Abstract
Patient registries are valuable tools helping to address significant challenges in research, care and policy. Registries, well embedded in many fields of medicine and public health, are relatively new in dementia. This systematic review presents the current situation in regards to dementia registries worldwide. We identified 31 dementia registries operating on an international, national or local level between 1986-2016. More than half of the registries aimed to conduct or facilitate research, including preclinical research registries and registries recruiting research volunteers. Other dementia registries collected epidemiological or quality of care data. We present evidence of practical and economic outcomes of registries for research, clinical practice and policy and recommendations for future development. Global harmonization of recruitment methods and minimum data would facilitate international comparisons. Registries provide a positive return on investment; their establishment and maintenance require ongoing support by government, policy makers, research funding bodies, clinicians, individuals with dementia and their caregivers.
1. Introduction
The prevalence of dementia is estimated to double every 20 years from 46.8 million in 2015 to nearly 131.5 million by 2050 [1,2]. The natural history of different dementias, the quality of diagnosis and care, the use of community services and long-term care, the costs of care and the effects on caregivers at a population level are largely unknown or rely on extrapolations from smaller samples. Such data would be beneficial in shaping policy and planning particularly in low and middle income countries where such information is even more wanting. Harnessing innovative approaches for effective prevention, treatment and care, and changes in policy could improve quality of care and quality of life for people with dementia and their families and help to minimize the financial costs of AD and other dementias [3,4]. Clinical registries are one approach to help recruitment for research and monitoring quality of care.
The many definitions and classifications of registries in medicine are typically embedded within broader frameworks and models of public health surveillance and reporting on the quality of health care [5]. In general, in epidemiology the term “register” refers to a “file of data concerning all cases of a particular disease or other health relevant condition in a defined population” and a “registry” is a “system of ongoing registration” [6 p211] (see Appendix 1 for Definitions and Classifications of Registries). The first patient registries were established in Scandinavian countries at the end of the 19th century. The increasing public health concern with chronic diseases during the 1950s led to a proliferation of registries [5]. The Scandinavian countries, such as Denmark, Finland and Sweden, remain world leaders in regards to extensive register networks and linkage of individual-level data from different sources (“an entire country [as] a cohort”) [7 p2398]. For instance, in Sweden in 2012 there were more than 100 healthcare registries [8]. National repositories of patient registries have been set up in Denmark [9], the UK [10] and the USA [11] to improve sharing of information about existing health data collection systems and, where possible, facilitate data sharing. Another type of registry – a research participant registry, aims to recruit people who are at increased risk of a condition, e.g. women with gestational diabetes, persons with family history of a disease [12]. Patient registries inform health policy and contribute to health care value. A 2011 health economics study in Sweden revealed that an annual investment of US$70m in registries could reduce the annual growth in health care spending by 0.6%, with the estimated cumulative return of more than US$7b over ten years – a $10 return on every dollar invested [13].
Despite popularity of registries in many fields of medicine since the 1950s, and their tangible outcomes, registries collecting dementia-related data are relatively new. The first dementia registries, which focused on diagnostic and clinicopathological data on AD, were established in USA, including the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) in 1986. The principal goal of CERAD was to standardize procedures for the evaluation and diagnosis at the Alzheimer Disease Centers (ADCs) [14]. A decade later, in 1999, the NIA funded the National Alzheimer’s Coordinating Center (NACC) [15] to develop and maintain a large database of clinical and neuropathological information collected by the ADCs. Other pioneering dementia registries, such as the IMAGE Project Population-Based Registry of AD in Quebec [16] and the Camberwell Dementia Case Register in the UK [17], focused on the study of genetic transmission patterns in AD, and the prevalence and natural history of AD.
Dementia registries [18–24] aim to advance dementia research by optimizing clinical trials for interventions in the pre-dementia phase of AD (i.e., preclinical registries), collecting epidemiological data, monitoring the quality of dementia care and recruiting volunteers for dementia studies (Table 1). Interest in dementia registries has been accompanied by expansion of international research consortia on AD, development of comprehensive national databases on healthy aging, international harmonization in sharing longitudinal datasets, and harnessing big data in dementia research [3,25]. There is considerable potential in linking data across health, care, research and administrative systems using electronic health records and dementia registries. Linkage of big data, i.e., “deep” biological and clinical data and “broad” population-based health and health care data, can advance the understanding of progression of dementia and assessing effectiveness of treatments and interventions [4].
Table 1.
Aims and categories of dementia registries
| Category of registry | Aims |
|---|---|
| Dementia research registry | • To support research into causes and risk factors for dementia. • To provide data on the natural history of dementia, determinants of progression, and their implications for clinical management. • To develop and measure effectiveness of interventions to reduce the risk and incidence of dementia, its treatment and management. • To evaluate and refine the diagnostic criteria for dementia, to standardize and validate screening instruments and diagnostic tests. |
| Subcategory: Preclinical dementia research registry | • To optimize conduct of clinical trials in preclinical stages of AD/dementia, to accelerate cohort development and trial recruitment. |
| Epidemiological dementia registry | • To collect epidemiological data on the prevalence, incidence, and risk of dementia. |
| Quality of dementia care registry | • To monitor the quality of dementia care. • To provide information on utilization and cost of health and aged care services and carer support, and to inform planning and development of dementia services. |
| Dementia research volunteer registry | • To identify people with dementia, their carers, and healthy volunteers who are willing to be involved in research studies and clinical trials. |
Systematic reviews have mapped registries in many areas of health care, such as renal replacement therapy [26] and trauma [27] but not yet for dementia-related data and their applications. Our study aims to address this gap and to present the current situation in the field including the benefits and outcomes of registries, as well as to inform their further development as tools for dementia research, care, prevention, and policy. This systematic review identifies and classifies dementia registries, including AD registries, operating around the world and reviews their characteristics and functions. We describe the outcomes of dementia registries in terms of research, epidemiology, quality of care, policy and service planning, and health economics, and present recommendations regarding practicalities of operating a dementia registry.
2. Method
2.1. Database search
The databases MEDLINE, EMBASE and SCOPUS were searched up until August 2016. There was no restriction on date of publication. The search combined the following terms: “dementia OR Alzheimer’s disease” and “registry OR register OR database” using “AND” relations. Subject headings and truncations were used when appropriate and supported by search engines. Results were limited to English language articles and abstracts published in peer-reviewed journals until August 2016. After removal of duplicates, the literature search in MEDLINE, EMBASE, SCOPUS, yielded 2,349 references. In addition, reference lists of retrieved articles were manually searched to find relevant publications not already covered in the search. An online search using Google search engine for dementia registry websites and grey literature was conducted in August 2016 with search terms used in database searches. Where contact details were provided in retrieved sources, an email was sent to a registry manager asking for additional peer reviewed publications and other resources, such as conference presentations and annual reports. Screening full text articles, combined with online searches and information sent by managers of individual registries yielded 57 dementia registries (38 identified through database search and 19 identified via search by hand of references, Google and grey literature), 31 of which met the review inclusion criteria presented below (Figure 1).
Figure 1.
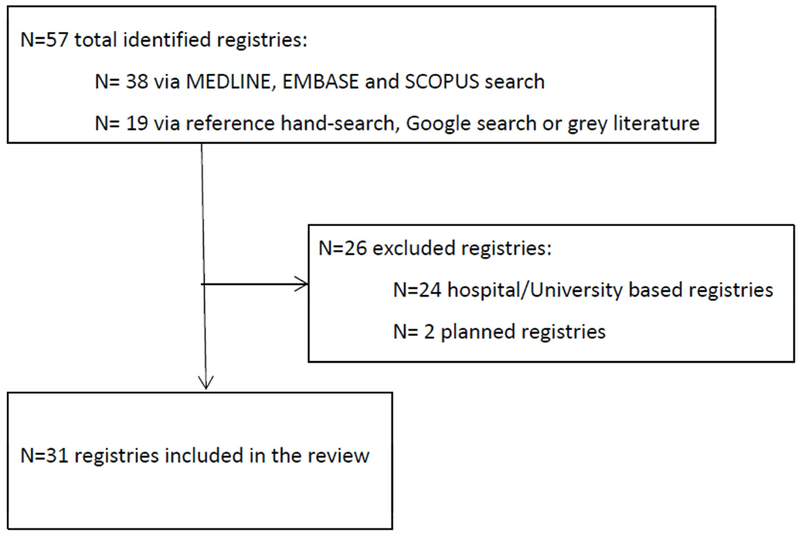
Study inclusion and exclusion criteria flowchart.
2.2. Inclusion and exclusion criteria
We included self-identified “dementia registries”, i.e., systems of ongoing registration of all cases of dementia in a defined population [6], regardless of the aims of a particular registry. Although definitions based on the aims of registries exist, mostly within national frameworks of quality of health care [28–30] and public health surveillance [31], we adopted a broad approach in order to explore the full range of existing dementia registries.
Our review included registries which a) collected information on patients with a diagnosis of AD/dementia, individuals at risk of developing dementia/AD, caregivers of people with AD/dementia, and/or control subjects without AD/dementia willing to be recruited into dementia studies; b) had been in operation at any time up to August 2016; and c) collected data at an international, national or local/regional/state-wide level. We excluded AD/dementia registries with restricted local coverage, such as hospital/clinic-based and university-based case registries, as higher levels of geographical coverage of a registry are more likely to be more representative of the whole eligible population [32]. We also excluded planned registries which had not been operational over the period covered by the review. To simplify presentation of results and discussion, the general term “dementia registry” (inclusive of AD-specific registries) is used in the text.
2.3. Data extraction
Based on retrieved peer-reviewed publications and grey literature, dementia registries meeting inclusion criteria were further analyzed to obtain information regarding ten characteristics (Table 2). These were the type of registry, registry coverage, language in which data are collected, year registry was established (or where applicable, period covered by registry data collection), most recent number of registered participants, dementia type, diagnostic system, recruitment setting, type of consent procedure, registry population and type of collected data. We included a descriptive review of benefits and outcomes of the dementia registries.
Table 2.
Data elements included in the review
| Category | Data elements |
|---|---|
| Type of registry | dementia research registry (including a preclinical dementia research registry), epidemiological dementia registry, quality of dementia care registry, dementia research volunteer registry |
| Registry coverage | international, national, local/state-wide |
| Language of collected data | Language of collected data |
| Year registry was established (or where applicable, period covered by data collection) | year, time period |
| Number (N) of registered participants | N (most recent) |
| Dementia type | dementia, AD (if other, specify) |
| Diagnostic system | ICD, DSM, NINCDS-ADRDA, not applicable |
| Recruitment setting | health care services, awareness campaign/general population, data linkage, other |
| Consent | opt-in, opt-out, other, info not available (if other, specify) |
| Registry population | patients with a diagnosis of AD/dementia, carers, family members of people with AD/dementia, individuals at risk of developing dementia/AD, normal (i.e., no diagnosis of dementia/AD) control subjects |
| Type of collected data | cognition, demographics, function, imaging and other biomarker measures, other (e.g., caregiver burden, BPSD symptoms) |
DSM - Diagnostic and Statistical Manual of Mental Disorders; ICD - International Classification of Diseases; NINCDS-ADRDA – the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria
Four categories of dementia registries, related to registry aims, were used in the review, based on the literature on patient/clinical registries [28–30] and dementia registries [20–24]: dementia research registries, epidemiological dementia registries, quality of dementia care registries and dementia research volunteer registries (Table 1). Preclinical dementia research registries, i.e. registries, which aim to optimize conduct of clinical trials for interventions in the pre-dementia phase of AD, were included as a subcategory of dementia research registries.
