Abstract
Objectives: Determine the prevalence of suboptimal peak inspiratory flow rate (PIFR) and associated patient characteristics and compare PIFR measurements obtained with spirometry and In-Check DIAL® device in ambulatory patients with COPD.
Methods: Patients underwent PIFR measurement with In-Check DIAL® device and pulmonary function testing with calibrated equipment. Group characteristics and lung function were compared for patients with suboptimal (≤ 60 L/min) and optimal (> 60 L/min) PIFR. Receiver operating curve analysis determined the best maximal forced inspiratory flow (FIF max) value in identifying optimal PIFR by gender and height.
Results: From July 1, 2016 to January 31, 2018, a total of 303 patients with chronic obstructive pulmonary disease (COPD) had PIFR and pulmonary function measurements. Group mean age was 65.5 ± 11.3 years with equal gender distribution. Suboptimal PIFR was observed in 61 (20.1%) patients. A significant correlation was observed between PIFR and FIF max, inspiratory capacity and residual volume (RV) to total lung capacity (TLC) ratio.
In the suboptimal PIFR group, mean FIF max measured by spirometry was significantly less compared with the optimal PIFR group; 178.5 ± 56.9 L/min and 263.4 ± 89.9 L/min, respectively (p<0.0001). Receiver operator curve analysis of FIF max to identify an optimal PIFR yielded an area under the curve of 0.79. Males < 65 inches had a suboptimal PIFR in 16.7 % of the male cohort, while females < 65 inches had a suboptimal PIFR in 27.4 % of the women.
Conclusions: Suboptimal PIFR was present in 1 in 5 stable patients with COPD and was more frequent in short statured females. Spirometry determined FIF max was associated with PIFR based on gender and height.
Keywords: copd, chronic obstructive pulmonary disease, peak inspiratory flow rate, inhalation therapy, dry powder inhaler, pulmonary function test
Introduction
Inhaled bronchodilators are frequently prescribed for symptomatic relief of dyspnea and to treat acute exacerbationsin patients with chronic obstructive pulmonary disease (COPD). Over the past decade, advances in the treatment of COPD have resulted in a proliferation of inhaled bronchodilators and anti-inflammatory agents and aerosol delivery devices, including metered dose inhalers, dry powder inhalers (DPI), soft mist inhalers and nebulizers. The expansion in the combinations of inhaled therapies and diversity of aerosol delivery devices presents a challenge to patients and providers. Patients must have the cognitive ability and manual dexterity to adequately perform specific inhalation maneuvers with a given aerosol delivery device according to the manufacturer’s specifications in order to achieve adequate lower respiratory tract deposition.1,2 Additionally, there is increasing awareness that aerosol delivery devices and patient factors can influence medication adherence and clinical outcomes.3,4,5 Hence, current Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines recommend that providers prescribe inhalation delivery devices based on an individual’s ability and preference.6
As a class of aerosol delivery devices, the DPI is effective and commonly used in the management of COPD. Its popularity is due to the compact size, convenience, ease of use and limited need for hand-breath coordination, such that the DPI accounts for 60% of the international market of inhaled medications.7 Currently, 10 commercial DPIs are available for delivery of long-acting bronchodilators and corticosteroids.8 However, lower respiratory tract delivery from a DPI is dependent on a patient’s inspiratory flow. The optimal peak inspiratory flow rate (PIFR) for adequate drug delivery from a Diskus DPI is reported to be greater than 60 L/min.5,7,8 Furthermore, measurement of PIFR allows objective evaluation of a patient’s ability to generate enough inspiratory force for effective drug delivery from a DPI and several commercially available devices are available to measure PIFR.9 Another assessment of peak inspiratory flow is the maximal forced inspiratory flow (FIF max) that is measured with spirometry. However, there is limited data comparing the measurement of peak inspiratory flow using different devices in ambulatory patients with COPD. Thus, the aims of the study were to 1) determine the prevalence of a suboptimal PIFR and describe the associated patient characteristics and 2) compare PIFR measurements obtained with an In-Check DIAL® device and a calibrated spirometer in stable, ambulatory patients with COPD.
