Currently, a dramatic increase in antibiotic resistance is driving researchers to find new antimicrobial agents. The large group of toxins called bacteriocins appears to be very promising from this point of view, especially because their narrow killing spectrum allows specific targeting against selected bacterial strains. Colicins are a subgroup of bacteriocins that act on Gram-negative bacteria. To date, some colicins are commercially used for the treatment of animals (1) and tested as a component of engineered species-specific antimicrobial peptides, which are studied for the potential treatment of humans (2). Here, we present a thorough single-molecule study of colicin U which leads to a better understanding of its mode of action. It extends the range of characterized colicins available for possible future medical applications.
KEYWORDS: colicin U, Shigella boydii, black lipid membrane, membrane pores, ion-selectivity
ABSTRACT
Colicin U is a protein produced by the bacterium Shigella boydii (serovars 1 and 8). It exerts antibacterial activity against strains of the enterobacterial genera Shigella and Escherichia. Here, we report that colicin U forms voltage-dependent pores in planar lipid membranes; its single-pore conductance was found to be about 22 pS in 1 M KCl at pH 6 under 80 mV in asolectin bilayers. In agreement with the high degree of homology between their C-terminal domains, colicin U shares some pore characteristics with the related colicins A and B. Colicin U pores are strongly pH dependent, and as we deduced from the activity of colicin U in planar membranes at different protein concentrations, they have a monomeric pore structure. However, in contrast to related colicins, we observed a very low cationic selectivity of colicin U pores (1.5/1 of K+/Cl− at pH 6) along with their atypical voltage gating. Finally, using nonelectrolytes, we determined the inner diameter of the pores to be in the range of 0.7 to 1 nm, which is similar to colicin Ia, but with a considerably different inner profile.
IMPORTANCE Currently, a dramatic increase in antibiotic resistance is driving researchers to find new antimicrobial agents. The large group of toxins called bacteriocins appears to be very promising from this point of view, especially because their narrow killing spectrum allows specific targeting against selected bacterial strains. Colicins are a subgroup of bacteriocins that act on Gram-negative bacteria. To date, some colicins are commercially used for the treatment of animals (1) and tested as a component of engineered species-specific antimicrobial peptides, which are studied for the potential treatment of humans (2). Here, we present a thorough single-molecule study of colicin U which leads to a better understanding of its mode of action. It extends the range of characterized colicins available for possible future medical applications.
INTRODUCTION
Colicins belong to a group of proteinaceous bacteriocins produced by certain Gram-negative enterobacteria, mainly by Escherichia coli (3). Their toxic activity against bacterial strains is limited to those which are closely related to the producer. This high colicin specificity limits the spectrum of target strains, but on the other hand, this could be advantageous for some applications, e.g., in the simultaneous administration of colicin and a probiotic strain. Colicins exhibit various modes of action, which can be divided into three basic mechanisms; the first is the ability of colicins to cleave DNA or RNA, such as colicins E2, E7, E8, E9, and others (4–7). The second is the formation of pores in the cytoplasmic membrane (colicins A, B, N, and others) (8–12). The third mechanism is a hydrolysis of peptidoglycan intermediates I and II in the periplasm, observed in colicin M (13). Most of the colicins discovered so far exert pore-forming activity in the cytoplasmic membrane of sensitive bacteria, which leads to a depolarization of the membrane and subsequent cell death. The most detailed studies were conducted with colicins A, B, N, E1, and Ia, summarized previously (3); also, the structures of their soluble forms were determined by X-ray crystallography (14–18).
Colicin U was discovered as a product of the bacterium Shigella boydii carrying the plasmid pColU (7.3 kb); subsequently, the plasmid from S. boydii M592 (serovar 8) was isolated and sequenced. Three colicin genes were identified, namely, the immunity gene cui (molecular weight, 20,688 Da); the lytic protein gene cul (molecular weight, 4,672 Da); and finally, the colicin U activity gene cua, which codes for a protein of 619 amino acids (molecular weight, 66,289 Da) (19). Recently, colicin U producers were found among Escherichia coli strains of human origin with a prevalence of up to 2% (20–23).
Colicin U has a 36% sequence identity to colicin A (19). However, since colicins, in general, usually consist of three domains (3, 15, 18, 24), the similarity and role of individual domains were also analyzed separately in detail among other colicins (25). The classification of colicins into evolutionarily closed groups depends strongly on the analyzed domain, the origin of which can be complex. For example, colicin B consists of three selectively independent segments (26, 27). Colicin U exhibits a similar mosaic pattern. Its N-terminal sequence (first 130 amino acids) has 54.2% identity to the corresponding part of bacteriocin 28b. On the other hand, the central region of colicin U shows some similarity to the pore-forming colicin K (34.6% identity in the region 280 to 386) (19). Finally, the C-terminal domain (CTD) of colicins is generally considered to be responsible for their lytic action (28–30); therefore, the analysis of CTD sequences is the most crucial for us. The last 200 amino acids of colicin U were found to exhibit 73% and 65% identity to the pore-forming CTDs of colicin B and colicin A, respectively. Therefore, colicin U was also expected to form pores (19). Also, the CTD exhibits 83% identity to colicin Y (31), which has not yet been tested for pore-forming activity.
In our study, we experimentally confirmed the pore-forming activity of colicin U. We analyzed the single-pore behavior of colicin U in black lipid membrane (BLM), and we demonstrated that colicin U pores are strictly voltage dependent and strongly influenced by the pH of the environment. Additionally, we determined an approximate pore diameter (0.7 to 1 nm) of colicin U using BLM measurements with nonelectrolytes.
RESULTS AND DISCUSSION
Single-pore characteristics.
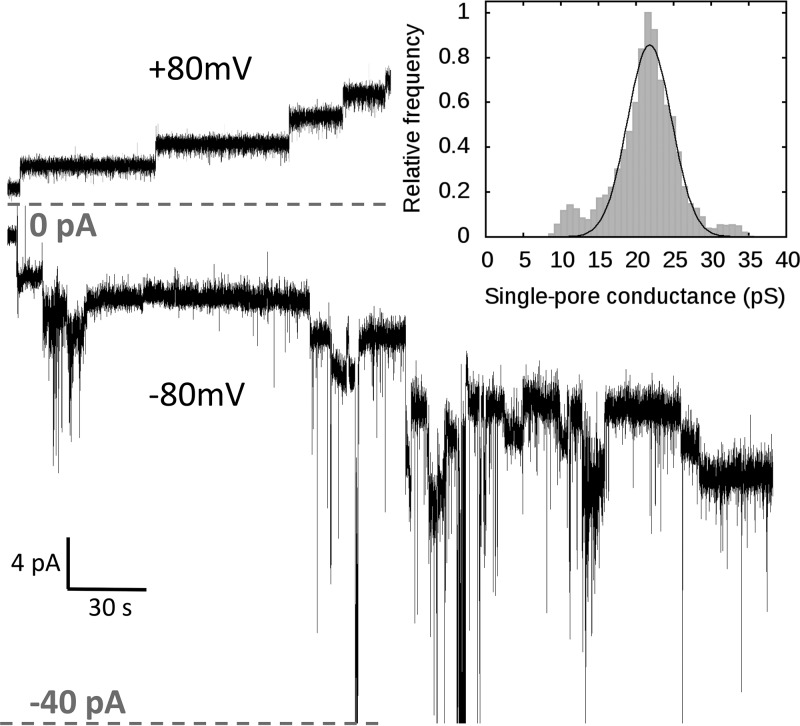
After the addition of colicin U to planar lipid membranes, we observed a step-like increase in the electrical current (Fig. 1, top). This is clear proof that colicin U forms discrete pores in planar lipid membranes with a quite uniform conductance of about 22 ± 3.5 pS under standard conditions (1 M KCl and 10 mM HEPES at pH 6 under 80 mV in membranes from soybean lipids). Under these conditions, the lifetime of the open pore is extremely long (minutes to hours) and cannot be precisely determined. We tested the single-pore behavior of colicin U under the opposite membrane potential (cis negative) and observed a highly unstable single-pore conductance (Fig. 1, bottom). Thus, we expect that colicin was inserted into the membrane in a “reversed” manner, as was suggested for another bacterial toxin (32). These pores inserted in a reversed manner, which had strongly variable single-pore conductance from 20 pS to 3 nS, were not further analyzed, as we consider them to be biologically irrelevant. Nevertheless, under such a nonphysiological (cis-negative) potential, we were able to register the closing of individual pores that previously opened under physiological membrane potential (cis positive), and we quantified their single-pore conductance.
FIG 1.
Representative recording of colicin U pores under +80 mV potential exhibiting typical behavior of opened membrane pores under standard conditions (top) and recording under −80 mV showing typical behavior of colicin inserted under nonphysiological membrane potential (cis negative, bottom). (Inset) The histogram shows the distribution of the single-pore conductance of colicin U under +80 mV. Colicin U (9 nM concentration) was added to the cis compartment in both cases. Measured on an asolectin membrane, in a solution of 1 M KCl and 10 mM HEPES at pH 6.
Effect of KCl concentration on single-pore conductance.
