Abstract
A PET study of 10 normal individuals was carried out to investigate the cerebral regions involved in the controlled updating of verbal working memory. Subjects viewed single concrete words on a computer monitor and detected occasional target words in an attended color. In the activating condition, a target was defined as a word that was identical to the previous word presented in the attended color. In the control condition, the target was a predesignated word. The same word lists, target probabilities, and target response demands were used for both conditions, with interword intervals constrained to ensure equivalence in the demand for target rehearsal. A comparison of the conditions found bilateral activation of dorsolateral prefrontal (middle frontal gyrus; MFG) and inferior parietal (supramarginal gyrus; SMG) cortical regions. Activation of the MFG is taken to reflect executive control by prefrontal regions over the working memory updating process linking posterior representations of the anticipated target stimulus to anterior representations of the planned response. It is proposed that the updating of the stimulus link is mediated via connections between the MFG and SMG. The role of the SMG as an amodal region binding the various modal representations in posterior association cortex of the word being retained in working memory is considered and reviewed. It is suggested that the combined activation of these regions is related to the executive control of goal‐setting in planned behavior. Hum. Brain Mapping 9:42–54, 2000. © 2000 Wiley‐Liss, Inc.
Keywords: PET, working memory, stimulus
INTRODUCTION
Verbal working memory systems are central to human thought processes that manipulate symbolic representations of abstract, concrete, and/or relational nature to secure meaning from contingent stimulation. To date, imaging studies of verbal working memory function have tended to employ abstract stimuli such as letters [Paulesu et al., 1993, 1995; Cohen et al., 1994, 1996; Desposito et al., 1995; Awh et al., 1996; Coull et al., 1996; Smith et al., 1996; Jonides et al., 1997a; see also McCarthy, 1995] and numbers [Petrides et al., 1993a]. The present study investigates working memory representations deriving from concrete word stimuli.
Functional neuroimaging studies clearly show that working memory function involves the cooperative activity of multiple distributed cortical regions [e.g., Cohen et al., 1994; Awh et al., 1996; Schumacher et al., 1996; Smith et al., 1996; Badgaiyan and Posner, 1997; Jonides et al., 1997; Ungerleider et al., 1998; Smith and Jonides, 1999]. Activity has been observed in the supplementary motor and premotor areas, the inferior and superior parietal lobes, the dorsolateral prefrontal cortex, Broca's area, the anterior cingulate, and the cerebellum. There has been considerable variability across such studies in the areas activated during working memory processing. This has been due in part to differential manipulation of particular stimulus (e.g., verbal, spatial, or object content) and/or demand (the executive processes maintaining and manipulating such content) characteristics, as well as variations in the areas of the brain imaged.
Studies concerned specifically with verbal working memory content have implicated the supramarginal gyrus of the left inferior parietal lobe (BA40) and the superior parietal lobe (BA7) bilaterally [see Awh et al., 1996; Smith et al., 1996; Jonides et al., 1997b]. Broca's area (BA44) and the left supplementary motor and premotor areas (BA6) have been implicated in phonological rehearsal of such content [see Paulesu et al., 1993; Awh et al., 1996; Smith et al., 1996; Smith et al., 1999]. The storage of verbal materials appears to involve Broca's area and the left hemisphere supplementary and premotor areas, whereas executive control appears to be mediated by the lateral prefrontal cortex and may also involve the anterior cingulate [Petrides 1993a, 1993b, 1995; Coull et al., 1996; Smith et al., 1996, 1999; Jonides et al., 1997a]. The specific regions involved include the middle frontal gyrus and some of its surrounding tissue (e.g., BA8, BA9, BA10, BA45, and BA46), although there is still uncertainty regarding the laterality and regional localisation of particular executive functions. It has been proposed that executive control is mediated via reciprocal connection of the prefrontal structures with other brain regions [see McCarthy, 1995; Jonides et al., 1997b], which, in the case of verbal working memory, presumably include the frontal and parietal regions listed earlier. An executive role for lateral prefrontal cortex has also been demonstrated in imaging studies using spatial and object based material [e.g., Petrides et al., 1993b; Cohen et al., 1994; Courtney et al., 1996; MCarthy et al., 1996; Smith et al., 1996], although the activations are predominantly in the right hemisphere or bilateral, and by lesion and electrophysiological work in nonhuman primates [see Goldman‐Rakic, 1987, 1994, 1995].
The present study focuses on verbal working memory processes deriving from natural world objects and to this end used concrete words as stimuli. For a number of reasons, it might be expected that the control and storage of working memory content deriving from concrete words would be different from that of letters and numbers. All stimulus types are symbolic objects that facilitate analytical processing of the world, but of the three only concrete words denote natural, physical objects within the world. It might be expected, therefore, that concrete words would additionally induce activation in regions of the brain concerned with memory for such objects. A number of positron emission tomography (PET) studies using word stimuli in non‐working memory paradigms have found activations bilaterally in BA40 [e.g., Tulving et al., 1994; Andreasen et al., 1995; Kapur et al., 1995], Similarly, PET studies using nonverbal stimuli [e.g., faces, concrete and abstract designs, objects) have also found activations of BA40 bilaterally [e.g., Sergent et al., 1992] or on the right side only [e.g., Kosslyn et al., 1994; Andreasen et al., 1996; Smith et al., 1996]. It has been argued that the mechanisms for working memory storage of visual and verbal material are similar to those for phonological material. Most notably, Baddeley [1986] has postulated the existence of a visuospatial scratchpad that works in parallel with a phonological loop and is involved in the storage and maintenance of certain nonphonological codes in working memory.
The present experiment used a target detection paradigm to manipulate the updating of working memory for words. A fixed target condition required the detection of a predesignated visual target word in an attended color from among a sequence of nontarget words presented one at a time on a computer monitor. This condition involved no updating of target identity. By contrast, a variable target condition required the detection of a repeated word in an attended color from among the same sequences. This condition forced a repeated updating of target identity. The design was constructed to respect a balance over task conditions of: (1) stimulus‐target comparison demands, (2) number of task‐related targets to be remembered, (3) target rehearsal requirements, (4) stimulus‐response characteristics, and (5) executive processes other than those concerned with the updating and storage of target word codes. It was expected, therefore, that the additional demands imposed by the constant updating condition would reveal areas concerned with the storage in working memory of representations induced by concrete words, as well as the areas concerned with the executive control of such storage.
