Abstract
Surprise is one of six emotions having a specific and universally recognized facial expression. Functional imaging and neuropsychologic studies have uncovered partly separable neural substrates for perceiving different facial expressions; however, the functional neuroanatomy of perceiving surprised faces has not yet been investigated. Using functional magnetic resonance imaging (fMRI), we aimed to identify the neural substrate of surprise perception from facial expressions. Based on the assumption of unexpectedness and novelty as elicitors of facial surprise reactions, we hypothesized recruitment of medial temporal lobe structures implicated in novelty detection during the perception of surprise in others. Healthy subjects were scanned while they were presented with surprised faces. As a control, they viewed faces depicting neutral or disgust expressions. Activations during the emotional conditions were contrasted with each other and with the neutral face condition. Compared to both control conditions, perception of surprised facial expressions yielded consistently increased signals in the parahippocampal region, an area associated previously with novelty detection. Our findings therefore suggest a close relation between perceiving surprise in others and the response to novel events. Additionally, we confirmed activation of the insula during perception of disgust expressions. Hum. Brain Mapping 23:181–187, 2004. © 2004 Wiley‐Liss, Inc.
Keywords: surprise, facial expression, fMRI, novelty, medial temporal lobe, disgust, insula
INTRODUCTION
Facial expressions provide important indicators of emotion and contribute to the appreciation of the physical environment. Six basic emotions (happiness, surprise, fear, sadness, anger, and disgust) have a distinct facial expression and are recognized universally [Ekman, 1992].
Recognition of facial expressions comprises multiple processes, including perceptual analysis of facial features and extraction of emotional meaning [Adolphs, 2002; Haxby et al., 2000]. The perceptual analysis of facial characteristics has been linked to the occipital and posterior temporal cortices [Hoffman and Haxby, 2000; Kanwisher et al., 1997; Sergent et al., 1992]. Extraction of emotional meaning from faces involves the orbitofrontal/inferior frontal cortex as shown by a neuropsychologic study [Hornak et al., 1996]. Consistent with this, functional imaging studies revealed orbitofrontal and inferior frontal activation while viewing emotional facial expressions [Nakamura et al., 1999; Narumoto et al., 2000; Sprengelmeyer et al., 1998].
More important, imaging and neuropsychologic studies have uncovered partly separable and specialized neural systems for recognizing different facial expressions. The amygdala is involved primarily during perception of fearful faces [Adolphs et al., 1994; Morris et al., 1996; Sprengelmeyer et al., 1999; Whalen et al., 1998; Young et al., 1995], whereas the processing of disgust has been linked to the insula and basal ganglia [Calder et al., 2000; Krolak‐Salmon et al., 2003; Phillips et al., 1997, 1998; Sprengelmeyer et al., 1996, 1998]. Moreover, the medial‐frontal cortex has been implicated in perceiving angry faces [Blair et al., 1999; Harmer et al., 2001]. Evidence concerning neural correlates of perceiving facial expressions of happiness and sadness is less clear‐cut. Functional imaging studies have pointed to activation of various neural structures during perception of happy faces, including the basal ganglia [Morris et al., 1996, 1998], inferior frontal cortex [Dolan et al., 1996], anterior cingulate cortex [Dolan et al., 1996; Kesler‐West et al., 2001], and the amygdala [Breiter et al., 1996; Pessoa et al., 2002]. A study investigating the neural substrate of perceiving sad faces suggests involvement of the temporal pole and amygdala [Blair et al., 1999].
The facial expression of surprise has been neglected in neuroscience, although this expression was described already by Charles Darwin in 1872, who proposed novelty and unexpectedness as its elicitors: “Attention, if sudden and close, graduates into surprise. […] Attention is shown by the eyebrows being slightly raised; and as this state increases into surprise, they are raised to a much greater extent with the eyes and mouth widely open. […] As surprise is excited by something unexpected or unknown, we naturally desire, when startled, to perceive the cause as quickly as possible …” [Darwin, 1999, p 278]. Psychological theories conceive surprise as an adaptive mechanism to restructure and extend cognitive concepts after analyzing an unexpected event [Schutzwohl, 1998]; however, the neural substrate involved in the perception of surprise and its significance to the observer has not yet been investigated.
