Abstract
Background
Behçet's disease is a chronic inflammatory vasculitis that can affect multiple systems. Mucocutaneous involvement is common, as is the involvement of many other systems such as the central nervous system and skin. Behç̧et's disease can cause significant morbidity, such as loss of sight, and can be life threatening. The frequency of oral ulceration in Behçet's disease is thought to be 97% to 100%. The presence of mouth ulcers can cause difficulties in eating, drinking, and speaking leading to a reduction in quality of life. There is no cure for Behçet's disease and therefore treatment of the oral ulcers that are associated with Behçet's disease is palliative.
Objectives
To determine the clinical effectiveness and safety of interventions on the pain, episode duration, and episode frequency of oral ulcers and on quality of life for patients with recurrent aphthous stomatitis (RAS)‐type ulceration associated with Behçet's disease.
Search methods
We undertook electronic searches of the Cochrane Oral Health Group Trials Register (to 4 October 2013); the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 9); MEDLINE via Ovid (1946 to 4 October 2013); EMBASE via Ovid (1980 to 4 October 2013); CINAHL via EBSCO (1980 to 4 October 2013); and AMED via Ovid (1985 to 4 October 2013). We searched the US National Institutes of Health trials register (http://clinicaltrials.gov) and the World Health Organization (WHO) Clinical Trials Registry Platform for ongoing trials. There were no restrictions on language or date of publication in the searches of the electronic databases. We contacted authors when necessary to obtain additional information.
Selection criteria
We included randomised controlled trials (RCTs) that looked at pre‐specified oral outcome measures to assess the efficacy of interventions for mouth ulcers in Behçet's disease. The oral outcome measures included pain, episode duration, episode frequency, safety, and quality of life. Trials were not restricted by outcomes alone.
Data collection and analysis
All studies meeting the inclusion criteria underwent data extraction and an assessment of risk of bias, independently by two review authors and using a pre‐standardised data extraction form. We used standard methodological procedures expected by Cochrane.
Main results
A total of 15 trials (n = 888 randomised participants) were included, 13 were placebo controlled and three were head to head (two trials had more than two treatment arms). Eleven of the trials were conducted in Turkey, two in Japan, one in Iran and one in the UK. Most trials used the International Study Group criteria for Behçet's disease. Eleven different interventions were assessed. The interventions were grouped into two categories, topical and systemic. Only one study was assessed as being at low risk of bias. It was not possible to carry out a meta‐analysis. The quality of the evidence ranged from moderate to very low and there was insufficient evidence to support or refute the use of any included intervention with regard to pain, episode duration, or episode frequency associated with oral ulcers, or safety of the interventions.
Authors' conclusions
Due to the heterogeneity of trials including trial design, choice of intervention, choice and timing of outcome measures, it was not possible to carry out a meta‐analysis. Several interventions show promise and future trials should be planned and reported according to the CONSORT guidelines. Whilst the primary aim of many trials for Behç̧et's disease is not necessarily reduction of oral ulceration, reporting of oral ulcers in these studies should be standardised and pre‐specified in the methodology. The use of a core outcome set for oral ulcer trials would be beneficial.
Plain language summary
Interventions for managing oral ulcers in Behçet's disease
Review question
This review has been conducted to assess the effects of different interventions, administered systemically or topically, for the prevention or treatment of oral ulcers in people with Behçet's disease. The interventions could be compared with an alternative intervention, no intervention or the administration of a placebo.
Background
Behçet's disease is a chronic disease characterised by a multitude of signs and symptoms including oral and genital ulcerations, skin lesions and inflammatory vascular involvement of the central nervous system and gastrointestinal tract. Although the underlying cause of Behçet’s disease is unknown it is thought to involve a genetic predisposition combined with environmental factors.
Behçet's disease most commonly presents in the third decade. The disease is rare in individuals older than age 50 years and during childhood. Although both sexes are equally affected, it is thought that the disease has a more severe course amongst men.
The oral ulceration that occurs in Behçet's disease can be painful and slow to heal. At its worst, this can cause significant difficulties in eating and drinking.
Study characteristics
Authors from Cochrane Oral Health carried out this review of existing studies and the evidence is current up to 4 October 2013. The review includes 15 studies published from 1980 to 2012 in which 888 participants were randomised. Eleven of the trials were conducted in Turkey, two in Japan, one in Iran, and one in the UK. Thirteen different interventions were assessed, administered either topically or systemically.
Topical interventions: sucralfate, interferon–alpha (different doses), cyclosporin A, triamcinolone acetonide ointment, phenytoin syrup mouthwash.
Systemic interventions: aciclovir, thalidomide (different doses), corticosteroids, rebamipide, etanercept, colchicine, interferon–alpha, cyclosporin.
Key results
There was insufficient evidence to support or refute the use of any included intervention with regard to pain, episode duration or episode frequency associated with oral ulcers, or the safety of the interventions.
Quality of the evidence
The quality of the evidence ranged from moderate to very low.
Summary of findings
Background
Description of the condition
Behçet's disease is a chronic, relapsing, multisystem inflammatory vasculitis (Chams‐Davatchi 2010). It affects both the large and small blood vessels (including veins and arteries) (Mat 2013). It is characterised by a multitude of systemic signs and symptoms. Oral and genital ulcerations, skin lesions, uveitis, and inflammatory vascular involvement of the central nervous system and gastrointestinal tract are common (Dalvi 2012). Although the aetiology of Behçet’s disease is unknown it is thought to involve a genetic predisposition combined with environmental factors (Yazici 2012).
The genetic risk factor most strongly associated with Behçet's disease is the human leukocyte antigen (HLA)‐B51 allele. HLA‐B51 occurs in around 60% of Behçet's disease patients (Gul 2007; Kose 2012; Yazici 1980).
Behçet's disease is more frequent in the countries along the 'Silk Road', an ancient trading route, where the prevalence of HLA‐B51 is relatively high compared with the other parts of the globe (Yurdakul 2010).
Behçet's disease most commonly presents in the third decade. The disease is rare in individuals older than age 50 years and during childhood. Although both sexes are equally affected, it is thought that the disease has a more severe course amongst men (Yazici 1984).
Diagnosis
Previously, the International Study Group (ISG) Guidelines for the Classification of Behçet's disease were generally accepted as a diagnostic tool (ISG 1990).
The criteria included recurrent oral 'aphthae' (at least three episodes within 12 consecutive months) plus two of the following: recurrent genital ulcers; uveitis or retinal vasculitis; skin lesions that are classified as erythema nodosum (EN)‐like lesions, acneiform lesions, pustulosis, or pseudofolliculitis; and a positive pathergy test.
More recently a large group involving people from 32 countries attempted to establish new international guidelines (Davatchi 2012). Following a prospective, international, multicentre, diagnostic accuracy study, data from over 2556 Behçet's patients from 27 different countries were reviewed. A new diagnostic scoring system was developed. As with the previous diagnostic criteria, oral lesions scored highly along with ocular and genital lesions. In fact 98% of Behçet's patients had oral aphthous ulceration as a feature (Davatchi 2004).
Oral ulceration in Behçet's disease
The oral ulceration that occurs in Behçet's disease resembles recurrent aphthous stomatitis (RAS). In the oral medicine and dental literature RAS is now commonly used as a term to indicate a primary condition where ulceration is not in association with a systemic disease such as Behçet's. Where a relevant systemic disease is present, various terms including RAS‐type ulceration would be used instead. In the general medical literature however, this division of nomenclature is not widely used and the oral ulceration in Behçet's is indistinguishable in appearance and natural history from RAS. It remains unclear whether the ulceration in RAS and RAS‐type ulceration shares a common pathogenesis. The term for oral ulceration in association with Behcet's disease in the international guidelines criteria includes oral aphthosis (ISG 1990) and oral aphthous lesions (Davatchi 2012). For the purposes of this review we have reviewed studies where aphthous ulceration is clearly the type of mouth ulceration being investigated, regardless of which precise terminology is used.
RAS is the most common form of oral ulceration with prevalence in the different populations ranging between 5% and 60% (Jurge 2006).
RAS‐type ulceration in association with a systemic disease is common. Systemic diseases featuring RAS‐type ulceration can include, but are not limited to, coeliac disease, Crohn's disease, vitamin B12 deficiency, iron deficiency anaemia, human immunodeficiency virus infection/acquired immunodeficiency syndrome (HIV/AIDS), cyclic neutropenia, systemic lupus erythematosus (SLE), and Behçet's disease (Baccaglini 2011).
The frequency of RAS‐type ulceration in Behçet's disease is 97% to 100% (Yurdakul 2008). There are a variety of Beḩcet's diagnostic criteria used over many years. Since 1990, the ISG have been most commonly but not exclusively used. Whichever criteria are used, RAS‐type ulceration is a major feature with high prevalence.
According to Bagan 1991, there are three recognised forms of RAS (and hence also RAS‐type ulceration).
Minor aphthae, typically round and less than 10 mm in diameter. These are generally pale in colour with an erythematous border and commonly affect non‐keratinised mucosa including the labial and buccal mucosa, the borders of the tongue, and the floor of the mouth. Minor aphthae can occur in isolation but multiple presentations are also common. Healing is spontaneous and usually takes 7 to 10 days. Episodes of ulceration are usually followed by an ulcer‐free period lasting a few days to several weeks before the next episode occurs (Thornhill 2007).
Major aphthae are similar to minor aphthae but are larger, usually exceeding 10 mm in diameter, and deeper. Consequently healing can take longer (20 to 30 days) and may result in scarring (Bagan 1991).
Herpetiform ulcers are less than 1 mm in diameter and often occur in multiples, from 1 to 100. There is a tendency for adjacent ulcers to merge.
In Behçet's disease, minor aphthae‐type lesions are the most commonly seen type, whereas major and herpetiform types are rare (Hamuryudan 1998; Melikoglu 2005; Yurdakul 2001). This mirrors the frequency of the different forms of aphthae in RAS patients.
Description of the intervention
The cause of RAS is not known; therefore the aims of treatment are primarily pain relief and the reduction of inflammation (Scully 2006). The cause of RAS‐type oral ulceration in Behçet's disease is also poorly understood and, therefore, treatment aimed primarily at the oral ulceration in Behç̧et's disease is also targeted at pain relief and the promotion of healing to reduce the duration of the disease or reduce the rate of recurrence. A variety of topical and systemic therapies have been utilised (Porter 1998) but few studies have demonstrated efficacy. Empirically, effective treatments include the use of corticosteroids, immunosuppressants, and topical barriers (Eisen 2001). Topical interventions can include mouthrinses, pastes, gels, sprays, injections, laser, and locally dissolving tablets. Many of the topical treatments are available without prescription. Systemic immunomodulators such as mycophenolate mofetil, pentoxyphylline, colchicine, dapsone, and thalidomide have also been used but with some caution due to their potential for adverse effects.
How the intervention might work
As the aetiopathogenesis of RAS‐type ulceration in Behçet's disease is not fully understood, the precise mechanisms of how the various topical and systemic interventions influence the disease process are unclear.
Topical interventions for oral ulceration range from inert barriers to active treatments. Providing a barrier for the ulcer (for example with a mucoadhesive paste) should allow the breach in the mucosa to temporarily be protected and therefore noxious stimulants are less likely to sensitise nerve endings. This in theory should provide pain relief. The addition of active compounds to the barrier can potentially give an immunomodulatory effect. Due to the nature of the mucosal layer, there is great variability in the penetration of active compounds through the mucosal barrier, and as such there is great variability in the efficacy of the topical treatments.
Systemic interventions include immunomodulators (colchicine, azathioprine, cyclosporin, thalidomide), corticosteroids, biological agents (interferon; anti‐TNF agents such as infliximab, etanercept, adalimumab), and other drugs such as dapsone and daclizumab. As previously stated, the precise mode of action of these interventions is often unclear.
Many of the systemic treatments used in Behçet's disease are given to control the underlying disease process and not primarily for the oral ulceration. Nevertheless, these systemic treatments may also improve the severity and frequency of episodes of RAS‐type ulceration in these patients. Where systemic treatments are being used primarily for control of RAS‐type ulceration, the risk‐benefit ratios are important and may be different to those when trying to control widespread or critical disease. In practice, a combination of systemic therapy for the underlying disease and topical therapy for symptomatic treatment of acute attacks of oral ulceration are frequently used.
Why it is important to do this review
All three clinical types of RAS and RAS‐type ulceration are associated with varying degrees of morbidity, including pain and difficulties in function. RAS (and RAS‐type) ulceration is a chronic episodic oral mucosal condition which can impact upon the experiences of daily life, such as physical health and functioning (Riordain 2011).
A recent Cochrane review (Brocklehurst 2012) evaluated the evidence for systemic interventions for RAS and an ongoing review by the same author group is evaluating topical interventions for RAS (Taylor 2013). Both of these reviews have specifically excluded trials of interventions for oral ulcers in patients with systemic disease. This therefore excludes trials involving Behçet's patients.
There is, therefore, a population of patients in whom oral ulcers are the most common presenting feature and for whom we have no formal evaluation of the evidence base on which to guide our clinical treatments for them. An evaluation of the evidence for interventions for oral ulcers in this group of patients is therefore essential.
Objectives
The objectives of this review are to determine the clinical effectiveness and safety of interventions on the pain, episode duration, and episode frequency of oral ulcers and on quality of life for patients with recurrent aphthous stomatitis (RAS)‐type ulceration associated with Behçet's disease.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) investigating the effects of interventions for the management of recurrent aphthous stomatitis (RAS)‐type ulceration in Behçet's disease were included. We also included RCTs of a cross‐over design provided that the trial included a suitable washout period and no carry‐over effects were evident. Split‐mouth studies were also to be included if it was apparent that there was no risk of contamination of the intervention from one part of the mouth to another (this would be more likely for any topical interventions that was physically applied and retained in one part of the mouth rather than a mouthwash for example).
Studies looking at interventions for the management of any systemic manifestations of Behçet's disease and which also reported on oral ulcers as an outcome measure were included. The oral outcome measures should have been pre‐specified in the methodology.
Types of participants
Participants with Behçet's disease with a history of recurrent oral aphthous‐type ulcers that were diagnosed clinically were included. Where additional systemic diseases were reported in studies, these were noted.
Types of interventions
Active treatment included any preventive, palliative, or curative interventions administered systemically or topically. Comparators were either standard care, no active treatment, or the administration of a placebo; head to head trials of different interventions were also included.
Types of outcome measures
Primary outcomes
Pain associated with oral ulcers.
Episode duration associated with oral ulcers.
Episode frequency associated with oral ulcers.
Safety of the intervention including adverse effects.
Secondary outcomes
Any patient‐reported outcomes that measured a change in the patients' quality of life or in morbidity (e.g. function), or both.
Search methods for identification of studies
For the identification of studies included or considered for this review, we developed detailed search strategies for each database searched. These were based on the search strategy developed for MEDLINE (Ovid) but revised appropriately for each database (Appendix 1). We combined this search strategy with the Cochrane highly sensitive search strategy (CHSSS) for identifying RCTs in MEDLINE: sensitivity maximising version, as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We linked the searches of EMBASE and CINAHL to the Cochrane Oral Health Group filters for identifying RCTs.
Electronic searches
The following electronic databases were searched:
Cochrane Oral Health Group Trials Register (to 4 October 2013) (see Appendix 2);
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 9) (see Appendix 3);
MEDLINE via Ovid (1946 to 4 October 2013) (see Appendix 1);
EMBASE via Ovid (1980 to 4 October 2013) (see Appendix 4);
CINAHL via EBSCO (1980 to 4 October 2013) (see Appendix 5);
AMED via Ovid (1985 to 4 October 2013) (see Appendix 6).
