Abstract
Standardized uptake values (SUVs) of 18F-fluorodeoxyglucose positron emission tomography (FDG PET) are widely used to help characterize pulmonary nodules. The purpose of this study is to assess the accuracy of the SUV corrected by blood glucose levels (SUVgluc), compared to four other commonly used semi-quantitative methods: maximal SUV normalized to body weight (SUVmax), ratio of SUV of nodule to cerebellum (SUVcer), SUV normalized to body surface area (SUVbsa) and SUV normalized to body mass index (SUVbmi). 52 patients with lung nodules had FDG PET scans, consecutively imaged between 7/1/2015 and 6/7/2016. Histopathologic result of the nodules, obtained within two months after the FDG PET scan, demonstrated 10 benign and 42 malignant lung nodules. The SUVgluc was defined as SUVmax × blood glucose level/100. The average SUVmax was 2.8 for benign nodules and 7.7 for malignant nodules. No significant difference in the receiver operating characteristic (ROC) area under the curves (AUCs) were found between the SUVmax (0.84) and the SUVcer (0.87) or SUVbsa (0.86), or SUVbmi (0.86) with p-values greater than 0.05; however, the ROC AUC for the SUVgluc (0.90) was larger than that for the SUVmax with p-value of 0.03. These results suggest that SUVgluc may assist in more accurately representing the glucose metabolism of malignant lung nodules by accounting for the patient’s blood glucose level (BGL). The simplicity of the SUVgluc method avoids an additional reference ROI, uses preexisting clinical data, i.e. pre-injection blood glucose level, and retains the familiar SUV reference values.
Keywords: Glucose-corrected SUV, PET, lung nodule, blood glucose level
Introduction
18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) is a commonly used imaging modality to assess the risk of benign versus malignant pulmonary nodules, noninvasively [1]. Although the accuracy of PET for diagnosing malignancy is heterogeneous, it is widely accepted for the clinical diagnosis and staging of lung cancer in patients with suspicious lung nodules [2]. Standardized uptake value (SUV) is a simple method to obtain semiquantitiative index of FDG uptake, however multiple factors can affect SUV, thus limit its reliability including: body surface area, lean body mass, blood glucose level, or other perturbing factors [3]. In this study, we compared accuracy of SUVmax (normalized by body weight) and four other corrected SUV parameters including: ratio of SUV of nodule to cerebellum (SUVcer), SUV normalized to body surface area (SUVbsa), SUV normalized to body mass area (SUVbmi) and SUV corrected by blood glucose level (SUVgluc).
Materials and methods
Patient selection
This study was designed as a retrospective single center study in the University of California San Diego medical center including Hillcrest and Thornton hospitals. It was approved by the institutional review board (IRB) and was Health Insurance Portability and Accountability Act (HIPAA) compliant. Documentation in our database was anonymous. Patients were considered eligible for this study if they underwent FDG PET-CT study between July 2015 and June 2016 and had a pathological diagnosis of the nodule within 2 months after the imaging. The various semi quantitative methods were compared to the “gold standard” which was defined as subsequent histological pathology confirmation.
FDG PET imaging
All patients were asked to fast for at least 6 hours prior to their scan. Blood glucose levels were measured immediately before the FDG injection. Patients were intravenously injected with 370-740 MBq FDG, within a 5-10 second interval. Following an uptake period of approximately 1 hour in a quiet room at rest, multi-station 3-dimensional (3D) PET acquisition with CT, for attenuation correction, was performed for approximately 60 min, using a GE Discovery VCT scanner. PET images were acquired, after the CT scan, at a rate of 2 minutes/bed position, in the 3D acquisition mode. CT images were then reconstructed onto a 512 × 512 matrix. PET images were reconstructed using a standard whole body 3D iterative reconstruction: 2 iterations; 28 subsets onto a 128 × 128 matrix with attenuation correction, decay correction, and scatter correction. The photon energy window was 425-650 keV. Slice thickness was 3.27 mm and reconstruction diameter was 70 cm. Pixel size was 5.47 mm × 5.47 mm with spatial resolution of 5 mm.
