Abstract
We used magnetoencephalography to study inter‐individual locational difference in the extrastriate region which responds to visual motion. Magnetic responses to visual motion onset from the right temporo‐occipital area were recorded from 12 subjects. All the subjects had clear responses to apparent or random dot coherent motion. The origins of these responses was investigated by use of the single equivalent current dipole model. The nearest scalp to the origin also was identified for each subject, which may be useful in transcranial stimulation studies. Although the magnetic responses of all the subjects should have the same functional properties; be related to neural activities synchronized exclusively to the onset of motion, the estimated origins varied greatly among the subjects. The location of origin could be classified as one of three types: temporo‐occipital, occipital, or parietal, according to the sulcal anatomy investigated in the individual's three‐dimensional magnetic resonance image. Temporo‐occipital types were found for seven subjects, and anatomically the regions were around human MT/V5. Two subjects had the occipital type, with regions posterior to the anatomical MT/V5 and corresponding to V3A anatomically. The other three subjects had origins classified as the parietal type dorso‐rostral to the anatomical MT/V5, with regions around the posterior end of the superior temporal sulcus. Although all these cortical regions appear to be related to the neural process of visual motion, whether they correspond functionally to the same names or migrated MT/V5 must now be determined. Hum. Brain Mapping 11:33–45, 2000. © 2000 Wiley‐Liss, Inc.
Keywords: MT/V5, V3A, superior temporal sulcus, apparent motion, random dot cinematogram, response time
INTRODUCTION
It is widely accepted that the human visual system has several functional subdivisions, each specific to the perception of form, color, motion, or depth [Livingstone and Hubel, 1988]. Two main streams, ventral and dorsal pathways, have been considered for these neural processes [Ungerleider and Haxby, 1994]. The ventral pathway, composed of V1, V2, V4, and inferior temporal areas, is responsible for perception of the color and form (shape) of an object. The dorsal pathway (V1, V2, MT/V5, MST, and the inferior parietal and superior temporal area) is involved in the analysis of such spatial aspects of visual stimuli as motion and pursuit eye movements.
This functional organization of the visual system has been studied extensively in monkey brains [Maunsell and Newsome, 1987]. Regions in the extrastriate cortex first were investigated [Dubner and Zeki, 1971; Allman and Kass, 1971], and the area now referred to as MT or V5 was proposed as the cardinal structure for visual motion process [Zeki, 1974]. In humans, similar functional localization of the visual system was first reported for a patient who had markedly selective impairment of visual motion perception [Zihl et al., 1983]. Although that patient's brain lesion extended over a wide portion of the bilateral occipito‐temporal area, a small area in a hemisphere was shown to impair motion perception to some extent in later studies [Thurston et al., 1988; Plant et al., 1993; Barton et al., 1996].
Recent methods for measuring brain functions that use positron emission and proton magnetic resonance permit non‐invasive investigation of normal human brain function in detail. A positron emission tomography (PET) study revealed the localization of human cortical areas that respond to visual motion stimuli as well as to color images [Zeki et al., 1991]. More details of the anatomical and functional structures of the human visual system have been shown by functional magnetic resonance imaging (fMRI) [Schneider et al., 1993] and visual motion‐related regions have been identified [Tootell et al., 1995a]. Both methods, however, measure regional blood flow or metabolic change rather than neural activity, and the assumption is that what they measure changes with neural activity in the region of interest. Because transient neural activity change may not induce related hemodynamic and metabolic changes, these methods may not be able to clarify rapid neural activity change even though future technological development ensure measurements of hemodynamic and metabolic changes in milliseconds.
Electroencephalography (EEG) was the only non‐invasive method to measure electrical activity originating from the human brain, until magnetoencephalography (MEG) was developed with the aim of localizing sources of measured brain signals [Hughes, 1983]. MEG measures magnetic fields outside of the head which are considered to originate from the internal current of the pyramidal neurons in the cerebral cortex [Hari and Lounasmaa, 1989; Hamalainen et al., 1993]. Because magnetic fields are less affected by the cerebrospinal fluid and skull than are the electrical fields that EEG measures, better estimation of the signal sources can be made with MEG [Hamalainen et al., 1993]. Recently, MEG has been used to determine the human cortical regions involved in the visual motion process and to assess their temporal activity patterns [ffytche et al., 1995a; Patzwahl et al., 1996; Anderson et al., 1996; Kaneoke et al., 1997; Ahlfors et al., 1999]. These studies measured magnetic responses to visual motion onset or direction change, and the locations identified for the visual motion process correspond to those found in PET and fMRI studies [Zeki et al., 1991; Tootell et al., 1995a] which measured regional activation during visual motion stimuli. When PET was used to identify the cortical activity induced by motion reversal and motion onset [Cornette et al., 1998], no significant activation in human MT/V5 occurred, whereas the area was found mainly to respond to such visual stimuli in the reported MEG studies. This discrepancy can be explained because magnetic responses are usually detected only by time domain averaging at the onset of an event, such as motion onset, due to the low signal to noise ratio. This procedure, however, permits measurements of neural activities only synchronized to an event onset even though it is too transient to induce metabolic or hemodynamic change. Use of MEG to investigate human brain therefore may provide physiological evidence not revealed by PET and fMRI studies as in the case that MEG detected activation of MT/V5 in dyslexic subjects though fMRI did not [Vanni et al., 1997].
The objective of this MEG study was to clarify those human cortical regions, in which neural activity is exclusively synchronous to the onset of motion and to show the localization of the individual regions by the use of individual sulcal anatomy with three‐dimensional MRI.
Abbreviations.
| AM | apparent motion |
| ECD | equivalent current dipole |
| EEG | electroencephalography |
| fMRI | functional magnetic resonance imaging or image |
| fT | femto Tesla |
| MEG | magnetoencephalography or magnetoencephalogram |
| MST | medial superior temporal area |
| MT | middle temporal area |
| Am | nano ampere meter |
| NS | nearest scalp |
| PET | positron emission tomography |
| pSTS | posterior end of superior temporal sulcus |
| RDC | random dot cinematogram |
| TMS | transcranial magnetic stimulation |
| V1 | primary visual cortex |
| V2 | secondary visual cortex |
| V3A | 3rd visual cortex accessory |
| V5 | 5th visual cortex |
MATERIALS AND METHODS
Subjects
Twelve healthy volunteers (two women and 10 men), who had visual acuity that was normal or corrected to normal participated in this study. Their ages were ranged from 25 to 40 years old. Except for two, all were right handed according to the Edinburgh MRC handedness scale [Oldfield, 1971].