There were no restrictions on language or date of publication in the searches of the electronic databases.
Searching other resources
Unpublished trials
We screened the bibliographies of included papers and relevant review articles were checked for studies not identified by the search strategies above.
We searched the following databases for ongoing trials (see Appendix 7):
US National Institutes of Health trials register (clinicaltrials.gov);
World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch/).
Data collection and analysis
Selection of studies
Two review authors (Jennifer Taylor (JT) and Anne‐Marie Glenny (AMG)) independently screened the titles and abstracts obtained from the initial electronic searches. Reports from the studies that fulfilled the inclusion criteria were obtained. When there were insufficient data in the study title to determine whether a study fulfilled the inclusion criteria, the full report was obtained and assessed independently by the same review authors. Disagreements were resolved by discussion.
Data extraction and management
At least two review authors (JT, AMG, Tanya Walsh (TW), Paul Brocklehurst (PB), and Philip Riley (PR)) independently extracted data from each included study using a tool developed for the review. All studies meeting the inclusion criteria underwent data extraction and an assessment of risk of bias using a pre‐standardised data extraction form. Studies rejected at this and subsequent stages were recorded in the table 'Characteristics of excluded studies'. Differences were resolved by discussion. If a single publication reported two or more separate studies, then the data from each study were extracted separately. If the findings of a single study were spread across two or more publications, then the publications were extracted as one. For each study with more than one control or comparison group for the intervention, the results were extracted for each intervention arm.
For each trial the following data were recorded.
Year of publication, country of origin, and source of study funding.
Details of the participants including demographic characteristics and criteria for inclusion.
Details on the type of intervention and comparisons.
Details on the study design.
Details on the outcomes reported, including methods and timings of assessments, and adverse outcomes.
Assessment of risk of bias in included studies
All review authors assessed the risk of bias in the included studies using the Cochrane's tool for assessing risk of bias. The domains that were assessed for each included study were:
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
completeness of outcome data (attrition bias);
selective outcome reporting (reporting bias);
risk of other potential sources of bias (other bias).
We tabulated a description of the above domains for each included trial, along with a judgement of the risk of bias (low, high, or unclear), based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
An example of a risk of bias judgement, based on allocation concealment only, is shown below:
low risk of bias (adequate concealment of the allocation (e.g. sequentially numbered, sealed, opaque envelopes or centralised or pharmacy‐controlled randomisation));
unclear risk of bias (unclear about whether the allocation was adequately concealed (e.g. where the method of concealment was not described or not described in sufficient detail to allow a definite judgement));
high risk of bias (inadequate allocation concealment (e.g. open random number lists or quasi‐randomisation such as alternate days, date of birth, or case record number)).
We provided a summary assessment of the risk of bias for the primary outcomes across the studies (Higgins 2011). For each study, we assessed the overall risk of bias according to the following rationales:
low risk, when there is a low risk of bias across all seven key domains;
unclear risk of bias, when there is an unclear risk of bias in one or more of the seven key domains;
high risk of bias, when there is a high risk of bias in one or more of the seven key domains.
If high risk of bias is present in one of the seven domains then it took precedence.
Measures of treatment effect
For dichotomous outcomes (for example presence or absence of pain), we expressed the estimate of effect of an intervention as risk ratio (RR) together with 95% confidence interval (CI). For continuous outcomes (for example pain on a visual analogue scale), we used mean differences (MDs) and 95% CIs to summarise the data; in the event that different studies measured outcomes using different scales, we would have expressed the estimate of effect of an intervention as standardised mean difference (SMD) and 95% CI.
Unit of analysis issues
If cluster randomised trials had been included, we would have undertaken data analysis, whenever feasible, at the same level as the randomisation, or at the individual level accounting for the clustering.
Analysis of cross‐over studies should take into account the two‐period nature of the data using, for example, a paired t‐test (Elbourne 2002). We would have entered log RRs or MDs (or SMDs) and standard errors into Review Manager (RevMan) software (Review Manager 2012) using the generic inverse variance method (Higgins 2011).
Dealing with missing data
We contacted trial authors for missing data if the report was published from the year 2000 or onwards. We considered it unfeasible to obtain data for trials published prior to this cut‐off date. We used methods as outlined in the Cochrane Handbook for Systematic Reviews of Interventions to estimate missing standard deviations (Higgins 2011).
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity. We further assessed the significance of any discrepancies in the estimates of the treatment effects from the different trials by means of Cochran's Chi2 test for heterogeneity; heterogeneity would have been considered significant if P < 0.1 (Higgins 2011). We also used the I2 statistic, which describes the percentage total variation across studies that is due to heterogeneity rather than chance, to quantify heterogeneity; with I2 over 50% being considered substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
If there had been a sufficient number of trials (more than 10) included in any meta‐analysis, we would have assessed publication bias according to the recommendations on testing for funnel plot asymmetry as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
If data had allowed, we would have performed meta‐analysis of studies assessing the same comparisons and outcomes. We would combine RRs for dichotomous outcomes, and MDs (or SMDs if different scales were used) for continuous outcomes, using a random‐effects model where there were four or more studies, or a fixed‐effect model if there were less than four studies. We would have included data from split‐mouth or cross‐over studies in any meta‐analysis using the generic inverse variance method described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), combining them with parallel studies using the methods described in Elbourne 2002. As meta‐analysis was not possible, we presented data in an additional table.
Sensitivity analysis
If the number of studies had allowed, we would have undertaken a sensitivity analysis for each intervention and outcome by limiting the analysis to studies at overall low risk of bias.
Presentation of main results
We developed a 'Summary of findings' table for the main outcomes. We assessed the quality of the body of evidence with reference to the overall risk of bias of the included studies, the directness of the evidence, the inconsistency of the results, the precision of the estimates, the risk of publication bias, and the magnitude of the effect. We categorised the quality of the body of evidence of each of the main outcomes as high, moderate, low, or very low.
Results
Description of studies
A total of 486 articles were identified through our search strategy. Of these, 38 articles appeared to be potentially relevant and full copies were obtained. Following screening of full papers, 15 studies were identified for inclusion (Figure 1). One trial had been completed but had not been fully published as yet (NCT00866359), one trial is ongoing (NCT01441076), and one trial was duplicated and linked to a subsequent included study (Masuda 1989). Twenty studies were excluded (Characteristics of excluded studies).
1.
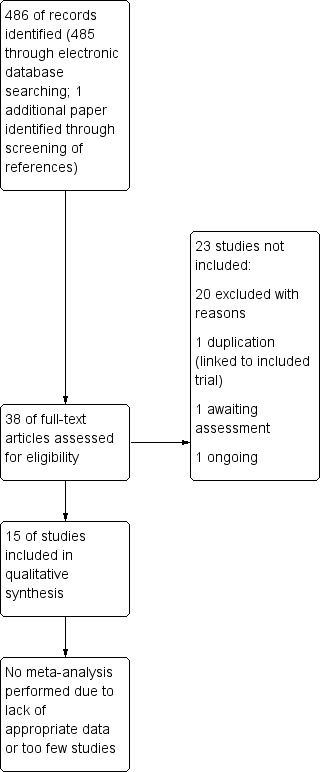
Study flow diagram.
Results of the search
A total of 15 trials were included in the review (n = 888 randomised participants; 780 evaluated) (Characteristics of included studies).
Included studies
The studies varied in sample size, ranging from 24 to 116. Eleven of the trials were conducted in Turkey, two in Japan, one in Iran, and one in the UK.
One trial was a cross‐over design with a washout period (Davies 1988) and the remaining 14 were designed as parallel studies.
The funding source was not stated in six of the trials. Six trials received pharmaceutical company funding and three further trials received funding from research facilities.
The diagnosis of Behçet's disease was not described clearly in all of the studies. Most trials used the International Study Group criteria for Behç̧et's disease (ISG 1990). The two studies from Japan used the Japanese diagnostic criteria, which were only described in detail in one of the trials, and one trial used the O'Duffy criteria (Aktulga 1980).
Six of the trials evaluated topical interventions applied directly to the ulcers and nine evaluated systemic interventions. The interventions used within the trials were diverse, and the mode of action with regard to the management of oral ulcers was often unclear.
Topical interventions
The six trials evaluating topical interventions assessed five main comparisons (five placebo controlled; two head to head).
Sucralfate versus placebo (Alpsoy 1999; Koc 1992).
Interferon–alpha (different doses) versus placebo (Hamuryudan 1991; Kilic 2009).
Cyclosporin A versus placebo (Ergun 1997).
Triamcinolone acetonide ointment versus phenytoin syrup mouthwash (Fani 2012).
Interferon–alpha 1000 IU versus interferon–alpha 2000 IU (Kilic 2009).
Systemic interventions
The nine trials evaluating systemic interventions assessed nine main comparisons (eight placebo controlled; one head to head).
Aciclovir versus placebo (Davies 1988).
Thalidomide (different doses) versus placebo (Hamuryudan 1998).
Corticosteroids versus placebo (Mat 2006).
Rebamipide versus placebo (Matsuda 2003).
Etanercept versus placebo (Melikoglu 2005).
Colchicine versus placebo (Aktulga 1980; Yurdakul 2001).
Interferon–alpha versus placebo (Alpsoy 2002).
Thalidomide 300 mg versus 100 mg versus placebo (Hamuryudan 1998).
Cyclosporin versus colchicine (Masuda 1989).
Six of the 15 studies were primarily looking at interventions for oral ulceration (Ergun 1997; Fani 2012; Hamuryudan 1991; Kilic 2009; Koc 1992; Matsuda 2003). Five studies had the management of Behç̧et's disease as the main aim (Aktulga 1980; Alpsoy 2002; Masuda 1989; Melikoglu 2005; Yurdakul 2001). The remaining studies looked at orogenital ulceration or genital ulceration (Alpsoy 1999; Davies 1988; Hamuryudan 1998; Mat 2006).
A wide range of outcomes were assessed, making comparison across trials difficult. For example, oral ulcers were measured using the following:
number of oral ulcers;
mean frequency of ulcers or number of episodes;
mean duration of ulcers;
ulcer healing time;
severity of ulcers;
size of ulcers;
time to initial response;
recurrence of ulcers;
time to recurrence of ulcers;
complete response, defined as absence of any oral ulcer of any size during treatment period;
percentage change in a patient's total ulcer burden;
monthly aphthae count.
Pain measurements included:
pain (e.g. scale of 0 to 3 (0: absent; 1: mild; 2: moderate; and 3: severe));
number of painful days;
ratio of painful days to days with ulcers;
total monthly pain scores.
Nine of the 15 studies did not report on pain as an outcome measure (Aktulga 1980; Ergun 1997; Fani 2012; Hamuryudan 1991; Masuda 1989; Mat 2006; Melikoglu 2005; Yurdakul 2001; Hamuryudan 1998).
The study by Kilic 2009 stated that pain was an outcome but did not describe how it would be measured and did not report any pain data in the results (Kilic 2009).
Other reported outcomes included measures of 'overall response' or 'positive response' (not specified), 'other disease features', laboratory abnormalities, number and severity of genital ulcers, response of eye disease to treatment, ocular complications, patient‐reported general well‐being, global disease severity, amount of suppression of pathergy and midstream specimen of urine (MSU) tests, and attacks of arthritis.
Three out of 15 trials failed to report adverse events (Fani 2012; Hamuryudan 1991; Koc 1992). None of the studies reported on issues of cost or reduction of morbidity. One trial described the use of a quality of life assessment tool but did not report any data on quality of life (Kilic 2009).
Excluded studies
Twenty trials were excluded (Characteristics of excluded studies). The main reason for exclusion was that following access to the full paper the study did not actually fulfil the criteria for being a RCT (eight studies) (lack of randomisation, no control group). Three cross‐over studies were excluded because of: lack of a washout period, one presented on topical only treatments for genital ulcers, and one reported oral ulcer outcomes but this was ad hoc reporting and not pre‐specified. Two papers was rejected as they were letters with insufficient information reported. Both letters were published longer than 10 years ago and we therefore did not obtain any further information from the authors (Convit 1984; Scheinberg 2002).
Risk of bias in included studies
A summary of the risk of bias for each study is presented in Figure 2 and the ‘Characteristics of included studies’ table.
2.
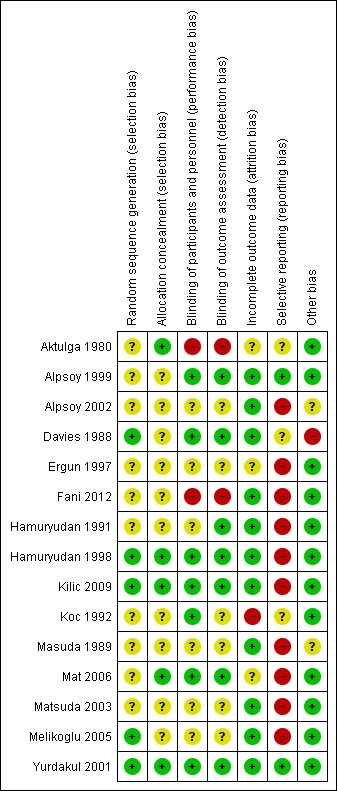
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Only one trial was assessed as being at low risk of bias (Yurdakul 2001). One of the trials was assessed as having overall unclear risk of bias (Alpsoy 1999). The remaining 13 trials were assessed as at overall high risk of bias.
Allocation
Three of the 15 trials were given an overall low risk of bias for selection bias (both for sequence generation and allocation concealment) (Hamuryudan 1998; Kilic 2009; Yurdakul 2001). Twelve of the 15 were assessed as at overall unclear risk of bias for allocation. Of these 12, the random sequence generation was at low risk of bias for two trials (Davies 1988; Melikoglu 2005) and allocation concealment was at low risk of bias for two (Aktulga 1980; Mat 2006).
Blinding
Six trials were shown to be at low risk of bias for blinding (Alpsoy 1999; Davies 1988; Hamuryudan 1998; Kilic 2009; Mat 2006; Yurdakul 2001). Seven trials had an overall unclear risk of bias, of which one had a low risk for detection bias (Hamuryudan 1991) and one had a low risk for performance bias (Koc 1992). Two trials had a high risk of bias for blinding as the interventions used were different in appearance and delivery method (Aktulga 1980; Fani 2012).
Incomplete outcome data
One trial was assessed as at high risk of bias due to incomplete data (Koc 1992) as all six dropouts were from the intervention arm and insufficient reasons were presented. Three trials were assessed as at unclear risk of bias (Aktulga 1980; Ergun 1997; Mat 2006). The remaining 11 trials were deemed low risk of bias.
Selective reporting
Only two of the 15 trials were given low risk of bias for selective reporting (Alpsoy 1999; Yurdakul 2001). Three trials (Aktulga 1980; Davies 1988; Koc 1992) were judged to be at unclear risk of bias and the remaining 10 trials were at high risk of bias. The most frequent reason for allocation of a high risk of bias was failure to report outcome data fully (Alpsoy 2002; Ergun 1997; Hamuryudan 1998; Masuda 1989; Matsuda 2003). Some trials presented only a selection of the pre‐specified outcome measures (Kilic 2009) or presented outcomes that were not pre‐specified (Mat 2006). One trial did not present the results for the outcomes pre‐specified in the trial protocol (Fani 2012). Some trials presented means with no standard deviations (Davies 1988; Kilic 2009) or only a P value (Davies 1988). Some trials carried out further analyses to support the findings, however the analyses were not presented (Hamuryudan 1991; Melikoglu 2005).
