Chronic Graft-versus-Host Disease (cGVHD) is the leading cause of long-term mortality and morbidity in patients after stem cell transplantation (SCT). Skin is the most commonly affected organ, involved in more than 90% of cGVHD cases1. Skin manifestations of cGVHD are broadly divided into two categories : erythema and sclerosis. Among patients being treated for cGVHD, 20% develop sclerosis within 3 years of transplant, leading to significant disability2.
The current standard for monitoring sclerosis is the NIH Skin Score, ranging from 0-3 based on body surface area (BSA) and sclerotic features3. However, this scoring system is subjective, coarse, and unreliable4. Measurement of sclerosis by exam is difficult due to ill-defined borders and paucity of reliable associated visible changes. Sclerosis scores among multiple observers rarely exhibit substantial agreement, and therefore the minimal change for reliable detection is 17 to 26% BSA5. These limitations have impeded the assessment of disease progression and treatment response4. Developing a quantitative and reproducible measurement of sclerosis was deemed a top priority by the 2014 NIH Consensus on Response Criteria for cGVHD6. Serial skin biopsy is not a practical option for long-term follow-up to assess treatment response, and histology results are generally non-specific7. This has motivated an interest in imaging tools for measuring sclerotic GVHD. Previously, a magnetic resonance imaging study of 15 cGVHD patients was able to identify abnormalities in the dermis, subcutaneous tissue, and muscle8. Ultrasound has shown differences in normalized shear wave speed in a single study of 4 healthy controls versus 5 sclerotic cGVHD patients9. Neither technology has yet advanced to large studies or widespread clinical use.
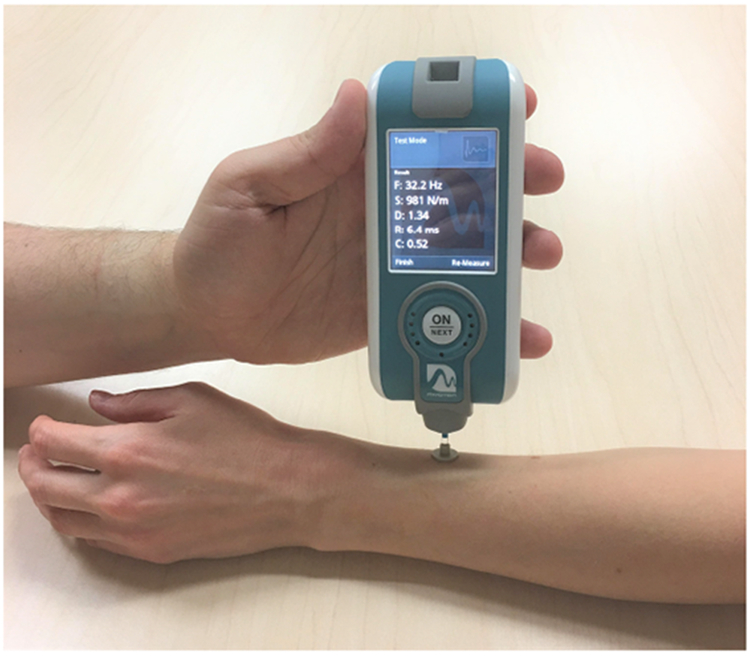
In the current study, we investigated the feasibility of using an affordable tool to rapidly and directly measure cutaneous sclerosis in cGVHD patients. We employed the Myoton (Figure 1), which is a commercially available, noninvasive handheld device developed to measure biomechanical properties of muscle. It has been applied in diverse fields including neurology and sports medicine10,11, but has not yet been used in dermal disorders. The device delivers a brief, constant mechanical impulse to which tissue responds with a damped natural oscillation. Biomechanical properties are automatically extracted from the oscillatory response curve as previously described12.
Figure 1.
Myoton, a noninvasive handheld device, modified to isolate cutaneous tissue.
The durometer is another handheld device that appears similar to the Myoton at first inspection. However, the underlying principle and function are in reality very different between the two devices. The durometer is an industrial tool designed for determining the hardness of a surface by measuring the amount of force required to produce an indentation. Therefore, the durometer reading is highly dependent on the amount of force applied by the individual conducting the measurement. By contrast, the Myoton applies a fixed, brief mechanical impulse, and calculates multiple biomechanical properties based on the tissue’s inherent response. The durometer has been used to measure skin hardness in patients with scleroderma, but no results have been published in GVHD patients13,14.
