Abstract
L-carnitine is a fundamental ammonium compound responsible for energy metabolism in all living organisms. It is an oxidative stress regulator, especially in bacteria and yeast and lipid metabolism in plants. Besides its metabolic functions, l-carnitine has detoxification and antioxidant roles in the cells. Due to the complex interrelationship of l-carnitine between lipid metabolism and salinity dependent oxidative stress, this study investigates the exogenous l-carnitine (1 mM) function on seed germination, cell division and chromosome behaviour in barley seeds (Hordeum vulgare L. cv. Bulbul-89) under different salt stress concentrations (0, 0.25, 0.30 and 0.35 M). The present work showed that l-carnitine pretreatment could not be successful to stimulate cell division on barley seeds under non-stressed conditions compared to stressed conditions. Depending on increasing salinity without pretreatment with l-carnitine, the mitotic index significantly decreased in barley seeds. Pretreatment of barley seeds with l-carnitine under salt stress conditions was found promising as a plant growth promoter and stimulator of mitosis. In addition, pretreatment of barley seeds with l-carnitine alleviated detrimental effects of salt stress on chromosome structure and it protected cells from the genotoxic effects of salt. This may be caused by the antioxidant and protective action of the l-carnitine. Consequently, this study demonstrated that the exogenous application of 1 mM l-carnitine mitigates the harmful effects of salt stress by increasing mitosis and decreasing DNA damage caused by oxidative stress on barley seedlings.
Subject terms: Cell-cycle exit, Abiotic
Introduction
L-carnitine (4-N-trimethylammonium-3-hydroxybutyric acid, LC)1 is an endogenous ammonium compound; animals, bacteria, some yeast and fungi and plants naturally synthesise it from l-lysine and l-methionine amino acids2–4. The compound which is involved in energy metabolism, hormonal action, adaptation to stress and detoxifying functions3,5–7 has an important role in transporting long chain fatty acids from the cytosol into the mitochondrial membrane. Mitochondria are the cell’s powerhouse, breaking down sugar and synthesising of ATP to provide energy. In mammals, energy is created from activated fatty acids by mitochondrial β-oxidation of acyl-CoA NADH and FADH2 which enters the citric acid cycle (CAC) carried by carnitine shuttle from cytosol to mitochondria membrane8,9. The electron transport chain needs oxygen and NADP for the synthesis of ATP through oxidative phosphorylation. At the end of the CAC, oxygen is reduced to H2O leading to a reduction in the concentration of oxygen and in turn reducing the formation of reactive oxygen species (ROS)5,10. Oxidative stress is reduced by l-carnitine and its esters, which also regulates the activity of enzymes that defend the cell against oxidative damage11 and the levels of nitric oxide that influences cellular respiration12. In addition to protecting catalase and superoxide dismutase against 3-nitropropionic acid (3-NPA) induced neurotoxicity, l-carnitine also safeguards the activity of the mitochondrial enzyme, succinate dehydrogenase13.
Many types of plants, such as cereals and legumes contain l-carnitine, which can be found in various locations such as in leaves, as well as dry and germinating seeds14. The level of enzymatic activity of carnitine acyltransferase has been measured in plant tissues and chloroplasts15–18. Several studies measured the carnitine transferase activity from mitochondria mostly in pea chloroplasts and mung-bean hypocotyl4,16,19–21. Quantification studies of acylcarnitines and free carnitine in Arabidopsis thaliana, Brassica napus, Linum usitatissinum and Nicotiana tobaccum6 show a link between carnitine and lipid metabolism in plants. Lipids are essential to regulating the permeability of cell membranes and may provide plants with an effective means to resist salt22–24. Indeed, a number of studies describe changes to fatty acid, polar lipids and sterols as being influential in salt stress4,6. Although there is no direct evidence between carnitine and fatty acid metabolism, carnitine acyltransferase enzymes is formed from carnitine esters from acyl CoAs which is responsible from the mitochondrial β-oxidation in the avocado plant and pea seedlings14,25–28.
Salinity presents a serious environmental problem as it affects life cycle parameters such as plant fertility, growth and production. Salinity affects approximately 20% of the world’s total agricultural land29–32. Salinity causes oxidative stress, which in turn modifies the composition of fatty acids and the lipid content of organisms. The ability of plants to adapt to different environmental conditions is directly affected by the composition of fatty acids and cell membrane lipids33–36. Depending on plant species and intensity of the salt stress to which the plant is exposed, lipids are key to regulating selective cell membrane permeability37,38. According to Shaya-Khmetova et al.39, after applying exogenous salt stress to wheat species, the total lipid content had decreased in a week. The change in the concentration of salt modulates the activity of ion-transporting proteins40 and the synthesis of osmolytes41, reflecting changes in the fatty acid composition of cell membrane lipids and lipid metabolism42. Furthermore, the levels of carnitine correlates with tolerance to abiotic stresses in plants30. Considering the positive effects of l-carnitine on lipid metabolism, fatty acid composition, cell membrane permeability and apoptosis under biotic and abiotic stress this study designed to determine how mitotic cycle is affected by l-carnitine and how l-carnitine alleviate detrimental effects of different concentrations of exogenous salt stress. The study was designed to determine whether l-carnitine could be effective to increase cell division and might be suppressor on programmed cell death under salt stress conditions; it also evaluates whether the compound can alleviate genotoxic effects of salt stress on DNA level and the chromosome structure in the meristematic root tip cells of barley (Hordeum vulgare L.).
