical results. Duncan post‐hoc test indicated that, compared to the movement execution, the movement observation induced a stronger N200 peak in centromedian, bilateral central, and left parietal‐occipital areas (P < 0. 0005), whereas the P300 peak prevailed in right central and parietal‐occipital areas (P < 0.05). In contrast, the centromedian P300 peak was stronger during the movement execution than observation (P < 0.05).
Figure 4.
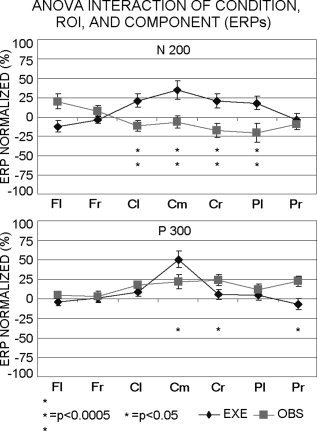
Group means (±SE) of the ERP amplitude (ANOVA). Means refer to a statistical interaction among the factors Condition (EXE, OBS), ROI (Fl, Fr, Cl, Cm, Cr, Pl, Pr), and Component (N200, P300). *Duncan post‐hoc testing, P < 0.05; **Duncan post‐hoc testing, P < 0. 0005. ROIs: Fl, left frontal; Fr, right frontal; Cl, left central; Cm, centromedian; Cr, right central; Pl, left parietal‐occipital; Pr, right parietal‐occipital.
Control Experiment
Figure 5 shows the group (five subjects) waveforms of the ERPs following the observation of the movement (OBS) and the displacement of a diode light (LIGHT). The diode light was presented by a ring‐device worn on the observed person. The waveforms refer to the main frontal (F3, F4), central (C3, Cz, C4), parietal (P3, P4), and occipital (O1, O2) electrodes of both hemispheres. It also represented the EMG activity of the observer, to emphasize the absence of involuntary finger movements during the movement and light observation. The time course of the N200 and P300 peaks can be clearly recognized during the movement observation, in line with the ERPs averaged across all subjects (Fig. 1). In contrast, the ERPs during the visual control condition presented very different features. They consisted of several transient variations of positivity and negativity showing an oscillatory nature rather than a clear sequence of N200 and P300 potentials. Of note, the same number of the EEG single trials was averaged for the movement observation and the displacement of the light.
Figure 5.
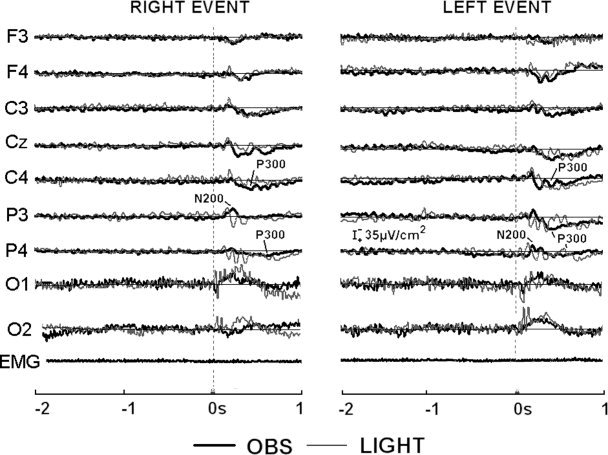
Data from five subjects. Grand average waveforms of ERPs for the conditions of movement observation (OBS) and of displacement of a diode light (LIGHT). The visual stimulus was a ring device worn on the observed person's right hand. The waveforms refer to the main frontal (F3, F4), central (C3, C4), parietal (P3, P4), and occipital (O1, O2) electrode sites of both hemispheres. The grand average waveform of the EMG activity of the observer during the movement observation (OBS) is represented.
