Abstract
Hypertension is a major determinant of cardiovascular morbidity and mortality and is highly prevalent in the general population. While the relationship between sleep apnea and increased blood pressure has been well documented, less recognized is emerging evidence linking sleep-related movement disorders such as restless legs syndrome/periodic limb movements of sleep and sleep-related bruxism with blood pressure (BP) dysregulation and hypertension. There is also recent literature linking narcolepsy-cataplexy with elevated BP and altered pressor responses, and there are data suggesting abnormal BP control in rapid eye movement sleep behavior disorder. It is thought that neural circulatory mechanisms, sympathetic activation in particular, comprise the predominant mediator underlying elevated BP in these neurological sleep disorders. There is very limited evidence that treating these sleep disorders may be beneficial in lowering BP primarily because this question has received very little attention. In this review, we discuss the potential pathophysiologic mechanisms underlying elevated BP in restless legs syndrome/periodic limb movements of sleep, sleep-related bruxism, narcolepsy-cataplexy and rapid eye movement sleep behavior disorder. We also examine the relationship between these sleep disorders and elevated BP and the impact of treatment of these conditions on BP control. Last, we discuss gaps in the literature evaluating the associations between these sleep disorders and elevated BP and identify areas for further research.
INTRODUCTION
Historically, research addressing the role of poor sleep in mediating the risk of hypertension has been largely dominated by studies on the effects of sleep disordered breathing on blood pressure (BP) regulation and consequent predisposition to hypertension. The association between obstructive sleep apnea (OSA) and hypertension has indeed been well described in several studies 1, 2. More recently, the impact of other sleep disorders on BP has been gaining attention. There is a growing body of literature linking sleep-related movement disorders including restless legs syndrome (RLS)/periodic limb movements of sleep (PLMS) and sleep-related bruxism with aberrant BP control and systemic hypertension 3, 4, 5. There is also literature suggesting a relationship between other sleep disorders such narcolepsy-cataplexy and rapid eye movement sleep behavior disorder (RBD) with abnormal BP responses 4, 5 In normal sleep, BP is lower than wakefulness levels and is sleep-stage dependent, increasing intermittently with arousals from non-rapid eye movement sleep and during rapid eye movement (REM) sleep 6-10. Autonomic mechanisms, specifically sympathetic predominance resulting from an imbalance between sympathetic and parasympathetic activity, is thought to play a key role in the pathophysiologic mechanisms underlying the relationship between these sleep disorders and hypertension, similar to that seen in the context of OSA 11-13. However, insufficient and disrupted sleep resulting from these conditions may play an important role in this association as well.
This review evaluates the evidence showing elevated BP in subjects with sleep-related movement disorders, narcolepsy-cataplexy and RBD, and examines possible underlying mechanisms. We further discuss the limited data regarding the effects of treatment of these sleep disorders on BP. The review is restricted to studies of adult subjects.
SLEEP-RELATED MOVEMENT DISORDERS AND HYPERTENSION
RLS/PLMS and hypertension
RLS is a fairly common condition, reported by 5-16% of the general population 14, 15. About 80% of patients with RLS have PLMS on polysomnography 16, 17. The latter are highly prevalent in the general population (21% in one study; 31% in men and 26% in women with 15 or more PLMS per hour of sleep in another study), and can be seen in patients without RLS as well, particularly in those older than 65 years 15, 18.
Evidence linking RLS with hypertension
A number of epidemiologic studies have shown an association between RLS and hypertension 3, 19, 20. Innes et al in their systematic review of 17 studies, most of which were cross-sectional in nature including the large Sleep Heart Health Study and the Wisconsin Sleep Cohort Study, found that in 10 studies there was a positive association between RLS and hypertension after accounting for common confounders such as BP, smoking status and sleep disturbances 15, 21, 22. RLS was positively associated with hypertension when symptom frequency exceeded more than half the days of the month 15, 23. It should be noted that these studies had significant variability in the definition of RLS, measurement of BP, and the use of RLS medications.
Some longitudinal studies that did not support a link between RLS and increased cardiovascular disease suggested a possible inverse relationship, with a trend toward or a significant increase in the incidence of RLS in those with hypertension 24. It is pertinent that two other large longitudinal studies, the Physician’s Health Study and the Women’s Health study, failed to show that RLS (based on a 3-question survey) was a risk factor for the development of cardiovascular disease, while another study, the Nurses’ Health Study, demonstrated a significant association between a diagnosis of RLS of more than 3 years duration made by a physician and incident myocardial infarction as well as fatal myocardial infarction 25. With regard to hypertension, in a large longitudinal study of enrollees in the Kaiser Permanente system, a significant association was noted between “primary” RLS and hypertension, although there was no association with overall cardiovascular disease 26.
Collectively, the literature available thus far suggests that a relationship between RLS and hypertension is likely, but the direction and magnitude of this relationship are unclear at this time. Additionally, the potential modification of this association by other factors including the etiology of RLS and presence of PLMS is unclear 3. Last, the day-night BP pattern carries significant clinical relevance. However, the distinction between daytime vs nighttime hypertension as an outcome is not always clear in the studies evaluating the effects of these sleep disorders on BP 27.
Evidence linking PLMS with hypertension
PLMS and hypertension are commonly comorbid; an older study estimated that 18% of subjects with essential hypertension had PLMS and 36.4% with stage 3 hypertension had PLMS 28.
