Abstract
Background: Metabolic syndrome is associated with preclinical cardiac disease and nonalcoholic fatty liver disease (NAFLD). It is uncertain whether preclinical cardiac disease is present in patients with NAFLD without metabolic syndrome (MetS). Objective: To explore preclinical cardiac disease in patients with NAFLD. Methods: A total of 64 patients with NAFLD, based on computed tomography scans liver attenuation, were identified. A control group, matched to age and gender, comprising of 94 patients was also drafted. Finally, two additional groups of patients with metabolic syndrome, with (n = 40) and without (n = 74) NAFLD, were also identified. Patients with hypertension, diabetes mellitus, and other concomitant liver diseases were excluded from the NAFLD group. Echocardiograms of all groups were reviewed. Results: Severe NAFLD compared to control was associated with a higher left ventricular mass after normalization for height2.7 (LVMHt2.7) (95% CI = 0.39, 12.92) and lower ratio of peak “E” (early) and “A” (late) diastolic ventricular filling velocities (E/A) - 0.39 (95% CI = -0.58, -0.19). Patients with metabolic syndrome (95% CI = 0.02, 0.09), metabolic syndrome with NAFLD (95% CI = 0.02, 0.08), or severe NAFLD (95% CI = 0.02, 0.09) compared to control was associated with a higher relative wall thickness (RWT). Conclusion: Healthy adults with NAFLD without metabolic syndrome, after adjusting for body mass index, demonstrated significant echocardiographic changes. Our results show that NAFLD is associated with preclinical cardiac disease, and this association is independent of traditional risk factors like systemic hypertension and diabetes mellitus.
Keywords: Nonalcoholic fatty liver disease, preclinical cardiac disease, diastolic dysfunction, metabolic syndrome, echocardiogram
Introduction
Nonalcoholic fatty liver disease (NAFLD) is characterized by an accumulation of fat in the liver, in the absence of secondary causes like alcohol, drugs and hereditary disorders. NAFLD has been increasingly identified as a leading cause of cryptogenic cirrhosis [1,2]. It comprises of a spectrum of clinicopathological conditions, ranging from simple fatty liver to nonalcoholic steatohepatitis, which can progress to cirrhosis and eventually hepatocellular cancer.
NAFLD is frequently associated with metabolic syndrome (MetS). Its association with metabolic syndrome is bidirectional [3]. Historically, NAFLD has been considered a hepatic manifestation of metabolic syndrome and its severity increases with increasing components of metabolic syndrome [4]. On the other hand, studies have also shown NAFLD as being a precursor and a risk factor for the future development of MetS [5].
There is evidence to suggest an association between NAFLD and cardiovascular events. Studies have demonstrated both increased cardiac events and diastolic dysfunction among NAFLD patients with one or more components of metabolic syndrome [6-8]. Few studies have shown an association between NAFLD and diastolic dysfunction [8-12]. However, it is unclear whether these cardiac abnormalities result from a direct effect of NAFLD or due to shared risk factors with metabolic syndrome. We aim to study this association and explore the relationship of these cardiac abnormalities with an increasing severity of NAFLD. Additionally, we also intend to evaluate the combined effect of MetS and NAFLD on cardiac abnormalities.
Materials and methods
Study population
We conducted a retrospective review of medical records of 11,036 patients who underwent computed tomography scans at Bronx Care Hospital, between a study period of January 2012 to July 2013. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in the Bronx Care Health System Institutional Review Board’s approval. Patients with atrial fibrillation, coronary artery disease, congestive heart failure, valvular heart disease and chronic kidney disease were excluded from the study. Further, patients with hypertension and diabetes mellitus were excluded from the NAFLD group but were included in the groups with metabolic syndrome. Patients with significant alcohol use (defined as more than 21 drinks per week in men and more than 14 drinks per week in women, over a 2-year period) and the use of steatogenic medications, such as methotrexate, valproate, tamoxifen, amiodarone and corticosteroids, were excluded. We also excluded patients with known liver diseases like cirrhosis, hepatitis B or C, autoimmune hepatitis and drug-induced hepatitis (Figure 1).
Figure 1.
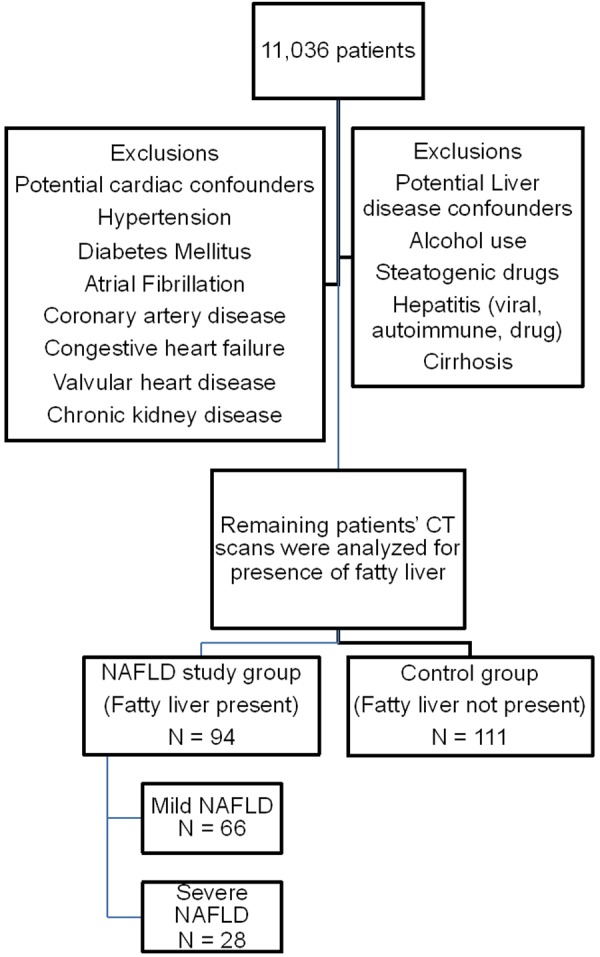
Flow diagram showing selection of patients.
We extracted information regarding patient’s age, gender, race and ethnicity in all study groups, as well as the control group. We also collected information about body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), and routine laboratory blood tests, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels.