DISCUSSION
A main issue of this study was the hemispherical representation of the ERPs characterizing the execution of a simple movement respect to the same movement carried out by others. To provide the same visual feedback in the two conditions, subjects looked at both own and observer's hand. The ERPs for the movement execution and observation showed remarkable spatiotemporal differences, reflecting the separate sensorimotor representations characterizing own movement respect to the others' movement. During the movement execution with the visual feedback, the MRPs (MRR1) peaked at about +110 msec over the contralateral somatomotor areas, whereas the P300 peaked at about +350 msec over the central midline. This centromedian positivity (P300) slowly recovered to baseline after the movement execution. Further studies should test the hypothesis that this long lasting positivity was a long‐lasting recovery specifically induced by the visuomotor transformations following movement execution (EXE) and if that positivity could be modulated by the motor features of the motor preparation. In contrast, the N200 and P300 accompanying the movement observation peaked later (+200 msec and +400 msec, respectively) and were distributed over parietal‐occipital other than somatomotor areas. The limited spatial resolution of the EEG technique did not allow a fine dissociation of contiguous posterior parietal and occipital responses, thus we considered a unique parietal‐occipital region. Present findings suggest a scarce cortical effect of the visual feedback concomitant with the movement execution, with the most important cortical responses being localized over somatomotor areas. However, the cortical responses accompanying the movement observation involved parietal‐occipital other than somatomotor areas, stressing the importance of the event‐related visuomotor processes. The parietal‐occipital areas may play a key role in the understanding of the motor act of another person, in accordance with the functions of cortical dorsal stream for the representation of the motor commands associated with visuospatial motor trajectory [Jeannerod et al., 1995; Goodale, 1998; Rizzolatti et al., 1997].
The ERPs characterizing the movement observation were maximum in left hemisphere (N200 peak at about +200 msec) and then lateralized to the right hemisphere (P300 peak at about +400 msec), regardless the side of the movement. These findings are consistent with a dynamical inter‐hemispherical representation of the ERPs characterizing the movement observation from execution. Furthermore, they extend the static prevalence of the right hemisphere for the movement observation as suggested previously by techniques having a low temporal resolution [Grèzes and Decety, 2001]. Regional cerebral blood flow studies have shown that, compared to the movement execution, the movement observation induced a prevalent activation of the right parietal or occipital areas [Decety et al., 1997; Iacoboni et al., 1999].
The polarity of the present ERPs might enlighten some peculiar functional aspects. The negative N200 accompanying the movement observation may reflect the balance between excitatory and inhibitory post‐synaptic activity [Rossi et al., 2000] in the negative MRPs [Babiloni et al., 1999; Chen et al., 1998; Deecke et al. 1976; Hallett, 1994; Leocani et al., 2000; Shibasaki et al. 1980]. In contrast, the positive P300 might mainly index cortical processes for the inhibition of irrelevant motor acts (i.e., imitation). These speculations agree with previous evidence showing a tight correlation between negative ERPs and readiness to respond [Brody et al., 1994] and between positive ERPs and focused motor responses [Chisholm and Karrer, 1988]. Other evidence comes from oddball P300 and Go/Nogo studies [Birbaumer et al., 1990; Brody et al., 1994; Gevins et al., 1989; Kok, 1986; Regan, 1989; Schupp et al., 1994; Woodward et al., 1991].
The present results agree with those of an influential MEG study [Nishitani and Hari, 2000] showing that the observation of a right precision pinching was characterized by an early occipital response. Furthermore, they only seem at variance of those showing responses of the left inferior prefrontal area during both movement execution and observation [Nishitani and Hari, 2000]. Indeed, the left inferior prefrontal cortex may contribute to the understanding of aimed and verbally coded actions, represented within a sort of pragmatic motor dictionary [Jeannerod et al., 1995; Rizzolatti et al., 1998, 1999]. In keeping with this, neuroimaging evidence disclosed a functional prevalence of the left hemisphere during the observation of aimed actions [Decety et al., 1997; Grafton et al., 1996; Grèzes et al., 1998 Rizzolatti et al., 1996b;]. The dominance of the left hemisphere suggests that the cortical system for the observation/execution matching may bridge “doing” and “communicating” functions, thus making actor and observer equivalent to sender and receiver of a message [Kohler et al., 2002; Nishitani and Hari 2000; Rizzolatti and Arbib, 1998]. An involvement of the left prefrontal cortex is linked less logically with the execution (with visual feedback) and observation of the present aimless finger movement, which is not goal‐oriented. It is reasonable that the observation of such a movement requires a stringent analysis of the kinematics and visio‐spatial parameters by parietal‐occipital areas. It is noteworthy that there was no dominance of the left hemisphere during the movement observation, in agreement a with a recent transcranial magnetic resonance (TMS) study [Aziz‐Zadeh et al., 2002]. This may be due to the fact that, in the present and in the mentioned study, subjects observed aimless finger movements of left or a right hand. At apparent odds with the present results, the TMS study showed an effect of the movement observation side [Aziz‐Zadeh et al., 2002]. When the TMS was applied to the left motor cortex, MEPs were larger during the observation of right than left hand movement and vice versa for the TMS of the right motor cortex. The differences are probably due to the different functional meaning of the TMS and ERP measurements. The TMS at hand primary motor cortex focused on the cortico‐spinal motor pathway contralateral to the stimulation, whereas the ERPs provided a rough index of excitatory and inhibitory post‐synaptic potentials at large cortical regions of interest of both hemispheres.