Other potential sources of bias
Twelve out of 15 trials were thought to have low risk for other potential sources of bias. Two trials were at unclear risk of bias (Alpsoy 2002; Masuda 1989). In one of these trials (Alpsoy 2002) it was unclear if both the intervention group and the control group received additional analgesia, which in turn could potentially affect the pain outcomes. In the other (Masuda 1989) it was unclear if additional topical therapies had been used. One trial was judged to be at high risk of bias due to concomitant systemic interventions being used (Davies 1988).
Effects of interventions
Summary of findings for the main comparison. Summary of findings: topical interventions.
| Topical interventions compared to placebo for managing oral ulcers in Behç̧et's disease | |
|
Patient or population: people with Behçet's disease Settings: primary care Intervention: topical interventions Comparison: placebo | |
| Outcomes | Comments |
| Pain associated with oral ulcers | 5 placebo‐controlled trials evaluated topical interventions (sucralfate suspension (2 trials), interferon–alpha (2 trials), cyclosporin A (1 trial). The quality of the evidence ranged from low to very low1 and there is insufficient evidence to support or refute the use of any evaluated intervention for oral ulcers in Behçet’s disease |
| Episode duration associated with oral ulcers | |
| Episode frequency associated with oral ulcers | |
| Safety of the intervention including adverse effects | |
1Studies downgraded for risk of bias and imprecision. Applicability, indirectness and publication bias were not considered to be of concern.
Summary of findings 2. Summary of findings: systemic interventions.
| Systemic interventions compared to placebo for managing oral ulcers in Behçet's disease | |
|
Patient or population: people with Behçet's disease Settings: primary care Intervention: systemic interventions Comparison: placebo | |
| Outcomes | Comments |
| Pain associated with oral ulcers | 8 placebo‐controlled trials evaluated topical interventions (aciclovir (1 trial), thalidomide (1 trial), corticosteroids (1 trial), rebamipide (1 trial), etanercept (1 trial), colchicine (2 trials), interferon–alpha (1 trial)). The quality of the evidence ranged from moderate1 to very low2 and there is insufficient evidence to support or refute the use of any evaluated intervention for oral ulcers in Behçet’s disease |
| Episode duration associated with oral ulcers | |
| Episode frequency associated with oral ulcers. | |
| Safety of the intervention including adverse effects | |
1Yurdakul 2001 downgraded for imprecision alone. 2Studies downgraded for risk of bias and imprecision. Applicability, indirectness and publication bias were not considered to be of concern.
Topical interventions
Six of the 15 included trials involved topical interventions. Five were placebo controlled (Table 1) and one made a head‐to‐head comparison.
Placebo‐controlled trials
Sucralfate
Two trials looked at sucralfate suspension versus placebo (Alpsoy 1999; Koc 1992). We were unable to pool the results as the mode of delivery of sucralfate differed between the studies (in one it was used as a mouthwash and in the other it was applied topically to ulcers). The trial by Alpsoy 1999 compared sucralfate suspension versus placebo suspension to be used as a mouthwash for two to four minutes after routine oral care and before bed. It included 40 participants and analysed results for 30. It had an unclear risk of selection bias (both for random sequence generation and allocation concealment). The results showed that sucralfate significantly decreased the mean frequency, healing time, and pain in comparison to baseline. However, no statistically significant differences were observed between the intervention and placebo for any of the oral ulcer outcomes at either end of treatment (three months) or end of follow‐up (six months) (Table 3). The trial by Koc 1992 included 41 participants (data evaluated for 35) and was at high risk of bias due to incomplete outcome data. It compared the sucralfate suspension and placebo suspension applied to oral ulcers four times a day. No statistically significant differences in number of painful days, number of episodes, or mean duration of episodes were seen at either the end of treatment or end of follow‐up (Table 3).
1. Sucralfate versus placebo (topical application).
| Sucralfate | Placebo | |||||||||
| Study | Outcome | Time point | Mean | Standard deviation | N | Mean | Standard deviation | N | Mean difference [95% CI] | P value |
| Alpsoy 1999 | Frequency of oral ulcer | End of treatment | 3.56 | 1.3 | 16 | 4.36 | 2.2 | 14 | ‐0.80 [‐2.12, 0.52] | 0.23 |
| End of follow up | 3.81 | 2.1 | 16 | 3.57 | 1.9 | 14 | 0.24 [‐1.19, 1.67] | 0.74 | ||
| Alpsoy 1999 | Healing time | End of treatment | 7.19 | 1.9 | 16 | 8.28 | 2.3 | 14 | ‐1.09 [‐2.61, 0.43] | 0.16 |
| End of follow up | 8.31 | 2.5 | 16 | 9.28 | 2.9 | 14 | ‐0.97 [‐2.92, 0.98] | 0.33 | ||
| Alpsoy 1999 | Pain | End of treatment | 0.69 | 0.5 | 16 | 1.07 | 0.8 | 14 | ‐0.38 [‐0.87, 0.11] | 0.12 |
| End of follow up | 1.47 | 0.5 | 16 | 1.28 | 0.6 | 14 | 0.19 [‐0.21, 0.59] | 0.35 | ||
| Koc 1992 | Number of painful days | End of treatment | 37.5 | 17.6 | 24 | 28.5 | 19.0 | 11 | 9.00 [‐4.25, 22.25] | 0.18 |
| End of follow up | 38.5 | 19.5 | 24 | 34.9 | 23.2 | 11 | 3.60 [‐12.17, 19.37] | 0.65 | ||
| Koc 1992 | Number of episodes | End of treatment | 6.4 | 2.5 | 24 | 5.0 | 2.0 | 11 | 1.40 [‐0.15, 2.95] | 0.08 |
| End of follow up | 6.5 | 2.0 | 24 | 5.5 | 1.3 | 11 | 1.00 [‐0.11, 2.11] | 0.08 | ||
| Koc 1992 | Mean duration of episodes | End of treatment | 10.3 | 8.3 | 24 | 11.3 | 5.6 | 11 | ‐1.00 [‐5.69, 3.69] | 0.68 |
| End of follow up | 8.9 | 6.9 | 24 | 8.2 | 2.99 | 11 | 0.70 [‐2.56, 3.96] | 0.68 | ||
There was insufficient evidence to support or refute the use of sucralfate suspension for oral ulcers in Behcet’s disease.
Interferon‐alpha
Two placebo‐controlled trials studied the effect of topical interferon‐alpha (Hamuryudan 1991; Kilic 2009). Both had a high risk of bias for selective reporting. The trial of 63 patients (61 evaluated) by Hamuryudan 1991 compared interferon–alpha 2c as a hydrogel versus placebo hydrogel. Patients applied a thin layer on any ulcer three times a day for 24 weeks. A similar application was made to the upper and lower lip mucosa irrespective of the presence of ulcers. No statistically significant difference was shown for the total number of ulcers throughout the treatment period (Table 4).
2. Interferon‐alpha versus placebo (topical).
| Interferon‐alpha | Placebo | |||||||||
| Study | Outcome | Time point | Mean | Standard deviation | n | Mean | Standard deviation | n | Mean difference [95% CI] | P value |
| Hamuryudan 1991 | Total ulcers | Duration of treatment (24 weeks) | 41.8 | 24.5 | 30 | 40.3 | 23.0 | 31 | 1.50 [‐10.43, 13.43] | 0.81 |
The Kilic 2009 trial compared two different dosages of interferon‐alpha lozenges (1000 IU versus 2000 IU) versus placebo in 84 patients. The data presented did not allow for analysis, however the authors reported no statistically significant difference between the total ulcer burden of the intervention (at either evaluated dosage) and placebo groups.
There was insufficient evidence to support the use of interferon‐alpha as a topical treatment for oral ulcers in Behçet’s disease.
Cyclosporin A
The trial by Ergun 1997 compared cyclosporin A in orabase (70 mg/g of orabase) versus placebo (orabase) in 24 patients. It had a high risk of reporting bias. No data were presented, however the authors reported no clinical improvement in the number, size, and healing time for either group. No adverse effects were seen.
There was insufficient evidence to support or refute the use of cyclosporin A in orabase as a treatment for oral ulcers in Behçet’s disease at the dose evaluated.
Head‐to‐head trials
The trial by Fani 2012 included 60 participants and it compared triamcinolone acetonide 0.1% ointment versus phenytoin syrup mouthwash (30 mg in 5 ml). The triamcinolone group applied the ointment to the lesions three times a day. The phenytoin group used two teaspoons of syrup in half a glass of warm water as a mouthwash for four to five minutes, three times a day. The trial had a high risk of reporting bias. The outcome measure of 'positive response' was not described, however a statistically significant difference was shown in favour of triamcinolone acetonide over phenytoin syrup (risk ratio (RR) 1.63, 95% confidence interval (CI) 1.13 to 2.34; P = 0.009) (Table 5).
3. Triamicinolone acetonide versus phenytoin.
| Triamicinolone acetonide | Phenytoin | |||||||
| Study | Outcome | Time point | Number with event | N | Number with event | N | RR (95% CI) | P value |
| Fani 2012 | Positive response | 7 days | 26 | 30 | 16 | 30 | 1.63 [1.13, 2.34] | 0.009 |
There was insufficient evidence from this single study to support or refute the use of either phenytoin syrup mouthwash or triamcinolone acetonide as a treatment for oral ulcers in Behçet’s disease.
Systemic interventions
Nine of the 15 trials evaluated systemic interventions. Eight out of the nine were placebo controlled (Table 2) and one trial was head to head.
Placebo‐controlled trials
Aciclovir
One trial of 36 patients compared acyclovir versus placebo (Davies 1988). The patients were given 800 mg of acyclovir five times a day for one week, followed by 400 mg twice a day for 11 weeks. Patients also received various concomitant treatments. The trial had a high risk of reporting bias. Data weren't presented in a usable format, however the authors reported no statistically significant difference in frequency of oral ulcers between groups.
There was insufficient evidence to support or refute the use of acyclovir as a treatment for oral ulcers in Behcet’s disease at the dose evaluated.
Thalidomide
One trial recruiting 101 patients compared thalidomide 300 mg daily versus 100 mg daily versus daily placebo (Hamuryudan 1998). It had a high risk of reporting bias. It included only male patients. The authors reported a significant effect on mean numbers of minor oral ulcers from week four of treatment in both thalidomide groups, however oral ulcer data were not presented separate from genital ulcer data. The treatment effect diminished on stopping treatment. There was no reported difference between the 100 mg and 300 mg dosages on oral ulcers. There were significant adverse effects including severe sedation, polyneuropathy, loss of libido, and weight gain. A greater number of adverse events was seen for the higher dose of thalidomide. Four patients withdrew from the study due to side effects (all from the intervention arm).
There was insufficient evidence to support or refute the use of thalidomide (at either 300 mg or 100 mg daily) as a treatment for oral ulcers in Behçet’s disease.
Corticosteroids
One trial compared intramuscular depot injections of corticosteroids versus saline placebo injections in 86 patients (Mat 2006). The primary aim of the study was to manage genital ulceration in Behçet’s disease however they did report on oral ulcers. Patients received 40 mg methylprednisolone by intramuscular injection versus a placebo intramuscular injection every 3 weeks for 27 weeks. The trial had a high risk of reporting bias. Various concomitant treatments were used including colchicine, amitriptyline, non‐steroidal anti‐inflammatory drugs, and thalidomide. No statistically significant difference between groups was shown with regard to oral ulceration (Table 6).
4. Depot corticosteroids versus placebo (systemic).
| Depot corticosteroids | Placebo | |||||||||
| Study | Outcome | Time point | Mean | Standard deviation | N | Mean | Standard deviation | N | Mean difference [95% CI] | P value |
| Mat 2006 | Mean number of oral ulcers | End of treatment (week 27) | 1.8 | 1.0 | 41 | 1.8 | 1.2 | 44 | 0.00 [‐0.47, 0.47] | 1.00 |
| End of follow‐up (week 35) | 1.9 | 1.6 | 34 | 2.0 | 2.3 | 40 | ‐0.10 [‐0.99, 0.79] | 0.83 | ||
There was insufficient evidence to support or refute the use of intramuscular depot injections of corticosteroids, at the dose evaluated, as a treatment for oral ulcers in Behçet’s disease.
Rebamipide
One trial of 35 patients compared 300 mg daily rebamipide versus placebo (Matsuda 2003). It had a high risk of reporting bias. Concomitant treatments were allowed but insufficient details were presented to allow full interpretation of the results. The authors stated that rebamipide improved the global evaluation aphthae count and global evaluation pain score in Behçet’s disease; data were not presented in a form to confirm or refute this statement.
There was insufficient evidence to support or refute the use of rebamipide, at the dose evaluated, as a treatment for oral ulcers in Behçet’s disease.
Etanercept
The trial by Melikoglu 2005 included 40 participants who received either etanercept 25 mg subcutaneously or placebo subcutaneously twice a week for four weeks. It included only males. There was a high risk of reporting bias. The authors reported a statistically significant difference in mean number of oral ulcers with etanercept (weeks one, two, three, and four). The data presented did not allow this statistically significant result to be confirmed at week four. The statistically significant effects disappeared in the follow‐up period.
There was insufficient evidence to support or refute the use of etanercept as a treatment for oral ulcers in Behçet’s disease at the dose evaluated.
Colchicine
Two trials compared colchicine with placebo. One trial of 116 patients compared colchicine (0.5 mg, dose adjusted per weight in kg) versus placebo (Yurdakul 2001). The trial was at overall low risk of bias. The trial authors provided additional data; and no significant difference was noted on the outcome of oral ulcers (Table 7). An earlier trial, of 35 patients compared 0.5 mg colchicine, three times a day for six months, with placebo. The colchicine capsule also contained 60 mg of amidone and 40 mg lactose. The placebo contained a diarrhoea producing agent, phenolphtalein. No difference was shown with regard to improvement in RAS (Table 7).
5. Colchicine versus placebo (systemic).
| Colchicine | Placebo | |||||||||
| Study | Outcome | Time point | Mean | Standard deviation | N | Mean | Standard deviation | N | Mean difference [95% CI] | P value |
| Yurdakul 2001* | Total number of ulcers | 24 months | 23.2 | 17.1 | 57 | 20.9 | 14.0 | 58 | 2.30 [‐3.42, 8.02] | 0.43 |
| Study | Outcome | Time point | Number with event | N | Number with event | N | RR (95% CI) | P value | ||
| Aktulga 1980 | Improvement in oral ulcer score | 6 months | 9 | 14 | 12 | 14 | 0.75 [0.48, 1.17] | 0.21 | ||
*data supplied by author
There was insufficient evidence to support or refute the use of colchicine as a treatment for oral ulcers in Behçet’s disease.
Interferon‐alpha
The trial by Alpsoy 2002 compared subcutaneous injections of interferon–alpha (6 x 106 IU) versus placebo subcutaneous injections in 50 patients. The treatment was given three times a week for three months and the patients were followed up for a further three months. The trial had a high risk of reporting bias. Data were not presented in a useable format, however the authors reported a statistically significant decrease in the duration and pain of oral ulceration. There was a high rate of adverse events including alopecia, leukopenia, and diarrhoea and 18 out of 23 patients experienced mild flu like symptoms in the treatment arm.
