Abstract
The applicability of functional magnetic resonance imaging (fMRI) in patients with Alzheimer's disease (AD) or schizophrenia is frequently limited by cognitive impairment, which prevents the adequate execution of complex tasks. An experimental design that puts only minor demands on the patients' cognitive ability but engages disease‐relevant brain structures would be of benefit. Novelty detection and repetition suppression are two basic components of memory that might be used to investigate specific brain areas under these conditions. Novelty detection has been related to hippocampal activation increases. Stimulus repetition related activation decreases (suppression) have been observed in the extrastriate cortex and have been related to perceptual priming. Both processes have been examined primarily in neuroimaging studies with complex cognitive tasks. We used event‐related fMRI to investigate novelty‐ and repetition‐related effects in an attended but passive picture‐viewing task in healthy subjects. The differential activation, detected in the novel vs. repeated contrast, was located in the bilateral anterior hippocampus and in bilateral occipital and inferior‐temporal areas. The hippocampal activation is of interest because medial temporal lobe lesions are key features in AD and schizophrenia. The repetition‐related activation decreases in the extrastriate areas are of potential value in investigating the conflicting results regarding perceptual priming impairment in both disorders. Our results indicate that activation of disease‐relevant brain regions under passive task conditions is possible. This might increase the utility of functional imaging in cognitively impaired patients. Hum. Brain Mapping 17:231–237, 2002. © 2002 Wiley‐Liss, Inc.
Keywords: hippocampus, extrastriate cortex, memory, priming, event‐related fMRI
INTRODUCTION
Functional neuroimaging has substantially contributed to basic cognitive neuroscience over the last decade. Its clinical application in neuropsychiatric disorders, however, remains limited. Complexity of the experimental tasks is one major limitation in the study of altered brain functions of cognitively impaired patients. Especially in dementia or in schizophrenia, patients are often not capable of following task instructions over the course of the experiment. The different activation patterns in patients compared with controls might, therefore, either be related to task specific neuronal dysfunction or to insufficient task comprehension with reduced behavioral compliance. To increase the applicability of functional neuroimaging in impaired patients, an experimental design that puts only minor demands on cognitive abilities while activating brain structures that are crucial for the specific disease would be of benefit. Two memory‐related neuronal processes of potential value in this context have been located in disease relevant brain areas. These processes are novelty detection and repetition suppression.
Novelty detection refers to the discrimination between novel and familiar stimuli. Lesion studies [Knight, 1996], intracranial ERP recordings [Grunwald et al., 1998], and functional imaging studies [Parkin, 1997] have highlighted the crucial role of the hippocampus in novelty detection. Brain imaging studies in humans have mostly reported novelty detection in the context of memory tasks, which put high demands on the participants' performance [Menon et al., 2000; Stern et al., 1996; Strange et al., 1999]. Unlike explicit encoding, however, novelty detection occurs automatically in the hippocampus [Martin, 1999].
Repetition suppression describes the reduced neuronal response after repeated presentation of identical stimuli. Functional neuroimaging studies have reported repetition suppression in the occipital (extrastriate cortex) and inferior temporal lobe and have related the decreased neuronal response to perceptual priming [Schacter and Buckner, 1998]. Most brain imaging studies that reported repetition suppression employed a semantic or recognition memory task to observe behavioral priming effects. As for novelty detection, however, there is evidence that repetition suppression occurs irrespective of task demands [Vogels et al., 1995].
We tested whether novelty related hippocampal activation and repetition suppression in the extrastriate cortex could be observed in attended picture viewing without a complex cognitive task.
SUBJECTS AND METHODS
Subjects
Eight healthy, right‐handed volunteers (5 male, 3 female; mean age, 24 years; SD = 1.7) were included in the study. All gave written informed consent before participation. The study was approved by the local ethic committee and is in accordance with the Declaration of Helsinki.