One may argue that the N200‐P300 accompanying the movement observation was to aspecific visual and attentional features rather than to visio‐motor transformations. To confute experimentally this explanation, the ERPs accompanying the movement observation and the displacement of a diode light were profoundly different. The movement observation was characterized by the N200 and P300, whereas the displacement of the diode light in the same subjects evoked potentials having a clear oscillatory nature. This different structure of the ERPs prevented any statistical comparison and indicated that the N200 and P300 accompanying the movement observation cannot be substantially explained in simple terms of aspecific attentional and visual variables. This conclusion is consistent with previous evidence demonstrating that generic visual stimuli and attentional variables do not mimic the effects of movement observation on sensorimotor cortical areas [Fadiga et al., 1995; Hari et al., 1998; Rizzolatti et al., 1996a].
CONCLUSIONS
In the present study, the ERPs modeled the hemispherical representation of the transient cortical responses characterizing the observation of brisk aimless movements with respect to the movement execution (similar visual feedback). A first potential accompanying the movement execution peaked at about +110 msec over the contralateral somatomotor areas. It was followed by a potential (P300) peaking at about +350 msec over the central midline. In contrast, the potentials accompanying the movement observation peaked later over parietal‐occipital other than somatomotor areas (N200 peak at +200 msec and P300 peak at +400 msec). Notably, the maximum N200 peak was in the left parietal area, whereas the maximum P300 peak was in the right parietal area, regardless the side of the movement.
The potentials accompanying the movement observation differed markedly by the potentials following the displacement of the diode light. These results suggest a rapid time evolution (about 200–400 msec) of the cortical responses characterizing the observation of aimless movements (as opposite to grasping or handling). The execution of these movements would mainly involve somatomotor cortical responses and would be scarcely founded on the visual feedback. In contrast, the observation of the same movements carried out by others would require dynamical responses of somatomotor and parietal‐occipital areas (especially of the right hemisphere), possibly for a stringent visuospatial analysis of the motor event.
Acknowledgements
We thank Prof. F. Eusebi, Chairman of the Biophysics Group of Interest of the Department of Human Physiology and Pharmacology, University of Rome La Sapienza, for his continuous support. Furthermore, we thank Prof. G. Rizzolatti and his coworkers for their constructive criticism and useful suggestions.
