Abstract
Lung transplant recipients have an increased risk of lung cancer that is poorly understood. Prior studies are largely descriptive and single-center, and have not examined risk factors or outcomes in this population. This registry-linkage study utilized matched transplant and cancer registry data from 17 US states/regions during 1987–2012. We used standardized incidence ratios (SIRs) to compare incidence with the general population, Poisson models to identify lung cancer risk factors, and Cox models to compare survival after diagnosis. Lung cancer risk was increased among lung recipients (SIR 4.8, 95%CI 4.1–5.5). Those with single lung transplant had 13-fold (95%CI 11–15) increased risk in the native lung. Native lung cancer risk factors included age, prior smoking, time since transplant, and idiopathic pulmonary fibrosis. Compared with cases in the general population, lung cancers in recipients were more frequently localized stage (p=0.02) and treated surgically (p=0.05). However, recipients had higher all-cause (adjusted HR 1.90, 95%CI 1.52–2.37) and cancer-specific mortality (adjusted HR 1.67, 95%CI 1.28–2.18). In conclusion, lung cancer risk is increased after lung transplant, especially in the native lung of single lung recipients. Traditional risk factors are associated with lung cancer in these patients. Lung cancer survival is worse among lung recipients despite earlier diagnosis.
1. Introduction
Lung transplant is increasingly utilized for patients with end-stage lung disease, with 13,000 US recipients alive with a functioning transplant in 2016.(1) Lung recipients face many long-term medical issues related to use of immunosuppressive medications, infection, and allograft dysfunction.(2–5) Among these risks, lung recipients have approximately twice the risk of cancer as the general population, and the incidence of lung cancer, specifically, may be even higher.(6–8) Excluding skin cancer, lung cancer represents the most common malignancy to arise after lung transplant.(8)
The increased risk of lung cancer following lung transplant is poorly understood, and may be attributable to immunosuppression, systemic inflammation, or lung infections.(9–11) For the large number of recipients with a single lung transplant (SLT) with a retained native lung, pre-existing pulmonary damage likely plays an important role as well.(12) Case series suggest that most incident lung cancers occur in the native lungs of SLT recipients, among former smokers, and among patients with pre-existing chronic obstructive pulmonary disease (COPD) or idiopathic pulmonary fibrosis (IPF).(13, 14) To date, there have been no studies systematically examining specific lung cancer risk factors and outcomes in patients after lung transplant.
To understand the epidemiology of lung cancer following lung transplant, we utilized data from the Transplant Cancer Match (TCM) Study, which links US transplant and cancer registries. Our primary objectives were three-fold: to evaluate lung cancer risk in lung recipients compared to the general population, distinguishing between cancers in the native and transplanted lungs; to evaluate risk factors for the development of lung cancer; and to compare lung cancers cases between lung recipients and other individuals with respect to histology, stage, treatment and survival.
2. Materials & Methods
2.1. Study Population
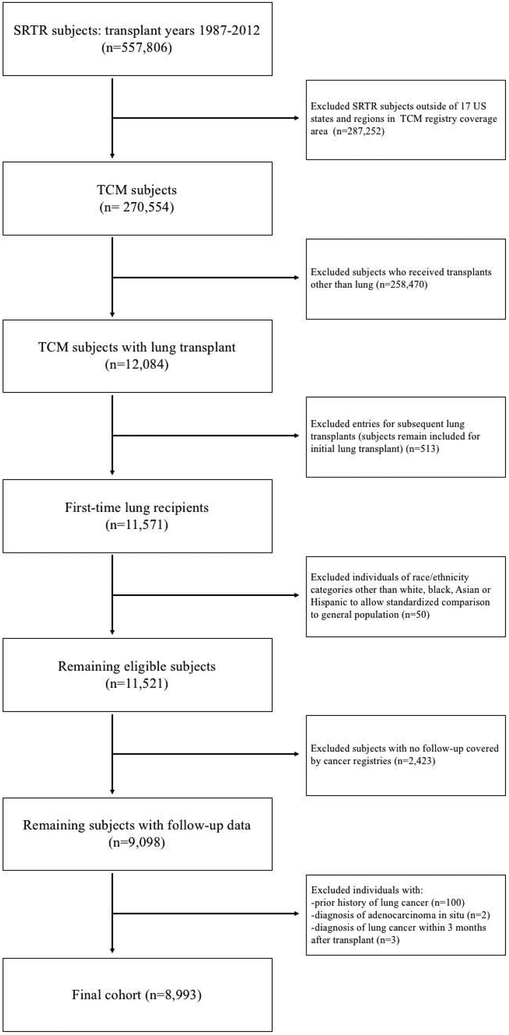
The TCM Study (www.transplantmatch.cancer.gov) links the US Scientific Registry of Transplant Recipients (SRTR) with 17 state and regional cancer registries (see Table 1 footnote).(6) The SRTR includes data on donors, candidates, and recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and is described elsewhere.(15) The Health Resources and Services Administration, US Department of Health and Human Services, provides oversight of the OPTN and SRTR. The TCM Study covers approximately half of US transplants during 1987–2012. We evaluated first-time lung recipients who resided in the area covered by a participating registry at waiting list entry or transplant for inclusion (n=11,571); after exclusions, the final cohort was comprised of 8,993 first-time lung recipients (see Figure 1).
Table 1.