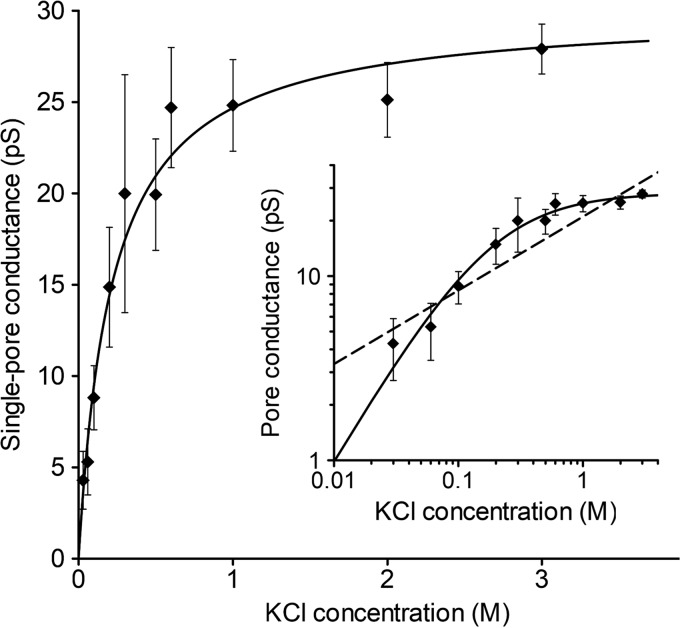
We analyzed the single-pore conductance of colicin U in KCl solutions with increasing molar strength (Fig. 2). We found a linear dependence at low electrolyte concentrations of up to 0.3 M KCl. It is worth noting that we were unable to detect individual pores at KCl concentrations below 0.03 M because of extremely low pore conductance. The single-pore conductance remains almost constant at KCl concentrations higher than 0.5 M; therefore, we suppose that the pore current reaches a saturation point at a single-pore conductance <30 pS.
FIG 2.
Single-pore conductance of colicin U as a function of KCl concentration. For every concentration point, a pore conductance was obtained from the conductance histogram fitted to a Gaussian function. The error bars correspond to the width at the half-maximum of the Gaussian fit. The data were fitted to the Michaelis-Menten equation with Gmax = 28.2 pS and Km = 0.19 M (solid line). (Inset) the same data in a double-logarithmic plot. Measured under 80 mV in a solution of KCl and 10 mM HEPES at pH 6. For comparison with colicin B (10), we also plotted the function y = G · xn, where n = 0.4 and G = 21 pS (inset, dashed line), which shows the lack of saturation at higher KCl concentrations.
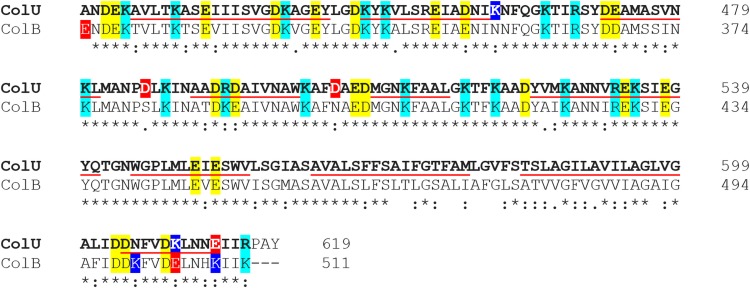
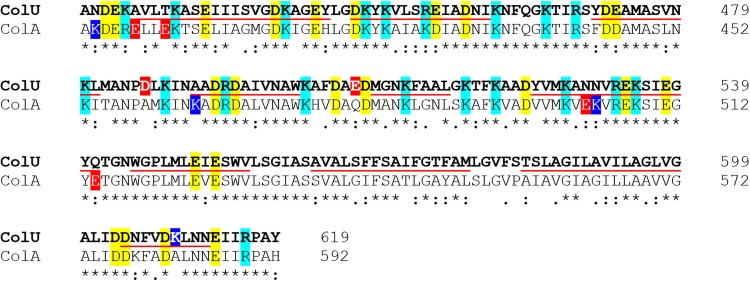
It seems that the number of ions passing through the pore at higher concentrations is limited by the size and shape of the pore but definitely not by the concentration of ions in solution. A similar trend in conductance was also measured in NaCl solution, with complete saturation at concentrations of >0.6 M NaCl (not shown). We fitted our data to the Michaelis-Menten equation and estimated that the colicin U pores under standard conditions are saturated at 28.2 pS, with an apparent Km at very low electrolyte concentrations of 0.19 M KCl. For an easier comparison with a highly related protein, we plotted a power function (y = G · x0.4) that describes the dependency of colicin B (Fig. 2, inset, dashed line) according to the previous study (10). Although the high sequence similarity (Fig. 3) of CTDs of colicin U and B (73% identity) (19) suggests that they should share some common pore properties, colicin U pores reach electrical current saturation at much lower KCl concentrations than the pores of colicin B. We can imagine the following two different explanations of this effect: (i) more stringent cation coordination by two aspartic acids (D486 and D505) of colicin U that are absent in colicin B (Fig. 3) or (ii) markedly different geometry of the pores formed by the two colicins. The first theory (cation coordination) would presume a high cation selectivity of the pore, which is not the case (see “Ion selectivity of colicin U” below). The pore geometry is discussed later (see “Inner diameter of colicin U pores” below).
FIG 3.
Sequence alignment of C-terminal 200 amino acids of colicin U and colicin B shows high sequence similarity. Asterisks mark identical residues; colons indicate similar amino acids. Negatively charged amino acids (glutamic and aspartic acid) are in yellow; positively charged (arginine and lysine) are in cyan. Red and blue highlights denote a difference in the charge of the two sequences. The underlined sequences correspond to the expected α-helices of the pore-forming domain (69). The amino acid sequence alignment was generated with Clustal Omega (70).
Lack of cooperativity of colicin U pores.
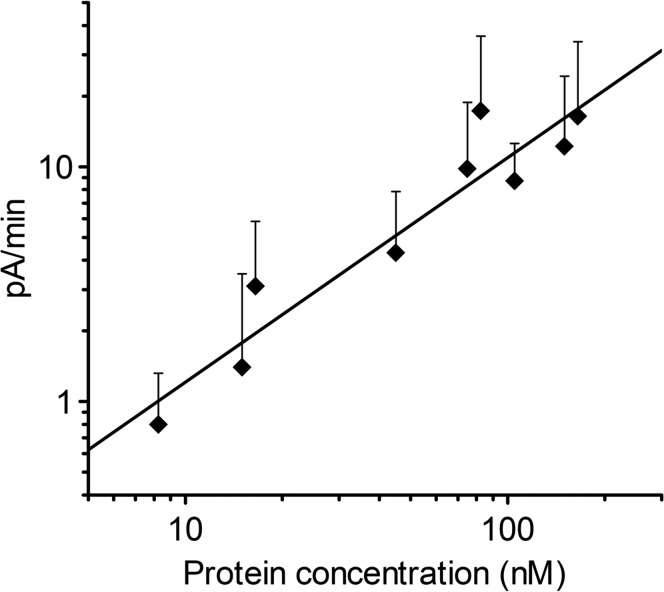
The stoichiometry of a pore is one of the crucial factors for determining the structure of pore-forming proteins in the membrane. To estimate the number of molecules creating the pore, we studied the pore-forming activity while varying the concentration of added colicin U (Fig. 4). Every single point on the graph represents colicin U activity expressed as an initial increase in electrical current after membrane formation. We fitted these activity data to a power function (y = k · xn), yielding an exponent of n = 0.95 ± 0.12. Note that the power function can be used instead of the Hill function if the concentration of the substance under study is very low compared with the saturation point (see “Estimation of pore stoichiometry” below). The exponent value being close to 1 (corresponding to Hill number n) reflects no cooperativity, which suggests that the colicin U pores are monomeric.
FIG 4.
Double-logarithmic plot of colicin U activity as a function of protein concentration. The activity was determined as an initial increase in electrical current after membrane formation (t < 400 s). The data were acquired at at least 10 membranes for every colicin concentration. The error bars represent standard deviation of the measurement. The line shows the fitted power function y = 0.128 · x0.85. Measured under 80 mV in a solution of 0.1 M KCl and 10 mM HEPES at pH 6.
Also, most of the previous works studying the molecularity of pore-forming colicins suggest that the colicin pore is formed by just a single molecule. This hypothesis is supported by studies on multilamellar vesicles with colicin E1 (33) or in planar lipid membranes with colicin E1 (34) and colicin A (11, 35). In contrast, a similar type of analysis implicated the oligomeric character of the nonrelated pore formed by molecules of CyaA toxin (36), proving the suitability of this approach on black lipid membranes.
Effect of pH on colicin U pores.
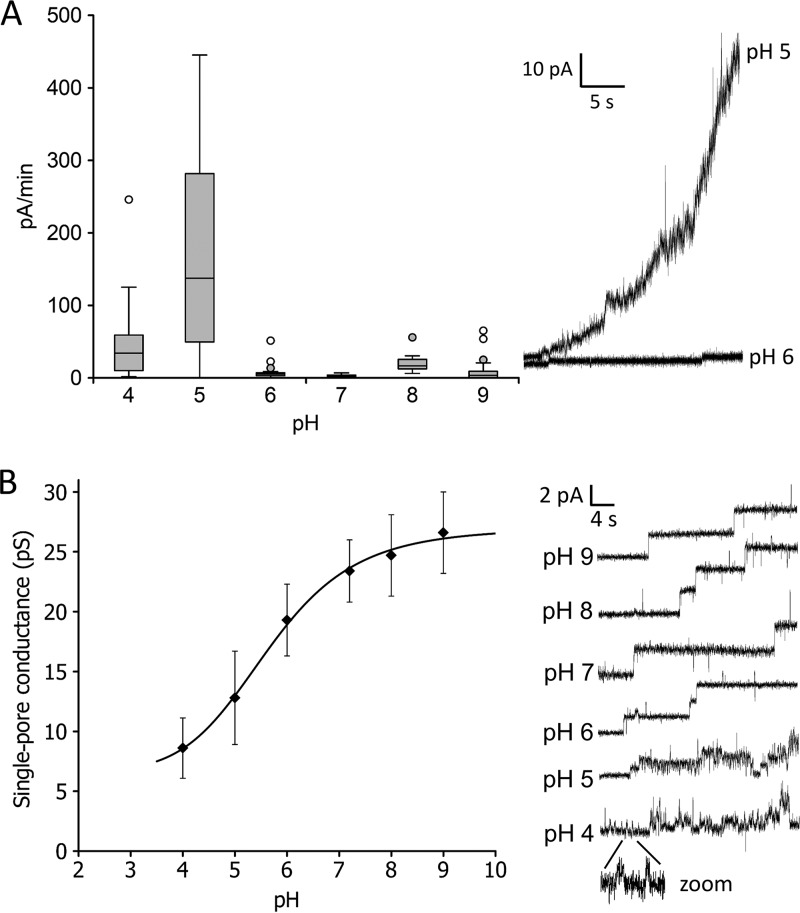
Since other colicins exhibited a strong pH dependence, we also focused on the influence of pH on colicin U activity in the membrane. In the pH range 6 to 9, colicin exhibited a low and steady activity (Fig. 5A). We observed that colicin activity increased with decreasing pH of the solution. The most dramatic increase was detected between pH 6 and 5 (Fig. 5A, right), indicating either an elevated tendency of colicin U to incorporate into a phospholipid bilayer or an enhanced pore opening of already-inserted protein molecules. However, by measuring on black lipid membranes, it is not possible to determine which of these two mechanisms applies here. At pH 4, the activity values (Fig. 5A) are probably underestimated due to the inherent membrane rupturing in the presence of colicin compared with the stable membranes without colicin, which makes the quantifications difficult.