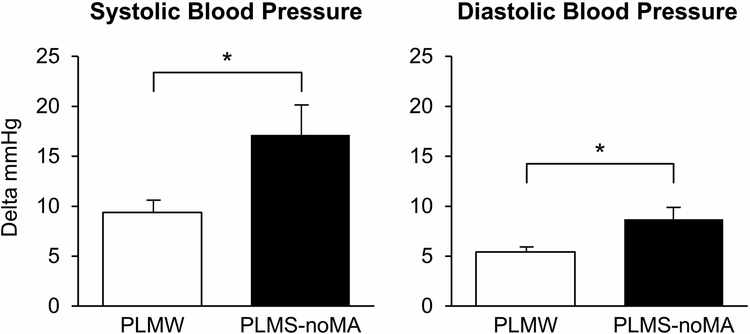
Individual PLMS are associated with increases of 25-30 mm Hg systolic BP and 10-15 mm Hg diastolic BP, from the start of the periodic limb movement (PLM) until 10-15 seconds after the end of the movement 29. Increases in heart rate (HR) followed by cortical arousal on electroencephalography may precede the PLM and persist during and shortly after the event 30-33. PLMS associated with arousals appear to increase the risk of hypertension compared to PLMS without arousals, possibly due to increased sympathetic activity associated with arousals (Figure 1) 34, 35.
Figure 1.
Mean SBP and DBP increments associated with PLMW and PLMS
(reproduced with permission from 35)
In one study, the PLM index (PLMI) was shown to be an independent predictor of cardiovascular and cerebrovascular risk scores in subjects with chronic kidney disease 36. In this study, moderate disease was defined as a PLMI of 15 per hour or greater and severe disease as a PLMI of 30 per hour or greater. In another study of older male subjects without prevalent hypertension at baseline, a PLMI of more than 30 per hour increased the risk of cardiovascular disease at 4 years; however, the risk of developing incident hypertension was not known 37. Koo et al in their multi-ethnic community study found that the PLMI and PLM arousal index were independently associated with prevalent hypertension, particularly in African-American subjects 34, possibly suggesting ethnic-dependent vulnerability to the hypertensive effect of PLMS.
Other studies have failed to demonstrate a significant association between PLMS and hypertension. In a study of subjects with RLS, those with a PLMI of greater than 35 per hour did not have a greater prevalence of hypertension but did have left ventricular hypertrophy 38. A study of subjects with OSA showed no difference in the prevalence of hypertension in those with and without concurrent PLMS (defined as a PLMI of 5 or more per hour) 39. Finally, one recent population-based cohort study did not find a significant association between PLMS and hypertension after accounting for confounders 40.
Possible mechanisms underlying RLS/PLMS and hypertension
First, sudden increases in BP seen with PLMS, probably due to sympathetic activation and vagal withdrawal, may be responsible for elevated BP in sleep seen in subjects with RLS and frequent PLMS 35, 41-43. As in subjects with OSA, this is thought to be an important mechanism underlying elevated BP in individuals with RLS/PLMS during the night (although the pattern and time frame may be different than that seen in subjects with OSA) and potentially during the daytime as well 44-46. Other autonomic disturbances, such as impaired BP and HR reduction in response to the head-up tilt test and Valsalva maneuvers, have been demonstrated in subjects with RLS 46. Second, difficulty with sleep initiation and/or maintenance with shortened sleep duration and sleep disruption secondary to RLS could conceivably lead to the development of hypertension 21. Third, sleep deprivation and PLMS have been shown to be associated with aortic stiffness and may serve to elevate cardiovascular risk, including that of hypertension, in patients with RLS and PLMS 47, 48. Fourth, increased evening cortisol is seen in the context of sleep deprivation and higher nighttime cortisol levels have been noted in subjects with RLS, suggesting involvement of inadequate sleep and of the hypothalamic-pituitary-adrenal axis 49, 50. Fifth, increased inflammation has been noted in subjects with RLS and may increase the risk of hypertension although it is unclear whether inflammation is the consequence or causation of RLS 51. Sixth, impaired vascular endothelial function has recently been implicated as a potential pathophysiologic mechanism for elevated BP in subjects with RLS 52. Seventh, metabolic dysfunction in the setting of sleep deprivation in RLS/PLMS could also contribute to the risk of hypertension 3. Last, iron deficiency, which is an etiologic factor for RLS, may be a cardiovascular risk factor in itself, and has been shown to be associated with diabetes mellitus type 2 and increased mortality in coronary artery disease; on the other hand, intravenous iron has been noted to improve functional status and quality of life and reduce hospitalizations in patients with heart failure 53. Central iron deficiency with increased dopaminergic tone in the basal ganglia may contribute to abnormalities in regulation of sympathetic tone in subjects with RLS/PLMS 54.
Effects of treatment of RLS/PLMS on blood pressure
There are a few studies examining the effects of treatment of RLS/PLMS on BP. One study showed that the use of dopamine agonist medications at night decreased the number of PLMS and PLM-related HR response in subjects with RLS but did not change tonic sympathetic-vagal regulation; however, it should be noted that these findings were noted acutely, after a single medication dose; hence the effects of chronic treatment are unknown 55. Another study reported that treatment of RLS for 3 months led to a borderline significant decline in supine BP with no change in autonomic balance during wakefulness 56. Last, a recent study of 37 subjects showed that treatment of RLS with the dopamine agonist rotigotine patch decreased the number of PLM-associated BP spikes in sleep as well as total nocturnal systolic and diastolic BP elevations 57.
Further epidemiological studies of cardiovascular markers in subjects with RLS and randomized controlled therapeutic trials are required to help determine cardiovascular risk in patients with RLS/PLMS and whether treatment of these conditions lowers BP and ameliorates overall cardiovascular risk 3.