Paradigm
Fifty novel pictures, 50 repeated pictures, 50 non‐pictorial stimuli, and 18 incidental targets were included in the study. The novel stimuli were 50 photographs of complex indoor and outdoor scenes, including pictures of objects and animals but excluding pictures of human faces. Ten of the novel pictures were randomly selected and repeated five times throughout the experiment, yielding 50 repeated pictures. To assess the cerebral activation related to the picture content and not to pure visual input, 50 non‐pictorial stimuli were included in the paradigm. These were created by randomly distributing the pixels of the 10 repeated pictures. This results in stimuli with equivalent color content as the repeated pictures, but without visual structure. Each non‐pictorial stimulus was presented five times throughout the experiment. To keep the attention focused, a picture with the instruction to rapidly press a response button was presented 18 times throughout the study. The cerebral response to this trial type was not assessed in the final analysis. A fixation cross was positioned in the middle of each stimulus.
The items were presented pseudo‐randomly with each item type occurring equally often in the first and second half of the experiment. The mean number of items between the repetition of a single picture was 19 (SD = 20.5). The presentation time of a single stimulus was 3 sec. The stimulus onset asynchrony (SOA) was 15 sec. A color picture with randomly distributed pixels (not one of the non‐pictorial stimuli) was presented between trials. The experiment was split in seven runs with 24 stimuli each. The SOA after item 1, 9, and 17 in each run were changed either to 16, 13, and 16 sec or to 14, 17 and 14 sec. This modification yielded a jitter with an effective hemodynamic response sampling rate of 1 time point/sec. The stimuli were projected into the MR‐scanner via a mirror system. The behavioral responses to the incidental targets were monitored during the experiment.
To assess the applicability of the design in a clinical population, the paradigm was presented to five patients with schizophrenia according to DSM IV [American Psychiatric Association, 1994] (3 male, 2 female; age, 32.6 years; SD = 6.2) and five control subjects (3 male, 2 female; age, 35 years; SD = 7.9) on a computer screen. All participants pushed the response button after all 18 incidental targets. The reaction time did not differ significantly (mean patients: 438 msec, SD = 108; mean controls: 439 msec, SD = 102) between the groups. This suggests that patients are capable of maintaining sufficient attention to perform equally well on the task as healthy control subjects. Only healthy subjects were examined with fMRI.
MR data acquisition
Whole‐brain fMRI was carried out on a 1.5 T system (Gyroscan ACS‐NT; Philips) using a single shot gradient echo planar imaging (EPI) sequence (TE = 50 msec, TR = 3 sec, FA = 90°, FOV = 256 mm, matrix = 64 × 64, slice thickness = 4 mm, 32 interleaved AC–PC slices). High‐resolution isotropic 3D T1‐weighted gradient echo images (1 × 1 × 1 mm) were acquired for anatomical coregistration.
MR data analysis
The fMR data analysis was carried out with SPM 99 [Friston, 1996]. All functional images were corrected for slice timing differences and aligned to the first volume in the time series. The images were normalized and resliced with a voxel size of 4 × 4 × 4 mm. Finally, all images were smoothed with an isotropic, full‐width, half maximum (FWHM) filter of 12 mm.
The stimulus specific regressors, e.g., the canonical hemodynamic response function (HRF) of SPM 99 and its first temporal derivative for each of the four item types, a subject‐ and a run‐specific function comprised a general linear model. Inferences were made with a fixed‐effects analysis and therefore related to the significance of activation in relation to the precision with which they were measured in this group. Differential responses were assessed with T statistics using contrasts of the parameter estimates of the hemodynamic response function (HRF). The contrasts novel vs. non‐pictorial stimuli, repeated vs. non‐pictorial stimuli, novel vs. repeated and repeated vs. novel were assessed and the t‐maps were Z‐transformed. The statistical threshold was set at P < 0.05 corrected for multiple comparisons (Z > 4.29) at a spatial extent threshold of five voxels.
The incidental targets were included in the statistical model, but did not contribute to the inferences about differential responses. In this sense the targets provided an incidental task to maintain the attentional set, whereas the focus of the study was the difference between repeated and non‐repeated pictorial stimuli.
The applied fixed‐effects model prevents the generalization of the results to a population beyond the participating subjects and does not allow the comparison of independent groups. The ultimate aim of our present study, however, is to establish a paradigm that can be used to compare clinical populations and control subjects. In a second analysis, we collapsed the data of single individuals to one map by calculating images of the novel‐repeated contrast. These images were introduced to a one‐sample t‐test. The results of this random effects analysis are presented in Table I in addition to the results of the fixed effects model.