Results
Effects of L-carnitine on cell division under salt stress
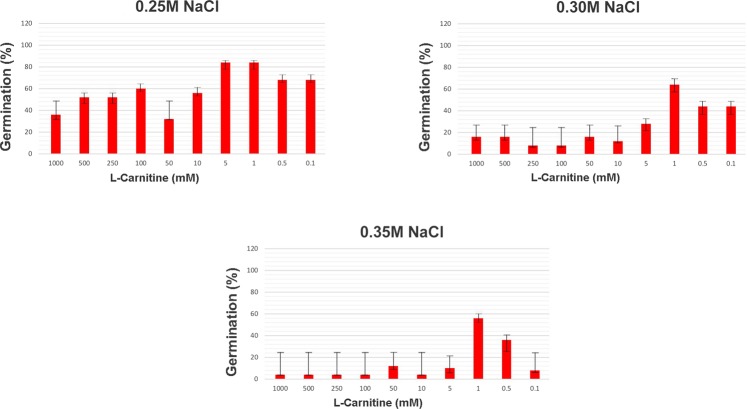
A preliminary study was performed to determine appropriate concentrations of NaCl to use; 0.25 M, 0.30 M and 0.35 M were deemed suitable to initiate salt stress responses in barley seeds. The salt concentrations preventing germination of seeds to a great extent (above 50% germination rate) were used to determine the NaCl concentrations. As a result of the preliminary study to identify which dose of the l-carnitine is effective for alleviating salt stress from 1000 mM to 0.1 mM concentrations were tested. As shown in the Fig. 1, the percentage of seed germination showed the same effect for 5 mM (84%) and 1 mM (84%) in 0.25 M salt. Depending on the increased salt stress concentration, the percentage of the germination regularly decreased in the 5 mM LC level (28% at 0.30 M and 10% at 0.35 M). Also, the experimental studies showed that l-carnitine solution must be prepared before used to get the same effects on seed germination. Finally, 1 mM l-carnitine concentration was found to be the most effective dose to offset the damage of salt stress stimulated to seed germination and the regulation of plant growth.
Figure 1.
Germination percentage of barley seeds with growth of cotyledons with respect to the total number of emerged seedlings after 7 days. Data were presented as the mean values obtained from three independent experiments covered with 50 seeds. The error bars indicate the standard deviation (±SD).
L-carnitine pretreatment (positive control, 1 mM LC) could not be successful to stimulate cell division on barley seeds by alone. Generally, mitotic index of barley seeds germinated in distilled water (negative control, 0 M NaCl) showed a higher rate (0.17) than pre-treated seeds group with l-carnitine (0.07) (Table 1). In addition, the number of anaphase and telophase cells were very low in both groups. However, most of the cells were observed in prophase stage in the negative control group. There is a statistically significant difference was determined in the prophase and metaphase indices between pre-treated and untreated groups with LC (p ≤ 0.05) (Table 1). Depending on the increasing salt stress concentration, the mitotic index significantly decreased in barley seeds. For instance, although the mitotic index value was calculated 0.17 in negative control group, it was 0.16 at 0.25 M NaCl, 0.14 at 0.30 M NaCl and 0.12 at 0.35 M NaCl concentrations. However, mitotic index of the pretreatment with l-carnitine under salt stress conditions was found promising as a plant growth promoter. In other words, pretreatment seeds with 1 mM l-carnitine to overcome detrimental effects of salt stress on cell division had effective results with the 0.28 mitotic index value at 0.25 M NaCl, 0.24 at 0.30 M NaCl and 0.14 at 0.35 M salinity conditions (Table 1). As seen from the phase indices after pretreatment with l-carnitine, the mitotic cycle was stimulated by increasing the prophase stage under salt stress conditions.
Table 1.
