Abstract
Heart failure with preserved ejection fraction (HFpEF) is a pathological complexity that decreases cardiac output and elevates the ventricular filling pressure. HFpEF is usually misdiagnosed and maltreated. HFpEF is usually correlated with excessive morbidity and mortality. The prevalence of HFpEF is growing, and there is a deficiency of evidence-based therapy, creating challenges for the physician with no effective management guidelines. Moreover, HFpEF is not equivalent to diastolic heart failure as previously thought, as diastolic dysfunction is not the only underlying mechanism related to HFpEF and sometimes may be absent. Several other mechanisms may work in concert to produce HFpEF syndrome, either cardiac related (chronotropic incompetence, a longitudinal left ventricular (LV) systolic dysfunction despite a normal ejection fraction) or extracardiac related (pulmonary hypertension, abnormal ventricular-arterial coupling, abnormal exercise-induced vasodilation, extracardiac volume overload). These complex pathophysiologic mechanisms indicate that HFpEF is heterogeneous and that this syndrome might be related to a vascular or an endothelial dysfunction or might be considered a cardiac manifestation of one or more systemic illnesses. The heterogeneity of HFpEF necessitates excluding many differential diagnoses. In addition, the multiple comorbidities that are inherent to this condition need to be controlled in order to achieve effective management. Taken together, these key mechanisms might contribute to the multiple difficulties in the management of HFpEF patients; these mechanisms also explain why medications used in patients with other heart conditions may or may not be successful in these patients. Novel therapies and clinical trials including paradigm shifts in therapeutic management are needed to effectively manage HFpEF. The current review article sheds light on novel paradigms related to pathologies, diagnoses, and strategies, along with some proposed recommendations and clinical options for effective management of HFpEF.
Keywords: Heart failure, new paradigm, clinical, management, HFpEF
Introduction
Heart failure is a clinical constellation of symptoms of dyspnea, fatigue, and fluid retention. Patients with diastolic dysfunction might progress to diastolic heart failure leading to unexplained dyspnea or exercise intolerance in elderly patients, who usually have isolated left atrial dilatation resulting from elevated left ventricular filling pressure. Heart failure with preserved ejection fraction (HFpEF) is discrete heart failure with a clinical constellation of symptoms, after exclusion of valvular, infiltrative and pulmonary disease [1]. Comorbidities including hypertension, coronary artery disease (CAD), diabetes, chronic kidney disease (CKD), anemia, obesity, and metabolic syndrome are contributing reasons for HFpEF. HFpEF was initially termed diastolic HF [2] or HF due to diastolic dysfunction. However, the condition is not synonymous with diastolic dysfunction, as other contributing mechanisms for this syndrome include endothelial dysfunction, chronotropic incompetence, impaired heart rate recovery, impaired vascular coupling, abnormal ventricular-vascular recovery, impaired vasodilator reserve, post capillary pulmonary hypertension, autonomic dysfunction, renin angiotensin aldosterone system (RAAS) and sympathetic systems up regulation [3]. In addition, at a molecular level, decreased activity of nitric oxide-cyclic guanosine monophosphate cGMP-protein kinase G (NO-cGMP-PKG), endothelial dysfunction, oxidative stress, and cardiac inflammation are often observed [4].
Clinically, HFpEF is defined as normal left ventricular (LV) systolic function, EF > 50%, left ventricular end-diastolic volume (LVEDV) < 97 ml/m2, left ventricular mass index (LVMI) ≥ 115 g/m2 in males and ≥ 95 g/m2 in females, diastolic dysfunction, mean tissue Doppler (e) < 9, elevated E/e ratio using the lateral wall > 13, left atrial (LA) volume index > 34 ml/m2, elevated N-terminal-pro-brain natriuretic peptide (NT-pro-BNP) biomarker, and elevated pulmonary artery pressure. Elevated pulmonary artery systolic pressure on echocardiography in the presence of a normal ejection fraction should bring prompt consideration of HFpEF [5].
The incidence of new cases of HFpEF in US is approximately 600,000-700,000 annually; HFpEF occurs in 40-60% of newly diagnosed heart failure (HF) cases and involves healthcare expenditures of $40 billion on HF according to a 2010 Center for Medicare and Medicaid Services reimbursement report. The annual mortality range is 5-30% [4].
HFpEF is a comorbidity driven by a systemic disease, manifested by cumulative risk factors that cause a loss of adaptation of both heart and vessels. HFpEF is associated with almost equivalent prevalence and clinical outcomes compared to heart failure with reduced ejection fraction (HFrEF), yet there is a different response to therapeutic drugs. This may be due to different pathophysiology, multiple comorbidities, and high non-cardiac causes [5]. Apart from the fact that while HFrEF starts from the heart and leads to the periphery, HFpEF has evolved from a ‘cardiocentric’ model which starts peripherally and ends with cardiac manifestation [6].
Phenotypes of heart failure with preserved ejection fraction and clinical implications (Figure 1)
Figure 1.

Phenotypes of diastolic dysfunction. HFpEF is a systemic syndrome with multiple phenotypes and contributing to diverse clinical representation, along with the degree of diastolic dysfunction and not necessarily the presence of right heart disease and pulmonary hypertension. Phenotype A is very common and is linked with few symptoms at rest including exercise induced left ventricle filling impairment and initial diastolic dysfunction. Phenotype B shows evident symptoms of heart failure, whereas phenotype C is related to overnight heart failure with severe pH. *HF: Heart Failure; LV: Left Ventricle; HFpEF: Heart Failure with preserved Ejection Fraction.
Patients with HFpEF are mostly older females [7]. Moreover, these patients have more obesity, anemia mostly related to iron deficiency, hypertension, atrial fibrillation (AF), and have less coronary artery disease compared with patients with HFrEF [8]. Since HFpEF patients are most likely to be older, the new endpoints, defined as functional class and quality of life, should be prioritized over the traditional end points [9]. It is important to note that the Bowditch effect, which is a physiological tachycardia leading to an increased force of contraction in a normal heart, but not in a failing heart, is manifested as exercise intolerance in patients with HFpEF. This results from tachycardia; thus, lowering heart rate in HFpEF is desirable to maintain an adequate force of contraction at an impaired relaxation level, but not in stiffer ventricles with higher grades of diastolic dysfunction [10]. Data from the I PRESERVE study suggested that the ideal hemodynamic parameters include a heart rate approximately 70 b/m (especially with advanced diastolic dysfunction), an ideal systolic blood pressure of 130-140 mmHg and an ideal diastolic blood pressure of 70-80 mmHg, an ideal central venous pressure (CVP) 12-15 cmH2O, along with the maintenance of an adequate renal blood flow by adequate mean perfusion pressure and cardiac output [11].
HFpEF is a diverse syndrome consisting of multiple pathophysiological mechanisms such as chronic volume overload phenotype (combined systolic and diastolic). Other phenotypes include associated right HF, and/or pulmonary hypertension, or the less commonly exercise-induced diastolic dysfunction phenotype [12].
Failure of clinical trials in HFpEF was mainly due to consideration of the one-size-fits-all concept, which turned out not to be true and confirmed that differentiation into different phenotypes is a must.
Based on phenotypic classifications, it is important to treat HFpEF patients by treating their comorbidities. Excessive reduction in the heart rate in HFpEF is not constantly preferable (although it is desirable in mild grades of diastolic dysfunction, but not in higher grades). It is crucial to consider uncommon diseases (“zebras”) as infrequent causes or risk factors for HFpEF during management [12].
Proposed mechanisms of diastolic dysfunction (Figure 2)
Figure 2.
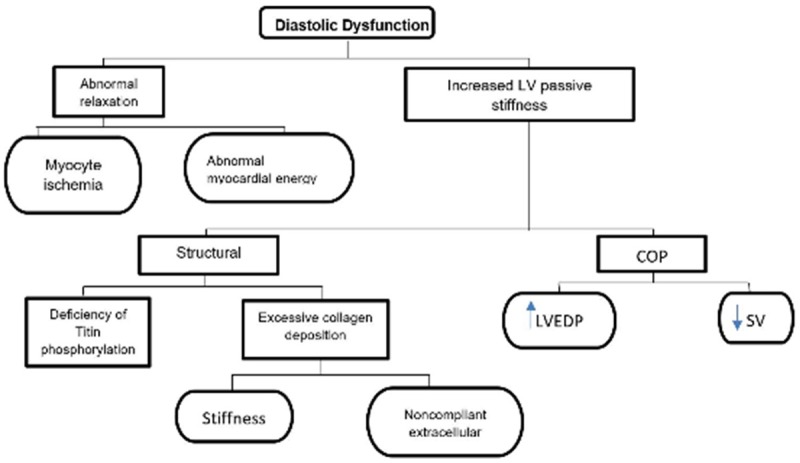
Proposed mechanisms of diastolic dysfunction. The mechanism of diastolic dysfunction is characterised by increased stiffness of left ventricle contributing to low cardiac output, thereby elevating diastolic pressure due to slow relaxation in early diastole and greater resistance in late diastole. Abnormal relaxation and increased stiffness in turn causes epicardial and microvascular ischemia, myocyte hypertrophy, diffuse fibrosis, fibro elastosis, pericardial constriction, capillary compression and venous engorgement. This causes volume overload of the contralateral ventricle and increased pulmonary pressure due to decreased stroke volume. *LV: Left Ventricle; SV: Stroke Volume; LVEDP: Left Ventricular End-Diastolic Pressure; COP: Center of Pressure.