Using functional magnetic resonance imaging (fMRI), we aimed to identify the neural substrate that mediates the perception of surprise in others. Based on the assumed association of surprise and novelty [Darwin, 1999; Schutzwohl, 1998], we hypothesized that besides temporal and occipital cortices subserving the perceptual analysis of facial features [Haxby et al., 2000, 2002], perception of surprised facial expressions involves the medial temporal lobes, which have been implicated previously in the response to contextually novel or distinctive stimuli [Gabrieli et al., 1997; Stern et al., 1996]. Furthermore, we aimed to validate our experimental paradigm by reproducing the association of disgust perception with insular activation [Phillips et al., 1997, 1998; Sprengelmeyer et al., 1998].
SUBJECTS AND METHODS
Subjects
The study subjects comprised 20 right‐handed, healthy individuals (10 men, 10 women; mean age 32.5 ± 8.3 years). All volunteers were free of neurologic or psychiatric diseases and gave written informed consent to take part in the experiment. The study was approved by the Ethical committee of the Technische Universität München.
Stimuli and Paradigm
Volunteers viewed grayscale pictures of faces from the Facial Expressions of Emotions: Stimuli and Test (FEEST) [Young et al., 2002], which displayed disgust, surprise or neutral expressions. As a neutral face, we used a morphed image with slightly happy expression (25% happy, 75% neutral) produced by computer graphic manipulation, because 100% neutral faces appear slightly cold and threatening [Phillips et al., 1997]. Each picture was presented individually against a gray background for 3 s with an interstimulus interval of 0.76 s. Eight faces (3 male/5 female) of the same expressions were ordered randomly and constituted one block. Ten blocks with alternating emotional (surprise or disgust) and neutral faces constituted one run. Participants carried out four separate runs in a counterbalanced order: two runs including surprised and neutral and the other two disgusted and neutral faces. In line with previous studies of emotion perception [Blair et al., 1999; Morris et al., 1996; Phillips et al., 1997, 1998; Sprengelmeyer et al., 1998], an implicit paradigm of facial expression perception was applied with volunteers pressing left or right response buttons in a gender decision task. To familiarize subjects with the stimuli, they viewed each picture once before fMRI scanning.
Image Acquisition and Analysis
Echoplanar MR brain images were acquired using a 1.5 Tesla Siemens Symphony Scanner (Erlangen, Germany) with a standard head coil. During each run, 110 T2*‐weighted images were acquired at each of 33 slices of 4 mm thickness parallel to the intercomissural line (AC–PC), covering the whole brain (TR = 3 s, TE = 50 ms, flip angle = 90 degrees, matrix = 64 × 64, field of view [FOV] = 200 mm). The first five volumes of each session were discarded, to allow time for the longitudinal magnetization to reach a steady state. High‐resolution T1‐weighted anatomic images were also acquired for each subject at the end of the sessions.
Statistical analysis was carried out using SPM software (SPM99; Wellcome Department of Cognitive Neurology, London, UK) based on the general linear model [Friston, 1997]. Images were realigned to the first scan of the session, stereotactically normalized into a standard space approximating that of Talairach and Tournoux [ 1988] and smoothed with an isotropic Gaussian kernel of 8 × 8 × 8 mm. Low frequency confounds were removed by a high pass filter with individually adjusted cutoffs. Data analysis was carried out by modelling the different conditions as reference waveforms, using boxcar functions convolved with a canonical hemodynamic response function (HRF).
A second‐level random effects approach was applied for statistical analysis. This approach takes into account between‐subject variability, allowing a more critical exploration of blood oxygenation level‐dependent (BOLD) responses than fixed‐effects models [Holmes and Friston, 1998]. On the first level, the four functional sessions were entered into an individual design matrix for each subject. Here, surprise and disgust conditions were defined explicitly with the neutral face conditions modelled implicitly. To show areas particularly involved in perception of a specific emotional expression, both emotion conditions were contrasted against two different baselines: (1) surprise and disgust conditions were compared to the neutral face condition (surprise vs. neutral, disgust vs. neutral); and (2) both emotion conditions were compared directly to each other (surprise vs. disgust, disgust vs. surprise). For a region to be denoted as specifically involved in perception of either surprise or disgust expressions, it thus had to fulfill the strict criterion of corresponding results across two different baseline conditions. For each of the four comparisons, individual contrast images were entered into a second level (random effects) analysis applying a one‐sample t‐test. Significance was accepted for voxels surviving a statistical threshold of P < 0.001, uncorrected. To avoid false positives, only clusters of 20 or more contiguous voxels were considered [Forman et al., 1995]. All coordinates reported are based on the Talairach atlas and were transformed by applying procedures developed by M. Brett (available online at http://www.mrc-cbu.cam.ac.uk/Imaging; accession date 10 April 2003).