Table I.
Z‐values and coordinates of local maxima of the novel‐repeated contrast in the fixed and random effects analysis
| Anatomical region | Fixed effects | Random effects | ||||||
|---|---|---|---|---|---|---|---|---|
| Z | MNI coordinates | Z | MNI coordinates | |||||
| x | y | z | x | y | z | |||
| Right hippocampus | 5.14 | 24 | −8 | −28 | 2.98 | 24 | −4 | −28 |
| Left hippocampus | 5.22 | −20 | −8 | −24 | 3.35 | −20 | −8 | −24 |
| Right superior occipital | >8 | 32 | −88 | 24 | — | — | — | — |
| Left superior occipital | >8 | −32 | −88 | 28 | — | — | — | — |
| Right medial occipital | 7.81 | 56 | −88 | 8 | 4.05 | 44 | −72 | 8 |
| Left medial occipital | 7.53 | −56 | −84 | 8 | 3.93 | −28 | −88 | 32 |
| Right inferior occipital | 7.23 | 28 | −84 | −24 | 4.52 | −32 | −84 | −16 |
| Left inferior occipital | 5.23 | −44 | −88 | −32 | — | — | — | — |
| Right inferior temporal | >8 | 32 | −44 | −28 | 5.09 | 32 | −40 | −24 |
| Left inferior temporal | >8 | −32 | −48 | −24 | 4.58 | −28 | −48 | −16 |
* Significance levels of the Z‐values are P < 0.05 corrected (fixed effects) and P < 0.001 uncorrected (random effects) for the brain volume.
RESULTS
All subjects pressed the response button to all incidental targets, which ensured that they were attending the stimuli.
In comparison to the non‐pictorial stimuli, the novel pictures elicited greater activation in the bilateral occipital region extending into the inferior parietal lobes, and in bilateral inferior temporal regions, extending in both medial temporal lobes. In addition, bilateral inferior frontal and left superior frontal areas were differentially activated in this contrast (Fig. 1a).
Figure 1.
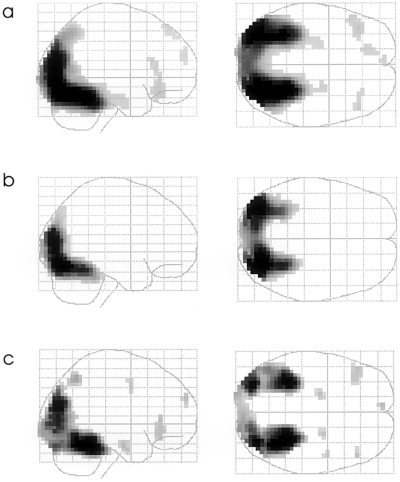
Glassbrain projections of the contrasts (a) novel pictures vs. non‐pictorial stimuli; (b) repeated pictures vs. non‐pictorial stimuli; (c) novel vs. repeated stimuli. The statistical threshold is set to P < 0.05, corrected for multiple comparisons (Z > 4.29). Clusters are depicted if they exceed five suprathreshold voxels.
The contrast repeated pictures vs. non‐pictorial stimuli showed greater activation in the bilateral occipital lobe, extending into the bilateral inferior temporal regions (Fig. 1b).
Novel compared to repeated pictures showed greater activation in the bilateral anterior hippocampi, in the occipital lobes extending into both inferior temporal regions and in the right parietal lobe. Furthermore, bilateral inferior frontal and right superior frontal regions were activated more in this contrast (Figs. 1c,2). Table I lists local maxima of this contrast in the fixed and random effects analysis in selected brain regions.
Figure 2.