Characteristics of cohort of first-time lung transplant recipients in the US Transplant Cancer Match Study (n=8993)*
| Characteristic | Transplant Recipient (number and %) |
|---|---|
| Sex | |
| Male | 4752 (52.8%) |
| Female | 4241 (47.2%) |
| Age at transplant, years | |
| 0–17 | 386 (4.3%) |
| 18–34 | 1210 (13.5%) |
| 35–49 | 1825 (20.3%) |
| 50–64 | 4640 (51.6%) |
| ≥65 | 932 (10.4%) |
| Race/ethnicity | |
| White, non-Hispanic | 7635 (84.9%) |
| Black, non-Hispanic | 624 (6.9%) |
| Hispanic | 590 (6.6%) |
| Asian/Pacific Islander | 144 (1.6%) |
| Highest education level | |
| None | 15 (0.2%) |
| Grade School | 334 (3.7%) |
| High School | 2690 (29.9%) |
| Attended college | 1817 (20.2%) |
| College degree | 1247 (13.9%) |
| Graduate degree | 509 (5.7%) |
| Not applicable (<5 years old) | 59 (0.7%) |
| Missing/unknown | 2322 (25.8%) |
| History of pre-transplant cigarette use | |
| Yes | 4410 (49.0%) |
| No | 2551 (28.4%) |
| Missing/Unknown | 2032 (22.6%) |
| Lung disease group | |
| COPD/A1AT | 3091 (34.4%) |
| IPF | 2212 (24.6%) |
| CF | 1401 (15.6%) |
| Other obstructive lung diseases | 522 (5.8%) |
| Inflammatory and fibrotic lung diseases | 748 (8.3%) |
| Airways diseases | 200 (2.2%) |
| Pulmonary hypertension and vascular diseases | 544 (6.1%) |
| Missing/Other | 275 (3.1%) |
| LAS grouped lung diagnosis | |
| Diagnosis group A | 4143 (46.1%) |
| Diagnosis group B | 525 (5.8%) |
| Diagnosis group C | 1407 (15.7%) |
| Diagnosis group D | 2767 (30.8%) |
| Missing/Other | 151 (1.7%) |
| Pre-transplant FEV1 %-predicted | |
| ≤30 %-predicted | 4227 (47.0%) |
| 31–50% predicted | 1722 (19.2%) |
| 51–80% predicted | 1330 (14.8%) |
| >80% predicted | 216 (2.4%) |
| Missing | 1498 (16.7%) |
| Pre-transplant FVC %-predicted | |
| ≤30% predicted | 878 (9.8%) |
| 31–50% predicted | 3423 (38.1%) |
| 51–80% predicted | 2762 (30.7%) |
| >80% predicted | 437 (4.9%) |
| Missing | 1493 (16.6%) |
| Transplant procedure type | |
| Single left lung | 2036 (22.6%) |
| Single right lung | 2036 (22.6%) |
| Bilateral sequential double lung | 4665 (51.9%) |
| En-Bloc double lung | 256 (2.9%) |
| Calendar year of transplant | |
| 1987–1994 | 964 (10.7%) |
| 1995–1999 | 1762 (19.6%) |
| 2000–2004 | 2341 (26.0%) |
| 2005–2009 | 3196 (35.5%) |
| 2010–2012 | 730 (8.1%) |
| Induction therapy | |
| None | 5471 (60.8%) |
| Anti-thymocyte globulin (ATG) | 811 (9.0%) |
| Anti-IL-2R antibody only | 2443 (27.2%) |
| Alemtuzumab only | 131 (1.5%) |
| Monoclonal antibody only (other than anti-IL2R and alemtuzumab) | 99 (1.1%) |
| Combination induction therapy | 38 (0.4%) |
| Calcineurin inhibitor maintenance† | |
| Tacrolimus | 4711 (52.4%) |
| Cyclosporine | 3385 (37.6%) |
| Both | 105 (1.2%) |
| Neither | 792 (8.8%) |
| Antimetabolite maintenance† | |
| Azathioprine | 4687 (52.1%) |
| Mycophenolate | 3537 (39.3%) |
| Both | 97 (1.1%) |
| Neither | 672 (7.5%) |
| Steroid maintenance† | |
| Yes | 8651 (96.2%) |
| No | 342 (3.8%) |
| mTOR inhibitor maintenance† | |
| Yes | 81 (0.9%) |
| No | 8912 (99.1%) |
The cohort includes lung transplant recipients in California, Colorado, Connecticut, Florida, Georgia, Hawaii, Illinois, Iowa, Kentucky, Michigan, New Jersey, New York, North Carolina, Pennsylvania, Texas, Utah and the Seattle-Puget Sound area of Washington state.
Maintenance regimens defined at baseline (initial discharge following transplant).
A1AT: alpha-1 antitrypsin deficiency, COPD: chronic obstructive pulmonary disease; IPF: idiopathic pulmonary fibrosis; CF: cystic fibrosis; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; LAS: Lung Allocation Score
LAS group A: obstructive lung diseases and others; group B: primary pulmonary hypertension and other pulmonary vascular diseases; group C: CF and immunodeficiency diseases; group D: IPF, fibrotic diseases and others
Figure 1.
Flow chart of selection of eligible study subjects. SRTR=Scientific Registry of Transplant Recipients. TCM=Transplant Cancer Match study
2.2. Data Collection and Variables
Characteristics of recipients and donors, transplant characteristics, and baseline immunosuppression and induction therapy were obtained from SRTR records. Recipient cigarette use was assessed using two SRTR variables: history of cigarette use or history of smoking >10 pack-years. Donor cigarette use was only reported in the dataset if >20 pack-years of lifetime use. Lung disease group was based on recipients’ primary listing diagnosis and categorized into 8 groups (see Table 1). We also assessed grouped diagnoses based on the 4 Lung Allocation Score (LAS) groups. We chose the 8 clinical disease groups for our analyses as we felt these better reflected clinicopathological as LAS grouping is partially based on waitlist mortality.(16) Pre-transplant spirometry testing values (forced expiratory volume in 1 second [FEV1], forced vital capacity [FVC]) were obtained from SRTR.
Incident lung cancers were ascertained using International Classification of Diseases for Oncology, 3rd edition codes (ICD-O-3) from the linked cancer registry records. Histologic subtype was classified as non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC) and other. NSCLC was further subdivided into adenocarcinoma, squamous cell carcinoma, and other NSCLC for some analyses. Clinical stage at cancer diagnosis was based on summary stage (localized, regional or distant). Cancer treatment (surgery, radiation, chemotherapy) and cause of death were ascertained from cancer registries, as described below.
2.3. Statistical Analyses
Follow-up of lung recipients for incident lung cancer began at 3 months after transplant (to avoid pre-existing cancers) and ended at the earliest of lung cancer diagnosis, death, graft failure/retransplant, loss to follow-up, or end of registry coverage (December 2012). We compared incidence among lung recipients to the general population using standardized incidence ratios (SIR, defined as observed cases/expected cases). Expected counts were obtained by applying general population lung cancer rates to person-time at risk, stratified by age, sex, race/ethnicity, calendar year, and registry area. We calculated overall SIRs and separate SIRs for cancers in the native and transplanted lungs by generating observed and expected counts separately for left and right lungs, then summing across recipients’ lungs based on the transplant procedure (left SLT, right SLT, bilateral transplant [BLT]). SIRs use the recipients’ demographic characteristics to calculate the expected number of cancers, but when the SIRs were calculated separately for transplanted lungs, we alternatively standardized by donor characteristics.
Poisson regression was utilized to calculate incidence rate ratios (IRRs) to examine risk factors for native lung cancers. Each SLT recipients’ relevant lung was used for this analysis. Risk factors assessed in univariate models for association with native lung cancer included recipient sex, age at transplant, education level, lung disease group, LAS diagnosis group, history of smoking, time since transplant, listing FEV1, listing FVC, baseline antimetabolite therapy, baseline calcineurin inhibitor, induction regimen and transplanted lung (left vs. right). LAS diagnosis group, baseline antimetabolite/calcineurin inhibitor and induction regimen were not included in our final multivariable model because of lack of significant association in univariate models. FEV1 and FVC demonstrated some differences in risk between categories of percent predicted values, but no significant trend. Thus, they were also not included. IRRs for lung disease group were based on effect coding, which compares incidence in each group to overall average incidence. In order to retain subjects with missing data in the models, “missing” was included as a category for each of these variables of interest. We also used Poisson regression to examine risk factors for lung cancers arising in donor lungs. Given a limited number of cases, we only assessed a few risk factors in univariate models including: donor and recipient age, single vs. bilateral lung transplant, donor and recipient smoking, and time since transplant.
