Abstract
We have used high spatial resolution (0.55 mm × 0.55 mm) functional magnetic resonance imaging (fMRI) to show that when stimulus duration is brief (<6 sec), the hyperoxic hemodynamic response to neural activity can resolve the columnar architecture of ocular dominance within the primary visual cortex of humans. Our fMRI maps of ocular dominance columns are strikingly similar in appearance, size, and orientation to those reported in the literature using optical imaging of intrinsic signals (OIS) in animal cortex and histology of post‐mortem human specimens. We also demonstrate that under brief visual stimulation conditions, our results are consistent over repeated experiments. This is not the case for long duration stimuli (≥10 sec). A simulated random data set exhibited the same response properties as maps obtained when using these prolonged visual stimuli. Our results suggest that brief visual stimulation is essential for fMRI to successfully resolve ocular dominance columns using the hyperoxic phase of the hemodynamic response to neural activity at our prescribed spatial resolution. Hum. Brain Mapping 14:210–217, 2001. © 2001 Wiley‐Liss, Inc.
Keywords: biology, brain, diagnostic imaging, neurosciences, ophthalmology, physiology
INTRODUCTION
The ability of current functional magnetic resonance imaging (fMRI) techniques to resolve the micro‐architecture of the cortex has been a topic of great debate [Kim et al., 2000b; Logothetis, 2000]. The source of this debate originates from the belief that the hyperoxic phase of the hemodynamic response (that which is typically measured in fMRI) spreads several millimeters beyond the site of increased neural activity [Das and Gilbert, 1995; Malonek and Grinvald, 1996]. Previous fMRI studies at a magnetic field strength of 1.5 Tesla (T) support this belief, demonstrating that the spatial resolution limit of fMRI within the visual cortex at 1.5 T is about 1.1 mm [Engel et al., 1997]. Current opinion is that only the initial hypo‐oxic phase of the hemodynamic response is spatially specific to the site of neural activity. Indeed, optical imaging of intrinsic signals (OIS) has repeatedly demonstrated the functional resolvability of this hypo‐oxic response at the cortical column level within animal visual cortex [Crair et al., 1997; Frostig et al., 1990; Grinvald et al., 1986; Maldonado et al., 1997; Malonek and Grinvald, 1996; Ohki et al, 2000; Shmuel and Grinvald, 2000]. As well, high spatial resolution fMRI studies at magnetic field strengths of 4.0 and 9.4 Tesla have recently demonstrated the detectability of the initial hypo‐oxic response in cat visual cortex and its ability to resolve iso‐orientation columns on a spatial scale approaching 100 μm [Kim et al., 2000a].
Studies demonstrating the poor resolution of fMRI using the hyperoxic phase of the hemodynamic response have typically employed long duration stimuli (≥10 sec) that saturate the signal profile [Engel et al., 1997; Kim et al., 2000a]. Although the shape and duration of the hemodynamic response to neural activity is somewhat variable between subjects, it is has been repeatedly demonstrated that the hyperoxic response saturates approximately 5–8 sec after the onset of stimulation when stimulation is maintained [Liu et al., 2000]. Under these conditions, the sustained elevation in cerebral blood flow and blood volume leads to a spatial spreading of the responding vasculature, and hence the measured MR signal, to areas well beyond the site of the excited neural tissue. This may be true for short duration stimuli as well. If stimulation is brief (less than 6 sec), however, the locus of neural activity in response to one stimulus may still be resolvable from an adjacent locus that responds to an orthogonal stimulus. Of course, as stimulus duration increases, the spatial spread of the ensuing hemodynamic response will make functional resolvability on a submillimeter scale unachievable. This issue has not been fully explored. Past studies, however, have demonstrated ocular dominance within human visual cortex using fMRI [Menon et al., 1997; Menon and Goodyear, 1999], and more recent efforts have resolved its columnar architecture even when visual stimulation was as long as 12 sec [Dechent and Frahm, 2000]. Although these studies produced convincing results, the question of reproducibility remains.