Visual Stimuli
We used two kinds of visual motion stimuli, apparent motion (AM) and coherent dots motion created by a random dot cinematogram (RDC), as in a previous study [Kaneoke et al., 1997] (Fig. 1). The visual stimulus used to induce AM consisted of two frames. Each frame had a green spot (0.2 degrees visual angle in diameter) as the fixation point and a white vertical bar (subtended 0.1 × 2.0 degrees) on the left of the fixation point. The bar was located 2.0 degrees and 1.0 degree offset from the fixation point respectively for Frames 1 and 2. The frames were presented alternately so that the subjects would see the horizontal to‐and‐fro motion of the bar between the places where the bars were presented. The duration of the presentation of each frame was randomized between 2 and 3 seconds with a step of 0.2 second to eliminate averaging late activities which might be evoked by the preceding stimulation and to prevent the subject's expectancy and adaptation to the stimuli [Anstis et al., 1985]. The interframe interval was about 16 milliseconds due to synchronization to the monitor frame flyback time. The respective measured luminances of the bar and the background of the screen were 224 and 4.3 lux.
Figure 1.
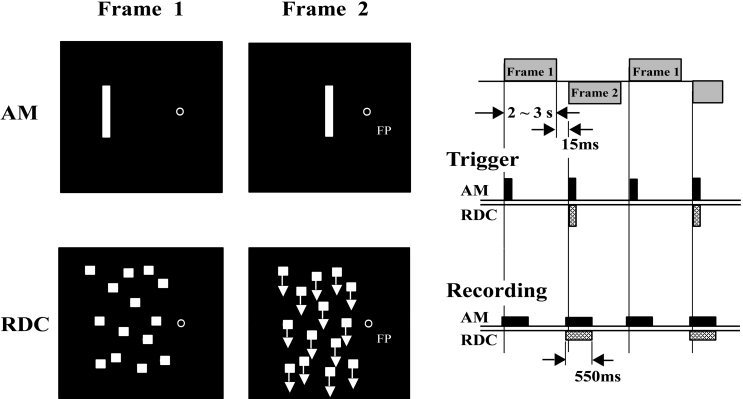
Schematic illustrations of the visual stimuli and a diagram of MEG acquisitions. Visual stimulus to induce apparent motion (AM) was created by the two frames; One (Frame 1) with a fixation point and a bar offset 2 degrees (visual angle) from that point, the other (Frame 2) with a fixation point at the same location as in Frame 1 and a bar offset 1 degree from that point. Two visual scenes were used for the random dot cinematogram (RDC), with stationary dots (Frame 1) the other with coherently moving dots (Frame 2). For both visual stimuli, Frames 1 and 2 were presented alternately for 2 to 3 seconds with an interstimulus interval (isi) of 16 ms. Trigger pulses for the averaging of magnetic responses occurred whenever the frame changed. Acquisition of the magnetic responses started 50 ms before and ended 500 ms after the trigger pulse.
RDC also consisted of two frames (10 × 10 degree visual angle), in which randomly placed dots were stationary or moved downward at a speed of 10 deg/s (Fig. 1). A fixation point, whose diameter was a 0.2 degree visual angle, was at the mid‐point of the inner edge of the stimulus. The two frames were presented alternately in each subject's left visual hemifield.
Four subjects underwent MEG studies with both AM and RDC. One of these two stimuli was used for the other eight subjects because they had larger magnetic responses to that stimulus. Both visual stimuli were generated by a VSG 2/3 (Cambridge Research Systems) and projected on a white screen in a magnetically shielded room with a video projector (BARCODATA 3100, BARCO) with a refresh rate of 62 Hz from outside through a small window. Measured phosphor persistence time was less than 20 ms. The distance from the subject's nasion to the screen was 150 cm.
MEG Acquisition
We used a 37 ch BTi Neuromagnetometer [Pantev et al., 1991]. The MEG recording procedure has been described in detail elsewhere [Kaneoke et al., 1997]. The subject lay on his/her right side on a non‐magnetized bed, in a dimly lit (10 lux), magnetically shielded room. The subject's head was placed on the top of a ground‐based dewer, in which 37 magnetic sensors were arranged concentrically. The center of the MEG sensors was aimed at the right lateral occipital scalp when recording from the subject's right temporo‐parieto‐occipital brain. After the subject's head was placed and fixed on the dewer, three points on the head (nasion, bilateral tips of tragi) were digitized using a sensor position indicator (SPI) of the BTi MEG system in order to register the spatial relation between the subject's head and the sensors.
Diagrams of the experimental procedures are shown in Figure 1. For the AM stimulus, two kinds of trigger pulses to average the evoked magnetic fields were given at the presentation of each frame. One was for the change from Frame 1 to 2, the other for the change from Frame 2 to 1. For RDC, there was a trigger at the time of change from a stationary to coherent motion stimulus. Recording of magnetic fields started 50 ms before and ended 500 ms after the trigger pulse. While the subject was looking at the fixation point, more than 200 magnetic responses to each event were collected and averaged, using only the epochs within ± 3000 fT. The waveforms then were filtered to the range of 1 to 80 Hz by the fast Fourier transform. In order to check visual fixation, horizontal and vertical eye movements were monitored by electro‐oculograms recorded from four gold‐coated electrodes placed around the right eye. The single equivalent current dipole (ECD) model [Sarvas, 1987] was used to estimate the underlying cerebral activities that generated the magnetic field evoked by the visual motion stimuli. Two criteria were utilized in the application of the single ECD model to a magnetic response: First, during the period of response the dipole location estimated with the ECD model must stay within ± 5 mm of each coordinate. Second, the correlation between the measured and expected magnetic field must remain more than 0.96 for more than 10 ms around the peak of the response. Spatio‐temporal source analysis (BESA) were also used to check the validity of the single ECD model [Probst et al., 1993]. The area in mm2 of the cortical region activated by the stimuli was estimated from the value of the current strength on the assumption that 1 nAm corresponds to an area of 4 mm2 [Hamalainen et al., 1993].
The mean coordinate of the dipole location during that period was calculated for each magnetic response. More than six MEG acquisitions were made for each subject to evaluate the reproducibility of the dipole location. The 95% confidence distance of the dipole location for each subject was calculated as the mean distance + 2 S.D. between the mean and each estimated dipole location. The coordinate system used for dipole location was as follows: The origin is the midpoint between the left and right tragus. The positive X‐axis extends from the origin through the nasion. The positive Z‐axis extends from the origin through the top of the head, such that it is perpendicular to the plane formed by the nasion and both tragi. The positive Y‐axis extends from the origin through the left side of the head, such that it is perpendicular to the X and Z axes.