3. Results
3.1. General overview of dementia registries
Thirty-one registries met inclusion criteria for the review (Table 3). We excluded 24 hospital-based or university-based registries: 14 in the USA, 2 in the UK, 2 in South Korea and individual registries in Canada, India, Italy, Pakistan, Japan and Taiwan because of their limited geographical coverage and two planned, but not operational national dementia registries (Cuba and the Netherlands). (The list of excluded registries is provided in Appendix 1).
Table 3.
Characteristics of dementia registries
| Registry name (country) | Coverage | Language of collected data | Established in year | N (most recent) | Dementia type | Diagnostic system | Recruitment setting | Consent | Registry population | Type of collected data |
|---|---|---|---|---|---|---|---|---|---|---|
| Dementia research registry | ||||||||||
| Prospective Dementia Registry Austria (PRODEM) (Austria) (Seiler et al., 2012) | National | German | 2008 (ongoing) | N=500+ (2014) | Dementia | DSM-IV | HCS | Opt-in | PWD, C | Cog, Dem, Funct, IBM, Other |
| the IMAGE Project Population-Based Registry of AD (Canada) (De Braekeleer et al., 1989) | Local (Quebec) | French | 1986 (no longer recruiting) | N=194 (definite) N=206 (probable) N=211 possible (1996) |
AD | NINCDS-ADRDA | HCS | Opt-in | PWD | Cog, Dem, Funct, IBM, Other |
| Bavarian Dementia Survey (BayDem) (Germany) (previously: the Erlangen Dementia Registry) (Schaller et al., 2015) | Local (Bavaria) | German | 2012 (ongoing) | N=91 (2012-2014) | Dementia | ICD-10 | HCS | Opt-in | PWD, C | Cog, Dem, Funct, Other |
| Health and Memory Study of Nord-Trøndelag (Norway) (Bergh et al., 2014) | Local (Nord-Trøndelag) | Norwegian | Data for 1995-2011 | N=1,434 | Dementia | ICD-10 | HCS | Opt-in | PWD | Cog, Dem, Funct, IBM, Other |
| Clinical Research Center for Dementia of South Korea (CREDOS) Study (Park et al., 2011) | National | Korean | 2005 (ongoing) | N=2,967 (2005-2012) |
Dementia | NINCDS-ADRDA/DSM-IV | HCS | Opt-in | PWD, C | Cog, Dem, Funct, IBM, Other |
| Camberwell Dementia Case Register (UK) (Holmes, 1996) | Local (Camberwell) | English | Operating 1993-1995 | N=530 | Dementia | NINCDS-ADRDA/DSM-III-R | HCS | Opt-in | PWD, C | Cog, Dem, Funct, IBM, Other |
| Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) (USA) (Fillenbaum et al., 2008) | National | English | Operating 1986-1994 | N=1,094 (AD), N=463 (control) | AD | NINCDS-ADRDA | HCS | Institutional Review Board approval | PWD, NC | Cog, Dem, Funct, IBM, Other |
| National Alzheimer’s Coordinating Center (NACC) Database (USA) (Beekly et al., 2004) | National | English | 1999 (ongoing) | N=74,397 (MDS), N=33,900 (UDS) (2016)** | AD/related disorders | NINCDR or DSM | HCS | Institutional Review Board approval | PWD | Cog, Dem, Funct, IBM, Other |
| Wisconsin Registry for Alzheimer Prevention (WRAP) (USA) (Sager et al., 2005) | Local (Wisconsin) | English | 2001 (ongoing) | N > 1,500 (2016) | AD | NINCDS-ADRDA | HCS | Opt-in | FAM, NC | Cog, Dem, Funct, IBM, Other |
| Preclinical dementia research registry | ||||||||||
| Alzheimer’s Prevention Initiative (API) Colombian Registry (Lopera et al., 2013; Reiman et al., 2011) | Local (Antioquia) | Spanish | 2010 (ongoing) | N=2,092 (2010-2012) | Autosomal Dominant AD | n/a | HCS | Opt-in | FAM | Cog, Dem, Funct, IBM, Other |
| European Prevention of Alzheimer’s Dementia (EPAD) (Ritchie et al., 2016) | International | International project | 2015 (ongoing) | Aim: N=24,000 (registry) N=6,000 (longitudinal cohort) N=1,500 (trials) |
AD | n/a | Other | Opt-in | AT-RISK | Cog, Dem, Funct, IBM, Other |
| Global Alzheimer Platform (GAP) Trial Ready Cohort for Preclinical/Prodromal Alzheimer’s Disease (TRC PAD) (USA) (Cummings et al., 2016) | National | English | 2013 (ongoing) | Aim: N=1,000 (preclinical) N=1,000 (prodromal) |
AD | n/a | Other | Opt-in | AT-RISK | Cog, Dem, Funct, IBM, Other |
| Dominantly Inherited Alzheimer Network (DIAN) Expanded Registry (Morris et al., 2012; Moulder et al., 2013) | International | International project | 2008 (ongoing) | N = 336 (2013) | Autosomal Dominant AD | n/a | HCP, AC/GP | Opt-in | FAM | Cog, Dem, Funct, IBM, Other |
| Epidemiological dementia registry | ||||||||||
| Cognitive Impairment Centralized Case Registry in Argentina (ReDeCAr) (Allegri, 2011) | National | Spanish | 2010 (pilot) | N=292 (2010) | Dementia | DSM-IV-TR/ICD-10 | HCS | Opt-in | PWD | Cog, Dem, Funct, Other |
| French National Alzheimer Database (Anthony et al., 2014) | National | French | 2009 (ongoing) | N=760,120 (since 2009) | AD/related disorders/Mild cognitive impairment | ICD-10 | HCS | Not required | PWD | Cog, Dem, Funct, Other |
| Experimental Registry for AD/other Dementias (Italy) (Francesconi et al., 2007) | Local (Tuscany) | Italian | Data for 1999-2005 | N=47,889 | Dementia | ICD-9 | DL | Info not available | PWD | Dem, Other |
| Registry of Dementia of Girona (ReDeGi) (Spain) (Garre-Olmo et al., 2009) | Local (Girona) | Spanish | 2007 (ongoing) | N=4,314 (2007-2012) | Dementia | DSM-IV-TR | HCS | Opt-in and opt-out | PWD | Cog, Dem, Funct, Other |
| AD/Related Dementia State Registry (USA) (Weeks Leonard et al., 2016) | State-wide (Georgia) | English | 2014 (ongoing) | N=112,430 (2013) | AD/related dementias | ICD-9, ICD-10 | DL | Not required | PWD | Dem, Other |
| New York State Department of Health AD/Other Dementias Registry (USA) (Lillquist, 2004) | State-wide (New York) | English | 1988 (ongoing) | Info not available | AD/dementia | ICD-9-CM | HCS | Info not available | PWD | Dem, Other |
| South Carolina AD Registry (USA) (Macera et al., 1991) | State-wide (South Carolina) | English | 1988 (ongoing) | N=225,938 | AD/related disorders | ICD-9-CM | DL | Info not available | PWD | Dem, Other |
| West Virginia AD Registry (USA) (Schreurs, 2010) | State-wide (West Virginia) | English | 2011 (ongoing) | N= 28,000 (2015) | AD/related disorders | ICD-9-CM, ICD-10-CM | HCS, DL | Not required | PWD | Cog, Dem, Funct, Other |
| Quality of dementia care registry | ||||||||||
| Danish Dementia Registry* (Johannsen et al., 2011) | National | Danish | 2005 (ongoing) | N=6,576 (2007-2012) | Dementia | ICD-10 | HCS | Not required | PWD | Cog, Dem, Funct, Other |
| Norwegian Dementia Registry* (NorKog) (Persson et al., 2015) | National | Norwegian | 2013 (ongoing) | N=3,633 (2014) | Dementia | ICD-10 | HCS | Opt-in | PWD, C | Cog, Dem, Funct, IBM, Other |
| Swedish Dementia Registry (SveDem) (Religa et al., 2015) | National | Swedish | 2007 (ongoing) | N=58,823 (2016) | Dementia | ICD-10 | HCS | Opt-out | PWD | Cog, Dem, Funct, Other |
| Swedish Behavioural and Psychologic al Symptoms of Dementia (BPSD) Registry (Mayer et al., 2014) | National | Swedish | 2010 (ongoing) | N=23,311 (2015) | Dementia | ICD-10 | HCS | Opt-out | PWD | Dem, Other |
| Dementia research volunteer registry | ||||||||||
| CHARIOT (Cognitive Health in Ageing Register: Investigational, Observational and Trial studies in dementia research) (UK) (Larsen et al., 2015) | Local (London) | English | Data for 2011-2014 | N > 25,000 (2016) | Dementia | n/a | HCS | Opt-in | NC | Dem, Other |
| Scottish Dementia Research Interest Register (SMRIR) (UK) (Russ et al., 2015) | Local (Scotland) | English | 2008 (2015: merged with JDR) | N=1,427 (carers), N=1,401 (patients) (2014) | Dementia | n/a | HCS, Other | Opt-in | PWD, C | Cog, Dem, Funct, Other |
| Dementia Register (DemReg) - Dementia Research Registry North Thames DeNDRoN and the EVIDEM (Evidence-based Interventions in Dementia) programme (UK) (Illife et al., 2011) | Local (North Thames) | English | 2009 (ongoing) | N=~ 250 (2010) | Dementia | ICD-10, DSM-IV, other | HCS | Opt-in | PWD | Cog, Dem, Funct, Other |
| Join Dementia Research (JDR) (UK) (https://www.joindementiaresearch.nihr.ac.uk/ | National | English | 2015 (ongoing) | N > 20,000 (2016) | Dementia | n/a | AC/GP | Opt-in | General population | Dem, Other |
| Alzheimer’s Prevention Registry (APR) (USA) (https://www.endalznow.org/) | National | English | 2012 (ongoing) | N > 200,000 (2016) | AD | n/a | AC/GP | Opt-in | General population | Dem, Other |
| Arizona Alzheimer Registry (USA) (Saunders et al., 2014) | State-wide (Arizona) | English | 2006 (2012: merged with APR | N=1,182 (2006-2011) | Cognitive impairment, dementia | n/a | AC/GP | Opt-in | General population | Cog, Dem, Funct, Other |
Originally established as a local registry;
MDS = Minimum Data Set, UDS = Uniform Data Set
Recruitment setting: DL = data linkage, AC/GP = awareness campaign/general population, HCS = health care setting, Other
Registry population: AT-RISK = individuals at risk of dementia/AD, C = carers, FAM = family members of patients with dementia/AD, PWD = patients with dementia/AD, NC = normal (i.e., no diagnosis of dementia/AD) control subjects
Type of data: Cog = Cognition, Dem = Demographic, Funct = Function, IBM= Imaging and other biomarker measures, Other, including caregiver burden, quality of life, behavioural and psychological symptoms of dementia
The 31 reviewed dementia registries were established in 14 countries, of which 24 were established between 2000 and 2015. They were in Europe (n=15), followed by North America (n=11), South America (n=2) and Asia (n=1). The review identified two international (both preclinical research) registries: a European collaboration (European Prevention of Alzheimer’s Dementia; EPAD) and a collaborative project involving countries in North America, South America, Europe, Asia and Australia (Dominantly Inherited Alzheimer Network (DIAN) Expanded Registry). In four countries there was more than one dementia registry - USA (n=10), UK (n=5), Sweden (n=2) and Norway (n=2) - with varying levels of coverage.