RESULTS
Mean accuracy of gender classification during scanning was 95% (SD = 7%) for 17 subjects (data of 3 subjects were not recorded due to technical problems). Contrasting surprise with the neutral face condition was associated with increased activations in the right parahippocampal gyrus, right cerebellum, middle temporal gyrus (Brodmann's area [BA] 21) and adjacent posterior superior temporal sulcus (STS) as well as in occipital regions (BA 18) (Table I, Fig. 1). The crucial role of the parahippocampal gyrus in the perception of surprise was underscored further by direct comparison of the “surprise” with the “disgust” condition, revealing significant activation exclusively in the right parahippocampal gyrus (Table I, Fig. 2).
Table I.
Activations in response to the perception of surprise expressions
| Region | Side | x | y | z | t * |
|---|---|---|---|---|---|
| Surprise versus neutral | |||||
| Parahippocampal gyrus (BA30/35) | Right | 16 | −31 | −7 | 3.74 |
| Middle temporal gyrus/STS (BA 21) | Right | 57 | −44 | 8 | 5.28 |
| Lingual gyrus (BA 18) | Left | −22 | −95 | −5 | 5.36 |
| Inferior occipital gyrus (BA 18) | Left | −24 | −88 | −7 | 4.64 |
| Cerebellum | Right | 12 | −35 | −8 | 5.24 |
| Surprise versus disgust | |||||
| Parahippocampal gyrus (BA 35) | Right | 20 | −24 | −14 | 4.47 |
Regions activated by surprise versus neutral condition and surprise versus disgust condition. Talairach coordinates refer to each regional cluster and the associated t‐values are shown.
P < 0.001, uncorrected, extent threshold = 20 voxels.
BA, Brodmann area; STS, superior temporal sulcus.
Figure 1.
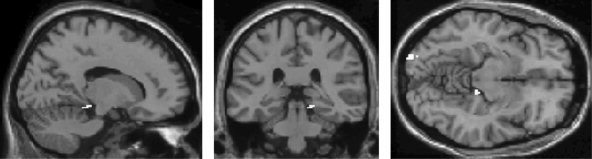
A statistical parametric map (SPM) showing significant activation of the right parahippocampal gyrus and left occipital gyrus in the surprise condition relative to the neutral condition (P < 0.001, uncorrected, extent threshold = 20 voxels).
Figure 2.
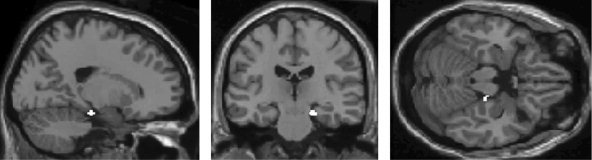
A statistical parametric map (SPM) showing significant activation of the right parahippocampal gyrus in the surprise condition relative to the disgust condition (P < 0.001, uncorrected, extent threshold = 20 voxels).
We found insular activations during perception of disgust for both contrasts (disgust vs. neutral and disgust vs. surprise). Perception of disgust when compared to the neutral face condition yielded additional activations in inferior frontal, postcentral, temporal, and occipital regions (Table II). Besides insular activation, the contrast of disgust with the surprise condition demonstrated further activations within the claustrum, inferior frontal gyrus, thalamus, postcentral and paracentral gyrus, inferior parietal gyrus, cerebellum, brainstem, and occipital cortices (Table II).
Table II.