FIG 5.
(A) Pore-forming activity of colicin U as a function of pH value, expressed as current increase per minute (left). Every experiment included measurements at at least 20 different membranes with a protein concentration of 70 nM added to the cis-positive compartment. The box plot shows the 25th and 75th percentiles, medians (middle lines), and outliers (circles). Current recordings at pH 5 and 6 (right) show the most significant difference in membrane activity. (B) pH dependence of single-pore conductance of colicin U (left) and typical current recordings (right) measured in 9 nM protein concentration. The single-pore conductance (G) was determined by fitting the conductance histograms to the Gaussian function. The error bars correspond to the width at the half-maximum of the Gaussian fit. Every G value was calculated from >100 events. Measured under 80 mV in a solution of 1 M KCl and 10 mM HEPES or Tris-HCl.
Interestingly, the single-pore conductance of colicin U was also found to be strongly affected by the pH of the solution but in the opposite direction than that of colicin U activity. A higher pH of solution induced a higher single-pore conductance of colicin, with an almost sigmoidal character of the dependence (Fig. 5B). The conductance increased 3-fold from pH 4 to pH 9. Another important factor influenced by pH is the dramatic increase in pore instability, which was detected below pH 6. Representative traces of single-pore current are shown in Fig. 5B (right).
The sigmoidal trend of the single-pore conductance presented here was also found for other colicins (10, 12, 37). These results indicate that negatively charged amino acid residues (pKa, ∼4) located in the pore (see “Voltage gating of colicin U pores” below) control the passage of ions across the membrane (12, 38).
As shown in Fig. 5A, colicin U reached the highest activity at acidic pH. Similarly, the insertion of colicins A and B into vesicles is also significantly higher at a pH lower than 5 (39). This strongly correlates with the fact that the C-terminal domains of colicins A and B undergo a molten globule state under acidic conditions. In contrast, the CTD of colicin N, another closely related colicin (19), is not affected by acidic pH (40). The difference could be caused by the pI values of the CTDs, which are different for colicins A and B compared with colicin N. Their calculated isoelectric points are 5.82, 5.48, and 10.25 for colicins A, B and N, respectively (41). The pI of the colicin U pore-forming domain (the final C-terminal 200 amino acids) is 4.89 (according to the isoelectric point calculator [42]). Therefore, we can suppose that a pH lower than 5 could cause a partial denaturation/unfolding of the colicin U CTD, enhancing its interaction with the membrane by a similar mechanism as for colicins A and B. This partial denaturation could explain the abrupt change in colicin U activity which was observed between pH 5 and 6. This, together with the different open-pore dynamics, in which the pores exhibit a shorter lifetime with lower single-pore conductance (Fig. 5B), suggests that there is an obvious change in the pore properties under acidic conditions.
Alternatively, the dramatic increase in colicin U activity at low pH could be caused by the fact that the pH of the surrounding solution is lower than the pI of the whole colicin U protein (pI, 6.4) (42). Under such conditions, the protein is positively charged and probably more attracted toward negatively charged phospholipids (∼30% of asolectin lipids are negatively charged due to the presence of phosphatidylinositol and phosphatidylserine) (43).
Voltage gating of colicin U pores.
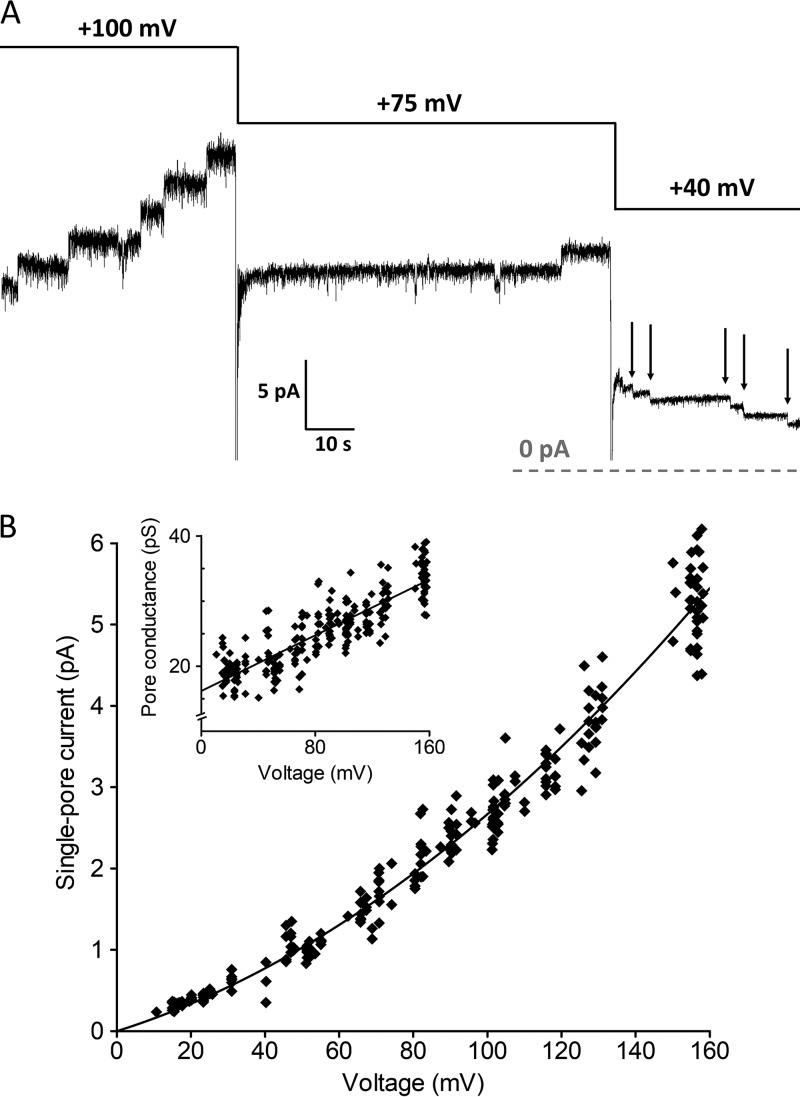
We observed that both the activity and the single-pore conductance of colicin U are voltage dependent. Generally, the higher the membrane potential, the higher the tendency of colicin to incorporate into the membrane and open the pore. We tested the effect of a cis-positive (physiological) voltage from 0 to 160 mV. Quite unexpectedly, at lower membrane potentials, the pores tend to close rapidly (Fig. 6A).
FIG 6.
(A) Representative current recording of colicin U measured with different membrane potentials. The pores which opened at 100 mV were closed later when the membrane potential was lower than 40 mV (arrows). The dashed line indicates zero current. (B) Dependence of single-pore current on applied membrane potential (fitted to the polynomial function y = 16x + 0.11x2). The inset shows the same data expressed as the single-pore conductance with the corresponding linear fit y = 16 + 0.11x. Every point represents one opening or closing of a pore (n = 270). Measured in a solution of 1 M KCl and 10 mM HEPES at pH 6.
From these recordings at different membrane potentials, we obtained a nonlinear current-voltage characteristic (IV curve) of individual colicin U pores (Fig. 6B). The single-pore conductance ranged from 16 to 33 pS when observed at low and high membrane potentials (10 and 160 mV, respectively), yielding a slope of 0.11 pS/mV.
Colicin U pores exhibit atypical behavior under physiological membrane potential (i.e., positive voltage in the cis compartment where colicin was added). Pores which opened under high cis-positive voltage closed spontaneously when the cis-positive voltage was lowered under 40 mV. For other colicins, pore closing under completely reversed potential is frequently reported (12, 44–46). Behavior similar to colicin U, in which the pores gradually closed under low physiological potential, was only found with colicin N (12).
Ion selectivity of colicin U.
In order to determine the ion selectivity of colicin U pores, we measured the single-pore current in a salt gradient (1 M KCl on the cis side and 0.1 M KCl on the trans side or vice versa) at different membrane potentials and estimated the reversal potential (Urev). The IV curve in the salt gradient can be fitted with a straight line within the limited interval from −60 to +60 mV (see Fig. S1 in the supplemental material). To obtain the single-pore current at a cis-negative voltage, we first reconstituted the pores under cis-positive voltage and then reversed the potential to negative values. This procedure led to a rapid closing of the pores, which was analyzed for different values of cis-negative potential. We determined Urev values of about +10 mV and −5 mV for the 0.1/1 M KCl and 1/0.1 M KCl gradients, respectively. The corresponding ion selectivity of colicin U pores is around 1.5/1 (K+/Cl−) according to the Goldmann-Hodgkin-Katz equation (47).