Diagnosis of nonalcoholic fatty liver disease
Diagnosis of NAFLD required concurrence of i) a positive criterion for hepatic steatosis (as seen on CT scan and explained below) and b) the absence of competing causes of liver disease. Among patients remaining after exclusions, attenuations of the right liver lobe, left liver lobe and spleen, as assessed on non-contrast computed tomography (CT) scans, were recorded. Liver to spleen (L:S) attenuation ratio was calculated by dividing mean attenuation of the right and left liver lobe by the attenuation of the spleen. It has been shown previously that an L:S attenuation ratio of less than 1.0 on CT scan can be effectively used to diagnose fatty liver [13]. Studies have also shown that a liver attenuation of less than 40 can reliably predict the presence of more than 30 percent fat in the liver [14]. In our study, patients with a liver attenuation of less than 40 were defined as severe NAFLD and patients with a liver attenuation ratio of more than 40 were defined as mild NAFLD.
An age and gender matched control group without NAFLD (L:S ratio > 1) was also identified. Additionally, two more groups - MetS only and MetS combined with NAFLD, were identified. MetS was defined by the National Cholesterol Education Program’s Adult Treatment Panel III criteria. It defined it as the presence of three or more of the following five: central adiposity, impaired fasting glucose, hypertension, elevated triglyceride (TG), and low high-density lipoprotein (HDL) [15].
Echocardiographic measurements
As per the standard practice of our institute, echocardiographic parameters of all patients were recorded as per the American Society of Echocardiography (ASE) recommendations [16,17]. The parasternal acoustic window was used to record 3-5 consecutive beats of 2-dimensional and M-mode recordings of the left ventricle (LV) internal diameter and wall thicknesses, at or just below the tips of the mitral valve leaflets, in long and short-axis views. Correct orientation of planes for imaging and doppler recordings were verified using standard procedures [18]. LV internal dimension and interventricular (septal and posterior wall) thickness were measured at end-diastole and end-systole, for 3 cycles [18]. Where the optimal orientation of the LV could not be obtained by M-mode, correctly oriented 2-D linear dimensions were made by utilizing the leading-edge convention [19,20]. A close correlation between these two methods has been previously reported [21]. End-diastolic LV dimensions were used to calculate left ventricular mass (LVM) by a formula that yields values closely related (r = 0.90, P < 0.001) to necropsy LV weight [22] and which showed excellent reproducibility (intraclass correlation coefficient = 0.93, P < 0.001) between two separate echocardiograms in 183 hypertensive patients [20].
LV hypertrophy was defined using ex-specific partition values in men and women for LV mass adjusted to its allometric relation to height (height2.7) [23-25]. Left ventricular mass after normalization for height2.7 (LVMHt2.7) prognostically validated partition values of > 46 g/m2.7 in men and > 44 g/m2.7 in women. These values were used to diagnose LV hypertrophy [26].
Relative wall thickness (RWT) was calculated as a ratio of twice the posterior wall thickness (PWT) to the left ventricular internal dimension in diastole (LVEDD). It was considered elevated if it was > 0.42, in both men and women [27]. Normal geometry was considered when both LV mass index and relative wall thickness were normal. A normal LV mass index with an increased relative wall thickness was classified as concentric remodeling, whereas, an increased LV mass index with a normal relative wall thickness was identified as eccentric LV hypertrophy. An increase in both variables was identified as concentric LV hypertrophy [19,28].
Transmitral flow velocities measured by pulsed doppler recordings at the mitral annulus and leaflet tips were traced along the black-white interface to record peak “E” (early) and “A” (late) diastolic ventricular filling velocities. The E/A ratio was then calculated [29].
Tissue doppler imaging (TDI) was used to calculate the early (e) and late (a) diastolic velocities from the mitral annular margins [30]. The ratio between early mitral inflow velocity and mitral annular early diastolic velocity (E/e’) was calculated among the study population [31].
Statistical analysis
Five study groups were formed based on liver disease status: Control, mild NAFLD, severe NAFLD, MetS only, NAFLD and MetS combined. Demographic information and clinical measurements were stratified across the study groups. Frequencies and percentages were reported for categorical variables. Mean and standard deviations were reported for numerical variables. Pearson’s chi-squared tests were used to assess the association between categorical variables and liver disease status. ANOVA tests were used to assess the association between continuous variables and liver disease status. Five echocardiogram measures, namely LVM, LVMHt2.7, RWT, E/A, E/e’, were stratified across liver disease status. Mean and standard deviations were reported. Post-hoc comparison using the Tukey honest significant difference (HSD) test was used to assess the inter group differences of the five echocardiogram measures, for our study groups. Four separate multivariable linear regressions were used to assess the association between liver disease status and echo variables (LVM, LVMHt2.7, RWT, E/A), controlling for age, gender, BMI, SBP and HR. Because E/e’ was not normally distributed, bootstrap multivariable linear regressions with 5000 times resampling was used to assess the association between liver disease status and E/e’, controlling for age, gender, BMI, SBP and HR.
Results
Table 1 compares confounders of the different liver disease status. Age, BMI, imputed waist circumstance, hypertension status, diabetes mellitus status, intake of anti-hypertensive and anti-diabetic medications, SBP, DBP, pulse pressure, HR, TG level, ALT and AST levels were all significantly associated with liver disease status.
Table 1.