Activations in response to the perception of disgust expressions
| Region | Side | x | y | z | t * |
|---|---|---|---|---|---|
| Disgust versus neutral | |||||
| Insula (BA 13) | Right | 38 | 5 | 15 | 4.64 |
| Inferior frontal gyrus (BA 45) | Right | 51 | 18 | 10 | 5.09 |
| Inferior frontal gyrus (BA 46) | Right | 50 | 43 | 9 | 4.88 |
| Postcentral gyrus (BA 3) | Right | 36 | −32 | 53 | 4.53 |
| Fusiform gyrus (BA 20) | Left | −38 | −41 | −13 | 4.56 |
| Middle temporal gyrus (BA 39) | Right | 51 | −73 | 20 | 5.63 |
| Inferior and middle occipital gyrus (BA 19) | Right | 46 | −80 | −3 | 6.00 |
| Middle occipital gyrus (BA 18/19) | Left | −42 | −81 | 15 | 4.16 |
| Inferior and middle occipital gyrus (BA 18) | Right | −38 | −93 | 0 | 5.56 |
| Cuneus (BA 18) | Left | −22 | −97 | 10 | 5.64 |
| Disgust versus surprise | |||||
| Insula (BA 13) | Right | 34 | −3 | 19 | 4.09 |
| Claustrum | Right | 30 | −3 | 17 | 5.02 |
| Inferior frontal gyrus (BA 46) | Right | 53 | 39 | 13 | 5.73 |
| Thalamus (medio‐dorsal) | Left | −6 | −13 | 6 | 5.34 |
| Postcentral gyrus (BA 2) | Left | −59 | −25 | 44 | 4.76 |
| Inferior parietal gyrus (BA 40) | Left | −36 | −35 | 39 | 5.98 |
| Paracentral gyrus (BA 5) | Left | −12 | −38 | 55 | 4.58 |
| Middle occipital gyrus (BA 37) | Right | 50 | −63 | −7 | 4.00 |
| Inferior occipital gyrus (BA 19) | Right | 44 | −78 | −3 | 5.67 |
| Cuneus (BA 19) | Left | −16 | −88 | 25 | 5.06 |
| Cerebellum | Right | 12 | −59 | −22 | 5.20 |
| Brainstem | Right | 12 | −19 | −29 | 4.34 |
Regions activated by disgust versus neutral condition and disgust versus surprise condition. Talairach coordinates are referring to each regional cluster and the associated t‐values are shown.
P < 0.001, uncorrected, extent threshold = 20 voxels.
BA, Brodmann area.
To explore whether gender had an influence on the neural responses within our regions of interest, we conducted post hoc two‐sample t‐tests on second level to show differences between men (n = 10) and women (n = 10) in perception of surprised and disgusted facial expressions when compared to the neutral face condition. These analyses revealed no significant gender effects for perception of surprised and disgusted facial expressions within the medial temporal lobes and insular cortex.
DISCUSSION
The results presented here provide evidence that the perception of surprised facial expressions consistently recruits structures within the medial temporal lobes, namely the right parahippocampal gyrus. Furthermore, they confirm the proposed association between the insula and facial expressions of disgust [Phillips et al., 1997, 1998; Sprengelmeyer et al., 1998]. Our findings thus corroborate further the notion of partly distinct neural systems for extracting meaning from different facial expressions. In support of this interpretation, activations within the parahippocampal gyrus and the insular cortex during processing of surprise and disgust expressions, respectively, satisfied our strict criterion of corresponding responses across two different high‐level baselines (neutral expressions and surprise/disgust expressions).
We also found support, however, for the idea of a common neural system for perceptual analysis of facial features, within temporal and occipital cortices [Haxby et al., 2000, 2002]. In line with previous studies [Phillips et al., 1997; Vuilleumier et al., 2001], activation of the temporal and occipital cortices was more pronounced when the emotional (surprise/disgust) conditions were compared to the neutral condition than when compared to each other. Increased activation of these areas may represent top‐down modulatory effects on the visual processing stream, reflecting attentional enhancement due to emotional significance [Pessoa et al., 2002; Vuilleumier et al., 2001]. Furthermore, the posterior part of the STS, as implicated during perception of surprised relative to neutral expressions, has been previously related to perception of static images of changeable aspects of the face [Hoffman and Haxby, 2000] including facial expression [Critchley et al., 2000; Kanwisher et al., 1997; Narumoto et al., 2001; Phillips et al., 1998].
Previous neuropsychologic and functional imaging studies have underscored the importance of the inferior frontal/orbitofrontal cortex in the extraction of emotional meaning from the face [Hornak et al., 1996; Nakamura et al., 1999; Narumoto et al., 2000; Sprengelmeyer et al., 1998]. The inferior frontal/orbitofrontal cortex has been suggested to play an essential role in social reinforcement processes [Rolls, 1996] and to be a common endpoint of networks of emotion recognition [Sprengelmeyer et al., 1998]. We found inferior frontal/orbitofrontal activation, however, during perception of facial expressions of disgust but not of surprise. This might be explained by the fact that surprise has no definite positive or negative valence as suggested by Ekman and Friesen [ 1975]: “…surprise itself is neutral in hedonic tone. It is rather the following emotion that gives it a positive or negative tone to the experience.”