Materials and Methods
Participant Selection
From July 2016 to January 2018, we enrolled consecutive ambulatory patients with COPD undergoing pulmonary function testing at the University of Texas Medical Branch. All patients underwent spirometry and lung volume measurements (Medgraphics, MGC Diagnostics Corp., Saint Paul, Minnesota) with daily-calibrated equipment. Lung volume measurements were determined by plethysmography. Pulmonary function testing was performed using American Thoracic Society (ATS)10 performance standards by registered pulmonary function technologists. Inclusion criteria were adult ambulatory patients with obstructive ventilatory defect defined as forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <0.70 without an exacerbation in the past 30 days. Exclusion criteria include pulmonary function testing with an FEV1/FVC 0.70, inability to follow verbal instructions, acutely worsening symptoms or testing that did not meet ATS performance criteria.
Measurements
As part of standard spirometry, a forced inspiratory vital capacity maneuver was performed through a low resistance spirometer that provided a measurement of FIF max.11 After pulmonary function testing, a respiratory therapist measured PIFR with an In-Check DIAL® device (Clement Clarke International Ltd, Harlow, United Kingdom) that was set to match the internal airflow resistance of a Diskus inhaler. The In-Check DIAL® device measures inspiratory flow from 15-120 L/min and the manufacturer specifications indicate the device is accurate to within 10% or 10 L/min. Patients were seated with a nose clip and instructed to exhale gently to functional residual capacity with a tight seal around the device followed by a rapid inhalation to maximal lung capacity. Patients were instructed to inhale as fast as possible and for as long as possible.2 The maneuver was performed 3 times and the greatest measurement recorded. The study was approved by the University of Texas Medical Branch Institutional Review Board (12-171) and written consent waived.
Statistical Analysis
All values represent mean ± standard deviation. Patients were classified according to PIFR measurements classed as optimal (> 60 L/min) or suboptimal (PIFR < 60 L/min). A comparison of patient characteristics with optimal and suboptimal PIFR was performed using an unpaired t-test. Chi square analysis was performed to compare categorical variables between the PIFR groups. Pearson correlation was performed to assess the relationship of PIFR with FEV1, FVC, inspiratory capacity and residual volume (RV) to total lung capacity (TLC) ratio. In addition, a receiver operating curve (ROC) analysis was performed to determine the best FIF max value in identifying optimal PIFR for the entire cohort and for subgroups based on gender and height using Youden’s Index. Logistic regression analysis was performed regarding the interaction of gender and height on PIFR. A p-value of ≤ 0.05 was considered statistically significant. The analysis was performed by using SAS version 12 (SAS Institute, Inc., Cary, North Carolina).
Results
Cohort Characteristics
Over a 19-month period, 303 unique patients with COPD underwent spirometry and PIFR measurements with an In-Check DIAL®. The group mean age was 65.5 ± 11.3 years with an even gender distribution (Table 1). Spirometry measurements for the entire group showed the presence of moderate airflow obstruction as determined by 2007 GOLD guidelines.12 Measurement of PIFR with In-Check DIAL® allowed separation of patients according to optimal (> 60 L/min) and suboptimal PIFR (≤ 60 L/min) (Table 1). Sixty-one patients (20.1%) had a suboptimal PIFR value.