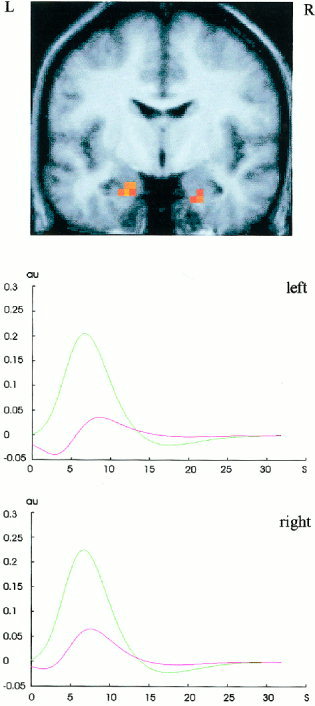
(a) Bilateral hippocampal activation of the contrast novel vs. repeated pictures projected on the normalized mean brain of the participants at P < 0.05, corrected for multiple comparisons (Z > 4.29). The peak differential activation on the left side (Z =5.22; 10 voxels) is located at the MNI coordinates (in brackets are the transformed Talairach coordinates) x = −20 (−20), y = −8 (−9), z = −24 (−20). The peak differential activation on the right side (Z = 5.14; 7 voxels) is located at x = 24 (24), y = −8 (−9), z = −28 (−23). (b) Fitted and adjusted hemodynamic responses to novel and repeated stimuli in bilateral hippocampal peak voxels expressed in adimensional units (au) against time (s).
There was no significant activation between repeated vs. novel pictures.
DISCUSSION
We tested whether novelty and repetition related activation changes can be observed in a passive picture viewing task under attended condition.
We observed stronger activation in both anterior hippocampi after the presentation of novel compared to repeated pictures. Early block designed PET studies have reported novelty‐related activation in the hippocampus [Tulving et al., 1994, 1996]. Later memory studies demonstrated that the hippocampus is primarily involved in the overall novelty of presented material, whereas the frontal cortex is involved in higher order memory formation [Dolan and Fletcher, 1997]. In agreement with these findings, Menon et al. [2000] reported that novelty processing accounts for much larger hippocampal activation than encoding. In partial agreement, Kirchhoff et al. [2000], reported that the right hippocampus is involved in both novelty detection and subsequent encoding. With respect to a functional segregation, Strange et al. [1999] reported that the anterior hippocampus is involved in novelty detection, whereas the posterior hippocampus is activated with increasing familiarity. In the present event‐related fMRI study we were able to demonstrate that significant novelty related anterior hippocampal activation can be observed even in the absence of any explicit memory task.
Hippocampal activation during explicit encoding is graded with respect to the depth of encoding [Martin, 1999] and explicit encoding is mediated by additional brain structures (e.g., frontal cortex). Therefore, hippocampal activation during encoding depends to a large extent on the subjects understanding, compliance and motivation during the experiment. Comparison of explicit encoding tasks with respect to hippocampal activation between cognitively impaired patients and healthy controls might be systematically confounded by these factors. Reduced activation in the patient group might be attributed to neuronal damage in the hippocampus, whereas it actually results from reduced task compliance. Passive activation of the anterior hippocampus based on novelty detection potentially reduces this systematic bias. This is of special relevance because the anterior hippocampus is of interest in Alzheimer disease (AD) and schizophrenia. The earliest AD‐related neuropathological changes, which account for the short‐term memory deficits as the first clinical symptoms, are located in this area [Braak and Braak, 1991]. With respect to schizophrenia, there is growing evidence from histopathological [Harrison, 1999] and neuroimaging studies [Shenton et al., 2001] of damage in medial temporal lobe structures. To what extent our approach can detect medial temporal lobe changes in disorders like AD or schizophrenia should be the topic of future investigations.
Repetition suppression has been reported in the occipital and inferior temporal region in a number of priming studies which included cognitive tasks, such as word completion [Badgaiyan et al., 1999, 2001; Blaxton et al., 1996; Buckner et al., 1995; Schacter et al., 1996; Squire et al., 1992], picture recognition [James et al., 1999, 2000], and picture identification with subliminal stimulation [Badgaiyan, 2000]. Event‐related fMRI studies have used face recognition [Jiang et al., 2000] and object classification tasks [Buckner et al., 1998; Koutstaal et al., 2001]. Grill‐Spector et al. [1999] observed repetition suppression during passive picture viewing in a block designed fMRI study but only scanned the occipital region. Henson et al. [2000] reported repetition suppression in the fusiform gyrus for repeated familiar faces and symbols in a passive but attended event‐related fMRI study. In the present study, which covered the entire brain, we observed widespread repetition suppression effects in bilateral occipital and inferior temporal regions under passive viewing condition.