Sleep-related bruxism and hypertension
Sleep-related bruxism is seen in 10-30% of the adult general population, is associated with stress, anxiety, alcohol and tobacco use, and commonly co-exists with OSA 58, 59. Bruxism is thought to arise from activation of subcortical structures, with possible abnormal interaction between the central and autonomic nervous systems 59, 60. Sympathetic activation may result from micro-arousals, and can start before the nocturnal bruxism episode and persist during the day 60-63. A dose-response relationship between duration of the bruxism episode and sympathetic activity has been reported 64. Increases in BP have been noted during bruxism episodes in sleep and in the daytime 5. Conversely, hypertension was shown to be an independent predictor of the intensity of sleep-related bruxism episodes in a recent study but the direction of the relationship remains uncertain 65. Medications used in the treatment of bruxism include beta-blockers and alpha-2 adrenergic agonists which are of limited benefit; their effects on sympathetic activity and BP in the context of bruxism have not been studied 58.
NARCOLEPSY-CATAPLEXY AND HYPERTENSION
Narcolepsy-cataplexy or narcolepsy type 1 is a relatively rare condition with an incidence of 25-50 cases per 100,000 in the general population, characterized by a deficiency of hypocretin or orexin in the central nervous system, resulting in excessive daytime sleepiness as the cardinal feature 66. OSA, obesity and other endocrine and psychiatric conditions are commonly seen to coexist in subjects with narcolepsy-cataplexy 67.
Narcolepsy-cataplexy and elevated nocturnal blood pressure
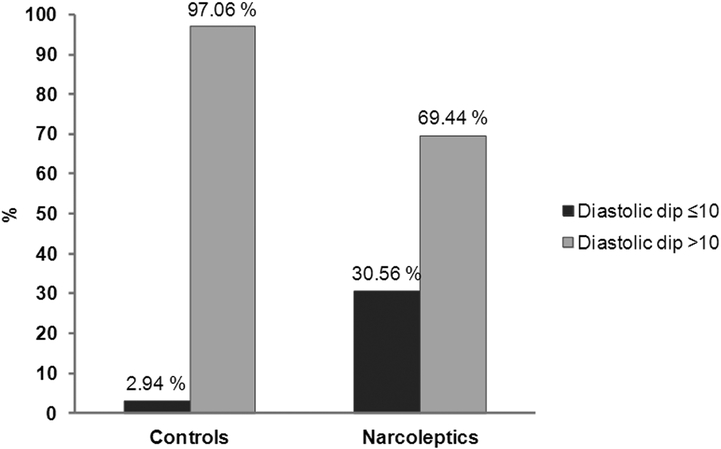
A study employing 24-hour BP monitoring showed nocturnal non-dipping status in one-third of patients with untreated narcolepsy-cataplexy versus 5% in controls (Figure 2) 68. Non-dipping diastolic BP status was significantly associated with narcolepsy-cataplexy (OR=12) and with percentage of time in REM sleep. Another study showed non-dipping BP in most of their cohort (n=35) with narcolepsy-cataplexy 69. Finally, a small case-control study using 24-hour beat by beat measurement of BP showed an increase in systolic BP during REM sleep in subjects with narcolepsy 70. It should be noted that these studies in subjects with narcolepsy-cataplexy have demonstrated non-dipping of BP at night, and perhaps more so in REM sleep, but they have not yielded evidence of an association with prevalent or incident hypertension. Also, the effect of comorbidities on this relationship is unknown 67.
Figure 2.
Non-dipping diastolic BP profile in patients with narcolepsy-cataplexy (n=36) and in controls (n=42)
(reproduced with permission from 68)
Abbreviations: noMA=no microarousals, SBP=systolic blood pressure, DBP=diastolic blood pressure, PLMW=periodic limb movements of wakefulness, PLMS= periodic limb movements of sleep
Possible mechanisms underlying narcolepsy-cataplexy and elevated blood pressure
In narcolepsy-cataplexy, there is sleep disruption with frequent awakenings 71, 72. Hypocretin (or orexin) is deficient in narcolepsy type 1, resulting in sleepiness, Hypocretin/orexin is also thought to play a role in modulating autonomic function, but it is worth noting that a recent study did not show a relationship between orexin levels and nondipping BP at night 69, 73-75. Decreased cardiovagal and sympathetic activity during wakefulness have been reported in subjects with narcolepsy 76, 77. Thus, mechanisms underlying elevated BP in subjects with narcolepsy-cataplexy are currently not entirely clear but may involve the orexin system and may also include sympathetic dysregulation secondary to sleep disruption; the latter possibly occurring as part of the disorder itself or secondary to PLMS that are commonly seen in these subjects 4.
Effects of treatment of narcolepsy-cataplexy on blood pressure
While orexin antagonism has recently been conceptualized as having potential antihypertensive effects, as noted above there are data suggesting elevated BP in subjects with narcolepsy and there are no human studies evaluating the effects of pharmacologic interventions targeting the orexin system on BP 78. Treatment of narcolepsy-cataplexy often requires the use of psychostimulants that can potentially adversely impact BP and other cardiovascular outcomes 79, 80. One recent study showed that subjects with narcolepsy-cataplexy treated with psychostimulants had higher diastolic BP and HR than those who were untreated 81. Furthermore, psychostimulants and anti-cataplectic medications had a synergistic effect on BP. Interestingly, while the percentage of REM sleep remained an independent predictor of 24-hour BP in treated and untreated subjects, endothelial function was similar in both of these groups. Thus, there is presently no evidence that treatment of narcolepsy/cataplexy lowers BP, but may in fact raise it due to the stimulant nature of the therapeutic agents.