Relationship Between PIFR and Pulmonary Function Tests
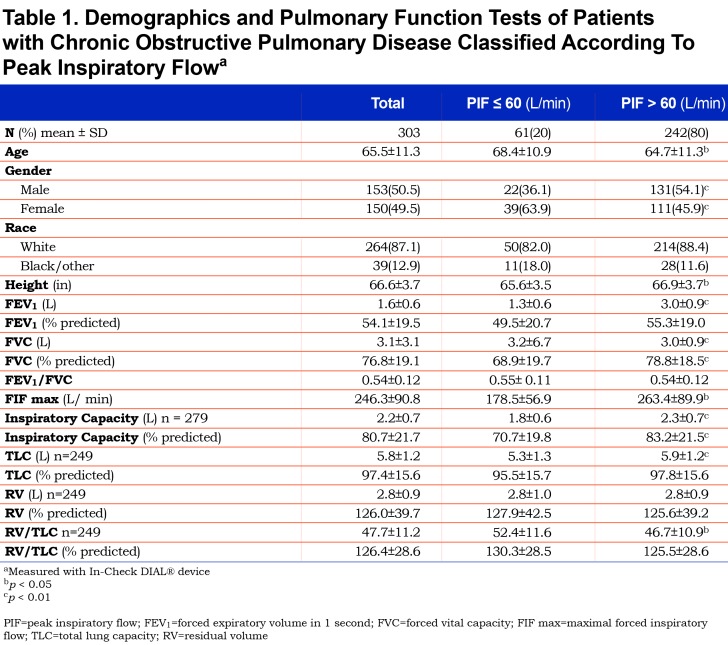
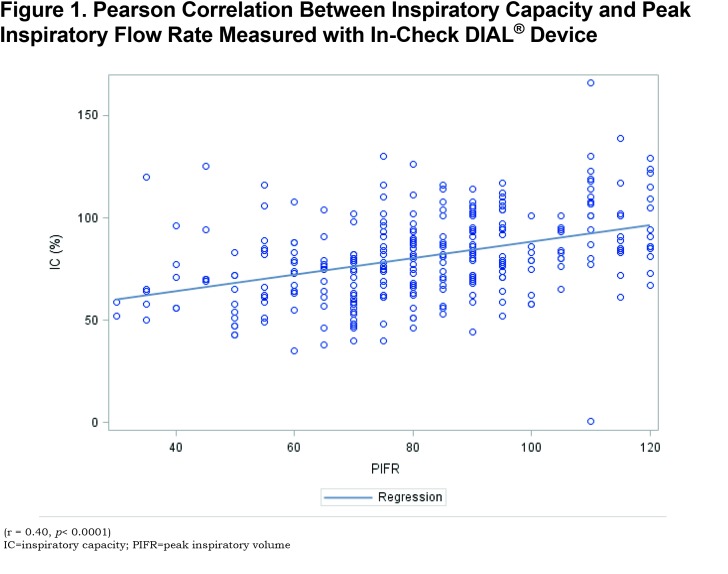
In the suboptimal PIFR group, lung function measurements demonstrated a significantly lower FEV1, total lung capacity (TLC) and inspiratory capacity compared to the optimal PIFR group. In addition, the RV/TLC was significantly greater in the suboptimal PIFR group. A significant correlation was demonstrated between PIFR and inspiratory capacity as well as residual volume (RV)/TLC (Figures 1 and 2). The correlation between PIFR and inspiratory capacity (r = 0.40, p< 0.0001) was stronger than the relationship between RV/TLC and PIFR (r = - 0.19, p = 0.002). Similarly, a strong correlation was observed between PIFR and FIF max (r = 0.65, p< 0.0001).
Receiver Operator Curve Analysis
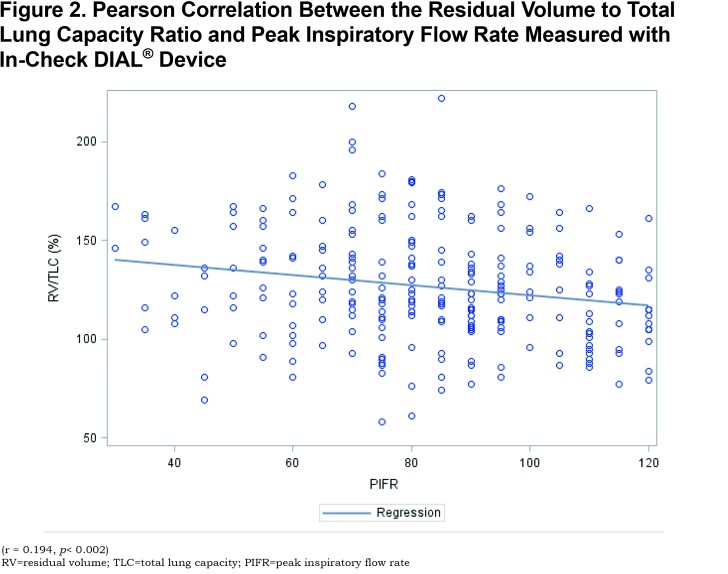
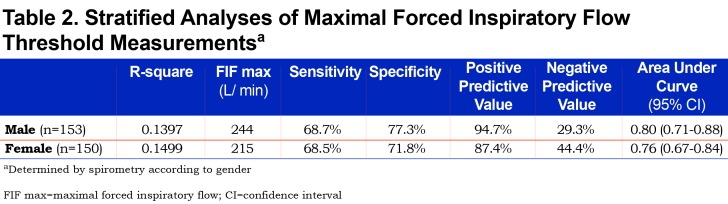
In the suboptimal PIFR group, the mean FIF max value obtained by spirometry was 178.5 ± 56.9 L/min, significantly lower than the FIF max value in the optimal PIFR group of 263.4 ± 89.9 L/min (p< 0.0001) (Table 1). ROC analysis allowed identification of an FIF max threshold value of 215 L/min with a positive predictive value of 92.2% to correctly identify patients with a PIFR > 60 L/min as assessed with In-Check DIAL®. (Sensitivity = 68.2%, Specificity = 77.1%, R-square = 0.1625). A ROC analysis of FIF max to identify an optimal PIFR provided an area under the curve (AUC) of 0.79 (Figure 3).
Influence of Gender and Height