We then compared lung cancer cases in lung recipients to cases in the general population, restricted to cancer registry areas that provided information on vital status (see Table 5 footnote). Hispanic ethnicity was inconsistently reported by cancer registries, so we excluded the few cases for which Hispanic ethnicity was indicated. We used chi-squared testing to compare characteristics of cases with or without a prior lung transplant. The probability of all-cause mortality following cancer diagnosis was calculated for transplant and non-transplant cases using Kaplan-Meier curves with log-rank testing. We used Cox proportional hazards regression to compare all-cause mortality and lung cancer-specific mortality following cancer diagnosis, adjusting for age at diagnosis, sex, race/ethnicity, year of diagnosis, stage, and treatment modality. Some multivariable models were restricted to registries that provided information on cause of death and cancer treatment (see Table 5 and 6 footnotes). Analyses are reported overall and for NSCLC cases, as SCLC is staged and treated differently.(17) A final survival model was created to compare transplant and non-transplant NSCLC cases with local or regional stage disease undergoing potentially curative therapy (either surgery or radiation).(18–20) To account for any differences in the relationship between lung transplant and survival over time, we developed survival models separately for 3 eras according to year of cancer diagnosis (1992–2002, 2003–2006, 2007–2012). We then tested an interaction term between year of cancer diagnosis and an indicator variable for having a lung transplant. Finally, among our cohort of lung transplant recipients, we compared survival between those who developed lung cancer and those who did not, treating the occurrence of lung cancer as a time-dependent risk factor.
Table 5.
Lung cancer cases in lung recipients compared to other lung cancer cases in the general population*
| Characteristics | Lung cancer cases in lung recipients | Other lung cancer cases in the general population | P-value |
|---|---|---|---|
| Total number of patients | 121 (100%) | 1,168,149 (100%) | |
| Age at cancer diagnosis, years | <0.001 | ||
| Median (IQR) | 62 (58–66) | 69 (61–76) | |
| <60 | 38 (31.4%) | 244,866 (21.0%) | |
| 60–69 | 68 (56.2%) | 342,912 (29.4%) | |
| ≥70 | 15 (12.4%) | 580,371 (49.7%) | |
| Sex | 0.70 | ||
| Male | 68 (56.2%) | 636,356 (54.5%) | |
| Female | 53 (43.8%) | 531,793 (45.5%) | |
| Race | 0.52 | ||
| White | 108 (89.3%) | 1,001,643 (85.8%) | |
| Black | 10 (8.3%) | 120,770 (10.3%) | |
| Asian/Pacific Islander | 3 (2.5%) | 45,736 (3.9%) | |
| Year of diagnosis | <0.001 | ||
| 1992–1996 | 5 (4.1%) | 262,446 (22.5%) | |
| 1997–2001 | 22 (18.2%) | 322,113 (27.6%) | |
| 2002–2005 | 41 (33.9%) | 262,703 (22.5%) | |
| 2006–2012 | 53 (43.8%) | 320,887 (27.5%) | |
| Lung cancer histology | 0.006§ | ||
| NSCLC | 111 (91.7%) | 983,838 (84.2%) | |
| Adenocarcinoma | 39 (32.2%) | 411,241 (35.2%) | |
| Squamous cell carcinoma | 42 (34.7%) | 250,318 (21.4%) | |
| Other NSCLC | 30 (24.8%) | 322,279 (27.6%) | |
| SCLC | 9 (7.4%) | 168,507 (14.4%) | |
| Other lung cancer | 1 (0.8%) | 15,804 (1.4%) | |
| Cancer summary stage | 0.019 | ||
| Localized | 33 (27.3%) | 214,272 (18.3%) | |
| Regional | 29 (24.0%) | 277,663 (23.8%) | |
| Distant | 55 (45.5%) | 570,530 (48.8%) | |
| Unstaged | 4 (3.3%) | 105,684 (9.0%) | |
| Surgical therapy† | 0.048 | ||
| Yes | 31 (36.9%) | 178,546 (26.0%) | |
| No | 53 (63.1%) | 498,319 (72.6%) | |
| Unknown | 0 | 9,927 (1.4%) | |
| Radiation therapy† | 0.007 | ||
| Yes | 19 (22.6%) | 254,221 (37.0%) | |
| No | 65 (77.4%) | 420,012 (61.2%) | |
| Unknown | 0 | 12,559 (1.8%) | |
| Chemotherapy† | 0.40 | ||
| Yes | 26 (30.9%) | 258,249 (37.6%) | |
| No | 53 (63.1%) | 398,341 (58.0%) | |
| Unknown | 5 (6.0%) | 30,202 (4.4%) | |
| Vital status | 0.06 | ||
| Alive | 8 (6.6%) | 141,301 (12.1%) | |
| Dead | 113 (93.4%) | 1,026,848 (87.9%) | |
| Vital status and cause of death‡ | |||
| Alive | 8 (7.6%) | 105,960 (11.2%) | |
| Dead | 98 (92.5%) | 841,165 (88.8%) | |
| Due to lung cancer | 60 (56.6%) | 555,592 (58.7%) | |
| Due to other causes | 38 (35.9%) | 285,573 (30.2%) |
Cohort is restricted to registry areas that provide information on vital status and stage: California, Colorado, Connecticut, Georgia, Hawaii, Iowa, Illinois, Kentucky, New Jersey, New York, Seattle-Puget Sound region, Texas, and Utah.
Analysis incudes the following registry areas that provide information on cancer treatment: California, Colorado, Connecticut, Georgia, Iowa, Kentucky (2001-), New Jersey, Texas (2003-)
Analysis includes the following registry areas that provide cause of death information: California, Colorado, Connecticut, Georgia, Iowa, Illinois, Kentucky, New Jersey, Seattle-Puget Sound region, Texas
p-value for chi-squared test of heterogeneity between squamous cell, adenocarcinoma, other NSCLC, SCLC, and other
NSCLC=non-small cell lung cancer, SCLC=small cell lung cancer
Table 6.