Image analysis
All PET images were reviewed and further analyzed using the Agfa Impax software by a board certified academic nuclear medicine physician. Focal activity corresponding to the pulmonary nodule on CT was manually identified on PET images. SUV of the dominant nodule was obtained by manually placing a circular ROI at the site of the maximum FDG uptake in the PET images and the maximal activity (SUVmax) was recorded. SUVmax was calculated as decay-corrected activity of tissue volume (kBq/mL)/injected FDG activity per body mass (kBq/g). SUVbmi was calculated from SUVmax by normalizing activity based on body mass index (BMI) = Weight (kg)/Height2 (m). SUVbsa was calculated from SUVmax by normalizing activity based on body surface area (BSA) = (Weight (kg) * Height (cm)/3600)1/2. The average SUV value of the cerebellum reference region was used to calculate the SUVcer, defined as the ratio of SUVmax divided by the average cerebellar SUV. Corrected SUV for the blood glucose level (SUVgluc) was calculated based on BGL immediately before the FDG injection (Table 1).
Table 1.
Definition and area under the curve (AUC) in the receiver operating characteristic (ROC) curve of SUV parameters
| SUV parameter | Definition | AUC in the ROC curve | p-value** |
|---|---|---|---|
| SUVmax | decay-corrected activity of tissue volume/injected activity per body mass | 0.84 | Not applicable |
| SUVbmi | (SUVmax/body weight) × BMI | 0.86 | 0.15 |
| SUVbsa | (SUVmax/body weight) × BSA | 0.86 | 0.43 |
| SUVcer | SUVmax/SUVcerebellum | 0.87 | 0.32 |
| SUVgluc | SUVmax × blood glucose level/100 | 0.90* | 0.03*** |
SUVgluc has the highest AUC among semiquantitative parameters.
p-value is in comparison to SUVmax.
SUVgluc is the only SUV parameter which significantly improves diagnostic accuracy of SUVmax in differentiating benign vs. malignant lung nodules (P = 0.03).
Statistical analysis
All data were expressed as mean ± SD (standard deviation). Differences were analyzed by the paired T-test and considered to be significant at a P-value less than 0.05. Since the sensitivity and specificity of a test depends on the selected threshold value, a more rigorous comparison of diagnostic accuracy was performed using ROC analysis using ROCKIT (1.1B2, University of Chicago, IL, USA).
Results
Histopathological and patient characteristics analysis
Following 18F-FDG PET, the final diagnosis was confirmed pathologically by subsequent biopsy within 2 months in all 52 patients. It revealed 42 malignant nodules, consisting of 20 adenocarcinoma, 15 squamous cell carcinoma, and 7 other malignancies. The other 10 nodules were benign. The average glucose level was 104.8 mg/dL (range 77 to 235). The rest of the patient characteristics are shown in Table 2. Weight, Height, BGL, BMI and BSA were not significantly different between benign and malignant pulmonary nodules (P>0.05). All five SUV parameters were significantly different between benign and malignant nodules (P<0.05) with SUVgluc having the smallest p value = 0.0005 (Table 2).
Table 2.
Patient characteristics and SUV values. Mean and Standard deviation (parentheses) values are reported
| All patient | Benign | Malignant | p-value | |
|---|---|---|---|---|
| N, number | 52 | 10 | 42 | |
| Weight, kg | 72.8±17.6 | 73.9±18.1 | 72.6±17.6 | 0.83 |
| Height, cm | 168.3±9.5 | 164.0±9.5 | 169.3±9.3 | 0.12 |
| Blood glucose level, mg/dL | 104.8 | 92.9±8.4 | 107.7±32.2 | 0.16 |
| Body Mass Index, kg/m2 | 25.6±5.4 | 27.4±6.4 | 25.2±5.1 | 0.24 |
| Body Surface Area, m2 | 1.83±0.25 | 1.82±0.25 | 1.84±0.25 | 0.88 |
| SUVmax | 6.8±4.8 | 2.8±1.7 | 7.7±4.8 | 0.003* |
| SUVbsa | 0.18±0.13 | 0.07±0.04 | 0.20±0.13 | 0.003* |
| SUVcer | 1.5±1.2 | 0.60±0.57 | 1.8±1.2 | 0.005* |
| SUVbmi | 2.4±1.7 | 1.0±0.6 | 2.7±1.7 | 0.003* |
| SUVgluc | 6.8±4.5 | 2.6±1.6 | 7.9±4.3 | 0.0005* |
Mean ± standard deviation (parentheses) values are reported.