Anatomical Investigation
T1 weighted (STAGE) sagittal magnetic resonance images (MRI) 1.5 mm thick were obtained with a 1.5 T MR imager (Shimadzu Medical Systems) and the three dimensional Fourier transform method. Three capsules of Vitamin D (5 mm in diameter) were attached to the subject's nasion and bilateral tragi in a MRI acquisition as landmarks in the superimposition of the brain image on the coordinate system used for dipole location. Three dimensional cortical surface images were produced by surface rendering from the MRI images obtained using a workstation (Indigo II, Silicon Graphics) with the aid of AVS (Advanced Visual Systems). The coordinate of Talairach and Tournoux [Talairach and Tournoux, 1988] was not used in this study because it does not fit the Japanese brain [Cheng et al., 1995]. Instead, we used each individual's sulcal anatomy to identify the area responsible for the magnetic response with the atlas of Ono et al. [1990] as the reference.
To determine the nearest point on the scalp (NS) to the cortical area activated by the visual motion stimuli, a virtual sphere whose center was at the mean dipole location and surface osculating to the scalp was generated and overlayed on the three dimensional MRI by AVS. The measured distance between NS and the dipole location was the radius of the sphere. The location of NS relative to the tragus was measured using the coordinate system.
RESULTS
Magnetic Responses to Visual Motion Stimuli
All our subjects perceived clear smooth motions for both visual stimuli. All the recorded MEG responses to AM stimuli had prominently large components in the peak latency range of 130 to 170 ms (mean ± S.D.: 151.2 ± 13.4 ms). The waveforms of MEG responses in Events 1 and 2 were very similar. The dipoles were estimated to be in the same region, therefore their dipole locations were averaged together. RDC also evoked a large first component, whose latency was, however, much longer than that of apparent motion (mean ± S.D.: 289.4 ± 21.8 ms). Figure 2 shows MEG waveforms, isocontour maps at the peaks of the first components, and the estimated dipole locations superimposed on the two‐dimensional MRI for both stimuli from a single subject (s04). Even though the response latencies differed by about 150 ms, the isocontour maps around the peak (see Fig. 2B) and estimated dipole locations were surprisingly similar. In this subject, the dipoles were located around the temporo‐occipital region (Fig. 2C). The magnetic responses for some subjects were more complex, having two to four components but the first component always was the largest.
Figure 2.
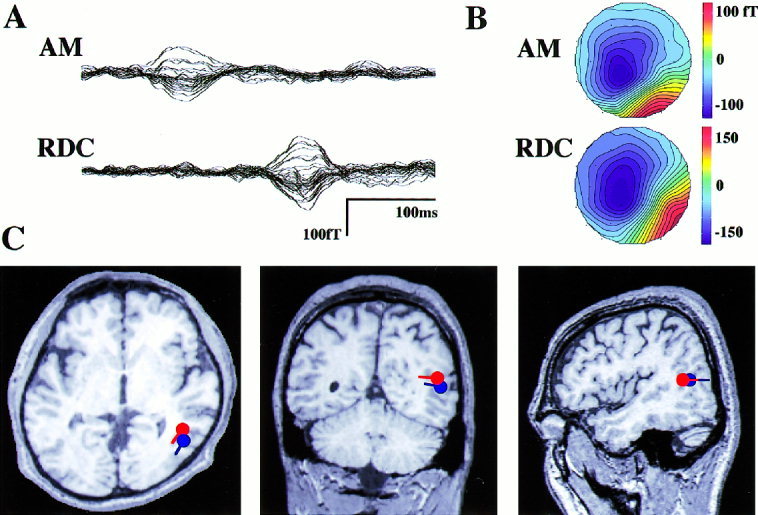
Magnetic response waveforms (A), their isocontour maps (B), and estimated origins of the responses (C) in one subject (s04) for both stimuli. All the 37 channels' magnetic response data are overlayed on the mean baseline before the trigger. The waveforms start at time 0 (trigger onset). Upward deflection of the magnetic waveforms and the positive values (red) in the isocontour map indicates the direction of magnetic field toward the sensor. Although the latencies of the responses differ markedly, the isocontour maps at the peaks are similar and have single dipole patterns. The origins estimated by the single ECD model are in the same cortical region, around the temporo‐occipital area in this subject. Locations and directions of the dipoles are shown on the two‐dimensional MRI in C (red: AM, blue: RDC).
Cortical Localization of the Magnetic Response Origin
All the subjects had magnetic responses to one of the types of stimuli which could be used to estimate the cortical origins with the single ECD model. These cortical origins could be classified in three groups based on the sulcal anatomy. In seven of the 12 subjects, the origins of the magnetic responses are located around the temporo‐occipital area, referred to as the temporo‐occipital type in this study. Origins for two subjects were located more posteriorly, designated the occipital type. The third, or parietal type, was found in the dorso‐anterior region of three subjects. Figure 3 shows the magnetic responses, isocontour maps, and dipole locations overlayed on a two‐dimensional MR image. Table I summarizes the dipole location and type by subject.
Figure 3.
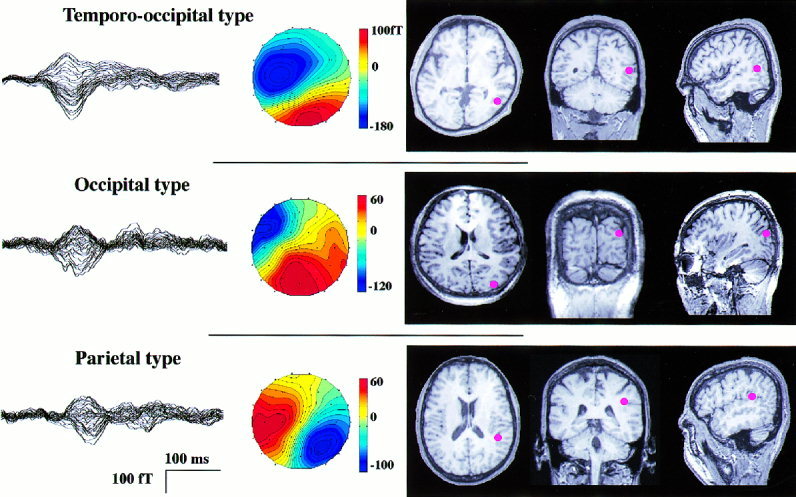
MEG waveforms, their isocontour maps at the peak, and origins of the responses on two‐dimensional MRI are shown by red spots for the three types of dipole locations. All are responses to AM. Waveforms are shown as in Figure 2. All the isocontour maps have a single dipole pattern, but their estimated origins differed. The origin of the occipital type is posterior to that of the temporo‐occipital type, and that of the parietal type is at the posterior end of the superior temporal sulcus.
Table I.