Clinical and biochemical parameters compared between various study groups
| Variable | Control (N = 94) | Mild NAFLD (N = 45) | Severe NAFLD (N = 19) | MetS only (N = 74) | NAFLD and MetS combined (N = 40) | Total (N = 272) | p-value |
|---|---|---|---|---|---|---|---|
| Age (years) (Mean ± SD) | 48.7 ± 12.6 | 44.6 ± 14.4 | 48.4 ± 14.5 | 65.6 ± 12.1 | 56.4 ± 12.4 | 53.7 ± 15.1 | < 0.001 |
| Gender M:F | 27:73 | 42:58 | 42:58 | 32:68 | 52:48 | 36:64 | 0.060 |
| BMI (kg/m2) (Mean ± SD) | 27.6 ± 5.9 | 29.9 ± 7.8 | 30.7 ± 5.9 | 28.9 ± 6.4 | 33.9 ± 8.6 | 29.6 ± 7.1 | < 0.001 |
| Waist Circumference (cm) (Mean ± SD) | 93.7 ± 13.1 | 99.4 ± 17.8 | 101.5 ± 11.9 | 99 ± 14.1 | 111.5 ± 20.3 | 99.3 ± 16.3 | < 0.001 |
| Smoker | 28 | 9 | 1 | 18 | 8 | 64 | 0.186 |
| Hypertension | 0 | 0 | 0 | 64 | 37 | 101 | < 0.001 |
| Anti-hypertensive medications | 0 | 0 | 0 | 62 | 37 | 99 | < 0.001 |
| Diabetes mellitus | 0 | 0 | 0 | 53 | 33 | 87 | < 0.001 |
| Anti-Diabetic medications | 0 | 0 | 0 | 49 | 33 | 82 | < 0.001 |
| Systolic blood pressure (mm of Hg) (Mean ± SD) | 118.1 ± 11.2 | 123.9 ± 10.7 | 121.9 ± 33.8 | 141.2 ± 22.8 | 131.4 ± 17.2 | 127.5 ± 20.2 | < 0.001 |
| Diastolic blood pressure (mm of Hg) (Mean ± SD) | 74.1 ± 8.10 | 73.5 ± 10.20 | 76.5 ± 22.1 | 80.5 ± 14.9 | 76.9 ± 7.3 | 76.3 ± 12.1 | 0.006 |
| Pulse pressure (mm of Hg) (Mean ± SD) | 43.9 ± 10.40 | 50.5 ± 9.0 | 45.4 ± 17.7 | 59.9 ± 20.4 | 54.5 ± 15.3 | 51.1 ± 16.1 | < 0.001 |
| Heart Rate (beats per minute) (Mean ± SD) | 75.9 ± 10.1 | 75.3 ± 10.5 | 80.9 ± 23.6 | 84.5 ± 15.9 | 85.0 ± 12.3 | 79.8 ± 14.1 | < 0.001 |
| Triglyceride level (mg/dL) (Mean ± SD) | 91.4 ± 51.3 | 115.7 ± 79.9 | 129.2 ± 150.7 | 165.8 ± 179.3 | 216.9 ± 122.9 | 135.9 ± 126.1 | < 0.001 |
| ALT (U/L) (Mean ± SD) | 16.6 ± 6.1 | 29.6 ± 27.2 | 41.4 ± 28.9 | 23.9 ± 27.0 | 41.2 ± 27.5 | 26.1 ± 24.1 | < 0.001 |
| AST (U/L) (Mean ± SD) | 19.3 ± 5.6 | 24.8 ± 14.9 | 48.7 ± 62.2 | 27.7 ± 25.7 | 36.4 ± 25.2 | 27.01 ± 25.3 | < 0.001 |
Abbreviations: BMI: body mass index, AST: aspartate aminotransferase, ALT: alanine aminotransferase.
A total of 94 patients were in the control group (mean age 48.7, 27% male). The NAFLD group had 64 patients, who were further divided into mild NAFLD (N: 45, Mean age 44.6, 42% males) and severe NAFLD (N: 19, Mean age 48.4, 42% males), after exclusions. The group of patients with metabolic syndrome included 74 patients (mean age 65.6, 32% males), while the group with NAFLD and metabolic syndrome combined (mean age 56.4, 52% males) had 40 patients (Table 1).
Waist circumference and transaminases levels were highest in patients with severe NAFLD and NAFLD with MetS combined. Patients with NAFLD and Mets combined had a significantly higher triglyceride level as compared to other groups (Table 1).
The most common indications for echocardiography in the control group were chest pain, shortness of breath and palpitations. Patients with a suspicion of cardiac disease underwent a cardiac stress test and troponin levels in order to rule out cardiac disease. The average time period between CT liver attenuation score and echocardiographic variables was less than 1 year.
Figure 2 compares echocardiographic variables across liver disease status. The mean LV mass was higher for the NAFLD and MetS combined group compared to both, the control group (195.24 vs. 154.84, P < 0.001) and the mild NAFLD group (195.24 vs. 155.80, P < 0.001). The LVMHt2.7 was also higher for the NAFLD and MetS combined group compared to both, the control group (49.90 vs. 40.77, P < 0.001) and the mild NAFLD group (49.9 vs. 39.71, P < 0.001). The LVMHt2.7 was higher in the severe NAFLD group compared to both, the control group (49.70 vs. 40.77, P = 0.05) and the mild NAFLD group (49.70 vs. 39.71, P = 0.04). The RWT was higher in the MetS only group, NAFLD and MetS combined group and the severe NAFLD group compared to the control group (MetS only vs. control: 0.47 vs. 0.42, P < 0.001; NAFLD and MetS combined vs. control: 0.46 vs. 0.42, P < 0.001; severe NAFLD vs. control: 0.47 vs. 0.42, P = 0.04). The E/A was lower for the MetS only group, NAFLD and MetS combined group and the severe NAFLD group compared to the control group (MetS only vs. control: 1.00 vs. 1.32, P < 0.001; NAFLD and MetS combined vs. control: 1.08 vs. 1.32, P = 0.01; severe NAFLD vs. control: 0.92 vs. 1.32, P < 0.001). The E/A for the mild NAFLD group was lower compared to the control group (1.28 vs. 1.32, P < 0.001). The E/A for the severe NAFLD group was lower compared to the mild NAFLD group (0.92 vs. 1.28, P = 0.01). The E/e’ was higher for the mild NAFLD group compared to the MetS only group (1.64 vs. 0.87, P = 0.03).
Figure 2.