The review identified registries with different levels of geographic coverage (international, national and regional/local/state-wide). National dementia registries were established in Argentina, Austria, France, South Korea and Sweden. In Canada, Colombia, Germany and Spain, dementia registries covered specific geographic and/or administrative regions within the country. In Norway, the UK and the USA, there were multiple registries operational on both national and local levels.
In addition, in Norway (initially the South-East and West Health Regions) and in Denmark (initially Copenhagen) local dementia registries over time evolved into national registries, and local registries in the UK (Scotland) and the USA (Arizona) merged with national registry initiatives. The characteristics of dementia registries included in the review are presented in Table 3. Figure 2 captures the registries ranging from the least specific (i.e. health aging/research volunteer registries) to the most specific (AD only registries).
Figure 2.
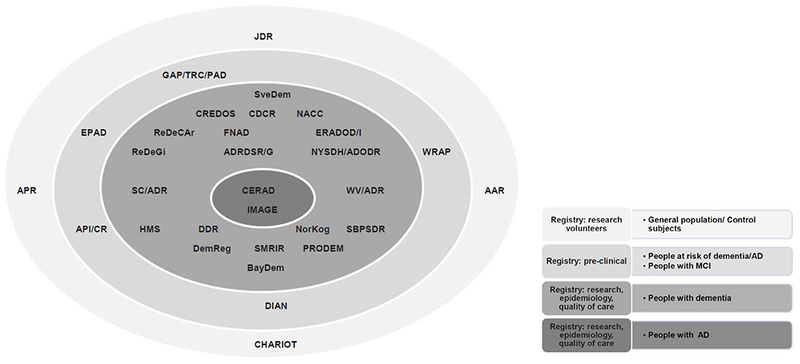
Populations captured in existing dementia registries.
AAR - Arizona Alzheimer Registry; AD/RDSR/G - AD/Related Dementia State Registry (Georgia); API/CR - Alzheimer’s Prevention Initiative Colombian Registry; APR - Alzheimer’s Prevention Registry; BayDem - Bavarian Dementia Survey; CDCR - Camberwell Dementia Case Register; CERAD - Consortium to Establish a Registry for Alzheimer’s Disease; CHARIOT - Cognitive Health in Ageing Register; CREDOS - Clinical Research Center for Dementia of South Korea; DemReg - Dementia Research Registry North Thames DeNDRoN/EVIDEM; DDR - Danish Dementia Registry; DIAN - Dominantly Inherited Alzheimer Network; EPAD - European Prevention of Alzheimer’s Dementia; ERADOD/I - Experimental Registry for AD/other Dementias in Italy; FNAD - French National Alzheimer Database; GAP/TRC/PAD - Global Alzheimer Platform Trial Ready Cohort for Preclinical/ Prodromal AD; HMS - Health and Memory Study of Nord-Trøndelag; JDR - Join Dementia Research; IMAGE - IMAGE Project Population-Based Registry of AD; NACC - National Alzheimer’s Coordinating Center; NorKog - Norwegian Dementia Registry; NYSDH/ADODR - New York State Department of Health AD/Other Dementias Registry; PRODEM - Prospective Dementia Registry Austria ; ReDeCAr - Cognitive Impairment Centralized Case Registry in Argentina; ReDeGi - Registry of Dementia of Girona; SC/ADR - South Carolina AD Registry; SveDem - Swedish Dementia Registry; SBPSDR - Swedish Behavioural and Psychological Symptoms of Dementia Registry; SMRIR - Scottish Dementia Research Interest Register; WV/ADR - West Virginia AD Registry; WRAP - Wisconsin Registry for Alzheimer Prevention
3.2. Research dementia registries
Thirteen dementia registries, including four preclinical research registries, focused on dementia research. Dementia research registries were the first dementia registries ever established: CERAD (data collected over 1986-1994) [14] and NACC (data collection ongoing since 1999) [15] in the USA, IMAGE in Canada (est. 1986, no longer recruiting) [16] and Camberwell Dementia Case Register in the UK (data collected over 1993-1995) [17]. CERAD’s aim was to develop standardized assessments for patients with AD based on longitudinal data collected from more than 1,000 dementia patients and almost 500 healthy control subjects recruited by the Alzheimer’s Disease Research Centers and university programs in the US. NACC is a central database in the USA collecting standardized information on all patients at the Alzheimer’s Disease Centers. Since its inception, NACC collected, first a cross-sectional, retrospective Minimum Data Set (MDS) (n~65,000), and then a prospective, standardized, and longitudinal clinical evaluation data, which still continues, the Uniform Data Set (UDS) (n~35,000); and also detailed neuropathological data, the Neuropathology Data Set (n~15,000 from both MDS and UDS).
Other dementia research registries were established from 2001 onwards in regions of Germany (Bavarian Dementia Survey – BayDem) [33], Norway (Health and Memory Study of Nord-Trondelag) [34], and USA (Wisconsin Registry for Alzheimer Prevention - WRAP) [35], and nationally in Austria (Prospective Dementia Registry Austria – PRODEM) [36] and South Korea (Clinical Research Center for Dementia of South Korea (CREDOS) Study) [37]. Almost half of the research registries were set up as longitudinal cohort studies. BayDem, CREDOS and PRODEM recruited patients with dementia and their caregivers through hospitals, specialist clinics, medical professionals, and aged care services, and collected data on demographics, cognition, function, bio-banking and other data, including BPSD and past medical history. Additionally, BayDem and PRODEM assessed caregiver burden related to dementia. The WRAP longitudinal registry recruited adults with a parent with autopsy-confirmed or probable AD and control participants (parents without AD/dementia) through health professionals, registry website or word of mouth. The WRAP registry collected data of similar type and scope as other longitudinal registry-based studies. Characteristics of research registries differed in the numbers of participants enrolled, diagnostic systems, and type of data collected (see Table 3). All dementia research registries actively seek consent (opt-in) from patients with dementia, and where applicable, their caregivers.
3.3. Preclinical dementia research registries
We identified four registries in this category: the Dominantly Inherited Alzheimer Network (DIAN) Expanded Registry [38], the Alzheimer’s Prevention Initiative (API) Colombian Registry [39], the European Prevention of Alzheimer’s Dementia (EPAD) project [40] and the Global Alzheimer Platform (GAP) Trial Ready Cohort for Preclinical/Prodromal Alzheimer’s Disease (TRC PAD) [41]. Preclinical registries are relatively new having been established from 2008 onwards. They recruit healthy individuals who are at high risk of developing symptomatic AD, including autosomal dominant Alzheimer’s disease (ADAD). Drawing on existing national and regional registers, EPAD develops a shared virtual registry, from which individuals are recruited into the Longitudinal Cohort Study and further, a trial of interventions that could delay (or even prevent) the onset of dementia [40]. Similarly, the TRC PAD GAP Cohort of preclinical and prodromal participants is identified primarily through existing dementia registries in USA, including the Alzheimer’s Prevention Registry [41]. The API Colombian Registry was established in the region of Antioquia, in close collaboration with the US-based Alzheimer’s Prevention Initiative, and GAP TRC PAD is a national project in the USA. DIAN and EPAD are international collaborations.
3.4. Epidemiological dementia registries
Eight registries collected epidemiological data on dementia, two with national coverage (Argentina and France) [42,43] and six which covered a particular region of the country (Italy, Spain and four registries in the USA) [44–49]. The first epidemiological dementia registries were set up in 1988 in USA and had state-wide coverage (New York State and South Carolina) and the other registries were established from 2005 onwards. Three registries identified patients with diagnosis of collaborations. dementia through a process of data linkage; the others identified eligible patients through healthcare services, including specialized clinics and general practitioners. Epidemiological registries focused primarily on collecting demographic and diagnostic data on patients with dementia/AD. Half of epidemiological registries also collected information on function, cognition, and other information, such as brief medical history, medication, or date of entry into residential care (where applicable). All registries, except for a pilot national registry in Argentina (Cognitive Impairment Centralized Case Registry in Argentina; ReDeCAr) and Registry of Dementia of Girona (ReDeGi), included over 10,000 patients with dementia, with numbers of registrants ranging between 28,000 (West Virginia, since 2011) and 225,938 (South Carolina, since 1988). Epidemiological dementia registries use different models of consent, including mandatory reporting (e.g., West Virginia), waiver of consent (e.g., the French National Alzheimer database) or informed consent (opt-in) (e.g., ReDeCAr and RedeGi).
3.5. Quality of dementia care registries
The review identified four registries collecting data on quality of care for dementia patients clustered in three Scandinavian countries: Denmark [50], Norway (NorKog; Norwegian Dementia Registry) [51] and Sweden (Swedish Dementia Registry – SveDem) [52]; and Swedish PBSD Registry [53]). These registries were established from 2005 onwards and in 2016 all registries collected data on a national level. Two registries (SveDem and the Danish Dementia Registry) evaluate the quality of dementia care based on adherence to national guidelines, using dementia-related quality indicators (eight indicators in Denmark and seven in Sweden). All registries include data on patients with dementia diagnosed and identified through health care and aged care services, including specialized clinics and nursing homes. Except for the Swedish BPSD Registry, all quality of care registries collect data on demographics, dementia diagnosis, function, cognition, as well as other data, such as medication, possession of driving license, and date of entry into residential care (if applicable). The Swedish BPSD Registry focuses on management of behavioral and psychological symptoms of dementia, and collects data using the Neuropsychiatric Inventory [54]. The numbers of registrants in quality of care registers range between approx. 3,500-6,500 (dementia registries in Denmark and Norway collecting data on regional level) and approx. 23,000-59,000 (two national registries in Sweden).
Quality of care dementia registries use various models of consent. The SveDem and the BPSD Registry operate within the framework of Swedish national quality registries [30] and require that patients are informed about collection of their data and have an option of actively opting-out. In Denmark, collection of data in clinical quality databases approved by the Danish National Board of Health, including the National Dementia Registry, does not require consent of an individual patient. In Norway, written informed consent from a patient diagnosed with dementia (or a relative, if the patient is not able to provide consent) is required to include patient’s data in the National Dementia Registry.
