Despite efforts to improve treatment of refractory cardiogenic shock (rCS), prognosis remains poor. Multidisciplinary Shock Teams have been proposed as a strategy to streamline care delivery and improve outcomes despite the lack of strong evidence1-5. This study sought to determine the feasibility and efficacy of the Shock Team approach for rCS at our tertiary care institution.
The Utah Cardiac Recovery Shock Team was established in April 2015 to evaluate patients in acute CS with a standardized comprehensive multidisciplinary assessment. Between April 2015 - August 2018, we prospectively identified 123 consecutive patients with rCS, treated with MCS, using the Team approach. We compared this cohort with the immediately preceding 121 rCS patients, treated with MCS, but without Shock Team evaluation (Control cohort) in a retrospective fashion. Post-cardiotomy patients and those requiring central extracorporeal membrane oxygenation (ECMO) were excluded. The study was approved by the University of Utah’s Institutional Review Board and participants gave written informed consent.
The Shock Team comprises a heart failure (HF) cardiologist, a HF cardiothoracic surgeon, an interventional cardiologist, and a Cardiovascular Intensive Care Unit (CVICU) attending physician. Once activated, all Team members participate in the decisions surrounding patient management and therapeutic options. Activations after hours or weekends, do not automatically bring all of the on-call staff in, however it initiates a discussion between the involved parties. The HF cardiologist performs the initial screening when clinical suspicion of CS exists, and serves as the central hub coordinating the whole process. Once a patient is deemed to have CS, empiric medical therapy is initiated and arterial line placement, right heart catheterization and coronary revascularization ensue as warranted, based on the clinical scenario. If despite optimal medical therapy the patient remains hypotensive, CI is <2.2 L/min/m2, PCWP or LVEDP is >15 mmHg, and/or has clinical signs of impaired end-organ perfusion, then escalation to short-term MCS is considered and device selection is made by consensus of all Team members. The patient continues to be managed by the Team until resolution of CS, or until a decision is made to de-escalate care respecting the patient’s or family’s wishes.
The primary endpoint was 30-day all-cause mortality. Secondary endpoints included shock-to-support time (as a surrogate of the feasibility of the Team approach), in-hospital survival, length of MCS support, escalation to surgically implantable durable VAD and length of ICU stay.
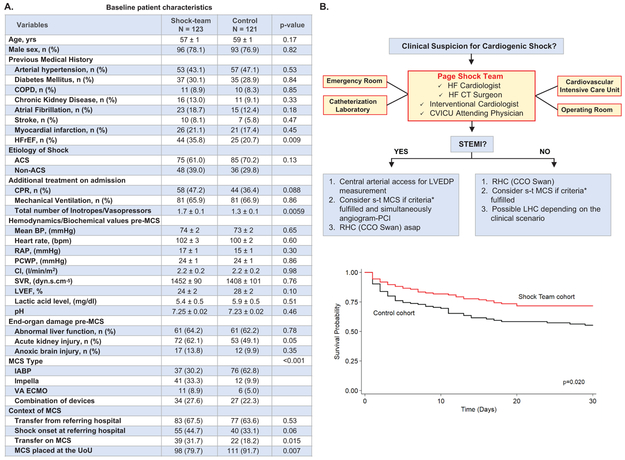
The baseline patient characteristics are presented in Figure, Panel A. After institution of the Shock Team, the primary outcome of 30-days all-cause mortality decreased in the univariate analysis and this effect persisted after controlling for relevant confounders (HR: 0.61 [95% CI 0.41 – 0.93]; Figure, Panel B). The multivariate modeling also identified acute coronary syndrome-related CS (HR: 2.76 [95% CI 1.69 – 4.50]), lactate level (HR: 1.14 [95% CI 1.10 – 1.18]) and acute kidney injury (HR: 2.12 [95% CI 1.36 – 3.32]) as independent risk factors at the time of MCS institution associated with 30-day mortality. Of note, device type was not associated with a survival benefit. Sensitivity analysis suggests no interaction on the effect of the Shock Team on 30-day mortality when examining the CS cause (STEMI, NSTEMI, ADHF, and other), the location of the onset of CS (i.e. referring vs University of Utah hospital) and the presence of CPR. A time series analysis to address a time trend was not possible, due to few time-points of data to reliably fit a model. In-hospital survival also favored the Shock Team (61.0% vs 47.9%; p=0.041). The secondary outcome of “Shock-to-support” time was comparable between the Shock Team and control (19±5 vs 25±8 hrs; p=0.52). Likewise, the mean length of MCS support was similar between the groups (121±13 vs 104±16 hrs). Among the 133 (54.5%) survivors to hospital discharge, 99 experienced improvement leading to MCS weaning, whereas 34 were bridged to a surgically implantable durable LVAD. The overall mean ICU stay was similar between the groups. No significant differences were seen between the groups in the rates of major bleeding, cerebrovascular accidents, rates of hemolysis and major vascular complication leading to surgical vascular repair, fasciotomy or amputation.