Perceptual priming and repetition suppression are of interest in AD and schizophrenia. Although some studies have reported disturbed repetition priming [e.g., Burke et al., 1994; Fleischman et al., 1999], others reported intact priming in AD [e.g., Heindel et al., 1998; Postle et al., 1996; Verfaellie et al., 2000]. A recent PET study demonstrated increased activation in the right occipital cortex during a word stem completion task in AD patients, which represents the reversed pattern compared to repetition suppression in healthy subjects [Backman et al., 2000]. This is interpreted as a disturbed shaping of the stimulus representation in the extrastriate region in AD. To what extent this observation is independent of the subjects' task performance can be investigated in a passive experimental design. Furthermore, the passive approach could detect repetition suppression and enhancement in severely demented subjects who cannot follow tasks like word‐stem completion.
In schizophrenia, behavioral studies have reported intact [e.g., Clare et al., 1993; Gras‐Vincendon et al., 1994; Perry et al., 2000], reversed [e.g., Williams, 1996], and increased repetition priming [Baving et al., 2001] under various task conditions. Functional neuroimaging of repetition suppression under passive task conditions represents an approach to elucidate the underlying neuronal mechanisms of these divergent results. It might be especially promising if different disease subgroups or stages of schizophrenia account for the divergent priming effects, as suggested by Williams [1996], because these variables are related to other cognitive abilities which might interfere with more complex task designs.
Here, novel compared to repeated pictures showed stronger activation in frontal areas. Because the frontal cortex hosts a variety of higher cortical functions and the interpretation of differential activation in this area under passive viewing conditions would be very speculative, these results cannot be further discussed in the context of our study.
Although the anatomical location of the results of our present study are in agreement with the current literature on novel/repeated differentiation, it should be highlighted that they were obtained after statistically correcting for the entire brain volume. Thus, they can be accepted without a prior anatomical hypothesis. They may form the basis for the generation of an anatomical hypothesis in studies with patient samples. This is of special importance because, the present results were obtained with a fixed effect analysis, which prevent the generalization beyond the participating subjects and prevents group comparisons. Group comparisons demand a random effect approach with usually lower statistical sensitivity than a fixed effect analysis. Based on results of the present study, analysis of group comparisons of a similar task can be restricted to specific anatomical region, e.g., the hippocampus, and do not have to be statistically corrected for the entire brain volume, which increases the likelihood of detecting significant activation differences between patient and controls. To give an idea of the effect strength of the present paradigm in a random effects model, results from the novel‐repeated contrast have been included in the present paper.
Our data do not allow the differentiation between novelty related activation increases and repetition suppression related decreases. In a recent review on the relationship of novelty detection and repetition suppression (neural priming), Habib [2001] points out that, theoretically, both represent distinct concepts. Novelty detection is believed to facilitate successful encoding, whereas neuronal priming is related to increased processing speed of repeated items. However, according to Habib [2001], functional imaging studies have thus far failed to clearly differentiate both concepts, mainly because individual studies focus on either of both topics but also because both concepts might essentially represent two sides of the same coin. A consistent finding in novelty studies, however, is the medial temporal lobe activation, which is not observed in priming studies. Therefore, it can be speculated that priming occurs irrespective of medial temporal lobe involvement, which is in agreement with intact priming in amnesic subjects.
Our goal was to create a fMRI paradigm that is independent of the subject's task compliance and at best independent of the subject's attention. Although a large body of literature has described perceptual priming as the correlate of repetition suppression to be independent of attention [e.g., Mulligan 1998], recent studies have reported effects of the allocation of attention on perceptual priming [e.g., Mulligan and Hornstein, 2000]. Furthermore, neuroimaging studies have showed pharmacological effects on repetition suppression [Thiel et al., 2001] and highlighted that repetition suppression occurs in implicit, but not explicit, visual memory tasks [Henson et al., 2002]. Patients with AD and schizophrenia suffer from attentional disturbances. This has to be taken into account when applying the present paradigm to one of the groups, even though the small group of schizophrenic patients, examined behaviorally in our present study, carried out as well on the task as healthy subjects. Attentional deficits will, however, also interfere with more complex cognitive task and represent a general problem in clinical research, which might even be best addressed with minor cognitive tasks. The potential modification of novelty detection and repetition suppression might be of value to the evaluation of pharmacological treatment.