The potential of brief stimuli for submillimeter resolution studies has been demonstrated in previous work showing the temporal progression of functional maps obtained by OIS [Frostig et al., 1990; Grinvald et al., 1986] and fMRI [Hu et al., 1997]. We have demonstrated recently that a magnetic field strength of 4 Tesla is aptly suited for single‐trial brief duration stimuli because the image signal‐to‐noise ratio (SNR) is sufficient at a spatial resolution of approximately 0.5 mm to detect the low percentage increases in MR signal (∼1–3%) associated with brief stimulus presentations [Menon and Goodyear, 1999]. In the present study, we attempted to demonstrate that when the hyperoxic hemodynamic response to neural activity does not saturate, blood oxygenation‐level‐dependent (BOLD) fMRI can achieve submillimeter functional resolution in human brain when adjacent functional units are orthogonal in their response. We show this by resolving the columnar architecture of ocular dominance within the primary visual cortex of humans. In addition, we show that when visual stimulation is prolonged such that the hemodynamic response saturates, the measured MR signals are the same as for a randomly generated data set.
MATERIALS AND METHODS
Subjects and Visual Stimuli
Four subjects were recruited from the academic environment of the University of Western Ontario. Each subject gave written informed consent before participating in an fMRI experiment approved by the Ethics Approval Board for Experimental Studies Involving Human Subjects at the University of Western Ontario. The visual stimulus was a vertical sinusoidal grating (2 cycles/degree, 66% contrast) drifting at 2 cycles/sec, reversing direction every second. The grating was contained within a circle that subtended 12° of visual angle. The remainder of the display was an isoluminant grey matched in mean luminance to the grating (60 cd/m2). The stimulus was projected onto a screen that was mounted onto the patient bed and was located 1.25 m from the subject's eyes. An angled adjustable mirror was positioned above the subject's eyes to provide an unobstructed view of the projection screen. The subject viewed the stimulus through a pair of liquid crystal shutter glasses (Translucent Technologies, Toronto, Ontario), the eyepieces of which were controlled manually by the experimenter outside the magnet room. Subjects were instructed to maintain fixation on a small red circular dot at the center of the display throughout the experiment. The subject's head was immobilized using a head vise that was well padded at the sides and back of the head, and had an additional padded bar placed across the forehead.
Data Acquisition
Anatomical and functional images were acquired with a Varian/Siemens Unity INOVA 4 Tesla whole‐body MR system (Varian, Palo Alto, CA; Siemens, Erlangen, Germany) using a 8‐cm diameter quadrature RF surface coil placed under the subject's occipital pole to transmit and receive the MR signal. T2*‐weighted images of three 3‐mm thick slices were acquired continuously and repeatedly during the experiment using a gradient‐recalled echo planar imaging sequence (0.55 mm × 0.55 mm prescribed in‐plane resolution, 14 cm field of view, 16 interleaved segments, repetition time (TR) = 4 sec, echo time (TE) = 15 msec, flip angle = 25°) with centric ordering of k‐space and a navigator echo for every segment to correct for physiological fluctuations due to respiration. This sequence provided a temporal resolution of 4 sec between acquired volumes. To regain a pseudo‐temporal resolution of 1 sec, images were reconstructed incorporating a ‘sliding window’ reconstruction every second [Menon and Goodyear, 1999; Riederer et al., 1988]. Image orientation was in a sagittal plane parallel to the medial bank of the visual cortex about the posterior calcarine sulcus, which was identified with the aid of sagittal T1‐weighted anatomical localizer images. For anatomical registration, high‐resolution (0.55 × 0.55 × 1.5 mm) T1‐weighted images were acquired using a 3D magnetization‐prepared fast low angle shot (FLASH) sequence (TR = 11 msec, TE = 6 msec, inversion time (TI) = 0.5 sec, flip angle = 11°).
