Abstract
Previous electrophysiological studies had found an early anterior negativity often with a maximum over the left hemisphere to correlate with the early detection of an error in the syntactic structure of a sentence. In this paper, the cortical structures involved in such early syntactic parsing processes were localized using MEG. Subjects were presented with acoustic sentences and asked to judge their syntactic correctness. The subjects' brain responses to syntactic violations were recorded with a 148‐channel whole‐head magnetometer. Dipole source localization was performed using a realistically shaped standard volume conductor model with fMRI constraints. The results show that the early syntactic parsing processes are supported by temporal regions, possibly the planum polare, as well as by fronto‐lateral regions. As indicated by the resultant dipole strengths, these regions are activated bilaterally with a dominance in the left hemisphere for four out of the five subjects. The contribution of the left temporal regions to the early syntactic processes seems to be larger than that of the left fronto‐lateral regions. Hum. Brain Mapping 11:1–11, 2000. © 2000 Wiley‐Liss, Inc.
Keywords: ELAN, MEG, ERP/ERF, dipole source localization, language comprehension, syntactic parsing
INTRODUCTION
Our knowledge about the cortical representation of language after being studied for more than 100 years through patients with circumscribed brain lesions [Broca, 1861; Caplan, 1992; Goodglass, 1993; Wernicke, 1874] has been enlarged quite recently through the use of brain imaging techniques such as position emission tomography [e.g., Démonet et al., 1992; Mazoyer et al., 1993; Petersen et al., 1989; Stromswold et al., 1996; Wise et al., 1991] and functional magnetic resonance imaging [e.g., Bavelier et al., 1997; Binder et al., 1994; Just et al., 1996]. Imaging studies at the sentence level using a visual presentation mode have suggested the inferior frontal cortex, in particular the Broca's area, to be active when syntactically complex sentences are processed [Bavelier et al., 1997; Caplan et al., 1998; Just et al., 1996; Stromswold et al., 1996]. Left frontal brain regions were also reported to be active during the processing of syntactically complex sentences in the auditory domain when aspects of working memory were involved, such as in a same/different task [Dapretto and Bookheimer, 1999]. Temporal regions were reported to be involved during syntactic processing in some visual studies [e.g., Just et al., 1996], and in most studies presenting their stimuli auditorily [e.g., Friederici et al., in press; Mazoyer et al., 1993; Müller et al., 1997]. The processing of lexical‐semantic information, in contrast, has been correlated with the middle temporal gyrus and with Brodmann's area 45/47 in the inferior frontal cortex [Démonet et al., 1992; Price et al., 1997; Shaywitz et al., 1995].
Although these studies allow a description of the neural network supporting language processes and syntactic processes in particular, they do not provide information necessary to describe the temporal parameters of the different subprocesses involved during language production and language comprehension. Behavioral and electrophysiological studies, however, indicate that the identification of the temporal structure of different subprocesses is crucial for the understanding of production [e.g., Levelt, 1989; Turennout et al., 1998] and comprehension processes [e.g., Frazier, 1987; Friederici et al., 1996]. An adequate description of the language brain relationship would thus require the specification of the temporal behavior of cortical activation during language processing. Electrophysiological studies suggest at least three temporally and topographically distinct subprocesses underlying language comprehension two of which are syntax‐related and one meaning‐related. During a first phase, the input is parsed into an initial syntactic structure. A second phase deals with lexical‐semantic access and integration processes. A third phase during which a final interpretation is assigned on the basis of lexical‐semantic and structural information, again involves syntactic aspects including syntactic reanalysis and repair whenever the final assignment fails [Friederici, 1995]. Different event‐related brain potentials (ERPs) are correlated with the three different subprocesses.
Lexical‐semantic integration processes are reflected in a centro‐parietally distributed negativity, called N400 [e.g., Chwilla et al., 1995; Kutas and Hillyard, 1983]. A recent attempt to localize the N400 using an MEG approach found cortical structures in the vicinity of the left auditory cortex to be implicated with the processing of contextually inappropriate words [Helenius et al., 1998].
Syntactic processes, in contrast, are indicated by an early anterior negativity mostly observed with a left maximum, called ELAN, and by a late centro‐parietally distributed positivity, called P600. These two components have been interpreted to reflect early first‐pass parsing processes and processes of syntactic reanalysis and repair respectively [Friederici, 1995; Friederici et al., 1996]. The functional distinction between the early syntactic processes reflected by the ELAN and the late syntactic processes has been shown recently in a study varying the probability of correct and syntactically incorrect sentences [Hahne and Friederici, 1999]. Although the late ERP component (P600) varied in amplitude as a function of probability, the early ERP component (ELAN) did not. This finding was taken to suggest that the early syntactic processes are highly automatic, whereas the late processes are more controlled. On the debate of whether the P600 or the early negativity is more likely to represent syntactic processes proper no final conclusion has been achieved [e.g., Coulson et al., 1998; Osterhout and Hagoort, 1999]. It seems, however, fair to say that the early negativity reflects early phrase structure building processes [e.g., Coulson et al., 1998; Friederici et al., 1996; Münte et al., 1998].