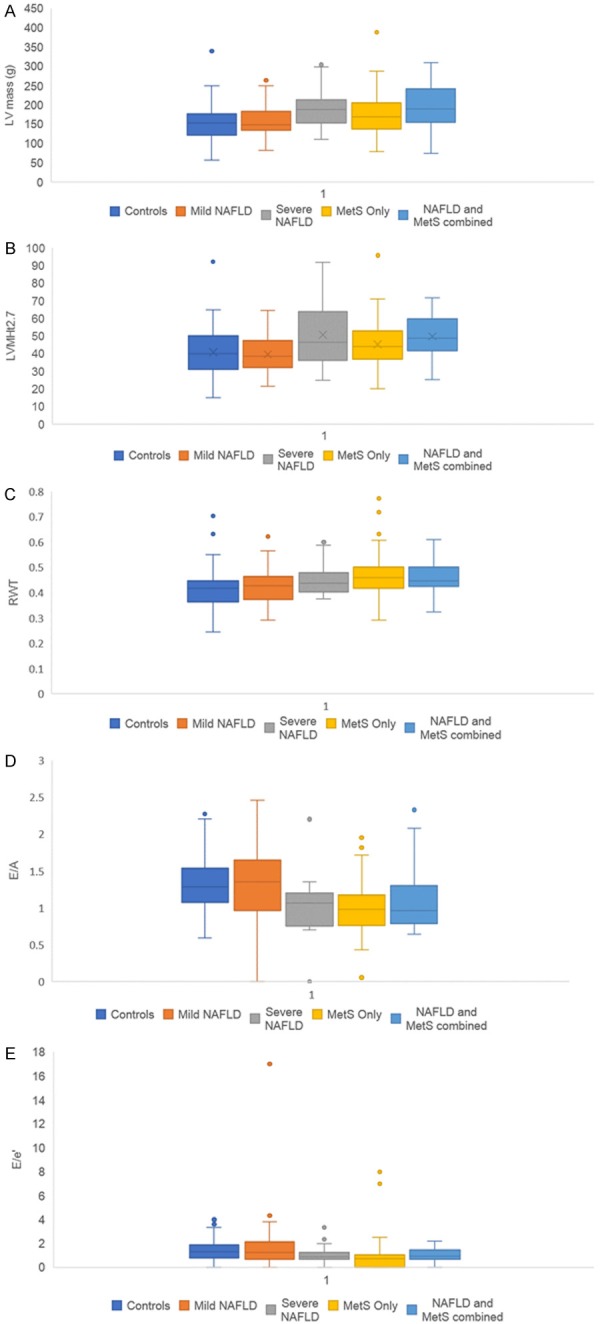
A. Box and whisker plots of mean left ventricular (LV) mass comparison across liver disease status for mild and severe NAFLD, MetS, NAFLD and MetS combined in comparison with control group. B. Box and whisker plots of left ventricular mass after normalization for height2.7 (LVMHt2.7) comparison across liver disease status for mild and severe NAFLD, MetS, NAFLD and MetS combined in comparison with control group. C. Box and whisker plots of relative wall thickness (RWT) comparison across liver disease status for mild and severe NAFLD, MetS, NAFLD and MetS combined in comparison with control group. D. Box and whisker plots of ratio between peak early (E) and late (A) diastolic ventricular filling velocities (E/A) comparison across liver disease status for mild and severe NAFLD, MetS, NAFLD and MetS combined in comparison with control group. E. Box and whisker plots of ratio between early mitral inflow velocity (E) and mitral annular early diastolic velocity (e’) (E/e’) comparison across liver disease status for mild and severe NAFLD, MetS, NAFLD and MetS combined in comparison with control group.
Table 2 displays results of regression analysis. Severe NAFLD compared to control group was associated with a higher LVMHt2.7 (CI = 0.39, 12.92). Patients with MetS only (CI = 0.02, 0.09), NAFLD and MetS combined (CI = 0.02, 0.08), or severe NAFLD (CI = 0.02, 0.09) had a higher RWT compared to control group. Severe NAFLD was associated with a lower E/A-0.39 (CI = -0.58, -0.19) compared to control group.
Table 2.
Regression results
| Control | LVM | LVMHt2.7 | RWT | E/A | E/e’ |
|---|---|---|---|---|---|
|
| |||||
| ref | ref | ref | ref | ref | |
| Mild NAFLD | -9.09 (-26.68, 8.50) | -2.09 (-6.69, 2.51) | 0.01 (-0.02, 0.04) | -0.08 (-0.22. 0.06) | 0.22 (-0.36, 1.08) |
| Severe NAFLD | 12.53 (-11.42, 36.47) | 6.66 (0.39, 12.92)* | 0.05 (0.02, 0.09)** | -0.39 (-0.58, -0.19)*** | -0.28 (-0.67, 0.12) |
| MetS only | 12.78 (-5.69, 31.25) | 0.99 (-3.84, 5.80) | 0.05 (0.02, 0.09)*** | -0.13 (-0.28, 0.01) | -0.16 (-0.52, 0.26) |
| NAFLD and MetS combined | 16.42 (-3.85, 36.68) | 3.98 (-1.32, 9.28) | 0.05 (0.02, 0.08)** | -0.14 (-0.30, 0.02) | -0.21 (-0.54, 0.12) |
Significance codes:
P < 0.001;
P < 0.01;
P < 0.05.
Abbreviations: NAFLD: Nonalcoholic fatty liver disease; MetS: Metabolic syndrome; LVM: Left ventricular mass; LVMHt2.7: Left ventricular mass after normalization for height2.7; RWT: Relative wall thickness; E/A: Ratio between peak early (E) and late (A) diastolic ventricular filling velocities; E/e’: Ratio between early mitral inflow velocity (E) and mitral annular early diastolic velocity (e’).
Discussion
Our study demonstrates the presence of preclinical cardiac manifestations, as detected by echocardiographic diastolic abnormalities, in healthy normotensive, non-diabetic individuals with NAFLD. We also compared this group of patients to those with metabolic syndrome, with and without NAFLD. To our knowledge, this is the first study of its kind that illustrates the gradual decline in diastolic cardiac function and worsening of LV geometry with progressively increasing amounts of fat accumulation in the liver.
NAFLD is the leading cause of liver disease in the west [32] and its prevalence is increasing, with worldwide estimates ranging from 6% to 35% (median prevalence of 20%), depending on the method used for diagnosis [33]. NAFLD is commonly associated with metabolic syndrome and this association not only predicts more severe necroinflammatory activity and fibrosis [4], but also has serious implications on overall cardiovascular health. It is not surprising that patients with NAFLD and metabolic syndrome have a greater carotid artery intimal medial wall thickness, which is a measure of subclinical atherosclerosis [34-37]. Metabolic syndrome when combined with NAFLD is also associated with a higher prevalence of both overt and covert cardiovascular disease. Targher et al. have shown a higher prevalence of cardiovascular, cerebrovascular and peripheral vascular diseases in NAFLD patients with type-1 [38] and type-2 [39] diabetes mellitus. Several studies have shown an increasing association between NAFLD and cardiovascular events, especially as the disease progresses, thereby leading to increased morbidity and mortality [40-46]. However, all these studies included patients with one or more components of metabolic syndrome. Due to the presence of these shared risk factors in such studies, it is unclear if NAFLD can directly lead to cardiovascular problems.