REFERENCES
- Aziz‐Zadeh L, Maeda F, Zaidel E, Mazziotta J, Iacoboni M (2002): Lateralization in motor facilitation during action observation. A TMS study. Exp Brain Res 144: 127–131. [DOI] [PubMed] [Google Scholar]
- Bell BD (1994): Pantomime recognition impairment in aphasia: an analysis of error types. Brain Lang 47: 269–278. [DOI] [PubMed] [Google Scholar]
- Babiloni F, Babiloni C, Carducci F, Fattorini L, Onorati P, Urbano A (1995): Performances of surface Laplacian estimators: a study of simulated and real scalp potential distributions. Brain Topogr 10: 33–45. [DOI] [PubMed] [Google Scholar]
- Babiloni F, Babiloni C, Carducci F, Fattorini L, Onorati P, Urbano A (1996): Spline Laplacian estimate of EEG potentials over a realistic magnetic resonance‐constructed scalp surface model. Electroencephalogr Clin Neurophysiol 98: 204–215. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Carducci F, Cincotti F, Rossini PM, Neuper C, Pfurtscheller G, Babiloni F (1999): Human movement‐related potentials vs. desynchronization of EEG alpha rhythm: a high‐resolution EEG study. Neuroimage 10: 658–665. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Babiloni F, Carducci F, Cincotti F, Cocozza G, Del Percio C, Moretti DV, Rossini PM (2002): Human cortical EEG rhythms during the observation of aimless movements: a high resolution EEG study. Neuroimage 17: 559–572. [PubMed] [Google Scholar]
- Birbaumer N, Elbert T, Canavan AG, Rockstroh B (1990): Slow potentials of the cerebral cortex and behavior. Physiol Rev 70: 1–41. [DOI] [PubMed] [Google Scholar]
- Blume WT, Buza RC, Okazaki H (1974): Anatomic correlates of the ten‐twenty electrode placement system in infants. Electroencephalogr Clin Neurophysiol 36: 303–307. [DOI] [PubMed] [Google Scholar]
- Botzel K, Plendl H, Paulus W, Scherg M (1993): Bereitschaftspotential: is there a contribution of the supplementary motor area? Electroencephalogr Clin Neurophysiol 89: 187–196. [DOI] [PubMed] [Google Scholar]
- Brody S, Rau H, Kohler F, Schupp H, Lutzenberger W, Birbaumer N (1994): Slow cortical potential biofeedback and the startle reflex. Biofeedback Self Regul 19: 1–11. [DOI] [PubMed] [Google Scholar]
- Chen R, Yaseen Z, Cohen LG, Hallett M (1998): Time course of corticospinal excitability in reaction time and self‐paced movements. Ann Neurol 44: 317–325. [DOI] [PubMed] [Google Scholar]
- Cochin S, Barthelemy C, Lejeune B, Roux S, Martineau J (1998): Perception of motion and qEEG activity in human adults. Electroencephalogr Clin Neurophysiol 107: 287–295. [DOI] [PubMed] [Google Scholar]
- Cochin S, Barthelemy C, Roux S, Martineau J (1999): Observation and execution of movement: similarities demonstrated by quantified electroencephalography. Eur J Neurosci 11: 1839–1842. [DOI] [PubMed] [Google Scholar]
- Chisholm RC, Karrer R (1988): Movement‐related potentials and control of associated movements. Int J Neurosci 42: 131–148. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cavalli A, Naldi P, Varrasi C, Cantello R (2000): Hemispheric asymmetries of cortico‐cortical connections in human hand motor areas. Clin Neurophysiol 111: 624–629. [DOI] [PubMed] [Google Scholar]
- Cui RQ, Deecke L (1999): High resolution DC‐EEG analysis of the Bereitschaftspotential and post movement onset potentials accompanying uni‐ or bilateral voluntary finger movements. Brain Topogr 11: 233–249. [DOI] [PubMed] [Google Scholar]
- Cui RQ, Huter D, Lang W, Deecke L (1999): Neuroimage of voluntary movement: topography of the Bereitschaftspotential, a 64‐channel DC current source density study. Neuroimage 9: 124–134. [DOI] [PubMed] [Google Scholar]
- Deecke L, Grozinger B, Kornhuber H (1976): Voluntary finger movements in man: cerebral potentials and theory. Biol Cybern 23: 99–119. [DOI] [PubMed] [Google Scholar]
- Decety J, Grezes J, Costes N, Perani D, Jeannerod M, Procyk E, Grassi F, Fazio F (1997): Brain activity during observation of actions. Influence of action content and subject's strategy. Brain 120: 1763–1777. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G (1992): Understanding motor events: a neurophysiological study. Exp Brain Res 91: 176–180. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G (1995): Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol 73: 2608–2611. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G (1996): Action recognition in the premotor cortex. Brain 119: 593–609. [DOI] [PubMed] [Google Scholar]