Experiment 1
Creation of ocular dominance maps
For all four subjects, one epoch of visual stimulation lasted 4 sec per 32‐sec trial, and either the left (L) or right (R) eye was stimulated monocularly (in random order) using the visual stimulus describe above during separate trials. For two subjects, each eye was stimulated four times during the experimental run. For one subject, each eye was stimulated five times, and for the remaining subject, each eye was stimulated six times.
Data Analysis
Images were corrected for subject head motion using BrainVoyager 2000 (Rainer Goebel and Max Planck Society) and data analysis was performed using Stimulate 5.7 (CMRR, University of Minnesota). To identify visual cortex, a copy of the image data set was spatially blurred (1‐mm Gaussian window) and a boxcar waveform time course (variable delay window of 4–8 sec) was then used as a cross‐correlation function to identify image voxels exhibiting a significant increase in MR signal (corrected P < 0.05, cluster size of six contiguous voxels) above baseline in response to all episodes of visual stimulation (i.e., both R and L trials). This created a mask of activated voxels within visual cortex to be used for analysis of the original unblurred data set. The image volumes (i.e., time points) corresponding to the peak of the fMRI response to visual stimulation and image volumes corresponding to baseline were then determined for each stimulation trial. For each and every voxel of the visual cortex mask, the percent change in MR signal above baseline during each L and R trial was calculated using the original 0.55‐mm resolution data set. Voxels were then separated into two populations to create a binary map of ocular dominance where yellow (blue) represented voxels exhibiting a greater percent change in MR signal during left (right) eye stimulation trials than during right (left) eye stimulation trials. The resulting map was then spatially interpolated to a final map resolution of 0.27 mm.
Experiment 2
The effect of stimulus duration
For two subjects, five separate experimental runs were performed using a stimulus duration of 2, 4, 6, 10, and 20 sec. For each map of ocular dominance (one for each stimulus duration), the ratio of the percent change in MR signal within a voxel during stimulation of the corresponding eye (i.e., a yellow voxel during stimulation of the left eye and a blue voxel during stimulation of the right eye) to the percent change in MR signal within the same voxel during stimulation of the fellow eye (i.e., a yellow voxel during stimulation of the right eye and a blue voxel during stimulation of the left eye) was calculated for all map voxels as a function of stimulus duration. In addition, two random data sets simulating the percent change in MR signal (i.e., selected from a population with a mean and standard deviation similar to that of the experimental image data) were created for each voxel and then sorted into two populations. One of the populations contained the larger of the two randomly generated numbers, and the other contained the smaller of the two randomly generated numbers. Each member of the first population simulated the fMRI response of a voxel during stimulation of the corresponding eye, and each member of the other population simulated the fMRI response of the same voxel during stimulation of the other eye. The ratio of the means of the two sorted populations was calculated to compare to the ratios calculated for the image data.
Experiment 3
Test/retest
Experiment 1 (i.e., 4‐sec stimulus) was repeated for all four subjects to assess the reliability of our maps of ocular dominance over repeated experiments. In addition, for the two subjects that participated in Experiment 2, the experimental run with a stimulus duration of 10 sec was also repeated. Using the image data from the repeated experiments, the average MR time courses of the right‐eye and left‐eye dominant map voxels of the first run were each plotted to determine the reproducibility of their MR signal time courses. As well, for the two subjects that participated in Experiment 2, the average MR time courses of the right‐eye and left‐eye dominant map voxels in the map for the 4‐sec stimulus were plotted for the 10‐sec stimulus image data.