RBD AND HYPERTENSION
RBD is a parasomnia characterized by intermittent loss of muscle atonia during REM sleep and dream enactment, manifested in the form of complex, often violent, motor activity. Estimated to occur in 0.4-0.5% of the general population 82, 83,84, 85. RBD generally presents with late onset and is more frequent among males 86. RBD ensues from degeneration of brainstem regions governing REM sleep, and is a well-established precursor of alpha-synucleinopathies, and mostly Parkinson’s disease 87. Manifestations of aberrant autonomic cardiovascular function have been well documented in RBD, suggesting altered sympathetic adrenergic and vagal control 88-91, 92. BP dysregulation is indicated by abnormal BP response to the orthostatic standing test, head-up tilt test, and Valsalva maneuver, as well as more frequent symptoms of orthostatic hypotension 89, 93-97. However, it is unclear if these changes represent part of the prodrome or the presence of the underlying alpha-synucleinopathies themselves, or whether they reflect the effects of RBD on BP. There appear to be no differences between patients with RBD and healthy subjects with regard to resting BP 89. Thus, despite the disrupted sleep occurring in RBD, there is currently a lack of evidence that RBD may be a risk factor for development of hypertension. Studies yielding data to support or refute such an association are needed.
CONCLUSIONS
In this review, we have presented contemporary data on the nexus between neurological sleep disorders, altered BP control and hypertension risk. Available literature favors an association between RLS/PLMS and hypertension. The relationship between sleep-related bruxism and elevated BP as well as between narcolepsy-cataplexy and elevated nighttime BP requires further study. Some pathophysiologic pathways underlying the association with hypertension may be common to all of these sleep disorders, such as autonomic imbalance with increased sympathetic activity, while other mechanisms may be more specific to the individual sleep disorders. It is notable that these disorders are highly prevalent in childhood although unlikely to be diagnosed and treated during that time; hence, autonomic dysfunction emerging in childhood may be a precursor state in these individuals manifesting with elevated BP later in life.
The mechanisms underlying elevated BP seen in subjects with RLS/PLMS may be multifactorial and include the effects of awakenings and insufficient sleep on BP, with elevated cortisol and iron deficiency potentially playing a role in addition to increased sympathetic outflow. The direction and strength of the relationship between RLS/PLMS and hypertension as well as the modifying effects of etiologic factors of sleep-related movement disorders on hypertension needs clarification. The impact of treatment of RLS/PLMS and treatment of bruxism on blood pressure also needs further study.
There is early evidence associating narcolepsy-cataplexy with elevated BP at night, particularly in REM sleep and with nocturnal non-dipping BP status. Autonomic mechanisms may be mediating factors but additional studies are required to confirm this relationship. Much needs to be clarified in terms of underlying pathophysiologic mechanisms and effects, if any, of treatment of narcolepsy-cataplexy on BP, particularly the effects of psychostimulant medication. With regard to other disorders of excessive sleepiness such as idiopathic hypersomnia, it is unknown whether there is any linkage with hypertension. Work addressing this gap is warranted.
In spite of abundant data on BP dysregulation in RBD, there is a dearth of published literature addressing whether this sleep disorder is associated with hypertension. The role of parasomnias in the development of hypertension also requires further study.
Supplementary Material
RESEARCH AGENDA.
Clarify the direction and strength of the relationship between RLS and hypertension in prospective longitudinal studies
Ascertain the mechanistic role of etiologic factors of RLS in this relationship
Determine whether and to what extent PLMS mediate the effects of RLS on daytime and 24-hour BP
Epidemiologic studies on cardiovascular risk biomarkers in patients with RLS and PLMS
Randomized controlled trials to assess whether treatment of RLS/PLMS decreases BP and overall cardiovascular risk
Evaluation of the effects of sleep-related bruxism and its treatment on BP
Determine whether narcolepsy is an independent risk factor for the onset or development of hypertension
Elucidate underlying pathophysiologic mechanisms for elevated BP in narcolepsy and effects of treatment of narcolepsy on BP and on control and progression of comorbid hypertension
Clarify the effects of RBD and other parasomnias on BP
Investigate the cross-sectional and longitudinal association between the above mentioned sleep disorders and resistant hypertension
Determine whether the postulated relationships are modulated by sex, race/ethnicity, and age
ACKNOWLEDGEMENTS
SOURCES OF FUNDING
Dr. Somers is supported by research grants from the National Institutes of Health (HL 65176, HL 134885).
DISCLOSURES
Dr. Mansukhani is the principal investigator on a research grant funded by ResMed Foundation evaluating the effects of adaptive servoventilation treatment of central apnea syndromes on healthcare utilization that is not relevant to the current work. Dr. Mansukhani is the recipient of the Paul and Ruby Tsai and Family Fund Career Development Award at Mayo Clinic.
Dr. Covassin is supported by a research grant from the American Heart Association (16SDG27250156) and the Mayo Clinic Marie Ingalls Research Career Development Fund.
Dr. Somers is a Consultant for Respicardia, ResMed, U-Health, GlaxoSmithKline, Roche and Bayer. He is an investigator on the SERVE-HF Steering Committee and is working with Mayo Health Solutions and their industry partners on intellectual property related to sleep and cardiovascular disease. The Philips Respironics Foundation has provided a gift to Mayo Foundation.