All SUV parameters are significantly different between the benign vs. malignant pulmonary nodules, whereas weight, height, BGL, or BMI are not.
Diagnostic value of five SUV parameters in differentiating benign and malignant pulmonary nodules
We found SUVmax to have the lowest diagnostic accuracy for detecting malignant lung nodules with area under the curve (AUC) = 0.84, in the receiver operating characteristic (ROC) curve, compared to other four semiquantitatitve corrected SUV parameters. The use of alternative semiquantitative methods including SUVbsa (AUC = 0.86), SUVbmi (AUC = 0.86), and SUVcer (AUC = 0.87), increased AUC in the ROC however this increase was not statistically significant (P>0.05), however only for SUVgluc (AUC = 0.88) this increase was statistically significant (P = 0.03) (Figure 1). Therefore AUC for SUVgluc showed the highest diagnostic accuracy for detecting malignant lung nodules. Figure 3 shows a representative FDG PET projection image of a patient with left upper lobe lung adenocarcinoma, BGL = 166 mg/dL, with SUVmax = 6.3 and SUVgluc = 10.5.
Figure 1.
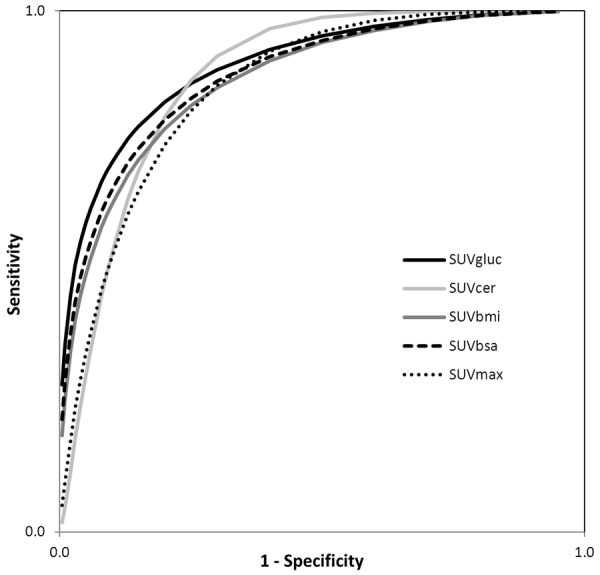
The ROC curves of SUVgluc, SUVcer, SUVbmi, SUVbsa and SUVmax. AUC for SUVcer, SUVbmi, and SUVbsa is not significantly different from SUVmax (P = 0.32, 0.15, and 0.43, respectively). The SUVgluc has the largest AUC, significantly different from SUVmax (AUC = 0.90 vs. 0.84, P = 0.03), thus the most accurate SUV parameter to distinguish malignant from benign pulmonary nodules.
Figure 3.
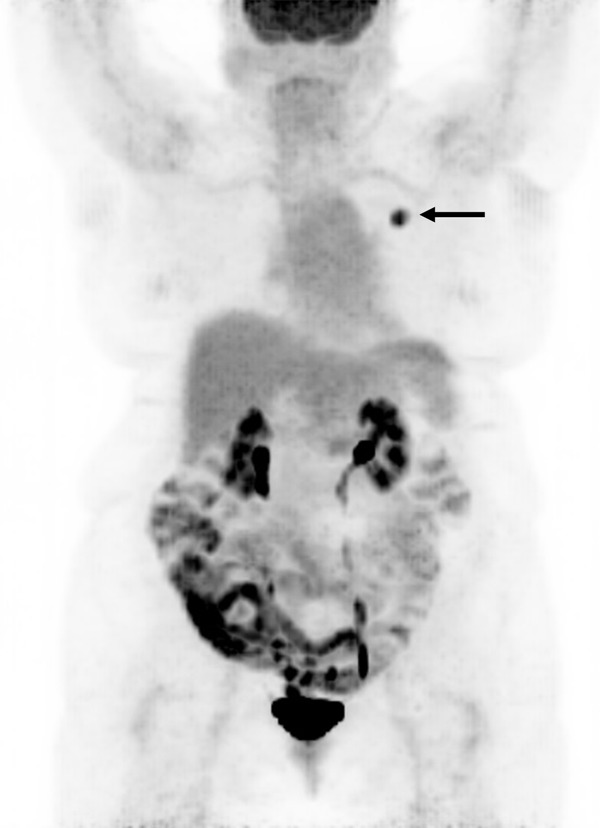
FDG PET projection image in a representative patient with left upper lobe lung adenocarcinoma (arrow), BGL = 166 mg/dL, SUVmax = 6.3 and SUVgluc = 10.5.