LV relaxation is an active process that consumes energy. It begins during the ejection phase of the systole and continues through IVR (isovolumic relaxation) and rapid filling phase. Calcium ions fluxes regulate the contraction and relaxation phases. Depolarization releases large amount of Ca into the cytosol to initiate contraction. A decrease in cytosolic Ca initiates relaxation through calmodulin mediated closure of L-type Ca channels and sarcoplasmic reuptake of Ca by SERCA (sarcoplasmic/endoplasmic reticulum calcium ATPase). Phosphorylation of phospholamban enhances calcium uptake. Phospholamban responds to B adrenergic stimulation mediated by protein kinase A (PKA). That is explained by the fact that impaired beta adrenergic signaling and inadequate ATP levels impair ventricular relaxation. The major factors influencing the relaxation process are calcium levels, which should fall to initiate relaxation (requires ATP and phosphorylation of phospholamban) and the inherent viscoelastic properties of myocardium, as in a hypertrophied heart there is an increase in fibrosis that leads to slower relaxation together with systolic load (the higher the systolic load, the faster the relaxation, and the poorer the compliance) [7]. The mechanism of the underlying diastolic dysfunction could be divided into two types of factors; intrinsic myocardial factors, which could be either cellular factors such as impaired Ca2+ homeostasis, changes in sarcomeric protein isotypes, abnormal cellular energy supply, or extracellular factors such as fibrosis (more evident in a hypertrophied heart), extrinsic factors such as RAAS, and the sympathetic nervous system [8]. Physiologically, diastolic function can be divided into active relaxation (equal to isovolumetric relaxation time [IVRT] and 100 ms of early diastole) and passive stiffness (compliance) [12].
Indices of left ventricular relaxation are isovolumetric pressure decay (max rate of LV pressure decline after aortic valve closure in IVR phase measured (peak neg. dP/dt)) affected by loading conditions. Time constant of relaxation (Tau) is a load-independent measure and depends on the rate of LV pressure decay during IVR phase (measured in catheterization laboratory). Factors that impair active relaxation include myocardial structural changes (ischemia, fibrosis), LV hypertrophy, LV contractility and afterload. Factors that impair LV passive property are almost the same and include LV chamber size [10].
Ventricular stiffness in HFpEF is mostly precipitated by activation of collagen crosslinking, reduction of metalloproteinase (MMP), and increased tissue inhibitor metalloproteinases (TIMPs) which will lead to accumulation of more of the stiffer collagen type I vs collagen type III, as well as a shift to more of the stiffer titin due to reduction of its phosphorylation and oxidation of titin cysteine residues [13].
Basic types of diastolic abnormalities can be divided into impaired ventricular relaxation (early diastole), increased myocardial stiffness (early to mid-diastole), or decrease myocardial compliance (late diastole) [14].
Temporal non-uniformities of relaxation can result in a push and pull situation between different regions of the ventricles, the result of which can often extend to the diastole and is manifested as post-systolic or proto-diastolic shortening [15]. The occurrence or persistence of regional contraction in the early diastole can influence diastolic pressure and volume transients, and therefore, contribute to diastolic dysfunction and lead to asynchrony phenomena [16].
Diastolic dysfunction of the left ventricle is an important abnormality that usually appears ahead of abnormal systolic function. The fundamental problems in HFpEF are abnormal relaxation, impaired LV filling and/or increased stiffness. These changes will lead to a higher level of diastolic pressure-volume relation with increased left ventricular end diastolic pressure (LVEDP), left atrial pressure (LAP), and pulmonary arterial wedge pressure (PAWP), manifested as congestive symptoms [17].
Symptomatology in HFpEF may be due to chronotropic incompetence, impaired vasodilatation during exercise, and metabolic derangement [18].
HFpEF is not just a disease, it is a multiplex of clinical conditions, involving not just diastolic dysfunction but other contributing pathophysiological mechanisms that will lead to uneasiness of management [19].
The sine qua non of HFpEF is abnormal relaxation and abnormal diastolic function. Diastolic dysfunction is considered not only a preclinical disorder but also stage-B of heart failure.
No single drug has proper lusitropic (relaxation) properties, with selective enhancement of relaxation, without affecting contractility and function. The optimal treatment of HFrEF might exacerbate HFpEF [20].
Proposed causes of HFpEF (Figure 3)
Figure 3.
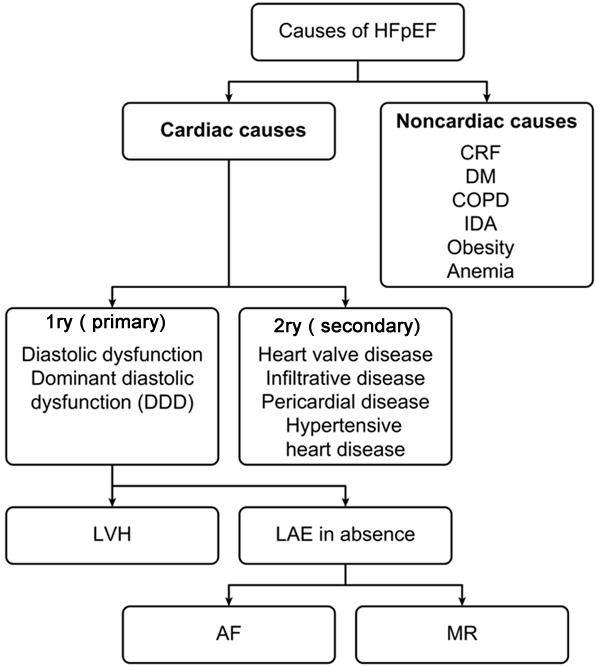
Proposed causes of HFpEF. The figure shows the schematic representation of the cardiac and non-cardiac causes of HFpEF. Cardiac causes basically represent the cellular and biological changes within the heart in the following years of the disease; lead to the left ventricular hypertrophy and left atrial enlargement leading to atrial fibrillation and mitral regurgitation. *CRF: Chronic Renal Failure; DM: Diabetes Mellitus; COPD: Chronic Obstructive Pulmonary Disease; IDA: Iron Deficiency Anemia; LVH: Left Ventricular Hypertrophy; LAE: Left Atrial Enlargement; AF: Atrial Fibrillation; MR: Mitral Regurgitation.
There are plenty of causative dilemmas for patients with HFpEF including misdiagnosis, incorrect assessment of ejection fraction, episodic LV systolic dysfunction, primary valvular disease, atrial myxoma, restrictive cardiomyopathies, pericardial constriction, obesity, diastolic dysfunction of uncertain origin, severe hypertension, myocardial ischemia, high-output failure, cor pulmonale, and pulmonary hypertension due to abnormal pulmonary vasculature [21].
Independent predictors for mortality in HFpEF include age, gender, NYHA (New York Heart Association) class, lower LVEF, the extent of CAD, peripheral arterial disease (PAD), diabetes mellitus (DM), renal dysfunction, stage of diastolic dysfunction, anemia, and increased red cell distribution width [22].
Precipitating factors for HFpEF include tachycardia, ischemia, hypertension (HTN), exercise, systemic stress (thyrotoxicosis, anemia, infection, and fever), arrhythmias (atrial fibrillation (AF), atrioventricular block (AVB)), increased salt intake, and nonsteroidal anti-inflammatory drugs (NSAIDs) [18].
Some risk factors such as smoking, obesity, and AF precede and are more prevalent in the diagnosis of new HFpEF compared to HFrEF [22].
Patients with HFpEF poorly withstand atrial fibrillation, as a loss of atrial contraction leads to a significant reduction in left ventricular filling due to a lack of atrial emptying with reduction in stroke volume and cardiac output. Tachycardia is poorly tolerated in HFpEF as it worsens the diastolic function via decreased relaxation time and time for fiber recoil, leading to elevated filling pressure.