The studies reviewed above suggest that the left inferior parietal cortex (BA40) and bilateral superior parietal cortex (BA7) would be involved in the storage of linguistic codes in working memory and right inferior parietal cortex (BA40) with the storage of nonlinguistic representations. It was also predicted that activation of dorsolateral prefrontal cortex would reflect executive control of the frequent updating requirements of the variable target condition. In a design similar to the present study, but using letter stimuli and involving executive processes over and above memory updating [Smith et al., 1996], areas BA9 and BA10 were activated bilaterally and BA45 and BA46 on the left. It is noteworthy that each of these prefrontal and parietal regions is connected reciprocally with central components of the hippocampal circuit (parahippocampal gyrus, perirhinal cortex, and entorhinal cortex) linked with short‐term memory function [see Squire et al., 1988]. Finally, it was predicted that regions of the frontal cortex associated with code rehearsal (i.e., BA6, BA44) would not be activated, since rehearsal of word codes was expected to be equivalent across task conditions.
METHODS
Ten right‐handed subjects with a mean age of 47.4 ± 3.3 years, including seven males and three females, participated after giving written informed consent. The age of the subjects reflects the constraints of a larger clinical study of which they were a part. Handedness was assessed using Annett's [1970] handedness questionnaire. All subjects had normal visual acuity [Newman, 1975], normal color vision [Ishihara, 1968], and were first‐language English speakers. Exclusion criteria for subjects was any history of head injury or loss of consciousness under unusual circumstances, any known psychological illness or neurological deficits, any recent major illness/recent hospitalization within the past 2 years, any use of a long‐term medication affecting the central nervous system, and any recreational drug use. Furthermore, participants were excluded if they consumed excessive amounts of cigarettes, coffee, or alcohol. Immediately prior to testing (at least 1 hour) participants were instructed not to consume any nicotine, caffeine, or a large meal and not to consume any alcohol at least 12 hours prior to testing. The group had 11.6 ± 2.2 years of education and demonstrated a mean estimated IQ of 109.4 ± 5.9 [Crawford, 1992].
Experimental task and procedure
Subjects were required to complete a task requiring the detection of target stimuli in an attended visual channel under fixed and variable target conditions, in an ABABAB 12‐block design with the condition of the first block counterbalanced across subjects. Stimuli were presented in blue and red, with subjects required to attend to only one color (the attended channel) in each block and depress a microswitch with their right index finger whenever a target stimulus was detected in that color. In the fixed target block, a target was defined a priori as a particular word in a nominated color (e.g., the word “bell” in blue). In a variable target block, a target was defined as a consecutive repeat of any word in the attended color, irrespective of whether a word in the unattended color intervened. Stimulus sequences were identical in structure and form in both conditions.
Task material was derived from a pool of 80 neutral, concrete words consisting of between four and seven letters and of no more than two syllables. Words were presented in Arial 48 point and grouped into presentation sets of 60 stimuli, representing six repeats of 10 words selected without replacement from the task material to minimize repetition effects. Half of the stimuli in each set were blue and the other half were red. Five of the repeats of each word were in one color and the remaining repeat was in the other color.
Stimuli were presented at fixed intensity for 200 ms on a computer monitor located 60 cm in front of the face, with a random stimulus onset asynchrony of 1.5–1.7 sec. The stimuli in each set were in pseudo‐random order. The constraints on ordering were that (1) no more than two nontarget stimuli preceded a target in the variable target condition, (2) no more than one target occurred consecutively in the variable target condition, and (3) one nontarget stimulus occurred immediately before a target in the fixed target condition. An attended channel target had a 16.7% probability of occurrence.
Following practice, subjects completed 24 presentation sets (12 fixed target, 12 variable target), with each set lasting 90 sec and being followed by a 2‐minute break. Speed and accuracy were emphasized equally in task instructions and recorded by the task computer. Analyses of these measures were conducted on means derived from all experimental blocks. Specific task instructions were presented verbally at the beginning of each block, with attended color counterbalanced over blocks.
PET and MRI scanning
Relative rCBF was measured by recording the distribution of cerebral radioactivity following intravenous injection of H2 15O into a small vein of the left forearm. Twelve PET scans were acquired for each subject from every second presentation set using a Siemens/CTI 951/31R scanner operating in high sensitivity 3D mode. Customized head molds were used during collection to restrict heads movement. The scans were reconstructed using a standard image reconstruction algorithm [Kinahan and Rogers, 1989] and a Hanning filter with a cutoff frequency of 0.45 cycles/pixel. Each scan consisted of two acquisition frames having durations of 30 sec (background frame) and 150 sec (foreground frame), respectively. Data from the background frame was forward decay corrected and used to correct the foreground frame for residual radioactivity from the preceding scan. The 80‐sec H2 15O infusion (mean activity per infusion 340 MBq) using a highly reproducible automated water generator [Clark and Tochon‐Danguy, 1992] produced a monotonically increasing brain countrate for ∼100 sec. The scanner acquisition was commenced in order to synchronize the foreground frame with the increasing brain countrate. The 100‐sec paradigm was commenced 5 sec prior to the commencement of the second acquisition frame. A high resolution MRI scan was also acquired for each subject (Siemens Magnotom 4000, magnetization prepared rapid acquisition gradient echo [MPRAGE] protocol; TR=12.5 msec, TE=5 msec, flip angle= 10 degrees). All subjects gave informed consent for this study, which was approved by the Austin & Repatriation Medical Centre Human Ethics Committee.
The reconstructed data volume measured 128 by 128 by 31 voxels, with a voxel size of 2.43 by 2.43 by 3.375 mm. An automated image registration algorithm (AIR 3.0, Woods, 1997a, 1997b) was used to align the second and subsequent PET image to the first PET image for each subject, using a rigid body transformation (six degrees of freedom [df]). An average PET image for each subject was formed by summing the 12 individual PET scans. Each subject's MRI image was edited to remove the scalp and other brain coverings using ANALYZE 7.5 (Biomedical Imaging Resource, Mayo Foundation, Rochester, MN). Each subject's average PET image was then aligned to their segmented MRI image using a six df linear alignment algorithm (AIR 3.0) since the voxel dimensions of the PET and MRI images had previously been independently measured.
Each subject's segmented MR image was aligned to a standard MR image using a 168 df nonlinear algorithm (AIR 3.0). The standard MR image was chosen from a group of normal MR scans because of its close similarity to the gross dimensions and features of the brain used in the Talairach and Tournoux atlas [1988]. The three alignment transformation matrices generated by AIR 3.0 were then combined and applied as a unique transformation for each PET image. The effect of this process was that each individual image for each subject was resliced only once in order to be transformed into Talairach space, sampled at a 2.0 by 2.0 by 4.0 mm voxel size. An average MR image of the 10 individual MR images after nonlinear transformation to Talairach space was also calculated.