For our study, the Myoton was modified for enhanced selection of cutaneous tissue and reduced interrogation of the muscle tissue for which it was designed. First, a 12 mm diameter disk was attached, thereby distributing the surface impulse over a 16 fold larger surface area than the standard 3 mm testing end (probe). During measurements, the probe rests perpendicular to the skin surface. The larger contact area decreases surface power density for selection of more superficial skin tissue and also reduces the amount of residual strain imparted during the measurement process. Second, impulse delivery time (tap time) was decreased from the default 15 ms to 7 ms. Decreased tap time results in a proportional decrease of total transferred mechanical energy, which translates into a smaller effective mass of natural tissue oscillation (i.e. selection of more superficial tissue).
In this cross-sectional study, cGVHD patients (n=8) with an NIH 2014 Skin Features Score of 3 (severe sclerosis) and healthy subjects (n=10) were recruited (Table 1). For each subject, the Myoton was used to measure skin stiffness bilaterally on 9 anatomic regions (shin, dorsal forearm, upper arm, shoulder, chest, abdomen, calf, upper back, lower back), resulting in 18 total measurement sites. Generally, cGVHD patients did not have skin involvement in all sites due to the heterogeneity of the disease. When undergoing Myoton measurements, subjects were instructed to relax in a supine position. They felt a slight, painless pressure for 7 ms at a time during the mechanical impulse. Twenty measurements with one second interval per site were conducted to minimize any possible measurement variation. A single measurer, JC, operated the Myoton after 80 hours of training by AV, the inventor of the device. Each subject's measurement session lasted approximately 30 minutes.
Table 1.
Subject Characteristics
| cGVHD Subjects (n=8) | Subject Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Summary* | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics & | Age | 55 | 52 | 40 | 28 | 72 | 59 | 47 | 47 | 50 [45 - 56] | ||
| Race | C | C | C | C | C | C | C | C | 100% Caucasian | |||
| Gender | M | M | M | F | M | M | M | F | 25% Female | |||
| BMI | 24 | 24 | 27 | 14 | 32 | 17 | 23 | 34 | 24 [21-28] | |||
| Disease Characteristics# | Disease Histology | AL | MD | MD | MD | AL | AL | LD | AL | 50% AL 38% MD 12% LD 0% Other |
||
| Transplant Characteristics$ | Transplant Source | BM | PB | PB | PB | PB | PB | PB | BM | 75% PB 0% CB 25% BM |
||
| cGVHD Characteristics at Study Entry | NIH Score BSA Involvement | >50% | >50% | 19 - 50% | >50% | 19 - 50% | >50% | 1-18% | 19 - 50% | 1% No BSA involved 12% 1-18%BSA 38% 19-50%BSA 50% >50%BSA |
||
| NIH Score Skin Feature% | DS | DS | DS | DS | DS | DS | DS | DS | 0% No sclerotic
features 0% SS 100% DS |
|||
| Myoton Stiffness measurements by Sites (N/m)^ | Shin L | 878 | 1012 | 631 | 1318 | 892 | 1123 | 1621 | 1853 | 1067 [888 - 1394] | ||
| Shin R | 726 | 1555 | 544 | 1050 | 696 | 1126 | 1129 | 1924 | 1088 [718 - 1235] | |||
| Dorsal Forearm L | 674 | 436 | 512 | 1438 | 426 | 400 | 758 | 800 | 593 [433 - 769] | |||
| Dorsal Forearm R | 898 | 451 | 593 | 1140 | 439 | 388 | 781 | 1247 | 687 [448 - 959] | |||
| Upper Arm L | 488 | 330 | 297 | 462 | 493 | 530 | 529 | 337 | 475 [336 - 502] | |||
| Upper Arm R | 412 | 331 | 368 | 415 | 514 | 581 | 468 | 351 | 414 [363 - 479] | |||
| Shoulder L | 399 | 790 | 480 | 491 | 598 | 1093 | 725 | 346 | 544 [460 - 741] | |||
| Shoulder R | 471 | 1191 | 627 | 798 | 555 | 591 | 938 | 397 | 609 [534 - 833] | |||