Sudden or persistent elevation of blood pressure as in cases of renovascular disease will cause abnormal diastolic relaxation and increase wall stress. Ischemic heart disease will lead to abnormal diastolic function, a rise in left atrial pressure and pulmonary venous congestion which results into respiratory congestion (angina equivalent) [2,3]. HFpEF patients are a heterogeneous group, as has been reported in a metanalysis. The group includes valvular heart disease in 11-22% of patients; pulmonary disease in 31-33%; left ventricular dilatation in 19%; and no LV hypertrophy, LA enlargement, or diastolic dysfunction (DD) in 30%; moreover, less than 2/3 of the patients did not have diagnostic criteria of DHF (diastolic heart failure) [23]. HFpEF is a disease of non-cardiac comorbidities including obesity, diabetes, anemia, chronic obstructive airway disease, peptic ulcer, cancer, and psychiatric disorders [24]. HFpEF is a substantial heterogeneous group of patients with a mixture of diagnoses including valvular, pulmonary, renal, myocardial and pericardial disease. There are many confounders with no purity [25].
HFpEF scores
The initial step of calculating an ESC HFA-PEF score is to detect pretest probability followed by performing a sophisticated echocardiographic assessment and natriuretic peptide level together with the presence or absence of atrial fibrillation. (Score < 2: likelihood of non-cardiac origin; 5-6: high likelihood of cardiac origin; 2-4: need further testing, consisting exercise testing in combination with echocardiography or invasive hemodynamics). The last step is to assess the underlying etiology and pathophysiology with cardiac magnetic resonance, laboratory measures and/or myocardial scintigraphy or myocardial biopsy [26].
The risk assessment tool using information from atherosclerotic risk assessment in community (ARIC), heart failure community surveillance for patients with acute heart failure admitted to the hospital included the following factors: white or black patient, more than 55 years old, and ejection fraction of more than 50%. It predicted risk of death within 28 days and one year from hospital admission, including the following variables: age, systolic blood pressure, BUN (mg/ml), Na (mEq/L), Hb (g/dL), HR (bpm), either BNP (pg/mL) or NT-pro-BNP (pg/mL), white race, BMI less than 18.5 (kg/m2), hypoxia (O2 sat less than 90), cerebrovascular stroke (TIA or stroke), COPD (chronic obstructive pulmonary disease), and atrial fibrillation or flutter [26].
An evidence based approach for diagnosis of HFpEF was developed by Reddy et al. [27], who created a composite score (H2FPEF score) ranging from 0-9 based on a range of predictive variables. The H2FPEF score simplified differentiating different causalities of HFpEF, in comparison to the ESC HFA-PEF score, which is a sophisticated score depending upon many variables [26]. The use of NT-pro-BNP levels did not incrementally add diagnostic ability to the H2FPEF score. Shown in Table 1.
Table 1.
Heart failure with preserved ejection fraction (H2FPEF) score
| Variables | Parameters | Description | Score |
|---|---|---|---|
| H* | Heavy | Body mass index more than 30 KG/M2 | 2 |
| H* | Hypertensive | 2 or more antihypertensive medication | 1 |
| F* | Atrial Fibrillation | Paroxysmal or persistent | 3 |
| P* | Pulmonary hypertension | sPAP more than 35 mmHg by ECHO Doppler | 1 |
| E* | Elder | More than 60 years old | 1 |
| F* | Filling pressure | Doppler ECHO E/e more than 9 | 1 |
*H2FPEF is used to diagnose heart failure with non-cardiac causes in patients with dyspnea, ranging from 0-9. *H = heart; F = failure; P = preserved; E = ejection; F = fraction.
General strategies and directives (Figure 4)
Figure 4.
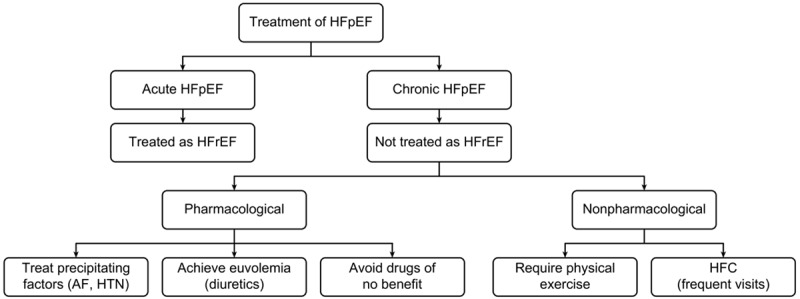
Proposed strategies in the management of HFpEF. The schematic representation of treatment options for HFpEF depicts that treatment for acute HFpEF is same as that of HFrEF. On the other hand, for chronic treatment options can be either pharmacologic or non-pharmacologic or may be both. In pharmacologic, treatment of precipitating factors to decrease the complications of the condition and diuretics are given to reduce the congestive symptoms associated with hypervolemia, whereas physical activity and frequent visits to HFC are recommended in non-pharmacologic management of HFpEF. *HFrEF: Heart Failure with Low Ejection Fraction; AF: Atrial Fibrillation; HTN: Hypertension; HFC: Heart Failure Consultant.
HFpEF patients have clusters of comorbidities, which all will conspire in such a complex multifaceted condition that needs multiple drug therapy with its negative impact on renal function. Quality of life estimation is a very important end point in such a complex syndrome, which therefore needs to be included in the studies, together with proper selection of similar phenotypes to avoid underpowered clinical trials [28].
HFpEF management is difficult. Any minor variation from normal hemodynamic parameters could lead to low cardiac output and lowering of blood pressure or pulmonary venous congestion [2]. As the left ventricle operates at barely adequate volume, if the volume is reduced, this will directly translate into significant hypotension. It is important to realize that it is difficult to achieve normal volume in HFpEF due to impaired relaxation. In spite of left ventricular volume, the left atrial pressure is high [4].
In a paradoxical phenomenon, patients with HFpEF are sensitive to hypovolemia (diuretic paradox), and insensitive to fluid infusion when the LAP (left atrial pressure) is already high. Acute renal failure leads to fluid retention and can trigger DHF [8]. Excessive fluid infusion can be a contributing factor for postoperative diastolic heart failure. Vasodilators are appropriate in cases of combined systolic and diastolic heart failure, and in cases of secondary diastolic dysfunction due to right ventricular dysfunction, unloading of RV (right ventricle) will reduce the effect of left ventricle compression. However, in cases of pure diastolic dysfunction vasodilator use can lead to hypotension (vasodilator paradox) [8]. Inodilators improve diastolic dysfunction in systolic and diastolic dysfunction, and biventricular failure to maintain stroke volume at lower left ventricular end diastolic volume and pressure, while this is not true in patients with HFpEF (Inotropic paradox) [7].
Therefore, the management of HFpEF is a fine tuning technique, requiring the maintenance of a euvolemic status and a fluid balance, together with controlling the triggering factors that can be tolerated in a normal heart but will not be in a heart with HFpEF, such as reduction of preload due to bleeding or vasodilatation [6]. The strategy of maintaining adequate cardiac output, while avoiding pulmonary congestion (warm the patient up while drying the patient out) [5], and correcting any predisposing factors, should be implemented. Close monitoring is mandatory to guide therapy and its response [1]. Proactive management by anticipating the problem before its occurrence is a better strategy. It is of utmost importance to target the ideal hemodynamic parameters together with the management of the reversible causes [3].
Managing common comorbidities
Hypertension heralds the diagnosis of HFpEF in 75-85% of cases, and shares its adverse outcomes as well as its acute and chronic symptoms [29]. Intensive diastolic BP reduction could reduce myocardial perfusion and promote myocardial ischemia, LV dilation, and subsequent HF. In HFpEF patients, there is an increase in both ventricular and vascular stiffness in excess due to associated aging or hypertension [30]. Therefore, excessive reduction in BP with vasodilation in HFpEF could potentially offset any benefit from the antagonism of pathological neuro-hormonal activation [31]. However, in spite of the potentially adverse effects of intensive BP lowering, the recent SPRINT (systolic blood pressure intervention trial) demonstrated that intensive BP lowering can significantly reduce the rate of development of acute decompensated HF [30]. While it is uncertain what proportion of these HF events were HFpEF versus HFrEF, it is likely that HFpEF was well-represented [32]. Both HFpEF and AF are related to adverse cardiovascular outcomes [33]. ACC/AHA (American College of cardiology/American Heart Association) guidelines recommend rate control for atrial fibrillation management; ESC guidelines, however, supported restoring rhythm control although strong evidence lacked [34]. Further studies are required to determine whether different rate control strategies or, indeed, rhythm control, will improve outcomes in management of HFpEF. Approximately 85% of elderly HFpEF patients are overweight or obese, and the HFpEF epidemic has largely paralleled the obesity outbreak [35]. Unfortunately, obesity has been overlooked as a factor in HFpEF pathophysiology and treatment [36].