Studies exploring the relationship between NAFLD and echocardiographic changes are not new. However, it remains unclear if these echocardiographic abnormalities can occur independently of metabolic syndrome. The pro-atherothrombotic state of metabolic syndrome causes cardiac remodeling resulting in subclinical cardiovascular abnormalities, such as increased left ventricular mass and subsequent systolic and diastolic dysfunction [19,47-49]. Endothelial dysfunction and eventual atherosclerosis is the main triggering event responsible for these effects and association between NAFLD and cardiac disease. It is well established in previous studies that an abnormal left ventricular geometry and function is present in patients with metabolic syndrome [24,47,49]. The progressive addition of metabolic risk factors is associated with a higher LV mass [50]. A higher prevalence of left ventricular diastolic dysfunction among patients with NAFLD and type-2 diabetes mellitus was demonstrated by Bonapace et al. [51]. In another study, de Simone et al. found that addition of multiple metabolic risk factors was associated with a higher LV mass normalized by height2.7 [50,52]. A small study by Goland et al. [53] showed echocardiographic abnormalities in 38 NAFLD patients, after excluding those with diabetes mellitus, hypertension and morbid obesity. Although, the study demonstrated an increased thickness of the intraventricular septum and posterior wall, as well as a larger LV mass and LV mass/height in NAFLD patients, it did not exclude patients with impaired fasting glucose, which has been shown to independently influence cardiac function [54]. Another small study by Fotbolcu et al. [11] involving 35 NAFLD patients, demonstrated significant differences in the diastolic interventricular septal thickness. They also showed that NAFLD patients have a higher LV mass and LV mass index to body surface area. Since obesity has been independently shown to be associated with left ventricular hypertrophy [55], LV mass indexing has been used to eliminate the effect of obesity on left ventricular mass and therefore avoid any misleading estimates. Among the various methods of indexing the LV mass, LV mass calculated using the Devereux formula [56] and indexed for height powered to 2.7, has shown to be superior to body surface area in predicting cardiovascular events [57]. However, in the study by Fotbolcu et al., indexing with body surface area was used which could have affected the results. Hence, in our study we chose to eliminate the effect of obesity by indexing for height powered to 2.7. Lin et al. found a higher prevalence of ischemic electrocardiographic changes in patients with NAFLD, but they also included patients with impaired fasting glucose and hypertension in their NAFLD cohort [58]. A few others have also studied the association between NAFLD and cardiac dysfunction, however they all either included patients with metabolic syndrome [10,59,60] or included patients with other confounding components of metabolic syndrome, such as hypertension, diabetes mellitus and dyslipidemia, alone or in combination [9,11,12,61-64]. Similarly, there have been other studies which have shown a relationship between NAFLD and coronary artery disease severity as seen on angiography, however these included patients with metabolic syndrome [65-67].
In our study, we precluded patients with hypertension and/or diabetes mellitus in the NAFLD groups - mild and severe, to see the true effect of NAFLD on echocardiographic variables. Findings of our study highlight, not only the existence of subclinical diastolic dysfunction among patients with NALFD, but also the declining diastolic dysfunction with a worsening severity of NAFLD. An increasing severity of NAFLD correlated with worsening cardiac structural disease, as measured by RWT. A gradual decline in the cardiac diastolic function, as measured by LV mass indexed for height2.7 and E/A ratio, was also observed with an increasing severity of NAFLD.
LV hypertrophy has been found to be associated with endothelial dysfunction which in turn leads to arterial stiffness [68]. Prior studies have shown that this effect is seen in long standing hypertension. In our study, we noted LV hypertrophy in patients with NAFLD and metabolic syndrome combined, yet interestingly LV hypertrophy was also present in the severe NAFLD group.
In our study, a higher degree of hepatic steatosis was associated with a higher RWT, which in itself is an indicator of concentric LV geometry occurring without LV hypertrophy. Concentric LV geometry has been shown to be associated with an increase in all-cause mortality in cardiovascular disease patients [69].
Ratio of transmitral “E” (early) and “A” (late) diastolic ventricular filling velocities (E/A) is a measure of LV filling pressures. As the ventricle gets stiff, E/A ratio declines suggesting a decline in LV diastolic function. We noticed a decline in E/A with an increasing severity of hepatic steatosis. A study from our institution by Bella et al. explains the effect of low or high E/A. In a sample of 3008 American Indian individuals, it was observed that mitral E/A > 1.5 at baseline doppler echocardiography was associated with a 2-fold increase in all-cause, and a 3-fold increase in cardiac related mortality. Whereas, mitral E/A < 0.6 was also associated with 2-fold increase in all-cause and cardiac mortality [29]. Decline in E/A ratio has also been shown to increase the risk of major cardiovascular events, as established in the Progetto Ipertensione Umbria Monitoraggio Ambulatoriale (PIUMA) study [70].
In our patient population, a widened pulse pressure was observed in the NAFLD and NAFLD with MetS combined group. Widened pulse pressure increases the arterial stiffness resulting in concentric left ventricular geometry and diastolic abnormalities [71]. It has also been reported by Palmieri et. al. that a greater pulse pressure/stroke volume index was associated with a higher mitral E-wave/A-wave ratio and a shorter isovolumetric relaxation time [72].
It is known that chronic hypertension results in a parallel adaptation of the cardiac and arterial system [73], and our study suggests a similar mechanism of cardiac and arterial remodeling in patients with NAFLD. Increased arterial stiffness and wave reflection have been found in individuals with asymptomatic left ventricular (LV) diastolic dysfunction, which in turn can progress to heart failure with reduce ejection fraction [74]. In our analysis, this phenomenon is also seen in patients with NAFLD. Wang et al. have studied and reported cardiovascular morbidities occurring as a result of the aforementioned process [75]. Based on our study results, we suggest that NAFLD leads to the development of subtle but significant changes in the LV structure and diastolic function, in these otherwise apparently healthy individuals without concomitant diabetes mellitus and/or hypertension.
Implications of the association between NAFLD and preclinical disease
We conclude that it is reasonable to screen for preclinical cardiac disease with echocardiography in patients with severe NAFLD given the sufficient evidence of association between the two. Screening would not only help to intervene at an earlier stage of the disease but would also reduce the long-term morbidity and mortality associated with cardiac diseases. Its clinical significance needs to be proven in prospective trials with long term follow up of patients. Further studies are needed to determine the prognostic significance of preclinical cardiac disease, and whether or not actual screening and interventions can reduce the risk of cardiovascular events in patients with NAFLD.
Study limitations
Our study did have a few limitations. The study design is retrospective and has its inherent limitations due to reliance on previously recorded medical information. This study does not represent ethnic groups other than Hispanics and African Americans. However, these two underserved ethnic groups have not been studied in the past. Diagnosis of NAFLD was made based on imaging study and not by liver biopsy, which remains the gold standard diagnostic technique. We included patients with prehypertension (mean systolic blood pressure in mild NAFLD and severe NAFLD was 124 and 122 mmHg, respectively), which may have had an influence on our results. American and International Society of Hypertension define the preceding values of minimally elevated systolic blood pressure as prehypertension; however, recommend no pharmacologic therapy for this group of people. Population-based studies are required to confirm our findings in this clinical cohort.