| Chest L | 456 | 332 | 362 | 391 | 649 | 1088 | 350 | - | 391 [356 - 552] | |||
| Chest R | 361 | 353 | 318 | 1479 | 484 | 1348 | 334 | - | 361 [344 - 916] | |||
| Abdomen L | 312 | 274 | 179 | 152 | 261 | 380 | 258 | 215 | 260 [206 - 284] | |||
| Abdomen R | 381 | 287 | 194 | 131 | 253 | 402 | 275 | 239 | 264 [228 - 310] | |||
| Calf L | 878 | 1084 | 383 | 867 | 878 | 1210 | 971 | 808 | 878 [852 - 999] | |||
| Calf R | 754 | 1769 | 390 | 820 | 657 | 1222 | 967 | 782 | 801 [729 - 1031] | |||
| Upper Back L | - | 491 | 682 | 1388 | 677 | 1574 | 700 | - | 691 [678 - 1216] | |||
| Upper Back R | - | 547 | 548 | 918 | 754 | 958 | 653 | - | 704 [575 - 877] | |||
| Lower Back L | - | 307 | 563 | 261 | - | 448 | 328 | 228 | 318 [273 - 418] | |||
| Lower Back R | - | 287 | 457 | 516 | - | 457 | 336 | 219 | 396 [299 - 457] | |||
| Control Subjects (n=10) | Subject Number | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | Summary* |
| Demographics& | Age | 66 | 68 | 28 | 49 | 23 | 28 | 35 | 25 | 26 | 22 | 28 [25-46] |
| Race | C | C | AA | A | C | A | A | AA | C | A | 40% Calucasian | |
| Gender | M | M | M | F | M | F | M | M | M | F | 30% Female | |
| BMI | 31 | 24 | 29 | 22 | 22 | 18 | 25 | 22 | 29 | 19 | 23 [22-28] | |
| Myoton Stiffness measurements by Sites (N/m) | Shin L | 639 | 660 | 494 | 524 | 525 | 652 | 541 | 1102 | 686 | 627 | 632 [529-658] |
| Shin R | 759 | 627 | 604 | 468 | 550 | 623 | 645 | 998 | 916 | 598 | 625 [600-731] | |
| Dorsal Forearm L | 459 | 330 | 364 | 377 | 381 | 471 | 328 | 392 | 572 | 480 | 386 [367-468] | |
| Dorsal Forearm R | 438 | 420 | 350 | 403 | 439 | 502 | 401 | 363 | 566 | 390 | 411 [393-439] | |
| Upper Arm L | 444 | 338 | 285 | 280 | 239 | 328 | 326 | 381 | 367 | 480 | 333 [295-377] | |
| Upper Arm R | 429 | 399 | 301 | 327 | 309 | 359 | 288 | 366 | 315 | 375 | 343 [310-372] | |
| Shoulder L | 586 | 520 | 404 | 1127 | 179 | 364 | 366 | 329 | 397 | 375 | 386 [364-491] | |
| Shoulder R | 540 | 457 | 347 | 834 | 329 | 309 | 326 | 326 | 279 | 249 | 328 [313-429] | |
| Chest L | 419 | 470 | 322 | 303 | 243 | 314 | 192 | 268 | 206 | 436 | 309 [249-395] | |
| Chest R | 399 | 538 | 277 | 343 | 282 | 327 | 217 | 314 | 237 | 342 | 320 [278-342] | |
| Abdomen L | 234 | 351 | 159 | 194 | 163 | 242 | 202 | 189 | 202 | 296 | 202 [190-240] | |
| Abdomen R | 234 | 332 | 169 | 169 | 187 | 241 | 193 | 191 | 199 | 257 | 196 [188-239] | |
| Calf L | 483 | 574 | 375 | 384 | 398 | 328 | 372 | 412 | 637 | 326 | 391 [372-465] | |
| Calf R | 561 | 728 | 425 | 411 | 388 | 332 | 314 | 363 | 673 | 253 | 400 [340-527] | |
| Upper Back L | 463 | 684 | 490 | 416 | 359 | 394 | 359 | 526 | 329 | 258 | 405 [359-483] | |
| Upper Back R | 559 | 692 | 496 | 566 | 428 | 478 | 389 | 414 | 396 | 271 | 453 [401-543] | |
| Lower Back L | 409 | 362 | 249 | 244 | 158 | 195 | 243 | 279 | 214 | 193 | 243 [200-271] | |
| Lower Back R | 311 | 271 | 262 | 292 | 159 | 206 | 236 | 197 | 199 | 183 | 221 [198-269] | |
Values are shown as Median [IQR] for continuous variables and n (%) for categorical variables.
Demographics: C: Caucasian, , A: Asian, AA: African American, M: Male, F: Female
Disease characteristics: AL: Acute Leukemia, MD: Myeloid Disorder (Multiple Myeloma was included in lymphoid disorders), LD: Lymphoid Disorder
Transplant Characteristics: PB: Peripheral Blood, CB: Core Blood, BM: Bone Marrow
NIH Score Skin Feature: SS: Superficial sclerotic feature, DS: Deep and other sclerotic feature
Only 7 cGVHD patients were measured on the chest and 6 were measured on upper and lower back for patient comfort considerations.