All-cause and lung cancer-specific mortality after lung cancer diagnosis in lung recipients compared with other lung cancer patients in the general population
| Model | Total lung cancer cases, n | Deaths, n (%) | Unadjusted HR (95% CI) | Lung cancer cases with treatment information, n § | Deaths, n (%)§ | Adjusted HR (95% CI) §|| |
|---|---|---|---|---|---|---|
| All-cause mortality* | ||||||
| All lung cancer | ||||||
| Lung recipients | 121 | 113 (94%) | 1.41 (1.17–1.69) | 84 | 78 (93%) | 1.90 (1.52–2.37) |
| Other patients | 1,168,149 | 1,026,848 (88%) | 1.0 (Ref) | 686,792 | 611,194 (89%) | 1.0 (Ref) |
| NSCLC | ||||||
| Lung recipients | 111 | 103 (93%) | 1.39 (1.15–1.69) | 76 | 70 (92%) | 1.84 (1.46–2.33) |
| Other patients | 983,939 | 860,563 (88%) | 579,440 | 513,240 (89%) | ||
| NSCLC curative treatment† | ||||||
| Lung recipients | 27 | 23 (85%) | 1.79 (1.20–2.69) | 27 | 23 (85%) | 2.18 (1.54–3.10) |
| Other patients | 190,629 | 140,887 (74%) | 1.0 (Ref) | 190,629 | 140,887 (74%) | 1.0 (Ref) |
| Lung cancer-specific mortality‡ | ||||||
| All lung cancer | 106 | 60 (57%) | 1.32 (1.03–1.69) | 1.67 (1.28–2.18) | ||
| Lung recipients | 947,125 | 555,592 (59%) | 1.0 (Ref) | 84 | 54 (64%) | 1.0 (Ref) |
| Other patients | 686,792 | 485,683 (71%) | ||||
| NSCLC | ||||||
| Lung recipients | 96 | 53 (55%) | 1.28 (0.98–1.67) | 76 | 48 (63%) | 1.62 (1.22–2.15) |
| Other patients | 795,129 | 459,592 (58%) | 1.0 (Ref) | 579,440 | 403,223 (70%) | 1.0 (Ref) |
| NSCLC curative treatment† | ||||||
| Lung recipients | 27 | 11 (41%) | 1.15 (0.64–2.08) | 27 | 11 (41%) | 1.83 (1.15–2.90) |
| Other patients | 190,629 | 96,294 (51%) | 1.0 (Ref) | 190,629 | 96,294 (51%) | 1.0 (Ref) |
Analyses of all-cause mortality included all registry areas that provide information on vital status: California, Colorado, Connecticut, Georgia, Hawaii, Iowa, Illinois, Kentucky, New Jersey, New York, Seattle-Puget Sound region, Texas, Utah
Analysis was restricted to patients with NSCLC with local or regional stage who underwent surgery and/or radiation as treatment. This analysis includes the following 8 registries that provided information on cancer treatment: California, Colorado, Connecticut, Georgia, Iowa, Kentucky (cases diagnosed in 2001 onward), New Jersey, Texas (2003 onward).
Analysis was restricted to the following registries that provided the cause of death information: California, Colorado, Connecticut, Georgia, Iowa, Illinois, Kentucky, New Jersey, Seattle, and Texas.
Analysis was restricted to the following 8 registries that provided information on cancer treatment: California, Colorado, Connecticut, Georgia, Iowa, Kentucky (cancers diagnosed in 2001 onward), New Jersey, Texas (2003 onward).
Models were adjusted for age at cancer diagnosis, sex, race, year of cancer diagnosis, cancer stage and treatment.
NSCLC=non-small cell lung cancer, HR=hazard ratio
The TCM Study was approved by human subjects committees of the National Cancer Institute and participating cancer registries. All statistical analyses were two-sided and performed using SAS (versions 9.3 and 9.4, SAS Institute, Cary, NC). A p-value of < 0.05 was considered statistically significant.
3. Results
3.1. Cohort description and lung cancer risk
The cohort of 8,993 first-time lung recipients were near evenly split between males and females, and the median age at transplant was 54 years (Table 1). The leading indications for transplant were COPD with or without alpha-1 antitrypsin deficiency (COPD/A1AT, 34%), IPF (25%) and cystic fibrosis (CF, 16%). Of recipients with available smoking data (77%), 64% had pre-transplant cigarette use. Forty-five percent received SLT and 55% received BLT. Donors were more likely to be male, more likely to be non-smokers, and were much younger than recipients (Table 2).
Table 2.
Characteristics of lung donors for first-time lung transplant recipients in the US Transplant Cancer Match Study (n=8,256)
| Characteristic | Lung Donor (number and %) |
|---|---|
| Sex | |
| Male | 5125 (62%) |
| Female | 3131 (38%) |
| Age at donation | |
| 0–17 | 1463 (18%) |
| 18–34 | 3514 (44%) |
| 35–49 | 2082 (25%) |
| 50–64 | 1054 (13%) |
| ≥65 | 43 (0.52%) |
| Race/ethnicity | |
| White, non-Hispanic | 5354 (65%) |
| Black, non-Hispanic | 1290 (16%) |
| Hispanic | 1370 (17%) |
| Asian/Pacific Islander | 242 (2.9%) |
| Lungs donated | |
| Single left lung | 1345 (16%) |
| Single right lung | 1341 (16%) |
| Both lungs | 5570 (67%) |
| Cigarette use >20 pack-years | |
| Yes | 1471 (18%) |
| No | 6055 (73%) |
| Missing/Unknown | 730 (8.8%) |
There were 183 incident lung cancers during follow-up (528 per 100,000 person-years). The median time from transplant to cancer was 3.9 years (interquartile range [IQR] 1.8–6.2) and was similar between those who developed lung cancer in a native (4.0 years, IQR 1.8–6.2) or a donor lung (3.5 years, IQR 0.93–6.9). We excluded all cases occurring in the first 3 months, and only 4 of 183 cases occurred in the subsequent 3 months. Most lung cancers occurred in SLT recipients (n=169, 92%) and, within this group, in the native lung (n=153, 91%). Overall, lung cancer risk was elevated almost 5-fold in lung recipients compared to the general population (SIR 4.8, 95% confidence interval [CI] 4.1–5.5) (Table 3). Risk of lung cancer in the native lung in SLT recipients was markedly high (SIR 13, 95%CI 11–15). Risk was not elevated in transplanted lungs when standardized by recipient characteristics (SIR 1.0, 95%CI 0.69–1.5). However, given the younger age of donors, when standardized by donor characteristics, the risk was strongly increased (SIR 5.0, 95%CI 3.3–7.3).
Table 3.
Standardized incidence ratios for lung cancer in lung recipients.
| Lung cancer grouping | SIR (95% CI)* |
|---|---|
| Lung cancer overall | 4.8 (4.1–5.5) |
| NSCLC | 5.2 (4.4–6.1) |
| Adenocarcinoma | 4.4 (3.3–5.7) |
| Squamous cell carcinoma | 8.1 (6.3–10) |
| SCLC | 2.6 (1.5–4.2) |
| Native lung cancer in SLT recipients | 13 (11–15) |
| Donor lung cancer | |
| Using recipient standardization | 1.0 (0.69–1.5) |
| Using donor standardization | 5.0 (3.3–7.3) |
SIRs compare observed lung cancer incidence in transplant recipients to general population incidence, standardized by age, sex, race/ethnicity, calendar year of transplant and registry area using recipient characteristics unless otherwise specified.