Up to now, it is not clear which cortical areas support the processes reflected in the ELAN. Studies from patients with brain lesions suggest that left anterior cortical, rather than subcortical, structures are crucially involved in these processes [Friederici et al., 1998, 1999], as patients with left anterior cortical lesions demonstrated a selective absence of the ELAN whereas patients with left anterior subcortical lesion do not. However, there are also findings that suggest an involvement of the temporal cortex in syntactic processes. In a lesion‐behavior study, Dronkers et al. [1995] reported that patients with temporal lesions including the anterior part of the superior temporal gyrus displayed a syntactic comprehension deficit. In a recent functional magnetic resonance imaging fMRI study from our own laboratory, the mid‐portion of the superior temporal gyrus was found to increase in activation as a function of syntactic correctness [Meyer et al., in press]. Other fMRI studies reported increased activation either in Wernicke's area in addition to Broca's area as a function of syntactic complexity [Just et al., 1996], or in the mid‐portion of the superior temporal gyrus in addition to the frontal operculum when processing sentences that only contained syntactic information (i.e., function words and inflectional morphology were presented, but content words were replaced by pseudo words) [Friederici et al., in press]. These findings indicate that both left frontal and temporal cortical areas support syntactic processing.
However, the reported imaging studies may reflect an additive effect of the early and the late syntactic processes due to low temporal resolution. Therefore, they do not allow specifying whether both frontal and temporal cortices are involved in early syntactic processes, or by either of these regions alone. Two recent MEG studies trying to define the generators underlying the ELAN component suggest that this component, although frontal in its distribution, involves temporal and frontal structures. Gross et al. [1998], using a twin MEG system with 37 channels, reported activation in left temporal and left frontal areas. However, due to limited coverage over the frontal regions, localization of the frontal generator was blurred. Knoesche et al. [1999] using a whole head 148 channel MEG system applied the so‐called Brain Surface Current Density (BSCD) method which allowed a localization of activity within eight defined regions of interest (i.e., left frontal, left parietal, left anterior temporal, left posterior temporal, and their right hemisphere homologue areas). Data revealed a significant difference between correct and syntactically incorrect sentences during an early time window 125–175 ms in frontal and in anterior and posterio‐temporal areas in the left and the right hemisphere.
The aim of the present study is to localize the early syntactic processes reflected in the early anterior negativity more precisely using a dipole modeling approach. Magnetoencephalography (MEG) and dipole localization techniques were utilized to reveal the loci and strengths of the ELAN generator, and thereby of early syntactic processes such as the detection of a phrase structure error. Critically, in this study, the number of items was very high (360) to increase the signal‐to‐noise ratio (SNR) to a level (>5.5) that allowed a good quality of dipole modeling. The dipole source localization was performed with fMRI constraints using a realistically shaped standard volume conductor model.
MATERIALS AND METHODS
Subjects
Six healthy German‐speaking adults (three males) volunteered as participants. Data from one of them were disregarded from further analysis because of the low signal‐to‐noise ratio. The remaining five subjects (three males, age 18–29 years; mean 23 years) were all right‐handed as measured by the Edinburgh Handedness Inventory [Oldfield, 1971]. All male subjects had a score of 100, subject #4 (female) of 90, and subject #5 (female) of 100. A score of 100 means totally right‐handed. All of them had no known hearing deficit.
Stimuli
There were two types of critical sentences, correct sentences, and syntactically incorrect sentences. Correct sentences consisted of a noun phrase, an auxiliary, and a past participle (e.g., Der Fisch wurde geangelt/The fish was caught). Incorrect sentences contained a phrase structure violation realized as a word category error (e.g., The fish was in caught). In these sentences, a preposition appears after the auxiliary requiring a noun phrase to follow. Instead, a past participle form followed the auxiliary, a word category error. There were a total of 65 correct and 65 incorrect sentences constituting two base lists. One hundred and thirty filler sentences were included to control for the presence of a preposition in correct and incorrect sentences. Sixty‐five filler sentences contained a correct prepositional phrase (e.g., Der Fisch wurde im See geangelt/The fish was in the sea caught). Sixty‐five additional filler sentences were incorrect to balance the number of correct and incorrect sentences. All critical sentence final past participles were regular German verb forms starting with the morpheme ge‐. Different lists containing an equal number of correct and incorrect sentences were constructed by quasi‐randomizing all correct and incorrect sentences from the two base lists. All sentences were spoken by a trained German speaker. Sentences were digitized and the onset of the critical past participle was determined by visual and auditory inspection of the speech wave for each item, allowing a precise time locking of the ERF to the critical element. To control for possible prosodic or other acoustic cues prior to the critical element in the incorrect condition, the speaker initially produced each sentence containing a preposition with a noun following the preposition. The incorrect version was constructed by splicing out the noun from the digitized speech signal using a speech‐editing tool. To ensure optimal splicing and to control for possible coarticulation impairments due to different onsets of the word following the preposition, those sentences in which the noun was spliced out only used nouns whose phonological transition was identical to the critical past participle form, that is both forms (noun and verb) started with the syllable ge‐. Acoustical analyses of the resulting incorrect sentences indicate that this procedure was successful. The sentence material used here was partly identical to that used by Hahne and Friederici [1999].