3.6. Dementia research volunteer registries
Six registries recruited volunteers for dementia studies; four in the UK: Dementia Register (DemReg) - Dementia Research Registry North Thames DeNDRoN and the EVIDEM (Evidence-based Interventions in Dementia) program [55], Scottish Dementia Research Interest Register [56], CHARIOT (Cognitive Health in Ageing Register: Investigational, Observational and Trial studies in dementia research) [57] and Join Dementia Research (https://www.joindementiaresearch.nihr.ac.uk); and two in the USA: Alzheimer’s Prevention Registry [58] and Arizona Alzheimer Registry [59]. These registries were established between 2006 and 2015, and over this time two local volunteer registries, operating in Scotland and Arizona, merged with new national registry initiatives (Join Dementia Research in the UK and Alzheimer’s Prevention Registry in the US, respectively).
Depending on the scope of intended dementia research, registries include patients with a diagnosis of dementia, their caregivers and/or healthy volunteers, i.e., individuals without a diagnosis of dementia. Eligible individuals are identified and recruited through primary and secondary care, social and community services, as well as community awareness campaigns, local champions, consumer organizations, user-friendly websites and social media. Registries recruiting through the general population collect, often through their website, demographic and contact information, and sometimes also basic information about medical and family history related to dementia. Registries recruiting via health providers usually collect more detailed data in regards to cognitive and functional status, as well as data on medications and medical investigations, such as imaging.
Depending on the size of the population targeted by the registry, recruitment strategy, and the type of registry population, number of research volunteers recruited to local registries range between 250 (DemReg) and over 25,000 (Arizona Alzheimer Registry). National dementia registries in the UK (Join Dementia Research) and the USA (Alzheimer’s Prevention Registry) recruited approx. 16,000 (2015-mid 2016) and approx. 215,000 (2012-mid 2016) research volunteers respectively.
3.7. Benefits and outcomes
Data collected by research registries and related longitudinal studies provide information on etiology and natural history of dementia, and the relative influence of environmental, biological and genetic factors [17,18,37]. Dementia research registries informed diagnostic and therapeutic practice [14,15], and help to assess physical, psychological and economic burden on caregivers [33,36]. Preclinical registries allow testing potential disease-modifying treatments, such as anti-amyloid drugs, in individuals at high risk of AD because of a genetic profile or a biomarker burden [2,40]. Research registry data have also been enriched by linkage to other research or administrative datasets [60]. In addition, the few operating quality of care, research, and volunteer registries actively survey caregivers of people with dementia and collect information on their needs and caregiving burden. Comparison of actual diagnostic and care practices against published recommended practices and quality indicators afforded an opportunity to highlight discrepancies and improve clinical practice [50,52]. Research volunteer registries raise community awareness of dementia research, care and prevention, and provide an opportunity for members of the public, including the “worried well” and caregivers, to participate in studies, and to the long-term knowledge translation resulting in improvement in dementia diagnosis, management and care.
Researchers have benefitted by having access to research-ready, and often prescreened, populations, to assess more accurately the feasibility of the study [55]. In addition, projects such as the Brain Health Registry in the USA (http://brainhealthregistry.org/) and the Neuroscience Research Australia (NeuRA) Research Volunteer Registry (https://www.neura.edu.au/volunteer/) helped in recruitment of research volunteers for prevention and treatment studies for a broad range of brain disorders, including AD, Parkinson’s disease, depression, schizophrenia and post-traumatic stress disorder. Prospective trial participants can have access to information about studies through registry websites or directly by email.
Dementia registries have proved useful epidemiological tools informing planning of services and health care policy, and complementing epidemiological studies, such as the Rotterdam Study [61]. Data linkage with health and/or administrative databases improves registration of people with dementia, although problems remain with sensitivity (i.e., completeness) and specificity of registry data [45]. For instance, the Experimental Registry for Alzheimer’s Disease and Other Dementias in Tuscany [45] enabled population-based analytical epidemiological studies and analyses of targeted health and social services for dementia.
Quality of care and epidemiological registries have proved to be important sources for local/national governments, policy makers, and health economists to assist with planning, provision and assessment of outcomes for service delivery in dementia [30]. Registry data have also provided information to calculate the costs of diagnostic procedures for dementia patients. In Sweden in 2010 the estimated average costs of dementia diagnosis per patient based on the registry data were US$968 in primary care and US$1,669 in specialist care [62]. In this context, it is important to consider the representativeness of the population of people with dementia captured by a registry.
4. Discussion
Our review reports the design, operation, recruitment, geographical coverage, and type and number of participants in 31 dementia registries world-wide. More than half of reviewed dementia registries aim at conducting or facilitating research, including clinical trials for interventions in the pre-dementia phase of AD and recruiting volunteers for dementia studies. Other registries collect epidemiological dementia data or data on quality of care. Due to their wide application and purposes, dementia registries facilitate better diagnosis, management and care of people with dementia, as well as caregiver support, across the course of the illness. Consequently, registries can significantly contribute to the reduced cost of dementia and improve standards of diagnosis and care [52]. For instance, an NACC data study comparing clinical diagnosis of AD with neuropathologic results at autopsy reported on inappropriate medication regimen in misdiagnosed patients, which could adversely affect their health outcomes and increase healthcare costs [63].
Registries provide well-established infrastructure for recruiting and following patients in randomized clinical trials and other types of studies in every-day healthcare environment [55,64]. They help to reduce barriers to research recruitment and its cost, which are major challenges to successful implementation of national initiatives, such as the US National Plan to Address Alzheimer’s Disease aiming to prevent and effectively treat AD by 2025 [19]. Recent developments in the broader field of patient registries also offer new and exciting possibilities for the use of registry data, including dementia registries. An example is a new clinical paradigm – a registry-based randomized controlled trial (RRCT) [64]. Through inclusion of a randomization module, the RRCT model combines the advantages of a prospective randomized trial and a large-scale clinical registry based on unselected consecutive enrolment. This novel paradigm was successfully applied in cardiology and was completed with over 90% cost saving, compared with a conventional RCT, due to limited number of patient visits, lower patient attrition and use of existing collaborative networks [65].
There is an ongoing need for improvements in the quality of assessment, diagnosis, management and care. The World Health Organisation (WHO) [66] stressed the role of clinical practice guidelines in helping to improve the average quality and consistency of care. Dementia registries are valuable tools for monitoring the uptake and quality of use of clinical practice guidelines in everyday practice. For instance, there have been significant improvements in the quality of dementia care related to operation of the Swedish Dementia Registry (SveDem); between 2011 and 2015. In primary health care centers which joined the registry, the percentage of complete basic investigations increased by 23% and diagnosis of “dementia not otherwise specified” decreased by 15% [67].
Cancer and cardiovascular disease registries have been trailblazers for dementia registries. The models and problems related to registries for cardiovascular diseases may be more closely related to dementia and AD than the model of a cancer registry [22]. The latter are usually successful as treatment usually occurs in hospitals and requires a pathologic or surgical diagnosis, which is available for all cases. Recent developments leading to more explicit understanding of the preclinical phase of AD and other dementias generate unique opportunities, harnessed by innovative registries optimizing conduct of clinical trials for interventions in the pre-dementia phase of AD, such as DIAN, API, EPAD and GAP TRC PAD. Nonetheless, a significant limitation of dementia registries is that over half of people with dementia [2] do not have a formal diagnosis, this being different from registries for research on cancer or stroke. Also, some dementia registries recruit research volunteers, including “worried well” older adults. This is in contrast to registries on other chronic conditions, which include persons with a formal diagnosis.
Despite these many tangible outcomes and significant progress in the field, three major deficiencies in regards to existing dementia registries stand out: no coverage in many countries, limited possibilities of data sharing between existing data collection systems (i.e., interoperability) and lack of available data on the costs of operating dementia registries and their cost-effectiveness.
Dementia registries cluster in North America, mostly the USA, and Western Europe, mostly in Scandinavia and the UK, with only three registries operational in other parts of the world (Argentina, Colombia, and South Korea). This is of concern as about 60% of people with dementia live in low and middle income countries (LMIC), and the proportionate rise in the prevalence of dementia between 2015 and 2050 is expected to be twice as high in these countries than in high income countries (264% and 116%; respectively) [1]. The WHO [66] recommends a range of actions to improve care and services for people with dementia and their caregivers worldwide. These include strengthening of health systems, developing and implementing dementia policies and plans, supporting dementia research, advocacy and awareness-raising, which can be addressed, among others, by dementia registries. In addition, the recently established WHO Global Dementia Observatory will promote planning and monitoring of strategic objectives, such as establishment of national dementia plans and policies, and will support surveillance systems providing data on the burden of dementia. Development of dementia registries in LMIC countries could support care systems and research, and help to put dementia on public health agenda.
There is also significant heterogeneity regarding the aims and characteristics of dementia registries, which rules out comparing registries in different categories, and often even registries within a particular category. Understandably, most registries were set up as individual data collection systems, designed to operate independently and not with the explicit aim of comparing datasets internationally. Nonetheless, as much as possible and feasible, standardization of registries and datasets is the sine qua non of truly international initiatives in dementia research in the era of big data. Data harmonization also allows comparisons between countries and regions with and without registries, providing the much needed, and currently limited, data on outcomes and cost-effectiveness of registries. International collaboration is facilitated by standardizing and integrating existing datasets, harmonization of ongoing international research initiatives (i.e., interoperability), and defining international data-sharing guidelines, including the challenges of informed consent [25]. The EPAD project [40] and the International Database for Longitudinal Studies on Aging and Dementia (IDAD) [4] are examples of initiatives aiming at integrating existing international datasets.
Set-up and running costs of existing dementia registries are not publicly available. One estimate from US is that the costs of establishing the Brain Health Registry and Alzheimer’s Prevention Initiative were millions of dollar and of maintenance, hundreds of thousands annually (Personal communication, M. Wiener, 2017). EPAD aims to recruit 24,000 participants from existing cohorts/registers (which reduces the costs of recruitment). The total cost of EPAD is €64 million/US$ 68 million over five years which includes €15 million in cash and €21 million in kind from pharmaceutical companies (Personal communication, C. Ritchie, 2017). The virtual EPAD register budget is approximately €1.5 million / US$ 1.6 million over 5 years from the European Union with 20% extra coming from pharmaceutical companies; a major cost is the development of the IT platform. In France the national AD bank derived from memory clinic intake approximately cost about €1.5 million (US$1.6) for data entry annually, €1 million (US$1.06) for development of the software for data base management and €300,000 pa for IT support queries and analyses (Personal communication, K. Ritchie and A. Gabelle, 2017). Based on the French data, a 50-year investment, the required total cost would be approximately US$ 97 million / €91 million, although costs could be 2-3 times higher and will depend on the size of the population covered and the comprehensiveness of the data base and follow-up intervals. While registries offer participants the opportunity to participate in scientific drug trials there is the possibility of ‘leakage’ to for-profit interests. Ethical guidelines are necessary, and some registries, such as SveDem, choose not to have sponsors from the pharmaceutical industry [52].