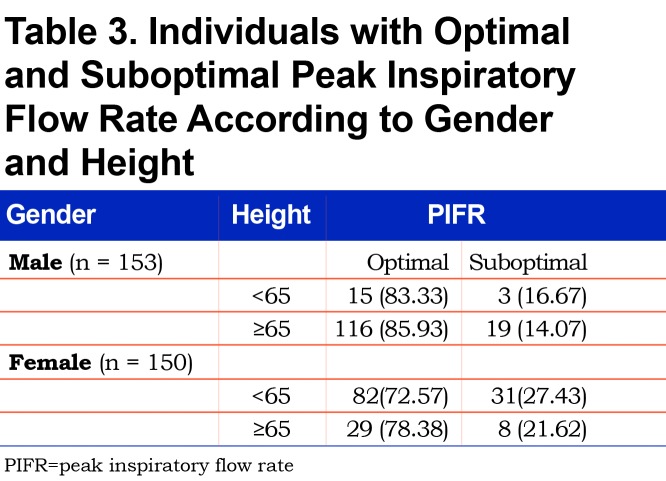
A ROC analysis by gender found that, in males, a FIF max value of 244 L/min provided a positive predictive value of 94.7% (Sensitivity = 68.7%, Specificity = 77.3%, R-square = 0.1397) (Table 2). In females, a FIF max value of 215 L/min yielded a positive predictive value of 87.4% (Sensitivity = 68.5%, Specificity = 71.8%, R-square = 0.1499) (Table 2). Further analysis of the cohorts by gender and height according to PIFR performance revealed that in males shorter than 65 in., a suboptimal PIFR measurement was observed in 16.7% of males. In females shorter than 65 in., a suboptimal PIFR was observed in 27.4% of women (Table 3). Both gender and height were independent predictors of suboptimal PIFR. However, when adding both to the same model, the effect was no longer significant suggesting the impact of gender was mediated through short stature.
Discussion
The findings of this study indicate that 1 in 5 ambulatory patients with COPD had suboptimal PIFR, as measured with an In-Check DIAL®. A greater proportion of individuals with suboptimal PIFR were females; shorter height; and had a higher RV/TLC ratio compared to the optimal PIFR group. Moreover, a significant linear correlation was demonstrated between PIFR measurements FIF max, inspiratory capacity and RV/TLC measurements. Measurements of FIF max with spirometry distinguished patients with optimal and suboptimal PIFR according to gender and height and the gender effect was mediated by height.