RESULTS
Figure 1 shows maps of ocular dominance obtained using high spatial resolution fMRI. Yellow represents voxels whose fMRI response (expressed as a percentage increase above baseline) was greater during left‐eye stimulation than during right‐eye stimulation. Blue represents voxels whose fMRI response was greater during right‐eye stimulation than during left‐eye stimulation. These maps in Figure 1 demonstrate the columnar or stripe‐like arrangement of ocular dominance about the calcarine sulcus where primary visual cortex, or visual area V1, is known to reside in humans. These stripes are arranged primarily perpendicular to the calcarine sulcus, consistent with ocular dominance column architectures demonstrated by optical imaging of intrinsic signals (OIS) in animal cortex [Crair et al., 1997; Frostig et al., 1990; Grinvald et al., 1986; Maldonado et al., 1997; Malonek and Grinvald, 1996; Ohki et al, 2000; Shmuel and Grinvald, 2000] and by histology of post‐mortem specimens of human visual cortex [Horton et al., 1990]. The stripe‐like appearance is lost in areas of the map where the cortex was interrogated in a more perpendicular orientation relative to the cortical surface. The cross‐sectional width of the stripes of ocular dominance within of our maps closely corresponds to the known width of ocular dominance columns in the primary visual cortex of humans (0.5–1.0 mm) [Horton et al., 1990].
Figure 1.
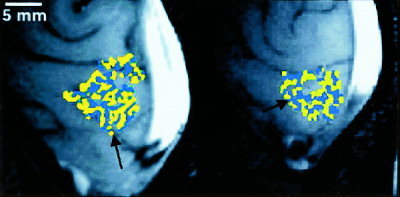
Maps of ocular dominance for two subjects. Blue (yellow) represents image voxels whose fMRI response was greater in magnitude during right (left) eye stimulation than during left (right) eye stimulation. Images have been cropped. The back of the head is to the right of each image, and the bright white area at the right of each image is the sagittal sinus. About the calcarine sulcus (indicated by black arrows), map voxels of ocular dominance are arranged as stripes, consistent with the known columnar architecture of ocular dominance.
Figure 2 shows ocular dominance maps from another subject. In Figure 2A, the map of ocular dominance was created using data from the first half of a 4‐sec stimulus run (i.e., 3 trials each of L and R stimulation). Figure 2B is a map created from the second half of the same 4‐sec stimulus run in a separate analysis. A significant portion of the map in Figure 2A (78% percent of all voxels) were reproduced in Figure 2B. Figures 2C and 2D were created from the first and second halves of a 10‐sec stimulus run, respectively. Only 56% of blue and yellow voxels in Figure 2C were reproduced in Figure 2D.
Figure 2.
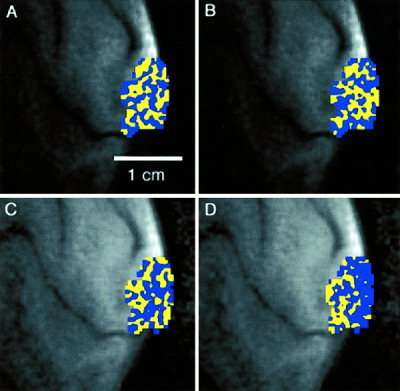
Maps of ocular dominance for one subject obtained from (A) the first half of a 4‐sec duration stimulus run; (B) the second half of a 4‐sec duration stimulus run; (C) the first half of a 10‐sec duration stimulus run; (D) the second half of a 4‐sec duration stimulus run.
Figure 3A shows the time courses of the MR signal within ocular dominance map voxels for one subject averaged over all stimulation trials. The stimulus duration was 4 sec. The peak magnitude of the fMRI response when the eye corresponding to the voxel designation was stimulated (i.e., a yellow voxel during left eye stimulation and a blue voxel during right eye stimulation) is nearly three times the peak magnitude of the fMRI response within the same voxel when the fellow eye was stimulated (i.e., a yellow voxel during right eye stimulation and a blue voxel during left eye stimulation). We term the ratio of these responses as the ‘fMRI response ratio.’ Notice, as well, that the magnitude of the fMRI response using a 4‐sec stimulus does not reach a plateau. Figure 3B shows the average time courses of the same map voxels that produced the plots in Figure 3A in a separate repeated experiment that was performed later in the same imaging session. Those voxels that were determined to be right‐eye dominant in the first experiment exhibited a greater fMRI response during right eye stimulation in the repeated experiment. As well, those voxels determined to be left‐eye dominant in the first experiment exhibited a greater fMRI response during left eye stimulation in the repeated experiment. When, however, the right‐eye and left‐eye dominant voxels of the first 4‐sec stimulus experiment were plotted for image data acquired when the stimulus duration was 10 seconds, as shown in Figure 3C, there was now no dissociation between the magnitude of the fMRI response during right eye or left eye stimulation.