The transformed PET images were smoothed using a 3D 12 mm FWHM Gaussian blurring function (AIR 3.0). The images were then masked to remove extracortical signal lying outside the cortical surface defined by each subject's MR image. Statistical parametric mapping [SPM95; Friston et al., 1995] was used to identify voxels that had statistically significant relative regional cerebral blood flow (rCBF) increases for the variable target condition relative to the fixed target condition. Significant activations were chosen after a Bonferroni‐like correction for the number of independent resolution elements (resels) throughout the brain volume. The resel calculation was determined using the SPM95 smoothness estimator.
The statistical images were color coded into six probability ranges using ANALYZE 7.5 to produce an approximate three‐bit image. The eight‐bit average MR image was inspected to determine the minimum acceptable dynamic range of voxel values that did not cause a loss of greyscale resolution. The average MR image was then rescaled to an approximate six‐bit image and multiplied using ANALYZE 7.5 with the three‐bit statistical image to produce a combined MR/PET image suitable for visualization. The anatomical location of the regions of brain activation was determined by reference to the Talairach co‐ordinates and by direct inspection of the combined MR/PET images.
RESULTS
The mean group reaction time (RT) to targets for the variable target condition was 529 (±61) ms and 519 (±44) ms for the fixed target condition. Subjects detected 88% (±14) of variable targets and 91% (±14) of fixed targets. There were no significant differences between conditions in either of these behavioral measures.
The major brain activations were observed bilaterally in the inferior parietal lobe and the middle frontal gyrus (Table 1, Figs. 1,2,3). Activation was observed in the left precentral frontal gyrus in the vicinity of the somatomotor cortex (Fig. 3). A region of rCBF change close to statistical significance was also observed in the right supplementary motor area (SMA), located at (x, y, z) = (+6, ‐4, +64) and having a Z‐score of 4.20 (P corr< 0.068). A small activation region in the right middle temporal lobe located at (x, y, z) = (+56, ‐30, ‐8) and having a Z‐score of 4.08 (P corr< 0.10) was also observed.
Table I.
Cortical areas associated with significant rCBF increases when performing the variable target condition relative to the fixed target condition
| Region | Adjusted rCBF (mL/100g/min) | Talairach co‐ordinates of peak activation | Z‐score | Brodmann areas covered | |
|---|---|---|---|---|---|
| Fixed | Variable | ||||
| L inferior parietal lobule | 56.6 | 58.2 | −38, −58, 40 | 5.71 | 40 |
| L precentral gyrus | 55.6 | 56.9 | −34, −10, 56 | 5.65 | 4 |
| L middle frontal gyrus | 56.3 | 57.5 | −36, −26, 40 | 5.34 | 8/9 |
| R inferior parietal lobule | 58.8 | 59.9 | −40, −60, 44 | 4.86 | 40 |
| R middle frontal gyrus | 57.9 | 59.1 | −38, −18, 44 | 4.66 | 8/9 |
Figure 1.
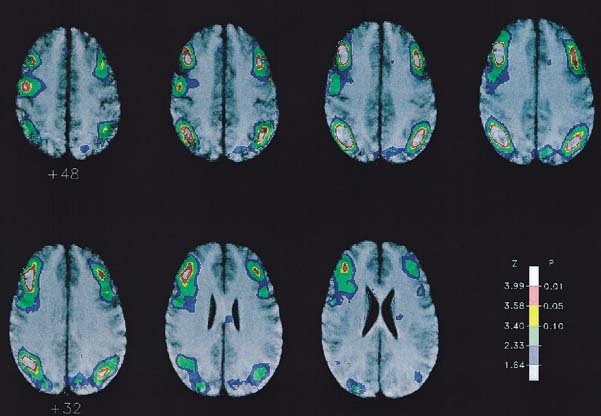
Cerebral activations determined for the variable target condition compared to the fixed target condition superimposed onto the average MR image of the subject group. Axial slices at 4 mm intervals and parallel with the AC‐PC plane are depicted from z = +48 mm to z = +24 mm. The color scale depicts the Z‐score range for the SPM image as indicated, with the associated probability limits shown after correction for the multiple nonindependent comparisons throughout the brain volume.
Figure 2.
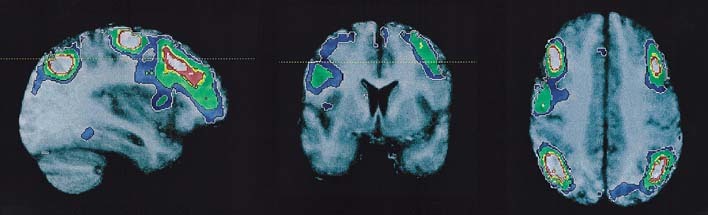
Orthogonal views located at (x, y, z) = (‐34, +8, +40) showing the anatomical extent of the left frontal and parietal activations superimposed on the average MR image of the subject group.
Figure 3.
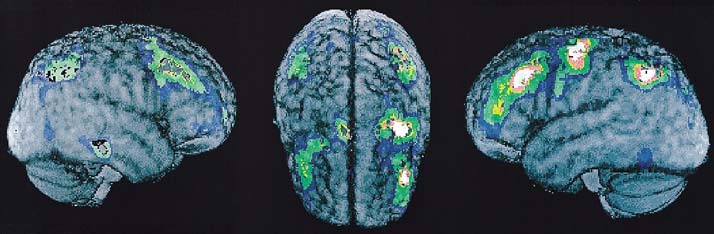
Surface rendered views showing activations on the left hemisphere, the right hemisphere, and from a top view of the cortex.
DISCUSSION
The primary prediction of a fronto‐parietal axis in the updating of working memory for words was supported in this study. These were obtained bilaterally in the supramarginal gyrus (SMG) of the inferior parietal cortex (BA40) and bilaterally in the middle frontal gyrus (MFG) of the lateral prefrontal cortex (BA8/9).
In contrast, the activation obtained in the left motor cortex (BA4) was unexpected. This activation is well localised and is consistent with right hand activation. The near‐significant activation of the right SMA was also unexpected, although notably, recent work has shown the co‐activation of this region with the left motor cortex following transmagnetic stimulation [Fox et al., 1997]. It could be argued that such effects may be the result of an artifact of data acquisition or analysis. But it is unlikely that such artefacts would induce specific effects that can be easily associated with task demands (i.e., right hand responding), particularly given that only a handful of task‐specific activations were obtained in the study and none that could not be explained. Further, counterbalancing controls precluded the ordering of task condition as a contaminating factor, the overt response required for targets was identical for both tasks and the response times obtained were equivalent across task conditions. We conclude, therefore, that the unexpected motor activations are related in some way to differential control between task conditions of the right hand movements required for target detection. It was assumed that the two tasks were matched in terms of response demand. However, the added requirement during the variable target condition to update target identity with each attended word may have forced subjects to increase response readiness across all task stimuli. Such a condition would presumably be reflected in the level of physiological activation measured by PET.