The inspiratory flow rate required by a patient to overcome the internal resistance varies according to the type of DPI used. Use of a Diskus inhaler requires a minimal inspiratory flow rate of 30 L/min and optimal flow rate greater than 60 L/min.8,13,14 Notably, reductions in PIFR due to patient error, supine position, air trapping and muscle weakness can result in suboptimal lower airway drug deposition in patients with COPD.15 In addition, older age,16-18 female gender19,20 and short stature20 have been associated with reductions in PIFR. One group examined inspiratory flow rates in 40 elderly patients with (n=26) and without (n=14) airflow obstruction and reported reductions in peak inspiratory flow in both groups irrespective of the presence of airflow obstruction.21 The authors reported a PIFR of less than 45 L/min in 20% of patients assessed using an In-Check DIAL® device with a resistance that simulated a Diskus inhaler. Similarly, Mahler et al reported a PIFR of less than 60 L/min in 19% of stable patients with COPD and that corresponded with reductions in FVC and inspiratory capacity.20 Furthermore, these investigators also reported that short stature and gender were independent predictors of a suboptimal PIFR. Interestingly, several reports have found little to no relationship with FEV1 and PIFR.5,17,18,20,21,22 In the current report, we observed a suboptimal PIFR in 20% of patients with COPD and identified women, shorter stature and air trapping to be factors associated with a suboptimal use of a DPI. Mechanisms to explain these observations invoke weak respiratory muscles and/or the presence of intrinsic positive end expiratory pressure that do not allow patients to achieve an adequate inspiratory flow.
Current guidelines offer no recommendations regarding use of delivery devices in specific patient populations to improve patient outcomes.6 Measurement of PIFR and identification of a suboptimal PIFR in each patient may allow transition from a DPI to a less flow-dependent delivery device, such as a nebulizer or a soft mist inhaler. Mahler et al examined the acute effects on lung function in a group of ambulatory patients with a mean PIFR of 53 ± 5 L/min that employed a randomized, open-label crossover design.23 Compared to baseline, nebulizer administration of a long acting beta2-agonist resulted in greater improvements in FVC and inspiratory capacity than DPI drug delivery. Another group retrospectively identified a suboptimal PIFR, defined as ≤ 60 L/min against no resistance, in 52% of patients hospitalized with an acute exacerbation of COPD.5 Patients with a suboptimal PIFR had a higher 30-day and 90-day readmission rate for COPD compared to the group with a PIFR > 60 L/min. Moreover, a multivariable analysis identified PIFR as the only significant variable associated with COPD readmissions. Furthermore, a cohort of 10 patients with a suboptimal PIFR prescribed nebulized bronchodilators upon discharge demonstrated reduced COPD-related 30-day and 90-day readmission rates compared with a similar group prescribed DPI delivered bronchodilators. However, to address the relationship between a suboptimal PIFR and clinical outcomes will require a prospective clinical trial comparing DPI and other drug delivery devices.
Investigators have attempted to correlate inspiratory flow measurements obtained with spirometry and other measuring devices. Earlier studies found positive correlations between PIFR assessments in smaller sample populations.17,18,22,24 In healthy individuals with asthma, COPD, neuromuscular disease, and non-respiratory disorders, Sehuelt et al reported that spirometry measurements of FIF max measured without resistance were moderately correlated with PIFR obtained with a device that simulated the internal resistance of the Diskus (R2 = 0.58).18 In patients with a PIFR > 60 L/min, 84% were correctly classified using spirometry measurement of FIF max with a cutoff value of 196 L/min.18 Our study also identified a sound relationship between spirometry and In-Check DIAL® device measurements of PIFR in ambulatory patients with COPD. Additionally, we were able to identify distinct threshold values, according to gender and height, which correspond with suboptimal PIFR values. Clinicians lacking easy access to an In-Check DIAL® device may use FIF max measurements obtained from spirometry to determine a given patient’s ability to adequately use a DPI and consider prescribing a flow independent delivery device.