MEG recordings
The MEG was recorded in a magnetically shielded room with a 148‐channel whole‐head magnetometer (MAGNES WHS 2500, Bti). For each subject, six experimental blocks were recorded. Before and after each experimental block, the position of the sensor array with respect to the nasion‐ear coordinate system was measured. A 148‐channel MEG, together with four electrodes of EOG and trigger markers, were recorded continuously. Triggers were added online at the beginning of each sentence and inserted offline to the onset of each following word. The signal was online bandpass filtered (0.1–100 Hz) and sampled at a rate of 508.63 Hz.
Data preprocessing
The MEG recordings were then epoched offline for a 700‐ms period (−200 to 500 ms with respect to the onset of the critical word). Prior to averaging, epochs were screened for eye movement and other artifacts. The epochs were excluded from averaging if (a) the standard deviation of the EOG within a sliding 200‐ms time window exceeded 30 μV, (b) the standard deviation of any MEG recording channel within a sliding 3‐sec time window exceeded 1100 fT, or (c) the drift of the MEG recording within the epoch was over 3000 fT. The data were first averaged over trials within each block, then transformed to a standard sensor array and averaged over all blocks.
One important issue for the processing of event‐related neuromagnetic data is the offset correction. In our study, because the stimuli are spoken sentences, there is no actually silent time window around the critical words, and the epoch prior to the sentence beginning is too far from the onset of critical words. To overcome this difficulty, high‐pass filtering is employed [Tervaniemi et al., 1999]. Because the main frequency of ELAN component as shown in Figure 1a is about 5 Hz, 2 Hz high‐pass filter was used to remove the baseline offset and drift.
Figure 1.

Effect of filtering. (a) Shows the signal waveforms of one typical channel from one subject. Solid line is the ERF signal without any offline filtering. ELAN component is the one between 100 ms and 200 ms with the half circle of about 100 ms. Dotted line refers to the signal after 2 Hz filtering. The baseline offset is effectively removed. Dashed line is the result of 2–10 Hz band pass filtering. (b) Is the power spectrum in fT2/Hz of ELAN effect calculated for the time window from 100 to 200 ms. The results of 148 channels were overlapped. Note that the main frequency band of ELAN effect is below 10 Hz.
Dipole source localization is quite sensitive to noise [Wang and Yang, 1995]. To improve the signal quality for dipole modeling, two additional measures were taken before averaging. The first one is low‐pass filtering. The power spectrum analysis of the ELAN effect (Fig. 1b) demonstrates that the main effect is located in the frequency band below 10 Hz. Because our focus is the localization of the ELAN effect, 10 Hz low pass filtering was adopted to improve the signal quality as well as to keep the ELAN component untouched. In combination, a band pass filter of 2–10 Hz was used. Figure 1a demonstrates the usefulness of such a filter both in removing baseline offset and in improving signal quality of the ELAN component. The second process is the bad channel rejection based on the correlation coefficient (CF) analysis. In principle, one sensor and the sensors in its neighborhood (distance < 40 mm) are supposed to pick up the signal from similar regions of the brain, and therefore the recordings from these sensors would be much correlated. If the median value of such a group CFs for one channel is lower than a specified limit, this channel has most probably been contaminated by strong noise and/or artifacts and will be rejected for further processing. In our analysis, the low limit of the CF was set to 0.7∼0.8, depending on the CF profiles.
Dipole modeling
The averaged responses for all the subjects resulted from the pre‐processing were fed into CURRY® (NEURO Scan Labs) for dipole modeling. The realistically shaped one‐compartment boundary element model for the interior of the skull from the standard Warp model in CURRY was adopted as the volume conductor [Hämäläinen and Sarvas, 1987]. The conductivity was set to 0.33 S/m.
From fMRI results of a study in which correct and incorrect sentences were presented acoustically [Meyer et al., 2000], two locations for each brain hemisphere were selected as the seed points for constrained dipole fitting (Table I). The coordinates in Table I are those of Talairach and Tournoux [1988] space. Considering the variation of the individual head from the standard volume conductor model and the difference between fMRI and MEG, we allowed the dipoles to move within a sphere region centered at the respective seed point with a radius of 10 mm. With this constraint, the dipole locations and orientations were fitted within a time interval of 20 ms around the ELAN peak.
Table I.