Peak latencies of the magnetic responses and dipole locations
| Subject | Handedness | Stimulation | Peak latency (ms) | Dipole location (cm) (X, Y, Z)* | 95% confidence distance (mm) | Dipole moment (nAm) | Estimated area (mm2) |
|---|---|---|---|---|---|---|---|
| Temporo‐occipital type | |||||||
| S01 | R | RDC | 267.0 | −1.3, −4.3, 6.4 | 8 | 7 | 28 |
| S02 | R | RDC | 286.4 | −0.8, −4.0, 6.4 | 11 | 18 | 72 |
| S03 | R | RDC | 256.3 | −2.8, −4.2, 6.4 | 11 | 39 | 154 |
| S04 | R | RDC | 299.8 | −2.2, −5.6, 6.4 | 6 | 39 | 154 |
| AM | 162.5 | −2.0, −4.5, 6.0 | 8 | 51 | 204 | ||
| S05 | R | RDC | 294.8 | −1.7, −5.0, 6.9 | 6 | 23 | 92 |
| AM | 150.8 | −2.6, −5.0, 6.8 | 9 | 35 | 140 | ||
| S06 | R | AM | 135.4 | −3.4, −3.3, 6.4 | 11 | 37 | 148 |
| S07 | L | AM | 151.2 | −3.2, −3.4, 7.5 | 6 | 12 | 48 |
| Mean (S.D.) | RDC 280.8 (18.5) | 8 (2) | 29 (15) | 116 (58) | |||
| AM 150.0 (11.1) | |||||||
| Occipital type | |||||||
| S08 | L | AM | 141.6 | −4.3, −3.1, 6.8 | 10 | 12 | 48 |
| S09 | R | AM | 130.0 | −3.3, −3.6, 7.5 | 6 | 8 | 32 |
| Mean | 135.8 | 8 | 10 | 40 | |||
| Parietal type | |||||||
| S10 | R | RDC | 320.5 | −1.8, −4.6, 8.0 | 6 | 18 | 72 |
| AM | 169.2 | −1.8, −4.3, 7.9 | 5 | 30 | 120 | ||
| S11 | R | RDC | 301.5 | −0.5, −3.3, 8.0 | 8 | 6 | 24 |
| AM | 164.7 | −0.2, −4.5, 9.3 | 6 | 12 | 48 | ||
| S12 | R | AM | 155.1 | −0.5, −4.9, 7.7 | 15 | 13 | 52 |
| Mean | RDC 311.0 | 8 | 16 (9) | 63 (36) | |||
| AM 163.0 | |||||||
See Materials and Methods for the explanation of the coordinates.
To validate application of the single ECD model, we checked whether the dipole locations were stable during the period of the response (Fig. 4). Furthermore, we performed the principal component analysis in BESA to estimate the number of components (dipoles) to explain the measured magnetic fields during the responses. More than 95% of the variance of the magnetic fields for all subjects' data could be explained by the first component when we used the analysis time of 40 ms around the peak of the responses. Thus the response around the peak can be considered to be mainly due to the single source, that is a localized small cortical area.
Figure 4.
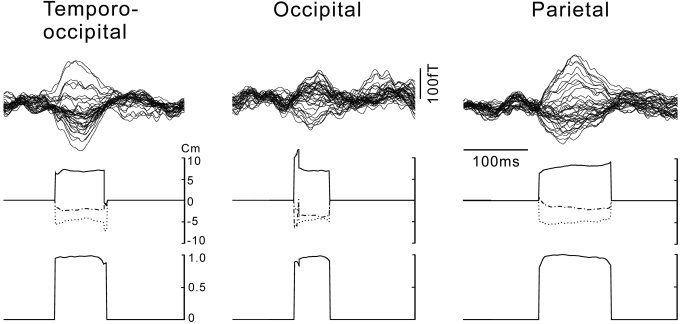
Waveforms of the responses to AM (top), time course of the coordinates of the dipole locations estimated by the single ECD model (middle), and the correlations between the measured and estimated magnetic fields (bottom) for the three subjects in different types, temporo‐occipital, occipital, and parietal type. Waveforms are shown as in Fig. 2. Note that the coordinates are quite stable and the correlation are close to 1 nearly entire durations of the responses. Principal component analysis also revealed that the magnetic fields during the same periods could be explained by the first component up to 99.1, 95.5, and 96.9% for the data of temporo‐occipital, occipital, and parietal type, respectively, that indicate the validity of the single dipole model. X: dash‐dot; Y: dot; Z: solid line. (See Materials and Methods for the explanation of the coordinate system used.)
Figure 5 shows the three‐dimensional MRI with the estimated dipole locations with a 95% confidence distance shown as the radius of the circle for all the subjects. Because the origins for both AM and RDC are quite similar (see Table I), the mean values are used for four subjects. For the seven subjects with the temporo‐occipital type, the cortical locations of the origins were around the meeting point of the lateral occipital sulcus (white line) and ascending limb of the inferior temporal sulcus (green line). Origins for the two subjects with the occipital type were posterior and around the transverse occipital sulcus (yellow line). The other three subjects with the parietal type, had origins around the angular gyrus, at the posterior end of the superior temporal sulcus (black line).
Figure 5.
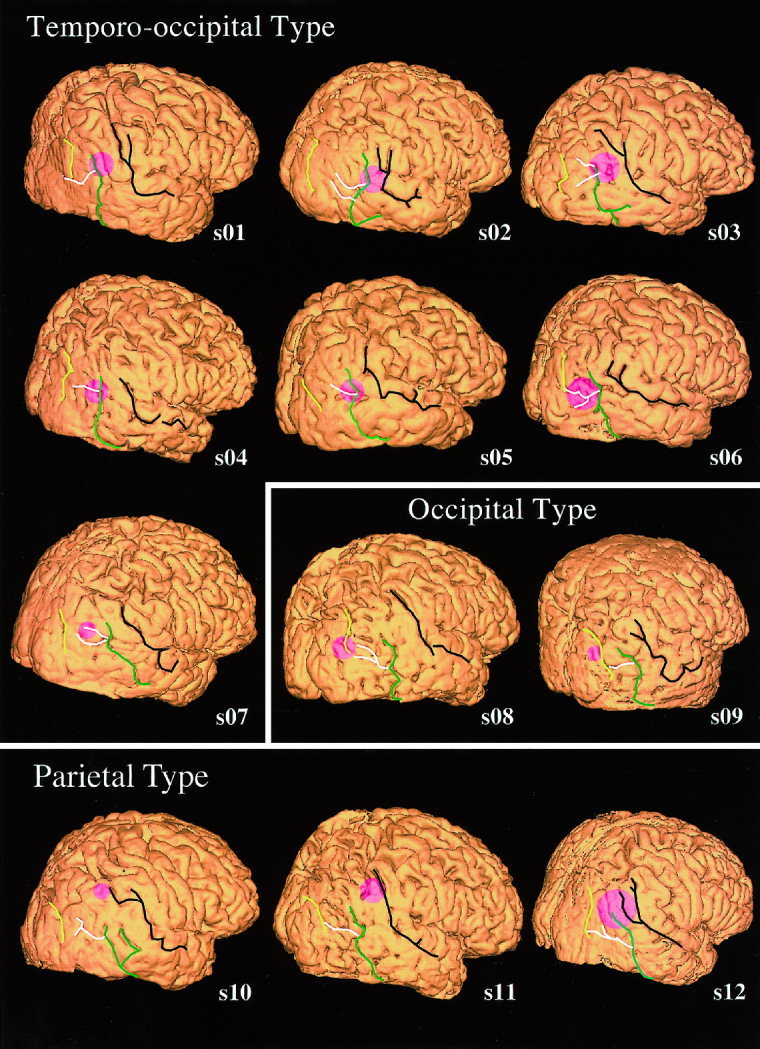
The dipole location and its 95% confidence distance is shown for each subject on the individual three‐dimensional MR image. The center of the pink circle indicates the nearest cortical surface to dipole location and the radius the 95% confidence distance (see methods). Seven subjects' origins were located around the meeting point of the ascending limb of the inferior temporal sulcus (green line) and the lateral occipital sulcus (white line). Origins for two subjects (occipital type) were located posterior to the temporo‐occipital type, around the transverse occipital sulcus (yellow line). Origins for three subjects with the parietal type were around the posterior end of the superior temporal sulcus (black line). The location for s12 was classified into this type because the center of the estimated area was closer to the posterior end of the superior temporal sulcus than the meeting point of the lateral occipital sulcus and the ascending limb of the inferior temporal sulcus.