SIR=standardized incidence ratio, SLT=single lung transplant, NSCLC=non-small cell lung cancer, SCLC=small cell lung cancer
Thirty-seven percent (n=67) of lung cancer diagnoses were squamous cell carcinoma, 32% (n=58) adenocarcinoma, and 22% (n=40) other NSCLC. Eight percent (n=15) were SCLC and 2% (n=3) were other histologic subtypes. The SIR for squamous cell cancer was elevated to 8.1 (95%CI 6.3–10.3) while the SIR for adenocarcinoma was 4.4 (95%CI 3.3–5.7). In contrast, the SIR for SCLC was lower at 2.6 (95%CI 1.5–4.2).
3.2. Risk factors for incident lung cancer
We examined risk factors for native lung cancer (n=153) in SLT recipients (n=4,072) (Table 4). Older age at transplant, lower education level, history of cigarette smoking prior to transplant, and longer time since transplant were significantly associated with increased risk in multivariable models. Ninety percent of cases of native lung cancers occurred in recipients with either COPD/A1AT or IPF. However, while an IPF diagnosis was associated with significantly higher risk of native lung cancer (adjusted IRR 1.6, 95%CI 1.0–2.6), risk in recipients with COPD/A1AT was similar to the average risk across disease groups. Lung cancer incidence was also higher in a native right lung compared to a native left lung. In unadjusted models to explore risk factors for lung cancer arising in the donor lung (n=26), donor and recipient age, recipient smoking, and having a single lung transplant were associated with incidence of donor lung cancer (Table S1).
Table 4.
Risk factors for native lung cancer in single-lung recipients (n=4,072)
| Characteristic | Cases | Unadjusted IRR (95% CI) | P-value | Adjusted IRR (95% CI)* | P-value |
|---|---|---|---|---|---|
| Gender | 0.55 | 0.99 | |||
| Male | 80 | 1.0 (Ref) | |||
| Female | 73 | 0.91 (0.66–1.3) | 1.0 (0.72–1.4) | ||
| Age at transplant, years | 0.001 | 0.02 | |||
| 35–49 | 14 | 0.44 (0.25–0.77) | 0.54 (0.30–0.96) | ||
| 50–64 | 117 | 1 | 1.0 (Ref) | ||
| ≥65 | 22 | 1.3 (0.84–2.1) | 1.5 (0.91–2.3) | ||
| Education level | 0.18 | 0.03 | |||
| None/grade school | 4 | 1.1 (0.41–3.1) | 1.2 (0.44–3.4) | ||
| High school | 48 | 1 | 1.0 (Ref) | ||
| Attended college | 31 | 1.0 (0.66–1.6) | 1.1 (0.68–1.7) | ||
| College degree | 10 | 0.56 (0.28–1.1) | 0.61 (0.30–1.2) | ||
| Graduate degree | 3 | 0.38 (0.12–1.2) | 0.34 (0.11–1.1) | ||
| Missing/unknown | 57 | 0.99 (0.68–1.5) | 1.4 (0.93–2.1) | ||
| Native lung (site of cancer) | 0.004 | 0.005 | |||
| Right | 91 | 1.6 (1.2–2.2) | 1.6 (1.2–2.2) | ||
| Left | 62 | 1 | 1.0 (Ref) | ||
| Lung disease group† | <0.001 | <0.001 | |||
| Idiopathic pulmonary fibrosis | 46 | 1.8 (1.1–2.7) | 1.6 (1.0–2.6) | ||
| COPD/A1AT | 92 | 1.6 (1.1–2.3) | 1.1 (0.69–1.6) | ||
| Cystic fibrosis | 0 | -- | -- | ||
| Other obstructive lung disease | 5 | 0.57 (0.26–1.3) | 0.57 (0.25–1.3) | ||
| Other inflammatory or fibrotic lung disease | 4 | 0.63 (0.26–1.5) | 0.67 (0.28–1.6) | ||
| Pulmonary hypertension and pulmonary vascular disease | 4 | 1.4 (0.58–3.3) | 1.9 (0.79–4.7) | ||
| Other‡ | 2 | 0.73 (0.22–2.4) | 0.79 (0.24–2.6) | ||
| History of cigarette smoking | <0.001 | <0.001 | |||
| Yes | 111 | 1 | 1.0 (Ref) | ||
| No | 12 | 0.50 (0.27–0.90) | 0.45 (0.28–0.72) | ||
| Missing/unknown | 30 | 0.53 (0.38–0.80) | 0.45 (0.24–0.85) | ||
| Time since transplant | 0.007 | <0.001 | |||
| 91 days – 1 year | 13 | 1 | 1.0 (Ref) | ||
| 1 – 2 years | 28 | 2.0 (1.0–3.8) | 2.0 (1.1–3.9) | ||
| 2 – 4 years | 37 | 1.8 (0.94–3.3) | 1.9 (1.0–3.6) | ||
| 4 – 6 years | 33 | 2.5 (1.3–4.8) | 2.8 (1.5–5.4) | ||
| >6 years | 42 | 2.8 (1.5–5.2) | 3.6 (1.9–6.7) |
Poisson regression model incidence rate ratios (IRRs) are mutually adjusted for the variables appearing in this table.
IRRs were calculated using effect coding, so that risk for each diagnosis group is compared to the average risk across diagnosis groups.
Other diseases also includes the category “other airways diseases.” No recipients had cystic fibrosis as a result of the restriction to single-lung recipients and age at transplantation to 35 years and older.