Some concern may arise from the fact that the mean age of the subjects was much greater (47 years) than that normally reported in working memory studies. A decline in the ability to learn and to remember new information is very typically reported as people enter middle age [e.g., Foos et al., 1992; Craik et al., 1995] and may be linked to a compromise of frontal executive processes [Rapp et al., 1994]. Physical correlates of such change include decreases in left and right temporal lobe gray matter volume as well as adjacent ventricular enlargement though no equivalent change in hippocampal measures [Sullivan et al., 1995]. Increases in heart rate and slowing of response time have also been reported with aging under conditions of high working memory demand [Jennings et al., 1990]. There are also age‐related changes in the pattern of alpha suppression during working memory function [Dujardin et al., 1994], in delta and theta power [Hartikainen et al., 1992] and in the amplitude, latency, and scalp topography of the P3 component of the event‐related potential that has been linked to working memory [e.g., Sandman et al., 1990; O'Donnell et al., 1992]. Significantly, both the middle frontal gyrus and inferior parietal lobe have been identified as possible sources of the P3 [McCarthy et al., 1997]. It should be considered, therefore, that there might be differential memory processing in young and older subjects. At this time, no functional imaging report addressing this issue has been identified. It should be noted, however, that the present study succeeds only in identifying structures involved in working memory processing and is silent on the dynamics of these processes. Certainly, the pattern of activations obtained in this study is consistent with earlier reports involving younger subjects and to this extent partially addresses the mooted concern.
The present study introduced a number of methodological manipulations intended to restrict activation to those regions concerned with the updating and storage in working memory of codes induced by concrete word stimuli. Experimental design was premised on the theoretical distinction [see Baddeley, 1986] between the deployment of stores for working memory of task‐related codes and a system for the rehearsal of the codes required to refresh the limited duration trace of such codes in these stores. It was assumed that both task conditions (fixed and variable targets) required the same degree of target rehearsal. This position was based on the finding that phonological material cannot be retained in working memory for longer than about 3 sec [see Baddeley, 1986] together with the fact that the interval between successive attended words exceeded this limit. Thus rehearsal demands would be equivalent for both task conditions, irrespective of differences in the rate of change in the material to be rehearsed. This presumption was supported since no significant activation was obtained in the frontal areas (BA44, the supplementary motor area and premotor cortex) associated with phonological rehearsal [see Paulesu et al., 1993; Awh et al., 1996; Smith et al., 1996]. It is conceivable that there may have been differences in rehearsal demands for non‐verbal components of working memory representations, although it is considered unlikely that such rehearsal would have occurred in the absence of phonological rehearsal.
It was also assumed that the task conditions differed only in the frequency of change in target identity. In the fixed target condition, targets changed only once every 60 words (task block). Thus, whereas word code rehearsal was required about 30 times per block, based on the estimated duration of the phonological store [see Baddeley, 1986], the word codes themselves would not change over the period of the task block. In contrast, target identity changed 25 times during each task block in the variable condition. This would necessarily require frequent updating of the working memory stores holding the codes for target words. Thus a comparison of the task conditions should reveal anatomical regions associated exclusively with this updating process. The behavioral results of the study indicated that the experimental and control study conditions were well balanced. Both the variable and the fixed target conditions elicited comparable speed and accuracy of target detection. This suggests that significantly different task activations were not related to differences in response processing demands. Further, since the same stimuli were used in both task conditions in counterbalanced task order, it is unlikely that the activations obtained could be related to task differences in stimulus processing demands.
The bilateral activation of BA40 in the present study is presumed to reflect the stores affected by the working memory updating process. It has been argued that the concrete word stimuli used in this study probably activated codes responsible for phonological as well as visuospatial modalities of information. To this extent, it can be argued that the study activated areas related to multiple, potentially independent types of working memory systems. If so, it would be less sensitive as a result to activations related to any one of these systems. To this extent, the interpretation of the data becomes more difficult. Nevertheless, sites of activation during working memory tasks for separate letter and visuospatial stimuli have already been reported in the literature. This is not the case for concrete word stimuli. Earlier studies requiring working memory for letters [see Petrides et al., 1993; Jonides et al., 1997a] only obtained activation of left BA40. This indicates that phonological systems involves left and not bilateral inferior parietal regions. Further, studies [Kosslyn et al., 1994; Andreasen et al., 1996; Smith et al., 1996] requiring working or episodic memory for visuospatial stimuli (designs, faces) have activated right but not left BA40. This suggests that visuospatial storage is restricted to the right. Finally and similar to the present study, a number of studies examining episodic memory for word stimuli have also found activation of BA40 bilaterally [Tulving et al., 1994; Andreasen et al., 1995; Kapur et al., 1995]. These results suggest that the activation of left and right BA40 in the present study reflects the updating of both phonological as well as visuospatial stores, respectively, induced by concrete word stimuli.
Contrary to expectations, the present study did not obtain activation of the superior parietal gyrus (BA7) in either cerebral hemisphere. This region has been activated bilaterally, along with left BA40, in a number of the studies by Jonides et al. [1997a], Smith et al. [1996], and Awh et al. [1996]. In many respects, the design of these earlier studies resembled that of the present study. However, as indicated elsewhere [see Jonides et al., 1997b], they required additional cognitive processes that might be expected to engage a number of other brain regions, including attentional processes involving structures that include BA7 of the superior parietal cortex. It is conceivable, therefore, that BA7 may not be involved directly in the storage of object working memory codes.