REFERENCES
- 1.Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, Malhotra A, Martinez-Garcia MA, Mehra R, Pack AI, Polotsky VY, Redline S and Somers VK. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J Am Coll Cardiol. 2017;69:841–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T, American Heart Association Council for High Blood Pressure Research Professional Education Committee CoCC, American Heart Association Stroke C, American Heart Association Council on Cardiovascular N and American College of Cardiology F. Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008;118:1080–111. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb DJ, Somers VK, Punjabi NM and Winkelman JW. Restless legs syndrome and cardiovascular disease: a research roadmap. Sleep medicine. 2017;31:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pepin JL, Borel AL, Tamisier R, Baguet JP, Levy P and Dauvilliers Y. Hypertension and sleep: overview of a tight relationship. Sleep Med Rev. 2014;18:509–19. [DOI] [PubMed] [Google Scholar]
- 5.Nashed A, Lanfranchi P, Rompre P, Carra MC, Mayer P, Colombo R, Huynh N and Lavigne G. Sleep bruxism is associated with a rise in arterial blood pressure. Sleep. 2012;35:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansukhani MP, Wang S and Somers VK. Chemoreflex physiology and implications for sleep apnoea: insights from studies in humans. Exp Physiol. 2015;100:130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG and Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation. 1998;97:943–5. [DOI] [PubMed] [Google Scholar]
- 8.Silvani A Physiological sleep-dependent changes in arterial blood pressure: central autonomic commands and baroreflex control. Clinical and experimental pharmacology & physiology. 2008;35:987–94. [DOI] [PubMed] [Google Scholar]
- 9.Silvani A and Dampney RA. Central control of cardiovascular function during sleep. Am J Physiol Heart Circ Physiol. 2013;305:H1683–92. [DOI] [PubMed] [Google Scholar]
- 10.Benarroch EE. Control of the cardiovascular and respiratory systems during sleep. Autonomic neuroscience : basic & clinical. 2019;218:54–63. [DOI] [PubMed] [Google Scholar]
- 11.Tamisier R, Weiss JW and Pepin JL. Sleep biology updates: Hemodynamic and autonomic control in sleep disorders. Metabolism: clinical and experimental. 2018;84:3–10. [DOI] [PubMed] [Google Scholar]
- 12.Calandra-Buonaura G, Provini F, Guaraldi P, Plazzi G and Cortelli P. Cardiovascular autonomic dysfunctions and sleep disorders. Sleep Med Rev. 2016;26:43–56. [DOI] [PubMed] [Google Scholar]
- 13.Miglis MG. Autonomic dysfunction in primary sleep disorders. Sleep medicine. 2016;19:40–9. [DOI] [PubMed] [Google Scholar]
- 14.Ohayon MM, O’Hara R and Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winkelman JW, Finn L and Young T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin Sleep Cohort. Sleep medicine. 2006;7:545–52. [DOI] [PubMed] [Google Scholar]
- 16.Zucconi M, Ferri R, Allen R, Baier PC, Bruni O, Chokroverty S, Ferini-Strambi L, Fulda S, Garcia-Borreguero D, Hening WA, Hirshkowitz M, Hogl B, Hornyak M, King M, Montagna P, Parrino L, Plazzi G and Terzano MG. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG). Sleep medicine. 2006;7:175–83. [DOI] [PubMed] [Google Scholar]
- 17.Trotti LM, Bliwise DL, Greer SA, Sigurdsson AP, Gudmundsdottir GB, Wessel T, Organisak LM, Sigthorsson T, Kristjansson K, Sigmundsson T and Rye DB. Correlates of PLMs variability over multiple nights and impact upon RLS diagnosis. Sleep medicine. 2009;10:668–71. [DOI] [PubMed] [Google Scholar]
- 18.Haba-Rubio J, Marti-Soler H, Marques-Vidal P, Tobback N, Andries D, Preisig M, Waeber G, Vollenweider P, Kutalik Z, Tafti M and Heinzer R. Prevalence and determinants of periodic limb movements in the general population. Annals of neurology. 2016;79:464–74. [DOI] [PubMed] [Google Scholar]
- 19.Ferini-Strambi L, Walters AS and Sica D. The relationship among restless legs syndrome (Willis-Ekbom Disease), hypertension, cardiovascular disease, and cerebrovascular disease. Journal of neurology. 2014;261:1051–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Y, Liu H, Dai T, Guan Y, Tu J and Nie H. Association between restless legs syndrome and hypertension: a meta-analysis of nine population-based studies. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2018;39:235–242. [DOI] [PubMed] [Google Scholar]
- 21.Innes KE, Selfe TK and Agarwal P. Restless legs syndrome and conditions associated with metabolic dysregulation, sympathoadrenal dysfunction, and cardiovascular disease risk: a systematic review. Sleep Med Rev. 2012;16:309–39. [DOI] [PubMed] [Google Scholar]
- 22.Winkelman JW, Shahar E, Sharief I and Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70:35–42. [DOI] [PubMed] [Google Scholar]
- 23.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, O’Connor GT, Boland LL, Schwartz JE and Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. [DOI] [PubMed] [Google Scholar]
- 24.Szentkiralyi A, Volzke H, Hoffmann W, Happe S and Berger K. A time sequence analysis of the relationship between cardiovascular risk factors, vascular diseases and restless legs syndrome in the general population. J Sleep Res. 2013;22:434–42. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Walters AS, Chiuve SE, Rimm EB, Winkelman JW and Gao X. Prospective study of restless legs syndrome and coronary heart disease among women. Circulation. 2012;126:1689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Den Eeden SK, Albers KB, Davidson JE, Kushida CA, Leimpeter AD, Nelson LM, Popat R, Tanner CM, Bibeau K and Quesenberry CP. Risk of Cardiovascular Disease Associated with a Restless Legs Syndrome Diagnosis in a Retrospective Cohort Study from Kaiser Permanente Northern California. Sleep. 2015;38:1009–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silvani A Sleep disorders, nocturnal blood pressure, and cardiovascular risk: A translational perspective. Autonomic neuroscience : basic & clinical. 2019;218:31–42. [DOI] [PubMed] [Google Scholar]