The substantial global cost of dementia, estimated at $818 billion [1], calls for development of cost-effective medical and social care interventions and evidence-based prevention strategies [62]. No study to evaluate the cost-effectiveness of dementia registries was located despite evidence from other areas of medicine, such as hip arthroplasty, cystic fibrosis, cancer, ophthalmology, and rheumatology that patient registries can significantly contribute to lowering the costs of health care [13,68]. For instance, in the USA, a cystic fibrosis registry contributed to the saving of US$23m p.a. in avoided infection-related costs, which equates to approximately 2% of the total costs of care for the condition [13]. Economic evaluation of five clinical quality registries in an Australian review [68] showed a relatively low cost for improvement in clinical practice, resulting in significant positive return on investment. The benefit-to-cost ratios for the return on investment ranged from 2:1 (Victorian Prostate Cancer Registry, net benefit AU$ 2.4 million / approx. US$ 1.8 million / Euro 1.7 million over 2009-2013) to 7:1 (Australia and New Zealand Dialysis and Transplantation Database, net benefit AU$ 49 million / approx. US$ 36.75 million / Euro 34.6 million over 2004-2013). If state-level registries could reach full national coverage, the minimum expected benefit-to-cost ratio was estimated to increase to 4:1 [68]. The Australian review emphasized that registries deliver significant value for money when “correctly implemented and sufficiently mature” [68 p3]. A caveat to drawing conclusions from other disease registries is that dementia unlike many other conditions has no disease modifying treatment yet.
The cost-effectiveness of dementia registries is linked to the need for sustainability in development and operation of registries, and efforts to attract (or leverage) registry resources to facilitate future self-financing. For instance, one of the aims of the EPAD Project is development of a sustainability business plan extending beyond the time frame of the project [69]. The business plan calls for an analysis of relevant public-private partnership collaborative models, and an analysis of stakeholders’ interests and incentives towards sustainability, Continuation of EPAD also requires development of sustainability procedures for the local trial delivery centers infrastructure. Similarly, in the US, the GAP TRC PAD initiative aims to establish a long-term sustainability business model involving two-fold financial support from pharmaceutical companies: upfront investment in the infrastructure and reimbursement based on trial enrollment [70].
5. Limitations
Several limitations of the systematic review are noted. First, the review included self-identified “dementia registries”, regardless of the aims of a registry. While this broad approach allowed exploring all categories of dementia registries, it resulted in heterogeneity. Secondly, to promote clarity, the review presented four mutually exclusive categories of dementia registries; in reality the situation is more complex. Although some registries operate within particular frameworks, which clearly define registry aims, e.g., the Scandinavian registries for the quality of care or the preclinical research registries, other registries, such as the Alzheimer’s Prevention Registry, can be included in more than one category (e.g., a preclinical registry and a research volunteer registry). Thirdly, the review is based on material retrieved through database search of peer-reviewed publications in English and grey literature identified through online searches and contact with registry managers. It is possible that the review excluded some eligible dementia registries not identified through the searches. Nonetheless, the search of resources available in English identified registries collecting original data in nine languages (Danish, English, French, German, Italian, Korean, Norwegian, Spanish and Swedish).
Fourth, registries vary in regards to the level of detail, completeness, and accuracy of the collected data. While methodology exists to evaluate coverage and accuracy of clinical databases [32] and surveillance systems [71], available data currently precludes such an analysis. Given the variable amount of information on methods used to construct different dementia registries presented in the peer-reviewed and grey literature, our review did not include an evaluation of existing registries. Also, methods of identification of patients with dementia and data collection (e.g., via health care services) may result in incomplete or unrepresentative sampling and limited geographic coverage often restricting the possibility of using registry data to provide estimates of incidence or prevalence [43]. For example, a registry which relies on enrolling patients who use health and aged care services, is more likely to have oversample advanced dementia and underrepresent and underestimate the numbers and needs of people in the earlier stages of dementia [40].
Fifth, we may have missed some studies because of the restriction to “registries” and “databases” since that term may not be used consistently for dementia/AD data repositories. Finally, we excluded hospital/clinic-based and university registries due to their limited geographic coverage. Many low and middle-income countries may successfully rely on these registries as the state/country level infrastructure is lacking.
6. Practicalities of hosting a dementia registry and recommendations
The technical, organizational and logistic challenges of establishing and maintaining registries are substantial. Critical elements in setting up a dementia registry follow [5,28,29]:
1. Ensure sufficient funding and sustainability of a registry.
Registries with good coverage and accuracy most likely will require significant and continuing funding. For instance, in Australia, the cost of establishing a major national registry (50,000 cases reported annually), including the cost of the IT systems, has been estimated in 2013 dollars at AU$ 750,000-1 million (approximately US$ 500,000 - 750,000 / Euro 470,000 – 700,000) and the cost of maintaining such a registry at AU$1-1.5 million p.a. (approximately US$ 0.75-1 million / Euro 470,000- 940,000) [28,72]. Specifically for dementia registries, costs vary depending on the cost of recruiting and screening subjects and of developing, launching and growing the registry. Typically registry costs include the online data system, developing and testing the minimum data-set, publicizing the registry, data-collection and reporting, outcome determination (via follow-up or data-linkage), system maintenance and statistical analysis. Funding is also required to cover the costs of the registry governance, collaboration with stakeholders, ethics approvals, and implementation of quality assurance procedures.
Given the high estimated cost of setting up and operating a dementia registry, including long term commitment of stakeholders and sustainability of funding, the essential question is whether a registry is the best method of collecting the data of interest, whether there are cheaper alternatives, and whether the returns on investment can be realized. Also, given the involvement of health care staff in data collection as a part of everyday clinical practice, the amount of mandatory information must be restricted to the minimum. The critical question is “can this [data collection] be done in any other way? If the answer is yes, then it is probable that the register is a luxury” [73 p227]; better recoding of routine hospital, specialist, and primary care consultations may be a much cheaper alternative.
2. Define and document the purpose of the registry.
The intended purpose and available funding determine design and scope of a dementia registry. There are many types of dementia registries, each serving particular purpose(s), and “no single [dementia] registry will be able to solve all problems” [22 p200]. Although categories often overlap, e.g., quality of care registries can provide research data, and epidemiological registries support clinical practice, the purposes of the registry have to be clearly defined and agreed upon by the registry stakeholders. Follow-up procedures, usually annually, need to be made clear at the outset. Also, prespecified indicators of a successful operation of a registry help to assess its effectiveness. For instance, registries monitoring quality of care for dementia patients operating in Scandinavian countries [50–53] monitor and report on adherence to clinical indicators stipulated by national guidelines.
3. Develop leadership/governance structure and registry policies.
Effective operation requires a multidisciplinary set of skills and involvement of a range of stakeholders, including clinicians, researchers, epidemiologists, statisticians, dementia patients and families, policy makers, patient advocacy groups, and regulatory agencies. A registry requires development of policy documents, which provide guidelines regarding ethics, data management, access, and security, communications, publications, reporting, and privacy.
4. Develop adequate infrastructure for data entry, storage and access.
It is recommended that registries use web-based infrastructure, as user-friendly online technology facilitates data collection, processing, and reporting [74]. Use of electronic databases supports data linkage, and where available, allows automatic data capture from existing e-health records and administrative data systems. National legislation and regulations should inform development of registry protocols for safe and secure collection, storage and transmission of electronic data, and address existing limitations of linkage to existing e-health records, such as capture of limited retrospective data and lack of formal diagnosis of dementia in many patients.
5. Identify data sources and data collection procedures.
Collection of data on dementia from multiple, diverse and often dispersed sites, including general practice, memory clinics, independent specialists, and residential aged care facilities, is a significant challenge. This can be addressed by standardizing the process of data collection and data entry through publishing eligibility criteria, metadata, and data dictionaries. A successful registry requires a sustainable workflow model, with minimal disruption, which can be easily integrated into the everyday practice of medical personnel contributing to a registry. The process of data collection must not place an unnecessary burden or financial cost on patients and other users of health services [28]. Integration of the registry infrastructure with the information and communications technology systems already in place within the national healthcare systems (where electronic health records or administrative systems exist) enables automatic data capture, which significantly reduces the burden of data entry.
6. Define the registry population, including case definition.
Use of reliable and valid diagnostic methods for dementia and/or cognitive impairment, such as DSM [75] or ICD [76], ensures collection of quality data. Depending on the aims, some registries may have a limited geographical coverage, limited time period for data collection, or may focus on particular patient attributes, such as a subtype of dementia or age group.
7. Decide on the type of consent process.
The majority of dementia registries actively seek consent from patients for inclusion of data, i.e., opt-in approach; others use an opt-out approach, in which consent for participation is presumed unless a patient expresses a wish not to participate. The latter can increase participation rate in a registry and to minimize recruitment bias, as data are collected from all groups of patients [77].
8. Decide on the scope of data collection and data collection instruments.
Data elements included in a dementia registry should reflect the goals and resources of the registry. Collected data should be acceptable to patients with dementia, their caregivers, and healthcare personnel, and should keep the burden of response and data collection to the minimum. Data collection instruments should be valid, reliable, and standardized across settings or regions.
9. Set up quality control procedures for a dementia registry.
A registry is only as good as the data it collects; it is essential that the registry data are of high quality and serve the registry purposes. Procedures and policies to ensure completeness and validity of data should be developed before the data collection commences, and reviewed at regular intervals. A range of factors ensures the quality of registry data: adherence to the procedures of data collection, clear definition and structure of data elements, sufficient training of the personnel involved in data collection, and good procedures for handling problems with data, such as data entry errors and missing data.
10. Continually re-evaluate purpose and operation of the registry.
The length of time for the planned operation of a registry should be taken into consideration during the initial stages of its development. In general, registries are long-term data repositories and to achieve their purpose(s), such as monitoring of quality of care or collecting data on the natural history of a disease, data must be collected over an extended period of time. This requires ensuring long-term sustainable funding and infrastructure, ideally, supported by policy makers and local/national governments. Registries with good coverage and accuracy require significant and continuing funding. Although registries can be expensive activities, which require considerable investment, their cost needs to be judged against improvements in the quality of healthcare and cost savings gained from the collected data. The Swedish government has recognized quality registries as a priority and has sought to increase necessary funding, which reached $US45m p.a. in 2013 [78]. The operation and value of a registry must be routinely examined to ensure that the purpose still holds and is being achieved. If registry objectives are not met, they “should be revised or the register closed. The least successful registers seem to be those which attempt to meet ill-defined needs” [73 p226]. Where applicable, a plan or criteria for closing a registry should be specified at the commencement of the project.
Several registry handbooks have been developed by professional and academic agencies and are freely available online [29,74,79]. These documents contain detailed information regarding setting up and operation of health registries. As the handbooks are based on national frameworks and policies, adaptation to local settings and circumstances may be required. Some countries, such as Australia [80], have developed operating principles for registries, which may inform national/regional dementia registries.