The results of our study further support the idea of partly independent neural representations for distinct basic emotions, mediating the processing of different information crucial for survival and environmental adaptation. Accordingly, we demonstrate activation of the parahippocampal gyrus during surprise, but not disgust perception. Functional neuroimaging studies have implicated the parahippocampal gyrus [Gabrieli et al., 1997] or posterior hippocampus and parahippocampal gyrus [Stern et al., 1996] in processing of novel compared to familiar visual stimuli. Consistent with this, Hunkin et al. [ 2002] found medial temporal lobe activations centered in the parahippocampal gyrus during processing of verbal associative novelty. Findings from lesion studies and single‐unit recordings in monkeys further support the role of the parahippocampal gyrus in novelty detection, showing perirhinal involvement during processing of contextual novelty [Brown and Aggleton, 2001]. In addition, the parahippocampal region receives prominent projections from unimodal and polymodal high‐level visual temporal and occipital cortices [Suzuki and Amaral, 1994], also implicated in the present study. The perception of facial expressions of surprise in others may therefore be related to detection or evaluation of novel stimuli in the environment, which is thought of as an initial step in memory formation subserved by the parahippocampal area [Fernandez et al., 1998]. This notion is in accordance with a psychological model of surprise, proposing an evolutionary old mechanism to analyze unexpected events to update knowledge for successful individual‐environmental transaction [Schutzwohl, 1998].
An alternative explanation would be that activation in the parahippocampal gyrus might have been caused merely by unfamiliar faces per se. We feel that this is unlikely, however, as no such activations occurred during the presentation of disgusted when compared to neutral faces and all individual faces were presented repeatedly. Another objection might be that surprised faces might have been confused with fearful faces. We therefore conducted a post hoc behavioral assessment using a set of morphed faces from the FEEST, each showing two of six basic emotions with different degrees of intensity [Young et al., 2002; for a detailed description please refer to the FEEST handbook]. Subjects were instructed to categorize each morphed face according to one of six basic emotions with a maximum score of 20 correct responses for each emotion. An error analysis of surprise recognition scores revealed only a few confusion errors with happiness (0.75 ± 0.91) and fear (1.65 ± 2.16), both being part of the surprise morphs. A paired t‐test revealed no more confusion of surprised with fearful than with happy expressions (t = −1,67; P = 0.11). Activations of the parahippocampal gyrus in response to surprised faces are thus unlikely due to confusion of surprised with fearful expressions. Moreover, the parahippocampal responses during surprise perception observed in our study can be distinguished clearly from amygdala activations implicated in the perception of fearful faces [Adolphs et al., 1994; Morris et al., 1996; Sprengelmeyer et al., 1999; Whalen et al., 1998; Young et al., 1995]. Even with clearly defined regions of interest for the bilateral amygdala (defined as spheres of 10‐mm radius centered at ±24, −4, −16, based on the location of the amygdala in the Talairach atlas) and small volume correction, we could not find significant responses within this region during perception of surprised faces when compared to either neutral or disgusted faces.
Insular activation found during perception of disgusted facial expressions converge with evidence from studies based on insular lesions [Calder et al., 2000], depth electrodes [Krolak‐Salmon et al., 2003], and functional imaging [Phillips et al., 1997, 1998; Sprengelmeyer et al., 1998], thus confirming the robustness and reliability of our findings. A metaanalysis carried out by Murphy et al. [ 2003], including four imaging studies that used facial expressions of disgust [Phillips et al., 1997, 1998, 1999; Sprengelmeyer et al., 1998], revealed that the insula was the only neural structure consistently activated across all studies. As the insular cortex has been implicated in gustatory processing [Frey and Petrides, 1999], recognition of disgust in others has been linked to the evaluation of distasteful stimuli [Phillips et al., 1997]. Accordingly, a recent fMRI study by Wicker and colleagues [ 2003] provided evidence for involvement of the insula during both perceiving facial expression of disgust and experiencing disgust. In our study, we further validated the association between the anterior insula and the processing of facial disgust, showing that the insula is also activated when the perception of disgusted faces is compared directly to the perception of a different emotion (surprise). By contrast, findings concerning the role of the basal ganglia in disgust are less consistent, given that imaging studies have revealed activation within different (sub)structures of the basal ganglia, i.e., putamen, globus pallidus and nucleus caudatus [Phillips et al., 1997, 1998, 1999; Sprengelmeyer et al., 1998]. Despite these heterogeneous results, the metaanalysis by Murphy et al. [ 2003] also pointed to a role of the globus pallidus in perception of facial disgust. Even at a more liberal statistical threshold, however, using small volume correction (P < 0.05) based on the coordinates provided by Murphy et al. [ 2003], we could not find significant neural responses within this region during the processing of disgusted faces when compared to either neutral or surprised faces.