Because of the small number of data no statistical analysis could be made, but the response latencies of the parietal type were longer than those of the other types for both types of visual stimuli (see Table I). The value for the 95% confidence distance varied from 6 to 15 mm (mean ± S.D.: 8.3 ± 2.7 mm). For most of the subjects therefore, the magnetic response origins could be identified within an error of 10 mm. The estimated dipole moments (current strength) varied markedly among the subjects. The mean value for the temporo‐occipital type (29 ± 15 nAm) was the largest of the three types. The corresponding activated cortical area calculated from the dipole moments ranged from 28 to 204 mm2.
Nearest Scalp to the Origin of the Magnetic Response
The nearest scalp (NS) to the estimated dipole location for each subject's magnetic response was determined by individual three‐dimensional MRI (Table II). The relative locations from the tragus for all subjects are shown on the scalp in Figure 6. The distance between NS and the dipole location varied from 1.3 to 3.5 cm (mean ± S.D.: 2.5 ± 0.6 cm), indicative that the dipole was located on the sulcus, not on the gyrus, because the distance between the scalp and the cortical surface usually is 1–1.5 cm [McGrath and Mills, 1984].
Table II.
Coordinates of nearest scalp (NS) to the estimated dipole location and from the right tragus
| Subject | Location of NS (cm) (X, Y, Z)* | Distance between NS and the dipole (cm) | Relative location of NS from the tragus (cm) | |
|---|---|---|---|---|
| Dorsal | Caudal** | |||
| Temporo‐occipital type | ||||
| S01 | −2.9, −6.2, 6.8 | 2.5 | 6.8 | 2.8 |
| S02 | −2.2, −7.2, 6.7 | 3.5 | 6.7 | 2.1 |
| S03 | −2.0, −7.2, 6.7 | 3.1 | 6.7 | 1.4 |
| S04 | −2.5, −7.1, 6.1 | 2.1 | 6.1 | 2.1 |
| S05 | −4.5, −6.2, 7.3 | 2.7 | 7.3 | 4.4 |
| S06 | −5.6, −4.9, 7.1 | 2.8 | 7.1 | 5.3 |
| S07 | −5.0, −5.0, 8.4 | 2.6 | 8.4 | 4.8 |
| Mean (S.D.) | 2.8 (0.4) | 7.0 (0.7) | 3.3 (1.5) | |
| Occipital type | ||||
| S08 | −6.6, −4.7, 5.5 | 3.1 | 5.5 | 6.6 |
| S09 | −4.3, −4.4, 7.8 | 1.3 | 7.8 | 4.3 |
| Mean | 2.2 | 6.7 | 5.5 | |
| Parietal type | ||||
| S10 | −2.4, −6.0, 8.7 | 1.8 | 8.7 | 2.0 |
| S11 | −0.8, −6.5, 10.0 | 2.9 | 10.0 | 0.6 |
| S12 | −1.6, −6.3, 8.7 | 2.0 | 8.7 | 1.3 |
| Mean | 2.2 | 9.1 | 1.3 | |
See Materials and Methods for the explanation of the coordinates.
The values show how high (dorsal) and how much posterior (caudal) it is from the tragus to the NS.
Figure 6.
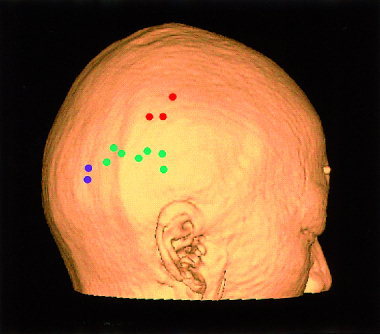
The nearest scalp points to the estimated dipole locations for all subjects. These are overlayed on the scalp created by three‐dimensional MRI for the single subject to show the relative locations. Green: temporo‐occipital; Red: parietal; Blue: occipital type.
We also measured the relative location of NS from the right tragus (preauricular point) for each subject (Table II). For the temporo‐occipital type, the mean NS location across the seven subjects' data was 7 (±0.7) cm dorsal from the plane determined by the bilateral tragi and nasion and 3.3 (±1.5) cm caudal to the tragus. This location always was dorsal to T6 in the ten‐twenty electrode system for electroencephalography [Jasper, 1958]. The NS for the occipital type was about 2 cm caudal to that for the temporo‐occipital type with the same dorsal distance. The NS for the parietal type was about 2 cm dorsal and 2 cm rostral to that of the temporo‐occipital type.
DISCUSSION
Using the 37 ch MEG system, we detected the magnetic responses to visual motion stimuli of 12 subjects. The origins of the first transient components were determined from the single ECD model, and their anatomical locations were investigated by individual three‐dimensional MRI.
Physiological Implication of the Magnetic Response
The visual stimulus used to induce AM consisted of the simple displacement of a bar. This raises the question of whether the magnetic response to AM is related to motion or merely due to luminance change [Uusitalo et al., 1997b]. We consider the response to be related to motion perception for the following reasons: First, in a previous study we found that the response latency changed with the distance of bar displacement [Kaneoke et al., 1997]. Second, the estimated origins were in the cortical region which responded to RDC [Kaneoke et al., 1997] and differed from the region which responded to color change [Kaneoke et al., 1996]. Because RDC does not change the global luminance, the only common feature in AM and RDC is motion to the visual system. Third, the amplitude of the response was related to the perception of AM when the interstimulus interval was changed systematically [Kawakami et al., 2000]. Fourth, the amplitude also changed with direction of motion when presented in the upper visual field [Naito et al., 2000].