A1AT=Alpha-1 antitrypsin deficiency; COPD: chronic obstructive pulmonary disease
3.3. Lung cancer stage and survival
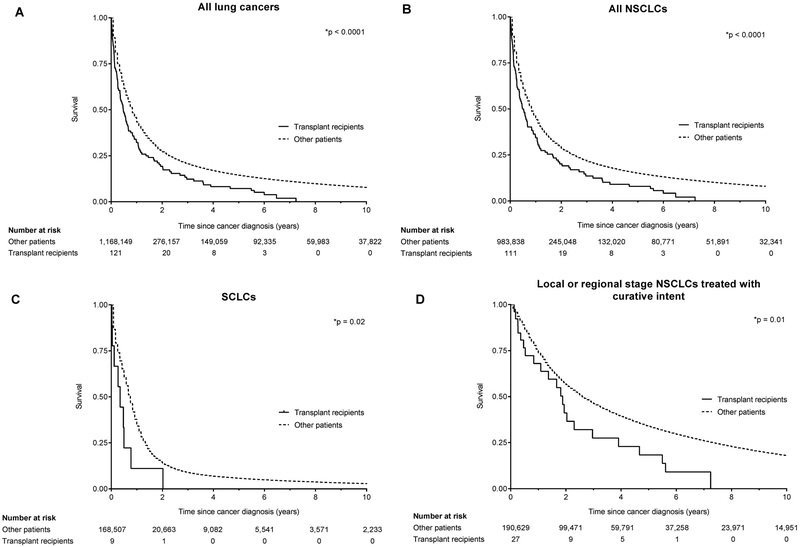
Lung cancer cases in lung recipients in the restricted cohort (n=121) were compared to 1,168,149 other lung cancer patients from the general population. Lung cancer patients with a lung transplant were younger (p<0.001) compared to other lung cancer patients (Table 5). Based on summary stage, lung cancers in lung recipients were more likely than other cases to be localized, both overall and for the subset of NSCLC (28% vs. 20%, p=0.04). Lung cancer patients with a lung transplant were more likely to receive surgery compared to other lung cancer patients (37% vs. 26%, p=0.05). Among NSCLC patients with localized/regional disease, lung recipients were also more likely to receive surgery (93% vs. 69%, p=0.01) and less likely to receive radiation (11% vs. 43%, p=0.001). Despite being more likely to present in localized stage, lung transplant patients had shorter survival after cancer diagnosis than other lung cancer patients (median 153 vs. 245 days, p<0.001; Figure 2A). Survival was also worse in lung transplant recipients when analysis was stratified to NSCLC (p<0.001, Figure 2B) or SCLC (p=0.02, Figure 2C).
Figure 2.
Kaplan-Meier curves comparing overall survival in lung transplant recipients to other lung cancer patients. P-values are based on the log-rank test. NSCLC=non-small cell lung cancer, SCLC=small cell lung cancer.
In Cox models adjusted for stage and cancer treatment, survival outcomes were worse, for all cancer cases and specifically for those with NSCLC (all-cause mortality: adjusted HR 1.84, 95%CI 1.46–2.33; cancer-specific mortality: adjusted HR 1.62, 95%CI 1.22–2.15) (Table 6). In analyses restricted to patients with NSCLC diagnosed at localized/regional stage who received potentially curative therapy (surgery and/or radiation), survival was also worse among lung recipients (p<0.001, Figure 1D; all-cause mortality: adjusted HR 2.18, 95%CI 1.54–3.10; cancer-specific mortality: adjusted HR 1.83, 95%CI 1.15–2.90). The association between transplantation and increased mortality appeared similar across the three time eras (Table S2), and interaction terms between year of lung cancer diagnosis and having a lung transplant were not significant for all-cause or lung cancer specific mortality.
Finally, among the cohort of lung transplant recipients, we compared survival between those who developed lung cancer in follow-up and those who did not, adjusted for age at transplant, sex, race/ethnicity and year of transplant. The risk of death was substantially higher among subjects who developed lung cancer compared to those who did not (unadjusted HR 7.25, 95% CI 6.17–8.53; adjusted HR 6.51, 95% CI 5.53–7.67).
4. Discussion
In this study, we present novel findings on incidence, risk factors, and outcomes of lung cancer among lung transplant recipients. We found that native lung cancer in SLT recipients occurred at a 13-fold higher rate than in the general population, and a number of established cancer risk factors were associated with increased risk. We also observed higher than expected lung cancer risk in transplanted lungs, when risk was standardized to characteristics of the lung donors. Finally, we demonstrated that mortality was elevated in lung recipients with lung cancer compared to other lung cancer patients, despite a higher likelihood of localized stage at diagnosis and surgical therapy.
A key finding of this study is that lung cancer risk is greatly increased in the native lungs of patients after SLT. These results are similar to a prior national cohort study which reported that 84% of all lung cancer cases occurred in SLT recipients. (8) Despite pre-existing damage in these native lungs, most appear to arise after transplant as: (1) patients undergo extensive evaluation prior to transplant (including chest computed tomography [CT]), which would detect many pre-existing cancers, (2) there were very few cases diagnosed within 6 months of transplant, and (3) the median time to lung cancer diagnosis in our population was 3.9 years after transplant. Nonetheless, pre-existing damage in the native lung is likely the most critical factor in the elevated risk for native lung cancer. The majority of patients who developed lung cancer in the native lung had listing diagnoses of COPD and IPF. An IPF diagnosis was associated with above-average risk, and this condition is a strong cancer risk factor in the general population. (21–23) Also, traditional risk factors, such as older age and smoking, were associated with further increased risk. Our finding of elevated risk of right-sided cancer confirmed the performance of our model, as the risk of right-sided lung cancer is increased by a similar magnitude in the general population, owing to relative size of the two lungs. (24, 25)
Our findings suggest that transplant-related factors also contribute to carcinogenesis. First, we found that native lung cancer risk increased with time from transplant, which may reflect cumulative effects of immunosuppression, local inflammation, and/or repeated pulmonary infections. Second, increased risk was not limited to native lung cancers alone. We initially observed that cancer risk in the transplanted lungs was similar to risk in the general population using the recipient’s characteristics. However, that comparison was somewhat misleading, because the transplanted lungs in the recipients come from donors who are, on average, much younger. Indeed, when we standardized by characteristics of the lung donor, a marked increased risk of cancer in the donor lung was readily apparent. Finally, there was a histology shift in the lung cancer cases after transplant, with an especially large increase in the risk for squamous cell lung cancer, suggesting unique processes leading to different tumor biology from that underlying lung cancers in the general population. As mentioned in the Methods, we did not find an association between specific baseline immunosuppression regimen or induction regimen and lung cancer (data not shown). The majority of patients are on a combination of an antimetabolite, a calcineurin inhibitor, and corticosteroids, so this may indicate that risk is related to immunosuppression in general, rather than a specific commonly-used agent.
We also found observations pertaining to stage of disease and treatment. More cancer cases in recipients were diagnosed at localized stage compared to lung cancers in other patients, which is likely attributable to increased surveillance. We found that lung recipients were more likely to receive more aggressive treatment, with 93% of recipients with localized or regional stage cancer undergoing surgery. Surgery is considered the gold standard for curative therapy (with the alterative being stereotactic body radiation therapy) for early stage NSCLC.(19, 20) This difference in treatment could reflect a number of possibilities, including more aggressive goals of care for transplant recipients, higher likelihood of surgical candidacy (with patients having previously undergone an extensive transplant evaluation), and lack of contribution of the native lung (where most cases occur) to transplant recipients’ gas exchange.