Seed points from fMRI study
| Left hemisphere (x, y, z) (mm) | Right hemisphere (x, y, z) (mm) | |
|---|---|---|
| Fronto‐lateral cortex (pars triangularis) | (−41, 10, 13) | (48, 11, 10) |
| Anterior STG (planum polare) | (−50, −8, 1) | (55, −8, 5) |
RESULTS
Averaged MEG data
The averaged MEG signals for the two different conditions from each of the five subjects are depicted in Figure 2. There are obvious differences between the two conditions. In the incorrect case and for all of the five subjects, the strong ELAN component reaches its peak from 130 ms to 160 ms. The ELAN peaks are labeled in Figure 2 with their corresponding latencies. The amplitudes of the ELAN components are around 100 fT. In the correct condition, there are no evident ELAN components and the signal is much weaker compared to the ELAN components in the incorrect condition. To show clearer ELAN effect between conditions, MEG signal waveforms of four typical channels (two channels from each hemisphere) from one subject (#4) are presented in Figure 3. The dipole modeling in the present study was focused on the ELAN components in the incorrect condition.
Figure 2.
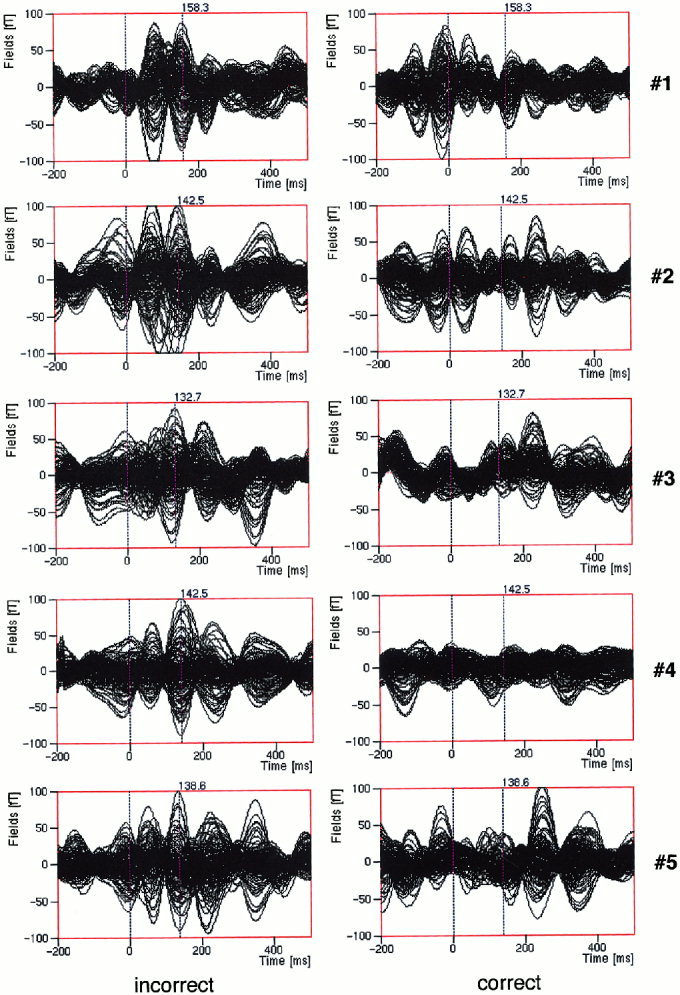
The overlaid time course of the averaged MEG data for two conditions for each of the five subjects. The ELAN peaks are labeled with their corresponding latencies.
Figure 3.

Time courses of four MEG channels over the left (A132, A78) and right (A147, A88) hemisphere for the conditions correct (dotted) and incorrect (solid) from one subject. A: anterior, P: posterior, L: left, R: right.
Dipole fitting
The dipole fitting results for all of the five subjects are listed in Table II. The coordinates in the table are those of Talairach space. Except for subject #1, the maximal goodness‐of‐fit for all the subjects is over 92%. For subject #1, this value is 81%. As one example, Figure 4 shows the dipole fitted magnetic field compared with the original averaged MEG measurement for one subject (#4). The small squares indicate the MEG sensors and the fitted dipoles (arrows in the figure) are projected and over plotted on the field contour maps.
Table II.