RDC also evoked a transient magnetic response, except the latency, similar to that for AM. The reason of the marked (around 150 ms) difference in the response latencies despite the origins were estimated to be the same is under investigation. In any case, the response to RDC should be related to the detection of motion onset. Visual motion stimuli created with RDC have been used in various psychological [Morgan and Ward, 1980; Siegel and Andersen, 1988] and physiological [Newsome et al., 1989] studies because they do not activate position‐sensitive mechanisms [Nakayama and Tyler, 1981]. A recent study revealed that the response latency changes with the preceding motion speed of RDC [Lam et al., 2000]. This response property of latency change with motion speed indicates that the magnetic response to motion in RDC is related to the detection of motion.
Cortical Localization of the Magnetic Response Origin
The origins of the first components of the magnetic responses to the visual motion stimuli used in this study were estimated for all the subjects using a single ECD model because their isocontour maps had clear single dipole patterns (see Figs. 2, 3, and 4). Our findings apparently contradict the fact that many cortical regions are reported to be involved in the process of visual motion perception in monkey experiments [Maunsell and Newsome, 1987] and human PET and fMRI studies [Dupont et al., 1994; Cheng et al., 1995; Tootell et al., 1995a; Sunaert et al., 1999]. Furthermore their activation timing may overlap based on single neural activity recordings in monkeys [Raiguel et al., 1989] and human fMRI studies [Anderson et al., 1996; Sunaert et al., 1999]. The magnetic response, however, is considered to be due to synchronized excitatory postsynaptic potentials (EPSPs) in the cortical pyramidal neurons [Hamalainen et al., 1993; Okada et al., 1997], whereas PET and fMRI measure regional metabolic or blood flow changes during a stimulus. Our findings therefore indicate the existence of cortical areas which get excitatory input synchronized to motion onset. Such areas must be localized in a small region because the origin of the magnetic response was fully estimated by the single ECD model.
Seven of the twelve subjects had estimated origins in the temporo‐occipital area (Fig. 5). This area includes the so‐called human homologue of MT/V5 because of its histological [Clarke and Miklossy, 1990; Tootell and Taylor, 1995] and functional [Zeki et al., 1991; Tootell et al., 1995a] similarity to monkey MT/V5. This region and the adjacent area (MT+) respond to various visual motion stimuli, including apparent motion [Kaneoke et al., 1997; Uusitalo et al., 1997a; Goebel et al., 1998], random dot coherent and incoherent motion [Cheng et al., 1995; McCarthy et al., 1995; McKeefry et al., 1997], motion after‐effect [Tootell et al., 1995b; He et al., 1998], illusory motion [Zeki et al., 1993], second‐order motion [Smith et al., 1998], motion‐from‐hue [ffytche et al., 1995b], and human eye movements [Puce et al., 1998]. Thus, this area can be considered the key structure in the visual motion process.
Individual cortical localization of area MT+ may vary [Watson et al., 1993; Patzwahl et al., 1996], therefore Watson et al. [Watson et al., 1993] used sulcal anatomy for the MT+ landmark; the meeting point of the lateral occipital sulcus and the ascending limb of the inferior temporal sulcus. This area corresponded to the origins of seven of our subjects (Fig. 5). Recent spatio‐temporal analysis of the motion process in the human brain by MEG and fMRI showed prominent transient activation peaking at about 170 ms in this area [Ahlfors et al., 1999]. The latency corresponds to our results for AM (Table I). These findings indicate that the origins of our seven subjects correspond to MT+ anatomically as well as functionally. Furthermore, the activated area estimated by the current strength (116 ± 58 mm2) also corresponds to the partial activation of human MT/V5 according to data from a histological investigation [Tootell and Taylor, 1995b] in which human MT made an ellipse of 1.5–2.0 × 1.0–1.4 cm, corresponding to an area of 118–220 mm2.
Our other five subjects had different cortical locations for their magnetic response origins based on the sulcal anatomy (Fig. 5). These variations can not be explained by the simple measurement errors because the sizes of their 95% confidence areas are similar to those for the temporo‐occipital type and the area does not include the meeting point of the lateral occipital sulcus and the ascending limb of the inferior temporal sulcus, which is the normal MT+ location for each subject.
The origin for the occipital type anatomically corresponds to V3A and that for the parietal type to the posterior end of the superior temporal sulcus (pSTS). Although both regions are related to the visual motion process [Cheng et al., 1995; Tootell et al., 1997], whether they correspond functionally to the same named regions is uncertain. Recent spatio‐temporal analysis [Ahlfors et al., 1999] showed that the activities in V3A and pSTS respectively peaked at about 230 and 260 ms; 60 and 90 ms later than that for MT+ (170 ms). Although we found that the response latencies for the parietal type were longer than those for the temporo‐occipital type, the difference was less than 20 ms. Furthermore, the response latencies for the occipital type were 10–20 ms shorter than those for the temporo‐occipital type. These findings raise the possibility that the cortical regions identified as the origins for the occipital and parietal types correspond functionally to MT+, but the results of the studies can not be directly compared because of different visual stimuli used. We, however, consider that this could occur because no histological localization of MT/V5 could not be made in the expected region in some humans [Sereno and Allman, 1991; Tootell and Taylor, 1995]. These facts suggest that MT+ in some humans migrate to cortical regions other than the expected temporo‐occipital area.
Transcranial magnetic stimulation (TMS) has been used to investigate the spatio‐temporal structure of brain function by means of transient stimulation or inactivation of the restricted cortical region [Ilmoniemi et al., 1997]. Although TMS studies done on the human visual motion area have found a functional significance of MT+ [Beckers and Homberg, 1992; Hotson et al., 1994; Beckers and Zeki, 1995; Walsh et al., 1998; Hotson and Anand, 1999], the results are not completely consistent. This could be due to a difference in the paradigms or visual stimuli used. Possibly, the discrepancy in the results came from no appropriate anatomical landmark on the skull for the stimulation of MT+ as well as inter‐individual variance of its localization as found in this study (see Fig. 6). Our findings should help in determining the precise location of the stimulation site and in clarifying the functional significance of human MT+ in TMS studies.
CONCLUSION
We found that the cortical region, whose the neural activities are considered responsible for the magnetic response to visual motion onset, varied markedly among 12 subjects tested, even when differences in their sulcal anatomies were taken into account. The locations could be classified as one of three types; a temporo‐occipital type for seven subjects, an occipital type for two subjects, and a parietal type for three subjects. Although these cortical locations respectively corresponded anatomically to MT+, V3A, and pSTS, whether these regions correspond functionally has yet to be confirmed.
Our study also has provided an external landmark for the nearest skull to these regions, which may be useful for TMS studies.