Management of acute HFpEF
Patients with acute HFpEF, like patients of HFrEF, should be treated with intravenous short-acting beta blocker, esmolol [37] as well as verapamil, a calcium channel blocker [38], and control atrial fibrillation in case of acute HFpEF [39]. Calcium channel blockers had a more beneficial effect on patients with hypertrophic cardiomyopathy [31] compared to other causes of diastolic dysfunction [20]. Up to date, there is no specific drug designed for diastolic dysfunction. Inodilators used in cases of combined systolic and diastolic heart failure and in cases of biventricular failure will improve the stroke volume without significant rise in left ventricular end diastolic volume and pressure [7]. Amiodarone reduces preoperative ventricular and supra-ventricular tachyarrhythmia’s (atrial fibrillation) when used after cardiac surgery [3,4].
Classic pharmacological therapy (Figure 5)
Figure 5.

Proposed therapies in HFpEF. The schematic representation depicts the current and future approach towards the therapeutic options of HFpEF. Future approaches can be either pharmacologic or non-pharmacologic. Pharmacologically promising drugs are Statin (lower cholesterol levels), PDEI5 (Inhibitor of Cyclic GMP in blood vessel lining) and NTG (blood vessels relaxation), however, the treatment options for HFpEF are now shifting towards new paradigm in which drugs are mainly anti-anginal. But, in future the focus will be mainly on treatment of comorbidities and precipitating factors. On the other hand, non-pharmacologic options include healthy diet, treatment of comorbidities and skilled persons for early diagnosis. *HFrEF: Heart Failure with Reduced Ejection Fraction; ACE1: Angiotensin Converting Enzymes 1; ARB: Angiotensin II Receptor Blockers; BB: Beta Blockers; HTN: Hypertension; AF: Atrial Fibrillation; MMP-1: Matrix Metalloproteinase-1; EPO alfa: Epoetin alfa.
Diuretics
Diuretic therapy will lead to hypovolemia and result in a reduction in the filling of a stiff ventricle, resulting in low cardiac output and hypotension as the ventricle is already working on a steep left ventricular end diastolic pressure-volume relationship. Daily assessment of patient fluid status is mandatory to avoid hypotension [2].
In addition to its diuretic effect, as part of the renin angiotensin-aldosterone system that is activated in heart failure, mineralocorticoid antagonists (aldosterone antagonists) reduce insulin resistance and the prevalence of atrial fibrillation [40]. The Aldo HF trial had a positive result while the TOPCAT trial (double blind randomized, multicenter) showed no significant difference in cardiovascular mortality, sudden cardiac death, or heart failure re-hospitalization. These ambiguous results may be related to geographical differences in patient enrollment, with some countries contributing comparatively healthier patients with fewer cardiovascular events resulting in dilution of the overall results [41].
Traditional pharmacological agents
Although drugs affecting myocardial remodeling, such as angiotensin converting enzymes inhibitors, angiotensin blockers, and beta blockers, are considered disease modifying agents in patients with HFrEF [42], there is a lack of evidence of effective use in patients with HFpEF, and the data from clinical trials are ambivalent [43]. Ad hoc trials were done in ACE-I (PEPCHF) [44] and angiotensin receptor antagonists (CHARM-Preserved Trial) [34], and the I-PRESEVE Trial with irbesartan was done in patients with heart failure and preserved systolic function ejection fraction where more than 45% showed that every 12.4 b/m increase in heart rate resulted in 13% higher risk of cardiovascular death and heart failure re-hospitalization [45].
The effect of non-dihydropyridine calcium channel blockers (verapamil) is still not clear [46]. Some trials showed improvement in diastolic function [47]. A sub study of the ASCOT trial (Anglo-Scandinavian cardiac outcome trial) demonstrated that patients getting an amlodipine-perindopril regimen had improved diastolic function as compared to atenolol-thiazide therapy [48]. Similarly, nifedipine and isosorbide dinitrate/hydralazine showed an improvement in diastolic function and exercise tolerance together with a reduction in soluble vascular cell adhesion Molecule-I with no reduction in left ventricular hypertrophy and pulmonary congestion [49].
Beta blockers work by reducing heart rate and prolonging the diastolic filling time, which improves filling of stiffed ventricles, however, the evidence still lacks [30]. Although a small, early study of propranolol suggested a reduction in total mortality, the patient group was atypical in that all were selected to have prior MI (myocardial infarction) and were mostly men, and the ejection fraction (EF) was as low as 40% [48]. In addition, there was no significant difference in cardiac deaths between these groups. Both carvedilol (the J-DHF study) and nebivolol (ELANDD study) had equivalent effects on the outcome in HFpEF patients [49]. Discharging HFpEF patients with beta blockers had no effect on one-year mortality or re-hospitalization as shown in the OPTIMIZE-HF registry [49].
Digoxin at a serum concentration of 0-0.9 ng/ml reduces heart failure re-hospitalization but has no effect on mortality or all cause re-hospitalization in patients with diastolic heart failure [50]. Digoxin when compared with placebo in the digitalis investigation group (DIG) trial, showed neutral results on mortality among patients with HFpEF [32]. A recent study showed a statistically significant effect of mortality and re-hospitalization in heart failure patients with either HFpEF or HFrEF treated with digoxin for two years as compared to placebo, however, at the end (3.2 year period) there was no difference between digoxin and placebo in both groups which may be due to higher digoxin dose or higher crossover as shown by Meyer and his coworkers. Recommendations suggest that the results of the DIG trial may provide support for the use of digoxin in patients who have HFpEF, because a trend towards reduction in hospitalizations for heart failure was observed with digoxin in the ancillary trial [50]. The DIG trial provided support of a beneficial effect of digoxin in patients with HFpEF, however, the current guideline does not support the usage as the main mechanism is defective relaxation not contraction [50].
Contemporary therapies with promise
Statins as anti-inflammatory agents
Statins, while important in management of CAD, still had a minimal role in patients with HFrEF [34]. In patients with HFpEF there are multiple studies showing that using statins in patients with HFpEF had a beneficial clinical effect. Fukuta et al. [51], reported that patients with heart failure and ejection fraction of more than 50% when treated with statins and followed for 21 months showed a significant reduction in mortality [52]. Another study showed that using statins is associated with improved survival [51]. In the EuroHeart Failure Survey analysis of 6800 heart failure patients of whom 46% had HFpEF, those using statins had a lower all-cause mortality over 12 weeks [50]. A meta-analysis of nearly 18,000 patients supported the use of statins for better survival in patients with HFpEF, although it is worth mentioning that statins did not show any significant effect in two mega trials in patients with HFrEF [53].
PDE-5 inhibitors: phosphodiesterase-5 (PDEI-5)
Phosphodiesterase-5 (PDE5) is highly explicit in pulmonary artery smooth muscle cells and involved in breakdown of cGMP leading to vasoconstriction. PDEI-5 sildenafil might have some benefits in patients with HFpEF [54]. As of now, end points in studies of PDE-5 inhibition in HFpEF are mainly improvement of symptoms and functional class [55] which still reflects on quality of life. The dilemma of end point selection is still ambiguous, such as in patients with HFrEF. For example, using inotropes improves symptoms but worsens survival, while using beta blockers worsens symptoms acutely but reduces mortality [55]. A small trial showed symptomatic improvements, however, the relax trial in patients with HFpEF reported no significant improvement in exercise capacity, quality of life or diastolic function, but the clinical benefits still need to be evaluated [54].
Isosorbide mononitrate
In the NEAT-HFpEF trial, isosorbide mononitrate did not improve 6-min walk distance (MWD), quality of life, or NT-pro B type natriuretic peptide (BNP) levels as compared to placebo [56]. Even though, this result is disappointing about nitric oxide deficiency in patients with endothelial dysfunction, this is because of continuous release of nitric oxide by the effect of therapeutic nitrate and there is no targeted release when needed together with occurrence of nitrate tolerance. Randomized studies revealed that inhaled or intravenous sodium nitrite increases stroke volume and cardiac output, and reduces ventricular filling and pulmonary artery pressure at rest and with exercise [56]. Beneficial effects are thought to be related to enhanced production of nitric oxide by nitrite in the setting of tissue hypoxia and acidosis [57].
Paradigm shifts in management
There is a huge requirement for management of HFpEF due to its increasing prevalence. Additionally, there is a need for new strategies aimed towards specific phenotypes and mechanisms of HFpEF.
The current therapeutic approach for management of HFrEF was not successful in management of patients with HFpEF, which is the main stimulus for targeting other pathophysiological mechanisms in the molecular structure [25] such as anti-fibrotic, anti-hypertrophic and anti-inflammatory drugs [58].
According to the PROMIS study, coronary microvascular dysfunction (CMD) was frequently found in patients with HFpEF despite no significant macrovascular coronary artery disease [25] which indicates that microvascular dysfunction may be a promising therapeutic target in HFpEF.