The selectivity of colicin pores seems to be strongly dependent on external conditions. Studies on other proteins suggest that ion selectivity is influenced not only by the composition of the membrane but also by the salt concentrations or by the surrounding pH (48). Generally, one would suppose that lowering the pH of the solution also decreases the preference of the pores for cations over anions. Indeed, our preliminary findings suggest that colicin U cation selectivity was lowered in a more acidic solution (data not shown). Nevertheless, the cation selectivity of the colicin U pore even under standard conditions is already very low compared with other colicins. For colicins E1 and B, the selectivity was found to be around 4.5/1 (Na+/Cl−) and for colicin A about 6.7/1 (K+/Cl−) (11, 12, 44, 49, 50). The reason for the low selectivity of colicin U pores compared with other colicins clearly stems from the differences in the sequences of their C-terminal domains, which otherwise exhibit a high degree of homology, and we expect very similar spatial arrangements of the membrane-inserted CTDs of these colicins. To improve our understanding, we focused on a comparison of colicin U with the two closely related colicins A and B, which have known ion selectivities. One of the mechanisms of cation selectivity is the attraction/coordination of cations by acidic amino acid residues in the pore, which is quite plausible for colicins. Accordingly, we would presume that those segments of colicin U, which lack negatively charged residues compared with other colicins, might be responsible for the lower ion selectivity of colicin U pores. Compared with the CTD of colicin A, only two such segments contain a lower number of acidic residues in the colicin U sequence, namely, from amino acids 425 to 428 (containing two fewer glutamic acids) and 530 to 541 (containing two less glutamic acids) (Fig. 7). This implies that these residues (within helices 1 and 6, according to reference 19) might play an important role in the ion selectivity of the pore. Interestingly, we did not find any negatively charged amino acid residues in the corresponding positions of the colicin B sequence (Fig. 3). Therefore, we hypothesize that the mechanism of cation selectivity might be different for colicins A and B. We can speculate that the possible binding of negatively charged phospholipids to colicin B could enhance the attraction of cations to the vicinity of the pore which, for an unknown reason, does not play a role in colicin U.
FIG 7.
Sequence alignment of C-terminal 200 amino acids of colicin U and colicin A shows high sequence similarity. The colicin U sequence includes two segments which might be responsible for the lower cation selectivity of colicin U pores, as they lack the acidic residues of colicin A from 425 to 428 (colicin A → colicin U differences are E398 → A425 and E401 → T428) and 530 to 541 (differences, E503 → N530 and E514 → Q541). Asterisks mark identical residues; colons mean similar amino acids. Negatively charged amino acids (glutamic and aspartic acid) are colored in yellow; positively charged (arginine and lysine) are in cyan. Red and blue highlighting denotes a difference in charge of the two sequences. The underlined sequences correspond to expected α-helices of the pore-forming domain (69). The amino acid sequence alignment was generated with Clustal Omega (70).
Permeability of colicin U pores for small inorganic cations.
To determine the inner pore size and its specificity, we observed the effect of various inorganic cations (Rb+, Cs+, NH4+, K+, Na+, and Li+) on the single-pore conductance of colicin U (Table 1). All these monovalent inorganic cations were expected to be small enough to fit into the colicin U pore. In these experiments the effect of Cl− was ignored, despite the low K+/Cl− selectivity, since we compared the pore conductance at identical Cl− concentrations (1 M). We found that the conductance was maximal for NH4Cl and minimal for LiCl solution. Except for Cs+ ions, all other cations decreased the pore conductance in accordance with their hydrated ion radius.
TABLE 1.
Single-pore conductance of colicin U in solutions of chloride and cations with various sizesa
We can conclude that most of the conductance data exhibit a linear correlation with the hydrodynamic radius of the cations. Pore conductance is in agreement with the mobility sequence of the ions, with the exception of Cs+ ions, which lowered the pore conductance more than could be presumed from their hydrodynamic radius (similar to K+ and Rb+). The reason for the low pore conductance in CsCl is not clear, but a comparable tendency was observed for Cs+ ions with colicins A, B, and N (10, 12). In summary, all the tested inorganic cations (with hydrated ionic radii from 0.105 to 0.216 nm) were small enough to pass through the colicin U pore.
Permeability of colicin U pores for organic cations.
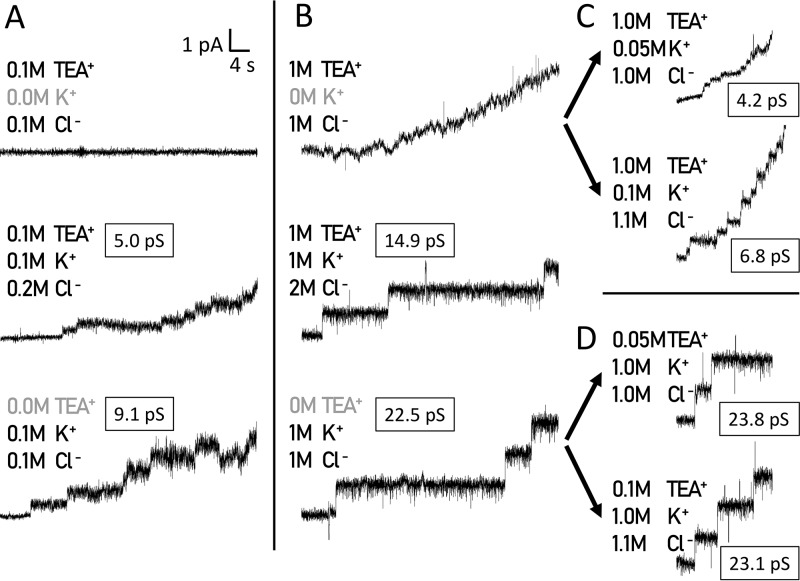
Subsequently, in order to study the effect of larger ions which, in general, could be excluded from the pore based on their size, we decided to study the influence of the organic cations TEA+ and Tris+ with hydrodynamic radii of 0.250 and 0.321 nm, respectively (51, 52). Both ions significantly affected the colicin U pores. We observed no increase in current with colicin U in a solution of 0.1 M TEACl (Fig. 8A, top line), while in 1 M TEACl, colicin U caused a continual current increase rather than an opening of the individual pores (Fig. 8B, top line). When we measured the activity of colicin U in a solution of 1 M TEACl together with 1 M KCl, colicin U formed pores similar to those in 1 M KCl but with significantly lowered single-pore conductance (14.9 versus 22.5 pS) (Fig. 8B). Simultaneously, we observed a similar relative decrease in single-pore conductance (5 versus 9 pS) in solutions with 10-fold lower molar strength (0.1 M TEACl plus 0.1 M KCl versus 0.1 M KCl) (Fig. 8A).
FIG 8.
Colicin U pore formation in the presence of TEA+ organic cations in solution. (A) Colicin U in 0.1 M TEACl does not permeabilize the membrane (top), while in the 0.1:0.1 M mixture of TEACl and KCl, the colicin forms pores with lower conductance (5 pS; middle) than in 0.1 M KCl (9.1 pS; bottom). (B) In the presence of 1 M TEACl, colicin U causes a gradual increase in current without any distinguishable events (top). In the 1:1 M mixture of TEACl and KCl, colicin U forms pores with decreased conductance (14.9 pS; middle) compared with 1 M KCl (22.5 pS; bottom). (C and D) The addition of KCl to 1 M TEACl induced the formation of detectable pores with low conductance (C), whereas the additions of TEACl to 1 M KCl did not change the single-pore properties of colicin U (D). The measurements were performed with the specified electrolytes and 10 mM HEPES at pH 6 under 80 mV. Representative traces are shown (n = 10).
As we did not observe any formation of discrete pores in TEACl alone, we hypothesized that the presence of K+ ions is crucial for the regular colicin U pore opening. To test this, we performed a titration of 1 M TEACl with KCl, and indeed, we observed a formation of discrete pores with an increasing single-pore conductance of about 4.2 pS (in 0.05 M K+), 6.8 pS (in 0.1 M K+), and 13 pS (in 0.5 M K+), clearly depending on K+ addition (Fig. 8C; see Table S1 in the supplemental material). Interestingly, in 1 M KCl, the addition of relatively large amounts of TEACl (0.05, 0.100, or 0.500 M) neither blocked the typical pore formation nor affected the single-pore conductance at all (Fig. 8D; Table S1).
The presence of TEA+ ions caused a gradual increase in membrane current but no clearly distinguishable pore openings. TEA+ was previously shown to exert a specific interaction with protein pores or with the membrane itself (53). In the presence of 0.1 M TEACl and 0.1 M KCl, the single-pore conductance decreased to ca. 50% of the value in 0.1 M KCl, despite the fact that the solution contained 0.2 M Cl− ions. The same effect was also observed at 10× higher concentrations. This is clear proof of pore blocking by TEA+. We can hypothesize about the TEA+ ion binding to the colicin U pore and obstructing the pore lumen for both K+ and Cl− ions. In this possible scenario, after increasing the K+ concentration, the TEA+ ion would be released from the pore, and a normal K+ (and Cl−) current should be observed with occasional blockages by TEA+. Interestingly, from these experiments, we could conclude that the presence of K+ or other alkali metal ions is probably necessary for the proper formation of the colicin pores, whereas the presence of Cl− ions alone is not sufficient.