There was insufficient evidence to support or refute the use subcutaneous Interferon–alpha as a treatment for oral ulcers in Behçet’s disease at the dose evaluated.
Head‐to‐head trials
One trial compared cyclosporin (10 mg/kg per day) to colchicine (1 mg daily) for 16 weeks for the management of ocular manifestations of Behçet’s disease (Masuda 1989). It included 96 patients (92 evaluated) and also reported on oral ulcers. It had a high risk of reporting bias. The results showed that cyclosporin alleviated oral aphthous ulceration in 70% compared to 20% in the colchicine group (RR 3.3, 95% CI 1.85 to 5.88; P < 0.0001). There were multiple adverse events in the cyclosporin arm and three patients withdrew and nine had a dose reduction as a result (Table 8).
6. Cyclosporin versus colchicine (systemic).
| Ciclosporin | Colchicine | |||||||
| Study | Outcome | Time point | Number with event | N | Number with event | N | RR (95% CI) | P value |
| Masuda 1989 | Alleviation of oral aphthous ulcers | Unclear | 33 | 46 | 10 | 46 | 3.3 [1.85, 5.88] | <0.0001 |
There was insufficient evidence to support or refute the use of cyclosporin (10 mg/kg per day) or colchicine (1 mg daily) as a treatment for oral ulcers in Behçet’s disease at the doses evaluated.
Discussion
Summary of main results
Fifteen randomised controlled trials (RCTs) were included in this review, evaluating the effectiveness of 13 different interventions for the management of oral ulcers in Behçet’s disease. There was considerable heterogeneity in the types of interventions evaluated and the way in which the interventions were used. Many of the trials specifically looked at oral ulcers as the primary outcome (six out of 15 trials), however for some of the studies the primary outcomes were related to other manifestations of Behçet's disease or the holistic management of Behçet's disease and the oral aspects were only reported as a secondary outcome.
The outcome measures evaluated and the timing of outcome measures varied widely. Some studies reported on individual ulcer data (size and number of ulcers), some on episodes (number of episodes, number of days with ulcers, number of days with no ulcers), and not all trials reported on pain as an outcome. Three of the 15 trials did not report adverse events or side effects and therefore the safety of the intervention used could not be assessed. Some studies reported data at the end of treatment and some at the end of follow‐up. This is of particular clinical relevance as many of the interventions were shown to be beneficial whilst actively on treatment, but on stopping treatment the positive results were not sustained. This would mean that patients would potentially require long‐term active treatment.
Twelve of the 15 trials were placebo controlled. There were three head‐to‐head trials. In the head‐to‐head trial by Fani et al (Fani 2012) no placebo was used. It is possible that the triamcinolone acetonide ointment was being used as an 'active placebo' or as a 'standard treatment' or 'usual treatment' arm, however there is no clear evidence from this review that triamcinolone is any better than placebo or no treatment for recurrent aphthous stomatitis (RAS)‐type ulceration in Behçet's disease. Without a placebo arm to the trial, it is theoretically possible that the benefit shown by the triamcinolone acetonide ointment was because the phenytoin syrup made the symptoms of the RAS‐type ulcers worse. Further evidence for the use of topical steroids including triamcinolone acetonide for the management of RAS will be available in the ongoing Cochrane review 'Topical interventions for the management of recurrent aphthous stomatitis' (Taylor 2013).
There was insufficient evidence to support or refute the use of any evaluated interventions for the management of oral ulcers in Behçet's disease.
Overall completeness and applicability of evidence
The diagnosis of Beḩcet's disease was not described clearly in all of the studies. Most trials used the International Study Group criteria for Behçet's disease (ISG 1990). The two studies from Japan used the Japanese diagnostic criteria, which were only described in detail in one of the trials. One trial used the O'Duffy criteria (Aktulga 1980).
Eleven of the trials were from Turkey and seven of these were from Istanbul, Turkey. Although this may represent the high prevalence of Behçet's disease in that area it also gives a heavy weighting to the evidence from one particular group.
As stated previously, many RCTs are carried out for the treatment of Behç̧et's disease and not all of them report on the oral outcomes. Where a study reported an oral outcome but this was not pre‐specified in the methodology, the study was excluded. This was to avoid ad hoc reporting of results after the trial was finished. It is important that as much information as possible is available for clinicians so they can plan their treatments appropriately. The heterogeneity of outcome measures used in trials for Behçet's disease was recently systematically reviewed by Hatemi et al (Hatemi, 2014). In the 18 included RCTs that they reviewed there were nine different oral outcome measurements used. This level of heterogeneity of outcome measures was also a feature in our review and meant that meta‐analysis was impossible.
There may be many treatments currently used for Behçet's disease which have a beneficial effect on oral ulcers, however until we have further evidence we can't recommend them for treating the oral ulcers of Behçet's disease.
There was a paucity of RCTs looking at anti‐TNF (tumour necrosis factor) treatments. Many of the anti‐TNF treatments have been evaluated in open studies. Anti‐TNF treatments have the potential to be used to manage the more serious and life threatening or organ threatening aspects of Behçet's disease. As a result of this, the oral aspects of Behçet's disease may not be reported as readily.
Oral ulceration is the most common sign and symptom of Behçet's disease and often pre‐dates other systemic involvement. As a result of this, many of the trials were primarily aimed at the management of oral ulceration (six out of the 15 trials). Of these six trials, five were for topical treatments. Whilst the oral aspects of Beḩcet's disease are not considered to be life threatening, they can cause significant morbidity and reduction of quality of life. None of the trials reported on these aspects.
Four of the studies looking at systemic interventions were aimed at orogenital disease and the remaining five studies were for the management of Behçet's disease. There are multiple trials of systemic treatments for Behçet's disease which did not fulfil our criteria for inclusion in this review as they did not report the oral aspects with pre‐specified oral outcome measures.
One trial has recently been completed and shows promising results for apremilast compared with placebo for oral ulcers (NCT00866359). Once fully available, the results of this trial will be incorporated in future updates of this review.
Quality of the evidence
One of the 15 trials was assessed as being at low risk of bias (Yurdakul 2001). One was at unclear risk of bias (Alpsoy 1999). The remainder were deemed as at high risk of bias. Of the 13 high risk of bias studies, 10 had a high risk of bias for reporting bias. The main issues with reporting bias were related to inadequate or incomplete reporting. Some studies did not report the pre‐specified outcomes, others reported a global evaluation of the outcomes but with no detailed data provided. Inappropriate use of graphs and tables which did not contain useable data was common. Some studies reported that separate analyses were carried out which confirmed their findings, but the separate analyses were never presented.
In previous times the space restrictions from some journals meant authors were required to condense their findings to conform to the limits stipulated. Fortunately in recent times there is the availability of an online supplement which means that all authors can make all the results available to the reader. In many of the studies a high risk of bias label for reporting bias could have been avoided if additional raw data had been available to this review group.
For topical interventions the quality of the evidence ranged from low to very low; for systemic interventions the quality of the evidence ranged from moderate to very low. The quality of the body of evidence was downgraded due to risk of bias and imprecision.
Potential biases in the review process
The review authors followed the guidelines for conducting this systematic review under the strictest of conditions (Higgins 2011). All abstracts were independently dual screened, and all papers were assessed and had the risk of bias assessment carried out by at least two independent authors. All papers were subsequently reviewed by five of the review authors. The findings were then discussed at a meeting with five of the authors including the three clinical authors.
Agreements and disagreements with other studies or reviews
A previous Cochrane review has looked at pharmacotherapy for Beḩcet's syndrome (Saenz 1998). They included five trials for oral ulceration, four of which are included in this review. The fifth trial (Benamour 1991) was considered to be a controlled clinical trial and therefore not eligible for inclusion in this review. The review by Saenz et al also concluded that there was "insufficient evidence either to support or to refute some of the classic treatments for Behçet's syndrome". The current recommendations for the management of Behçet's disease were written by the EULAR (European League Against Rheumatism) group and published in 2008 (Hatemi, 2008). This multidisciplinary expert committee carried out a systematic review and presented their findings and recommendations according to the system involved in the disease. The management of oral ulcers is contained in the mucocutaneous section and states that "oral ulcers may be managed with topical preparations". The RCTs included in this review are all noted by the group, and additionally they make recommendations based on various open studies. Colchicine is a readily used systemic treatment in Beḩcet's disease and the authors state "Colchicine is widely used without any solid proof of its efficacy", which confirms the findings of the colchicine study included in this review (Yurdakul 2001)
Another recent review, 'Behçet's syndrome: Facts and Controversies' (Mat 2013), summarises the RCTS available and comments on the EULAR recommendations. Many of the RCTs described were carried out in the same department that the authors are from (Cerrahpsa medical facility, Istanbul). They also report that data from the open studies on the use of biologic agents is promising (interferon‐alpha, anti‐TNF). They conclude that "Local treatment for oral and genital ulcers is sufficient".
The findings of our systematic review indicate uncertainty on the effectiveness and safety of local and systemic treatment for oral ulcers.
Authors' conclusions
Implications for practice.
Whilst there is no 'gold standard treatment' for the management of oral ulcers in Behçet's disease, there are a number of treatments which are currently used in practice.
In practice, topical treatments are generally used as first line therapy, however it is often necessary to consider systemic treatments for many patients. When patients have manifestations of Behçet's disease that may cause severe morbidity (for example blindness) or they have multiple morbidities, or they are life threatening, the clinical reasoning for stepping up the treatment to include potentially harmful systemic interventions may be justified. It may be a secondary beneficial outcome that the patient's oral symptoms also improve in these cases. However, there are some patients who do not have this level of severity of Behçet's disease but they do have severe oral ulceration which can cause significant morbidity and reduction of quality of life (eating, drinking, and speaking). For these patients, it is important that we have the best evidence to guide our clinical decision making.
This review found insufficient evidence to support or refute the use of any of the included topical or systemic interventions for the management of oral ulcers in Behçet's disease.
Implications for research.
This review was limited by the poor methodology of many of the trials, which in turn led to huge heterogeneity of outcome measures and timing of outcome measures, and inadequate reporting of results.Future trials for Beḩcet's disease should be appropriately planned, executed, and reported according to the CONSORT guidelines (www.consort‐statement.org). The interventions evaluated should be clinically relevant and the controls used should be appropriate. Cross‐over trials should have a washout period.
As oral ulcers are the most common feature of Behçet's disease, appropriate pre‐specified oral outcome measures should be used for all trials of interventions for Beḩcet's disease. The development of a set of standardised outcome measures for oral ulcer trials is registered with COMET (www.comet‐initiative.org). The use of an oral ulcer core outcome set when planning trials will allow homogeneity of outcomes for future systematic reviews. Hatemi et al are leading a group who are currently developing a core set of outcome measures for Behçet's disease (Hatemi, 2014), however there is no planned involvement of an oral ulcer related specialty in that group (that is oral medicine, oral surgery, or dentistry).
The inclusion of a quality of life assessment tool such as the Chronic Oral Mucosal Diseases Questionnaire (Riordain 2011) would be advantageous.
As many of the patients will require long term active treatment, the inclusion of an appropriate economic evaluation of the interventions would be appropriate. This could assess the cost effectiveness of treatments.
Further research into the following interventions is warranted:
thalidomide;
rebapamide;
etanercept;
and interferon‐alpha.
Further research would most likely change current clinical practice.
What's new
| Date | Event | Description |
|---|---|---|
| 21 November 2019 | Review declared as stable | This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future. |
History
Protocol first published: Issue 3, 2014 Review first published: Issue 9, 2014
| Date | Event | Description |
|---|---|---|
| 12 August 2014 | Amended | Following peer review the wording around the definition of recurrent aphthous stomatitis (RAS) and RAS‐type ulceration has been clarified. This is an amendment to the protocol. |
Notes
This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future
Acknowledgements
The review team would like to acknowledge Anne Littlewood, Jo Weldon, and Janet Lear for their help with the management of this review. We would also like to thank authors of trials for additional data provided.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1. Behcet syndrome/ 2. (Behcet adj2 (syndrome$ or disease)).ti,ab. 3. ("triple‐complex syndrome$" or "triple‐complex disease$").ti,ab. 4. or/1‐3 5. Stomatitis, aphthous/ 6. ((aphthous or apthous or mouth$ or oral$) adj3 (ulcer$ or lesion$ or stomatitis)).ti,ab.˜ 7. (aphthae or apthae).ti,ab. 8. "canker sore$".ti,ab. 9. "herpetiform ulcer$".ti,ab. 10. "periadenitis mucosa necrotica recurrens".ti,ab. 11. or/5‐10 12. 4 and 11
The above subject search was linked with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of theCochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011] (Higgins 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 2. The Cochrane Oral Health Group Trials Register search strategy
(((Behcet* and disease*) or (Behcet* and syndrome*)):ti,ab) AND (INREGISTER)
((("triple‐complex syndrome*" or "triple‐complex disease*")):ti,ab) AND (INREGISTER)
MeSH DESCRIPTOR Behcet Syndrome
(#1 or #2 or #3) AND (INREGISTER)
Appendix 3. The Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 [mh "Behcet syndrome"] #2 (behcet near/2 syndrome*) or (behcet near/2 disease*) #3 ("triple‐complex syndrome*" or "triple‐complex disease*") #4 {or #1‐#3} #5 [mh "Stomatitis, aphthous"] #6 ((aphthous or apthous or mouth* or oral*) and (ulcer* or lesion* or stomatitis)) #7 (aphthae or apthae) #8 "canker sore*" #9 "herpetiform ulcer*" #10 "periadenitis mucosa necrotica recurrens" #11 {or #5‐#10} #12 #4 and #11
Appendix 4. EMBASE (Ovid) search strategy
1. Behcet disease/ 2. (Behcet adj2 (syndrome$ or disease)).ti,ab. 3. ("triple‐complex syndrome$" or "triple‐complex disease$").ti,ab. 4. or/1‐3 5. Stomatitis, aphthous/ 6. ((aphthous or apthous or mouth$ or oral$) adj3 (ulcer$ or lesion$ or stomatitis)).ti,ab. 7. (aphthae or apthae).ti,ab. 8. "canker sore$".ti,ab. 9. "herpetiform ulcer$".ti,ab. 10. "periadenitis mucosa necrotica recurrens".ti,ab. 11. or/5‐10 12. 4 and 11
The above subject search was linked to the Cochrane Oral Health Group filter for identifying RCTs in EMBASE via OVID:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 16. 14 NOT 15
Appendix 5. CINAHL (EBSCO) search strategy
S1 (MH "Beḩcet's Syndrome") S2 (behcet N2 syndrome*) or (behcet N2 disease*) S3 ("triple‐complex syndrome*" or "triple‐complex disease*") S4 S1 or S2 or S3 S5 (MH "Stomatitis, Aphthous") S6 ((aphthous or apthous or mouth* or oral*) and (ulcer* or lesion* or stomatitis)) S7 (aphthae or apthae) S8 "canker sore*" S9 "herpetiform ulcer*" S10 "periadenitis mucosa necrotica recurrens" S11 S5 or S6 or S7 or S8 or S9 or S10 S12 S4 and S11
The above subject search was linked to the Cochrane Oral Health Group filter for identifying RCTs in CINAHL via EBSCO:
S1 MH Random Assignment or MH Single‐blind Studies or MH Double‐blind Studies or MH Triple‐blind Studies or MH Crossover design or MH Factorial Design S2 TI ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or AB ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or SU ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") S3 TI random* or AB random* S4 AB "latin square" or TI "latin square" S5 TI (crossover or cross‐over) or AB (crossover or cross‐over) or SU (crossover or cross‐over) S6 MH Placebos S7 AB (singl* or doubl* or trebl* or tripl*) or TI (singl* or doubl* or trebl* or tripl*) S8 TI blind* or AB mask* or AB blind* or TI mask* S9 S7 and S8 S10 TI Placebo* or AB Placebo* or SU Placebo* S11 MH Clinical Trials S12 TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial) S13 S1 or S2 or S3 or S4 or S5 or S6 or S9 or S10 or S11 or S12
Appendix 6. AMED (Ovid) search strategy
1. ((behcet and syndrome$) or (behcet and disease$)).ti,ab. 2. ("triple‐complex syndrome$" or "triple‐complex disease$").ti,ab. 3. or/1‐2 4. ((aphthous or apthous or mouth$ or oral$) adj3 (ulcer$ or lesion$ or stomatitis)).ti,ab. 5. (aphthae or apthae).ti,ab. 6. "canker sore$".ti,ab. 7. "herpetiform ulcer$".ti,ab. 8. "periadenitis mucosa necrotica recurrens".ti,ab. 9. or/4‐8 10. 3 and 9
Appendix 7. US National Institutes of Health Trials Register (ClinicalTrials.gov) and WHO Clinical Trials Registry Platform search strategy
Behcet* and oral and ulcer* Behcet* and mouth and ulcer* Behcet* and stomatitis
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aktulga 1980.