7. Conclusions
Over the last two decades dementia registries have proven to be versatile tools, which have significantly contributed to dementia research, clinical practice and health policy. Recent developments, such as availability of big data, international collaborations calling for harmonization of research projects and datasets, and increasing focus on preclinical stages of AD, create new and exciting opportunities. In addition, development of dementia registries in low and middle income countries could be a part of dementia awareness rising and could provide tools for improvement in research and clinical services in these regions of the world. Although almost 2 in 3 people with dementia in the world live in low and middle income countries, there is a scarcity of registries in these regions.
Given the tangible practical and economic outcomes for research, clinical practice and policy, further development and growth of dementia registries can be expected and should be supported by policy makers, research funding bodies, clinicians and consumers. The recent evolution in clinical research on AD, i.e., trials of candidate drugs in pre-symptomatic at-risk populations, requires engagement of cohorts identified through existing registers or recruited via research volunteer databases. Standardization of procedures including ethical principles and security of privacy and minimum data sets will assist international collaboration and comparisons.
Patient registries are valuable tools helping to address significant challenges in research, care and policy. Registries, well embedded in many fields of medicine and public health, are relatively new in dementia. This systematic review presents the current situation in regards to dementia registries worldwide. We identified 31 dementia registries operating on an international, national or local level between 1986-2016. More than half of the registries aimed to conduct or facilitate research, including preclinical research registries and registries recruiting research volunteers. Other dementia registries collected epidemiological or quality of care data. We present evidence of practical and economic outcomes of registries for research, clinical practice and policy and recommendations for future development. Global harmonization of recruitment methods and minimum data would facilitate international comparisons. Registries provide a positive return on investment; their establishment and maintenance require ongoing support by government, policy makers, research funding bodies, clinicians, individuals with dementia and their caregivers.
ACKNOWLEDGEMENT:
A Scoping Project for a National Dementia Registry in Australia has been funded by the Dementia Collaborative Research Centre - Assessment and Better Care, University of New South Wales (UNSW), Australia, with financial support from Alzheimer Disease International and The Global CEO Initiative on Alzheimer’s Disease CEOi. The study is supported in kind by Centre for Healthy Brain Ageing, UNSW Sydney Australia.
Appendix 1. Definitions and Classifications of Registries
The many definitions and classifications of registries [1] are typically embedded within broader frameworks and models of public health surveillance and reporting on the quality of health care (Box 1). In general, in epidemiology the term “register” refers to a “file of data concerning all cases of a particular disease or other health relevant condition in a defined population” and a “registry” is a “system of ongoing registration” [2 p211]. Different terms, such as “patient registries”, “clinical registries”, “clinical data registries”, “disease registries”, and “medical registries” have been used internationally to describe registries focused on health information. Terms such as “clinical databases”, “outcomes registries”, “quality registries”, “clinical quality registries”, have been used in reference to registries aiming specifically at clinical audit, i.e., “the routine assessment of the quality of care of health care providers” [3 p131]. In addition, “patient-powered patient registries” (also called “patient-generated”, “patient-run”, “patient-powered, and participant controlled” registries) are data sets managed or controlled by patients and family members affected by a particular medical condition, who also set the research agenda, and decide on the translation and dissemination of the study outcomes based on the collected data [4](Workman, 2013).
Box 1. Examples of frameworks and models of public health surveillance and reporting on the quality of health care.
The US Agency for Healthcare Research and Quality
The US Agency for Healthcare Research and Quality (AHRQ) defines a patient registry as an “organized system that uses observational study methods to collect uniform data (clinical and other) to evaluate specified outcomes for a population defined by a particular disease, condition, or exposure, and that serves one or more predetermined scientific, clinical, or policy purposes” [5 p13]. According to the AHRQ, there are three categories of patient registries: a) disease or condition registries where inclusion is related to a diagnosis of a disease or a condition, b) product registries, which include patients exposed to a health care product, such as a drug or a device, and c) health service registries, which relate health outcome to exposure to healthcare services. Many registries are a combination of these three categories.
The Framework for Australian Clinical Quality Registries
In Australia clinical registers are defined as “databases that systematically collect health-related information within an overall governance and management structure on individuals who are: treated with a particular surgical procedure, device or drug, e.g. joint replacement; diagnosed with a particular illness, e.g. stroke; or managed via a specific healthcare resource, e.g. treated in an intensive care unit.”1 Similarly to the US AHRQ, three (overlapping) categories of clinical registries are described: a) condition/disease registries (collecting diagnostic details on patients with specific disease/condition), b) drug/device/product registries (monitoring safety of devices/drugs/products, such as blood), and c) clinical quality registries (monitoring outcomes and reporting on quality of care).
The Framework for Australian Clinical Quality Registries further defines Australian clinical quality registers as “organisations that systematically monitor the quality (appropriateness and effectiveness) of health care, within specific clinical domains, by routinely collecting, analysing and reporting health-related information. The information is used to identify benchmarks, significant outcome variance, and inform improvements in healthcare quality” [6 p7].
The Swedish Patient Data Act
The Swedish Healthcare Quality Registries have been defined as “an automated and structured collection of personal data that were initiated with the purpose to systematically and continuously develop and safeguard quality of care. A national or regional quality registry refers to a quality registry in which personal data have been collected from several caregivers and which allows for comparisons within healthcare at a national or regional level” [7 p108].
References
- [1].Richesson R, Vehik K. Patient registries: utility, validity and inference. Adv Exp Med Biol 2010;686:87–104. [DOI] [PubMed] [Google Scholar]
- [2].Porta M (Ed.). A dictionary of epidemiology. Oxford: Oxford University Press; 2014. [Google Scholar]
- [3].Black N, Tan S. Use of national clinical databases for informing and for evaluating health care policies. Health Policy 2013;109(2):131–6 (Richesson & Vehik, 2010) [DOI] [PubMed] [Google Scholar]
- [4].Workman TA. Engaging patients in information sharing and data collection: The role of patient-powered registries and research networks [Internet]. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- [5].Gliklich RE, Dreyer NA, Leavy MB (Eds). Registries for Evaluating Patient Outcomes: A User’s Guide. Rockville MD: Agency for Healthcare Research and Quality (US); 2014. Apr. [PubMed] [Google Scholar]
- [6].Australian Commission on Safety and Quality in Health Care. Framework for Australian clinical quality registries. Sydney: ACSQHC; 2014. [Google Scholar]
- [7].Emilsson L, Lindahl B, Koster M, Lambe M, Ludvigsson JF. Review of 103 Swedish Healthcare Quality Registries. J Intern Med 2015;277(1):94–136. [DOI] [PubMed] [Google Scholar]
Appendix 2. Hospital-based and university-based dementia registries.
Canada
University of Western Ontario Dementia Study Registry [1]
India
Nizam’s Institute of Medical Sciences Hospital Dementia Registry [2]
Italy
Treviso Dementia (TREDEM) Registry [3]
Japan
Hyogo Institute Hospital for Aging Brain and Cognitive Disorders Registry [4]
Pakistan
Shifa International Hospital, Islamabad Registry [5]
South Korea
Konkuk Dementia Registry, Konkuk University [6]
Hyoja Dementia Registry, Hyoja Geriatric Hospital [7]
Taiwan
Lin-Shin Dementia Registry Project [8]
UK
Maudsley Biomedical Research Center Dementia Case Registry at King’s Health Partners [9]
Newcastle and Tyneside Area Case Register [10]
USA
Alzheimer’s Disease Prevention Registry, Duke University [11]
Alzheimer’s Disease Patient Registry, Prototype Alzheimer’s Collaborative Team (PACT) [12]
Boston University Alzheimer’s Disease Center Registry [13]
East Boston Alzheimer’s Disease Registry [14]
Massachusetts Alzheimer’s Disease Research Center at Massachusetts General Hospital Registry [15]
Mayo Clinic Alzheimer’s Disease Patient Registry [16]
Rhode Island Hospital Alzheimer Prevention Registry [17]
University Alzheimer Center, Case Western Reserve University and University Hospitals of Cleveland Registry [18]
University of California at Los Angeles Alzheimer’s and Dementia Care Program Registry [19]
University of Iowa Prototype Alzheimer’s Disease Registry [20]
University of Pittsburgh Alzheimer’s Disease Patient Registry [21]
University of Rochester Alzheimer’s Disease Research Center Neuropathology Database [22]
University of South California Alzheimer’s Disease Research Center Registry [23]
University of Washington Alzheimer’s Disease Patient Registry [24]
References
- [1].Bowler JV, Munoz DG, Merskey H, Hachinski V. Fallacies in the pathological confirmation of the diagnosis of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 1998. January 1;64(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alladi S, Mekala S, Chadalawada SK, Jala S, Mridula R, Kaul S. Subtypes of dementia: a study from a memory clinic in India. Dement Geriatr Cogn Dis 2011. August 10;32(1):32–8. [DOI] [PubMed] [Google Scholar]
- [3].Gallucci M, Spagnolo P, Aricò M, Grossi E. Predictors of Response to Cholinesterase Inhibitors Treatment of Alzheimer’s Disease: Date Mining from the TREDEM Registry. J Alzheimers Dis 2016. February 22;50(4):969–79. [DOI] [PubMed] [Google Scholar]