Next, we tested the effect of another organic cation, namely, Tris+, with a larger ionic radius (0.321 nm) (52). Interestingly, the interaction of the pores with TEA+ and Tris+ was strikingly different. When a solution of 1 M Tris-Cl was used, stable colicin U pores were formed but with extremely low conductance (1.7 pS) (Table S1), which suggests that some of the Tris+ and/or Cl− ions are able to pass through the pore under these conditions. We again tested the permeability for other ions by additions of KCl. At a molar concentration ratio of 0.6:0.8:1.4 (Tris+/K+/Cl−), the pore conductance increased but only to 8.8 pS (Table S1). Such a low conductance (compared with mixtures of KCl and TEACl) (Table S1) could be explained by the binding of the Tris+ ion to the pore lumen and by blocking the passage of other solutes, including K+ and Cl−. Such an explanation is supported by the following experimental data: in 1 M KCl (G, 22.5 pS) (Table 1) the expected “contribution” of Cl− to single-pore conductance is about 9 pS, when assuming a 1.5/1 permeability ratio for K+ and Cl− [9 pS = 22.5 pS/(1.5 + 1)] (see “Ion selectivity of colicin U” below). However, in a 1 M Tris-Cl solution, the actual single-pore conductance is five times lower. Thus, the Tris+ ion must effectively block the Cl− flow through the pore. These experiments with Tris+ also demonstrate that alkali cations are definitely not indispensable for colicin U activity, as was indicated above. Therefore, the Tris+ ion probably binds to the colicin U pore and, thus, limits the passage of other ions. This effect cannot be reversed by equimolar concentration of K+. On the other hand, the TEA+ ion, which probably also binds to colicin, prevents a certain step in pore formation. However, TEA+ can be outcompeted by an excess of K+ that allows the normal pore-forming action of colicin U.
Inner diameter of colicin U pores.
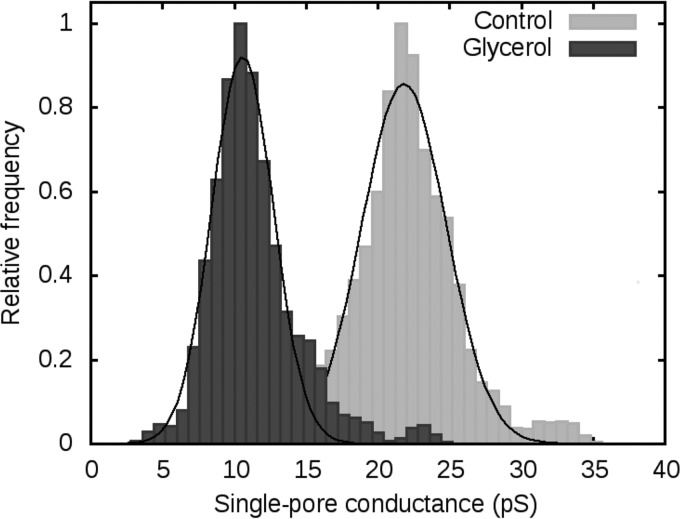
As the experiments with an organic cation did not led to unequivocal conclusions, we decided to estimate the inner radius of colicin U pores using nonelectrolytes (NEs). We tested the single-pore conductance of colicin U under standard conditions (1 M KCl and 10 mM HEPES [pH 6]) with added NE at a concentration of 20% (wt/vol). Molecules of NEs smaller than the inner size of the pore should be able to enter the pore and decrease pore conductance (mediated by K+ and Cl−), while bigger NE molecules should not have any effect. We used molecules of ethylene glycol, glycerol, arabinose, sorbitol, maltose, polyethylene glycol 300 (PEG 300), PEG 400, and PEG 1000, ordered according to their size from the smallest to the biggest molecule. Figure 9 shows histograms of the single-pore conductance of colicin U in the absence and presence of glycerol, which produced the greatest decrease in pore conductance. Glycerol lowered the average pore conductance by 10 pS. The effect of other NEs is summarized in Table 2.
FIG 9.
Distribution of single-pore conductance of colicin U in the absence (Control) and presence of 20% (wt/vol) glycerol (n > 100). In both experiments, the solution contained 1 M KCl and 10 mM HEPES at pH 6 under 80 mV. The conductivities of the solutions used were 102.5 mS/cm in the absence and 68.6 mS/cm in the presence of glycerol.
TABLE 2.
Single-pore conductance in the presence of nonelectrolytesa and pore filling
| Nonelectolyte, 20% (wt/vol) | Radius of nonelectrolyte (nm)b | Conductance ± HWHM (pS)d | Conductivity of solution (mS/cm) | F | F(%)c |
|---|---|---|---|---|---|
| None | 21.9 ± 3.5 | 102.5 | 0 | 0 | |
| Ethylene glycol | 0.26 | 18.1 ± 2.0 | 66.3 | 0.38 | 17.8 |
| Glycerol | 0.31 | 10.6 ± 2.5 | 68.6 | 2.16 | 100.0 |
| Arabinose | 0.34 | 10.6 ± 3.0 | 64.0 | 1.77 | 82.2 |
| Sorbitol | 0.39 | 16.9 ± 2.2 | 64.2 | 0.50 | 23.0 |
| Maltose | 0.5 | 20.8 ± 4.5 | 74.0 | 0.14 | 6.4 |
| PEG 300 | 0.6 | 20.2 ± 2.4 | 55.0 | 0.10 | 4.5 |
| PEG 400 | 0.7 | 18.3 ± 3.5 | 57.1 | 0.25 | 11.4 |
| PEG 1000 | 0.94 | 19.7 ± 3.4 | 56.0 | 0.13 | 6.2 |
n > 100.
The radii of the nonelectrolytes were reported previously (54).
The values of F(%) are normalized to the highest filling value observed in the presence of glycerol.
Measured in 20% (wt/vol) NE, 1 M KCl, and 10 mM HEPES at pH 6 under 80 mV.
The data in Table 2 indicate that glycerol (diameter of 0.62 nm) and arabinose (0.68 nm) decreased the conductance of pores, whereas maltose (1 nm) and PEG molecules had no significant effect. Surprisingly, ethylene glycol molecules caused almost no decrease in conductance, even though it was the smallest molecule analyzed. Such unexplained anomalous behavior led us to exclude the data with ethylene glycol from our further calculations.
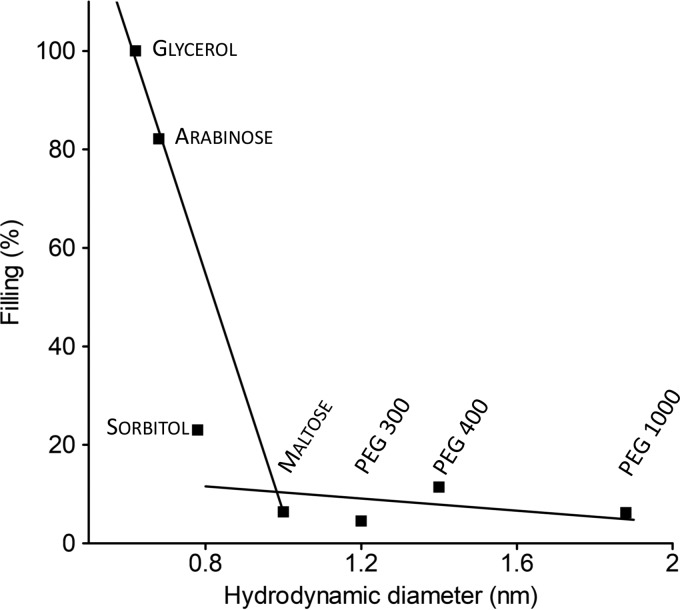
Single-pore conductance data influenced by NEs were used for estimating colicin U pore size. To express our results more precisely, we used a “filling” factor F which provides information about the concentration of NE in the pore. This approach uses an assumption that the smallest NEs are close to completely filling the pore (54), which allows the percentage filling [F(%)] to be calculated (Fig. 10).
FIG 10.
Dependence of filling [F(%)] of the pore on hydrodynamic diameter of nonelectrolytes. The solid lines are visual guides. NEs were ordered according to their size (from left to right), namely, glycerol, arabinose, sorbitol, maltose, PEG 300, PEG 400, and PEG 1000. For calculation of F(%), see “Determination of the pore size” below.
It is obvious that the size of sorbitol molecules (0.78 nm) is limiting. Sorbitol molecules lower pore conductance just slightly (Fig. 10) and this results from just a partial filling of the pore (23%), while maltose (1 nm) or PEG 300 (1.2 nm) hardly affects the pore conductance at all (6.4% and 4.5% filling, respectively). Therefore, we conclude that the diameter of colicin U pores is between 0.7 and 1 nm.
Additionally, in order to provide a more thorough description of the inner structure of the pores, we measured single-pore conductance in the presence of various nonelectrolytes on the cis and trans sides. Our aim was to investigate the sizes of the cis and trans entrances of the pore separately. First, we chose arabinose, which was the biggest molecule that passed through the pore. We tested 20% arabinose on individual sides of the membrane and observed single-pore conductances of about 9.5 pS (±2.5 pS) and 12.0 pS (±2.6 pS) when arabinose was used on the cis and trans sides, respectively. These values are very close to the conductance when the arabinose is placed on both sides (10.6 pS) of the membrane. If the pore was spatially asymmetrical, we would presume to find differences in conductance under the tested conditions, which was not the case. Therefore, we conclude that arabinose fills the pore from both sides relatively symmetrically.
We did not carry out measurements of pore asymmetry for nonelectrolytes with a bigger radius, as we did not expect any effect. For example, the dynamic range of the asymmetry detection drops 4-fold from arabinose (82% filling) to sorbitol (with only 23% filling); therefore, thanks to the quite broad pore-conductance distribution, the pore asymmetry would be barely detectable.