| Methods | Study design: RCT parallel Trial MD: NS Conducted in: Turkey Number of centres: 1 Recruitment period: NS Sample size calculation undertaken and met: not mentioned |
|
| Participants | Source of recruitment: NS Age GrA: 34.2 years ±7.2 Age GrB: 33 years ±12.8 Gender (overall sample): 6F/22M Gender GrA: 5F/9M Gender GrB: 1F/13M Inclusion criteria: well defined Behcet’s disease according to the O’Duffy criteria Exclusion criteria: NS Number randomised: 35 Number evaluated: 28 |
|
| Interventions |
Comparison: colchicine versus placebo GrA (n = 14): capsules containing colchicine 0.5 mg, lactose 40 mg, amidone, 60 mg taken tds GrB (n = 14): placebo capsules containing phenolphthalein 60 mg, lactose 40 mg tds (decreased to bd if diarrhoea a problem) |
|
| Outcomes | Primary outcomes (patients seen monthly): semiquantitative assessment of 0‐3 for aphthous stomatitis Carried out monthly for 6 months No reporting of adverse events, quality of life or cost |
|
| Funding | Supported in part by Turkish and Technical research council (TAG 386) | |
| Notes | Comparable groups at baseline: no information in study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomised" |
| Allocation concealment (selection bias) | Low risk | Quote: "the code was known to a local pharmacist who dispensed the medication according to our instructions" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "identical capsules" Placebo group were informed to decrease dose from 3 times a day to twice daily if diarrhoea a problem |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Given different instructions provided to the 2 treatment arms, blinding unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7 dropouts no details given (evenly distributed) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details given for outcome measurements, who was assessing and interassessor calibration |
| Other bias | Low risk | None apparent |
Alpsoy 1999.
| Methods | Study design: RCT (parallel) Trial ID: NS Conducted in: Turkey Number of centres: NS Recruitment period: NS Sample size calculation undertaken and met: NS |
|
| Participants | Source of recruitment: NS Age (overall sample): 33.4 years (SD 7.61) Age GrA: 33.0 years (SD 9.0) Age GrB: 34.1 years (SD 5.3) Gender (overall sample): 14 F/16 M Gender GrA: 8 F/8 M Gender GrB: 6 F/8 M Inclusion criteria: diagnosed according to the criteria of International Study Group for Behçet's disease Exclusion criteria: active eye disease or organ involvement requiring systemic therapy or received recent systemic therapy for at least 12 weeks and topical therapy for at least 4 weeks prior to the study Number randomised: 40 (20:20) Number evaluated: 30 (16:14) |
|
| Interventions |
Comparison: sucralfate suspension versus placebo GrA (n = 16): 5 mL of sucralfate to use as an oral rinse for 1 to 2 minutes after routine mouth care and before sleep GrB (n = 14): as for sucralfate 3 months treatment; 3 months follow‐up |
|
| Outcomes | Mean frequency of lesion Healing time Pain (scale of 0 to 3 (0, absent; 1, mild; 2, moderate; and 3, severe)) Adverse events No reporting of quality of life or cost |
|
| Funding | NS | |
| Notes | Comparable groups at baseline: yes Treatment for oral and genital lesions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The clinical investigator (H.E.) and patients were unaware of the specific drugs that the patients were taking during the course of the study" Comment: placebo identical in appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The clinical investigator (H.E.) and patients were unaware of the specific drugs that the patients were taking during the course of the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The clinical investigator (H.E.) and patients were unaware of the specific drugs that the patients were taking during the course of the study" |
| Selective reporting (reporting bias) | Low risk | 10 patients (4 sucralfate‐treated patients and 6 placebo‐treated patients) failed to complete the study. In all patients, medication use was well tolerated, and no patients were withdrawn from the study because of adverse events. High dropout but reasons and numbers similar across groups |
| Other bias | Low risk | None apparent |
Alpsoy 2002.
| Methods | Study design: RCT (parallel) Trial ID: NS Conducted in: Turkey Number of centres: NS Recruitment period: June 1996 to March 2000 Sample size calculation undertaken and met: NS |
|
| Participants | Source of recruitment: NS Age (overall sample): 32.38 (SD 7.94) Age GrA: 32.82 years (SD 8.17) Age GrB: 31.89 years (7.85) Gender (overall sample): 17 F/27 M Gender GrA: 7 F/16 M Gender GrB: 10 F/11 M Inclusion criteria: Behcet’s disease as defined by the International Study Group for Behcet’s disease Exclusion criteria: those with hepatic, renal, cardiovascular, infectious or other autoimmune disease; those who had received recent systemic therapy for at least 4 weeks; pregnant or lactating women Number randomised (overall and by group): 50 (25:25) Number evaluated (overall and by group): 44 (23:21) |
|
| Interventions |
Comparison: interferon alfa‐2a versus placebo GrA (n = 23): interferon alfa‐2a, 6 × 106 IU, or placebo subcutaneously 3 times a week GrB (n = 21): as for interferon alfa‐2a 3 months treatment; 3 months follow‐up |
|
| Outcomes | Mean frequency and duration of lesions (patient level) Pain (scale of 0 to 3 (0 indicates absent; 1, mild; 2, moderate; and 3, severe)) Overall response Patients were examined clinically at weekly intervals and were followed up for another 3 months after the treatment Adverse events No reporting of quality of life or cost |
|
| Funding | NS | |
| Notes | Comparable groups at baseline: yes Co‐interventions: subjects were given oral acetaminophen, 1000 mg before injections and 500 mg after 6 hours, during the first month of the therapy. Unclear if for both groups Treatment for treatment of Behcet’s disease and not specifically oral ulceration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind" Comment: identical placebo, however, unclear if both groups or just those in treatment group received paracetamol during first month of study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "observed and assessed by an investigator (E.A.) who was blinded to the test medication being used" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/25 in the interferon‐alfa 2a and 4/25 in placebo group not included in analysis |
| Selective reporting (reporting bias) | High risk | Data not fully reported in text |
| Other bias | Unclear risk | Unclear if both groups or just those in treatment group received paracetamol during first month of study, which may affect pain scores |
Davies 1988.
| Methods | Study design: RCT (cross‐over) Trial ID: NS Conducted in: UK Number of centres: NS Recruitment period: NS Sample size calculation undertaken and met |
|
| Participants | Source of recruitment: NS Age (overall sample): mean 43 years (range 18 to 55 years) Gender (overall sample): 15 F/7 M Inclusion criteria: patients with recurrent oral and genital ulceration and other disease features fulfilling the Mason and Barnes diagnostic criteria for Bechet syndrome Exclusion criteria: patients with life‐threatening or severe complications such as active uveitis were excluded Number randomised: 22 Number evaluated: 18 Comorbidities: not stated but "All patients had serum creatinine concentrations within the normal range" |
|
| Interventions |
Comparison: aciclovir versus placebo After 1 month of baseline observations patients were randomly assigned to a 3‐month period of treatment with acyclovir or placebo and after a further 1 month of 'wash‐out' observation, they received 3 months' treatment with acyclovir or placebo GrA (n = 18): active treatment consisted of oral acyclovir 800 mg 5 times daily for 1 week, followed by 400 mg twice daily for 11 weeks GrB (n = 18): matched dummy tablets were used during the period of placebo treatment |
|
| Outcomes | Number and severity of oral (and genital) ulcers were recorded. Severity 0 (no symptoms) to 3 (severe discomfort) (Clinical assessment) ‘Other disease features’ were similarly assessed Frequency (number of new ulcers in each treatment period) and severity of oral and genital ulcers and the pattern of other disease features (patient self‐assessment) Adverse events No reporting of quality of life or cost. |
|
| Funding | "We are grateful to Dr Angela Gilbert and Dr Karen Ditchfield of Burroughs Wellcome for help in devising the trial protocol and for supplying drugs and placebo material" | |
| Notes | Comparable groups at baseline: predominantly female. No difference in disease severity in pretrial 1 month Co‐interventions: other drug therapy "Six patients were receiving systemic prednisolone (mean dose of 6 mg daily, range 1.25‐12.5 mg). One patient were receiving 150 mg azathioprine daily and a second patient 4.8 g aspirin and 400 mg tolmetin sodium (Tolectin) daily. These drug doses were not changed during the trial" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The order in which patients received acyclovir or placebo was randomly determined by computer generated code" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Matched dummy tablets were used during the period of placebo treatment"; "double blind"; matched placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patients were assessed by one clinician (U.M.D.) on a blind basis before and after each phase of the trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/22 patients dropped out in the first phase of treatment. Both were on active therapy. 1 had unacceptably active disease and the other could not swallow the tablets. 2 other patients kept inadequate records. Total dropout 4/22, 18% |
| Selective reporting (reporting bias) | Unclear risk | Frequency of oral ulcers (patient reported 0 not fully reported (missing paired sd). Severity of oral ulcers (patient reported) not reported. Frequency and severity of oral ulcers (clinical assessment) given as P value only (P = 0.45) |
| Other bias | High risk | Other systemic interventions were given, unclear as to which group these participants were in Order effects assessed ‐ cross‐over trial Quote: "As the order in which the patients received acyclovir or placebo did not influence the outcome, the results have been pooled" The order in which patients received treatment or placebo was randomly assigned, however the data or results were not presented separately |
Ergun 1997.
| Methods | Study design: RCT (parallel) Trial ID: NS Conducted in: (country) NS Number of centres: NS Recruitment period: NS Sample size calculation undertaken and met: NS |
|
| Participants | Source of recruitment: NS Age (overall sample): mean 36.5 years (range 22 to 54 years SD 6.5 years) Gender (overall sample): 19 F/5 M Inclusion criteria: Beḩcet's syndrome according to the criteria of the International Study Group (ISG), and having had more than 8 ulcers within the previous 8‐week period Exclusion criteria: patients with eye, joint or visceral involvement, hepatic, renal, hematological disorders, hypertension, pregnancy, or lactation were excluded Number randomised (overall and by group): 24 (12: 12) Number evaluated (overall and by group): 20 (NS:NS) Comorbidities: NS |
|
| Interventions |
Comparison: cyciosporine‐A versus placebo GrA (n = 12): topical cyciosporine‐A 70 mg per g of orabase GrB (n = 12): orabase as a placebo 8‐week treatment period |
|
| Outcomes | Primary outcomes: number, size and healing time Secondary outcomes: side effects or lab abnormalities (4 and 8 weeks) Adverse events No reporting of quality of life or cost |
|
| Funding | NS | |
| Notes | Comparable groups at baseline: predominantly female Co‐interventions: NS |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ‘"Patients were assigned randomly" Comment: method not stated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "orobase as a placebo" Comment: insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Time of assessment: 4/24 did not complete the study. Distribution across the 2 groups not given; no reasons given for non‐failure to complete |
| Selective reporting (reporting bias) | High risk | Incomplete reporting of all outcomes |
| Other bias | Low risk | None apparent |
Fani 2012.
| Methods | Study design: RCT (parallel, 2 arms) Trial ID: IRCT201107036920N2 Conducted in: Iran Number of centres: 1 Recruitment period: NS Sample size calculation undertaken and met: NS |
|
| Participants | Source of recruitment: NS Age (overall sample): range = 15 to 65 years Age GrA: 35.47 years (SD 8.85), range = 15 to 57 years Age GrB: 38.77 years (SD 9.4), range = 17 to 65 years Gender (overall sample): 44 F/16 M Gender GrA: 22 F/8 M Gender GrB: 22 F/8 M Inclusion criteria: first visit; not taking any medication for the disease Exclusion criteria: NS Number randomised (overall and by group): 60 (30:30) Number evaluated (overall and by group): 60 (30:30) Comorbidities: NS |
|
| Interventions |
Comparison: 0.1% triamcinolone acetonide (TA) ointment versus phenytoin syrup GrA (n = 30): TA ointment applied to the lesions 3 times per day (advised not to eat or drink for 30 min after application) GrB (n = 30): 2 teaspoons of phenytoin syrup in half a glass of warm water used as a mouthwash for 4‐5 min 3 times per day (advised not to eat or drink for 30 min after application) Interventions were taken for 1 week |
|
| Outcomes | Positive response (no definition supplied) (outcome recorded at participant level) No reporting of adverse events, quality of life or cost |
|
| Funding | NS | |
| Notes | Comparable groups at baseline: yes Co‐interventions: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly treated" Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly treated" Comment: insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions were different in appearance and delivery. Participants and personnel would be aware of what intervention each participant received |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Interventions were different in appearance and delivery. Participants and personnel would be aware of what intervention each participant received |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | No definition is given for the only outcome 'positive response'. Also, trial protocol states different outcome measures from publication |
| Other bias | Low risk | None apparent |
Hamuryudan 1991.
| Methods | Study design: RCT (parallel, 2 arms) Trial ID: NS Conducted in: Turkey Number of centres: presumed to be 1 (but unclear from the letter) Recruitment period: NS Sample size calculation undertaken and met: NS |
|
| Participants | Source of recruitment: NS Age (overall sample): NS Age GrA: NS Age GrB: NS Gender (overall sample): 37 F/26 M (randomised) Gender GrA: 14 F/16 M (analysed) Gender GrB: 22 F/9 M (analysed) Inclusion criteria: Behcet’s disease patients with active oral ulcers Exclusion criteria: receiving systemic drug therapy Number randomised (overall and by group): 63 (31:32) Number evaluated (overall and by group): 61 (30:31) Comorbidities: NS |
|
| Interventions |
Comparison: interferon‐α‐2c hydrogel versus placebo GrA (n = 31): interferon‐α‐2c hydrogel (1 x 10⁵ U/g) to be applied in a thin layer on any ulcer 3 times per day for 24 weeks. A similar application to upper and lower lip mucosa irrespective of the presence of ulcers GrB (n = 32): placebo hydrogel (same regimen as GrA) All other topical medications were withheld during the study |
|
| Outcomes | Number of ulcers (by examiner every 2 weeks) (ulcer level) Type of ulcers (by examiner every 2 weeks) (ulcer level) Number and type of ulcers occurring and healing between visits (by patient) No reporting of adverse events, quality of life or cost. |
|
| Funding | Boehinger Ingelheim Zentrale, GmbH, Germany supplied the active intervention | |
| Notes | Comparable groups at baseline: no (gender imbalance although not clear if statistically significant) Co‐interventions: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized double blind trial" Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomized double blind trial" Comment: insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind" Comment: no information of taste/appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "double blind" and "seen by the same blind observer every second week" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 dropout per group |
| Selective reporting (reporting bias) | High risk | Separate analysis carried out on gender imbalance but data not presented |
| Other bias | Low risk | None apparent |
Hamuryudan 1998.