Our study has several limitations with one being that this was a single center, cross-sectional, exploratory study of ambulatory patients with COPD with an observed 20% prevalence of suboptimal PIFR measurements. However, other investigators have reported similar prevalence.5,17,18,20,21 Another limitation involved assessment of PIFR performance solely against a simulated internal resistance of the Diskus and did not assess PIFR performance of other DPI devices. Reports reveal that other DPI devices, such as Turbuhaler and Handihaler, have a higher internal resistance than the Diskus, thus the prevalence of a suboptimal PIFR, defined as < 60 L/min, may be greater with other DPI devices. However, the Diskus is a frequently used delivery device and the results of this study are applicable to a wide number of patients prescribed this device. Another study limitation involves our selection of a suboptimal PIFR measurement of less than 60 L/min. While the respirable fraction of an inhaled drug is greater with higher inspiratory flow rates, a PIFR > 30 L/min has been reported as the minimal flow rate to provide a clinical effect14,25 and the effect of a PIFR between 30 and 60 L/min is not clear. Our use of an optimal PIFR of > 60 L/min was based on previous clinical reports.5,7,8,15,19,20
In summary, 1 in 5 stable, ambulatory patients with COPD had suboptimal PIFR. Suboptimal PIFR measurements were identified more frequently in females, short stature individuals and those with air trapping. Spirometry determined values of FIF max corresponded with PIFR measurements according to gender and height. Spirometry allows identification of patients with a decreased FIF max based on gender and height that can be used as a physiologic threshold value for future interventional studies.
Abbreviations
peak inspiratory flow rate, PIFR; maximal forced inspiratory flow, FIF max; chronic obstructive pulmonary disease, COPD; residual volume, RV; total lung capacity, TLC; dry powder inhaler, DPI; Global initiative for chronic Obstructive Lung Disease, GOLD; American Thoracic Society, ATS; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; receiver operating curve, ROC; total lung capacity, TLC; area under the curve, AUC
Funding Statement
This work was supported by the Clinical and Translational Science Award (UL1TR001439) from the National Center for Advancing Translational Sciences, the Claude D. Pepper Older Americans Independence Center (5P30-AG024832), and grants from the Agency for Healthcare Research and Quality (R01-HS020642 and R24-HS022134). The University of Texas Medical Branch (UTMB) institutional review board (IRB) project approval number is 12-171.
References
- 1. Dolovich MB,Ahrens RC,Hess DR,et al. Device selection and outcomes of aerosol therapy: evidence based guidelines. Chest. 2005;127(1):335-371. doi: https://doi.org/10.1378/chest.127.1.335 [DOI] [PubMed] [Google Scholar]
- 2. Laube BL,Janssens HM,de Jongh FHC,et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Resp J. 2011;37(6):1308-1331. doi: https://doi.org/10.1183/09031936.00166410 [DOI] [PubMed] [Google Scholar]
- 3. Bourbeau J,Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831-838. doi: https://doi.org/10.1136/thx.2007.086041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dhand R,Mahler DA,Carlin Bw,et al. Results of a patient survey regarding COPD knowledge, treatment experiences and practices with inhalation devices. Resp Care. 2018;63(7):833-839. doi: https://doi.org/10.4187/respcare.05715 [DOI] [PubMed] [Google Scholar]
- 5. Loh CH,Peters SP,Lovings TM,Ohar JA. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all-cause readmissions. Ann Am Thorac Soc. 2017;14(8):1305-1311. doi: https://doi.org/10.1513/AnnalsATS.201611-903OC [DOI] [PubMed] [Google Scholar]
- 6. Vogelmeier CF,Criner GJ,Martinez FJ,et al. Global strategy for the diagnosis,management, and prevention of chronic obstructive lung disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557-582. doi: https://doi.org/10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 7. Ghosh S,Ohar J,Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease; implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30(6):381-287. doi: https://doi.org/10.1089/jamp.2017.1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mahler DA. Peak Inspiratory Flow Rate as a criterion for dry powder inhaler use in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14(7):1103-1107. doi: https://doi.org/10.1513/AnnalsATS.201702-156PS [DOI] [PubMed] [Google Scholar]