In conclusion, our findings demonstrate robust medial temporal lobe activations, during perception of surprised facial expressions, that are focused in the parahippocampal gyrus and further corroborate the role of the insula in the emotion of disgust. We suggest that surprise perception in others subserves a specific adaptive function, related to novelty detection. Furthermore, these findings support the concept of partly distinct neural system for perceiving different emotional facial expressions.
REFERENCES
- Adolphs R (2002): Neural systems for recognizing emotion. Curr Opin Neurobiol 12: 169–177. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A (1994): Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372: 669–672. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Morris JS, Frith CD, Perrett DI, Dolan RJ (1999): Dissociable neural responses to facial expressions of sadness and anger. Brain 122: 883–893. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR (1996): Response and habituation of the human amygdala during visual processing of facial expression. Neuron 17: 875–887. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP (2001): Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci 2: 51–61. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manes F, Antoun N, Young AW (2000): Impaired recognition and experience of disgust following brain injury. Nat Neurosci 3: 1077–1078. [DOI] [PubMed] [Google Scholar]
- Critchley H, Daly E, Phillips M, Brammer M, Bullmore E, Williams S, Van Amelsvoort T, Robertson D, David A, Murphy D (2000): Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Hum Brain Mapp 9: 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C (1999): The expression of the emotions in man and animals. London: Fontana Press; p 278. [Google Scholar]
- Dolan RJ, Fletcher P, Morris J, Kapur N, Deakin JF, Frith CD (1996): Neural activation during covert processing of positive emotional facial expressions. Neuroimage 4: 194–200. [DOI] [PubMed] [Google Scholar]
- Ekman P (1992): An argument for basic emotions. Cogn Emot 6: 169–200. [Google Scholar]
- Ekman P, Friesen WV. 1975. Unmasking the face. Englewood Cliffs, NJ: Prentice‐Hall. [Google Scholar]
- Fernandez G, Weyerts H, Schrader‐Bolsche M, Tendolkar I, Smid HG, Tempelmann C, Hinrichs H, Scheich H, Elger CE, Mangun GR, Heinze HG (1998): Successful verbal encoding into episodic memory engages the posterior hippocampus: a parametrically analyzed functional magnetic resonance imaging study. J Neurosci 18: 1841–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Frey S, Petrides M (1999): Re‐examination of the human taste region: a positron emission tomography study. Eur J Neurosci 11: 2985–2988. [DOI] [PubMed] [Google Scholar]
- Friston K (1997): Analysis of brain images: principles and overviews In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC, editors. Human brain function. San Diego: Academic Press; p 22–41. [Google Scholar]
- Gabrieli JD, Brewer JB, Desmond JE, Glover GH (1997): Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science 276: 264–266. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Thilo KV, Rothwell JC, Goodwin GM (2001): Transcranial magnetic stimulation of medial‐frontal cortex impairs the processing of angry facial expressions. Nat Neurosci 4: 17–18. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI (2000): The distributed human neural system for face perception. Trends Cogn Sci 4: 223–233. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI (2002): Human neural systems for face recognition and social communication. Biol Psychiatry 51: 59–67. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Haxby JV (2000): Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat Neurosci 3: 80–84. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ (1998): Generalizability, random effects and population inference. Neuroimage 7: 480. [Google Scholar]
- Hornak J, Rolls ET, Wade D (1996): Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia 34: 247–261. [DOI] [PubMed] [Google Scholar]
- Hunkin NM, Mayes AR, Gregory LJ, Nicholas AK, Nunn JA, Brammer MJ, Bullmore ET, Williams SC (2002): Novelty‐related activation within the medial temporal lobes. Neuropsychologia 40: 1456–1464. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM (1997): The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler‐West ML, Andersen AH, Smith CD, Avison MJ, Davis CE, Kryscio RJ, Blonder LX (2001): Neural substrates of facial emotion processing using fMRI. Brain Res Cogn Brain Res 11: 213–226. [DOI] [PubMed] [Google Scholar]