- [4].Imamura T, Hirono N, Hashimoto M, Shimomura T, Tanimukai S, Kazui H, Hanihara T, Mori E. Clinical diagnosis of dementia with Lewy bodies in a Japanese dementia registry. Dement Geriatr Cogn Dis 1999. May 11;10(3):210–6. [DOI] [PubMed] [Google Scholar]
- [5].Ahmad A, Khatri I, Siddiqui M, Khan N, Kamal S, Mehboob N. Dementia in Pakistan: initial results from our registry at a tertiary care hospital. Alzheimers Dement 2011. July 31;7(4):S767. [Google Scholar]
- [6].Moon Y, Kim H, Kim SH, Han SH. Asymptomatic stroke can aggravate the severity of apathy in patients with Alzheimer’s disease. Alzheimers Dement 2012. July 31 ;8(4):P557. [Google Scholar]
- [7].Kwak YT, Suk SH. Clinical course of small vessel dementia patients according to cholinesterase inhibitor treatment. Eur J Neurol 2009. September 1;16:350. [Google Scholar]
- [8].Chiu PY, Tsai CT, Chen PK, Liu YL, Lai TJ. Frequency of Early and Late-onset Dementias in a Taiwanese Dementia Clinic: First Report on the Lin-Shin Dementia Registry Project. Taiwanese J Psychiatry 2015. March 1;29(1):29–39. [Google Scholar]
- [9].Kiddle SJ, Sattlecker M, Proitsi P, Simmons A, Westman E, Bazenet C, Nelson SK, Williams S, Hodges A, Johnston C, Soininen H. Candidate blood proteome markers of Alzheimer’s disease onset and progression: a systematic review and replication study. J Alzheimers Dis 2014. January 1;38(3):515–31. [DOI] [PubMed] [Google Scholar]
- [10].Ballard C, O’Brien J, Gray A, Cormack F, Ayre G, Rowan E, Thompson P, Bucks R, McKeith I, Walker M, Tovee M. Attention and fluctuating attention in patients with dementia with Lewy bodies and Alzheimer disease. Arch Neurol 2001. June 1;58(6):977–82. [DOI] [PubMed] [Google Scholar]
- [11].Romero HR, Welsh-Bohmer KA, Gwyther LP, Edmonds HL, Plassman BL, Germain CM, McCart M, Hayden KM, Pieper C, Roses AD. Community engagement in diverse populations for Alzheimer disease prevention trials. Alzheimer Dis Assoc Disord 2013. December;28(3):269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cohen D, Paveza G, Levy PS, Ashford JW, Brody JA, Eisdorfer C, Gorelick P, Hirschman R, Luchins D, Trozzolo T, Shaw H. An Alzheimer’s disease patient registry: the Prototype Alzheimer Collaborative Team (PACT). Aging Clin Exp Res 1990. September 1 ;2(3):312–6. [DOI] [PubMed] [Google Scholar]
- [13].Jefferson AL, Lambe S, Chaisson C, Palmisano J, Horvath KJ, Karlawish J. Clinical research participation among aging adults enrolled in an Alzheimer’s Disease Center research registry. J Alzheimers Dis 2011. January 1 ;23(3):443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Evans DA, Scherr PA, Smith LA, Albert MS, Funkenstein HH. The east Boston Alzheimer’s disease registry. Aging Clin Exp Res 1990. September 1;2(3):298–302. [DOI] [PubMed] [Google Scholar]
- [15].Fong TG, Jones RN, Shi P, Marcantonio ER, Yap L, Rudolph JL, Yang FM, Kiely DK, Inouye SK. Delirium accelerates cognitive decline in Alzheimer disease. Neurology 2009. May 5;72(18):1570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Petersen RC, Kokmen E, Tangalos E, Ivnik RJ, Kurland LT. Mayo Clinic Alzheimer’s Disease Patient Registry. Aging Clin Exp Res 1990. December 1;2(4):408–15. [DOI] [PubMed] [Google Scholar]
- [17].Daiello LA, Cizginer S, Pelosi MA, Ott BR. Psychoactive medication and memory impairment among members of an Alzheimer prevention registry. Alzheimers Dement 2015. July 1;11(7):P734. [Google Scholar]
- [18].Mizrahi EH, Fritsch T, Geldmacher DS, Soas AH, Friedland RP, Lerner AJ. Medication use in Alzheimer’s Disease and healthy aging: Results from a research registry. Clin Gerontol 2001. December 1;24(1-2):75–84. [Google Scholar]
- [19].Reuben DB, Evertson LC, Wenger NS, Serrano K, Chodosh J, Ercoli L, Tan ZS. The University of California at Los Angeles Alzheimer’s and Dementia Care Program for Comprehensive, Coordinated, Patient-Centered Care: Preliminary Data. J Am Geriatr Soc 2013. December 1 ;61 (12):2214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kohout FJ. The University of Iowa Prototype Alzheimer’s Disease Registry: Plans and progress. Aging Clin Exp Res 1990. September 1;2(3):308–12. [DOI] [PubMed] [Google Scholar]
- [21].Kuller LH, Ganguli M, Ratcliff GG, Huff FJ, Belle SH, Detre KM. The University of Pittsburgh Alzheimer’s Disease Patient Registry: the Monongahela Valley Independent Elders Survey (MoVIES). Aging Clin Exp Res 1990. September 1;2(3):302–5. [DOI] [PubMed] [Google Scholar]
- [22].Papka M, Rubio A, Schiffer RB, Cox C. Lewy body disease: can we diagnose it?. J Neuropsychiatry Clin Neurosci 1998. November;10(4):405–12.1998 Nov;10(4):405-12. [DOI] [PubMed] [Google Scholar]
- [23].Andel R, McCleary CA, Murdock GA, Fiske A, Wilcox RR, Gatz M. Performance on the CERAD Word List Memory task: a comparison of university-based and community-based groups. Int J Geriatr Psychiatry 2003. August 1;18(8):733–9. [DOI] [PubMed] [Google Scholar]
- [24].Larson EB, Kukull WA, Teri L, McCormick W, Pfanschmidt M, Van Belle G, Sumi M. University of Washington Alzheimer’s Disease Patient Registry (ADPR): 1987–8. Aging Clin Exp Res 1990. December 1;2(4):404–8. [PubMed] [Google Scholar]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Prince M,Wimo A, Guerchet M, Ali GC,Wu Y-T, Prina M. World Alzheimer Report 2015. The Global Impact of Dementia An analysis of prevalence, incidence, costs and trends. London: Alzheimer’s Disease International; 2015. [Google Scholar]
- [2].Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, et al. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol 2016;15(5):455–532. [DOI] [PubMed] [Google Scholar]
- [3].Khachaturian ZS, Petersen RC, Snyder PJ, Khachaturian AS, Aisen P, de Leon M, et al. Developing a global strategy to prevent Alzheimer’s disease: Leon Thal Symposium 2010. Alzheimers Dement 2011. March;7(2):127–32. [DOI] [PubMed] [Google Scholar]
- [4].Organisation for Economic Co-operation and Development (OECD). Addressing Dementia: The OECD Response OECD Health Policy Studies. Paris: OECD Publishing; 2015. [Google Scholar]
- [5].Richesson R, Vehik K. Patient registries: utility, validity and inference. Adv Exp Med Biol 2010;686:87–104. [DOI] [PubMed] [Google Scholar]
- [6].Porta M (Ed.). A dictionary of epidemiology. Oxford: Oxford University Press; 2014. [Google Scholar]
- [7].Frank L When an Entire Country Is a Cohort. Science 2000;287(5462):2398–9. [DOI] [PubMed] [Google Scholar]
- [8].Emilsson L, Lindahl B, Koster M, Lambe M, Ludvigsson JF. Review of 103 Swedish Healthcare Quality Registries. J Intern Med 2015;277(1):94–136. [DOI] [PubMed] [Google Scholar]
- [9].Sortso C, Thygesen LC, Bronnum-Hansen H. Database on Danish population-based registers for public health and welfare research. Scand J Public Health 2011. ;39(7 Suppl):17–9. [DOI] [PubMed] [Google Scholar]
- [10].Black N, Barker M, Payne M. Cross sectional survey of multicentre clinical databases in the United Kingdom. BMJ 2004;328(7454):1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Heger M A registry of registries? The US backs the idea for patients. Nat Med 2011. January;17(1):4. [DOI] [PubMed] [Google Scholar]
- [12].Harris PA, Scott KW, Lebo L, Hassan N, Lightner C, Pulley J. ResearchMatch: a national registry to recruit volunteers for clinical research. Acad Med 2012;87(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Larsson S, Lawyer P, Garellick G, Lindahl B, Lundstrom M. Use of 13 disease registries in 5 countries demonstrates the potential to use outcome data to improve health care’s value. Health Aff 2012;31(1):220–7. [DOI] [PubMed] [Google Scholar]
- [14].Fillenbaum GG, van Belle G, Morris JC, Mohs RC, Mirra SS, Davis PC, et al. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): the first twenty years. Alzheimers Dement 2008. March;4(2):96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Beekly DL, Ramos EM, van Belle G, Deitrich W, Clark AD, Jacka ME, et al. The National Alzheimer’s Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord 2004;18(4):270–7. [PubMed] [Google Scholar]
- [16].De Braekeleer M, Cholette A, Mathieu J, Boily C, Robitaille Y, Gauvreau D. Familial factors in Alzheimer’s disease (IMAGE project). A case-control study in the Saguenay-Lac-St-Jean region (Quebec, Canada). Eur Neurol 1989;29 Suppl 3:2–8. [DOI] [PubMed] [Google Scholar]
- [17].Holmes C The Camberwell dementia case register. Int J Geriatr Psychiatry 1996;1(4):369–375. [Google Scholar]
- [18].Ertekin-Taner N Genetics of Alzheimer disease in the pre- and post-GWAS era. Alzheimers Res Ther 2010;2(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fargo KN, Carrillo MC, Weiner MW, Potter WZ, Khachaturian Z. The crisis in recruitment for clinical trials in Alzheimer’s and dementia: An action plan for solutions. Alzheimers Dement 2016. November; 12(11):1113–5. [DOI] [PubMed] [Google Scholar]
- [20].Barnhart RL, van Belle G, Edland SD, Kukull W, Borson S, Raskind M, et al. Geographically overlapping Alzheimer’s disease registries: comparisons and implications. J Geriatr Psychiatry Neurol 1995;8(4):203–8. [DOI] [PubMed] [Google Scholar]
- [21].Hughes JP, van Belle G, Kukull W, Larson EB, Teri L. On the uses of registries for Alzheimer disease. Int J Geriatr Psychiatry. 1989. Winter;3(4):205–17. [PubMed] [Google Scholar]
- [22].Kuller L Establishing and implementing the goals of a registry for dementing diseases. Aging 1990. June;2(2):200–7. [DOI] [PubMed] [Google Scholar]
- [23].Leach J, Levy R. Dementia case registers. Int J Geriatr Psychiatry 1993;8(3):197–201. [Google Scholar]
- [24].Radebaugh TS, Khachaturian ZS, Mortimer JA, Madison RE. Methods and problems in the development of large area registries for dementing disease. Aging 1990;2(1):79. [Google Scholar]
- [25].Khachaturian AS, Meranus DH, Kukull WA, Khachaturian ZS. Big data, aging, and dementia: pathways for international harmonization on data sharing. Alzheimers Dement 2013;9(5 Suppl):S61–2. [DOI] [PubMed] [Google Scholar]
- [26].Liu FX, Rutherford P, Smoyer-Tomic K, Prichard S, Laplante S. A global overview of renal registries: a systematic review. BMC Nephrol 2015;16:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Porgo TV, Moore L, Tardif PA. Evidence of data quality in trauma registries: A systematic review. J Trauma Acute Care Surg 2016. April;80(4):648–58. [DOI] [PubMed] [Google Scholar]
- [28].Australian Commission on Safety and Quality in Health Care. Framework for Australian clinical quality registries. Sydney: ACSQHC; 2014. [Google Scholar]
- [29].Gliklich RE, Dreyer NA, Leavy MB (Eds). Registries for Evaluating Patient Outcomes: A User’s Guide.. Rockville MD: Agency for Healthcare Research and Quality (US); 2014. Apr. [PubMed] [Google Scholar]