- 28.Espinar-Sierra J, Vela-Bueno A and Luque-Otero M. Periodic leg movements in sleep in essential hypertension. Psychiatry and clinical neurosciences. 1997;51:103–7. [DOI] [PubMed] [Google Scholar]
- 29.Winkelman JW. The evoked heart rate response to periodic leg movements of sleep. Sleep. 1999;22:575–80. [DOI] [PubMed] [Google Scholar]
- 30.Sasai T, Matsuura M and Inoue Y. Change in heart rate variability precedes the occurrence of periodic leg movements during sleep: an observational study. BMC neurology. 2013;13:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allena M, Campus C, Morrone E, De Carli F, Garbarino S, Manfredi C, Sebastiano DR and Ferrillo F. Periodic limb movements both in non-REM and REM sleep: relationships between cerebral and autonomic activities. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2009;120:1282–90. [DOI] [PubMed] [Google Scholar]
- 32.Ferri R, Zucconi M, Rundo F, Spruyt K, Manconi M and Ferini-Strambi L. Heart rate and spectral EEG changes accompanying periodic and non-periodic leg movements during sleep. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2007;118:438–48. [DOI] [PubMed] [Google Scholar]
- 33.Sieminski M, Pyrzowski J and Partinen M. Periodic limb movements in sleep are followed by increases in EEG activity, blood pressure, and heart rate during sleep. Sleep & breathing = Schlaf & Atmung. 2017;21:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koo BB, Sillau S, Dean DA 2nd, Lutsey PL and Redline S. Periodic limb movements during sleep and prevalent hypertension in the multi-ethnic study of atherosclerosis. Hypertension. 2015;65:70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pennestri MH, Montplaisir J, Colombo R, Lavigne G and Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–8. [DOI] [PubMed] [Google Scholar]
- 36.Lindner A, Fornadi K, Lazar AS, Czira ME, Dunai A, Zoller R, Veber O, Szentkiralyi A, Kiss Z, Toronyi E, Mucsi I, Novak M and Molnar MZ. Periodic limb movements in sleep are associated with stroke and cardiovascular risk factors in patients with renal failure. J Sleep Res. 2012;21:297–307. [DOI] [PubMed] [Google Scholar]
- 37.Koo BB, Blackwell T, Ancoli-Israel S, Stone KL, Stefanick ML and Redline S. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) study. Circulation. 2011;124:1223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirza M, Shen WK, Sofi A, Jahangir A, Mori N, Tajik AJ and Jahangir A. Frequent periodic leg movement during sleep is associated with left ventricular hypertrophy and adverse cardiovascular outcomes. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2013;26:783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Alawi A, Mulgrew A, Tench E and Ryan CF. Prevalence, risk factors and impact on daytime sleepiness and hypertension of periodic leg movements with arousals in patients with obstructive sleep apnea. J Clin Sleep Med. 2006;2:281–7. [PubMed] [Google Scholar]
- 40.Haba-Rubio J, Marti-Soler H, Tobback N, Andries D, Marques-Vidal P, Vollenweider P, Preisig M and Heinzer R. Clinical significance of periodic limb movements during sleep: the HypnoLaus study. Sleep medicine. 2018;41:45–50. [DOI] [PubMed] [Google Scholar]
- 41.Bertisch SM, Muresan C, Schoerning L, Winkelman JW and Taylor JA. Impact of Restless Legs Syndrome on Cardiovascular Autonomic Control. Sleep. 2016;39:565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sforza E, Pichot V, Barthelemy JC, Haba-Rubio J and Roche F. Cardiovascular variability during periodic leg movements: a spectral analysis approach. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2005;116:1096–104. [DOI] [PubMed] [Google Scholar]
- 43.Guggisberg AG, Hess CW and Mathis J. The significance of the sympathetic nervous system in the pathophysiology of periodic leg movements in sleep. Sleep. 2007;30:755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S and Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2007;118:1923–30. [DOI] [PubMed] [Google Scholar]
- 45.Pennestri MH, Montplaisir J, Fradette L, Lavigne G, Colombo R and Lanfranchi PA. Blood pressure changes associated with periodic leg movements during sleep in healthy subjects. Sleep medicine. 2013;14:555–61. [DOI] [PubMed] [Google Scholar]
- 46.Izzi F, Placidi F, Romigi A, Lauretti B, Marfia GA, Mercuri NB, Marciani MG and Rocchi C. Is autonomic nervous system involved in restless legs syndrome during wakefulness? Sleep medicine. 2014;15:1392–7. [DOI] [PubMed] [Google Scholar]
- 47.Sunbul M, Kanar BG, Durmus E, Kivrak T and Sari I. Acute sleep deprivation is associated with increased arterial stiffness in healthy young adults. Sleep & breathing = Schlaf & Atmung. 2014;18:215–20. [DOI] [PubMed] [Google Scholar]
- 48.Drakatos P, Higgins S, Pengo MF, Kent BD, Muza R, Karkoulias K, Leschziner G and Williams A. Derived Arterial Stiffness is Increased in Patients with Obstructive Sleep Apnea and Periodic Limb Movements during Sleep. J Clin Sleep Med. 2016;12:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leproult R and Van Cauter E. Role of sleep and sleep loss in hormonal release and metabolism. Endocrine development. 2010;17:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schilling C, Schredl M, Strobl P and Deuschle M. Restless legs syndrome: evidence for nocturnal hypothalamic-pituitary-adrenal system activation. Movement disorders : official journal of the Movement Disorder Society. 2010;25:1047–52. [DOI] [PubMed] [Google Scholar]