Mitotic index and phase indices of root tip cells of H. vulgare L. after pretreatment with 1 mM l-carnitine and unpretreated group germinated in distilled water (negative control; 0 M NaCl) were shown under various salt stress concentrations.
| Treatment | Mitotic Index (MI) | Mitotic Index (%) | Prophase Indice (Ip) | Metaphase Indice (IM) | Anaphase Indice (IA) | Telophase Indice (IT) |
|---|---|---|---|---|---|---|
| 0 M NaCl (Negative Control) | *0.17 ± 0.04b | 17 | 0.12 ± 0.04bc | 0.04 ± 0.02d | 0.00 ± 0.00a | 0.00 ± 0.00a |
| 0.25 M NaCl | 0.16 ± 0.01ab | 16 | 0.09 ± 0.04b | 0.02 ± 0.01abc | 0.00 ± 0.00a | 0.00 ± 0.00a |
| 0.30 M NaCl | 0.14 ± 0.01ab | 14 | 0.05 ± 0.01a | 0.01 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| 0.35 M NaCl | 0.12 ± 0.01a | 12 | 0.03 ± 0.01a | 0.01 ± 0.01ab | 0.00 ± 0.00a | 0.00 ± 0.00a |
| 0 M NaCl + 1 mM LC (Positive Control) | 0.07 ± 0.01a | 7 | 0.04 ± 0.01a | 0.02 ± 0.01c | 0.00 ± 0.00a | 0.00 ± 0.00a |
| 0.25 M NaCl + 1 mM LC | 0.28 ± 0.08c | 28 | 0.19 ± 0.05d | 0.02 ± 0.01bc | 0.01 ± 0.01a | 0.01 ± 0.01a |
| 0.30 M NaCl + 1 mM LC | 0.24 ± 0.01c | 24 | 0.16 ± 0.02 cd | 0.02 ± 0.01c | 0.02 ± 0.01b | 0.02 ± 0.01c |
| 0.35 M NaCl + 1 mM LC | 0.14 ± 0.02ab | 14 | 0.05 ± 0.01a | 0.01 ± 0.00abc | 0.01 ± 0.01a | 0.01 ± 0.01b |
*Values with insignificant difference (p ≤ 0.05) for each column are indicated with same letters (means ± SD).
Effects of L-carnitine on chromosome structure under salt stress
The negative control (distilled water, 0 M NaCl) group showed normal mitotic cells (Table 2, Fig. 2) and regular formation of mitotic chromosome number (2n = 2x = 14) while different types of chromosomal aberrations were observed in the root tips of positive control (1 mM LC + 0 M NaCl) group cells with the rate of 0.08. However, barley seeds exposed to salt stress (0.25, 0.30, 0.35 M NaCl) exhibited with different types of chromosome aberration indices in different phases of mitosis (Table 2, Figs 3–5). The genotoxicity index (IG) depending on increasing concentration of salt stress was significantly (p ≤ 0.05) higher than both control groups. The frequency of chromosomal aberrations was 0.23 at 0.25 M, 0.51 at 0.30 M and 0.72 at 0.35 M NaCl concentrations. Pretreatment barley seeds with 1 mM l-carnitine showed regenerative impact on chromosome aberrations under salt stress. Genotoxicity index was completely decreased depending on increasing salt stress concentration (Table 2). These aberrations were found 0.06 (6%) at 0.25 M, (20%) at 0.30 M and 0.15 (15%) at 0.35 M. In all tested salt concentrations, pretreatment with l-carnitine showed effective results to alleviate mutational effects on the chromosome structure, and l-carnitine pretreatment was significantly successful to inhibit detrimental effects of salt stress on chromosome behaviour.
Table 2.
Genotoxicity index (IG) and chromosome aberrations of the root tip cells of H. vulgare L. after pretreatment with 1 mM l-carnitine and unpretreated group germinated in distilled water (negative control; 0 M NaCl) were shown under various salt stress concentrations.
| Treatment | Genotoxicity Index (IG) | Disorderly Prophase | Stickiness | Ring Chromosome | Micronucleus | Alignment Anaphase | Fault polarization | Anaphase /Telophase Bridge | Vagrant Chromosome | Multinucleated cells |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 M NaCl (Negative Control) | *0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| 0.25 M NaCl | 0.23 ± 0.08d | 0.07 ± 0.01a | 0.00 ± 0.00a | 0.01 ± 0.01b | 0.00 ± 0.00ab | 0.00 ± 0.01ab | 0.00 ± 0.01ab | 0.00 ± 0.01a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| 0.30 M NaCl | 0.51 ± 0.03e | 0.12 ± 0.01b | 0.00 ± 0.00a | 0.02 ± 0.01c | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.01ab | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| 0.35 M NaCl | 0.72 ± 0.07e | 0.09 ± 0.02b | 0.00 ± 0.00a | 0.01 ± 0.01c | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00b | 0.00 ± 0.01a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| 0 M NaCl + 1 mM LC (Positive Control) | 0.08 ± 0.01ab | 0.01 ± 0.01a | 0.01 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.06 ± 0.00bc |
| 0.25 M NaCl +1 mM LC | 0.06 ± 0.04a | 0.01 ± 0.02a | 0.04 ± 0.01b | 0.00 ± 0.00a | 0.01 ± 0.01b | 0.00 ± 0.00a | 0.01 ± 0.01ab | 0.01 ± 0.01a | 0.01 ± 0.01b | 0.04 ± 0.02b |
| 0.30 M NaCl +1 mM LC | 0.20 ± 0.04cd | 0.01 ± 0.01a | 0.03 ± 0.01b | 0.01 ± 0.01b | 0.01 ± 0.00b | 0.02 ± 0.00b | 0.01 ± 0.01b | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.16 ± 0.04d |
| 0.35 M NaCl +1 mM LC | 0.15 ± 0.05bc | 0.02 ± 0.01a | 0.04 ± 0.00b | 0.01 ± 0.00b | 0.01 ± 0.00b | 0.02 ± 0.01a | 0.01 ± 0.01b | 0.01 ± 0.02a | 0.01 ± 0.01b | 0.09 ± 0.02c |
*Values with insignificant difference (P ≤ 0.05) for each column are indicated with same letters (means ± SD).