| Methods | Study design: RCT (parallel, 3 arms) Trial ID: NS Conducted in: Turkey Number of centres: 1 Recruitment period: October 1993 to April 1996 Sample size calculation undertaken and met: undertaken but not met |
|
| Participants | Source of recruitment: Behcet’s Syndrome Research Center at the University of Istanbul Age GrA: 27.8 years (95% CI 25.9 to 29.6) Age GrB: 27.6 years (95% CI 25.7 to 29.4) Age GrC: 26.7 years (95% CI 24.8 to 28.6) Gender: all males Inclusion criteria: males aged 18 to 35; active mucocutaneous disease (occurrence of at least 2 episodes of oral or genital ulceration within 3 months before the study started) Exclusion criteria: moderate or severe eye disease resulting in any decrease in visual acuity; organ involvement requiring immunosuppressive therapy; previous immunosuppressive therapy; clinical neuropathy Number randomised (overall and by group): 96 (32:32:32) Number evaluated (overall and by group): 95 (32:32:31) Comorbidities: NS |
|
| Interventions |
Comparison: thalidomide (higher dose) versus thalidomide (lower dose) versus placebo GrA (n = 32): 300 mg thalidomide per day (each patient given 3 bottles containing 100 mg tablets and instructed to take 1 tablet from each; 1 in the morning and 2 in the evening) GrB (n = 32): 100 mg thalidomide per day (each patient given 3 bottles (the morning and 1 evening bottle containing identical placebo tablets and the second evening one containing 100 mg tablets) and instructed to take 1 tablet from each; 1 in the morning and 2 in the evening) GrC (n = 32): identical placebo tablets (1 in the morning and 2 in the evening) 24 weeks of treatment, final follow‐up was 4 weeks later (28 weeks). Participants seen every 4 weeks or when they had a problem |
|
| Outcomes | Primary outcomes: number of new lesions (ulcer level); complete response, defined as absence of any oral or genital ulcer of any size during the 24‐week treatment period Secondary outcomes: changes in the number of mucocutaneous lesions, and response of eye disease to treatment, defined as the absence of uveitis activations and any decrease in visual acuity in either eye No reporting of adverse events, quality of life or cost |
|
| Funding | Funding source: Grunenthal GmbH, Aachen, Germany | |
| Notes | Comparable groups at baseline: yes Co‐interventions: patients permitted to use topical lidocaine for pain relief when required |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A simple, computer‐generated, random‐number list was prepared by a person not involved in the trial" |
| Allocation concealment (selection bias) | Low risk | Quote: "The code was kept in an opaque, sealed envelope by the senior author of the study and was opened only after all data had been entered into a computer for analysis" Comment: the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind" Comment: active and placebo tablets were identical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind" Comment: active and placebo tablets were identical |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant was not analysed (due to adverse event); unlikely to influence results |
| Selective reporting (reporting bias) | High risk | Data for oral and genital ulcers not reported separately. Graph of number of oral ulcers over time presented but no raw data |
| Other bias | Low risk | None apparent |
Kilic 2009.
| Methods | Study design: RCT (parallel, 3 arms) Trial ID: NCT00483184 (protocol available on ClinicalTrials.gov) Conducted in: Turkey Number of centres: 4 Recruitment period: 20 months Sample size calculation undertaken and met: yes but not met |
|
| Participants | Source of recruitment: rheumatology or dedicated Behcet’s disease outpatient clinics of 4 medical schools Age (overall sample): 37 (SD 9.4), range = 20 to 57 years Age GrA: 36 (SD 9.4), range = 22 to 56 years Age GrB: 36 (SD 8.6), range = 20 to 50 years Age GrC: 37 (SD 10.2), range = 21 to 57 years Gender (overall sample): 59 F/25 M Gender GrA: 19 F/7 M Gender GrB: 20 F/7 M Gender GrC: 20 F/11 M Inclusion criteria: aged 18 to 75 years; fulfilling Behcet’s disease International Study Group criteria; presence of active oral ulcers within previous year; at least 2 oral ulcers accessible to measurement with a total diameter of ≥ 4 mm Exclusion criteria: having disease features requiring any form of IFN or other immune suppressive medication within 30 days of screening; hypersensitive to IFN‐α; pregnant/lactating/childbearing potential and not using a medically acceptable contraceptive method during the study Number randomised (overall and by group): 84 (26:27:31) Number evaluated (overall and by group): 72 (23:22:27) Comorbidities: none noted |
|
| Interventions |
Comparison: interferon‐α lozenges (2000 IU/day) versus placebo versus interferon‐α lozenges (1000 IU/day) GrA (n = 26): 2 x 500 IU IFN‐α lozenges twice daily for 12 weeks GrB (n = 27): 2 placebo lozenges twice daily for 12 weeks GrC (n = 31): 1 x 500 IU IFN‐α lozenge plus 1 placebo lozenge twice daily for 12 weeks |
|
| Outcomes | Subjects were monitored weekly over an initial 4 weeks of the treatment and then bi‐weekly over an additional 8 weeks of treatment. Oral lesions were counted and measured at each study visit Primary outcomes: percentage change in a pt’s total ulcer burden (from baseline) Secondary outcomes: oral ulcer initial response at weeks 1‐10; time to initial response; oral ulcer sustained response at weeks 3‐10; oral ulcer recurrence at weeks 2‐12; time to recurrence; patient‐reported pain associated with oral lesions; patient‐reported general well‐being; global disease severity (both patient‐ and investigator‐reported); safety assessment/adverse events No reporting of cost |
|
| Funding | Supported by Nobel Ilac San. Ve Tic. A.S., Istanbul, Turkey | |
| Notes | Comparable groups at baseline: yes Co‐interventions: no Treatment for oral ulcers associated with Behcet’s |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using randomly permuted blocks" |
| Allocation concealment (selection bias) | Low risk | Quote: "Using randomly permuted blocks" Comment: also active and placebo lozenges prepared by pharma, and bottling and preparation of randomization boxes was carried out remotely by Dilan Laboratories in Canada |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind" Comment: active and placebo lozenges were identical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind" Comment: active and placebo lozenges were identical |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14% total attrition (A: 12%, B: 19%, C: 13%) with reasons reported in full, which were similar across groups |
| Selective reporting (reporting bias) | High risk | Most outcomes were not presented as stated in methods. Measures of variance not provided |
| Other bias | Low risk | None apparent |
Koc 1992.
| Methods | Study design: RCT parallel Trial ID: NS Conducted in: (country) Turkey Number of centres: 1 Recruitment period: NS Sample size calculation undertaken and met: NS |
|
| Participants | Source of recruitment: patients with oral aphthous ulceration and a diagnosis of Behcets’ attending Hacettepe University Age (overall sample): mean 31 (18‐50) Age GrA: 31.1 years Age GrB: 32.5 years Gender (overall sample) : 17/24 Gender GrA male to female ration 1.4 Gender GrB male to female ratio 0.6 Inclusion criteria: Behcet’s disease diagnosed by ‘international criteria’ no reference given Oral ulcers Not taking immunosuppression or cytotoxic therapy Exclusion criteria: NS Number randomised (overall and by group): 41 Number evaluated (overall and by group): 35 Comorbidities: NS |
|
| Interventions |
Comparison: sucralfate suspension versus placebo GrA (n = 24): sucralfate suspension applied to ulcers 4x day with applicator for up to 12 weeks GrB (n = 11): placebo suspension applied to ulcers 4x day with applicator for up to 12 weeks Following 12 weeks treatment, all interventions stopped and patients followed for 12 weeks ‘no treatment’ |
|
| Outcomes | Number of ulcers (NU): sum of oral ulcers observed in 12‐week period Number of days with oral ulcers: sum of days with oral ulcers in 12‐week period Number of episodes with oral ulcers Mean duration of ulcer episodes Number of painful days Ratio of painful days to days with ulcers Size of oral ulcers No reporting of adverse events, quality of life or costs |
|
| Funding | Research grant from Bilim pharmaceutical company | |
| Notes | Comparable groups at baseline: insufficient information Co‐interventions: patients taking colchicine were allowed to continue – but no allowance for this made in the results (i.e. we do not know how many patients in each group were also taking colchicine) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the study was prospective double blind randomized" Comment: insufficient details |
| Allocation concealment (selection bias) | Unclear risk | No information on randomisation or concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "prepared by the manufacturer and were similar in taste and colour" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details re blinding of clinicians |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 dropouts ‐ all from intervention group, insufficient reasons given |
| Selective reporting (reporting bias) | Unclear risk | Reports all outcomes however it is unclear how many patients are actually making up the results table (i.e. was it 41 then 35 evaluable then 6 dropouts in intervention, or were the dropouts before – either way it means the arms were not balanced) |
| Other bias | Low risk | None apparent |
Masuda 1989.
| Methods | Study design: RCT (parallel) (double masked and double dummy) Trial ID: NS Conducted in: Japan Number of centres: 1 Recruitment period: NS Sample size calculation undertaken and met: NS |
|
| Participants | Source of recruitment: NS Age: NS Gender: NS Inclusion criteria: complete or incomplete Behcet’s disease, visual acuity 20/40 or less, at least 2 episodes of ocular attack during the 16 weeks before study selection Exclusion criteria: renal or hepatic dysfunction, neurological Behcet’s and /or hypertension Number randomised (overall and by group): 96 (47:49) Number evaluated (overall and by group): 92 (46:46) Comorbidities: NS |
|
| Interventions |
Comparison: cyclosporin versus colchicine GrA (n = 47): cyclosporin 10 mg/kg GrB (n = 49): colchicine 1 mg Both given once per day for 16 weeks (if side effects then dose reduced or treatment stopped) |
|
| Outcomes | Primary outcomes: ocular; immunological bloods Secondary outcomes: non‐ocular complications (oral aphthous, dermal and genital ulceration) were classified into 4 grades 0‐3 based on frequency and number of lesions. No information as to what specific oral outcome measures were used, when they were used or whether it was patient‐reported or clinician Adverse events No reporting of quality of life or costs |
|
| Funding | Supported by the Japan Society for Promotion of Science and the National Eye Institute, Bethesda, USA | |
| Notes | Comparable groups at baseline: not stated regarding baseline oral ulcer history Co‐interventions: not stated if any topical measures used in addition Treatment of ocular disease associated with Behcet’s |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized" Comment: no information given |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double masked and double dummy" Comment: however no description of appearance/taste of interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/97 dropouts; equally balanced |
| Selective reporting (reporting bias) | High risk | Outcome data not fully reported |
| Other bias | Unclear risk | Not stated if any topical measures used in addition |
Mat 2006.
| Methods | Study design: RCT parallel Trial ID: NS Conducted in: Turkey Number of centres: 1 Recruitment period: February 2001 to March 2002 Sample size calculation undertaken and met: yes, but not met due to dropouts |
|
| Participants | Source of recruitment: multidisciplinary Behcet's Syndrome outpatient clinic at the Cerrahpasa Medical Facility Istanbul Age GrA: 31.7 years ± 7 Age GrB: 29.4 years ± 6 Gender (overall sample): 43F/ 43M Gender GrA: 21F/ 21M Gender GrB: 22F/ 22M Inclusion criteria: 18 to 45, active genital disease, live in Istanbul Exclusion criteria: previous immunosuppression in last month, previous steroids greater than 5 mg/day, severe organ involvement, or eye disease, or DM, active infection, peptic ulcer, hypertension, pregnancy Number randomised (overall and by group): 86 (42:44) Number evaluated (overall and by group): 72 (34:38) Comorbidities: patients continued their colchicine, low dose aspirin, amitriptyline, acetaminophen. Topical treatment as well as additional systemic drugs such as thalidomide were also permitted for oral and genital lesions. Only systemic immunosuppressives were withheld |
|
| Interventions |
Comparison: corticosteroid injections versus placebo GrA (n = 42): depot corticosteroid intramuscular injections (40 mg methylepred) GrB (n = 44): depot injection as above but with 1 ml saline Injections given every 3 weeks for 27 weeks |
|
| Outcomes | Primary outcomes: difference in the mean number of genital ulcers Secondary outcomes: difference in the mean numbers of other mucocutaneous lesions and attacks of arthritis reported as mean number of lesions Adverse events No reporting of quality of life or costs |
|
| Funding | Supported by association for the rheumatology section at Cerrahpsa Medical Facility | |
| Notes | Comparable groups at baseline: no detailed baseline ulcer information given Co‐interventions: 4 in each arm received colchicine, 3 in each arm amitryptilne, 9 in each arm NSAIDS, 1 in each arm low dose aspirin and 1 in each arm thalidomide |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "random number generated by a computer" Comment: simple randomisation has resulted in equally balanced groups according to gender and additional medication |
| Allocation concealment (selection bias) | Low risk | Quote: "a study nurse, not involved in data collection, kept the randomization list and injected the drug or placebo….the randomization code was not opened until the data had been entered" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "syringe covered with a label to conceal the milky solution" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the physicians involved in patient assessment were blinded to the treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Presents attrition data but no explanation as to why there were "lost to follow‐ups" |
| Selective reporting (reporting bias) | High risk | Patients receiving additional therapies not presented separately but as part of the arms Results also presented male/female and this was not a pre‐specified outcome |
| Other bias | Low risk | None apparent |
Matsuda 2003.
| Methods | Study design: RCT (parallel) Trial ID: NS Conducted in: Japan Number of centres: 6 Recruitment period: August 1994 to December 1996 Sample size calculation undertaken and met: NS |
|
| Participants | Source of recruitment: various clinics Age: NS Age GrA: NS Age GrB: NS Gender (overall sample): NS Gender GrA: NS Gender GrB: NS Inclusion criteria: Behcet’s disease diagnosed using the Japanese criteria (reference given) presence of aphthae for more than 7 days in the month before the study Exclusion criteria: use of any other drugs to treat gastric ulcer or gastritis Number randomised (overall and by group): 35 Number evaluated (overall and by group): 31 Comorbidities: NS |
|
| Interventions |
Comparison: rebamipide versus placebo GrA (n = 19): rebamipide 300 mg tablet to be taken 3x day after meals GrB (n = 16): placebo tablet to be taken 3x day after meals Treatment period: 6 months |
|
| Outcomes | Primary outcomes: aphthae count (patient reported on daily basis); pain score (patient reported on daily basis); monthly aphthae count; total monthly pain scores; global evaluation of above rated by investigator at the end of the study on 6‐point scale Secondary outcomes: monthly bloods Adverse events No reporting of quality of life or costs |
|
| Funding | Pharma funded (Otsuka pharmaceutical company) | |
| Notes | Comparable groups at baseline: states yes Co‐interventions: yes, patients remained on existing treatment including immunosuppression and steroids |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "the study controller created a random allocation list" and "patients were randomly assigned" Comment: insufficient evidence of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "placebo tablets which were 'indistinguishable from the active" Comment: no other details given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 from each group and reasons stated |
| Selective reporting (reporting bias) | High risk | Does not report the individual outcome measures – groups them together in the observer global score (subjective?) |
| Other bias | Low risk | None apparent |
Melikoglu 2005.