- [30].Jacobsson-Ekman G, Lindahl B, Nordin A (Eds). National quality registries in Swedish health care. Stockholm: Karolinska Institutet University Press; 2016. [Google Scholar]
- [31].German RR, Lee LM, Horan JM, Milstein RL, Pertowski CA, Waller MN. Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep 2001. ;50(RR-13): 1–35. [PubMed] [Google Scholar]
- [32].Black N, Payne M. Directory of clinical databases: improving and promoting their use. Qual Saf Health Care 2003;12(5): 348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schaller S, Marinova-Schmidt V, Setzer M, Schnetzer I-M, Thron C, Popp S, et al. The use of real world data to tailor community services for persons with dementia and informal caregivers: The Bavarian Dementia Survey (BayDem) [Internet]. Alzheimer Europe; 2015. Sept. Available from: http://www.alzheimer-europe.org/Conferences/Previous-conferences/2015-Ljubljana/Detailed-programme-abstracts-and-presentations/PO2-Policies-and-strategies
- [34].Bergh S, Holmen J, Gabin J, Stordal E, Fikseaunet A, Selbaek G, et al. Cohort profile: the Health and Memory Study (HMS): a dementia cohort linked to the HUNT study in Norway. Int J Epidemiol 2014;43(6):1759–68. [DOI] [PubMed] [Google Scholar]
- [35].Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. J Geriatr Psychiatry Neurol 2005;18(4):245–9. [DOI] [PubMed] [Google Scholar]
- [36].Seiler S, Schmidt H, Lechner A, Benke T, Sanin G, Ransmayr G, et al. Driving cessation and dementia: results of the prospective registry on dementia in Austria (PRODEM). PloS One 2012;7(12):e52710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Park HK, Na DL, Han SH, Kim JY, Cheong HK, Kim SY, et al. Clinical characteristics of a nationwide hospital-based registry of mild-to-moderate Alzheimer’s disease patients in Korea: a CREDOS (Clinical Research Center for Dementia of South Korea) study. J Korean Med Sci 2011;26(9): 1219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Morris JC, Aisen PS, Bateman RJ, Benzinger TL, Cairns NJ, Fagan AM, et al. Developing an international network for Alzheimer research: The Dominantly Inherited Alzheimer Network. Clin Investig 2012;2(10):975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Reiman EM, Langbaum JB, Fleisher AS, Caselli RJ, Chen K, Ayutyanont N, et al. Alzheimer’s Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis 2011;26 Suppl 3:321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ritchie CW, Molinuevo JL, Truyen L, Satlin A, Van der Geyten S, Lovestone S. Development of interventions for the secondary prevention of Alzheimer’s dementia: the European Prevention of Alzheimer’s Dementia (EPAD) project. Lancet Psychiatry 2016;3(2):179–86. [DOI] [PubMed] [Google Scholar]
- [41].Cummings P, Aisen R, Barton J, Bork R, Doody J, Dwyer … & GAP-NET. Re-engineering Alzheimer clinical trials: Global Alzheimer’s Platform Network. J Prev Alzheimers Dis 2012; 3(2):114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Allegri RF. Primer Registro Centralizado de Patologias Cognitivas en Argentina (ReDeCAr) Resultados del Estudio Piloto Año 2011 [First Centralized Register of cognitive disease in Argentina (ReDeCAr), Results from the pilot study]. Buenos Aires: Publicación del Ministerio de Salud; 2011. [Google Scholar]
- [43].Anthony S, Pradier C, Chevrier R, Festraëts J, Tifratene K, Robert P. The French National Alzheimer Database: A Fast Growing Database for Researchers and Clinicians. Dement Geriatr Cogn Dis 2014;38(5-6):271–80. [DOI] [PubMed] [Google Scholar]
- [44].Calvo-Perxas L, Aguirregomozcorta M, Casas I, Flaque M, Hernandez M, Linares M, et al. Rate of dementia diagnoses according to the degree of aging of the population. Int Psychogeriatr 2015;27(3):419–27. [DOI] [PubMed] [Google Scholar]
- [45].Francesconi P, Gini R, Roti L, Bartolacci S, Corsi A, Buiatti E. The Tuscany experimental registry for Alzheimer’s disease and other dementias: how many demented people does it capture? Aging Clin Exp Res 2007;19(5):390–3. [DOI] [PubMed] [Google Scholar]
- [46].Lillquist PP. Challenges in surveillance of dementias in New York State. Prev Chronic Dis 2004;1(1):A08. [PMC free article] [PubMed] [Google Scholar]
- [47].Macera CA, Still CN, Brandes DA, Abramson RK, Davis DR. The South Carolina Alzheimer’s disease patient registry: A progress report. Am J Alzheimers Dis Other Demen 1991. ;6(1): 35–38. [Google Scholar]
- [48].Schreurs BG. The West Virginia Alzheimer’s Disease Registry: helping West Virginia cope. W V Med J 2011;107(3):44–5. [PubMed] [Google Scholar]
- [49].Leonard EW, Bu R, Brown AA. Best Practices for Establishing Georgia’s Alzheimer’s Disease Registry. Minn J L Sci & Tech 2016;17(1): 221–275. [Google Scholar]
- [50].Johannsen P, Jorgensen K, Korner A, Elmo EG, Lauesen LB, Utzon J. Development of a dementia assessment quality database. Aging Ment Health 2011. January;15(1):40–6. [DOI] [PubMed] [Google Scholar]
- [51].Persson K, Braekhus A, Selbaek G, Kirkevold O, Engedal K. Burden of Care and Patient’s Neuropsychiatric Symptoms Influence Carer’s Evaluation of Cognitive Impairment. Dement Geriatr Cogn Disord 2015;40(5-6):256–67 [DOI] [PubMed] [Google Scholar]
- [52].Religa D, Fereshtehnejad SM, Cermakova P, Edlund AK, Garcia-Ptacek S, Granqvist N, et al. SveDem, the Swedish Dementia Registry - a tool for improving the quality of diagnostics, treatment and care of dementia patients in clinical practice. PloS One 2015;10(2):e0116538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mayer S, Granvik E, Minthon L, Nägga K. Improved quality of life by active intervention with the Swedish BPSD registry. Alzheimers Dement 2014;10(4):P139–P40. [Google Scholar]
- [54].Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44(12):2308–14. [DOI] [PubMed] [Google Scholar]
- [55].Illife S, Curry L, Kharicha K, Rait G, Wilcock J, Lowery D, et al. Developing a Dementia Research Registry: a descriptive case study from North Thames DeNDRoN and the EVIDEM programme. BMC Med Res Methodol 2011. January 27;11(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Russ TC, Parra MA, Lim AE, Law E, Connelly PJ, Starr JM. Prediction of general hospital admission in people with dementia: cohort study. Br J Psychiatry 2015;206(2):153–9. [DOI] [PubMed] [Google Scholar]
- [57].Larsen ME, Curry L, Mastellos N, Robb C, Car J, Middleton LT. Development of the CHARIOT Research Register for the Prevention of Alzheimer’s Dementia and Other Late Onset Neurodegenerative Diseases. PloS One 2015;10(11):e0141806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Langbaum JB, High N, Reiman EM, Aisen PS, Albert MS, Comer M, et al. Alzheimer’s prevention registry: A shared resource to the scientific community to facilitate enrollment in studies. Alzheimers Dement 2015;11(7):P479. [Google Scholar]
- [59].Saunders KT, Langbaum JB, Holt CJ, Chen W, High N, Langlois C, et al. Arizona Alzheimer’s Registry: Strategy and Outcomes of a Statewide Research Recruitment Registry. J Prev Alzheimers Dis 2014. September;1(2):74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Skogen JC, Bergh S, Stewart R, Knudsen AK, Bjerkeset O. Midlife mental distress and risk for dementia up to 27 years later: the Nord-Trondelag Health Study (HUNT) in linkage with a dementia registry in Norway. BMC Geriatr 2015. March 10;15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hofman A, Brusselle GG, Darwish Murad S, van Duijn CM, Franco OH, Goedegebure A, et al. The Rotterdam Study: 2016 objectives and design update. Eur J Epidemiol 2015. August;30(8):661–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wimo A, Jonsson L, Bond J, Prince M, Winblad B. The worldwide economic impact of dementia 2010. Alzheimers Dement 2013. January;9(1 ):1–11 e3. [DOI] [PubMed] [Google Scholar]
- [63].Gaugler JE, Ascher-Svanum H, Roth DL, Fafowora T, Siderowf A, Beach TG. Characteristics of patients misdiagnosed with Alzheimer’s disease and their medication use: an analysis of the NACC-UDS database. BMC Geriatr 2013. December 19;13:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].James S, Rao SV, Granger CB. Registry-based randomized clinical trials—a new clinical trial paradigm. Nat Rev Cardiol 2015. May;12(5):312–6. [DOI] [PubMed] [Google Scholar]
- [65].Frobert O, Lagerqvist B, Gudnason T, Thuesen L, Svensson R, Olivecrona GK, et al. Thrombus Aspiration in ST-Elevation myocardial infarction in Scandinavia (TASTE trial). A multicenter, prospective, randomized, controlled clinical registry trial based on the Swedish angiography and angioplasty registry (SCAAR) platform. Study design and rationale. Am Heart J 2010. December;160(6):1042–8. [DOI] [PubMed] [Google Scholar]
- [66].WHO. Dementia: a public health priority. Geneva, Switzerland: WHO; 2012. [Google Scholar]
- [67].SveDem [Internet]. Primärvård Resultat - Bättre liv för sjuka äldre [Betterlife for tye ill elderly]; 2016. Available from: http://www.ucr.uu.se/svedem/component/edocman/primarvardsres2011-2015.
- [68].Australian Commission on Safety and Quality in Health Care. Economic evaluation of clinical quality registries. Sydney: ACSQHC; 2016. [Google Scholar]
- [69].European Prevention of Alzheimer’s Dementia [Internet]. EPAD Project Structure: Work Package 7: Business Model and Sustainability; 2015. Available from: http://ep-ad.org/project-structure/
- [70].Global Alzheimer’s Platform [Internet]. Trial-Ready Cohort; 2017. Available from: http://globalalzplatform.org/about/trial-ready-cohort/
- [71].Calba C, Goutard FL, Hoinville L, Hendrikx P, Lindberg A, Saegerman C, Peyre M. Surveillance systems evaluation: a systematic review of the existing approaches. BMC Public Health 2015. May 1;15(1):448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Monash University. Registry science handbook. Melbourne: Monash University; 2013. [Google Scholar]
- [73].Weddell JM. Registers and registries: a review. Int J Epidemiol 1973;2(3):221–8. [DOI] [PubMed] [Google Scholar]
- [74].Lundström M (ed.), Albrecht S, Serring I, Svensson K, Wendel E. (2005). Handbook for establishing quality registries. Karlskrona: EyeNet. [Google Scholar]
- [75].American Psychiatric Association. Diagnostic and statistical manual of mental disorders [5th ed.]. Washington, DC: APA; 2013. [Google Scholar]
- [76].World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: WHO; 1992. [Google Scholar]
- [77].Evans SM, Loff B, Cameron PA. Clinical registries: the urgent need to address ethical hurdles. Med J Aust 2013. February 18;198(3): 134–5. [DOI] [PubMed] [Google Scholar]
- [78].Wilkins S, Best RL, Evans SM. Need for a roadmap for development of a coordinated national registry programme. Intern Med J 2015. November;45(11):1189–92. [DOI] [PubMed] [Google Scholar]
- [79].Australian Commission on Safety and Quality in Health Care. Operating Principles and Technical Standards for Australian Clinical Quality Registries. Sydney: ACSQHC; 2008. [Google Scholar]