Figure 2.
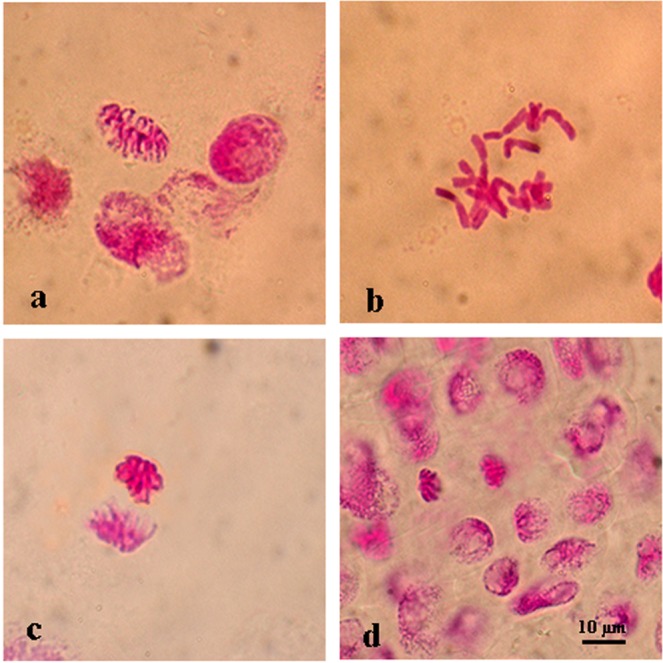
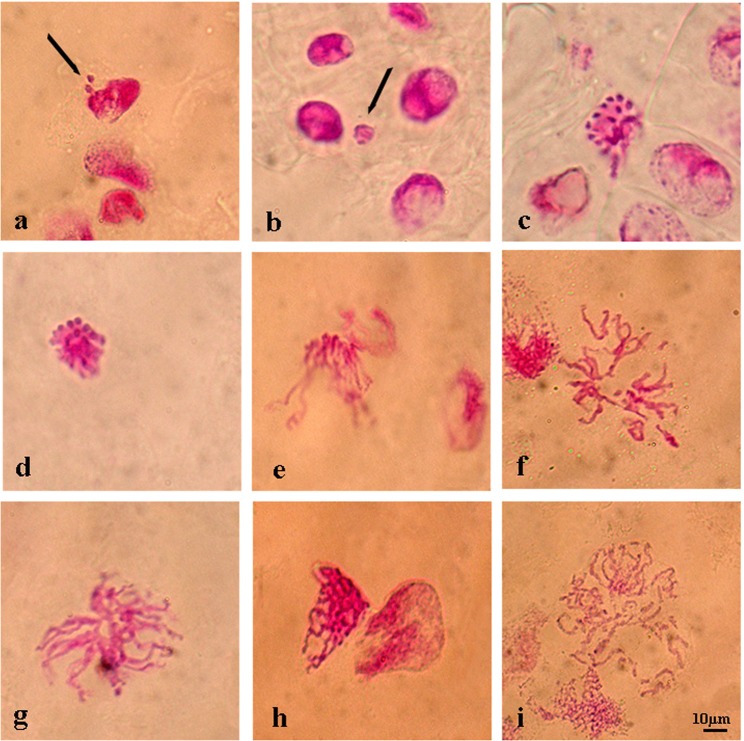
Normal mitotic chromosome structure in the meristematic root tip cells of barley (H. vulgare L. cv. Bulbul-89) seeds without pretreatment with l-carnitine under different salt stress concentrations. Prophase (a), metaphase 2n = 24 (b), anaphase (c), telophase (d). Scale bar: 10 µm.