| Methods | Study design: RCT parallel Trial ID: NS Conducted in: Turkey Number of centres: 1 Recruitment period: NS Sample size calculation undertaken and met: NS |
|
| Participants | Source of recruitment: Behcet’s syndrome research centre Cerrahpsa Medical Faculty, Istanbul Age GrA: 28.5 years ±5.3 Age GrB: 30.8 years ±6.2 Gender: all male Inclusion criteria: males, age 18 to 45, presence of at least 1 of a list of criteria including oral ulcers, genital ulcers, nodular lesions, swollen joints, positive pathergy and MSU test Exclusion criteria: serious organ involvement e.g. eye and cns and major arterial disease, systemic or local infection in yb, previous use of study drug 4 weeks prior, abnormal bloods (specifically stated) Number randomised (overall and by group): 40 (20:20) Number evaluated (overall and by group): 38 (19:19) Comorbidities: NS |
|
| Interventions |
Comparison: etanercept versus placebo GrA (n = 19): etanercept 25 mg dissolved in 1 ml distilled water subcutaneous twice weekly GrB (n = 19): placebo 25 mg vial dissolved in 1 ml distilled water and delivered as above. |
|
| Outcomes | Primary outcomes: amount of suppression of pathergy and MSU tests Secondary outcomes: differences between the mean number of mucocutaneous lesions and swollen joints between the study arms at each weekly visit; mean number of oral ulcers Adverse events No reporting of quality of life or costs |
|
| Funding | NS | |
| Notes | Comparable groups at baseline: yes all baseline data presented Co‐interventions: additional drugs used prednisolone, indomethacin, paracetamol, ornidazole, azathioprine, naproxen, topoca cs Treatment for Behcet’s disease |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "equal number of cards mixed drawn and placed sequentially on a list" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "code was opened only after data had been entered into computer" Comment: states that vials were distributed to patients by a study nurse who was not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "characteristics of the study drug and placebo were identical and the clinical assessors and patients were blinded to the preparation being administered" Comment: unclear how robust blinding mechanisms was as one patient withdrew following discovery they were on placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear how robust blinding mechanisms was as one patient withdrew following discovery they were on placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/40 dropouts, equally distributed |
| Selective reporting (reporting bias) | High risk | Includes results for all patients even those on additional treatments. States "separate analysis done" "our findings remain robust" "agree" but separate analysis not presented |
| Other bias | Low risk | None apparent |
Yurdakul 2001.
| Methods | Study design: RCT, double‐blind, placebo‐controlled study Trial ID: NS Conducted in: Turkey Number of centres: 1 Recruitment period: "Consecutive patients attending the center were recruited into the study between November 1991 and December 1995. The recruitment period was 24 months" Sample size calculation undertaken and met: NS |
|
| Participants | Source of recruitment: '"multidisciplinary Beḩcet's Syndrome Research Center at the Cerrahpasa Medical School" Inclusion criteria: patients fulfilled the criteria for the diagnosis of Beḩçet's syndrome and be consecutive patients (male or female); be 18‐35 years of age; have active disease; have a disease duration of 2 years; and live at reasonable travelling distance from centre Active disease was defined as the minimum presence of oral or genital ulceration or erythema nodosum occurring at least 3 times within the preceding 6 months Exclusion criteria: patients who had received immunosuppressive agents, steroids or colchicine within the preceding 6 months; had organ involvement requiring immunosuppression; had eye disease, especially with retinal involvement during the recruitment period Number randomised: 116 (58:58) Number evaluated monthly: 84 by study end |
|
| Interventions |
Comparison: colchicine versus placebo GrA (n = 58): 0.5 mg colchicine tablets. Dose adjusted per weight in kg GrB (n = 58): placebo tablets that were identical to the active drug in appearance and taste |
|
| Outcomes | Primary outcomes: oral ulceration; genital ulcers; erythema nodosum; follicular lesions; arthritis Secondary outcomes: adverse events No reporting of quality of life or costs |
|
| Funding | NS | |
| Notes | Comparable groups at baseline: yes Co‐interventions: placebo group given various additional treatments thalidomide, pulsed methylpred, systemic prednisolone and NSAID; "the patients were permitted to use treatment for oral and genital ulceration and acetaminophen or NSAID for joint disease, if needed" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "equal numbers of cards that were assigned to either the active drug or the placebo arm were mixed, drawn, and placed sequentially on a list by a secretary not involved in running the trial" |
| Allocation concealment (selection bias) | Low risk | Quote: "The code was kept in a sealed envelope by one of the authors (HY) and was opened only after all data had been entered into the computer for analysis. The allocation to the study and the dispensing of the medications were done by a research assistant" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "At each visit, the patients received a bottle containing either 0.5 mg colchicine or placebo tablets that were identical to the active drug in appearance and taste" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All participating physicians were blinded to the patients’ allocation to the study arms" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotes: "One hundred twenty consecutive patients were eligible for the study. Four women declined to participate" and "Eighty‐four patients (72%; 45 male, 39 female) completed the 24‐month study (Figure 1)" Dropouts equal in both sides; reasons given for dropouts 2 year trial – not unreasonable dropout considering length of trial |
| Selective reporting (reporting bias) | Low risk | Author provided addition raw ulcer data |
| Other bias | Low risk | None apparent |
bd = twice per day; CI = confidence interval; min = minute; NS = not specified; RAS = recurrent aphthous stomatitis; RCT = randomised controlled trial; SD = standard deviation; tds = 3 times per day.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 1998 | Not RCT |
| Azizlerli 1995 | Although mentions placebo 'chosen at random' it is unclear how active intervention arms were allocated |
| Bacanli 2006 | Not RCT |
| Ben Slama 2002 | Not RCT |
| Calguneri 1996 | Results presented for whole study not clear at which point the final outcomes were measured i.e. not at point that randomisation stopped (this paper does not present an RCT, it presents RCT+ follow on open study combined) |
| Chams‐Davatchi 2010 | Topical application – genital ulcers only |
| Convit 1984 | Letter (possibly linked to Convit 1972) |
| Davatchi 2009 | Cross‐over design (4 months each phase, active colchine) but no washout period stated |
| Lee 2008 | Open label descriptive study comparing BDRAS versus RAS ‐ no control |
| Nanke 2008 | Not RCT |
| NCT01211977 | No oral outcomes |
| NCT01693640 | Not RCT |
| NCT01693653 | Trial terminated |
| O'Duffy 1998 | Not RCT |
| Pizarro 2000 | Not RCT |
| Scheinberg 2002 | Letter |
| Sharquie 2002 | No washout period |
| Sharquie 2013 | Insufficient washout demonstrated; no oral ulcer outcome measures |
| Sun 2009 | Not RCT |
| Yazici 1990 | Primary aim ocular manifestations not oral; reject no prespecified oral outcome measures (oral outcomes not described in methods). No trial register registered to check |
RAS = recurrent aphthous stomatitis; RCT = randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00866359.
| Methods | A phase 2, multicenter, randomised, double‐blind, placebo‐controlled, parallel‐group study |
| Participants | 18 years or older Diagnosis of Behçet disease. At the time of diagnosis, subjects must meet the international study group criteria for Behçet Disease Females of childbearing potential (FCBP) must have negative pregnancy tests and agree to use 2 forms of contraception throughout the study Males must use barrier contraception (latex condoms) when engaging in reproductive sexual activity with FCBP Laboratory criteria: Hgb ≥ 9 g/dL, WBC count ≥ 3000/μL and ≤14,000/μL, platelet count ≥ 100,000/μL, serum creatinine ≤ 1.5 mg/dL (≤ 132.6 μmol/L), total bilirubin ≤ 2.0 mg/dL, AST and ALT ≤ 1.5 X ULN 2 or more oral ulcers over the 28 day period before screening, with or without current treatment 2 or more oral ulcers at the time of randomisation (Visit 2, Baseline) |
| Interventions | Apremilast (CC‐10004) Treatment phase Days 1‐7: titration from 10 mg BID apremilast tablets to 30 mg BID apremilast Day 8‐84: maintenance of 30 mg BID apremilast. Dose reductions to 20 mg BID apremilast permitted Extension phase: all subjects will be given active drug Days 85‐91 Day 92‐169: maintenance of 30 mg BID apremilast or dose reductions to 20 mg BID apremilast Placebo |
| Outcomes | Primary outcome measures: the change in the number of oral ulcers from baseline to Day 85; early termination will be compared between the apremilast and the placebo treatment groups Secondary outcome measures: pain of oral/genital ulcers; number of ulcer‐free subjects and those with ≥ 50% reduction; Behcet’s disease Current Activity Form score; safety (ECG, AE, Labs); new Behcet’s disease manifestations PRO questionnaires |
| Notes | Data taken from clinicaltrials.gov/show/NCT00866359 Abstract published 2013; awaiting full paper |
Characteristics of ongoing studies [ordered by study ID]
NCT01441076.
| Trial name or title | A pilot study of anakinra in Behcet's disease (BD) |
| Methods | Double‐blind randomised controlled trial |
| Participants | People who have active Behcet's disease, with an oral or genital ulcer within the past month, or 3 or more flares of eye disease in the past 6 months |
| Interventions | Anakinra 100 mg subcutaneously daily with dose escalation up to 200 mg daily for 3 months versus placebo |
| Outcomes | Primary outcome: assess the time to disease flare in patients randomised to continuation of anakinra versus withdrawal in Behcet's disease for 6 months |
| Starting date | September 2011 |
| Contact information | clinicaltrials.gov/show/NCT01441076 |
| Notes | Ongoing but not recruiting |
Differences between protocol and review
The order of authors has been altered to reflect degree of contribution, however, all authors listed on the protocol remain authors on the full review and all have provided input into its production.
Following peer review the wording around the definition of recurrent aphthous stomatitis (RAS) and RAS‐type ulceration has been clarified. This is an amendment to the protocol.
Contributions of authors
Development of protocol based on the latest Cochrane guidance: Jennifer Taylor (JT), Philip Riley (PR), Paul Brocklehurst (PB), Mike Pemberton (MP), Anne‐Marie Glenny (AMG), Tanya Walsh (TW), and Rachel Gorodkin (RG).
Identification of studies: JT, AMG. Data extraction: JT, PB, AMG, TW, PR. Assessment of risk of bias: JT, PB, AMG, TW, PR. Data input and synthesis: JT, AMG, TW, PB, PR. Writing of initial report: JT, AMG, PB, TW, MP, AMG, PR, RG. Addressing feedback following peer review: JT, AMG.
Sources of support
Internal sources
-
MAHSC, UK.
Cochrane Oral Health is supported by the Manchester Academic Health Sciences Centre (MAHSC) and the NIHR Manchester Biomedical Research Centre.
The University of Manchester, UK.