- 51.Trotti LM and Rye DB. Restless legs syndrome. Handbook of clinical neurology. 2011;100:661–73. [DOI] [PubMed] [Google Scholar]
- 52.Koh SY, Kim MS, Lee SM, Hong JM and Yoon JH. Impaired vascular endothelial function in patients with restless legs syndrome: a new aspect of the vascular pathophysiology. Journal of the neurological sciences. 2015;359:207–10. [DOI] [PubMed] [Google Scholar]
- 53.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F and Anker SD. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. European heart journal. 2015;36:657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Earley CJ, Connor J, Garcia-Borreguero D, Jenner P, Winkelman J, Zee PC and Allen R. Altered brain iron homeostasis and dopaminergic function in Restless Legs Syndrome (Willis-Ekbom Disease). Sleep medicine. 2014;15:1288–301. [DOI] [PubMed] [Google Scholar]
- 55.Manconi M, Ferri R, Zucconi M, Clemens S, Rundo F, Oldani A and Ferini-Strambi L. Effects of acute dopamine-agonist treatment in restless legs syndrome on heart rate variability during sleep. Sleep medicine. 2011;12:47–55. [DOI] [PubMed] [Google Scholar]
- 56.Rocchi C, Albanese M, Placidi F, Romigi A, Lauretti B, Marfia GA, Liguori C, Marciani MG, Mercuri NB and Izzi F. Chronic dopaminergic treatment in restless legs syndrome: does it affect the autonomic nervous system? Sleep medicine. 2015;16:1071–6. [DOI] [PubMed] [Google Scholar]
- 57.Bauer A, Cassel W, Benes H, Kesper K, Rye D, Sica D, Winkelman JW, Bauer L, Grieger F, Joeres L, Moran K, Schollmayer E, Whitesides J, Carney HC, Walters AS, Oertel W and Trenkwalder C. Rotigotine’s effect on PLM-associated blood pressure elevations in restless legs syndrome: An RCT. Neurology. 2016;86:1785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mesko ME, Hutton B, Skupien JA, Sarkis-Onofre R, Moher D and Pereira-Cenci T. Therapies for bruxism: a systematic review and network meta-analysis (protocol). Systematic reviews. 2017;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lavigne GJ, Khoury S, Abe S, Yamaguchi T and Raphael K. Bruxism physiology and pathology: an overview for clinicians. Journal of oral rehabilitation. 2008;35:476–94. [DOI] [PubMed] [Google Scholar]
- 60.Kato T, Rompre P, Montplaisir JY, Sessle BJ and Lavigne GJ. Sleep bruxism: an oromotor activity secondary to micro-arousal. Journal of dental research. 2001;80:1940–4. [DOI] [PubMed] [Google Scholar]
- 61.Marthol H, Reich S, Jacke J, Lechner KH, Wichmann M and Hilz MJ. Enhanced sympathetic cardiac modulation in bruxism patients. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2006;16:276–80. [DOI] [PubMed] [Google Scholar]
- 62.Huynh N, Kato T, Rompre PH, Okura K, Saber M, Lanfranchi PA, Montplaisir JY and Lavigne GJ. Sleep bruxism is associated to micro-arousals and an increase in cardiac sympathetic activity. J Sleep Res. 2006;15:339–46. [DOI] [PubMed] [Google Scholar]
- 63.Seraidarian P, Seraidarian PI, das Neves Cavalcanti B, Marchini L and Claro Neves AC. Urinary levels of catecholamines among individuals with and without sleep bruxism. Sleep & breathing = Schlaf & Atmung. 2009;13:85–8. [DOI] [PubMed] [Google Scholar]
- 64.Nukazawa S, Yoshimi H and Sato S. Autonomic nervous activities associated with bruxism events during sleep. Cranio : the journal of craniomandibular practice. 2018;36:106–112. [DOI] [PubMed] [Google Scholar]
- 65.Martynowicz H, Dymczyk P, Dominiak M, Kazubowska K, Skomro R, Poreba R, Gac P, Wojakowska A, Mazur G and Wieckiewicz M. Evaluation of Intensity of Sleep Bruxism in Arterial Hypertension. Journal of clinical medicine. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.American Academy of Sleep M. International classification of sleep disorders. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 67.Cohen A, Mandrekar J, St Louis EK, Silber MH and Kotagal S. Comorbidities in a community sample of narcolepsy. Sleep medicine. 2018;43:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dauvilliers Y, Jaussent I, Krams B, Scholz S, Lado S, Levy P and Pepin JL. Non-dipping blood pressure profile in narcolepsy with cataplexy. PloS one. 2012;7:e38977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sieminski M, Chwojnicki K, Sarkanen T and Partinen M. The relationship between orexin levels and blood pressure changes in patients with narcolepsy. PloS one. 2017;12:e0185975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grimaldi D, Calandra-Buonaura G, Provini F, Agati P, Pierangeli G, Franceschini C, Barletta G, Plazzi G, Montagna P and Cortelli P. Abnormal sleep-cardiovascular system interaction in narcolepsy with cataplexy: effects of hypocretin deficiency in humans. Sleep. 2012;35:519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Donadio V, Plazzi G, Vandi S, Franceschini C, Karlsson T, Montagna P, Vetrugno R, Bugiardini E, Mignot E and Liguori R. Sympathetic and cardiovascular activity during cataplexy in narcolepsy. J Sleep Res. 2008;17:458–63. [DOI] [PubMed] [Google Scholar]
- 72.Dauvilliers Y, Arnulf I and Mignot E. Narcolepsy with cataplexy. Lancet (London, England). 2007;369:499–511. [DOI] [PubMed] [Google Scholar]