There is a new paradigm shift away from the traditional emphasis on afterload excess pathway (RAAS) and towards inflammation as the primary stimulus for hypertrophy and diastolic dysfunction [59]. Declining protein kinase G (PKG) pathway activity accelerates pro-hypertrophic signaling and increases myocyte stiffness by promoting hypophosphorylation of titin, enhancing diastolic dysfunction and ventricular stiffening (NO-cGMP-PKG signaling pathway) [60].
Verciguat
This molecule was found to stimulate the soluble cGMP pathway in patients with HFpEF and is currently being studied in the SOCRATES trial (Soluble Guanylate Cyclase Stimulator Heart Failure Study). In a prespecified secondary analysis, a dose-related effect on the primary endpoint change in NT-proBNP levels was observed. Moreover, vericiguat was not associated with any deleterious effects on heart rate, blood pressure, renal function, or troponin release [61]. Reduction in NT-proBNP in the highest dose arm was associated with improved LVEF and trended towards fewer clinical events at 12 weeks [62].
LCZ696
LCZ696 is a first-in-class ARNI (angiotensin receptor neprilysin inhibitors) that is a complex molecule derived from the combination of the neprilysin inhibitor (neprilysin degrades biologically active natriuretic peptides that reduce the production of cGMP) and the angiotensin receptor blocker valsartan. LCZ696 additionally stimulates natriuretic peptide signals through transmembrane receptor guanylyl cyclase, which acts as an antihypertrophic and antifibrotic, and improves diastolic function (lusitropic effect) by stimulating the activity of PKG [63].
LCZ696 has been demonstrated to decrease NT-proBNP in a phase II trial in patients with HFpEF (LVEF ≥ 45%) [55]. LCZ696 is currently being tested on a large scale in a phase III trial PARAGON-HF [64].
Ranolazine
Ranolazine causes late sodium current inhibition, and a verification of a study (Ranolazine for the Treatment of Diastolic Heart Failure [RALI-DHF]) in 20 patients with HFpEF indicated an improvement in hemodynamic parameters but no effect on diastolic function. Further clinical studies are needed to address the efficacy of ranolazine in patients with HFpEF [65].
Ivabradine
Inhibitor ivabradine, when used for heart rate reduction in HFpEF, was found to have an effect as an anti-hypertrophic, anti-fibrotic, anti-inflammatory and anti-apoptotic agent as compared to metoprolol (a selective B1 receptor blocker) as shown in a preliminary and experimental study [65].
These results testify that the addition of ivabradine to conventional therapy in patients with HFpEF can improve LV diastolic function, as evaluated by 2D and tissue Doppler-echocardiographic patterns. These Doppler-echocardiographic results match with the clinical improvement of patients evaluated [66]. Ivabradine improves diastolic function and decreases aortic stiffness and fibrosis in a diabetic mouse model with HFpEF [67].
Anakinra
Pro-inflammatory cytokines IL-1 and IL-1B (Interleukin-1) contribute to ventricular and vascular remodeling in cases of pressure overload induced cardiac hypertrophy. Anakinra, an IL-1 antagonist used in management of rheumatoid arthritis, was shown in the D-HART Pilot Study in HFpEF patients to have led to a significant reduction in C-reactive protein (CRP) and resulted in significant improvement in oxygen consumption [23].
In response to chronic cardiac stress as in heart failure, there is higher level of ST2 (interleukin 1 receptor-like), and the receptor for IL-33 (IL 33 is a member of IL-1 family) together with upregulation of IL-33/ST2 pathway which causes hypertrophy and fibrosis [24]. Aging is associated with increased metallo-proteinase-9 (MMP-9) levels that precedes the development of diastolic dysfunction, this lead to use MMP-9 inhibitor in experimental mice study, transcription factor STATS3 (Signal transducer and activator of transcription 3) had a cardiac protection and anti-remolding, decreased the effect of angiotensin and improved endothelial function and antioxidant. Further studies are warranted for evidence based medicine [25].
Advanced glycation end-products (AGEs) breaker
Advanced glycation end-products (AGEs) are formed when glucose interacts non-enzymatically with proteins. AGEs cause myocardial stiffness through either direct cross linking of collagen and elastin fibers or indirectly stimulating oxidative stress via promoting production of stiffer collagen together with reduction of nitric oxide [68].
Advanced glycation end-products breaker (Algebrium) may have a beneficial effect in the management of HFpEF [69]. Algebrium reduces left ventricular mass with reducing left ventricular filling pressure with improvement in quality of life [70]. In spite of failure in a proof of concept in patients HFrEF further studies are needed in patients with HFpEF [69].
SGLT2
Studies are underway to test the efficacy of inhibitors of sodium-glucose cotransporter type 2 (SGLT2) in HFpEF patients. Recently, empagliflozin resulted in reduction of hospitalization and cardiovascular mortality together with a reduction in blood pressure and body weight in heart failure patients mostly due to glucosuria and osmotic diuresis. Empagliflozin, which showed a valuable effect in patients with and without heart failure, is currently being evaluated in the EMPEROR-Preserved study while the HF-PRESERVED trial, will evaluate the effect of dapagliflozin on biomarkers, symptoms, and functional status in HFpEF patients with type-2 diabetes or prediabetes [71].
Sitaxsentan
Sitaxsentan, a selective endothelin type-A (ETA) receptor antagonist, provides a modest increase in treadmill exercise time [62] in HFpEF patients, but did not improve any of the secondary endpoints such as left ventricular mass or diastolic function. Further studies will be necessary to determine the effect of ETA receptor antagonists on HFpEF.
Other strategies that can be used include myostatin-blocking antibodies and MicroRNAs. However, well designed proof of conceptual studies is still needed for further confirmation. A Phase-I trial evaluating the safety and tolerability of MTP-131 (elamipretide, a novel mitochondria-targeting peptide) in mild to moderate HF patients is currently under investigation [25].
Device therapy
CardioMEMS
CardioMEMS is a wireless device using a radio frequency transmitter inserted in the distal pulmonary artery during right heart catheterization to monitor daily hemodynamic parameters [72]. In the single-blind randomized CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients) trial, 119 HFpEF patients were implanted with a microelectro-mechanical pressure sensor during right heart catheterization [55]. Patients were randomized to sensor guided treatment or regular clinical guided treatment and were followed for up to 18 months. Those with sensor guided treatment had a 50% reduction in hospitalization for heart failure. Further studies are warranted [73].
Interatrial shunt
Heart failure symptoms in HFpEF are usually preceded by a rise in LA pressure and pulmonary venous congestion, so creating a controlled or unidirectional shunt between atria to allow left atrial decompression will relieve symptoms in patients with HFpEF. Hemodynamic modeling based on clinical measurements suggests that an appropriately sized iatrogenic atrial septal defect could reduce exercise-induced increase in LA pressure in patients with HFpEF. Subsequently, an open-label study (REDUCE LAP heart failure trial) showed an exercise reduction in left atrial pressure with symptomatic improvement in functional tolerance and quality of life during a 6 month follow up period [74]. During one year of follow up, device patency was maintained and there was an improvement in functional class and exercise tolerance together with an improvement in quality of life and in pulmonary arterial wedge pressure.
Microventricular assist device
A device implanted via mini-thoracotomy in the right subclavian area to drain the blood from the left atrium to the right subclavian artery had a significant value on hemodynamics, increased cardiac output, provided mild increase in blood pressure, and reduced left atrial and pulmonary artery pressure in patients with HFrEF, however, in HFpEF, it still needs to be evaluated [75].
Baroreflex activation therapy
Carotid sinus stimulation will intensify the parasympathetic tone in patients with HFpEF for their autonomic dysfunction [76]. BAT (baroreflex activation therapy) was studied in HOP4HF (a randomized outcome trial to evaluate the clinical safety and efficacy of BAT in HFpEF patients) for HFpEF (HYHA III) in addition to guideline medical therapy. It was found to be safe, with improvement in exercise tolerance, quality of life and reduction of heart failure hospitalization [77].
Rate-adaptive pacing (RAP)
Rate-adaptive pacemakers showed considerable benefits to patients with mild to moderate HFpEF for treatment of chronotropic incompetence, as shown in the RESET trial [75].
Left atrial (LA) pacing therapy
Pacing of the left atrium had a valuable effect, demonstrating improvement of left ventricular filling and reduction of left atrial pressure, which will have a clinical effect in patients with HFpEF due to the presence of interatrial dyssynchrony [74]. Cardiac resynchronization therapy might be beneficial in HFpEF and is currently under investigation [78].
Cardiac contractility modulation (CCM)
CCM used in patients with HFpEF with reduced EF less than 35% and with normal or slightly wider QRS complex did not fulfill the indication of cardiac synchronization therapy. CCM emits biphasic high voltage bipolar signal to the right ventricular septum during the absolute refractory period resulting in improvement in myocardial contraction with subsequent improvement in quality of life and exercise capacity [79].