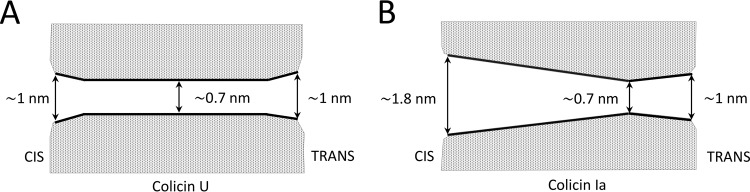
The determined diameter of colicin U pores is very similar to the pore size found for colicin E1 (0.9 nm) (55) or colicin Ia (diameter estimated as 0.7 nm in the constricted site of the pore and 1 and 1.8 nm at its entrances) (54). This estimated pore diameter of colicin U pores (0.7 to 1 nm) is in strong contrast with its very low single-pore conductance (∼22 pS in 1 M KCl). The calculation using Ohm’s law and a 1-nm pore diameter would yield a pore conductance many times higher. Such a discrepancy has been noticed not only for other colicins (34, 54–57) but also for some other pore-forming molecules, such as syringomycin E, where the authors hypothesize that electrostatic interactions between ions and the pore hinder ion movement within the pore via unknown mechanisms (58). When comparing the inner profiles of colicin U and colicin Ia, important differences might be observed. The constriction and broad entrances seen in colicin Ia pores were not detected in colicin U pores (Fig. 11).
FIG 11.
Model of pore lumen of colicin U and Ia. (A) Our proposed model of the inner profile of colicin U. The profile is deduced from the results of pore filling by NEs. Molecules of glycerol (0.62 nm) can enter the pore without limitations from both sides of the membrane, whereas the size of arabinose (0.68 nm diameter) is comparable to the pore diameter. Sorbitol molecules (0.78 nm) can only occupy the pore entrances, while the bigger molecules, such as maltose (1.0 nm), cannot enter the pore at all. (B) The geometrical profile of the colicin Ia pore exhibits a strikingly asymmetrical shape. The parameters were taken from Krasilnikov and colleagues (54).
Instead, the inner profile of the colicin U pore is relatively symmetrical without any detected constriction. Such a simple pore profile is in accordance with its unequivocal saturability by high electrolyte concentrations. We suppose the pores to be quite narrow (about 0.7 nm) in diameter throughout the whole length, with only mildly broadened entrances (Fig. 11), as implied by the partial sorbitol filling. This quite narrow cylindrical profile nicely correlates with the fact that colicin U has a lower single-pore conductance (22 pS) than the 44 pS of colicin Ia under the same conditions (59).
Conclusions.
In summary, we demonstrate here that colicin U forms pores with nonohmic behavior in phospholipid bilayers under physiological membrane potential. Certain properties of the pores are similar to the closely related colicins A and B. The molecule of colicin U probably undergoes a conformational change at acidic pH, which is especially apparent in the altered pore dynamics and pore-forming activity. Additionally, colicin U pores are probably formed by protein monomers. However, some properties of colicin U pores differ greatly from other colicins. The voltage gating of colicin U pores is rather unexpected; the pores that opened under high physiological membrane potential are actively closed when the value of membrane potential approaches zero. The single-pore current of colicin U reaches its Km at very low electrolyte concentrations (0.19 M KCl), and the cationic selectivity of pores is very low compared with the selectivity of other colicins. Finally, our results suggest that colicin U forms cis-trans symmetrical pores of an inner diameter of 0.7 to 1 nm, with only mildly broadened entrances. Such an inner profile of the pore, where specific residues slow down ions passing through the pore, can explain the quite low conductance of colicin U pores.
MATERIALS AND METHODS
Preparation of colicin U.
Colicin U was expressed and purified as described elsewhere with minor modifications (60, 61). Briefly, the PCR product containing colicin activity (cua) and colicin immunity (cui) genes (2.4 kb) was amplified with specific primers (Col-U-XhoI-F, AGGACTCGAGATGCCTGGATTTAATTATGGTGGACAT; and ImmUU, AGGACTCGAGATGCAAAAAGAGCATAATGA) and PfuI polymerase and cloned to a pBAD-A vector (Thermo Fischer Scientific, USA) using restriction enzyme XhoI (New England BioLabs [NEB]) and T4 ligase (NEB), where the cua was fused at its 5′end to DNA encoding the His tag (the fused sequence from the pBAD-A vector contained the His tag MGGSHHHHHHGMASMTGGQQMGRDLYDDDDKDRWGSELE). The resulting pDS1066 plasmid was then transformed into the expression Escherichia coli TOP10F´ strain. Colicin U was expressed from overnight cultures by induction with 0.02% arabinose (wt/vol). The prepurification of colicin U was performed by Ni-nitrilotriacetic acid (Ni-NTA)–agarose chromatography (Qiagen, The Netherlands). An AKTA fast protein liquid chromatography (FPLC) System (GE Healthcare, USA) with a Superdex 75 10/300 GL column was used for the final purification step. The N-terminal His tag in recombinant colicin U does not interfere with its pore-forming properties.
Black lipid membrane measurements.
Planar lipid membrane experiments were performed as described previously (62, 63).
The electrolyte solution contained 1 M KCl and 10 mM HEPES (pH 6) (or otherwise indicated in the figure legends). Colicin U (9 nM concentration) was added to the cis compartment with a positive electrode, whereas the trans compartment was grounded, unless indicated otherwise. This corresponds technically to the situation in vivo; colicin is added externally to the cells with negative membrane potential. Planar lipid membranes were formed across a 0.5-mm aperture by painting the soybean lipids (3% wt/vol, asolectin-type II; Merck KGaA, Germany) dissolved in n-decan and butanol (9:1). The membrane current was registered using Ag/AgCl electrodes with salt bridges connected to an LCA-200-100G amplifier (Femto, Germany) and digitized with a KPCI-3108 16-Bit A/D card (Keithley Instruments, USA) with a 1-kHz sampling rate.
Single pore analysis.
Single-pore recordings were processed in the program QuB (64). Most of the data were then filtered with a 40-Hz low-pass filter (Gaussian filter in the frequency domain) to visualize individual events. For the determination of ion selectivity, we estimated the reversal potential (Urev) by fitting a linear function to the current-voltage dependence of individual pore openings (or closings) in the presence of a KCl gradient across the membrane (cis, 1 M KCl; trans, 0.1 M KCl; or opposite). The K+/Cl− selectivity was then calculated as the ion permeability ratio of pores using an equation derived from the Goldmann-Hodgkin-Katz equation (47, 65, 66)
where Pk and Pa are the permeability of cations and anions, respectively. c1 and c2 are the concentrations of electrolytes on the two sides of the membrane, R is the universal gas constant, T is the absolute temperature, and F is the Faraday constant.
Estimation of pore stoichiometry.
To estimate the pore stoichiometry, we tested colicin U activity in terms of electrical current increase (picoamperes per minute) after membrane formation at different protein concentrations. In theory, such data could be fitted to the Hill function
where Θ is the current increase (picoamperes per minute), [C] is the colicin concentration, KA is the concentration of colicin producing the half-maximal current increase, and n is the Hill coefficient. The parameter A relates to the area of the bilayer, the membrane voltage, and other experimental conditions.
When [C] is ≪KA the data can be fitted to a simplified power function in the form
where q represents an empirical constant describing the effectivity of colicin pore opening.
In practice, we fitted the activity data (pA/min) at different colicin concentrations (nanomolar) to a straight line on a double logarithmic plot using the software Gnuplot 5.0 (http://www.gnuplot.info). The slope of this line corresponds to the Hill number n. The uncertainty of this parameter value was quantified during the fitting as the asymptotic standard error.
Determination of the pore size.
A pore size estimation using the measurement of single-pore conductance (G) in the presence of nonelectrolytes (NE) was described previously (67, 68). For measurements with nonelectrolytes, we used a 1 M KCl and 10 mM HEPES (pH 6) solution with added 20% (wt/vol) nonelectrolyte; ethylene glycol, glycerol, arabinose, sorbitol, maltose, poly(ethylene glycol) (PEG) 300, PEG 400, and PEG 1000 were all purchased from Merck KGaA.
For a more precise determination of the opened pore dimensions, we used a “channel filling” parameter (F), which was described previously (54). The filling is characterized by the equation
where g0 is the single-pore conductance in solution without nonelectrolyte (1 M KCl), gi is the single-pore conductance in solution with nonelectrolyte (20% wt/vol), χ0 is the conductivity of the solution without NE, and χi is the conductivity of the solution with 20% (wt/vol) NE.
We can express the filling of the pore in terms of a percentage [F(%)]
where Fi is the filling of the pore in solution with the measured NE; FS represents the filling solution with the smallest analyzed NE. The radius of the constriction should be close to the radius of the smallest NE molecule, which only partially fills the pore. The conductivity of solutions, χ (micro-Siemens per centimeter), was measured with a PC 5000 L pH/conductivity meter (VWR International, USA) under standard conditions at 25°C. For measurements of cis-trans asymmetry, we always used the tested NE with 20% PEG 1000 on the opposite side of the membrane.
Statistics and data analysis.
For single-pore experiments, we used three independently produced and purified colicin U preparations that gave highly reproducible results. Here, we present the data measured on the single preparation. The values of single-pore conductance were obtained from the conductance histogram (from >50 events measured on 10 to 30 individual membranes) fitted to a Gauss function. The error bars in graphs correspond to the half-width of the Gaussian function (HWHM), and they reflect the variability of single-pore conductance rather than the error of the measurement. Histograms of single-pore conductance were constructed by kernel density estimation (with the Gaussian kernel of 1-pS width) to more accurately determine the position of the maximum and to overcome bin edge effects. The data were fitted using the software Gnuplot 5.0.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by project SVV-260426 and grant no. 390115 from the Grant Agency of Charles University to T.D. This work was also supported by the Czech Science Foundation (GA16-21649S) to D.Š.