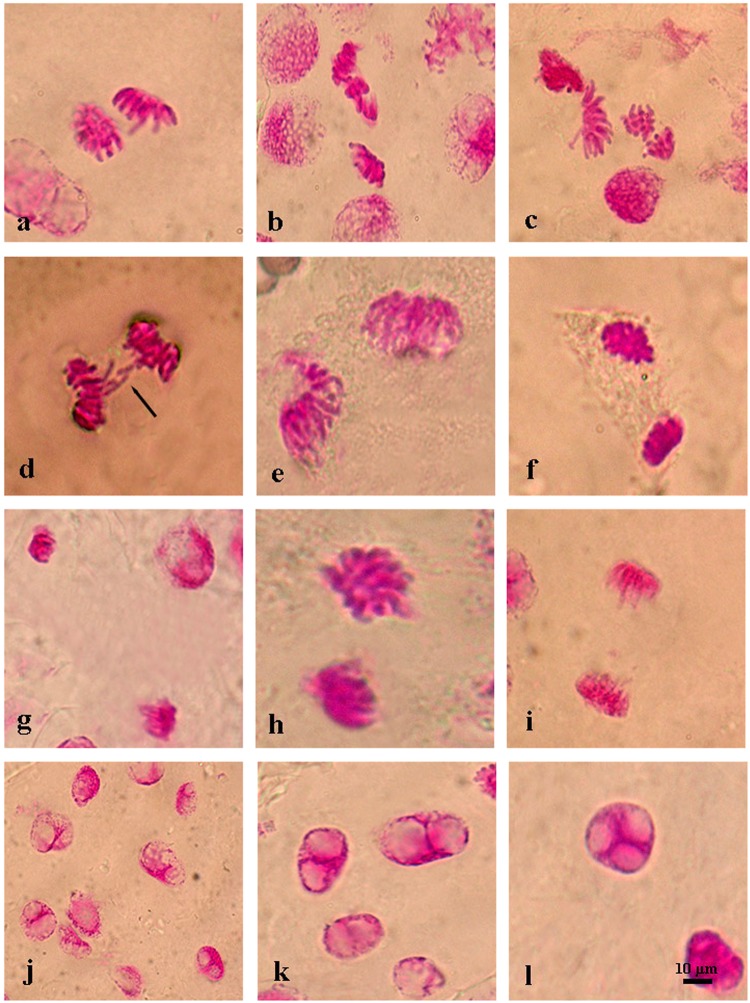
Figure 3.
Representative photos of prophase level in the meristematic root tip cells of barley (H. vulgare L. cv. Bulbul-89) seeds pretreatment with and without l-carnitine under different salt stress concentrations. Micronucleus (a,b), granulation (c,d), disorderly prophase, (e–i). Scale bar: 10 µm.
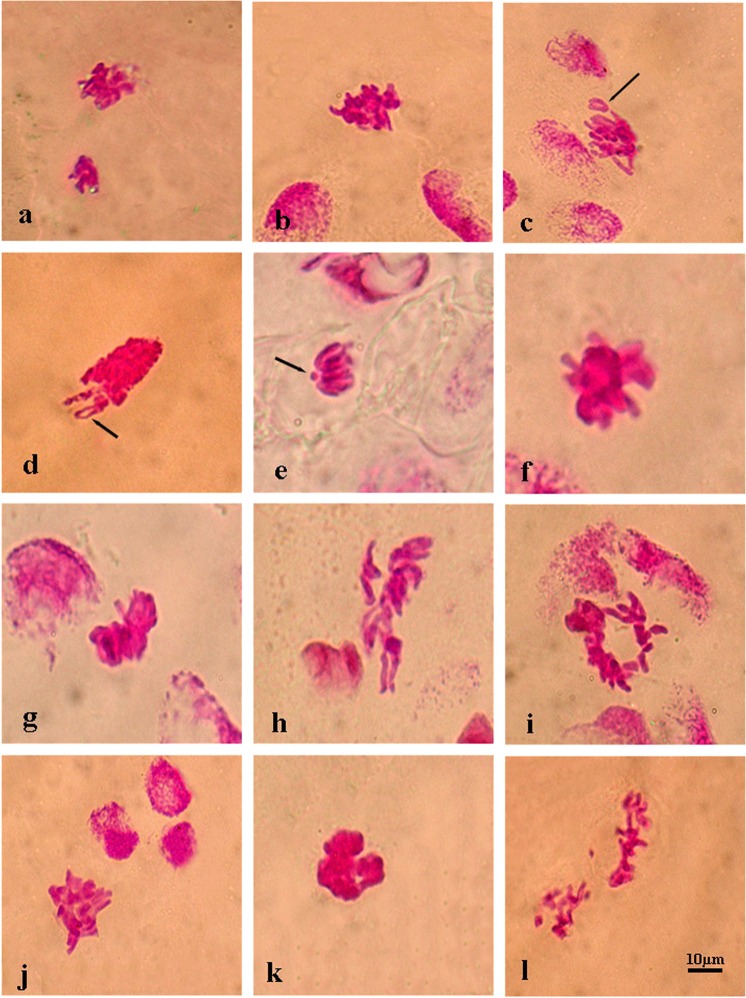
Figure 5.