- Gevins AS, Bressler SL, Morgan NH, Cutillo BA, White RM, Greer DS, Illes J (1989): Event‐related covariances during a bimanual visuomotor task. I. Methods and analysis of stimulus‐ and response‐locked data. Electroencephalogr Clin Neurophysiol 74: 58–75. [DOI] [PubMed] [Google Scholar]
- Gevins A, Leong H, Smith M, Le J, Du R (1995): Mapping cognitive brain function with modern high‐resolution electroencephalography. Trend Neurosci 18: 429–436. [DOI] [PubMed] [Google Scholar]
- Goodale MA (1998): Vision for perception and vision for action in the primate brain. Novartis Found Symp 218: 21–34. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G (1996b): Localization of grasp representations in humans by positron emission tomography. Exp Brain Res 112: 103–111. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Costes N, Decety J (1998): Top down effect of the strategy to imitate on the brain areas engaged in perception of biological motion: a PET investigation. Cogn Neuropsychol 15: 553–582. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Decety J (2001): Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta‐analysis. Hum Brain Mapp 12: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M (1994): Movement‐related cortical potentials. Electromyogr Clin Neurophysiol 34: 5–13. [PubMed] [Google Scholar]
- Homan RW, Herman J, Purdy P (1987): Cerebral location of international 10‐20 system electrode placement. Electroencephalogr Clin Neurophysiol 66: 376–382. [DOI] [PubMed] [Google Scholar]
- Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G (1998): Activation of human primary motor cortex during action observation: a neuromagnetic study. Proc Natl Acad Sci U S A 95: 15061–15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G (1999): Cortical mechanisms of human imitation. Science 286: 2526–2528. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Luders HO, Burgess R, Shibasaki H (1992): Movement‐related potentials recorded from supplementary motor area and primary motor area. Brain 115: 1017–1043. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Luders HO, Shibasaki H, Collura TF, Burgess R, Morris HH, Hamano T (1995): Movement‐related potentials associated with bilateral simultaneous and unilateral movements recorded from human supplementary motor area. Electroencephalogr Clin Neurophysiol 95: 323–334. [DOI] [PubMed] [Google Scholar]
- Jeannerod M (2001): Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage 14: 103–109. [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Arbib MA, Rizzolatti G, Sakata H (1995): Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci 18: 314–320. [PubMed] [Google Scholar]
- Kim S, Ashe J, Hendrich K, Ellermann J, Merkle H, Ugurbil K, Georgopulos A (1993): Functional magnetic resonance imaging of motor cortex: hemispherical asymmetry and handedness. Science 261: 615–617. [DOI] [PubMed] [Google Scholar]
- Kohler E, Keysers C, Umilta MA, Fogassi L, Gallese V, Rizzolatti G. (2002): Hearing sounds, understanding actions: action representation in mirror neurons. Science 297: 846–848. [DOI] [PubMed] [Google Scholar]
- Kok A (1986): Effects of degradation of visual stimulation on components of the event‐related potential (ERP) in go/nogo reaction tasks. Biol Psychol 23: 21–38. [DOI] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M (2000): Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain 123: 1161–1173. [DOI] [PubMed] [Google Scholar]
- Mayberry RI, Jaques J, DeDe G (1998): What stuttering reveals about the development of the gesture‐speech relationship. New Dir Child Dev 79: 77–87. [DOI] [PubMed] [Google Scholar]
- Netz J, Ziemann U, Homberg V (1995): Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res 104: 527–533. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Hari R (2000): Temporal dynamics of cortical representation for action. Proc Natl Acad Sci U S A 97: 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez P, Silberstein RB, Cadiush PJ, Wijesinghe J, Westdorp AF, Srinivasan R (1994): A theoretical and experimental study of high resolution EEG based on surface Laplacians and cortical imaging. Electroencephalogr Clin Neurophysiol 90: 40–57. [DOI] [PubMed] [Google Scholar]
- Nunez P (1995): Neocortical dynamics and human EEG rhythms. New York: Oxford University Press. [Google Scholar]