Conclusion
In conclusion, our results indicate that NAFLD is associated with preclinical cardiac disease and this association is independent of traditional risk factors like systemic hypertension and diabetes mellitus. The differences in relative wall thickness and E/A ratio were independent of age, sex and body size. Further prospective studies are necessary to validate these results and to determine whether detection of NAFLD predicts future cardiovascular disease.
Disclosure of conflict of interest
None.
References
- 1.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–669. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 2.Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000;32:689–692. doi: 10.1053/jhep.2000.17894. [DOI] [PubMed] [Google Scholar]
- 3.Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018;68:335–352. doi: 10.1016/j.jhep.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 5.Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. 2015;47:181–190. doi: 10.1016/j.dld.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, Arcaro G. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119–2121. doi: 10.2337/dc07-0349. [DOI] [PubMed] [Google Scholar]
- 7.Fallo F, Dalla Pozza A, Sonino N, Lupia M, Tona F, Federspil G, Ermani M, Catena C, Soardo G, Di Piazza L, Bernardi S, Bertolotto M, Pinamonti B, Fabris B, Sechi LA. Non-alcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in essential hypertension. Nutr Metab Cardiovasc Dis. 2009;19:646–653. doi: 10.1016/j.numecd.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Kovalic AJ, Satapathy SK. The role of nonalcoholic fatty liver disease on cardiovascular manifestations and outcomes. Clin Liver Dis. 2018;22:141–174. doi: 10.1016/j.cld.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Granér M, Nyman K, Siren R, Pentikäinen MO, Lundbom J, Hakkarainen A, Lauerma K, Lundbom N, Nieminen MS, Taskinen MR. Ectopic fat depots and left ventricular function in nondiabetic men with nonalcoholic fatty liver disease. Circ Cardiovasc Imaging. 2014;8 doi: 10.1161/CIRCIMAGING.114.001979. [DOI] [PubMed] [Google Scholar]
- 10.VanWagner LB, Wilcox JE, Colangelo LA, Lloyd-Jones DM, Carr JJ, Lima JA, Lewis CE, Rinella ME, Shah SJ. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: a population-based study. Hepatology. 2015;62:773–783. doi: 10.1002/hep.27869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fotbolcu H, Yakar T, Duman D, Karaahmet T, Tigen K, Cevik C, Kurtoglu U, Dindar I. Impairment of the left ventricular systolic and diastolic function in patients with non-alcoholic fatty liver disease. Cardiol J. 2010;17:457–463. [PubMed] [Google Scholar]
- 12.Goland S, Shimoni S, Zornitzki T, Knobler H, Azoulai O, Lutaty G, Melzer E, Orr A, Caspi A, Malnick S. Cardiac abnormalities as a new manifestation of nonalcoholic fatty liver disease: echocardiographic and tissue Doppler imaging assessment. J Clin Gastroenterol. 2006;40:949–955. doi: 10.1097/01.mcg.0000225668.53673.e6. [DOI] [PubMed] [Google Scholar]
- 13.Piekarski J, Goldberg HI, Royal SA, Axel L, Moss AA. Difference between liver and spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology. 1980;137:727–729. doi: 10.1148/radiology.137.3.6934563. [DOI] [PubMed] [Google Scholar]
- 14.Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, Ha HK, Lee MG, Hwang S, Lee SG, Yu ES, Cho EY. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–112. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 15.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 16.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 17.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American society of echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Apridonidze T, Shaqra H, Ktaich N, Liu JE, Bella JN. Relation of components of the metabolic syndrome to left ventricular geometry in hispanic and non-hispanic black adults. Am J Cardiovasc Dis. 2011;1:84–91. [PMC free article] [PubMed] [Google Scholar]
- 20.Palmieri V, Dahlof B, DeQuattro V, Sharpe N, Bella JN, de Simone G, Paranicas M, Fishman D, Devereux RB. Reliability of echocardiographic assessment of left ventricular structure and function: the PRESERVE study. prospective randomized study evaluating regression of ventricular enlargement. J Am Coll Cardiol. 1999;34:1625–1632. doi: 10.1016/s0735-1097(99)00396-4. [DOI] [PubMed] [Google Scholar]
- 21.Devereux RB, de Simone G, Pickering TG, Schwartz JE, Roman MJ. Relation of left ventricular midwall function to cardiovascular risk factors and arterial structure and function. Hypertension. 1998;31:929–936. doi: 10.1161/01.hyp.31.4.929. [DOI] [PubMed] [Google Scholar]
- 22.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 23.de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–1062. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 24.Chinali M, Devereux RB, Howard BV, Roman MJ, Bella JN, Liu JE, Resnick HE, Lee ET, Best LG, De Simone G. Comparison of cardiac structure and function in American Indians with and without the metabolic syndrome (the strong heart study) Am J Cardiol. 2004;93:40–44. doi: 10.1016/j.amjcard.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Wachtell K, Bella JN, Liebson PR, Gerdts E, Dahlöf B, Aalto T, Roman MJ, Papademetriou V, Ibsen H, Rokkedal J, Devereux RB. Impact of different partition values on prevalences of left ventricular hypertrophy and concentric geometry in a large hypertensive population: the LIFE study. Hypertension. 2000;35:6–12. doi: 10.1161/01.hyp.35.1.6. [DOI] [PubMed] [Google Scholar]
- 26.McKimmie RL, Daniel KR, Carr JJ, Bowden DW, Freedman BI, Register TC, Hsu FC, Lohman KK, Weinberg RB, Wagenknecht LE. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study. Am J Gastroenterol. 2008;103:3029–35. doi: 10.1111/j.1572-0241.2008.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Ganau A, Devereux RB, Roman MJ, De Simone G, Pickering TG, Saba PS, Vargiu P, Simongini I, Laragh JH. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–1558. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 29.Bella JN, Palmieri V, Roman MJ, Liu JE, Welty TK, Lee ET, Fabsitz RR, Howard BV, Devereux RB. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults: the Strong Heart Study. Circulation. 2002;105:1928–1933. doi: 10.1161/01.cir.0000015076.37047.d9. [DOI] [PubMed] [Google Scholar]