Dipole localization results
| Subject (sex) | Dipole location | Deviation from seed points (L/R) (mm) | Dipole strength left/right (nAm) | Goodness‐ of‐fit | LR | |
|---|---|---|---|---|---|---|
| Left/right (mm) | ||||||
| x y z x y z | ||||||
| #1 (M) | fronto‐lateral | (−39.5 10.5 17.8)/(— — —) | 5.0/— | 7.8/— | 81% | 0.175 |
| planum polare | (−53.1 −10.7 8.6)/(55.0 −10.4 9.6) | 8.7/5.2 | 37/26 | |||
| #2 (M) | fronto‐lateral | (−41.8 7.7 14.7)/(46 11 18.8) | 3.0/9.0 | 28/27 | 92% | 0.247 |
| planum polare | (−48.6 −10.0 5.3)/(47.0 −8.4 1.0) | 5.0/9.1 | 53/32 | |||
| #3 (M) | fronto‐lateral | (−44.8 9.7 13.7)/(46.4 14.9 7.4) | 3.9/5.0 | 20/16 | 92% | 0.294 |
| planum polare | (−47.6 −7.6 4.3)/(49.8 −5.0 −3.0) | 4.1/10 | 33/18 | |||
| #4 (F) | fronto‐lateral | (−37.3 8.9 11.5)/(43.4 9.5 18.8) | 4.1/10 | 19/14 | 95% | 0.518 |
| planum polare | (−59.2 −9.2 4.8)/(51.4 −16.9 7.8) | 10/10 | 41/13 | |||
| #5 (F) | fronto‐lateral | (−41.3 10.6 6.9)/(38.8 14.3 12.0) | 6.1/10 | 27/29 | 93% | −0.263 |
| planum polare | (−57.0 −6.6 8.0)/(48.7 −12.5 11.3) | 10/10 | 35/60 | |||
| Mean | fronto‐lateral | (−41.0 9.5 12.9)/(43.6 12.5 14.2) | 0.5/6.3 | 90.6% | 0.193 | |
| planum polare | (−53.1 −8.8 6.2)/(50.0 −10.6 5.3) | 6.1/5.3 | ||||
Figure 4.
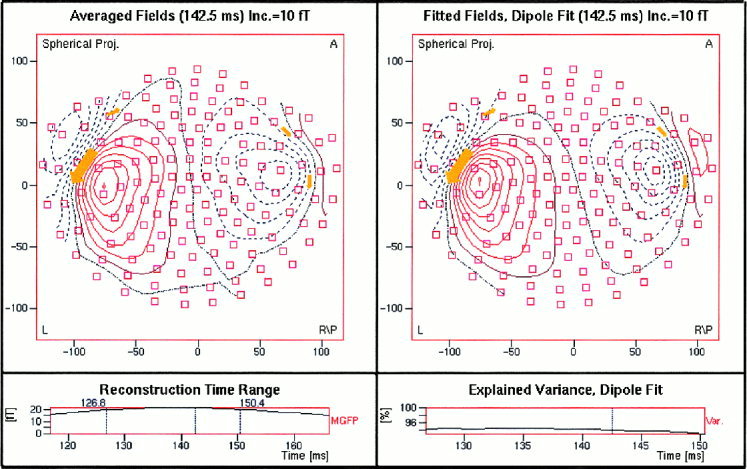
ELAN magnetic field distribution and dipole fitted field for an individual subject (A = anterior; L = left; R/P = right posterior). The small squares indicate the MEG sensors. The resultant dipoles are projected and over plotted on the contour maps as arrows. The left‐lower panel gives the mean global field curve and the right‐lower panel shows the goodness‐of‐fit.
For each subject, four dipoles were fitted with fMRI constraints. The resultant dipoles in each hemisphere are presented in Figure 5. In this figure, the dipoles are overlaid with the averaged standard brain cortex. For subject #1, only three dipoles were fitted because the right frontal dipole showed an activation strength unlikely to be explained by neuronal currents [Freeman, 1975] and was therefore excluded from the final dipole solution.
Figure 5.
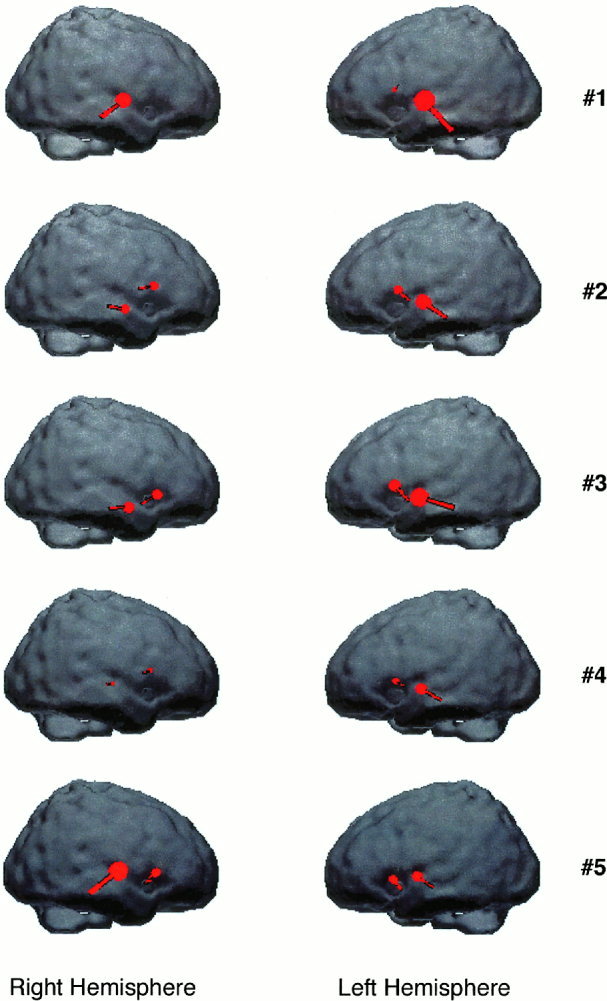
Dipole localization results for all the five subjects. The dipoles are overlaid with the corresponding hemisphere.