- Pfurtscheller G, Neuper C (1994): Event‐related synchronization of mu rhythm in the EEG over the cortical hand area in man. Neurosci Lett 174: 93–96. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva F (1999): Functional meaning of event‐related desynchronization (ERD) and ‐synchronization (ERS). Event‐related desynchronization and related oscillatory phenomena of the brain In: Pfurtscheller G, Lopes da Silva F, editors. Handbook of electroencephalography and clinical neurophysiology. Amsterdam: Elsevier; p 51–66. [Google Scholar]
- Rektor I, Feve A, Buser P, Bathien N, Lamarche M (1994): Intracerebral recording of movement related readiness potentials: an exploration in epileptic patients. Electroencephalogr Clin Neurophysiol 90: 273–283. [DOI] [PubMed] [Google Scholar]
- Regan DM (1989): Human brain electrophysiology. Evoked potentials and evoked magnetic fields in science and medicine. New York: Elsevier; p 236–244. [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L (1996a): Premotor cortex and the recognition of motor actions. Brain Res Cogn 3: 131–141. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Perani D, Fazio F (1996b): Localization of cortical areas responsive to the observation of hand grasping movements in humans: a PET study. Exp Brain Res 111: 246–252. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V (1997): Parietal cortex: from sight to action. Curr Opin Neurobiol 7: 562–567. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA (1998): Language within our grasp. Trends Neurosci 21: 188–194. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M (1998): The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol 106: 283–296. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L (1999): Resonance behaviors and mirror neurons. Arch Ital Biol 137: 85–100. [PubMed] [Google Scholar]
- Rossi S, Pasqualetti P, Rossini PM, Feige B, Ulivelli M, Glocker FX, Battistini N, Lucking CH, Kristeva‐Feige R (2000): Effects of repetitive transcranial magnetic stimulation on movement‐related cortical activity in humans. Cerebral Cortex 10: 802–808. [DOI] [PubMed] [Google Scholar]
- Rossi S, Tecchio F, Pasqualetti P, Ulivelli M, Pizzella V, Romani GL, Passero S, Battistini N, Rossini PM (2002): Somatosensory processing during movement observation in humans. Clin Neurophysiol 113: 16–24 [DOI] [PubMed] [Google Scholar]
- Schroeder J, Wenz F, Schad LR, Baudendistel K, Knopp MV (1995): Sensorimotor cortex and supplementary motor area changes in schizophrenia, a study with functional magnetic resonance imaging. J Psychol 167: 197–201. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Barrett G, Halliday E, Halliday AM (1980): Components of the movement‐related cortical potential and their scalp topography. Electroencephalogr Clin Neurophysiol 49: 213–222. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Lutzenberger W, Rau H, Birbaumer N (1994): Positive shifts of event‐related potentials: a state of cortical disfacilitation as reflected by the startle reflex probe. Electroencephalogr Clin Neurophysiol 90: 135–144. [DOI] [PubMed] [Google Scholar]
- Towle VL, Bolanos J, Suarez D, Tan K, Grzeszczuk R, Levin DN, Cakmur R, Frank SA, Spire JP (1993): The spatial location of EEG electrodes: locating the best‐fitting sphere relative to cortical anatomy. Electroencephalogr Clin Neurophysiol 86: 1–6. [DOI] [PubMed] [Google Scholar]
- Urbano A, Babiloni C, Onorati P, Babiloni F (1996): Human cortical activity related to unilateral movements. A high resolution EEG study. Neuroreport 8: 203–206. [DOI] [PubMed] [Google Scholar]
- Urbano A, Babiloni C, Onorati P, Carducci F, Ambrosini A, Fattorini L, Babiloni F (1998): Responses of human primary sensorimotor and supplementary motor areas to internally‐triggered unilateral and simultaneous bilateral one‐digit movements. A high resolution EEG study. Eur J Neurosci 10:765–770. [DOI] [PubMed] [Google Scholar]
- von Stein A, Sarnthein J (2000): Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol 38: 301–313. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Brown WS, Marsh JT, Dawson ME (1991): Probing the time‐course of the auditory oddball P3 with secondary reaction time. Psychophysiology 28: 609–618. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Hallett M (2001): Hemispheric asymmetry of ipsilateral motor cortex activation during unimanual motor tasks: further evidence for motor dominance. Clin Neurophysiol 112: 107–113. [DOI] [PubMed] [Google Scholar]