Acknowledgements
We thank Mr. O. Nagata, and Mr. Y. Takeshima for their technical support, Dr. M. Hoshiyama and Dr. T. Nihashi for BESA analysis. We gratefully acknowledge the expertise of Dr. M. Furuse and Mr. A. Izawa of Nakatsugawa Municipal Hospital in MRI acquisition, and Dr. K. Ito of Institute for Longevity Sciences for the MEG data acquisition in part.
Edited by: Ritta Hari, Associate Editor
REFERENCES
- Ahlfors SP, Simpson GV, Dale AM, Belliveau JW, Liu AK, Korvenoja A, Virtanen J, Huotilainen M, Tootell RBH, Aronen HJ, Ilmoniemi RJ (1999): Spatiotemporal activity of a cortical network for processing visual motion revealed by MEG and fMRI. J Neurophysiol 82: 2545–2555. [DOI] [PubMed] [Google Scholar]
- Allman JM, Kass JH (1971): A representation of the visual field in the caudal third of the middle temporal gyrus of the owl monkey (Aotus trivirgatus). Brain Res 31: 85–105. [DOI] [PubMed] [Google Scholar]
- Anderson SJ, Holliday IE, Singh KD, Harding GFA (1996): Localization and functional analysis of human cortical area V5 using magneto‐encephalography. Proc R Soc Lond B Biol Sci 263: 423–431. [DOI] [PubMed] [Google Scholar]
- Anstis S, Giaschi D, Cogan AI (1985): Adaptation to apparent motion. Vision Res 25: 1051–1062. [DOI] [PubMed] [Google Scholar]
- Barton JJS, Sharpe JA, Raymond JE (1996): Directional defects in pursuit and motion perception in humans with unilateral cerebral lesions. Brain 119: 1535–1550. [DOI] [PubMed] [Google Scholar]
- Beckers G, Homberg V (1992): Cerebral visual motion blindness: transitory akinetopsia induced by transcranial magnetic stimulation of human area V5. Proc R Soc Lond B Biol Sci 249: 173–178. [DOI] [PubMed] [Google Scholar]
- Beckers G, Zeki S (1995): The consequences of inactivating areas V1 and V5 on visual motion perception. Brain 118: 49–60. [DOI] [PubMed] [Google Scholar]
- Cheng K, Fujita H, Kanno I, Miura S, Tanaka K (1995): Human cortical regions activated by wide‐field visual motion: an H2(15)O PET study. J Neurophysiol 74: 413–427. [DOI] [PubMed] [Google Scholar]
- Clarke S, Miklossy J (1990): Occipital cortex in man: organization of callosal connections, related myelo‐ and cytoarchitecture, and putative boundaries of functional visual areas. J Comp Neurol 298: 188–214. [DOI] [PubMed] [Google Scholar]
- Cornette L, Dupont P, Spileers W, Sunaert S, Michiels J, Van Hecke P, Mortelmans L, Orban GA (1998): Human cerebral activity evoked by motion reversal and motion onset. A PET study. Brain 121: 143–157. [DOI] [PubMed] [Google Scholar]
- Dubner R, Zeki SM (1971): Response properties and receptive fields of cells in an anatomically defined region of the superior temporal sulcus in the monkey. Brain Res 35: 528–532. [DOI] [PubMed] [Google Scholar]
- Dupont P, Orban GA, De Bruyn B, Verbruggen A, Mortelmans L (1994): Many areas in the human brain respond to visual motion. J Neurophysiol 72: 1420–1424. [DOI] [PubMed] [Google Scholar]
- ffytche DH, Guy CN, Zeki S (1995a): The parallel visual motion inputs into areas V1 and V5 of human cerebral cortex. Brain 118: 1375–1394. [DOI] [PubMed] [Google Scholar]
- ffytche DH, Skidmore BD, Zeki S (1995b): Motion‐from‐hue activates area V5 of human visual cortex. Proc R Soc Lond B Biol Sci 260: 353–358. [DOI] [PubMed] [Google Scholar]
- Goebel R, Khorram‐Sefat D, Muckli L, Hacker H, Singer W (1998): The constructive nature of vision: direct evidence from functional magnetic resonance imaging studies of apparent motion and motion imagery. Eur J Neurosci 10: 1563–1573. [DOI] [PubMed] [Google Scholar]
- Hamalainen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV (1993): Magnetoencephalography‐theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys 65: 413–497. [Google Scholar]
- Hari R, Lounasmaa OV (1989): Recording and interpretation of cerebral magnetic fields. Science 244: 432–436. [DOI] [PubMed] [Google Scholar]
- He S, Cohen ER, Hu X (1998): Close correlation between activity in brain area MT/V5 and the perception of a visual motion aftereffect. Curr Biol 8: 1215–1218. [DOI] [PubMed] [Google Scholar]
- Hotson J, Braun D, Herzberg W, Boman D (1994): Transcranial magnetic stimulation of extrastriate cortex degrades human motion direction discrimination. Vision Res 34: 2115–2123. [DOI] [PubMed] [Google Scholar]
- Hotson JR, Anand S (1999): The selectivity and timing of motion processing in human temporo‐parieto‐occipital and occipital cortex: a transcranial magnetic stimulation study. Neuropsychologia 37: 169–179. [DOI] [PubMed] [Google Scholar]
- Hughes JR (1983): Magnetoencephalography‐update and future hopes. Am J EEG Technol 23: 179–189. [Google Scholar]
- Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Naatanen R, Katila T (1997): Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport 8: 3537–3540. [DOI] [PubMed] [Google Scholar]
- Jasper H (1958): Ten‐twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol 10: 371–375. [PubMed] [Google Scholar]
- Kaneoke Y, Bundou M, Koyama S, Suzuki H, Kakigi R (1997): Human cortical area responding to stimuli in apparent motion. Neuroreport 8: 677–682. [DOI] [PubMed] [Google Scholar]
- Kaneoke Y, Koyama S, Kakigi R (1996): Human cortical region which responds to the color change. Soc Neurosci Abstr 22: 400. [Google Scholar]
- Kawakami O, Kaneoke Y, Kakigi R (2000): Perception of apparent motion is related to the neural activity in the human extrastriate cortex as measured by magnetoencephalography. Neurosci Lett 285: 135–138. [DOI] [PubMed] [Google Scholar]
- Lam K, Kaneoke Y, Gunji A, Yamasaki H, Matsumoto E, Naito T, Kakigi R (2000): Magnetic response of human extrastriate cortex in the detection of coherent and incoherent motion. Neuroscience 97: 1–10. [DOI] [PubMed] [Google Scholar]
- Livingstone M, Hubel D (1988): Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science 240: 740–749. [DOI] [PubMed] [Google Scholar]
- Maunsell JHR, Newsome WT (1987): Visual processing in monkey extrastriate cortex. Annu Rev Neurosci 10: 363–401. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Spicer M, Adrignolo A, Luby M, Gore J, Allison T (1995): Brain activation associated with visual motion studied by functional magnetic resonance imaging in humans. Hum Brain Mapp 2: 234–243. [Google Scholar]
- McGrath P, Mills P (1984): Atlas of sectional anatomy: head, neck, and trunk. New York: Karger; 238p. [Google Scholar]