- 9. Capstick TGD,Clifton IJ. Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma. Expert Rev Respir Med. 2012;6(1):91-101. doi: https://doi.org/10.1586/ers.11.89 [DOI] [PubMed] [Google Scholar]
- 10. Brusasco V,Crapo R,Viegi G,et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511-522. doi: https://doi.org/10.1183/09031936.05.00035005 [DOI] [PubMed] [Google Scholar]
- 11. Miller MR,Hankinson J,Brusasco V,et al. Standardisation of spirometry. Eur Resp J. 2005;26(2):319-338. doi: https://doi.org/10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 12. Rabe KF,Hurd S,Anzueto A,et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease executive summary. Am J Respir Crit Care Med. 2007;176(6):532-555. doi: https://doi.org/10.1164/rccm.200703-456SO [DOI] [PubMed] [Google Scholar]
- 13. Clark AR,Hollingworth AM. The relationship between powder inhaler resistance and peak inspiratory conditions in healthy volunteers--implications for in vitro testing. J Aerosol Med. 1993;6(2):99-110. doi: https://doi.org/10.1089/jam.1993.6.99 [DOI] [PubMed] [Google Scholar]
- 14. Haidl P,Heindl S,Siemon K,Bernacka M,Cloes RM. Inhalation device requirements for patients' inhalation maneuvers. Respir Med. 2016;118:65-75. doi: https://doi.org/10.1016/j.rmed.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 15. Pleasants RA,Hess DR. Aerosol delivery devicse for obstructive lung diseases. Resp Care. 2018;63(6):708-733. doi: https://doi.org/10.4187/respcare.06290 [DOI] [PubMed] [Google Scholar]
- 16. Jarvis S,Ind PW,Shiner RJ. Inhaled therapy in elderly COPD patients; time for re evaluation?. Age Ageing. 2007;36(2):213-218. doi: https://doi.org/10.1093/ageing/afl174 [DOI] [PubMed] [Google Scholar]
- 17. Malmberg LP,Rytilä P,Happonen P,Haahtela T. Inspiratory flows through dry powder inhaler in chronic obstructive pulmonary disease: age and gender rather than severity matters. Int J Chron Obstruct Pulmon Dis. 2010;5:257-262. doi: https://doi.org/10.2147/COPD.S11474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seheult JN,Costello S,Tee KC,et al. Investigating the relationship between peak inspiratory flow rate and volume of inhalation from a Diskus™ Inhaler and baseline spirometric parameters: a cross-sectional study. Springerplus. 2014;3:496. doi: https://doi.org/10.1186/2193-1801-3-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sharma G,Mahler DA,Mayorga VM,Deering KL,Harshaw O,Ganapathy V. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD Exacerbation. Chronic Obstr Pulm Dis. 2017;4(3):217-224. doi: https://doi.org/10.15326/jcopdf.4.3.2017.0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mahler DA,Waterman LA,Gifford AH. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus® dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2013;26(3):174-179. doi: https://doi.org/10.1089/jamp.2012.0987 [DOI] [PubMed] [Google Scholar]
- 21. Janssens W,VandenBrande P,Hardeman E,et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Respir J. 2008;31(1):78-83. doi: https://doi.org/10.1183/09031936.00024807 [DOI] [PubMed] [Google Scholar]
- 22. Derom E,Strandgården K,Schelfhout V, Borgström L,Pauwels R. Lung deposition and efficacy of inhaled formoterol in patients with moderate to severe COPD. Respir Med. 2007;101(9):1931-1941. doi: https://doi.org/10.1016/j.rmed.2007.04.013 [DOI] [PubMed] [Google Scholar]
- 23. Mahler DA,Waterman LA,Ward J,Gifford AH. Comparison of dry powder versus nebulized beta-agonist in patients with COPD who have suboptimal peak inspiratory flow rate. J Aerosol Med Pulm Drug Deliv. 2014;27(2):103-109. doi: https://doi.org/10.1089/jamp.2013.1038 [DOI] [PubMed] [Google Scholar]
- 24. Broeders ME,Molema J,Hop WC,Folgering HT. Inhalation profiles in asthmatics and COPD patients: reproducibility and effect of instruction. J Aerosol Med. 2003;16(2):131-141. doi: https://doi.org/10.1089/089426803321919898 [DOI] [PubMed] [Google Scholar]
- 25. Chrystyn H. Is inhalation rate important for a dry powder inhaler? Using the In-Check Dial to identify these rates. Respir Med. 2003;97(2):181-187. doi: https://doi.org/10.1053/rmed.2003.1351 [DOI] [PubMed] [Google Scholar]