The bilateral activation of the MFG is interpreted to reflect executive processes controlling the updating of working memory. The activation peaked in BA8 and extended bilaterally into adjacent region BA9 (see Fig. 3). Most imaging studies [e.g., Petrides 1993a, 1995; Coull et al., 1996; Smith et al., 1996; Jonides et al., 1997a] investigating verbal working memory have not obtained activation of BA8, but only of adjacent regions BA9, BA10, BA45, and BA46. An exception is the fMRI study by Cohen et al. [1997] that found activation in right but not left BA8 as a function of working memory load. There are, however, critical differences between the present study and these previous studies. First, the previous studies used letter or number rather than concrete word stimuli. Thus the variation in regional activation may reflect differences in the character of the information to be remembered. Concrete word stimuli are likely to induce semantic and object word codes not obtained with more abstract letters and numbers. Second, whereas some of the previous studies manipulated the updating of working memory codes, this was only done in conjunction with the manipulation of other executive processes. One study [Smith et al., 1996] provides the closest match with the present study. In the experimental condition of that study, subjects were required to attend to a sequence of letter stimuli and detect stimuli that matched the letter presented three back in the sequence. This forces the subject to keep the mental list in temporal order of the last three stimuli and update the list with each new stimulus by adding the latest letter and removing the oldest. The control condition required subjects to assess whether letters matched any of three presented a priori at the beginning of each test block. These two tasks differed, therefore, in two main respects: updating the contents of working memory and updating their temporal tags. That study obtained bilateral activation of BA9, BA10, and left BA45 and left BA46. Comparison with the present study, therefore, suggests that areas BA45 and BA10 are involved in the updating of stores holding information about the temporal order of stimulus codes to be retained in working memory, whereas BA9 and BA46 are involved in the updating of working memory stores that retain these stimulus codes. Earlier suggestions have been made that temporal order processes are mediated by dorsolateral prefrontal activity [e.g., Jonides et al., 1997; Moscovitch et al., 1992].
The activation of dorsolateral rather than ventrolateral prefrontal cortex in the present study bears on the debate about the functional organization of working memory processes in human lateral frontal cortex [see Cohen et al., 1997; Owen et al., 1997]. Goldman‐Rakic [1987, 1994, 1995] has argued that these processes are organized according to type (e.g., modality) of information being processed, with dorsolateral regions being principally concerned with memory for spatial material and ventrolateral regions with non‐spatial material. The results of the present study do not support this position. An alternative view has been put [Petrides, 1995, 1996] according to which the lateral frontal regions are organized according to the nature of processing required — with the ventrolateral cortex concerned principally with the active organization of response sequences relevant to information received from posterior association systems and dorsolateral cortex subserving executive processing related to the active manipulation and monitoring of information within working memory. For two reasons, the present study appears to support this position. First, dorsolateral activation obtained in the present study appears unequivocally related to the updating within working memory of the sensory representation of anticipated target stimuli. Second, the present study did not obtain any activation in ventrolateral prefrontal cortex. This appears consistent with the model of Petrides [1995, 1996], since the present study did not require any reorganization of response sequences relative to anticipated targets; rather, the response requirements for both the fixed and variable target conditions were invariant and identical. Thus a clear prediction that arises from the Petrides model is that ventrolateral prefrontal cortex should activate if response requirements varied during task performance.
Working memory provides the primary mechanism in humans for the mapping of stimulus to response. Adult humans are primarily active responders to the environment, so that stimuli tend to be sought out or anticipated rather than passively received, and the response to such stimuli usually prepared in advance of their detection. Thus most human behavior is driven by plans and goals that facilitate intentionality. According to Shallice [1988], goal‐oriented behavior is mediated in working memory via the activation of “schemata” that reflect clusters of learned, contextually related links between stimulus and response representations. Much of human behavior can be explained in terms of the operation of such schemata. In familiar, well‐learned contexts, the relevant schemata will activate and provide an infrastructure for the automatic control of behavior. Thus contextually relevant stimuli will automatically generate contextually appropriate responses. In unfamiliar contexts, however, goal‐related behavior must be guided by control mechanisms, e.g., the supervisory attentional system of Shallice [1988] or the central executive of Baddeley [1986]. These mechanisms control the selection, evaluation, and association of stimulus and response and may develop or modify relevant schemata.
The present experiment can be conceptualized in such terms. The goal of both experimental tasks was to make an appropriate response to relevant stimulus words. Executive control was needed to ensure that the relevant stimulus and required response were identified, stored, and associated in working memory. By design, the tasks differed only in the frequency with which the identity of the relevant stimulus (i.e., target) was updated. Thus the activations obtained in the study implicate structures involved in the updating of the working memory stores that prescribe stimulus relevance and stimulus‐response contingency; in this case, the middle frontal gyri (MFG) of the dorsolateral prefrontal cortex (DLPFC) and the supramarginal gyri (SMG) of the inferior parietal lobe.
A number of studies [Petrides, 1995, 1996; see also Owen, 1997; Smith and Jonides, 1999] have already implicated the DLPFC (areas 9 and 46) in task management, particularly in relation to the active manipulation and monitoring of information required for planned action. It would seem feasible, therefore, that at least one function of the area is to link working memory representations of goal‐related, stimulus and response contingencies during controlled processing. There is evidence from a number of primate functional anatomy studies for the existence of bidirectional pathways that might support such a mapping function, including connections between dorsolateral PFC, ventrolateral PFC, and premotor cortex on the one hand and between dorsolateral PFC and the inferior parietal cortex on the other [e.g., Jones et al., 1970; Mesulam et al., 1977; Goldman‐Rakic, 1987; Pandya et al., 1996; see also Damasio et al., 1993; Arnold, 1994;Goldman‐Rakic, 1996; Petrides, 1996]. In terms of the study paradigm, a nodal link in the MFG would point to the representation in posterior cortex of the target to be detected and to the representation in frontal cortex of the planned finger response. This would provide an economical mechanism for planned stimulus‐response mapping that satisfies a number of important functional requirements. First, it provides independent executive control for maintaining a working activation of the representations relevant to a current goal (i.e., goal‐setting). Second, it does not preclude alternative and/or concurrent mappings involving any given stimulus or response representation. This provides scope for multiple contextual representations involving overlapping stimulus‐response sets and hence for multitasking. Third, it provides a trigger mechanism by which a planned action can be released following activation of the representation of an anticipated stimulus during sensory processing [see Goldman‐Rakic, 1996].
Neuroanatomical and neurophysiological evidence provides general support for the view that the supramarginal gyrus of the inferior parietal is an amodal region important for stimulus representation. The left and right supramarginal gyri appear to be associated, respectively, with linguistic [see Petrides et al., 1993; Jonides et al., 1997a] and nonlinguistic [Kosslyn et al., 1994; Andreasen et al., 1996; Smith et al., 1996] codes. It is likely, therefore, that these posterior regions are critical to the storage in working memory of task‐related, stimulus representations. Further, and consistent with the discussion above, there are bidirectional connections between this region and the MFG [see also Arnold, 1994; Kolb and Wishaw, 1995]. But whereas this suggests the role for this region in working memory function, it does not clarify its role in stimulus representation. One possibility has been suggested by Damasio [1989a, 1989b], who proposes that the region is a multimodal sensory convergence zone relevant to perceptual binding and memory retrieval. Certainly, the region receives multiple convergent inputs from modal‐specific sensory regions. Damasio et al. [1989a, 1989b] propose that the region acts to bind the modal fragments of sensory features, providing an efficient and adaptive, amodal record of the feature combinations that moderate perceptual experience. Notably, the region is commonly activated in studies involving the retention of sensory information in working memory, irrespective of stimulus modality and stimulus type [e.g., Kosslyn et al., 1994; Tulving et al., 1994; Andreasen et al., 1995; Awh et al., 1996; Kapur et al., 1995; Jonides et al., 1997b; Smith et al., 1996].