A novel aspect of this study is our examination of survival, comparing lung cancer outcomes in patients with lung transplant to the general population. Despite the increase in surgical treatment, survival was worse for lung cancer cases arising in lung transplant recipients. When we assessed NSCLC patients with local and regional stages who received potentially curative treatment, we again observed higher cancer-specific mortality in lung recipients. All-cause mortality following lung cancer diagnosis was high. There is likely a component of mortality which is due to allograft dysfunction, related to pulmonary toxicity of cancer treatments and/or reduction in immunosuppression in response to a cancer diagnosis. Nonetheless, the added deaths attributed to lung cancer may also reflect more aggressive cancer biology, lack of effective immune control of tumor progression, or simply death attributed to cancer with major contributions from transplant-related comorbid illness. This finding of worse survival is consistent with previous studies that suggest worse prognosis for certain malignancies after solid organ transplant. (26, 27) A previous study of NSCLC presenting after any solid organ transplant demonstrated that these patients present at earlier stages but had worse overall survival than the general population.(28) Lung cancer has the lowest 5-year survival of all common malignancies.(29) In our analysis, mortality was 6.5-fold elevated among recipients who developed lung cancer, demonstrating the high lethality attributable to this cancer among lung recipients.
These findings have important implications. We define the high risk of lung cancer in native lungs of SLT recipients, and the high mortality in these patients despite a higher likelihood of presenting in early stage. The results may provide some evidence for a benefit of BLT over SLT among at-risk recipients, such as those with IPF. Our results also have implications for counseling and post-transplant surveillance for lung cancer in patients receiving SLT. While the frequency of post-transplant clinical surveillance typically decreases after a few years, we found an increasing incidence of lung cancer with further time since transplant. There are no data on screening with annual chest CT in the transplant population as is currently recommended for high-risk current and former heavy smokers in the general population. (30, 31) We caution that screening in lung recipients may by complicated by interpretation of imaging given pre-existing lung disease, and given the lower survival of early stage cancers demonstrated here, screening may detect more cancer without an associated survival benefit. Additional research in this area will be needed.
This study has notable strengths. This is the first study to comprehensively compare lung cancer after lung transplant to other lung cancers in the general population. The study cohort includes approximately half of all lung transplants performed in the US over the study period, with data on lung recipients and donors (from the SRTR) and largely complete ascertainment of lung cancers (from cancer registries). There are limitations as well. Some analyses were limited by missing data or lack of granular details. For example, smoking status was missing for 23% of recipients and we did not have detailed information on the exposure such as pack-years of tobacco use. This limits our analysis where, particularly for native lung cancer, smoking is expected to be a strong and dose-dependent risk factor. We would expect heavy smoking to be a much more significant risk factor for post-transplant cancer than we determined for smoking overall. We also lacked longitudinal information on details of immunosuppression regimens, other than baseline regimen and induction, and post-transplant complications. This prevented us from evaluating the impact of post-transplant risk factors which likely contribute to carcinogenesis in both native and donor lungs. It is possible that these factors contribute to development of lung cancer, but the absence of data in our study would not bias associations with the baseline factors that we assessed. The small number of cases of donor lung cancers limited us to an exploratory analysis, and we could not assess whether post-transplant events, such as infections and chronic rejection, contribute to cancers in the donor lung. The study is also limited by lack of additional data beyond December 2012. Finally, stage is most completely recorded in the cancer registries as a summary stage (localized, regional or distant), but this does not completely align with TNM staging used to guide NSCLC treatment. (20)
In conclusion, we found a nearly 5-fold increase in risk in lung cancer among lung transplant recipients compared to the general population, with a 13-fold increase for native lung cancer among SLT recipients. Traditional cancer risk factors, such as age, smoking and an IPF diagnosis, were associated with increased risk in these recipients. While our findings point to an additional influence of post-transplant factors on lung cancer risk, the unique contribution of specific factors such as immunosuppressants, and role of post-transplant infections on risk of lung cancer will require future examination. Survival is poor following a lung cancer diagnosis, and despite earlier stage at diagnosis and more aggressive treatment, lung recipients had worse overall and cancer-specific mortality than other lung cancer patients. These findings provide important insights into the epidemiology of lung cancer after lung transplant, suggest a potential benefit of BLT over SLT, and may be useful in understanding which lung transplant recipients are at highest risk for lung cancer.
Supplementary Material
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Cancer Institute. The authors gratefully acknowledge the support and assistance provided by individuals at the Health Resources and Services Administration (Monica Lin), the SRTR (Ajay Israni, Bertram Kasiske, Paul Newkirk, Jon Snyder), and the following cancer registries: the states of California (Tina Clarke), Colorado (Jack Finch), Connecticut (Lou Gonsalves), Florida (Brad Wohler), Georgia (Rana Bayakly), Hawaii (Brenda Hernandez), Iowa, Illinois (Lori Koch), Kentucky (Jaclyn Nee), Michigan (Glenn Copeland), New Jersey (Xiaoling Niu), New York (Amy Kahn), North Carolina (Chandrika Rao), Texas (Leticia Nogueria), and Utah (Janna Harrell), and the Seattle-Puget Sound area of Washington (Margaret Madeleine). We also thank Kelly Yu at the National Cancer Institute for study management, and analysts at Information Management Services for programming support (David Castenson, Matthew Chaloux, Michael Curry, Ruth Parsons).
The SRTR is currently operated under contract number HHSH250201500009C (Health Resources and Services Administration) by the Minneapolis Medical Research Foundation, Minneapolis, MN. Previously the SRTR was managed under contracts HHSH250201000018C and HHSH234200537009C. The following cancer registries were supported by the SEER Program of the National Cancer Institute: California (contracts HHSN261201000036C, HHSN261201000035C, and HHSN261201000034C), Connecticut (HHSN261201300019I), Hawaii (HHSN261201000037C, N01-PC-35137, and N01-PC-35139), Iowa (HSN261201000032C and N01-PC-35143), New Jersey (HHSN261201300021I, N01-PC-2013–00021), Seattle-Puget Sound (N01-PC-35142), and Utah (HHSN2612013000171). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: California (agreement 1U58 DP000807–01), Colorado (U58 DP000848–04), Georgia (5U58DP003875–01), Illinois (5U58DP003883–03), Maryland (U58DP12–1205 3919–03), Michigan (5U58DP003921–03), New Jersey (NU58DP003931-05-00), New York (U58DP003879), North Carolina (U58DP003933) and Texas (5U58DP000824–04). Additional support was provided by the states of California, Colorado, Connecticut, Illinois, Iowa, Massachusetts (Massachusetts Cancer Prevention and Control Cooperative Agreement 5458DP003920), New Jersey, New York (including the Cancer Surveillance Improvement Initiative), Texas, Utah, and Washington, as well as the University of Utah and Fred Hutchinson Cancer Research Center in Seattle, WA.
The views expressed in this paper are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute, Health Resources and Services Administration, SRTR, cancer registries, or their contractors.
Dr. Triplette was supported by a training grant from the NHLBI/NIH (T32 HL007287) during the conduct of this study.