Also listed in Table II are the deviations of the resultant dipole locations from the seed points (the planum polare and the fronto‐lateral cortex). Although it varies from subject to subject, the grand averaged deviation is within 6.3 mm.
As shown in Figure 5, for all the subjects, the dipoles located in the temporal cortical areas show the stronger activation strength than those located in the frontal cortical areas. Four out of five subjects show much stronger activity in the left hemisphere than in the right hemisphere as indicated by the corresponding dipole strength. However, in spite of being totally right‐handed, subject #5 shows stronger activity in the right hemisphere. Such an asymmetry was measured by one laterality index [see also Segalowitz and Bryden, 1983] defined as follows:
Where L and R denote the strength of dipole located at the left temporal areas and that at the right temporal areas, respectively. With this definition, the index ranges between 1 and −1 with positive values for the left lateralization and negative values for the right lateralization. The laterality indices for different subjects are listed in Table II. It clearly shows the dominance of the left hemisphere.
DISCUSSION
This paper aimed at a precise localization of early syntactic parsing processes. Earlier electrophysiological studies had found an early anterior negativity often with a maximum over the left hemisphere to correlate with the early detection of an error in the syntactic structure of a sentence [e.g., Friederici et al., 1993, 1996; Hahne and Friederici, 1999; Neville et al., 1991]. Brain lesions involving the left anterior cortex caused an absence of this component, whereas left anterior subcortical lesions did not [Friederici et al., 1998, 1999]. These data suggested that left anterior cortical regions might house the generator for the early left anterior negativity. However, fMRI data from a study comparing the processing of correct sentences and syntactically incorrect sentences [Meyer et al., 2000] as well as a brain surface current source density MEG study [Knoesche et al., 2000] indicated an involvement of temporal regions as well. However, the fMRI and data did not allow to temporally separate between early and late syntactic processes, the current source density approach did not allow precise spatial conclusions.
The present data in which dipole fitting was constrained by fMRI results provide a clear indication that syntactic processes in the early stage are supported by tissue at or in the vicinity of the planum polare as well as brain tissue in the fronto‐lateral cortex bilaterally, with a dominance in the left hemisphere. A clear left hemisphere dominance was obtained for three out of three male subjects. A similar left asymmetry was observed for one female subject, whereas another female subject displayed a right asymmetry. Although the few number of subjects does not allow drawing firm conclusions about gender differences with respect to the language processes under investigation, the present results may be connected to earlier studies claiming a larger asymmetry for language processes in male as compared to female subjects. Evidence for the view that language processes are less lateralized in the female than in the male [Bryden, 1979; McGlone, 1980] comes from lesion studies [e.g., McGlone, 1978], from dichotic listening studies [e.g., Lake and Bryden, 1976] as well as from brain imaging studies [e.g., Jaeger et al., 1998]. But for each of these classes of studies, there are also examples reporting no differences between male and female subjects [e.g., Carr, 1969; Frost et al., 1999; Hécaen et al., 1981]. In earlier EEG studies using sentence material similar to the present study, no systematic differences were reported between males and females [e.g., Friederici et al., 1993, 1996; Hahne and Friederici, 1999]. However, a recent MEG study using similar material testing only females found a bilateral to right asymmetric distribution of the early syntactic component. The hypothesis that a clearer left asymmetry is present in males compared to females is now being systematically investigated in an additional MEG study.
With respect to the cortical structures involved in early parsing processes, the present findings clearly show that these processes are supported by temporal regions, possibly the planum polare, as well as by fronto‐lateral regions. Thus, the early syntax‐related ERP component with its left anterior distribution [e.g., Friederici et al., 1993, 1996; Hahne and Friederici, 1999; Neville et al., 1991] is not due to a left frontal generator alone, but seems to evoke from both a frontal and a temporal generator. The contribution of the left temporal region even seems to be larger than the contribution of the left fronto‐lateral regions as indicated by the dipole strength. The present finding can be connected to lesion studies reporting a syntactic comprehension deficit for those patients with temporal lesions in which the lesion extended to the anterior part of the superior temporal gyrus [Dronkers et al., 1995]. Moreover, this finding is in agreement with recent fMRI results showing either no significant activation in frontal regions as a function of syntactic incorrectness [Meyer et al., 2000; Müller et al., 1997] or only minimal activation in frontal areas when syntax is in focus [Friederici et al., in press], but a clear increase of activation in the planum polare as a function of both these factors during auditory language comprehension.
Acknowledgements
We thank Anja Hahne for providing the stimulus material for the present study, and Martin Meyer for his cooperation with respect to the fMRI data.