Figure 4 attempts to distill these ideas into a modular, testable model of working memory that respects conceptual contributions from cognitive psychology [e.g., Shallice, 1988, 1996; Moscovitch, 1992; Baddeley et al., 1996], cognitive neuroimaging [e.g., Goldman‐Rakic, 1987, 1988, 1995, 1996; Petrides, 1995, 1996; Owen, 1997; Smith et al., 1997, 1999; Rushworth et al., 1998] and functional neuroanatomy [Jones et al., 1970; Mesulam et al., 1977; Damasio, 1989a, 1989b; Damasio et al., 1993; Arnold, 1994; Pandya et al., 1996; Fuster, 1997]. The central design principle is that regions responsible for executive control of working memory function link together regions holding perceptual and response representations relevant to controlled, goal‐oriented behavior. Thus it incorporates discrete systems for stimulus representation, response representation and executive control. Anterior cortical regions are taken to be responsible for the executive control of working memory and for response representations, whereas posterior cortical regions are taken to be responsible for stimulus representations. Based on the present and earlier studies, it is proposed that the controlled linking of an expected stimulus to planned response (i.e., goal‐setting) is handled (no doubt among other things) by the middle frontal gyrus. This linkage is effected by parallel connections from the MFG to the inferior parietal cortex for sensory representations and, presumably, to other regions of the frontal cortex for motor representations. It is taken that the inferior parietal cortex is an amodal region binding the modal, sensory fragments of perceptual objects. It is proposed that the ventrolateral prefrontal cortex assumes the analogous amodal role for response representations. Whereas there is no evidence from the present study for this role, it has nevertheless been shown [e.g., Petrides, 1995, 1996] that areas 45 and 46 of the ventrolateral PFC are associated with the organisation of response sequences based on the activation of posterior sensory systems. Further, these regions in the ventrolateral PFC are connected with the premotor cortex (e.g., Brodmann areas 4, 6, 8, and 44) that are likely to hold the modal motor fragments necessary for an integrated response [see also Arnold, 1994; Fuster, 1997].
Figure 4.
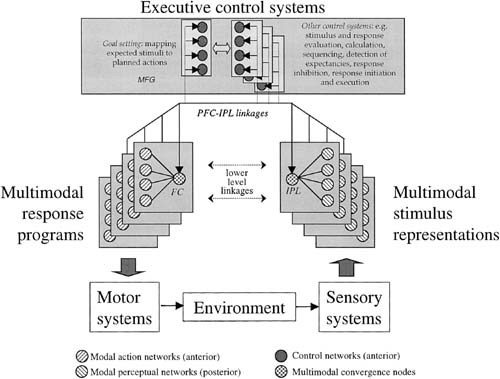
Model describing a hierarchical system for controlled mapping of stimulus and response representations via an executive control module within working memory. The key points of the model are: (1) Executive control of working memory is handled by regions in the prefrontal cortex. Based on the present and earlier studies, it is proposed that goal‐setting involving the controlled linking of an expected stimulus to a planned response is handled by regions in the middle frontal gyrus (MFG). (2) This linkage is mediated by parallel connections from the MFG to amodal convergence zones in the posterior parietal cortex (IPL) for sensory representations and in the frontal cortex (FC) for motor representations. Such convergence zones are conceived to bind together and hold on line the modal fragment records of feature‐based sensory activity (“multimodal sensory representations”) and motor activity (“multimodal response programs”), respectively. The model suggests linkages that may provide a basic working memory configuration representing task‐related goals and form the structural basis for mental sets upon which other task‐related executive processes in the dorsolateral prefrontal cortex (e.g., stimulus evaluation, calculation, sequencing, and response initiation and execution) may be performed. The model suggests other possible (and interconnecting) executive control systems. The multiple nodes within each such system are intended to reflect multitasking.
A residual anomaly in the present study is the extensive activation obtained bilaterally in area 8. It is possible that the activation may be related to silent word reading, as obtained in the recent study by Price et al. [1994]. Less effort may have been expended by subjects in the fixed target condition, which required processing of each stimulus word only to assess whether it matched the target held in working memory. The variable target condition additionally required that the word be retained in working memory for subsequent processing. This may have required more careful scanning and reading of each word, as would be the case for the reading of text. This account is consistent with the close anatomical and physiological links of BA8 with the visual and oculomotor systems [Bruce et al., 1985; Pandya et al., 1987]. As pointed out by Petrides et al. [1987, 1993b], this area has been implicated in the cue‐based selection of visual stimuli to become the target of visual search. The use of word color in the present experiment to indicate whether a word was to be attended or not would have provided such a cue in the variable target condition.
In summary, this study has demonstrated that bilateral activation of the middle frontal gyrus of the dorsolateral prefrontal cortex (BA 8/9) and the supramarginal gyrus of the inferior parietal (BA40) cortex are involved in the updating of verbal working memory for which a controlled response is required. The activation of the middle frontal gyrus is argued to reflect executive control over the updating process linking anticipated stimulus to planned response. In this regard, it is seen to facilitate goal‐setting during intentional behavior. It is further proposed that the updating of goal‐related, stimulus representations is mediated via connections between the middle frontal gyrus and the supramarginal gyrus. In this regard, the supramarginal gyrus may represent an amodal convergence zone binding modal representations of sensory information being retained in working memory [see Damasio, 1989a, b]. The involvement of BA8 in the present study is unclear but may reflect cueing processes facilitating the selection of the visual word stimulus for further processing.
Acknowledgements
The support of the NH&MRC Neuroimaging Consortium is acknowledged. We thank the anonymous reviewers for their valuable comments on the manuscript.