REFERENCES
- American Psychiatric Association (1994): Diagnostic and statistical manual of mental disorders. 4th Edition Washington, DC: American Psychiatric Association. [Google Scholar]
- Backman L, Almkvist O, Nyberg L, Andersson J (2000): Functional changes in brain activity during priming in Alzheimer's disease. J Cogn Neurosci 12: 134–141. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD, Schacter DL, Alpert NM (1999): Auditory priming within and across modalities: evidence from positron emission tomography. J Cogn Neurosci 11: 337–348. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD (2000): Neuroanatomical organization of perceptual memory: an fMRI study of picture priming. Hum Brain Mapp 10: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgaiyan RD, Schacter DL, Alpert NM (2001): Priming within and across modalities: exploring the nature of rCBF increases and decreases. Neuroimage 13: 272–282. [DOI] [PubMed] [Google Scholar]
- Baving L, Wagner M, Cohen R, Rockstroh B (2001): Increased semantic and repetition priming in schizophrenic patients. J Abnorm Psychol 110: 67–75. [DOI] [PubMed] [Google Scholar]
- Blaxton TA, Bookheimer SY, Zeffiro TA, Figlozzi CM, Gaillard WD, Theodore WH (1996): Functional mapping of human memory using PET: comparisons of conceptual and perceptual tasks. Can J Exp Psychol 50: 42–56. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E (1991): Neuropathological staging of Alzheimer‐related changes. Acta Neuropathol (Berl) 82: 239–259. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Petersen SE, Ojemann JG, Miezin FM, Squire LR, Raichle ME (1995): Functional anatomical studies of explicit and implicit memory retrieval tasks. J Neurosci 15: 12–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen B, Dale AM (1998): Functional‐anatomic correlates of object priming in humans revealed by rapid presentation event‐related fMRI. Neuron 20: 285–296. [DOI] [PubMed] [Google Scholar]
- Burke J, Knight RG, Partridge FM (1994): Priming deficits in patients with dementia of the Alzheimer type. Psychol Med 24: 987–993. [DOI] [PubMed] [Google Scholar]
- Clare L, McKenna PJ, Mortimer AM, Baddeley AD (1993): Memory in schizophrenia: what is impaired and what is preserved? Neuropsychologia 31: 1225–1241. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Fletcher PC (1997): Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature 388: 582–585. [DOI] [PubMed] [Google Scholar]
- Fleischman DA, Gabrieli JD, Gilley DW, Hauser JD, Lange KL, Dwornik LM, Bennett DA, Wilson RS (1999): Word‐stem completion priming in healthy aging and Alzheimer's disease: the effects of age, cognitive status, and encoding. Neuropsychology 13: 22–30. [DOI] [PubMed] [Google Scholar]
- Friston KJ (1996): Statistical parametric mapping and other analyses of functional imaging data In: Toga AW, Mazziotta JC, editors. Brain mapping. The methods. San Diego, New York: Academic Press; p 363–386. [Google Scholar]
- Gras‐Vincendon A, Danion JM, Grange D, Bilik M, Willard‐Schroeder D, Sichel JP, Singer L (1994): Explicit memory, repetition priming and cognitive skill learning in schizophrenia. Schizophr Res 13: 117–126. [DOI] [PubMed] [Google Scholar]
- Grill‐Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R (1999): Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron 24: 187–203. [DOI] [PubMed] [Google Scholar]
- Grunwald T, Lehnertz K, Heinze HJ, Helmstaedter C, Elger CE (1998): Verbal novelty detection within the human hippocampus proper. Proc Natl Acad Sci U S A 95: 3193–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib R (2001): On the relation between conceptual priming, neural priming, and novelty assessment. Scand J Psychol 42: 187–195. [DOI] [PubMed] [Google Scholar]
- Harrison PJ (1999); The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain 122: 593–624. [DOI] [PubMed] [Google Scholar]
- Heindel WC, Salmon DP, Fennema‐Notestine C, Chan AS (1998): Repetition priming with nonverbal stimuli in patients with dementia of the Alzheimer type. Neuropsychology 12: 43–51. [DOI] [PubMed] [Google Scholar]
- Henson R, Shallice T, Dolan R (2000): Neuroimaging evidence for dissociable forms of repetition priming. Science 287: 1269–1272. [DOI] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Gorno‐Tempini ML, Dolan RJ (2002): Face repetition effects in implicit and explicit memory tests as measured by fMRI. Cereb Cortex 12: 178–186. [DOI] [PubMed] [Google Scholar]