External sources
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; and the Swiss Society for Endodontology, Switzerland.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed are those of the authors and not necessarily those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Declarations of interest
There are no financial conflicts of interest and the review authors declare that they do not have any associations with any parties who may have vested interests in the results of this review.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Aktulga 1980 {published data only}
- Aktulga E, Altaç M, Müftüoglu A, Ozyazgan Y, Pazarli H, Tüzün Y, et al. A double blind study of colchicine in Behçet's disease. Haematologica 1980;65(3):399‐402. [PubMed] [Google Scholar]
Alpsoy 1999 {published data only}
- Alpsoy E, Er H, Durusoy C, Yilmaz E. The use of sucralfate suspension in the treatment of oral and genital ulceration of Behcet disease: a randomized, placebo‐controlled, double‐blind study. Archives of Dermatology 1999;135(5):529‐32. [DOI] [PubMed] [Google Scholar]
Alpsoy 2002 {published data only}
- Alpsoy E, Durusoy C, Yilmaz E, Ozgurel Y, Ermis O, Yazar S, et al. Interferon alfa‐2a in the treatment of Behcet disease: a randomized placebo‐controlled and double‐blind study. Archives of Dermatology 2002;138(4):467‐71. [DOI] [PubMed] [Google Scholar]
Davies 1988 {published data only}
- Davies UM, Palmer RG, Denman AM. Treatment with acyclovir does not affect orogenital ulcers in Behcet's syndrome: a randomized double‐blind trial. British Journal of Rheumatology 1988;27(4):300‐2. [DOI] [PubMed] [Google Scholar]
Ergun 1997 {published data only}
- Ergun T, Gurbuz O, Yurdakul S, Hamuryudan V, Bekiroglu N, Yazici H, et al. Topical cyclosporine‐A for treatment of oral ulcers of Behcet's syndrome [letter]. International Journal of Dermatology 1997;36(9):720. [DOI] [PubMed] [Google Scholar]
Fani 2012 {published data only}
- Fani MM, Ebrahimi H, Pourshahidi S, Aflaki E, Shafiee Sarvestani S. Comparing the effect of phenytoin syrup and triamcinolone acetonide ointment on aphthous ulcers in patients with Behcet's syndrome. Iranian Red Crescent Medical Journal 2012;14(2):74‐7. [PMC free article] [PubMed] [Google Scholar]
Hamuryudan 1991 {published data only}
- Hamuryudan V, Yurdakul S, Rosenkaimer F, Yazici H. Inefficacy of topical alpha interferon in the treatment of oral ulcers of Behcet's syndrome: a randomized, double blind trial. British Journal of Rheumatology 1991;30(5):395‐6. [DOI] [PubMed] [Google Scholar]
Hamuryudan 1998 {published data only}
- Hamuryudan V, Mat C, Saip S, Ozyazgan Y, Siva A, Yurdakul S, et al. Thalidomide in the treatment of the mucocutaneous lesions of the Behcet syndrome. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1998;6:443‐50. [DOI] [PubMed] [Google Scholar]
Kilic 2009 {published data only}
- Kilic H, Zeytin HE, Korkmaz C, Mat C, Gul A, Cosan F, et al. Low‐dose natural human interferon‐alpha lozenges in the treatment of Behcet's syndrome. Rheumatology 2009;48(11):1388‐91. [DOI] [PubMed] [Google Scholar]
Koc 1992 {published data only}
- Koc Y, Akpek G, Akpolat T, Gullu I, Kansu E, Kiraz S, et al. Topical sucralfate therapy for oral ulcers in Behcet's disease: a randomized double‐blind study. Journal of Dermatological Treatment 1992;3(4):197‐9. [Google Scholar]
Masuda 1989 {published data only}
- Masuda K, Nakajima A, Urayama A, Nakae K, Kogure M, Inaba G. Ciclosporin treatment for Behcet's disease ‐ double‐masked group comparative study. Rinsho Hyoka 1989;14:437‐61. [Google Scholar]
- Masuda K, Nakajima A, Urayama A, Nakae K, Kogure M, Inaba G. Double‐masked trial of cyclosporin versus colchicine and long‐term open study of cyclosporin in Behcet's disease. Lancet 1989;1(8647):1093‐6. [DOI] [PubMed] [Google Scholar]
Mat 2006 {published data only}
- Mat C, Yurdakul S, Uysal S, Gogus F, Ozyazgan Y, Uysal O, et al. A double‐blind trial of depot corticosteroids in Behçet's syndrome. Rheumatology (Oxford, England) 2006;45(3):348‐52. [DOI] [PubMed] [Google Scholar]
Matsuda 2003 {published data only}
- Matsuda T, Ohno S, Hirohata S, Miyanaga Y, Ujihara H, Inaba G, et al. Efficacy of rebamipide as adjunctive therapy in the treatment of recurrent oral aphthous ulcers in patients with Behcet's disease: A randomised, double‐blind, placebo‐controlled study. Drugs in R&D 2003;4(1):19‐28. [DOI] [PubMed] [Google Scholar]
Melikoglu 2005 {published data only}
- Melikoglu M, Fresko I, Mat C, Ozyazgan Y, Gogus F, Yurdakul S, et al. Short‐term trial of etanercept in Behcet's disease: a double blind, placebo controlled study. Journal of Rheumatology 2005;32(1):98‐105. [PubMed] [Google Scholar]
Yurdakul 2001 {published and unpublished data}
- Yurdakul S, Mat C, Tüzün Y, Ozyazgan Y, Hamuryudan V, Uysal O, et al. A double‐blind trial of colchicine in Behçet's syndrome. Arthritis and Rheumatism 2001;44(11):2686‐92. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anonymous 1998 {published data only}
- Anonymous. Thalidomide: new preparation, for well‐defined indications. Prescrire International 1988;7(37):131‐4. [PubMed] [Google Scholar]
Azizlerli 1995 {published data only}
- Azizlerli G, Sarica R, Kose AA, Ovul C, Erkan F, Singer R, et al. Results of acyclovir treatment in Behcet's disease. Nouvelles Dermatologiques 1995;14(7):428‐31. [Google Scholar]
Bacanli 2006 {published data only}
- Bacanli A, Yerebakan Dicle O, Parmaksizoglu B, Yilmaz E, Alpsoy E. Topical granulocyte colony‐stimulating factor for the treatment of oral and genital ulcers of patients with Behcet's disease. Journal of the European Academy of Dermatology and Venereology 2006;20(8):931‐5. [DOI] [PubMed] [Google Scholar]
Ben Slama 2002 {published data only}
- Ben Slama L, Djemil M. [Colchicine]. Revue de Stomatologie et de Chirurgie Maxillo‐faciale 2002;103(2):128‐9. [PubMed] [Google Scholar]
Calguneri 1996 {published data only}
- Calguneri M, Ertenli I, Kiraz S, Erman M, Celik I. Effect of prophylactic benzathine penicillin on mucocutaneous symptoms of Behcet's disease. Dermatology (Basel, Switzerland) 1996;192(2):125‐8. [DOI] [PubMed] [Google Scholar]
Chams‐Davatchi 2010 {published data only}
- Chams‐Davatchi C, Barikbin B, Shahram F, Nadji A, Moghaddassi M, Yousefi M, et al. Pimecrolimus versus placebo in genital aphthous ulcers of Behcet's disease: a randomized double‐blind controlled trial. International Journal of Rheumatic Diseases 2010;13(3):253‐8. [DOI] [PubMed] [Google Scholar]
Convit 1984 {published data only}
- Convit J, Goihman‐Yahr M, Rondon‐Lugo AJ. Effectiveness of dapsone in Behcet's disease. British Journal of Dermatology 1984;111(5):629‐30. [DOI] [PubMed] [Google Scholar]
Davatchi 2009 {published data only}
- Davatchi F, Sadeghi Abdollahi B, Tehrani Banihashemi A, Shahram F, Nadji A, Shams H, et al. Colchicine versus placebo in Behcet's disease: randomized, double‐blind, controlled crossover trial. Modern Rheumatology / the Japan Rheumatism Association 2009;19(5):542‐9. [DOI] [PubMed] [Google Scholar]
Lee 2008 {published data only}
- Lee JH, Jung JY, Bang D. Hyaluronic acid gel on recurrent oral ulcers: comparison between recurrent aphthous ulcers and the oral ulcers of Behcet's disease. Journal of the European Academy of Dermatology and Venereology 2008;22(5):590‐5. [DOI] [PubMed] [Google Scholar]
Nanke 2008 {published data only}
- Nanke Y, Kamatani N, Okamoto T, Ogiuchi H, Kotake S. Irsogladine is effective for recurrent oral ulcers in patients with Behcet's disease: an open‐label, single‐centre study. Drugs in R&D 2008;9(6):455‐9. [DOI] [PubMed] [Google Scholar]
NCT01211977 {published data only}
- NCT01211977. A Pilot Study of XOMA 052 in Familial Cold Autoinflammatory Syndrome (FCAS) / Muckle‐Wells Syndrome (MWS) and Behcet's Disease (BD). http://clinicaltrials.gov/show/NCT01211977.
NCT01693640 {published data only}
- NCT01693640. A Pilot Study of the Safety and Efficacy of Abatacept Injections in the Treatment of Mucocutaneous Manifestations of Behcet's Syndrome. http://clinicaltrials.gov/show/NCT01693640. [Google Scholar]
NCT01693653 {published data only}
- NCT01693653. Tocilizumab for the Treatment of Behcet's Syndrome. http://clinicaltrials.gov/show/NCT01693653.
O'Duffy 1998 {published data only}
- O'Duffy JD, Calamia K, Cohen S, Goronzy JJ, Herman D, Jorizzo J, et al. Interferon‐alpha treatment of Behcet's disease. Journal of Rheumatology 1998;25(10):1938‐44. [PubMed] [Google Scholar]
Pizarro 2000 {published data only}
- Pizarro A, Herranz P, Garcia‐Tobaruelaa A, Casado M. [Pentoxifylline in the treatment of orogenital aphthosis and Behcet's syndrome]. Medicina Clinica 115(17):678. [DOI] [PubMed] [Google Scholar]
Scheinberg 2002 {published data only}
- Scheinberg MA. Treatment of recurrent oral aphthous ulcers with etanercept. Clinical and Experimental Rheumatology 2002;20(5):733‐4. [PubMed] [Google Scholar]
Sharquie 2002 {published data only}
- Sharquie KE, Najim RA, Abu‐Raghif AR. Dapsone in Behçet's disease: a double‐blind, placebo‐controlled, cross‐over study. Journal of Dermatology 2002;29(5):267‐79. [DOI] [PubMed] [Google Scholar]
Sharquie 2013 {published data only}
- Sharquie KE, Helmi RM, Noiami AA, Al‐Hayani RK, Kadhom MA. The therapeutic role of isotretinoin in the management of Behçet's disease: a single‐blinded, controlled therapeutic study. Journal of Drugs in Dermatology 2013;12(4):68‐73. [PubMed] [Google Scholar]
Sun 2009 {published data only}
- Sun A, Wang YP, Chia JS, Liu BY, Chiang CP. Treatment with levamisole and colchicine can result in a significant reduction of IL‐6, IL‐8 or TNF‐alpha level in patients with mucocutaneous type of Behcet's disease. Journal of Oral Pathology & Medicine 2009;38(5):401‐5. [DOI] [PubMed] [Google Scholar]
Yazici 1990 {published data only}
- Yazici H, Pazarli H, Barnes CG, Tuzun Y, Ozyazgan Y, Silman A, et al. A controlled trial of azathioprine in Behçet's syndrome. The New England Journal of Medicine 1990;322(5):281‐5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00866359 {published data only}
- NCT00866359. A Phase 2, Multi‐center, Randomized, Double‐blind, Placebo‐controlled, Parallel‐group Study Followed by an Active‐Treatment Extension to Evaluate the Efficacy and Safety of Apremilast(CC‐10004) in the Treatment of Behçet Disease. http://clinicaltrials.gov/show/NCT00866359.
References to ongoing studies
NCT01441076 {published data only}
- NCT01441076. A Pilot Study of Anakinra in Behcet's Disease (BD). http://clinicaltrials.gov/show/NCT01441076.
Additional references
Baccaglini 2011
- Baccaglini L, Lalla RV, Bruce AJ, Sartori‐Valinotti JC, Latortue MC, Carrozzo M, et al. Urban legends: recurrent aphthous stomatitis. Oral Diseases 2011;17(8):755‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baccaglini 2013
- Baccaglini L. There is limited evidence that topical lavender oil is effective for palliative treatment of recurrent aphthous stomatitis. The Journal of Evidence‐Based Dental Practice 2013;13(2):47‐9. [DOI] [PubMed] [Google Scholar]
Bagan 1991
- Bagan JV, Sanchis JM, Milián MA, Peñarrocha M, Silvestre FJ. Recurrent aphthous stomatitis. A study of the clinical characteristics of lesions in 93 cases. Journal of Oral Pathology & Medicine 1991;20(8):395‐7. [DOI] [PubMed] [Google Scholar]
Benamour 1991
- Benamour S, Bennis R, Messoudi M, Zaoui A, Amor B. Behcet's disease and desensitization by autologous saliva. Revue de Medecine Interne 1991;12(5):339‐42. [DOI] [PubMed] [Google Scholar]
Brocklehurst 2012
- Brocklehurst P, Tickle M, Glenny AM, Lewis MA, Pemberton MN, Taylor J, et al. Systemic interventions for recurrent aphthous stomatitis (mouth ulcers). Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD005411.pub2] [DOI] [PubMed] [Google Scholar]
Chams‐Davatchi 2010
- Chams‐Davatchi C, Barikbin B, Shahram F, Nadji A, Moghaddassi M, Yousefi M, et al. Pimecrolimus versus placebo in genital aphthous ulcers of Behcet's disease: a randomized double‐blind controlled trial. International Journal of Rheumatic Diseases 2010;13(3):253‐8. [DOI] [PubMed] [Google Scholar]
Dalvi 2012
- Dalvi SR, Yildirim R, Yazici Y. Behçet's Syndrome. Drugs 2012;72(17):2223‐41. [DOI] [PubMed] [Google Scholar]
Davatchi 2004
- Davatchi F. New and innovative therapies for Behçet's disease. APLAR Journal of Rheumatology 2004;7(2):141‐5. [Google Scholar]
Davatchi 2012
- Davatchi. Diagnosis/Classification Criteria for Behcet's Disease. Pathology Research International 2012;2012:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisen 2001
- Eisen D, Lynch DP. Selecting topical and systemic agents for recurrent aphthous stomatitis. Cutis 2001;68:201‐6. [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- GRADEpro. Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2008.
Gul 2007
- Gul A. Standard and novel therapeutic approaches to Behcet's disease. Drugs 2007;67(14):2013‐22. [DOI] [PubMed] [Google Scholar]
Hamuryudan 1998
- Hamuryudan V, Mat C, Saip S, Ozyazgan Y, Siva A, Yurdakul S, et al. Thalidomide in the treatment of the mucocutaneous lesions of the Behçet syndrome. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1998;128(6):443‐50. [DOI] [PubMed] [Google Scholar]
Hatemi, 2008
- Hatemi G, Silman A. EULAR recommendations for the management of Behcet's disease. Annals of the Rheumatic Diseases 2008;67:1656‐62. [DOI] [PubMed] [Google Scholar]
Hatemi, 2014
- Hatemi G, Merkel PA, Hamuryudan V, Boers M, Direskeneli H, Aydin SZ, Yazici H. Outcome measures used in clinical trials for Behcet's syndrome: a systematic review. Journal of Rheumatology 2014;41(3):599‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ISG 1990
- International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s disease. Lancet 1990;335:1078. [PubMed] [Google Scholar]
Jurge 2006
- Jurge S, Kuffer R, Scully C, Porter SR. Mucosal disease series. Number VI. Recurrent aphthous stomatitis. Oral Diseases 2006;12(1):1‐21. [DOI] [PubMed] [Google Scholar]
Kose 2012
- Kose O. Development of immunopathogenesis strategies to treat Behçet's disease. Pathology Research International 2012;Article ID: 261989:7 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mat 2013
- Mat C, Yurdakul S, Sevim A, Ozyazgan Y, Tuzun Y. Behcet's syndrome: facts and controversies. Clinics in Dermatology 2013;31(4):352‐61. [DOI] [PubMed] [Google Scholar]
Melikoglu 2005
- Melikoglu M, Fresko I, Mat C, Ozyazgan Y, Gogus F, Yurdakul S, et al. Short‐term trial of etanercept in Behçet's disease: a double blind, placebo controlled study. Journal of Rheumatology 2005;32(1):98‐105. [PubMed] [Google Scholar]
Porter 1998
- Porter SR, Scully C, Pederson A. Recurrent aphthous stomatitis. Current Reviews in Oral Biology and Medicine 1998;9(3):306‐21. [DOI] [PubMed] [Google Scholar]
Review Manager 2012
- Review Manager (RevMan) [Computer program]. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Riordain 2011
- Riordain RN, Meaney S, McCreary C. Impact of chronic oral mucosal disease on daily life: preliminary observations from a qualitative study. Oral Diseases 2011;17(3):265‐9. [DOI] [PubMed] [Google Scholar]
Saenz 1998
- Saenz A, Ausejo M, Shea B, Wells GA, Welch V, Tugwell P. Pharmacotherapy for Behcet's syndrome. Cochrane Database of Systematic Reviews 1998, (Issue 2). [DOI: 10.1002/14651858.; CD001084] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scully 2006
- Scully C. Clinical practice. Aphthous ulceration. The New England Journal of Medicine 2006;355(2):165‐72. [DOI] [PubMed] [Google Scholar]
Taylor 2013
- Taylor J, Brocklehurst P, Glenny AM, Walsh T, Tickle M, Lewis MA, et al. Topical interventions for recurrent aphthous stomatitis (mouth ulcers). Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD010881] [DOI] [PubMed] [Google Scholar]
Thornhill 2007
- Thornhill MH, Baccaglini L, Theaker E, Pemberton MN. A randomized, double‐blind, placebo‐controlled trial of pentoxifylline for the treatment of recurrent aphthous stomatitis. Archives of Dermatology 2007;143(4):463‐70. [DOI] [PubMed] [Google Scholar]
Yazici 1980
- Yazici H, Chamberlain MA, Schreuder I, D'Amaro J, Muftuoglu M. HLA antigens in Behçet's disease: a reappraisal by a comparative study of Turkish and British patients. Annals of the Rheumatic Diseases 1980;39(4):344‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yazici 1984
- Yazici H, Tüzün Y, Pazarli H, Yurdakul S, Ozyazgan Y, Ozdoğan H, et al. Influence of age of onset and patient's sex on the prevalence and severity of manifestations of Behçet's syndrome. Annals of the Rheumatic Diseases 1984;43(6):783‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yazici 2012
- Yazici H, Ugurlu S, Seyahi E. Behçet syndrome: is it one condition?. Clinical Reviews in Allergy and Immunology 2012;43(3):275‐80. [DOI] [PubMed] [Google Scholar]
Yurdakul 2001
- Yurdakul S, Mat C, Tüzün Y, Ozyazgan Y, Hamuryudan V, Uysal O, et al. A double‐blind trial of colchicine in Behçet's syndrome. Arthritis and Rheumatism 2001;44(11):2686‐92. [DOI] [PubMed] [Google Scholar]
Yurdakul 2008
- Yurdakul S, Yazici H. Behçet’s syndrome. Best Practice and Research. Clinical Rheumatology 2008;22(5):793‐809. [DOI] [PubMed] [Google Scholar]
Yurdakul 2010
- Yurdakul S, Yazici Y. Epidemiology of Behçet's syndrome and regional differences in disease expression. In: Yurdakul S, Yazici Y editor(s). Behçet's Syndrome. 1st Edition. New York: Springer, 2010:35‐52. [Google Scholar]