REFERENCES
- Andreassen NC, O'Leary DS, Arndt S, Cizadlo T, Hurtig R, Rezai K, Watkins GL, Boles Ponto L, Hichwa RD. 1995. Short term and long term verbal memory: a positron emission tomography study. Proc Natl Acad Sci USA 92:5111–5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annett M. 1970. A classification of hand preference by association analysis. Br J Psychol 61:303–321. [DOI] [PubMed] [Google Scholar]
- Arnold SE. 1994. Neuroanatomy In: Fraser A, Molinoff PB, Winokur A, editors. Biological bases of brain function and disease. New York: Raven Press; p 1–18. [Google Scholar]
- Awh E, Jonides J, Smith E, Schumacher E, Koeppe R, Katz S. 1996. Dissociation of storage and rehearsal in verbal working memory: evidence from positron emission tomography. Psychological Sci 7:25–31. [Google Scholar]
- Baddeley A. 1986. Working memory. Oxford: Clarendon Press. [Google Scholar]
- Baddeley A, Della Sala S. 1996. Working memory and executive control. Phil Trans R Soc Lond. B 351:1397–1404. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD, Posner MI. 1997. Time course of cortical activations in implicit and explicit recall. J Neurosci 17:4904–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Chertkow H, Bub D, Murtha S, Dixon R, Evans A. 1997. The neural substrate for concrete, abstract and emotional word lexica: a positron emission tomography study. J Cog Neurosci 9:441–461. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. 1985. Primate frontal eye fields. 1. Single neurons discharging before saccades. J Neurophysiol 53:603–635. [DOI] [PubMed] [Google Scholar]
- Clark J, Tochon‐Danguy HJ. 1992. “R2D2,” a bedside [oxygen‐15] water infuser. Proc. 6th Inter Work on Targetry and Target Chemistry, 91 ed. Weinreich R, PSI, Switzerland.
- Cohen J, Forman S, Braver T, Casey B, Servan‐Schreiber D, Noll D. 1994. Activation of the prefrontal cortex in a nonspatial working memory task with functional MRI. Hum Brain Mapping 1:293–304. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith ES. 1997. Temporal dynamics of brain activation during a working memory task. Nature 386:604–608. [DOI] [PubMed] [Google Scholar]
- Cohen M, Kosslyn S, Breiter H, DiGirolamo G, Thompson W, Anderson A, Bookheimer S, Rosen B, Belliveau J. 1996. Changes in cortical activity during mental rotation: a mapping study using functional MRI. Brain 119:89–100. [DOI] [PubMed] [Google Scholar]
- Coull J, Frith C, Frakowiak R, Grasby P. 1996. A fronto‐parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia 34:1085–1095. [DOI] [PubMed] [Google Scholar]
- Courtney S, Underleider L, Keil K, Haxby J. 1996. Object and spatial visual working memory activate separate neural systems in human cortex. Cereb Cortex 6:39–49. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Anderson ND, Kerr SA, Li KZH. 1995. In: Baddeley AD, Wilson BA. et al., editors. Handbook of memory disorders. Chichester, UK: John Wiley & Sons; p 211–241). [Google Scholar]
- Crawford J. 1992. Current and premorbid intelligence measures in neuropsychological assessment In: Crawford J, Parker D, McKinlay W, editors. A handbook of neuropsychological assessment. Hove, UK: Erlbaum; p 21–49. [Google Scholar]
- Damasio AR. 1989a. Time‐locked multiregional retroactivation: a systems‐level proposal for the neural substrates of recall and recognition. Cognition 33:25–62. [DOI] [PubMed] [Google Scholar]
- Damasio AR. 1989b. The brain binds entities and events by multiregional activation. Neural Computation 1:123–132. [Google Scholar]
- Damasio AR, Anderson SW. 1993. The frontal lobes In: Heilman K. Valentstein E, editors. Clinical neuropsychology, 3rd ed. New York: Oxford University Press; p 409–460. [Google Scholar]
- Desposito MD, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. 1995. The neural basis of the central executive of working memory. Nature 378:279–281. [DOI] [PubMed] [Google Scholar]
- Dujardin K, Bourriez JL, Guieu JD. 1994. Event‐related desynchronization (ERD) patterns during verbal memory tasks: effect of age. Inter J Psychophysiol 16:17–27. [DOI] [PubMed] [Google Scholar]
- Foos PW, Wright L. 1992. Adult age differences in the storage of information in working memory. Experimental Aging Res 18:51–57. [DOI] [PubMed] [Google Scholar]
- Fox P, Ingham R, George MS, Mayberg H, Ingham J, Roby J, Martin C, Jerebek P. 1997. Imaging human intra‐cerebral connectivity by PET during TMS. NeuroReport 8:2787–2791. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J‐P, Frith CD, Frackowiak RSJ. 1995. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2:189–210. [Google Scholar]
- Fuster J. 1997. Network memory. Trends Neurosci 20:451–459. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic PS. 1987. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory In: Plum F, editor. Handbook of physiology, the nervous system, higher functions of the brain, section 1, vol. 5: part 1. Bethesda, MD: Am Physiol Soc, p 373–417. [Google Scholar]
- Goldman‐Rakic PS. 1988. Topography of cognition: parallel distributed networks in primate association cortex. Ann Rev Neurosci 11:137–156. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic PS. 1994. The issue of memory in the study of prefrontal functions In: Theiry AM, Glowinski J, Goldman‐Rakic PS, Christen Y, editors. Motor and cognitive functions of the prefrontal cortex. Berlin: Springer‐Verlag; p 112–122. [Google Scholar]
- Goldman‐Rakic PS. 1995. Architecture of the prefrontal cortex and the central executive. Ann NY Acad Sci 769:71–83. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic PS. 1996. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Phil Trans R Soc Lond B 351:1445–1453. [DOI] [PubMed] [Google Scholar]
- Hartikainen P, Soininen H, Partanen J, Helkala EL, Riekkinen P. 1992. Aging and spectral analysis of EEG in normal subjects: a link to memory and CSF AChE. Acta Neurologica Scandinavica. 86:148–155. [DOI] [PubMed] [Google Scholar]
- Hinke RM, Xu H, Stillman AE, Kim S, Merkle H, Salmi R, Ugurbil K. 1993. Functional magnetic resonance imaging of Broca's area during internal speech. Neuroreport 4:675–678. [DOI] [PubMed] [Google Scholar]
- Ishihara S. 1968. Tests for color‐blindness, 38 plates ed. Tokyo: Kanehara Shuppan. [Google Scholar]
- Jennings JR, Nebes RD, Yovetich NA. 1990. Aging increases the energetic demands of episodic memory: a cardiovascular analysis. J Exp Psychol 119:77–91. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TPS. 1970. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain 93:793–820. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber EJ, Awh E, Minoshima S, Koeppe RA. 1997b. Verbal working memory load affects regional brain activation as measured by PET. J Cog Neurosci 9:462–475. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE. 1997a. The architecture of working memory In: Rugg M, editor. Cognitive Neuroscience. Sussex, UK: Psychology Press; p 243–276. [Google Scholar]
- Kapur S, Craik FIM, Jones C, Brown GM, Houle S, Tulving E. 1995. Functional role of the prefrontal cortex in memory retrieval: a PET study. Neuroreport 6:1880–1884. [DOI] [PubMed] [Google Scholar]