Representative photos of anaphase level in the meristematic root tip cells of barley (H. vulgare L. cv. Bulbul-89) seeds pretreatment with and without l-carnitine under different salt stress concentrations. Vagrant chromosomes (a,c,d), spindle abnormalities in anaphase (b,c), anaphase bridge (d), fault polarization (e–h), alignment anaphase (i), binucleated cells (j,k), multinucleated cell (l) (→ represent aberrant chromosome). Scale bar: 10 µm.
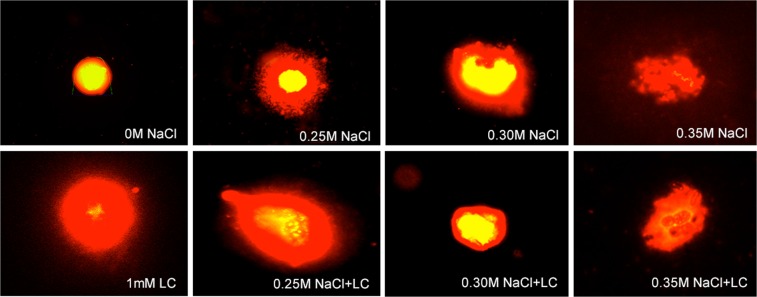
The most common monitored aberrations were also disorderly prophase (Fig. 3), stickiness (Fig. 4) and multinucleated cells (Fig. 5j–l) in seeds pre-treated with l-carnitine and different salt stress concentrations. All multinucleated cells were encountered in the positive control and pre-treated seeds with l-carnitine while seeds germinated in salt stress concentrations and distilled water did not showed any multinucleated cells. In addition, micronucleus (Fig. 3a,b), granulation (Fig. 3c,d), disrupted equatorial plate (Fig. 4a,h), ring chromosomes (Fig. 4c,d), vagrant chromosomes (Fig. 5a,c,d) and spindle abnormalities in anaphase (Fig. 5b,c), anaphase and telophase bridges (Fig. 5d), fault polarization (Fig. 5e–h), alignment anaphase (Fig. 5i) were also observed and noticed as statistically significant (p ≤ 0.05) compared to most common chromosome aberrations. Also, screening the DNA damage on nuclei to find more proof about genotoxicity, all threated samples were examined using Comet Assay (Table 3). Based on the genotoxicity index, depending on the salt stress concentration from 0 to 0.35 M, the percentage of tail DNA was increased from 0.17 ± 0.02 to 74.24 ± 2.70 (Fig. 6). Indeed, the 0 M NaCl concentration showed no head DNA (99.83%) damage. However, 0.35 M NaCl concentration showed significant differences on head DNA level (25.76%) (p ≤ 0.05). On the other hand, the application of 1 mM l-carnitine to barley seeds showed almost no damage on tail DNA (0.45%). Furthermore, the most protective role of l-carnitine on DNA level on barley seeds was observed in the highest concentration (0.35 M) of salt, after pretreatment with l-carnitine the percentage of tail DNA decreased almost three-fold from 74.24 ± 2.70 to almost 27.03 ± 1.30. Figure 7 also shows the changes on the nucleus on DNA damage due to the concentration levels.
Figure 4.
Representative photos of metaphase level in the meristematic root tip cells of barley (H. vulgare L. cv. Bulbul-89) seeds pretreatment with and without l-carnitine under different salt stress concentrations. Disrupted equatorial plate (a,h,l), ring chromosomes (c–e), stickiness (b,f,g,i–k) (→ represent aberrant chromosome). Scale bar: 10 µm.
Table 3.
Comet assay (head DNA %, tail DNA %) scores of the root tip cells of H. vulgare L. after pretreatment with 1 mM l-carnitine and unpretreated group germinated in distilled water (negative control; 0 M NaCl) were shown under various salt stress concentrations.
| Treatment | Head DNA (%) | Tail DNA (%) |
|---|---|---|
| 0 M NaCl (Negative Control) | 99.83 ± 2.22a | 0.17 ± 0.02a |
| 0.25 M NaCl | 37.34 ± 2.77abc | 62.70 ± 1.37b |
| 0.30 M NaCl | 34.89 ± 2.08ab | 65.11 ± 2.77b |
| 0.35 M NaCl | 25.76 ± 4.72ab | 74.24 ± 2.70c |
| 0 M NaCl + 1 mM LC (Positive Control) | 99.55 ± 7.84a | 0.45 ± 0.08ab |
| 0.25 M NaCl + 1 mM LC | 65.66 ± 2.82bcd | 34.34 ± 1.09ab |
| 0.30 M NaCl + 1 mM LC | 65.92 ± 2.81bcd | 34.08 ± 1.56ab |
| 0.35 M NaCl + 1 mM LC | 72.97 ± 1.03cd | 27.03 ± 1.30ab |
*Values with insignificant difference (p ≤0.05) for each column are indicated with same letters (means ± SD).
Figure 6.