Abbreviations
- A1AT
alpha-1 antitrypsin deficiency
- BLT
bilateral lung transplant
- CF
cystic fibrosis
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CT
computed tomography
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- IPF
idiopathic pulmonary fibrosis
- IRR
incidence rate ratio
- LAS
Lung Allocation Score
- NSCLC
non-small cell lung cancer
- OPTN
Organ Procurement and Transplantation Network
- SCLC
small cell lung cancer
- SIR
standardized incidence ratio
- SLT
single lung transplant
- SRTR
Scientific Registry of Transplant Recipients
- TCM
Transplant Cancer Match
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr. Lynch discloses funds from the National Cancer Institute to cover costs of Iowa Cancer Registry linkage to the SRTR. The other authors have no conflicts of interest to disclose.
Supporting Information
Additional supplementary materials may be found online in the Supporting Information section for this article.
References
- 1.Valapour M, Lehr CJ, Skeans MA, Smith JM, Carrico R, Uccellini K et al. OPTN/SRTR 2016 Annual Data Report: Lung. American Journal of Transplantation 2018. Suppl 1: 363–433. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad S, Shlobin OA, Nathan SD. Pulmonary complications of lung transplantation. Chest 2011;139(2):402–411. [DOI] [PubMed] [Google Scholar]
- 3.Kotloff RM, Ahya VN. Medical complications of lung transplantation. The European Respiratory Journal 2004;23(2):334–342. [DOI] [PubMed] [Google Scholar]
- 4.Lyu DM, Zamora MR. Medical complications of lung transplantation. Proceedings of the American Thoracic Society 2009;6(1):101–107. [DOI] [PubMed] [Google Scholar]
- 5.Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proceedings of the American Thoracic Society 2009;6(1):79–93. [DOI] [PubMed] [Google Scholar]
- 6.Engels EA, Pfeiffer RM, Fraumeni JF Jr., , Kasiske BL, Israni AK, Snyder JJ et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011;306(17):1891–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. American Journal of Transplantation 2010;10(8):1889–1896. [DOI] [PubMed] [Google Scholar]
- 8.Magruder JT, Crawford TC, Grimm JC, Kim B, Shah AS, Bush EL et al. Risk Factors for De Novo Malignancy Following Lung Transplantation. American Journal of Transplantation 2017; 17(1): 227–238. [DOI] [PubMed] [Google Scholar]
- 9.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Review of Anticancer Therapy 2008;8(4):605–615. [DOI] [PubMed] [Google Scholar]
- 10.Olland AB, Falcoz PE, Santelmo N, Kessler R, Massard G. Primary lung cancer in lung transplant recipients. The Annals of Thoracic Surgery 2014;98(1):362–371. [DOI] [PubMed] [Google Scholar]
- 11.Billups K, Neal J, Salyer J. Immunosuppressant-driven de novo malignant neoplasms after solid-organ transplant. Progress in Transplantation 2015;25(2):182–188. [DOI] [PubMed] [Google Scholar]
- 12.Dickson RP, Davis RD, Rea JB, Palmer SM. High frequency of bronchogenic carcinoma after single-lung transplantation. The Journal of Heart and Lung Transplantation 2006;25(11):1297–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathew J, Kratzke RA. Lung cancer and lung transplantation: a review. Journal of Thoracic Oncology 2009;4(6):753–760. [DOI] [PubMed] [Google Scholar]
- 14.Raviv Y, Shitrit D, Amital A, Fox B, Rosengarten D, Fruchter O et al. Lung cancer in lung transplant recipients: experience of a tertiary hospital and literature review. Lung Cancer 2011;74(2):280–283. [DOI] [PubMed] [Google Scholar]
- 15.Leppke S, Leighton T, Zaun D, Chen SC, Skeans M, Israni AK et al. Scientific Registry of Transplant Recipients: collecting, analyzing, and reporting data on transplantation in the United States. Transplantation Reviews 2013;27(2):50–56. [DOI] [PubMed] [Google Scholar]
- 16.Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB et al. Development of the new lung allocation system in the United States. American Journal of Transplantation 2006;6(5 Pt 2):1212–1227. [DOI] [PubMed] [Google Scholar]
- 17.vVn Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011;378(9804):1741–1755. [DOI] [PubMed] [Google Scholar]
- 18.Dan T, Williams NL. Management of Stage I Lung Cancer with Stereotactic Ablative Radiation Therapy. Surgical Oncology Clinics of North America 2017;26(3):393–403. [DOI] [PubMed] [Google Scholar]
- 19.Shah JL, Loo BW Jr., Stereotactic Ablative Radiotherapy for Early-Stage Lung Cancer. Seminars in Radiation Oncology 2017;27(3):218–228. [DOI] [PubMed] [Google Scholar]
- 20.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. Journal of Thoracic Oncology 2016;11(1):39–51. [DOI] [PubMed] [Google Scholar]
- 21.Koshiol J, Rotunno M, Consonni D, Pesatori AC, De Matteis S, Goldstein AM et al. Chronic obstructive pulmonary disease and altered risk of lung cancer in a population-based case-control study. PloS One 2009;4(10):e7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang P, Sun Z, Krowka MJ, Aubry MC, Bamlet WR, Wampfler JA et al. Alpha1-antitrypsin deficiency carriers, tobacco smoke, chronic obstructive pulmonary disease, and lung cancer risk. Archives of Internal Medicine 2008;168(10):1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. American Journal of Respiratory and Critical Care Medicine 2000;161(1):5–8. [DOI] [PubMed] [Google Scholar]
- 24.Garland LH, Beier RL, Coulson W, Heald JH, Stein RL. The apparent sites of origin of carcinomas of the lung. Radiology 1962;78:1–11. [DOI] [PubMed] [Google Scholar]
- 25.Parkash O Lung cancer. A statistical study based on autopsy data from 1928 to 1972. Respiration 1977;34(5):295–304. [DOI] [PubMed] [Google Scholar]
- 26.Johnson EE, Leverson GE, Pirsch JD, Heise CP. A 30-year analysis of colorectal adenocarcinoma in transplant recipients and proposal for altered screening. Journal of Gastrointestinal Surgery 2007;11(3):272–279. [DOI] [PubMed] [Google Scholar]
- 27.Miao Y, Everly JJ, Gross TG, Tevar AD, First MR, Alloway RR et al. De novo cancers arising in organ transplant recipients are associated with adverse outcomes compared with the general population. Transplantation 2009;87(9):1347–1359. [DOI] [PubMed] [Google Scholar]
- 28.Sigel K, Veluswamy R, Krauskopf K, Mehrotra A, Mhango G, Sigel C et al. Lung Cancer Prognosis in Elderly Solid Organ Transplant Recipients. Transplantation 2015;99(10):2181–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018;124(13):2785–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Lung Screening Trial Research T, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England Journal of Medicine 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moyer VA, Force USPST. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine 2014;160(5):330–338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.