Figure. Shock Team approach appears to be feasible and beneficial in the management of refractory cardiogenic shock.
A. Baseline characteristics of study population. Data expressed as mean ± standard error of the mean (SE) or n (%). COPD: Chronic obstructive pulmonary disease; HFrEF: Heart failure reduced ejection fraction; ACS: Acute coronary syndrome; CPR: Cardiopulmonary resuscitation; MCS: Mechanical circulatory support; BP: Blood pressure; RAP: Right atrial pressure; PCWP: Pulmonary capillary wedge pressure; CI: Cardiac index; SVR: Systemic vascular resistance; LVEF: Left ventricular ejection fraction; IABP: Intra-aortic balloon pump; VA-ECMO: Veno-arterial extracorporeal membrane oxygenation; UoU: University of Utah hospital;
B. Shock Team algorithm and adjusted Kaplan Meier 30-day survival. *Criteria for considering s-t MCS: a. Low Systemic Blood Pressure: SBP <90 mmHg OR MAP <50 mmHg for >30 mins OR needed IV vasoactive agents infusion to maintain SBP>90 mmHg or MAP>50 mmHg, plus ONE of the following: PCWP or LVEDP>15 mmHg and CI<2.2 L/min/m2, OR signs of pulmonary edema, OR impaired end-organ perfusion defined as: altered mental status; cold, clammy skin and extremities; urine output <30 ml/h
HF: Heart failure; CT: Cardiothoracic; CVICU: Cardiovascular intensive care unit; STEMI: ST-elevation myocardial infarction; LVEDP: Left ventricular end-diastolic pressure; s-t MCS: Short-term mechanical circulatory support; PCI: Percutaneous coronary intervention; RHC: Right heart catheterization; CCO: Continuous cardiac output; LHC: Left heart catheterization
In this study the multidisciplinary Shock Team approach for the treatment of rCS decreased in-hospital and 30-day all-cause mortality. This strategy may constitute an opportunity to improve the management of this condition, for which multiple interventions and devices have failed to show a survival benefit. Importantly, our finding on the secondary outcome of “Shock to Support” time addresses the concern of delaying care with increasing number of providers comprising the Shock Team. Indeed, a multidisciplinary approach did not delay the implementation of critical decisions, while ensuring appropriate level of support and planning in case escalation was needed. After 4 years of implementation in our institution, the Shock Team initiative remains fully operational, suggesting the sustainability of such programs in clinical practice. These encouraging findings warrant validation by prospective large-scale randomized controlled trials.
Acknowledgements
We are thankful to Greg Stoddard MPH, MBA, for statistical support. Also, we are thankful to ABIOMED® for providing the required funding for the first year of our prospective Cardiogenic Shock Registry.
Sources of Funding
Financial support to Dr Drakos was provided by the American Heart Association Heart Failure Strategically Focused Research Network, 16SFRN29020000, NHLBI R01 HL135121-01, NHLBI R01 HL132067-01A1 and the Nora Eccles Treadwell Foundation, Salt Lake City, Utah, USA. ABIOMED® funded the first year of our prospective registry.
Footnotes
The data that support the findings of this study are available from the corresponding author upon reasonable request
Disclosures
Dr. Drakos is a consultant to Abbott.
References
- 1.Tchantchaleishvili V, Hallinan W and Massey HT. Call for Organized Statewide Networks for Management of Acute Myocardial Infarction-Related Cardiogenic Shock. JAMA Surg. 2015;150:1025–6. [DOI] [PubMed] [Google Scholar]
- 2.van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG, American Heart Association Council on Clinical C, Council on C, Stroke N, Council on Quality of C, Outcomes R and Mission L. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 2017;136:e232–e268. [DOI] [PubMed] [Google Scholar]
- 3.Rab T, Ratanapo S, Kern KB, Basir MB, McDaniel M, Meraj P, King SB 3rd, and O’Neill W Cardiac Shock Care Centers: JACC Review Topic of the Week. J Am Coll Cardiol. 2018;72:1972–1980. [DOI] [PubMed] [Google Scholar]
- 4.Takayama H, Truby L, Koekort M, Uriel N, Colombo P, Mancini DM, Jorde UP and Naka Y. Clinical outcome of mechanical circulatory support for refractory cardiogenic shock in the current era. J Heart Lung Transplant. 2013;32:106–11. [DOI] [PubMed] [Google Scholar]
- 5.Tehrani BN, Truesdell AG, Sherwood MW, Desai S, Tran HA, Epps KC, Singh R, Psotka M, Shah P, Cooper LB, Rosner C, Raja A, Barnett SD, Saulino P, deFilippi CR, Gurbel PA, Murphy CE and O’Connor CM. Standardized Team-Based Care for Cardiogenic Shock. J Am Coll Cardiol. 2019;73:1659–1669. [DOI] [PubMed] [Google Scholar]