We thank Lucie Jánská for excellent technical assistance and to Tomáš Wald for his kind help with protein purification.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00493-19.
REFERENCES
- 1.Gillor O, Nigro LM, Riley MA. 2007. Potential application of bacteriocins as antimicrobials, p 73–74. In Riley MA, Gillor O (ed), Research and applications in bacteriocins. Horizon Scientific Press, Norfolk, United Kingdom. [Google Scholar]
- 2.Qiu X-Q, Wang H, Lu X-F, Zhang J, Li S-F, Cheng G, Wan L, Yang L, Zuo J-Y, Zhou Y-Q, Wang H-Y, Cheng X, Zhang S-H, Ou Z-R, Zhong Z-C, Cheng J-Q, Li Y-P, Wu GY. 2003. An engineered multidomain bactericidal peptide as a model for targeted antibiotics against specific bacteria. Nat Biotechnol 21:1480–1485. doi: 10.1038/nbt913. [DOI] [PubMed] [Google Scholar]
- 3.Cascales E, Buchanan SK, Duché D, Kleanthous C, Lloubès R, Postle K, Riley M, Slatin S, Cavard D. 2007. Colicin biology. Microbiol Mol Biol Rev 71:158–229. doi: 10.1128/MMBR.00036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaller K, Nomura M. 1976. Colicin E2 is DNA endonuclease. Proc Natl Acad Sci U S A 73:3989–3993. doi: 10.1073/pnas.73.11.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsia K-C, Chak K-F, Liang P-H, Cheng Y-S, Ku W-Y, Yuan HS. 2004. DNA binding and degradation by the HNH protein ColE7. Structure 12:205–214. doi: 10.1016/j.str.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Pommer AJ, Cal S, Keeble AH, Walker D, Evans SJ, Kühlmann UC, Cooper A, Connolly BA, Hemmings AM, Moore GR, James R, Kleanthous C. 2001. Mechanism and cleavage specificity of the H-N-H endonuclease colicin E9. J Mol Biol 314:735–749. doi: 10.1006/jmbi.2001.5189. [DOI] [PubMed] [Google Scholar]
- 7.James R, Penfold CN, Moore GR, Kleanthous C. 2002. Killing of E. coli cells by E group nuclease colicins. Biochimie 84:381–389. doi: 10.1016/S0300-9084(02)01450-5. [DOI] [PubMed] [Google Scholar]
- 8.Bourdineaud JP, Boulanger P, Lazdunski C, Letellier L. 1990. In vivo properties of colicin A: channel activity is voltage dependent but translocation may be voltage independent. Proc Natl Acad Sci U S A 87:1037–1041. doi: 10.1073/pnas.87.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakey JH, Slatin SL. 2001. Pore-forming colicins and their relatives. Curr Top Microbiol Immunol 257:131. doi: 10.1007/978-3-642-56508-3_7. [DOI] [PubMed] [Google Scholar]
- 10.Pressler U, Braun V, Wittmann-Liebold B, Benz R. 1986. Structural and functional properties of colicin B. J Biol Chem 261:2654–2659. [PubMed] [Google Scholar]
- 11.Schein SJ, Kagan BL, Finkelstein A. 1978. Colicin K acts by forming voltage-dependent channels in phospholipid bilayer membranes. Nature 276:159–163. doi: 10.1038/276159a0. [DOI] [PubMed] [Google Scholar]
- 12.Wilmsen HU, Pugsley AP, Pattus F. 1990. Colicin N forms voltage- and pH-dependent channels in planar lipid bilayer membranes. Eur Biophys J 18:149–158. doi: 10.1007/BF02427374. [DOI] [PubMed] [Google Scholar]
- 13.Patin D, Barreteau H, Auger G, Magnet S, Crouvoisier M, Bouhss A, Touzé T, Arthur M, Mengin-Lecreulx D, Blanot D. 2012. Colicin M hydrolyses branched lipids II from Gram-positive bacteria. Biochimie 94:985–990. doi: 10.1016/j.biochi.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Elkins P, Bunker A, Cramer WA, Stauffacher CV. 1997. A mechanism for toxin insertion into membranes is suggested by the crystal structure of the channel-forming domain of colicin E1. Structure 5:443–458. doi: 10.1016/s0969-2126(97)00200-1. [DOI] [PubMed] [Google Scholar]
- 15.Hilsenbeck JL, Park H, Chen G, Youn B, Postle K, Kang C. 2004. Crystal structure of the cytotoxic bacterial protein colicin B at 2.5 Å resolution. Mol Microbiol 51:711–720. doi: 10.1111/j.1365-2958.2003.03884.x. [DOI] [PubMed] [Google Scholar]
- 16.Parker MW, Postma JPM, Pattus F, Tucker AD, Tsernoglou D. 1992. Refined structure of the pore-forming domain of colicin A at 2.4 Å resolution. J Mol Biol 224:639–657. doi: 10.1016/0022-2836(92)90550-4. [DOI] [PubMed] [Google Scholar]
- 17.Vetter IR, Parker MW, Tucker AD, Lakey JH, Pattus F, Tsernoglou D. 1998. Crystal structure of a colicin N fragment suggests a model for toxicity. Structure 6:863–874. doi: 10.1016/s0969-2126(98)00088-4. [DOI] [PubMed] [Google Scholar]
- 18.Wiener M, Freymann D, Ghosh P, Stroud RM. 1997. Crystal structure of colicin Ia. Nature 385:461–464. doi: 10.1038/385461a0. [DOI] [PubMed] [Google Scholar]
- 19.Šmajs D, Pilsl H, Braun V. 1997. Colicin U, a novel colicin produced by Shigella boydii. J Bacteriol 179:4919–4928. doi: 10.1128/jb.179.15.4919-4928.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Micenková L, Bosák J, Štaudová B, Kohoutová D, Čejková D, Woznicová V, Vrba M, Ševčíková A, Bureš J, Šmajs D. 2016. Microcin determinants are associated with B2 phylogroup of human fecal Escherichia coli isolates. MicrobiologyOpen 5:490–498. doi: 10.1002/mbo3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Micenková L, Bosák J, Vrba M, Ševčíková A, Šmajs D. 2016. Human extraintestinal pathogenic Escherichia coli strains differ in prevalence of virulence factors, phylogroups, and bacteriocin determinants. BMC Microbiol 16:218. doi: 10.1186/s12866-016-0835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Micenková L, Beňová A, Frankovičová L, Bosák J, Vrba M, Ševčíková A, Kmeťová M, Šmajs D. 2017. Human Escherichia coli isolates from hemocultures: septicemia linked to urogenital tract infections is caused by isolates harboring more virulence genes than bacteraemia linked to other conditions. Int J Med Microbiol 307:182–189. doi: 10.1016/j.ijmm.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Micenková L, Frankovičová L, Jaborníková I, Bosák J, Dítě P, Šmarda J, Vrba M, Ševčíková A, Kmeťová M, Šmajs D. 2018. Escherichia coli isolates from patients with inflammatory bowel disease: ExPEC virulence- and colicin-determinants are more frequent compared to healthy controls. Int J Med Microbiol 308:498–504. doi: 10.1016/j.ijmm.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Baty D, Frenette M, Lloubès R, Geli V, Howard SP, Pattus F, Lazdunski C. 1988. Functional domains of colicin A. Mol Microbiol 2:807–811. doi: 10.1111/j.1365-2958.1988.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 25.Zakharov SD, Cramer WA. 2002. Colicin crystal structures: pathways and mechanisms for colicin insertion into membranes. Biochim Biophys Acta 1565:333–346. doi: 10.1016/S0005-2736(02)00579-5. [DOI] [PubMed] [Google Scholar]
- 26.Riley MA, Gordon DM. 1996. The ecology and evolution of bacteriocins. J Ind Microbiol Biotechnol 17:151–158. doi: 10.1007/BF01574688. [DOI] [Google Scholar]
- 27.Riley MA. 1993. Molecular mechanisms of colicin evolution. Mol Biol Evol 10:1380–1395. doi: 10.1093/oxfordjournals.molbev.a040081. [DOI] [PubMed] [Google Scholar]
- 28.Cleveland MV, Slatin S, Finkelstein A, Levinthal C. 1983. Structure-function relationships for a voltage-dependent ion channel: properties of COOH-terminal fragments of colicin E1. Proc Natl Acad Sci U S A 80:3706–3710. doi: 10.1073/pnas.80.12.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh P, Mel SF, Stroud RM. 1993. A carboxy-terminal fragment of colicin Ia forms ion channels. J Membr Biol 134:85–92. doi: 10.1007/bf00232745. [DOI] [PubMed] [Google Scholar]
- 30.Martinez MC, Lazdunski C, Pattus F. 1983. Isolation, molecular and functional properties of the C-terminal domain of colicin A. EMBO J 2:1501–1507. doi: 10.1002/j.1460-2075.1983.tb01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Šmajs D, Doležalová M, Macek P, Žídek L. 2008. Inactivation of colicin Y by intramembrane helix–helix interaction with its immunity protein. FEBS J 275:5325–5331. doi: 10.1111/j.1742-4658.2008.06662.x. [DOI] [PubMed] [Google Scholar]
- 32.Knapp O, Maier E, Mašín J, Šebo P, Benz R. 2008. Pore formation by the Bordetella adenylate cyclase toxin in lipid bilayer membranes: role of voltage and pH. Biochim Biophys Acta 1778:260–269. doi: 10.1016/j.bbamem.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 33.Peterson AA, Cramer WA. 1987. Voltage-dependent, monomeric channel activity of colicin E1 in artificial membrane vesicles. J Membr Biol 99:197–204. doi: 10.1007/bf01995700. [DOI] [PubMed] [Google Scholar]
- 34.Slatin SL. 1988. Colicin E1 in planar lipid bilayers. Int J Biochem 20:737–744. doi: 10.1016/0020-711x(88)90058-4. [DOI] [PubMed] [Google Scholar]