- McKeefry DJ, Watson JDG, Frackowiak RS, Fong K, Zeki S (1997): The activity in human areas V1/V2, V3, and V5 during the perception of coherent and incoherent motion. Neuroimage 5: 1–12. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Ward R (1980): Conditions for motion flow in dynamic visual noise. Vision Res 20: 431–435. [DOI] [PubMed] [Google Scholar]
- Naito T, Kaneoke Y, Osaka N, Kakigi R (2000): Asymmetry of the human visual field in magnetic response to apparent motion. Brain Res 865: 221–226. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Tyler CW (1981): Psychophysical isolation of movement sensitivity by removal of familiar position cues. Vision Res 21: 427–433. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Britten KH, Movshon JA (1989): Neuronal correlates of a perceptual decision. Nature 341: 52–54. [DOI] [PubMed] [Google Scholar]
- Okada YC, Wu J, Kyuhou S (1997): Genesis of MEG signals in a mammalian CNS structure. Electroencephalogr Clin Neurophysiol 103: 474–485. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathey CD (1990): Atlas of the cerebral sulci. New York: Thieme Medical Publishers; 218p. [Google Scholar]
- Pantev C, Gallen C, Hampson S, Buchanan S, Sobel D (1991): Reproducibility and validity of neuromagnetic source localization using a large array biomagnetometer. Am J EEG Technol 31: 83–101. [Google Scholar]
- Patzwahl DR, Elbert T, Zanker JM, Altenmuller EO (1996): The cortical representation of object motion in man is interindividually variable. Neuroreport 7: 469–472. [DOI] [PubMed] [Google Scholar]
- Plant GT, Laxer KD, Barbaro NM, Schiffman JS, Nakayama K (1993): Impaired visual motion perception in the contralateral hemifield following unilateral posterior cerebral lesions in humans. Brain 116: 1303–1335. [DOI] [PubMed] [Google Scholar]
- Probst T, Plendl H, Paulus W, Wist ER, Scherg M (1993): Identification of the visual motion area (area V5) in the human brain by dipole source analysis. Exp Brain Res 93: 345–351. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Bentin S, Gore JC, McCarthy G (1998): Temporal cortex activation in humans viewing eye and mouth movements. J Neurosci 18: 2188–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiguel SE, Lagae L, Gulyas B, Orban GA (1989): Response latencies of visual cells in macaque areas V1, V2 and V5. Brain Res 493: 155–159. [DOI] [PubMed] [Google Scholar]
- Sarvas J (1987): Basic mathematical and electromagnetic concepts of the biomagnetic inverse problem. Phys Med Biol 32: 11–22. [DOI] [PubMed] [Google Scholar]
- Schneider W, Noll DC, Cohen JD (1993): Functional topographic mapping of the cortical ribbon in human vision with conventional MRI scanners. Nature 365: 150–153. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Allman JM (1991): Cortical visual areas in mammals In: Leventhal A.G., editor. The neural basis of visual function. London: Macmillan, P 160. [Google Scholar]
- Siegel RM, Andersen RA (1988): Perception of three‐dimensional structure from motion in monkey and man. Nature 331: 259–261. [DOI] [PubMed] [Google Scholar]
- Smith AT, Greenlee MW, Singh KD, Kraemer FM, Hennig J (1998): The processing of first‐ and second‐order motion in human visual cortex assessed by functional magnetic resonance imaging (fMRI). J Neurosci 18: 3816–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunaert S, Van Hecke P, Marchal G, Orban GA (1999): Motion‐responsive regions of the human brain. Exp Brain Res 127: 355–370. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planner stereotaxic atlas of the human brain. New York: Thieme Medical; 121p. [Google Scholar]
- Thurston SE, Leigh RJ, Crawford T, Thompson A, Kennard C (1988): Two distinct deficits of visual tracking caused by unilateral lesions of cerebral cortex in humans. Ann Neurol 23: 266–273. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Mendola JD, Hadjikhani NK, Ledden PJ, Liu AK, Reppas JB, Sereno MI, Dale AM (1997): Functional analysis of V3A and related areas in human visual cortex. J Neurosci 17: 7060–7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Rosen BR, Belliveau JW (1995a): Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci 15: 3215–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Taylor JB (1995): Anatomical evidence for MT and additional cortical visual areas in humans. Cereb Cortex 5: 39–55. [DOI] [PubMed] [Google Scholar]
- Tootell RBH, Reppas JB, Dale AM, Look RB, Sereno MI, Malach R, Brady TJ, Rosen BR (1995b): Visual motion aftereffect in human cortical area MT revealed by functional magnetic resonance imaging. Nature 375: 139–141. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV (1994): ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol 4: 157–165. [DOI] [PubMed] [Google Scholar]
- Uusitalo MA, Jousmaki V, Hari R (1997a): Activation trace lifetime of human cortical responses evoked by apparent visual motion. Neurosci Lett 224: 45–48. [DOI] [PubMed] [Google Scholar]
- Uusitalo MA, Virsu V, Salenius S, Nasanen R, Hari R (1997b): Activation of human V5 complex and rolandic regions in association with moving visual stimuli. Neuroimage 5: 241–250. [DOI] [PubMed] [Google Scholar]
- Vanni S, Uusitalo MA, Kiesila P, Hari R (1997): Visual motion activates V5 in dyslexics. Neuroreport 8: 1939–1942. [DOI] [PubMed] [Google Scholar]
- Walsh V, Ellison A, Battelli L, Cowey A (1998): Task‐specific impairments and enhancements induced by magnetic stimulation of human visual area V5. Proc R Soc Lond B Biol Sci 265: 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JD, Myers R, Frackowiak RS, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S (1993): Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex 3: 79–94. [DOI] [PubMed] [Google Scholar]
- Zeki S, Watson JDG, Frackowiak RSJ (1993): Going beyond the information given: the relation of illusory visual motion to brain activity. Proc R Soc Lond B Biol Sci 252: 215–222. [DOI] [PubMed] [Google Scholar]
- Zeki S, Watson JDG, Lueck CJ, Friston KJ, Kennard C, Frackowiak RSJ (1991): A direct demonstration of functional specialization in human visual cortex. J Neurosci 11: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki SM (1974): Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J Physiol 236: 549–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zihl J, von Cramon D, Mai N (1983): Selective disturbance of movement vision after bilateral brain damage. Brain 106: 313–340. [DOI] [PubMed] [Google Scholar]