Sites affected with sclerotic cGVHD are in bold
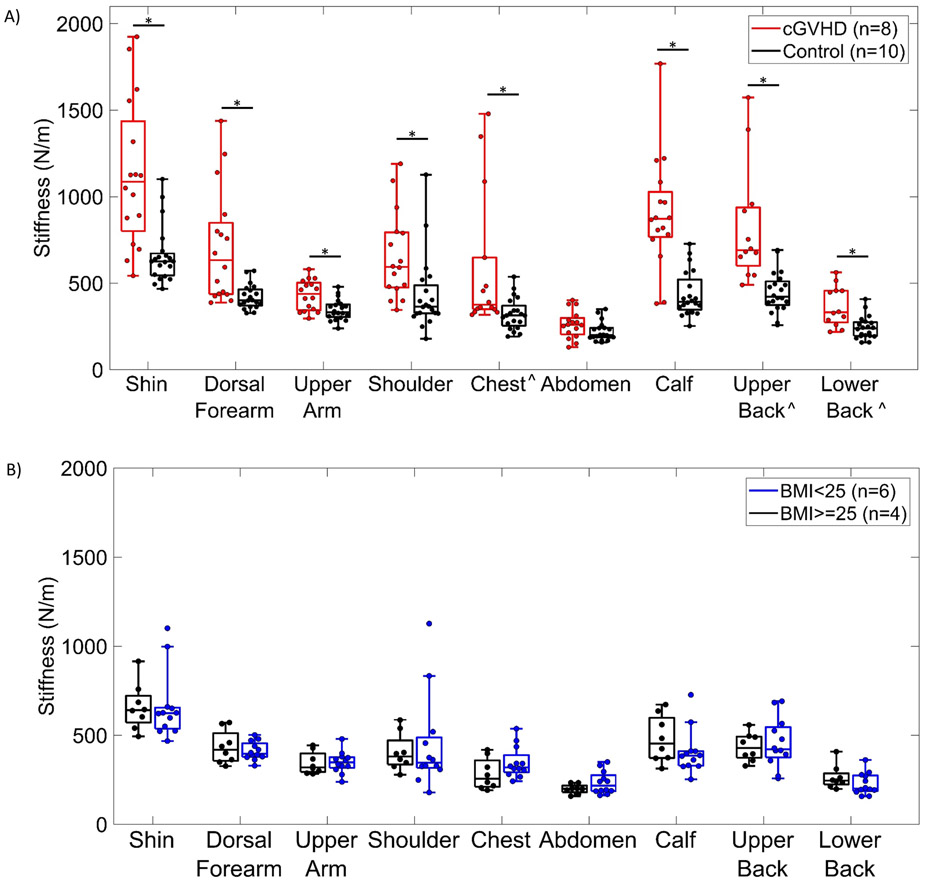
Stiffness measurements of cGVHD patients (n=8) were compared to healthy controls (n=10) by anatomic region (Figure 1A). By a Wilcoxon rank sum test (α=.05), in 8 of the 9 measured regions (representing 16 of 18 measurement sites), cGVHD patients demonstrated significantly higher skin stiffness compared to healthy subjects (p<.05). The only site without statistically significant differences was the abdomen, likely because the participating patients were not affected at the periumbilical measurement location. When the average stiffness over all 9 anatomic regions was compared between patients and controls, cGVHD patients had significantly higher overall skin stiffness (657 ± 387 N/m vs. 392 ± 171 N/m, respectively, p<0.0001). The variation of skin stiffness measurements is generally higher in cGVHD patients compared to controls, as evidenced by the large interquartile range in Figure 2A. This reflects the heterogeneous presentation of the disease in terms of anatomic regions affected in an individual patient.
Figure 2.
A) Box-and-whisker plot of skin stiffness of 8 cGVHD patients and 10 healthy controls, measured with the Myoton device in 9 anatomic regions. *Statistically significant differences (p<.05). ^Only 7 cGVHD patients were measured on the chest and 6 were measured on upper and lower back for patient comfort considerations. B) Box-and-whisker plot of skin stiffness measured with Myoton in 9 anatomic regions on 10 healthy controls, divided by BMI above 25 and BMI below 25.
An additional analysis divided the healthy subjects into normal (<25 kg/m2) or high (>=25 kg/m2) body mass index (BMI) groups to investigate BMI as a potential confounder. Within these controls, no measurement sites had significant differences when stratified by normal or high BMI (Figure 2B).