- 30.Quiñones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. Recommendations for quantification of doppler echocardiography: a report from the doppler quantification task force of the nomenclature and standards committee of the American society of echocardiography. J Am Soc Echocardiogr. 2002;15:167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 31.Garcia MJ, Thomas JD, Klein AL. New Doppler echocardiographic applications for the study of diastolic function. J Am Coll Cardiol. 1998;32:865–875. doi: 10.1016/s0735-1097(98)00345-3. [DOI] [PubMed] [Google Scholar]
- 32.de Alwis NM, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. J Hepatol. 2008;48(Suppl 1):S104–112. doi: 10.1016/j.jhep.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim HC, Kim DJ, Huh KB. Association between nonalcoholic fatty liver disease and carotid intima-media thickness according to the presence of metabolic syndrome. Atherosclerosis. 2009;204:521–525. doi: 10.1016/j.atherosclerosis.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Brea A, Mosquera D, Martin E, Arizti A, Cordero JL, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study. Arterioscler Thromb Vasc Biol. 2005;25:1045–1050. doi: 10.1161/01.ATV.0000160613.57985.18. [DOI] [PubMed] [Google Scholar]
- 36.Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol. 2008;49:600–607. doi: 10.1016/j.jhep.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, Erbel R, Blankstein R, Feldman T, Al-Mallah MH, Santos RD, Budoff MJ, Nasir K. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230:258–267. doi: 10.1016/j.atherosclerosis.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 38.Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Pichiri I, Sorgato C, Zenari L, Bonora E. Prevalence of non-alcoholic fatty liver disease and its association with cardiovascular disease in patients with type 1 diabetes. J Hepatol. 2010;53:713–718. doi: 10.1016/j.jhep.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 39.Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–1218. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- 40.Fracanzani AL, Tiraboschi S, Pisano G, Consonni D, Baragetti A, Bertelli C, Norata D, Valenti L, Grigore L, Porzio M, Catapano A, Fargion S. Progression of carotid vascular damage and cardiovascular events in non-alcoholic fatty liver disease patients compared to the general population during 10 years of follow-up. Atherosclerosis. 2016;246:208–213. doi: 10.1016/j.atherosclerosis.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Mellinger JL, Pencina KM, Massaro JM, Hoffmann U, Seshadri S, Fox CS, O’Donnell CJ, Speliotes EK. Hepatic steatosis and cardiovascular disease outcomes: An analysis of the framingham heart study. J Hepatol. 2015;63:470–476. doi: 10.1016/j.jhep.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pickhardt PJ, Hahn L, Muñoz del Rio A, Park SH, Reeder SB, Said A. Natural history of hepatic steatosis: observed outcomes for subsequent liver and cardiovascular complications. AJR Am J Roentgenol. 2014;202:752–758. doi: 10.2214/AJR.13.11367. [DOI] [PubMed] [Google Scholar]
- 43.Pisto P, Santaniemi M, Bloigu R, Ukkola O, Kesäniemi YA. Fatty liver predicts the risk for cardiovascular events in middle-aged population: a population-based cohort study. BMJ Open. 2014;4:e004973. doi: 10.1136/bmjopen-2014-004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boddi M, Tarquini R, Chiostri M, Marra F, Valente S, Giglioli C, Gensini GF, Abbate R. Nonalcoholic fatty liver in nondiabetic patients with acute coronary syndromes. Eur J Clin Invest. 2013;43:429–438. doi: 10.1111/eci.12065. [DOI] [PubMed] [Google Scholar]
- 45.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, Okuda J, Ida K, Yoshikawa T. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–84. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 47.Palmieri V, Bella JN. Metabolic syndrome and left ventricular structure and functional abnormalities. Am J Hypertens. 2006;19:206–207. doi: 10.1016/j.amjhyper.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Palmieri V, Okin PM, de Simone G, Bella JN, Wachtell K, Gerdts E, Boman K, Nieminen MS, Dahlöf B, Devereux RB. Electrocardiographic characteristics and metabolic risk factors associated with inappropriately high left ventricular mass in patients with electrocardiographic left ventricular hypertrophy: the life study. J Hypertens. 2007;25:1079–1085. doi: 10.1097/HJH.0b013e3280825638. [DOI] [PubMed] [Google Scholar]
- 49.de Simone G, Kizer JR, Chinali M, Roman MJ, Bella JN, Best LG, Lee ET, Devereux RB Strong Heart Study Investigators. Normalization for body size and population-attributable risk of left ventricular hypertrophy: the strong heart study. Am J Hypertens. 2005;18:191–196. doi: 10.1016/j.amjhyper.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 50.de Simone G, Palmieri V, Bella JN, Celentano A, Hong Y, Oberman A, Kitzman DW, Hopkins PN, Arnett DK, Devereux RB. Association of left ventricular hypertrophy with metabolic risk factors: the HyperGEN study. J Hypertens. 2002;20:323–331. doi: 10.1097/00004872-200202000-00024. [DOI] [PubMed] [Google Scholar]
- 51.Bonapace S, Perseghin G, Molon G, Canali G, Bertolini L, Zoppini G, Barbieri E, Targher G. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care. 2012;35:389–395. doi: 10.2337/dc11-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bella JN, Devereux RB, Roman MJ, O’Grady MJ, Welty TK, Lee ET, Fabsitz RR, Howard BV. Relations of left ventricular mass to fat-free and adipose body mass: the strong heart study. The Strong Heart Study Investigators. Circulation. 1998;98:2538–2544. doi: 10.1161/01.cir.98.23.2538. [DOI] [PubMed] [Google Scholar]
- 53.Goland S, Shimoni S, Zornitzki T, Knobler H, Azoulai O, Lutaty G, Melzer E, Orr A, Caspi A, Malnick S. Cardiac abnormalities as a new manifestation of nonalcoholic fatty liver disease: echocardiographic and tissue Doppler imaging assessment. J Clin Gastroenterol. 2006;40:949–955. doi: 10.1097/01.mcg.0000225668.53673.e6. [DOI] [PubMed] [Google Scholar]