- 73.Nunez A, Rodrigo-Angulo ML, Andres ID and Garzon M. Hypocretin/Orexin neuropeptides: participation in the control of sleep-wakefulness cycle and energy homeostasis. Current neuropharmacology. 2009;7:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Plazzi G, Moghadam KK, Maggi LS, Donadio V, Vetrugno R, Liguori R, Zoccoli G, Poli F, Pizza F, Pagotto U and Ferri R. Autonomic disturbances in narcolepsy. Sleep Med Rev. 2011;15:187–96. [DOI] [PubMed] [Google Scholar]
- 75.Berteotti C and Silvani A. The link between narcolepsy and autonomic cardiovascular dysfunction: a translational perspective. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2018;28:545–555. [DOI] [PubMed] [Google Scholar]
- 76.Ferini-Strambi L, Spera A, Oldani A, Zucconi M, Bianchi A, Cerutti S and Smirne S. Autonomic function in narcolepsy: power spectrum analysis of heart rate variability. Journal of neurology. 1997;244:252–5. [DOI] [PubMed] [Google Scholar]
- 77.Donadio V, Liguori R, Vandi S, Pizza F, Dauvilliers Y, Leta V, Giannoccaro MP, Baruzzi A and Plazzi G. Lower wake resting sympathetic and cardiovascular activities in narcolepsy with cataplexy. Neurology. 2014;83:1080–6. [DOI] [PubMed] [Google Scholar]
- 78.Sieminski M, Szypenbejl J and Partinen E. Orexins, Sleep, and Blood Pressure. Curr Hypertens Rep. 2018;20:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morgenthaler TI, Kapur VK, Brown T, Swick TJ, Alessi C, Aurora RN, Boehlecke B, Chesson AL Jr., Friedman L, Maganti R, Owens J, Pancer J and Zak R. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30:1705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mansukhani MP, Kolla BP and Park JG. Risks associated with use of stimulant medications in patients with obstructive sleep apnea and cardiomyopathy: a case-control study. Sleep medicine. 2017;32:171–175. [DOI] [PubMed] [Google Scholar]
- 81.Bosco A, Lopez R, Barateau L, Chenini S, Pesenti C, Pepin JL, Jaussent I and Dauvilliers Y. Effect of psychostimulants on blood pressure profile and endothelial function in narcolepsy. Neurology. 2018;90:e479–e491. [DOI] [PubMed] [Google Scholar]
- 82.Chiu H, Wing Y, Lam L, Li S, Lum C, Leung T and Ho CK. Sleep-related injury in the elderly--an epidemiological study in Hong Kong. Sleep. 2000;23:513–517. [PubMed] [Google Scholar]
- 83.Ohayon MM and Schenck CH. Violent behavior during sleep: prevalence, comorbidity and consequences. Sleep medicine. 2010;11:941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kang SH, Yoon IY, Lee SD, Han JW, Kim TH and Kim KW. REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. Sleep. 2013;36:1147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haba-Rubio J, Frauscher B, Marques-Vidal P, Toriel J, Tobback N, Andries D, Preisig M, Vollenweider P, Postuma R and Heinzer R. Prevalence and Determinants of REM Sleep Behavior Disorder in the General Population. Sleep. 2017; 41(2). [DOI] [PubMed] [Google Scholar]
- 86.Olson EJ, Boeve BF and Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123:331–339. [DOI] [PubMed] [Google Scholar]
- 87.Howell MJ and Schenck CH. Rapid eye movement sleep behavior disorder and neurodegenerative disease. JAMA neurology. 2015;72:707–712. [DOI] [PubMed] [Google Scholar]
- 88.Miyamoto T, Miyamoto M, Inoue Y, Usui Y, Suzuki K and Hirata K. Reduced cardiac 123I-MIBG scintigraphy in idiopathic REM sleep behavior disorder. Neurology. 2006;67:2236–2238. [DOI] [PubMed] [Google Scholar]
- 89.Rocchi C, Placidi F, Liguori C, Del Bianco C, Lauretti B, Diomedi M, Pisani A, Mercuri NB and Izzi F. Daytime autonomic activity in idiopathic rapid eye movement sleep behavior disorder: a preliminary study. Sleep Medicine. 2018;52:163–167. [DOI] [PubMed] [Google Scholar]
- 90.Ferini-Strambi L, Oldani A, Zucconi M and Smirne S. Cardiac autonomic activity during wakefulness and sleep in REM sleep behavior disorder. Sleep. 1996;19:367–369. [DOI] [PubMed] [Google Scholar]
- 91.Lanfranchi PA, Fradette L, Gagnon J-F, Colombo R and Montplaisir J. Cardiac autonomic regulation during sleep in idiopathic REM sleep behavior disorder. Sleep. 2007;30:1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chiaro G, Calandra-Buonaura G, Cecere A, Mignani F, Sambati L, Loddo G, Cortelli P and Provini F. REM sleep behavior disorder, autonomic dysfunction and synuclein-related neurodegeneration: where do we stand? Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2018;28:519–533. [DOI] [PubMed] [Google Scholar]
- 93.Wan Y, Luo Y, Gan J, Hu R, Zhou M and Liu Z. Clinical markers of neurodegeneration in Chinese patients with idiopathic rapid eye movement sleep behavior disorder. Clinical neurology and neurosurgery. 2016;150:105–109. [DOI] [PubMed] [Google Scholar]
- 94.Postuma R, Gagnon J, Vendette M and Montplaisir J. Markers of neurodegeneration in idiopathic rapid eye movement sleep behaviour disorder and Parkinson’s disease. Brain. 2009;132:3298–3307. [DOI] [PubMed] [Google Scholar]
- 95.Postuma RB, Gagnon JF, Pelletier A and Montplaisir J. Prodromal autonomic symptoms and signs in Parkinson’s disease and dementia with Lewy bodies. Movement Disorders. 2013;28:597–604. [DOI] [PubMed] [Google Scholar]
- 96.Ferini-Strambi L, Oertel W, Dauvilliers Y, Postuma RB, Marelli S, Iranzo A, Arnulf I, Birgit H, Manni R and Miyamoto T. Autonomic symptoms in idiopathic REM behavior disorder: a multicentre case–control study. Journal of neurology. 2014;261:1112–1118. [DOI] [PubMed] [Google Scholar]
- 97.Frauscher B, Nomura T, Duerr S, Ehrmann L, Gschliesser V, Wenning GK, Wolf E, Inoue Y, Högl B and Poewe W. Investigation of autonomic function in idiopathic REM sleep behavior disorder. Journal of neurology. 2012;259:1056–1061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.