Figure 3.
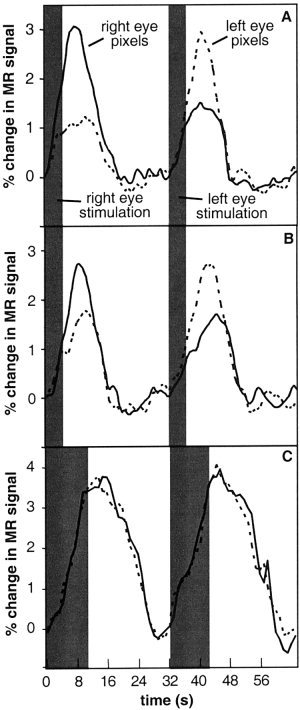
Average time‐course of MR signal over one trial (expressed as a percentage change from baseline) within ocular dominant image voxels for one subject. Corresponding (fellow) eye stimulation refers to blue (yellow) voxels during right eye stimulation and to yellow (blue) voxels during left eye stimulation. The shaded gray region indicates the duration of the visual stimulus. A: The average time‐course of MR signal over one right‐eye stimulation and one left‐eye stimulation trial for a right eye voxel (i.e., blue) and left eye voxel (i.e., yellow) of an ocular dominance map created for a stimulus duration of 4 sec. B: The average time‐course of MR signal for the same voxels as in (A) for a repeated experiment within the same imaging session. The right‐eye dominance of the right eye voxels of the first experiment remains observed during the repeated experiment. Likewise, the left eye voxels of the first experiment exhibit left‐eye dominance during the repeated experiment. C: The average time‐course of MR signal for the same voxels in (A) for a repeated experiment within the same imaging session with a stimulus duration of 10 sec. The dissociation between right eye and left eye dominance has been lost due to the spatial spread of the hemodynamic response to a long‐duration stimulus.
Figure 4 shows the fMRI response ratio as a function of stimulus duration for one subject averaged over all voxels in each ocular dominance map (one map for each stimulus duration). As the duration of the stimulus increases, the fMRI response ratio approaches the value of 1.96 predicted by the randomly generated set of data simulating fMRI response magnitude. Only for stimulus durations 4 sec or less is the fMRI response ratio significantly greater (P < 0.05) than the ratio for a random data set.
Figure 4.
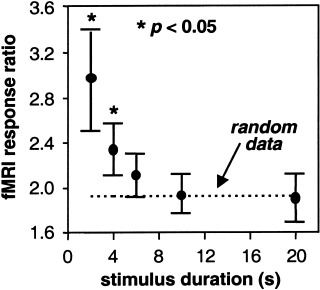
The ratio of the average magnitude of the fMRI response within a blue (yellow) voxel during right (left) eye stimulation to the average magnitude of the fMRI response within the same voxel during left (right) eye stimulation (termed the “fMRI response ratio”) as a function of stimulus duration. The dashed line indicates the fMRI response ratio for a randomly generated data set selected from a population with a mean and standard deviation similar to the experimental data. The asterisk indicates a fMRI response ratio that is statistically greater than that for the random data. Error bars indicate SE of the mean for all voxels in each functional map.