CCM was used experimentally in 2 patients with HFpEF and resulted in improvement of functional class, exercise capacity, quality of life, and diastolic function. In HFpEF, there is low phosphorylation of titin resulting in stiffness and fibrosis. CCM improves phosphorylation of titin with subsequent reduction in fibrosis and collagen expression [79].
Exercise training
Exercise rehabilitation programs in patients with HFpEF had a valuable effect, either due to a cardiac effect through reversing atrial remodeling and reducing diastolic dysfunction or due to peripheral mechanisms through improving endothelial dysfunction in skeletal vasculature [80]. Making lifestyle changes to incorporate more activity is recommended in stable patients with HFrEF. In patient with HFpEF, Ex-DHF (exercise training in diastolic heart failure) showed an improvement in quality of life mainly by improving cardiac function [81], while on the other hand Haykosky et al. [82], showed that the improvement in exercise capacity was mainly due to improvement in skeletal muscle vasculature. Kitzman et al. [83], showed that after 16 weeks of exercise there is an increase in peak VO2 (Volume of Oxygen) that was dissociated from endothelial dependent arterial dilatation [73]. In contrast, Fujimoto et al. [84], found that one year of exercise training failed to improve cardiac output. Overall, exercise training improves exercise tolerance and quality of life in patients with HFpEF. Cardiac rehabilitation programs are warranted and need further evaluation [42].
Nutritional strategies
Dietary modifications have a valuable effect, such as the DASH (sodium restricted dietary approach to stop hypertension) diet for treatment of hypertensive HFpEF, which showed an improvement in diastolic function along with reduced ventricular and arterial stiffness [85]. Beetroot juice (rich in inorganic nitrate) daily for one week significantly improved submaximal aerobic endurance and blood pressure in elderly HFpEF patients [86]. Zamani et al. [86], found that a single dose of inorganic nitrate (beetroot juice) given to the patients with HFpEF improves peak oxygen consumption via reduction of systemic vascular resistance. Inorganic nitrate converts to nitrite by the effect of oral flora, which in turn converts into nitrate by the effect of hypoxia that will cause vasodilation and increase blood flow with increase in oxygen delivery to the skeletal muscle [83]. Kitzman et al. [83], showed that caloric restriction in addition to exercise training in elderly HFpEF patients significantly improves exercise tolerance and quality of life [87].
Current guidelines
ACC/AHA and the European Society of Cardiology (ESC) guidelines have similar recommendations. The guidelines for the management of HFpEF advocate controlling of blood pressure, management of comorbidities, and adjustment of volume status with a fitting diuretic dose to relieve hypervolemia without causing hypotension [18].
Perspectives in management
No treatment has yet been shown to reduce morbidity and mortality in patients with HFpEF. There is a deficiency of evidence in management of HFpEF, in contrast to the evidences in management of HFrEF [88].
This could be due to variation in patient selection, dropout rates, phenotypes/stages of the disease, etc. (The absence of evidence from clinical trials does not mean evidence of absence) [89].
HFpEF syndrome is a superimposition of HF over a systemic disease which needs proper phenotypic differentiation and control of the associated comorbidities [90]. Molecular structural changes, left atrial hypertension and elevated pulmonary artery pressure contribute in a majority of the symptoms. Therefore, the main strategy should be pulmonary decongestion and control pulmonary artery pressure [16]. There is an intense need to control comorbidities, improve reserve through exercise rehabilitation programs and restore the balance of oxidative stress due to various factors [91]. It is important to avoid inotropic constraint, achieve euvolemic status and control heart rate (ivabradine). There is therapeutic potential in soluble cyclic guanylyl cyclase activators, tetrahydrobiopterin (BH4) cofactor essential for production of nitric oxide, PKG modulators, nitric oxide (nNOS) activators, and NADPH oxidase 2 (NOX2) inhibitors. (NOX2 stimulation will lead to cardiomyopathy and oxidative stress in case of sepsis) [92]. Patients with chronic HFpEF should not be treated as though they have chronic HFrEF (however, acute HFpEF and HFrEF are treated similarly). It is advisable to avoid drugs that do not have benefit especially those which are considered for the treatment of HFrEF [89]. When there is pulmonary hypertension, functional NYHA classification is necessary along with a 6 minute walk test, at the start of the treatment and on follow up, to detect the hemodynamic response [93].
Conclusion
HFpEF is a clinical syndrome of multiple pathophysiological mechanisms, differential diagnoses, comorbidities, misdiagnoses, and maltreatments are associated with clinical outcomes similar to HFrEF. It is challenging to deal with such patients, their frequent emergency room visits and hospital readmissions. Diagnostic modalities such as hemodynamic assessment, elevated pulmonary artery pressure, and assessment for coronary artery disease are significant addition to the usual diagnostic modalities, where B-type natriuretic peptide is less specific. There are few evidence-based therapies with no stringent guidelines. Management of comorbidities and phenotypic consideration will help in improving clinical outcomes. It is also essential that further trials regarding therapeutic innovations be conducted to benefit patients with HFpEF.
Disclosure of conflict of interest
None.
References
- 1.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 3.How to diagnose diastolic heart failure. European Study Group on Diastolic Heart Failure. Eur Heart J. 1998;19:990–1003. doi: 10.1053/euhj.1998.1057. [DOI] [PubMed] [Google Scholar]
- 4.Paulus WJ, Van Ballegoij JJ. Treatment of heart failure with normal ejection fraction: an inconvenient truth. J Am Coll Cardiol. 2010;55:526–537. doi: 10.1016/j.jacc.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 5.Maurer MS, Hummel SL. Heart failure with a preserved ejection fraction: what is in a name? J Am Coll Cardiol. 2011;58:275–277. doi: 10.1016/j.jacc.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 6.Yu CM, Lin H, Yang H, Kong SL, Zhang Q, Lee SW. Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation. 2002;105:1195–1201. doi: 10.1161/hc1002.105185. [DOI] [PubMed] [Google Scholar]
- 7.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 9.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the Urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 10.Ennezat PV, Lefetz Y, Marechaux S, Six-Carpentier M, Deklunder G, Montaigne D, Bauchart JJ, Mounier-Vehier C, Jude B, Neviere R, Bauters C, Asseman P, de Groote P, Lejemtel TH. Left ventricular abnormal response during dynamic exercise in patients with heart failure and preserved left ventricular ejection fraction at rest. J Card Fail. 2008;14:475–480. doi: 10.1016/j.cardfail.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 12.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurer MS, Burkhoff D, Fried LP, Gottdiener J, King DL, Kitzman DW. Ventricular structure and function in hypertensive participants with heart failure and a normal ejection fraction: the cardiovascular health study. J Am Coll Cardiol. 2007;49:972–981. doi: 10.1016/j.jacc.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 14.Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss HP, Tschope C. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117:2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 15.Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A, Frenneaux M. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009;54:402–409. doi: 10.1016/j.jacc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Prasad A, Hastings JL, Shibata S, Popovic ZB, Arbab-Zadeh A, Bhella PS, Okazaki K, Fu Q, Berk M, Palmer D, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. Characterization of static and dynamic left ventricular diastolic function in patients with heart failure with a preserved ejection fraction. Circ Heart Fail. 2010;3:617–626. doi: 10.1161/CIRCHEARTFAILURE.109.867044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanhecke TE, Kim R, Raheem SZ, McCullough PA. Myocardial ischemia in patients with diastolic dysfunction and heart failure. Curr Cardiol Rep. 2010;12:216–222. doi: 10.1007/s11886-010-0101-1. [DOI] [PubMed] [Google Scholar]
- 18.Guidelines for the evaluation and management of heart failure. Report of the American college of cardiology/American heart association task force on practice guidelines (Committee on evaluation and management of heart failure) Circulation. 1995;92:2764–2784. doi: 10.1161/01.cir.92.9.2764. [DOI] [PubMed] [Google Scholar]