- Krolak‐Salmon P, Henaff MA, Isnard J, Tallon‐Baudry C, Guenot M, Vighetto A, Bertrand O, Mauguiere F (2003): An attention modulated response to disgust in human ventral anterior insula. Ann Neurol 53: 446–453. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ (1998): A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 121: 47–57. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ (1996): A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383: 812–815. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo‐Smith I, Lawrence AD (2003): Functional neuroanatomy of emotions: a meta‐analysis. Cogn Affect Behav Neurosci 3: 207–233. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Ito K, Sugiura M, Kato T, Nakamura A, Hatano K, Nagumo S, Kubota K, Fukuda H, Kojima S (1999): Activation of the right inferior frontal cortex during assessment of facial emotion. J Neurophysiol 82: 1610–1614. [DOI] [PubMed] [Google Scholar]
- Narumoto J, Okada T, Sadato N, Fukui K, Yonekura Y (2001): Attention to emotion modulates fMRI activity in human right superior temporal sulcus. Brain Res Cogn Brain Res 12: 225–231. [DOI] [PubMed] [Google Scholar]
- Narumoto J, Yamada H, Iidaka T, Sadato N, Fukui K, Itoh H, Yonekura Y (2000): Brain regions involved in verbal or non‐verbal aspects of facial emotion recognition. Neuroreport 11: 2571–2576. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG (2002): Neural processing of emotional faces requires attention. Proc Natl Acad Sci USA 99: 11458–11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Williams L, Senior C, Bullmore ET, Brammer MJ, Andrew C, Williams SC, David AS (1999): A differential neural response to threatening and non‐threatening negative facial expressions in paranoid and non‐paranoid schizophrenics. Psychiatry Res 92: 11–31. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, Gray JA, David AS (1997): A specific neural substrate for perceiving facial expressions of disgust. Nature 389: 495–498. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, Williams SC, Bullmore ET, Brammer M, Gray JA (1998): Neural responses to facial and vocal expressions of fear and disgust. Proc R Soc Lond B Biol Sci 265: 1809–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET (1996): The orbitofrontal cortex. Philos Trans R Soc Lond B Biol Sci 351: 1433–1443. [DOI] [PubMed] [Google Scholar]
- Schutzwohl A (1998): Surprise and schema strength. J Exp Psychol Learn Mem Cogn 24: 1182–1199. [DOI] [PubMed] [Google Scholar]
- Sergent J, Ohta S, MacDonald B (1992): Functional neuroanatomy of face and object processing. A positron emission tomography study. Brain 115: 15–36. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H (1998): Neural structures associated with recognition of facial expressions of basic emotions. Proc R Soc Lond B Biol Sci 265: 1927–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengelmeyer R, Young AW, Calder AJ, Karnat A, Lange H, Homberg V, Perrett DI, Rowland D (1996): Loss of disgust. Perception of faces and emotions in Huntington's disease. Brain 119: 1647–1665. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Young AW, Schroeder U, Grossenbacher PG, Federlein J, Buttner T, Przuntek H (1999): Knowing no fear. Proc R Soc Lond B Biol Sci 266: 2451–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR (1996): The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA 93: 8660–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG (1994): Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol 350: 497–533. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. (1988). Co‐planar stereotactic atlas of the human brain. Stuttgart: Thieme. [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ (2001): Effects of attention and emotion on face processing in the human brain: an event‐related fMRI study. Neuron 30: 829–841. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA (1998): Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G (2003): Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron 40: 655–664. [DOI] [PubMed] [Google Scholar]
- Young AW, Aggleton JP, Hellawell DJ, Johnson M, Broks P, Hanley JR (1995): Face processing impairments after amygdalotomy. Brain 118: 15–24. [DOI] [PubMed] [Google Scholar]
- Young AW, Perrett D, Calder A, Sprengelmeyer R, Ekman P (2002): Facial expressions of emotions: stimuli and test (FEEST). Thurstone: Thames Valley Test Company. [Google Scholar]