Discussion
The radiopharmaceutical tracer FDG follows a three compartment model with a net uptake rate of K = k1k3/(k2+k3) (Figure 2). SUVmax is proportional to this uptake rate (K) whereas glucose metabolism rate (GMR) is proportional to K × [Glucose] [3,4]. The rational for SUVgluc is analogous to the calculation of the GMR which involves the scaling of the FDG uptake rate with the blood glucose level. Therefore, SUVgluc defined as SUVmax × BGL (mg/dL)/100 is a better marker of GMR, especially in higher blood glucose levels, where SUVmax is of limited value. Glucose and 18F-FDG compete to enter the cells using the same glucose transporters. High BGL reduces 18F-FDG uptake in the tissue by competitive inhibition and by altered the biodistribution of FDG. The reason for fasting prior to the start of the PET study is to achieve low blood glucose levels for better target-to-background image contrast [5-7]. The end effect is the high blood glucose falsely reduces SUVmax, but not SUVgluc, due to competitive inhibition of FDG uptake. Although prior publications have shown the advantage of SUVgluc over SUVmax in evaluation of lymphoma patients [8], predicting the prognosis of pancreatic cancer [8], and brain tumors [9], one study found no advantage of SUVgluc over SUVmax on SUV-survival correlation in esophageal cancer [10]. It is unclear whether glucose normalization improves diagnosis accuracy or treatment response monitoring of malignant tumors [11]. To our knowledge, only one study has reported the application of the SUVgluc in lung nodules, where the SUVgluc was found not to be beneficial in differentiating lung nodules; however, only patients with glucose levels less than 150 mg/dl were studied [12]. We speculate that limiting the patients in that study to those with BGL<150 mg/dL masked the statistical significance of the advantage of SUVgluc over SUVmax because this advantage is related to BGL as SUVgluc = SUVmax × BGL/100 (Table 1). In fact, patients with normal BGL do not need glucose correction and those with high BGL will benefit from SUVgluc. Therefore, to overcome this shortcoming, we included all the patients with BGL ranging from 77 to 235 mg/dL. Another study suggests that for BGL<200 mg/dL, correction of blood glucose is not necessary however the accuracy of SUVgluc versus other SUV indicators for differentiating lung nodules was not evaluated [13]. Limitations of our study include the retrospective nature and having a single center study; however, it helps the uniformity of the data. Also, we did not evaluate the SUV in the same patient after injection of glucose, to raise BGL and study the effect of BGL on the same patient’s pulmonary nodule.
Figure 2.
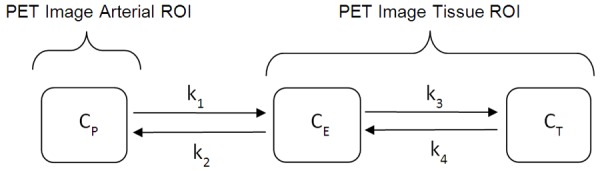
FDG tracer kinetic model, follows a three compartment model with a net uptake rate of K = k1k3/k2+k3. Cp = plasma concentration of FDG tracer, CE = extracellular concentration, CT = Tissue concentration.
In summary, our study demonstrates that the SUVgluc is the most accurate semiquantitative method to differentiate malignant from benign lung nodules. The method is relatively simple to adopt clinically since the BGL is readily available as part of routine PET protocols, and there is no need for an additional reference ROI to define.
Acknowledgements
Amin Haghighat Jahromi is supported by National Institute of Health grant (NIH T32 Grant 4T32EB005970-09).
Disclosure of conflict of interest
None.