- 54.Miyazato J, Horio T, Takishita S, Kawano Y. Fasting plasma glucose is an independent determinant of left ventricular diastolic dysfunction in nondiabetic patients with treated essential hypertension. Hypertens Res. 2002;25:403–409. doi: 10.1291/hypres.25.403. [DOI] [PubMed] [Google Scholar]
- 55.Zhou BF. Effect of body mass index on all-cause mortality and incidence of cardiovascular diseases--report for meta-analysis of prospective studies open optimal cut-off points of body mass index in Chinese adults. Biomed Environ Sci. 2002;15:245–252. [PubMed] [Google Scholar]
- 56.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 57.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Cataliotti A, Seminara G, Stancanelli B, Malatino LS CREED Investigators. Prognostic impact of the indexation of left ventricular mass in patients undergoing dialysis. J Am Soc Nephrol. 2001;12:2768–2774. doi: 10.1681/ASN.V12122768. [DOI] [PubMed] [Google Scholar]
- 58.Lin YC, Lo HM, Chen JD. Sonographic fatty liver, overweight and ischemic heart disease. World J Gastroenterol. 2005;11:4838–4842. doi: 10.3748/wjg.v11.i31.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Psychari SN, Rekleiti N, Papaioannou N, Varhalama E, Drakoulis C, Apostolou TS, Iliodromitis EK. Epicardial fat in nonalcoholic fatty liver disease: properties and relationships with metabolic factors, cardiac structure, and cardiac function. Angiology. 2016;67:41–48. doi: 10.1177/0003319715576672. [DOI] [PubMed] [Google Scholar]
- 60.Kim NH, Park J, Kim SH, Kim YH, Kim DH, Cho GY, Baik I, Lim HE, Kim EJ, Na JO, Lee JB, Lee SK, Shin C. Non-alcoholic fatty liver disease, metabolic syndrome and subclinical cardiovascular changes in the general population. Heart. 2014;100:938–943. doi: 10.1136/heartjnl-2013-305099. [DOI] [PubMed] [Google Scholar]
- 61.Fallo F, Dalla Pozza A, Sonino N, Lupia M, Tona F, Federspil G, Ermani M, Catena C, Soardo G, Di Piazza L, Bernardi S, Bertolotto M, Pinamonti B, Fabris B, Sechi LA. Non-alcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in essential hypertension. Nutr Metab Cardiovasc Dis. 2009;19:646–653. doi: 10.1016/j.numecd.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 62.Hallsworth K, Hollingsworth KG, Thoma C, Jakovljevic D, MacGowan GA, Anstee QM, Taylor R, Day CP, Trenell MI. Cardiac structure and function are altered in adults with non-alcoholic fatty liver disease. J Hepatol. 2013;58:757–762. doi: 10.1016/j.jhep.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 63.Karabay CY, Kocabay G, Kalayci A, Colak Y, Oduncu V, Akgun T, Kalkan S, Guler A, Kirma C. Impaired left ventricular mechanics in nonalcoholic fatty liver disease: a speckle-tracking echocardiography study. Eur J Gastroenterol Hepatol. 2014;26:325–331. doi: 10.1097/MEG.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 64.Fotbolcu H, Yakar T, Duman D, Karaahmet T, Tigen K, Cevik C, Kurtoglu U, Dindar I. Impairment of the left ventricular systolic and diastolic function in patients with non-alcoholic fatty liver disease. Cardiol J. 2010;17:457–63. [PubMed] [Google Scholar]
- 65.Alper AT, Hasdemir H, Sahin S, Onturk E, Akyol A, Nurkalem Z, Cakmak N, Erdinler I, Gurkan K. The relationship between nonalcoholic fatty liver disease and the severity of coronary artery disease in patients with metabolic syndrome. Turk Kardiyol Dern Ars. 2008;36:376–381. [PubMed] [Google Scholar]
- 66.Wong VW, Wong GL, Yip GW, Lo AO, Limquiaco J, Chu WC, Chim AM, Yu CM, Yu J, Chan FK, Sung JJ, Chan HL. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60:1721–1727. doi: 10.1136/gut.2011.242016. [DOI] [PubMed] [Google Scholar]
- 67.Acikel M, Sunay S, Koplay M, Gündoğdu F, Karakelleoğlu S. Evaluation of ultrasonographic fatty liver and severity of coronary atherosclerosis, and obesity in patients undergoing coronary angiography. Anadolu Kardiyol Derg. 2009;9:273–279. [PubMed] [Google Scholar]
- 68.Olsen MH, Wachtell K, Hermann KL, Bella JN, Dige-Petersen H, Rokkedal J, Ibsen H. Left ventricular hypertrophy is associated with reduced vasodilatory capacity in the brachial artery in patients with longstanding hypertension. A LIFE substudy. Blood Press. 2002;11:285–292. doi: 10.1080/080370502320779494. [DOI] [PubMed] [Google Scholar]
- 69.Milani RV, Lavie CJ, Mehra MR, Ventura HO, Kurtz JD, Messerli FH. Left ventricular geometry and survival in patients with normal left ventricular ejection fraction. Am J Cardiol. 2006;97:959–963. doi: 10.1016/j.amjcard.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 70.Schillaci G, Pasqualini L, Verdecchia P, Vaudo G, Marchesi S, Porcellati C, de Simone G, Mannarino E. Prognostic significance of left ventricular diastolic dysfunction in essential hypertension. J Am Coll Cardiol. 2002;39:2005–2011. doi: 10.1016/s0735-1097(02)01896-x. [DOI] [PubMed] [Google Scholar]
- 71.Roman MJ, Devereux RB, Kizer JR, Okin PM, Lee ET, Wang W, Umans JG, Calhoun D, Howard BV. High central pulse pressure is independently associated with adverse cardiovascular outcome: the Strong Heart Study. J Am Coll Cardiol. 2009;54:1730–1734. doi: 10.1016/j.jacc.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palmieri V, Bella JN, Roman MJ, Gerdts E, Papademetriou V, Wachtell K, Nieminen MS, Dahlöf B, Devereux RB. Pulse pressure/stroke index and left ventricular geometry and function: the LIFE Study. J Hypertens. 2003;21:781–787. doi: 10.1097/00004872-200304000-00022. [DOI] [PubMed] [Google Scholar]
- 73.Roman MJ, Saba PS, Pini R, Spitzer M, Pickering TG, Rosen S, Alderman MH, Devereux RB. Parallel cardiac and vascular adaptation in hypertension. Circulation. 1992;86:1909–1918. doi: 10.1161/01.cir.86.6.1909. [DOI] [PubMed] [Google Scholar]
- 74.Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. doi: 10.1161/HYPERTENSIONAHA.112.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA, Ting CT, Najjar SS, Lakatta EG, Yin FC, Chou P, Chen CH. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension. 2010;55:799–805. doi: 10.1161/HYPERTENSIONAHA.109.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]