DISCUSSION
When a visual stimulus is presented, many types of neurons within the visual cortex respond, and the response of the ocular dominance columns cannot be singled out (at least not using our stimulus paradigm). The consistency of time courses across repeated runs, however, and the reproducibility of a significant portion of our maps lead us to believe that the fMRI signal is predominantly originating from ocular dominance columns. Our maps showing that the columnar architecture of ocular dominance can be revealed using only 4 trials each of left‐eye and right‐eye monocular stimulation demonstrates the power of our technique (the fifth and sixth trials that were presented to two subjects were discarded due to head motion). We attribute the significance of our results to the enhanced signal‐to‐noise ratio that quadrature surface coils and slice‐interleaved k‐space segments can provide (see Materials and Methods). As well, high static magnetic field strength (4 Tesla) provides greater MR signal and BOLD contrast [Gati et al., 1997] permitting the analysis of single trials rather than averaging over multiple trials that could be contaminated by head motion.
Upon first inspection of Figures 2A and 2B, it appears that the map in Figure 2B is not a satisfactory reproduction of the map in Figure 2A. In fact, 78% of the voxels in Figure 2A were reproduced in Figure 2B with the correct color designation. Careful inspection of the two maps reveals that many localized patterns, or at least single voxels, are indeed the same. When as few as one voxel switches from a left‐eye column designation to a right‐eye column designation between runs, however, it gives the appearance that a columnar stripe has a different orientation because the connectivity of voxels is altered. The sensitivity of our technique to noise necessitates more trials to increase the number of reproducible voxels. The inability to maintain head fixation for more than 2 to 3 min, however, makes this a difficult task because we require 3 or 4 trials each of left‐eye and right‐eye stimulation to get one map plus an additional 3 or 4 trials to acquire a second map for comparison. Upon inspection of the image data in a movie loop, we concluded that head motion was minimal for approximately the first two‐thirds of the experimental run. We feel that the map in Figure 2A (created from the first half of the data set) is a more accurate representation of the ocular dominance column mosaic within the primary visual cortex of this subject, and that the map in Figure 2B is more contaminated by head motion ‘noise.’
The maps in Figures 2C and 2D, created using a 10‐sec stimulus, do not demonstrate columnar‐like arrangements. Only 56% of voxels in Figure 2C were reproduced with the same color designation in Figure 2D. This percentage is at chance levels. Perhaps some columnar architecture is noticeable in Figure 2C, however, compared to Figure 2A, the map of Figure 2C does not exhibit a consistent alternating pattern of stripes.
As shown in Figure 3, the magnitude of the fMRI response within an ocular dominant voxel is greater than zero when the other eye is stimulated. This may be due to horizontal connections between columns in the upper layers of the cortex that inhibit the activity of the adjacent columns when one eye is being stimulated [Das and Gilbert, 1995]. This inhibitory activity is most likely an aerobic process, and thus leads to a detectable fMRI response. A more plausible explanation would be that even though the locus of the hemodynamic response may be within an ocular dominance column corresponding to the eye being stimulated, there remains a spill‐over of this response into adjacent cortex corresponding to the other eye. As Figure 4 shows, however, the magnitude of this spill‐over is less than half when the stimulus is less than 6 sec in duration. This makes the fMRI response to short duration stimuli a robust mapping signal. Figure 4 also shows that as the duration of visual stimuli increases, the spill‐over and spread of the vascular response produces a mapping signal that is identical to that predicted by a random dataset, making functional resolution at our prescribed resolution unlikely. The mapping signal predicted by ‘random’ data is specific to our imaging parameters and will of course change when imaging parameters are modified.