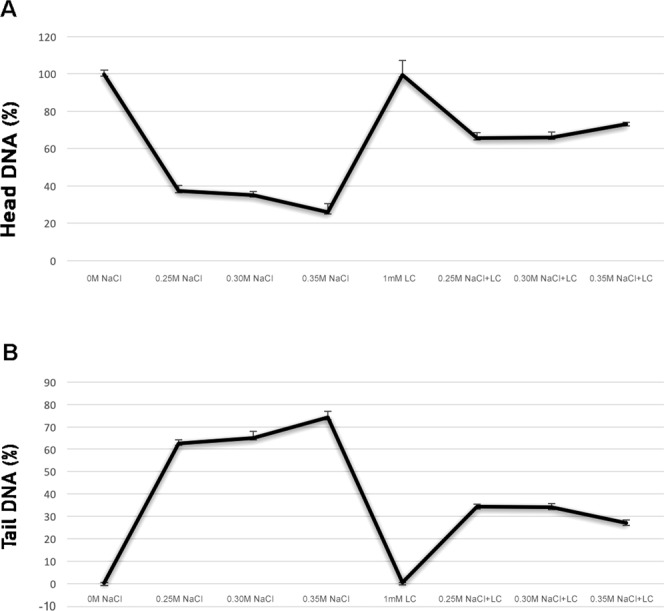
Comparison of DNA damage using Comet assay in the meristematic root tip cells of barley (H. vulgare L. cv. Bulbul-89) seeds pretreatment with and without l-carnitine under different salt stress concentrations. (A) Percentage of head DNA, (B) Percentage of tail DNA. Error bars indicated standard deviation (±SD), n = 200, (*p ≤ 0.05).
Figure 7.
Representative photos of DNA comets in the meristematic root tip cells of barley (H. vulgare L. cv. Bulbul-89) seeds observed in comet assay pretreatment with and without l-carnitine under different salt stress concentrations.
Discussion
In the present study, cytogenetic and genotoxic effects of l-carnitine as a plant growth promoter germinated in different salt stress concentrations (0, 0.25, 0.30 and 0.35 M) was reported by determining the putative effects on seed germination, cell division and DNA damage in the meristematic root tip cells of H. vulgare L.
Pretreatment of barley seeds with l-carnitine made a mitodeppresive effect on cell cycle compared to the negative control group germinated seeds in distilled water. While mitotic index was counted 17% in the negative control group cells it was observed in the positive control group cells as 7% (see Tables 1 and 2). Chromosomal alterations were not observed in the root tip cells of barley seeds germinated in distilled water and the genotoxicity index was 0.00% (see Table 2, Fig. 2). However, the barley seeds pre-treated with 1 mM l-carnitine showed 8% genotoxicity index and different types of chromosome aberrations such as multinucleated cells and disorderly prophase (see Table 2, Fig. 3). According to the comet assay genotoxicity results, percentage of tail DNA in positive control group cells increased 2.65-fold compared with the negative control group cells. Furthermore, 1 mM concentration of l-carnitine showed more genotoxic effects under unstressed conditions which might be cytotoxic on the mitotic chromosome structure in barley seeds. Otherwise, there are limited reports about l-carnitine levels in plant metabolism43,44. The only report for Panter and Mudd45 presented endogenous l-carnitine levels in some higher plants and it was observed that barley seeds did not contain any level of l-carnitine. Hence, barley seeds were used to determine the exogenous effects in all studied parameters pre-treated with l-carnitine for 24 hours. These results indicated that exogenous l-carnitine was not effective to stimulate cell cycle for mitosis in the absence of stress (see Table 1).
Though there are numerous studies of exogenous l-carnitine uptake under stress conditions in yeast46,47, bacteria48–50 and animals51, in plants most of the studies were performed about the endogenous level of l-carnitine content4,29,52,53, whereas this study reported for the first-time alleviative effects of exogenous l-carnitine on cell division and DNA damage under salt stress conditions.
Cytogenetic monitoring and Comet Assay are the most effective method to determine species-specific effects on DNA level. These allows the researchers to understand the cytogenetic changes via mutagenesis. The mitotic index also is a cytogenetic monitoring test which measures the proliferation of mitotic cells (M phase) in the cell cycle and its inhibition could be scored as cellular death54,55. There are numerous studies showing that l-carnitine decreased cell apoptosis, and death and chromosomal alterations in mammals56,57 and mice58. Furthermore, cytoprotective effects of l-carnitine was frequently investigated in human metabolism under oxidative stress conditions57,59.