- James TW, Humphrey GK, Gati JS, Menon RS, Goodale MA (1999): Repetition priming and the time course of object recognition: an fMRI study. Neuroreport 10: 1019–1023. [DOI] [PubMed] [Google Scholar]
- James TW, Humphrey GK, Gati JS, Menon RS, Goodale MA (2000): The effects of visual object priming on brain activation before and after recognition. Curr Biol 10: 1017–1024. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Haxby JV, Martin A, Ungerleider LG, Parasuraman R (2000): Complementary neural mechanisms for tracking items in human working memory. Science 287: 643–646. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE (2000): Prefrontal‐temporal circuitry for episodic encoding and subsequent memory. J Neurosci 15: 6173–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R (1996); Contribution of human hippocampal region to novelty detection. Nature 383: 256–259. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL (2001): Perceptual specificity in visual object priming: functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia 39: 184–199. [DOI] [PubMed] [Google Scholar]
- Martin A (1999): Automatic activation of the medial temporal lobe during encoding: lateralized influences of meaning and novelty. Hippocampus 9: 62–70. [DOI] [PubMed] [Google Scholar]
- Menon V, White CD, Eliez S, Glover GH, Reiss AL (2000): Analysis of a distributed neural system involved in spatial information, novelty, and memory processing. Hum Brain Mapp 11: 117‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan NW (1998): The role of attention during encoding in implicit and explicit memory. J Exp Psychol Learn Mem Cogn 24: 27–47. [DOI] [PubMed] [Google Scholar]
- Mulligan NW, Hornstein SL (2000): Attention and perceptual priming in the perceptual identification task. J Exp Psychol Learn Mem Cogn 26: 626–637. [DOI] [PubMed] [Google Scholar]
- Parkin AJ (1997): Human memory: novelty, association and the brain. Curr Biol 7: 768–769. [DOI] [PubMed] [Google Scholar]
- Perry W, Light GA, Davis H, Braff DL (2000): Schizophrenia patients demonstrate a dissociation on declarative and non‐declarative memory tests. Schizophr Res 46: 167–174. [DOI] [PubMed] [Google Scholar]
- Postle BR, Corkin S, Growdon JH (1996): Intact implicit memory for novel patterns in Alzheimer's disease. Learn Mem 3: 305–312. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS (1996): Conscious recollection and the human hippocampal formation: evidence from positron emission tomography. Proc Natl Acad Sci U S A 93: 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL (1998): Priming and the brain. Neuron 20: 185–195. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW (2001): A review of MRI findings in schizophrenia. Schizophr Res 49: 1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Ojemann JG, Miezin FM, Petersen SE, Videen TO, Raichle ME (1992): Activation of the hippocampus in normal humans: a functional anatomical study of memory. Proc Natl Acad Sci U S A 89: 1837–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR (1996): The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci U S A 93: 8660–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ (1999): Segregating the functions of human hippocampus. Proc Natl Acad Sci U S A 96: 4034–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel CM, Henson RN, Morris JS, Friston KJ, Dolan RJ (2001): Pharmacological modulation of behavioral and neuronal correlates of repetition priming. J Neurosci 21: 6846–6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Kapur S, Habib R, Houle S (1994): Novelty encoding networks in the human brain: positron emission tomography data. Neuroreport 5: 2525–2528. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Craik FE, Habib R, Houle S (1996): Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex 6: 71–79. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Keane MM, Johnson G (2000): Preserved priming in auditory perceptual identification in Alzheimer's disease. Neuropsychologia 38: 1581–1592. [DOI] [PubMed] [Google Scholar]
- Vogels R, Sary G, Orban GA (1995): How task‐related are the responses of inferior temporal neurons? Vis Neurosci 12: 207–214. [DOI] [PubMed] [Google Scholar]
- Williams LM (1996): Cognitive inhibition and schizophrenic symptom subgroups. Schizophr Bull 22: 139–151. [DOI] [PubMed] [Google Scholar]