The hypo‐oxic phase of the hemodynamic response (commonly referred to as the ‘initial dip’ in fMRI parlance) as used in optical imaging and in recent fMRI studies of animal cortex provides a response ratio that is nearly 6:1 [Kim et al., 2000a; Malonek and Grinvald, 1996]. The magnitude of this ‘initial dip,’ however, is about an order of magnitude smaller than the hyperoxic phase even at 4 Tesla, and thus relies on averaging over multiple trials. This may not be practical for awake and behaving animals, including humans, because subject motion during lengthy high‐resolution scans becomes problematic. Because our image voxel time courses consist essentially of time points that are averages over a 4‐sec time period, the ‘initial dip’ is most likely obscured (this is also why in our time courses the fMRI signal begins to rise so quickly after stimulus onset). Nonetheless, fMRI has recently demonstrated that the ‘initial dip’ is detectable using high spatial resolution fMRI and can be used to localize neural activity to the iso‐orientation columns of cat visual cortex in an anesthesia model [Kim et al., 2000a]. Our experiences in high‐resolution functional imaging of human brain have shown that it is difficult to keep the head motionless for many minutes at a time even when employing a bite‐bar. This is especially true between scans when subjects tend to relax and adjust their head position. We have chosen to demonstrate the reproducibility of portions of our ocular dominance maps by dividing an experimental run into two halves rather than comparing one run to another. Three‐dimensional motion correction of surface coil images based on image edge detection is problematic due to the signal drop off with distance from the coil and hence lack of image ‘edge’ opposite the coil. It has been our experience that most head motion (at least with our head vise immobilization device) is due to either nodding of the head, causing an in‐plane rotation of a sagittal image, or sinking of the head into the head holder as padding becomes compressed over time, causing an in‐plane translation of a sagittal image. For sagittal image orientations, a 2D head‐motion correction scheme may suffice.
Using our imaging technique and short duration stimuli we were also able to show that the ocular dominance of the fMRI response within a voxel is reproducible in a repeated experiment, as shown in Figure 3B. The magnitude of the fMRI response, however, was different from one scan to the next. This was probably due to imperfect head motion correction across scans leading to partial‐voluming. Hence, we were unable to show the identical map from experiment to experiment. If image signal‐to‐noise ratio was sufficient to permit a resolution of 0.1–0.2 mm, slight head motion between scans would result in a simple rotation and translation of the whole map. Unfortunately, the image signal‐to‐noise of our system at present is not sufficient for 0.1–0.2 mm resolution, although efforts would be best concentrated on improving this shortfall. Portions of the map, however, were reproducible (as shown in Figure 2A,B) when a map obtained from the first half of one experimental run was compared to a map obtained from the second half of the experimental run.
It is important to stress that we do not claim that the hyperoxic phase of the hemodynamic response is as spatially specific as the initial hypo‐oxic phase. Rather, our results suggest that a non‐saturated hyperoxic response is capable of resolving adjacent and functionally different areas of neural activity. Using the ‘initial dip’ is most likely better suited for localizing (i.e., determining where a functional unit is and where it isn't) because the relative difference between the magnitude of signal between the active and non‐active state is much higher and is independent of stimulus duration. We did not detect the ‘initial dip’ in our data. As mentioned above, this may be due to poor temporal resolution because each data point is an average over 4 sec, or it may simply be that we did not detect it. With hardware improvements in gradient switching speed and with techniques to further improve the image signal‐to‐noise ratio, the direct comparison of maps created using the ‘initial dip’ and the unsaturated hyperoxic response will become feasible.
CONCLUSIONS
In support of our findings from previous studies [Menon and Goodyear, 1999], we have demonstrated than an unsaturated hyperoxic response to neural activity can achieve submillimeter functional resolution by demonstrating the columnar architecture of ocular dominance within human visual cortex. More importantly, we have shown that our ocular dominance maps are consistent with known columnar architecture, and that the fMRI response to monocular visual stimulation is significantly greater than that predicted by random data and consistent over repeated experiments. These findings provide the first demonstration of the reproducible high‐spatial resolution capabilities of fMRI in humans when using short duration stimuli and a stable high magnetic field system equipped with specialized hardware.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) grant 1RO1EY11551 and the Medical Research Council of Canada (MRC).