References
- 1.Christensen JA, Nathan MA, Mullan BP, Hartman TE, Swensen SJ, Lowe VJ. Characterization of the solitary pulmonary nodule: 18F-FDG PET versus nodule-enhancement CT. AJR Am J Roentgenol. 2006;187:1361–1367. doi: 10.2214/AJR.05.1166. [DOI] [PubMed] [Google Scholar]
- 2.Deppen SA, Blume JD, Kensinger CD, Morgan AM, Aldrich MC, Massion PP, Walker RC, McPheeters ML, Putnam JB Jr, Grogan EL. Accuracy of FDG-PET to diagnose lung cancer in areas with infectious lung disease: a meta-analysis. JAMA. 2014;312:1227–1236. doi: 10.1001/jama.2014.11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol. 2010;195:310–320. doi: 10.2214/AJR.10.4923. [DOI] [PubMed] [Google Scholar]
- 4.Busing KA, Schonberg SO, Brade J, Wasser K. Impact of blood glucose, diabetes, insulin, and obesity on standardized uptake values in tumors and healthy organs on 18F-FDG PET/CT. Nucl Med Biol. 2013;40:206–213. doi: 10.1016/j.nucmedbio.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Claeys J, Mertens K, D’Asseler Y, Goethals I. Normoglycemic plasma glucose levels affect F-18 FDG uptake in the brain. Ann Nucl Med. 2010;24:501–505. doi: 10.1007/s12149-010-0359-9. [DOI] [PubMed] [Google Scholar]
- 6.Hadi M, Bacharach SL, Whatley M, Libutti SK, Straus SE, Rao VK, Wesley R, Carrasquillo JA. Glucose and insulin variations in patients during the time course of a FDG-PET study and implications for the “glucose-corrected” SUV. Nucl Med Biol. 2008;35:441–445. doi: 10.1016/j.nucmedbio.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Turcotte E, Leblanc M, Carpentier A, Benard F. Optimization of whole-body positron emission tomography imaging by using delayed 2-deoxy-2-[F-18] fluoro-D: -glucose injection following I.V. insulin in diabetic patients. Mol Imaging Biol. 2006;8:348–354. doi: 10.1007/s11307-006-0064-1. [DOI] [PubMed] [Google Scholar]
- 8.Lee SM, Kim TS, Lee JW, Kim SK, Park SJ, Han SS. Improved prognostic value of standardized uptake value corrected for blood glucose level in pancreatic cancer using F-18 FDG PET. Clin Nucl Med. 2011;36:331–336. doi: 10.1097/RLU.0b013e31820a9eea. [DOI] [PubMed] [Google Scholar]
- 9.Nozawa A, Rivandi AH, Kesari S, Hoh CK. Glucose corrected standardized uptake value (SUVgluc) in the evaluation of brain lesions with 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2013;40:997–1004. doi: 10.1007/s00259-013-2396-9. [DOI] [PubMed] [Google Scholar]
- 10.van Heijl M, Omloo JM, van Berge Henegouwen MI, van Lanschot JJ, Sloof GW, Boellaard R. Influence of ROI definition, partial volume correction and SUV normalization on SUV-survival correlation in oesophageal cancer. Nucl Med Commun. 2010;31:652–658. [PubMed] [Google Scholar]
- 11.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA, Stroobants S, Delbeke D, Donohoe KJ, Holbrook S, Graham MM, Testanera G, Hoekstra OS, Zijlstra J, Visser E, Hoekstra CJ, Pruim J, Willemsen A, Arends B, Kotzerke J, Bockisch A, Beyer T, Chiti A, Krause BJ European Association of Nuclear Medicine (EANM) FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Degirmenci B, Wilson D, Laymon CM, Becker C, Mason NS, Bencherif B, Agarwal A, Luketich J, Landreneau R, Avril N. Standardized uptake value-based evaluations of solitary pulmonary nodules using F-18 fluorodeoxyglucose-PET/computed tomography. Nucl Med Commun. 2008;29:614–622. doi: 10.1097/MNM.0b013e3282f9b5a0. [DOI] [PubMed] [Google Scholar]
- 13.Eskian M, Alavi A, Khorasanizadeh M, Viglianti BL, Jacobsson H, Barwick TD, Meysamie A, Yi SK, Iwano S, Bybel B, Caobelli F, Lococo F, Gea J, Sancho-Muñoz A, Schildt J, Tatcı E, Lapa C, Keramida G, Peters M, Boktor RR, John J, Pitman AG, Mazurek T, Rezaei N. Effect of blood glucose level on standardized uptake value (SUV) in 18F-FDG PET-scan: a systematic review and meta-analysis of 20,807 individual SUV measurements. Eur J Nucl Med Mol Imaging. 2019;46:224–237. doi: 10.1007/s00259-018-4194-x. [DOI] [PubMed] [Google Scholar]