- 19.González A, López B, Querejeta R, Zubillaga E, Echeverría T, Díez J. Filling pressures and collagen metabolism in hypertensive patients with heart failure and normal ejection fraction. Hypertension. 2010;55:1418–1424. doi: 10.1161/HYPERTENSIONAHA.109.149112. [DOI] [PubMed] [Google Scholar]
- 20.Franssen C, Hamdani N, Ottenheijm CA, Paulus WJ. Relative importance of titin and collagen for myocardial stiffness in metabolic risk-induced heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;61:E696. [Google Scholar]
- 21.Díez J, Querejeta R, López B, González A, Larman M, Martínez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–2517. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 22.Weber KT, Brilla CG, Janicki JS. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res. 1993;27:341–348. doi: 10.1093/cvr/27.3.341. [DOI] [PubMed] [Google Scholar]
- 23.Heymans S, Hirsch E, Anker SD, Aukrust P, Balligand JL, Cohen-Tervaert JW, Drexler H, Filippatos G, Felix SB, Gullestad L, Hilfiker-Kleiner D, Janssens S, Latini R, Neubauer G, Paulus WJ, Pieske B, Ponikowski P, Schroen B, Schultheiss HP, Tschope C, Van Bilsen M, Zannad F, McMurray J, Shah AM. Inflammation as a therapeutic target in heart failure? A scientific statement from the translational research committee of the heart failure association of the European society of cardiology. Eur J Heart Fail. 2009;11:119–129. doi: 10.1093/eurjhf/hfn043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschope C. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4:44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451. [DOI] [PubMed] [Google Scholar]
- 25.Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, Beussink-Nelson L, Ljung Faxén U, Fermer ML, Broberg MA, Gan LM, Lund LH. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J. 2018;39:3439–3450. doi: 10.1093/eurheartj/ehy531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Ghamdi B, Shafquat A, Mallawi Y. Cardiac contractility modulation therapy: are there superresponders? HeartRhythm Case Rep. 2017;3:229–232. doi: 10.1016/j.hrcr.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138:861–870. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 29.Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, Lopez-Sendon J, Teerlink JR, White M, McMurray JJ, Komajda M, McKelvie R, Ptaszynska A, Hetzel SJ, Massie BM, Carson PE I-Preserve Investigators. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in heart failure with preserved ejection fraction study (I-Preserve) trial. Circulation. 2010;121:1393–1405. doi: 10.1161/CIRCULATIONAHA.109.909614. [DOI] [PubMed] [Google Scholar]
- 30.Drazen JM, Morrissey S, Campion EW, John A, Jarcho A. Randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2174–2175. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boonyasirinant T, Rajiah P, Setser RM, Lieber ML, Lever HM, Desai MY, Flamm SD. Aortic stiffness is increased in hypertrophic cardiomyopathy with myocardial fibrosis: novel insights in vascular function from magnetic resonance imaging. J Am Coll Cardiol. 2009;54:255–262. doi: 10.1016/j.jacc.2009.03.060. [DOI] [PubMed] [Google Scholar]
- 32.Phan TT, Shivu GN, Abozguia K, Davies C, Nassimizadeh M, Jimenez D, Weaver R, Ahmed I, Frenneaux M. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:29–34. doi: 10.1161/CIRCHEARTFAILURE.109.877720. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Kurrelmeyer KM, Torre-Amione G, Nagueh SF. Systolic and diastolic dyssynchrony in patients with diastolic heart failure and the effect of medical therapy. J Am Coll Cardiol. 2007;49:88–96. doi: 10.1016/j.jacc.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 34.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 35.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J, Shi H, Zhang J, Lu Y, Fu M, Ge J beta-PRESERVE Study Investigators. Rationale and design of the beta-blocker in heart failure with normal left ventricular ejection fraction (beta-PRESERVE) study. Eur J Heart Fail. 2010;12:181–185. doi: 10.1093/eurjhf/hfp193. [DOI] [PubMed] [Google Scholar]
- 38.Yip GW, Wang M, Wang T, Chan S, Fung JW, Yeung L, Yip T, Lau ST, Lau CP, Tang MO, Yu CM, Sanderson JE. The Hong Kong diastolic heart failure study: a randomised controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart. 2008;94:573–580. doi: 10.1136/hrt.2007.117978. [DOI] [PubMed] [Google Scholar]
- 39.Thorvaldsen T, Claggett BL, Shah A, Cheng S, Agarwal SK, Wruck LM, Chang PP, Rosamond WD, Lewis EF, Desai AS, Lund LH, Solomon SD. Predicting risk in patients hospitalized for acute decompensated heart failure and preserved ejection fraction: the atherosclerosis risk in communities study heart failure community surveillance. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.117.003992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 41.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 42.Hogg K, McMurray J. Neurohumoral pathways in heart failure with preserved systolic function. Prog Cardiovasc Dis. 2005;47:357–366. doi: 10.1016/j.pcad.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Von Lueder TG, Sangaralingham SJ, Wang BH, Kompa AR, Atar D, Burnett JC Jr, Krum H. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail. 2013;6:594–605. doi: 10.1161/CIRCHEARTFAILURE.112.000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mangiafico S, Costello-Boerrigter LC, Andersen IA, Cataliotti A, Burnett JC Jr. Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. Eur Heart J. 2013;34:886c–893c. doi: 10.1093/eurheartj/ehs262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the randomized aldosterone antagonism in heart failure with preserved ejection fraction trial (RAAM-PEF) J Card Fail. 2011;17:634–642. doi: 10.1016/j.cardfail.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Bergstrom A, Andersson B, Edner M, Nylander E, Persson H, Dahlstrom U. Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish doppler-echocardiographic study (SWEDIC) Eur J Heart Fail. 2004;6:453–461. doi: 10.1016/j.ejheart.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Conraads VM, Metra M, Kamp O, De Keulenaer GW, Pieske B, Zamorano J, Vardas PE, Böhm M, Dei Cas L. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail. 2012;14:219–225. doi: 10.1093/eurjhf/hfr161. [DOI] [PubMed] [Google Scholar]
- 48.van Veldhuisen DJ, Cohen-Solal A, Bohm M, Anker SD, Babalis D, Roughton M, Coats AJ, Poole-Wilson PA, Flather MD SENIORS Investigators. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: data from SENIORS (Study of effects of nebivolol intervention on outcomes and rehospitalization in seniors with heart failure) J Am Coll Cardiol. 2009;53:2150–2158. doi: 10.1016/j.jacc.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto K, Origasa H, Hori M J-DHF Investigators. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese diastolic heart failure study (J-DHF) Eur J Heart Fail. 2013;15:110–118. doi: 10.1093/eurjhf/hfs141. [DOI] [PubMed] [Google Scholar]
- 50.Van Veldhuisen DJ, McMurray JJ. Pharmacological treatment of heart failure with preserved ejection fraction: a glimpse of light at the end of the tunnel? Eur J Heart Fail. 2013;15:5–8. doi: 10.1093/eurjhf/hfs194. [DOI] [PubMed] [Google Scholar]
- 51.Fukuta H, Sane DC, Brucks S, Little WC. Statin therapy may be associated with lower mortality in patients with diastolic heart failure: a preliminary report. Circulation. 2005;112:357–363. doi: 10.1161/CIRCULATIONAHA.104.519876. [DOI] [PubMed] [Google Scholar]
- 52.Davis BR, Kostis JB, Simpson LM, Black HR, Cushman WC, Einhorn PT, Farber MA, Ford CE, Levy D, Massie BM, Nawaz S ALLHAT Collaborative Research Group. Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation. 2008;118:2259–2267. doi: 10.1161/CIRCULATIONAHA.107.762229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kjekshus J, Pedersen TR, Olsson AG, Faergeman O, Pyörälä K. The effects of simvastatin on the incidence of heart failure in patients with coronary heart disease. J Card Fail. 1997;3:249–254. doi: 10.1016/s1071-9164(97)90022-1. [DOI] [PubMed] [Google Scholar]
- 54.Lam CS, Brutsaert DL. Endothelial dysfunction: a pathophysiologic factor in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2012;60:1787–1789. doi: 10.1016/j.jacc.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–174. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 56.Kitzman DW, Rich MW. Age disparities in heart failure research. JAMA. 2010;304:1950–1951. doi: 10.1001/jama.2010.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zakeri R, Levine JA, Koepp GA, Borlaug BA, Chirinos JA, LeWinter M, Van Buren P, Dávila-Román VG, de Las Fuentes L, Khazanie P, Hernandez A, Anstrom K, Redfield MM. Nitrate’s effect on activity tolerance in heart failure with preserved ejection fraction trial: rationale and design. Circ Heart Fail. 2015;8:221–228. doi: 10.1161/CIRCHEARTFAILURE.114.001598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maeder MT, Kaye DM. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2009;53:905–918. doi: 10.1016/j.jacc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 59.Van Heerebeek L, Hamdani N, Falcão-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–839. doi: 10.1161/CIRCULATIONAHA.111.076075. [DOI] [PubMed] [Google Scholar]