REFERENCES
- Crair MC, Ruthazer ES, Gillespie DC, Stryker MP (1997): Ocular dominance peaks at pinwheel center singularities of the orientation map in cat visual cortex. J Neurophysiol 77: 3381–3385. [DOI] [PubMed] [Google Scholar]
- Das A, Gilbert CD (1995): Long‐range horizontal connections and their role in cortical reorganization revealed by optical recording of cat visual cortex. Nature 375: 780–784. [DOI] [PubMed] [Google Scholar]
- Dechent P, Frahm J (2000): Direct mapping of ocular dominance columns in human primary visual cortex. Neuroreport 11: 3247–3249. [DOI] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA (1997): Retinotopic organization in human visual cortex and the spatial precision of fMRI. Cereb Cortex 7: 181–192. [DOI] [PubMed] [Google Scholar]
- Frostig RD, Lieke EE, Ts'o DY, Grinvald A (1990): Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high‐resolution optical imaging of intrinsic signals. Proc Natl Acad Sci USA 87: 6082–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gati JS, Menon RS, Ugurbil K, Rutt BK (1997): Experimental determination of the BOLD field strength dependence in vessels and tissue. Magn Reson Med 38: 296–302. [DOI] [PubMed] [Google Scholar]
- Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN (1986): Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature 324: 361‐364. [DOI] [PubMed] [Google Scholar]
- Horton JC, Dagi LR, McCrane EP, de Monasterio FM (1990): Arrangement of ocular dominance columns in human visual cortex. Arch Ophthalmol 108: 1025–1031. [DOI] [PubMed] [Google Scholar]
- Hu X, Le TH, Ugurbil K (1997): Evaluation of the early response in fMRI in individual subjects using short stimulus duration. Magn Reson Med 37: 877–884. [DOI] [PubMed] [Google Scholar]
- Kim DS, Duong TQ, Kim SG (2000a): High resolution mapping of iso‐orientation columns by fMRI. Nat Neurosci 3: 164–169. [DOI] [PubMed] [Google Scholar]
- Kim DS, Duong TQ, Kim SG (2000b): Reply to “Can current fMRI techniques reveal the micro‐architecture of cortex? ” Nat Neurosci 3: 414. [DOI] [PubMed] [Google Scholar]
- Liu HL, Pu Y, Nickerson LD, Liu Y, Fox PT, Gao JH (2000): Comparison of the temporal response in perfusion and BOLD‐based event‐related functional MRI. Magn Reson Med 43: 768–772. [DOI] [PubMed] [Google Scholar]
- Logothetis N (2000): Can current fMRI techniques reveal the micro‐architecture of cortex? Nat Neurosci 3: 413–414. [DOI] [PubMed] [Google Scholar]
- Maldonado PE, Godecke I, Gray CM, Bonhoeffer T (1997): Orientation selectivity in pinwheel centers in cat striate cortex. Science 276: 1551–1555. [DOI] [PubMed] [Google Scholar]
- Malonek D, Grinvald A (1996): Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science 272: 551–554. [DOI] [PubMed] [Google Scholar]
- Menon RS, Ogawa S, Strupp JP, Ugurbil K (1997): Ocular dominance in human V1 demonstrated by functional magnetic resonance imaging. J Neurophysiol 77: 2780–2787. [DOI] [PubMed] [Google Scholar]
- Menon RS, Goodyear BG (1999): Submillimeter functional localization in human striate cortex using BOLD contrast at 4 Tesla: implications for the vascular point‐spread function. Magn Reson Med 41: 230–235. [DOI] [PubMed] [Google Scholar]
- Ohki K, Matsuda Y, Ajima A, Kim DS, Tanaka S (2000): Arrangement of orientation pinwheel centers around area 17/18 transition zone in can visual cortex. Cereb Cortex 10: 593–601. [DOI] [PubMed] [Google Scholar]
- Riederer SJ, Tasciyan T, Farzaneh F, Lee JN, Wright RC, Herfkens RJ (1988): MR fluoroscopy: technical feasibility. Magn Reson Med 8: 1–15. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Grinvald A (2000): Coexistence of linear zones and pinwheels within orientation maps in cat visual cortex. Proc Natl Acad Sci USA 97: 5568–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]