REFERENCES
- Bavelier D, Corina D, Jezzard P, Padmanabhan S, Clark VP, Karni A, Prinster A, Braun A, Lalwani A, Rauschecker JP, Turner R, Neville H (1997): Sentence reading: a functional MRI study at 4 Tesla. J Cog Neurosci 9: 664–686. [DOI] [PubMed] [Google Scholar]
- Binder JR, Rao SM, Tammeke TA (1994): Functional MRI of human auditory cortex. Ann Neurol 35: 662–672. [DOI] [PubMed] [Google Scholar]
- Broca P (1861): Remarques sur le siége de la faculté du langage articulé, suivies d'une observation d'aphémie. (parte de la parole). Bull Soc Anat Paris 6: 330–357. [Google Scholar]
- Bryden MP (1979): Evidence for sex‐related differences in cerebral organization In: Wittig M, Petersen AC, editors. Sex‐related differences in cognitive functioning: developmental issues. New York: Academic Press. [Google Scholar]
- Caplan D (1992): Language: structure, processing and disorders. Cambridge, MA: MIT Press. [Google Scholar]
- Caplan D, Alpert N, Waters G, Olivieri A (2000): Activation of Broca's area by syntactic processing under conditions of concurrent articulation. Human Brain Mapp 9: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr BM (1969): Ear effect variables and order of report in dichotic listening. Cortex 5: 63–68. [DOI] [PubMed] [Google Scholar]
- Chwilla DJ, Brown C, Hagoort, P (1995): The N400 as a function of the level of processing. Psychophysiology 32: 274–285. [DOI] [PubMed] [Google Scholar]
- Coulson S, King J, Kutas M (1998): Expect the unexpected: event‐related brain responses of morphosyntactic violations. Lang Cog Proc 13: 21–58. [Google Scholar]
- Dapretto M, Bookheimer SY (1999): Form and content: dissociating syntax and semantics in sentence comprehension. Neuron 24: 427–432. [DOI] [PubMed] [Google Scholar]
- Démonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak RSJ (1992): The anatomy of phonological and semantic processing in normal subjects. Brain 115: 1753–1768. [DOI] [PubMed] [Google Scholar]
- Dronkers NE, Redfern BB, Ludy CA (1995): Lesion localization in chronic Wernicke's aphasia. Brain Lang 51: 62–65. [Google Scholar]
- Frazier L (1987): Sentence processing: a tutorial view In: Coltheart M, editor. Attention and performance XII: the psychology of reading. London: Lawrence Erlbaum. [Google Scholar]
- Freeman W (1975): Action in the nervous system. New York: Academic Press. [Google Scholar]
- Friederici AD (1995): The time course of syntactic activation during language processing: a model based on neuropsychological and neurophysiological data. Brain Lang 50: 259–284. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Pfeifer E, Hahne A (1993): Event‐related brain potentials during natural speech processing: effects of semantic morphological and syntactic violations. Cog Brain Res 1: 183–192. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Mecklinger A, Hahne A (1996): The temporal structure of syntactic parsing: early and late event‐related brain potential effects elicited by syntactic anomalies. J Exp Psych: Learn Mem Cog 22: 1219–1248. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Hahne A, von Cramon DY (1998): First‐pass versus second‐pass parsing processes in a Wernicke's and a Broca's aphasic: electro‐physiological evidence for a double dissociation. Brain Lang 62: 311–341. [DOI] [PubMed] [Google Scholar]
- Friederici AD, von Cramon DY, Kotz SA (1999): Language related brain potentials in patients with cortical and subcortical left hemisphere lesions. Brain 122: 1033–1047. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Meyer M, von Cramon DY in press. Auditory language comprehension: an event‐related fMRI study on the processing of syntactic and lexical information. Brain Lang. [PubMed] [Google Scholar]
- Frost JA, Binder JR, Springer JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW (1999): Language processing is strongly left lateralized in both sexes—evidence from functional MRI. Brain 122: 199–208. [DOI] [PubMed] [Google Scholar]
- Goodglass H (1993): Understanding aphasia. San Diego, CA: Academic Press. [Google Scholar]
- Gross J, Ioannides AA, Dammers J, Maess B, Friederici AD, Müller‐Gärtner H‐W (1998): Magnetic field tomography analysis of continuous speech. Brain Top 10: 273–281. [DOI] [PubMed] [Google Scholar]
- Hahne A, Friederici AD (1999): Electrophysiological evidence for two steps in syntactic analysis: early automatic and late controlled processes. J Cog Neurosci 11: 193–204. [DOI] [PubMed] [Google Scholar]
- Hämäläinen MS, Sarvas J (1987): Feasibility of the homogenous head model in the interpretation of neuromagnetic data. Phys Med Biol 32: 91–97. [DOI] [PubMed] [Google Scholar]
- Hécaen H, DeAgostini M, Monzon‐Montes A (1981): Cerebral organization in lefthanders. Brain Lang 12: 261–284. [DOI] [PubMed] [Google Scholar]