- 60.Van Heerebeek L, Franssen CP, Hamdani N, Verheugt FW, Somsen GA, Paulus WJ. Molecular and cellular basis for diastolic dysfunction. Curr Heart Fail Rep. 2012;9:293–302. doi: 10.1007/s11897-012-0109-5. [DOI] [PubMed] [Google Scholar]
- 61.Butler J, Filippatos G, Lam CS, Maggioni AP, Ponikowski P, Shah SJ, Solomon SD, Kraigher-Krainer E, Samano ET. Oral sGC stimulator vericiguat in patients with worsening chronic heart failure and reduced ejection fraction - the soluble guanylate cyclase stimulator in heart failure patients with reduced EF (SOCRATES-REDUCED) study. JAMA. 2015;314:2251–2262. doi: 10.1001/jama.2015.15734. [DOI] [PubMed] [Google Scholar]
- 62.Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, Ohba K, Matsubara J, Maeda H, Horibata Y, Sakamoto K, Sugamura K, Yamamuro M, Sumida H, Kaikita K, Iwashita S, Matsui K, Kimura K, Umemura S, Ogawa H. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1778–1786. doi: 10.1016/j.jacc.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 63.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ Prospective comparison of ARNI with ARB on Management of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 64.Maier LS, Layug B, Karwatowska-Prokopczuk E, Belardinelli L, Lee S, Sander J, Lang C, Wachter R, Edelmann F, Hasenfuss G, Jacobshagen C. Ranolazine for the treatment of diastolic heart failure in patients with preserved ejection fraction: the RALI-DHF proof-of-concept study. JACC Heart Fail. 2013;1:115–122. doi: 10.1016/j.jchf.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 65.Reil JC, Hohl M, Reil GH, Granzier HL, Kratz MT, Kazakov A, Fries P, Müller A, Lenski M, Custodis F, Gräber S, Fröhlig G, Steendijk P, Neuberger HR, Bohm M. Heart rate reduction by If-inhibition improves vascular stiffness and left ventricular systolic and diastolic function in a mouse model of heart failure with preserved ejection fraction. Eur Heart J. 2013;34:2839–2849. doi: 10.1093/eurheartj/ehs218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cacciapuoti F, Magro VM, Caturano M, Lama D, Cacciapuoti F. The role of ivabradine in diastolic heart failure with preserved ejection fraction. A doppler-echocardiographic study. J Cardiovasc Echogr. 2017;27:126–131. doi: 10.4103/jcecho.jcecho_6_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCaffrey DJ, McDonald K. If channel blockade with ivabradine in patients with diastolic heart failure. Eur Heart J. 2010;32:670–679. [Google Scholar]
- 68.Hartog JW, Voors AA, Bakker SJ, Smit AJ, van Veldhuisen DJ. Advanced glycation end-products and heart failure: pathophysiology and clinical implications. Eur J Heart Fail. 2007;9:1146–1155. doi: 10.1016/j.ejheart.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 69.Little WC, Zile MR, Kitzman DW, Hundley WG, O’Brien TX, DeGroof RC. The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J Card Fail. 2005;11:191–195. doi: 10.1016/j.cardfail.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 70.Hartog JW, Willemsen S, van Veldhuisen DJ, Posma JL, van Wijk LM, Hummel YM, Hillege HL, Voors AA BENEFICIAL investigators. Effects of alagebrium, an advanced glycation endproduct breaker, on exercise tolerance and cardiac function in patients with chronic heart failure. Eur J Heart Fail. 2011;13:899–908. doi: 10.1093/eurjhf/hfr067. [DOI] [PubMed] [Google Scholar]
- 71.Rastogi A, Bhansali A. SGLT2 inhibitors through the windows of EMPA-REG and CANVAS trials: a review. Diabetes Ther. 2017;8:1245–1251. doi: 10.1007/s13300-017-0320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS CHAMPION Trial Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 73.Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935–944. doi: 10.1161/CIRCHEARTFAILURE.113.001229. [DOI] [PubMed] [Google Scholar]
- 74.Feldman T, Mauri L, Kahwash R, Litwin S, Ricciardi MJ, van der Harst P, Penicka M, Fail PS, Kaye DM, Petrie MC, Basuray A, Hummel SL, Forde-McLean R, Nielsen CD, Lilly S, Massaro JM, Burkhoff D, Shah SJ REDUCE LAP-HF I Investigators and Study Coordinators. Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction REDUCE LAP-HF: a phase 2, randomized, sham-controlled trial. Circulation. 2018;137:364–375. doi: 10.1161/CIRCULATIONAHA.117.032094. [DOI] [PubMed] [Google Scholar]
- 75.Laurent G, Eicher JC, Mathe A, Bertaux G, Barthez O, Debin R, Billard C, Philip JL, Wolf JE. Permanent left atrial pacing therapy may improve symptoms in heart failure patients with preserved ejection fraction and atrial dyssynchrony: a pilot study prior to a national clinical research programme. Eur J Heart Fail. 2013;15:85–93. doi: 10.1093/eurjhf/hfs150. [DOI] [PubMed] [Google Scholar]
- 76.Olshansky B, Sabbah HN, Hauptman PJ, Colucci WS. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation. 2008;118:863–871. doi: 10.1161/CIRCULATIONAHA.107.760405. [DOI] [PubMed] [Google Scholar]
- 77.Kass DA, Kitzman DW, Alvarez GE. The restoration of chronotropic competence in heart failure patients with normal ejection fraction (RESET) study: rationale and design. J Card Fail. 2010;16:17–24. doi: 10.1016/j.cardfail.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Penicka M, Kocka V, Herman D, Trakalova H, Herold M. Cardiac resynchronization therapy for the causal treatment of heart failure with preserved ejection fraction: insight from a pressure-volume loop analysis. Eur J Heart Fail. 2010;12:634–636. doi: 10.1093/eurjhf/hfq068. [DOI] [PubMed] [Google Scholar]
- 79.Paulus WJ. A properly validated diagnostic algorithm for heart failure with preserved ejection fraction. Circulation. 2018;138:871–873. doi: 10.1161/CIRCULATIONAHA.118.035711. [DOI] [PubMed] [Google Scholar]
- 80.Keteyian SJ. Exercise training in patients with heart failure and preserved ejection fraction: findings awaiting discovery. J Am Coll Cardiol. 2013;62:593–594. doi: 10.1016/j.jacc.2013.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Edelmann F, Stahrenberg R, Gelbrich G, Durstewitz K, Angermann CE, Dungen HD, Scheffold T, Zugck C, Maisch B, Regitz-Zagrosek V, Hasenfuss G, Pieske BM, Wachter R. Contribution of comorbidities to functional impairment is higher in heart failure with preserved than with reduced ejection fraction. Clin Res Cardiol. 2011;100:755–764. doi: 10.1007/s00392-011-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–74. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fujimoto N, Prasad A, Hastings JL, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive endurance exercise training in patients with heart failure with preserved ejection fraction. Am Heart J. 2012;164:869–877. doi: 10.1016/j.ahj.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC, Chirinos JA. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–380. doi: 10.1161/CIRCULATIONAHA.114.012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Little WC, Brucks S. Therapy for diastolic heart failure. Prog Cardiovasc Dis. 2005;47:380–388. doi: 10.1016/j.pcad.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 88.Handoko ML, Paulus WJ. Polishing the diastolic dysfunction measurement stick. Eur J Echocardiogr. 2008;9:575–577. doi: 10.1093/ejechocard/jen181. [DOI] [PubMed] [Google Scholar]
- 89.Elshaer F, Hassan W, Fawzy ME, Lockyer M, Kharabsheh S, Akhras N, Shahid M, Elwidaa H, Elkum N, Canver C. The prevalence, clinical characteristics, and prognosis of diastolic heart failure: a clinical study in elderly Saudi patients with up to 5 years follow-up. Congest Heart Fail. 2009;15:117–122. doi: 10.1111/j.1751-7133.2008.00043.x. [DOI] [PubMed] [Google Scholar]
- 90.Phan TT, Shivu GN, Abozguia K, Gnanadevan M, Ahmed I, Frenneaux M. Left ventricular torsion and strain patterns in heart failure with normal ejection fraction are similar to age-related changes. Eur J Echocardiogr. 2009;10:793–800. doi: 10.1093/ejechocard/jep072. [DOI] [PubMed] [Google Scholar]
- 91.AlHabib KF, Elasfar AA, AlBackr H, AlFaleh H, Hersi A, AlShaer F, Kashour T, AlNemer K, Hussein GA, Mimish L. Design and preliminary results of the heart function assessment registry trial in Saudi Arabia in patients with acute and chronic heart failure. Eur J Heart Fail. 2011;13:1178–1184. doi: 10.1093/eurjhf/hfr111. [DOI] [PubMed] [Google Scholar]
- 92.Joseph LC, Kokkinaki D, Valenti MC, Kim GJ, Barca E, Tomar D, Hoffman NE, Subramanyam P, Colecraft HM, Hirano M, Ratner AJ, Madesh M, Drosatos K, Morrow JP. Inhibition of NADPH oxidase 2 (NOX2) prevents sepsis-induced cardiomyopathy by improving calcium handling and mitochondrial function. JCI Insight. 2017;2 doi: 10.1172/jci.insight.94248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aronow WS, Ahn C, Kronzon I. Effect of propranolol versus no propranolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction ≥ 40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol. 1997;80:207–209. doi: 10.1016/s0002-9149(97)00320-2. [DOI] [PubMed] [Google Scholar]