- Kinahan PE, Rogers JG. 1989. Analytic 3D image reconstruction using all detected events. IEEE Trans Nucl Sci 36:964–968. [Google Scholar]
- Kolb B, Wishaw Q. 1996. Fundamentals of neuropsychology. New York: Freeman. [Google Scholar]
- Kosslyn SM, Alpert NM, Thompson WL, Chabris CE, Rauch SL, Anderson AE. 1994. Identifying objects seen from different viewpoints: a PET investigation. Brain 117:1055–1071. [DOI] [PubMed] [Google Scholar]
- McCarthy G. 1995. Functional neuroimaging of memory. Neuroscientist 1:155–163. [Google Scholar]
- McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, Goldman‐Rakic P. 1996. Activation of human prefrontal cortex during spatial and non‐spatial working memory tasks measured by functional MRI. Cereb Cortex 6:600–611. [DOI] [PubMed] [Google Scholar]
- McCarthy G, 1997. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol 77:1630–1634. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, van Hoesen GW, Pandya DN, Geschwind N. 1977. Limbic and sensory projections of the inferior parietal lobule (area PG) in the rhesus monkey: a study with a new method for horseradish peroxidase histochemistry. Brain Res 136:393–414. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. 1992. Memory and working‐with‐memory: a component process model based on modules and central systems. J Cog Neurosci 4:257–267. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. 1992. The neuropsychology of memory and ageing In: Craik FIM, Salthouse TA, editors. The handbook of ageing and cognition. Hillsdale, NJ: Erlbaum; p 315–372. [Google Scholar]
- Newman M. 1975. Visual acuity In: Moses RA, editor. Adler's physiology of the eye: clinical application, 6th ed. Saint Louis: Mosby; p 500–528. [Google Scholar]
- O'Donnell BF, Friedman S, Swearer JM, Drachman DA. 1992. Active and passive P3 latency and psychometric performance: influence of age and individual differences. Inter J Psychophysiol 12:187–195. [DOI] [PubMed] [Google Scholar]
- Owen AM. 1997. The functional organisation of working memory processes within human lateral frontal cortex: the contribution of functional neuroimaging. Eur J Neurosci 9:1329–1339. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Barnes CL. 1987. Architecture and connections of the frontal lobes In: Perecman E, editor. The frontal lobes revisited. Hillsdale, NJ: Erlbaum; p 41–72. [Google Scholar]
- Pandya DN, Yeterian EH. 1996. Comparison of prefrontal architecture and connections. Phil Trans R Soc Lond B 351:1423–1432. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Connelly A, Frith C, Friston K, Heather J, Myers R, Gadian D, Frackowiak R. 1995. Functional MR imaging correlations with positron emission tomography In: Drayer BP, editor. Neurosurgery clinics of North America: functional neuroimaging (journal?) 5:207–225. [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS. 1993. The neural correlates of the verbal component of working memory. Nature 362:342–345. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. 1989. Positron emission tomographic studies of the processing of single words. J Cog Neurosci 1:153–169. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Snyder AZ, Raichle ME. 1990. Activation of extrastriate and frontal cortical areas by visual words and word‐like stimuli. Science 249:1041–1044. [DOI] [PubMed] [Google Scholar]
- Petrides M. 1987. Conditional learning and the primate frontal cortex In: Perecman C, editor. The frontal lobes revisited. Hillsdale, NJ: Erlbaum; p 91–108. [Google Scholar]
- Petrides M. 1995. Functional organization of the human frontal cortex for mnemonic processing. Ann NY Acad Sci 769:85–96. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivistos B, Evans A, Meyer E. 1993b. Dissociation of human mid‐dorsolateral from posterior dorsolateral frontal cortex in memory processing. Proc Natl Acad Sci USA 90:873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Alivistos B, Meyer E, Evans A. 1993a. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci USA 90:878–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. 1996. Specialised systems for the processing of mnemonic information within the primate frontal cortex. Phil Trans R Soc Lond B 351:1455–1462. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE, Fox PT, Raichle ME. 1988. Localisation of cognitive operations in the human brain. Science 240:1627–1631. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJ, Watson JD, Patterson K, Howard D, Frackowiak RS. 1994. Brain activity during reading: the effects of exposure duration and task. Brain 117:1255–1269. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F. 1998. Words in the brain's language. Behavioural Brain Sci (in press). [Google Scholar]
- Rapp PR, Heindel WC. 1994. Memory systems in normal and pathological aging. Current Opin Neurol 7:294–298. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Owen AM. 1998. The functional organisation of the lateral frontal cortex: conjecture or conjuncture in the electrophysiology literature? Trends Cognitive Sci 2:46–53. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Donnelly JF, O'Halloran JP, Isenhart R. 1990. Age‐related change in P3 amplitude as a function of predictable and unpredictable rare events. Inter J Neurosci 52:189–199. [DOI] [PubMed] [Google Scholar]
- Sergent J, Ohta S, MacDonald B. 1992. Functional neuroanatomy of face and object processing. Brain 115:15–36. [DOI] [PubMed] [Google Scholar]
- Shallice T. 1988. From neuropsychology to mental structure. Cambridge: Cambridge University Press. [Google Scholar]
- Shallice T, Burgess P. 1996. The domain of supervisory processes and temporal organisation of behavior. Phil Trans R Soc Lond B 351:1405–1412. [DOI] [PubMed] [Google Scholar]
- Smith E, Jonides J, Koeppe R. 1996. Dissociating verbal and spatial working memory using PET. Cereb Cortex 6:11–20. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. 1997. Working memory: a view from neuroimaging. Cognitive Psychol 33:5–42. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. 1999. Storage and executive processes in the frontal lobes. Science 283:1657–1661. [DOI] [PubMed] [Google Scholar]
- Squire L, Zola‐Morgan S. 1988. Memory: brain systems and behavior. Trends Neurosci 11:170–175. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. 1995. Age‐related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiol Aging. 16:591–606. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 1988. Co‐planner stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Tulving E, Kapur S, Markowitsch HJ, Craik FM, Habib R, Houle S. 1994. Neuroanatomical correlates of retrieval in episodic memory: auditory sentence recognition. Proc Natl Acad Sci USA 91:2012–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. 1997a. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comp Assist. Tomog 22:141–154. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JDG, Sicotte NL, Mazziotta JC. 1997b. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comp Assist Tomog 22:154–165. [DOI] [PubMed] [Google Scholar]