- Helenius P, Salmelin R, Service E, Connolly JF (1998): Distinct time courses of word and context comprehension in the left temporal cortex. Brain 121: 1133–1142. [DOI] [PubMed] [Google Scholar]
- Jaeger JJ, Lockwood AH, Vanvalin RD, Kemmerer DL, Murphy BW, Wack DS (1998): Sex differences in brain regions activated by grammatical and reading tasks. NeuroReport 9: 2803–2807. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR (1996): Brain activation modulated by sentence comprehension. Science 274: 114–116. [DOI] [PubMed] [Google Scholar]
- Knoesche T, Maess B, Friederici AD (1999): Processing of syntactic information monitored by brain surface current density mapping based on MEG. Brain Top 12: 75–87. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA (1983): Event‐related potentials to grammatical errors and semantic anomalies. Mem Cog 11: 539–550. [DOI] [PubMed] [Google Scholar]
- Lake DA, Bryden MP (1976): Handedness and sex differences in hemispheric asymmetry. Brain Lang 3: 266–282. [DOI] [PubMed] [Google Scholar]
- Levelt W (1989): Speaking: from intention to articulation. Cambridge, MA: MIT Press. [Google Scholar]
- Mazoyer BM, Tzourio N, Frak V, Syrota A, Murayama N, Levrier O, Salamon G, Dehaene S, Cohen L, Mehler J (1993): The cortical representation of speech. J Cog Neurosci 5: 467–479. [DOI] [PubMed] [Google Scholar]
- McGlone J (1978): Sex differences in functional brain asymmetry. Cortex 14: 122–128. [DOI] [PubMed] [Google Scholar]
- McGlone J (1980): Sex differences in human brain organization: a critical survey. Behav Brain Sci 3: 215–227. [Google Scholar]
- Meyer M, Friederici AD, von Cramon DY (2000): Neurocognition of auditory sentence comprehension: event‐related fMRI reveals sensitivity to syntactic violations and task demands. Cog Brain Res 9: 19–33. [DOI] [PubMed] [Google Scholar]
- Müller R‐A, Rothermel RD, Behen ME, Muzik O, Mangner TJ, Chugani HT (1997): Receptive and expressive language activations for sentences: a PET study. NeuroReport 8: 3767–3770. [DOI] [PubMed] [Google Scholar]
- Münte TF, Heinze H‐J, Matzke M, Wieringa BM, Johannes S (1998): Brain potentials and syntactic violations revisited: no evidence for specificity of the syntactic positive shift. Neuropsych 36: 217–226. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Nicol J, Barss A, Forster KI, Garrett MF (1991): Syntactically based sentence processing classes: evidence from event‐related brain potentials. J Cog Neurosci 3: 151–165. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychology 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Osterhout L, Hagoort P (1998): A superficial resemblance does not necessarily mean that you are a part of the family: counterarguments to Coulson, King and Kutas (1998) in the P600/ SPS‐P300 debate. Lang Cog Proc 14: 1–14. [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintum M, Raichle ME (1989): Positron emission tomographic studies of the processing of single words. J Cog Neurosci 1: 153–170. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Wise RJS (1997): Segregating semantic from phonological processes during reading. J Cog Neurosci 9: 727–733. [DOI] [PubMed] [Google Scholar]
- Segalowitz SG, Bryden MP (1983): Individual differences in hemispheric representation of language In: Segalowitz SG, editor. Language functions and brain organization. New York: Academic Press, p 341–372. [Google Scholar]
- Shaywitz BA, Pugh KR, Constable RT, Shaywitz SE, Bronen RA, Fulbright RK, Shankweiler DP, Katz L, Fletcher JM, Skudlarski P, Gore JC (1995): Localization of semantic processing using functional resonance imaging. Human Brain Mapp 2: 149–158. [Google Scholar]
- Stromswold K, Caplan D, Alpert N, Rauch S (1996): Localization of syntactic comprehension by positron emission tomography. Brain Lang 52: 452–473. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Tervaniemi M, Kujala A, Alho K, Virtanen J, Ilmoniemi RJ, Näätänen R (1999): Functional specialization of the human auditory cortex in processing phonetic and musical sounds: a Magnetoencephalographic (MEG) study. NeuroImage 9: 330–336. [DOI] [PubMed] [Google Scholar]
- van Turennout M, Hagoort P, Brown CM (1998): Brain activity during speaking: from syntax to phonology in 40 milliseconds. Science 280: 572–574. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yang F (1995): Dynamic extraction of visual evoked potentials through spatial analysis and dipole localization. IEEE Trans Biomed Eng 42: 762–768. [DOI] [PubMed] [Google Scholar]
- Wernicke C (1874): Der aphasische symptomenkomplex. Breslau: Cohn and Weigert. [Google Scholar]
- Wise R, Chollet F, Hadar U, Friston K, Hoffner E, Frackowiak R (1991): Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain 114: 1803–1817. [DOI] [PubMed] [Google Scholar]