Our results demonstrate that a commercially available biomechanical measurement device can objectively distinguish between healthy subjects and patients with severe cGVHD. One limitation of this preliminary study is small sample size and patient homogeneity (Caucasian, NIH score of 3), which makes it difficult to assess the generalizability of the results. While our results indicate that skin stiffness measurements are significantly greater in patients with the most severe sclerotic cGVHD when compared to healthy controls, further investigation is required to determine whether this carries over to patients with mild-moderate disease. Further study and device development are also needed to assess the degree to which the Myoton is isolating skin and subcutaneous tissue. In this study, one observer performed measurements on all patients, so the results do not speak to the interobserver reproducibility of the device. Further investigation is also necessary to answer the critical question as to whether the Myoton can distinguish meaningful longitudinal changes in sclerosis of individual patients.
We have taken the first step towards developing a new method to objectively measure sclerotic GVHD disease. Reproducibility, generalizability, and the relative contributions of different soft tissue layers to the Myoton signal remain to be determined. If prospective longitudinal studies can correlate changes in stiffness values to clinical disease progression, the Myoton can become an important tool for monitoring patient course and treatment response in sclerotic cGVHD.
3. Acknowledgements
Dr. Tkaczyk is grateful for support from NIH K12 CA090625. This study is also supported by Baltic-American Freedom Foundation. This study is also supported in part by Career Development Award Number IK2 CX001785 from the United States Department of Veterans Affairs Clinical Science R&D (CSRD) Service.
Funding/Support: NIH K12 CA090625, Baltic-American Freedom Foundation
Footnotes
Conflict of interest disclosures: None
Competing Interests
There is no competing financial interests in relation to the work described.
References
- 1.Rodgers CJ, Burge S, Scarisbrick J, Peniket A. More than skin deep? Emerging therapies for chronic cutaneous GVHD. Bone Marrow Transplantation. 2012June;48(3):323–37. [DOI] [PubMed] [Google Scholar]
- 2.Inamoto Y, Storer BE, Petersdorf EW, Nelson JL, Lee SJ, Carpenter PA, et al. Incidence, risk factors, and outcomes of sclerosis in patients with chronic graft-versus-host disease. Blood. 2013January;121(25):5098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biology of Blood and Marrow Transplantation. 2015;21(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Socie G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014September;124(3):374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell SA, Jacobsohn D, Powers KET, Carpenter PA, Flowers ME, Cowen EW, et al. A Multicenter Pilot Evaluation of the National Institutes of Health Chronic Graft-versus-Host Disease (cGVHD) Therapeutic Response Measures: Feasibility, Interrater Reliability, and Minimum Detectable Change. Biology of Blood and Marrow Transplantation. 2011;17(11):1619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring Therapeutic Response in Chronic Graft-versus-Host Disease. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2014 Response Criteria Working Group Report. Biology of Blood and Marrow Transplantation. 2015;21(6):984–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen CV, Querfeld C, Miller DD. Histopathology of Cutaneous Graft-Versus-Host Disease. Atlas of Graft-versus-Host Disease. 2016;:43–56. [Google Scholar]
- 8.Clark J, Yao L, Pavletic SZ, Krumlauf M, Mitchell S, Turner ML, et al. Magnetic Resonance Imaging in Sclerotic-Type Chronic Graft-vs-Host Disease. Archives of Dermatology. 2009January;145(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SY, Cardones AR, Doherty J, Nightingale K, Palmeri M. Preliminary Results on the Feasibility of Using ARFI/SWEI to Assess Cutaneous Sclerotic Diseases. Ultrasound in Medicine & Biology. 2015;41(11):2806–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gervasi M, Sisti D, Amatori S, Andreazza M, Benelli P, Sestili P, et al. Muscular viscoelastic characteristics of athletes participating in the European Master Indoor Athletics Championship. European Journal of Applied Physiology. 2017;117(8):1739–46. [DOI] [PubMed] [Google Scholar]
- 11.Fröhlich-Zwahlen A, Casartelli N, Item-Glatthorn J, Maffiuletti N. Validity of resting myotonometric assessment of lower extremity muscles in chronic stroke patients with limited hypertonia: A preliminary study. Journal of Electromyography and Kinesiology. 2014;24(5):762–9. [DOI] [PubMed] [Google Scholar]
- 12.Vain A, inventor; Myoton As, assignee. Device and method for real-time measurement of parameters of mechanical stress state and biomechanical properties of soft biological tissue. United States patent application US 13/977,873 2011. July 7.
- 13.Falanga V, Bucalo B. Use of a durometer to assess skin hardness. Journal of the American Academy of Dermatology. 1993;29(1):47–51. [DOI] [PubMed] [Google Scholar]
- 14.Seyger MM, Hoogen FHVD, Boo TD, Jong EMD. Reliability of two methods to assess morphea: Skin scoring and the use of a durometer. Journal of the American Academy of Dermatology. 1997;37(5):793–6. [DOI] [PubMed] [Google Scholar]