- 35.Luria SE. 1982. The mistaken identity of colicin A. J Bacteriol 149:386–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osicková A, Osicka R, Maier E, Benz R, Sebo P. 1999. An amphipathic alpha-helix including glutamates 509 and 516 is crucial for membrane translocation of adenylate cyclase toxin and modulates formation and cation selectivity of its membrane channels. J Biol Chem 274:37644–37650. [PubMed] [Google Scholar]
- 37.Collarini M, Amblard G, Lazdunski C, Pattus F. 1987. Gating processes of channels induced by colicin A, its C-terminal fragment and colicin E1 in planar lipid bilayers. Eur Biophys J 14:147. doi: 10.1007/BF00253839. [DOI] [PubMed] [Google Scholar]
- 38.Nestorovich EM, Rostovtseva TK, Bezrukov SM. 2003. Residue ionization and ion transport through OmpF channels. Biophys J 85:3718–3729. doi: 10.1016/S0006-3495(03)74788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez-Manas JM, Lakey JH, Pattus F. 1992. Brominated phospholipids as a tool for monitoring the membrane insertion of colicin A. Biochemistry 31:7294–7300. doi: 10.1021/bi00147a013. [DOI] [PubMed] [Google Scholar]
- 40.Evans LJA, Goble ML, Hales KA, Lakey JH. 1996. Different sensitivities to acid denaturation within a family of proteins: implications for acid unfolding and membrane translocation. Biochemistry 35:13180–13185. doi: 10.1021/bi960990u. [DOI] [PubMed] [Google Scholar]
- 41.Lakey JH, Parker MW, González‐Mañas JM, Duché D, Vriend G, Baty D, Pattus F. 1994. The role of electrostatic charge in the membrane insertion of colicin A. Eur J Biochem 220:155–163. doi: 10.1111/j.1432-1033.1994.tb18610.x. [DOI] [PubMed] [Google Scholar]
- 42.Kozlowski LP. 2016. IPC—isoelectric point calculator. Biol Direct 11:55. doi: 10.1186/s13062-016-0159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masin J, Osickova A, Sukova A, Fiser R, Halada P, Bumba L, Linhartova I, Osicka R, Sebo P. 2016. Negatively charged residues of the segment linking the enzyme and cytolysin moieties restrict the membrane-permeabilizing capacity of adenylate cyclase toxin. Sci Rep 6:29137. doi: 10.1038/srep29137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bullock JO, Armstrong SK, Shear JL, Lies DP, McIntosh MA. 1990. Formation of ion channels by Colicin B in planar lipid bilayers. J Membr Biol 114:79–95. doi: 10.1007/bf01869387. [DOI] [PubMed] [Google Scholar]
- 45.Bullock JO, Cohen FS, Dankert JR, Cramer WA. 1983. Comparison of the macroscopic and single channel conductance properties of colicin E1 and its COOH-terminal tryptic peptide. J Biol Chem 258:9908–9912. [PubMed] [Google Scholar]
- 46.Qiu XQ, Jakes KS, Kienker PK, Finkelstein A, Slatin SL. 1996. Major transmembrane movement associated with colicin Ia channel gating. J Gen Physiol 107:313–328. doi: 10.1085/jgp.107.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hodgkin AL, Katz B. 1949. The effect of sodium ions on the electrical activity of the giant axon of the squid. J Physiol 108:37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aguilella VM, Queralt-Martín M, Aguilella-Arzo M, Alcaraz A. 2011. Insights on the permeability of wide protein channels: measurement and interpretation of ion selectivity. Integr Biol (Camb) 3:159–172. doi: 10.1039/c0ib00048e. [DOI] [PubMed] [Google Scholar]
- 49.Bullock JO. 1992. Ion selectivity of colicin E1: modulation by pH and membrane composition. J Membr Biol 125:255–271. doi: 10.1007/bf00236438. [DOI] [PubMed] [Google Scholar]
- 50.Nardi A, Slatin SL, Baty D, Duché D. 2001. The C-terminal half of the colicin A pore-forming domain is active in vivo and in vitro. J Mol Biol 307:1293–1303. doi: 10.1006/jmbi.2001.4524. [DOI] [PubMed] [Google Scholar]
- 51.Castellan GW. 1983. The ionic current in aqueous solutions, p 769–780. In Physical chemistry, 3rd ed Addison Wesley, Boston, MA. [Google Scholar]
- 52.Maier E, Reinhard N, Benz R, Frey J. 1996. Channel-forming activity and channel size of the RTX toxins ApxI, ApxII, and ApxIII of Actinobacillus pleuropneumoniae. Infect Immun 64:4415–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khodakhah K, Melishchuk A, Armstrong CM. 1997. Killing K channels with TEA+. Proc Natl Acad Sci U S A 94:13335–13338. doi: 10.1073/pnas.94.24.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krasilnikov OV, Da Cruz JB, Yuldasheva LN, Varanda WA, Nogueira RA. 1998. A novel approach to study the geometry of the water lumen of ion channels: colicin Ia channels in planar lipid bilayers. J Membr Biol 161:83–92. doi: 10.1007/s002329900316. [DOI] [PubMed] [Google Scholar]
- 55.Raymond L, Slatin SL, Finkelstein A. 1985. Channels formed by colicin E1 in planar lipid bilayers are large and exhibit pH-dependent ion selectivity. J Membr Biol 84:173–181. doi: 10.1007/bf01872215. [DOI] [PubMed] [Google Scholar]
- 56.Bullock JO, Kolen ER, Shear JL. 1992. Ion selectivity of colicin E1: II. Permeability to organic cations. J Membr Biol 128:1–16. doi: 10.1007/bf00231866. [DOI] [PubMed] [Google Scholar]
- 57.Kayalar C, Düzgüneş N. 1986. Membrane action of colicin E1: detection by the release of carboxyfluorescein and calcein from liposomes. Biochim Biophys Acta 860:51–56. doi: 10.1016/0005-2736(86)90497-9. [DOI] [PubMed] [Google Scholar]
- 58.Malev VV, Schagina LV, Gurnev PA, Takemoto JY, Nestorovich EM, Bezrukov SM. 2002. Syringomycin E channel: a lipidic pore stabilized by lipopeptide? Biophys J 82:1985–1994. doi: 10.1016/S0006-3495(02)75547-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kienker PK, Jakes KS, Finkelstein A. 2000. Protein translocation across planar bilayers by the colicin Ia channel-forming domain. J Gen Physiol 116:587–598. doi: 10.1085/jgp.116.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bosák J, Laiblová P, Smarda J, Dedicová D, Smajs D. 2012. A novel colicin FY of Yersinia frederiksenii inhibits pathogenic Yersinia strains via YiuR-mediated reception, TonB import and cell membrane pore-formation. J Bacteriol 194:1950–1959. doi: 10.1128/JB.05885-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bosák J, Micenková L, Doležalová M, Šmajs D. 2016. Colicins U and Y inhibit growth of Escherichia coli strains via recognition of conserved OmpA extracellular loop 1. Int J Med Microbiol 306:486–494. doi: 10.1016/j.ijmm.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Seydlová G, Pohl R, Zborníková E, Ehn M, Šimák O, Panova N, Kolář M, Bogdanová K, Večeřová R, Fišer R, Šanderová H, Vítovská D, Sudzinová P, Pospíšil J, Benada O, Křížek T, Sedlák D, Bartůněk P, Krásný L, Rejman D. 2017. Lipophosphonoxins II: design, synthesis, and properties of novel broad spectrum antibacterial agents. J Med Chem 60:6098–6118. doi: 10.1021/acs.jmedchem.7b00355. [DOI] [PubMed] [Google Scholar]
- 63.Masin J, Roderova J, Osickova A, Novak P, Bumba L, Fiser R, Sebo P, Osicka R. 2017. The conserved tyrosine residue 940 plays a key structural role in membrane interaction of Bordetella adenylate cyclase toxin. Sci Rep 7:9330. doi: 10.1038/s41598-017-09575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nicolai C, Sachs F. 2013. Solving ion channel kinetics with the OuB Software. Biophys Rev Lett 08:191–211. doi: 10.1142/S1793048013300053. [DOI] [Google Scholar]
- 65.Goldman DE. 1943. Potential, impedance, and rectification in membranes. J Gen Physiol 27:37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benz R, Janko K, Läuger P. 1979. Ionic selectivity of pores formed by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta 551:238–247. doi: 10.1016/0005-2736(89)90002-3. [DOI] [PubMed] [Google Scholar]
- 67.Holz R, Finkelstein A. 1970. The water and nonelectrolyte permeability induced in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. J Gen Physiol 56:125–145. doi: 10.1085/jgp.56.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krasilnikov OV, Yuldasheva LN, Nogueira RA, Rodrigues CG. 1995. The diameter of water pores formed by colicin Ia in planar lipid bilayers. Braz J Med Biol Res 28:693–698. [PubMed] [Google Scholar]
- 69.Geli V, Lazdunski C. 1992. An alpha-helical hydrophobic hairpin as a specific determinant in protein-protein interaction occurring in Escherichia coli colicin A and B immunity systems. J Bacteriol 174:6432–6437. doi: 10.1128/jb.174.20.6432-6437.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high‐quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.