Salinity is a global and major problem reducing agricultural crop production all over the world. It is reported that ~50% of the world’s arable land will be affected from the increasing soil salinity by 205060,61. Salinity also has a very strong relationship with osmotic and oxidative stress acting osmolytes, reactive oxygen species and membrane proteins53,62–64. Salt accumulation in the root systems generates osmotic stress by inhibiting water and essential elements uptake65 and high concentration of salt stress cause repressive effects on DNA, RNA and protein synthesis, cell cycle progression, seed germination, plant growth and production66,67. To survive under salt stress, plants adapt their mechanism via activating genes and protein kinases associated with stresses, signal transduction involved in cell division24,68. To increase plant resistance to salt stress, researchers or breeders focus on identifying new nutrients and fertilizers given to plants exogenously for improving plant production. As an exogenous supplement in plant metabolism, these findings suggest that l-carnitine could be a potential salt stress inhibitor stimulating progression of cell cycle via increasing mitosis under salt stress conditions (see Table 1), because l-carnitine has antioxidant action by activating antioxidant enzymes responsible from the synthesis of protective molecules reported by numerous studies in mammals under stress conditions59. Similarly, antioxidants influence specific cell cycle transitions to control cell cycle and protect plant cells from oxidative stress damages53,69–71. According to Charrier et al.29 exogenous carnitine stimulates development and protects Arabidopsis thaliana seedlings from the oxidative and salt stress damage29. Likewise, the results of this study, as a new plant growth stimulator by promoting seed germination, increasing cell division and decreasing the damage on DNA caused by salt stress, 1 mM l-carnitine is recommended as the appropriate dose to protect cells from the oxidative damage.
On the other hand, increasing salt stress concentrations breaks down the chromosomal structure in the root tip cells of barley seeds. Numerous cytogenetic studies reported that mutagenic effects of high levels salt stress on plant cell metabolism result from possible structural or numerical abnormalities on chromosomes72–74. Similarly, the highest chromosome alteration percentage was observed in the highest salt stress concentration (84%, 0.35 M) in this study. The sensitivity of the genotype to salt stress was statistically significant and different. Disorderly prophase (12 and 9%, respectively in 0.30 and 0.35 M NaCl) was the most common chromosome aberration in barley seeds germinated in salt stresses. Moreover, ring chromosomes (2%) (see Table 2, Fig. 4c,d) were the second significant aberration in 0.30 M salt stress concentration. Salinity side effects on chromosome structure were dependent to salt stress level, where a low level of salt stress has fewer side effects in contrast to high level stress application in all studied treatment group cells (see Table 2). Pretreatment with l-carnitine also significantly decreased the percentage of the genotoxicity index or chromosome aberrations and DNA damage on tail DNA in the meristematic root tips cells of barley (by ~5-fold in 0.25 M, ~4-fold in 0.30 M and ~6-fold in 0.35 M NaCl) compared to the absence of l-carnitine under salt stress (see Table 3). This could also be due to the regulation of the protective effect as well as DNA repair capability of l-carnitine58,75,76. These findings showed that l-carnitine was effective at different degrees to alleviate detrimental effects of salt stress on chromosome structure (see Table 2). However, different types of chromosomal alterations were also counted in pretreatment with 1 mM l-carnitine such as micronucleus, stickiness, alignment anaphase, fault polarization, anaphase/telophase bridges, vagrant chromosomes and multinucleated cells in interphase cells in the meristematic root tip cells of barley seeds under salt stress conditions. Interestingly, multinucleated cells were only observed in the pre-treated l-carnitine groups both the absence of salt stress and under salt stress concentrations (see Table 2). So, it may result from the l-carnitine and may cause formation of multinucleated cells in interphase level. Similarly, some researchers reported that a significant decrease in DNA damage and chromosome aberrations pre-treated with l-carnitine alleviates negative effects of oxidative stress56,77 and inhibition of tumor growth78,79 and cancer cell death80 in animal cells. According to Missihoun et al.81 the regulation of carnitine biosynthesis was affected by the salt stress levels in A. thaliana wild and transgenic plants that expression level of the betaine aldehyde dehydrogenase genes has an important role on the early growing stage of the seedlings to mitigate the salt stress in young tissues. Compared to the results of this study, it was determined that l-carnitine might have a cytoprotective effect to balance the overexpression level of the genes depending on increasing levels of salt stress. Due to the physiological functions of the carnitine and other betaines such as protection of enzyme structure and increasing membrane stability depending on the stress level have been reported as non-toxic even in high concentrations82. Otherwise, positive control group (1 mM l-carnitine) found to be genotoxic on the barley chromosome structure compared to the negative control group (0 mM l-carnitine). However, the presented study here suggests that l-carnitine has a protective effect on chromosome structure and DNA level against the genotoxic effects under the salt stress especially in high concentrations in barley cells.
In summary, the results of this study imply that the level of l-carnitine pretreatment (1 mM) was species specific under the tolerance levels to salt stress of H. vulgare L. Testing the putative cytoprotective effects of l-carnitine as an exogenous supplement were identified on cell cycle proliferation, genotoxicity and the level of DNA damage under salinity. A deeper understanding of these protective role of l-carnitine on DNA level related to putative salt stress sensors might help marker assisted breeding and genetic engineering to cope with various abiotic stresses.
Methods
Plant materials, experimental design and cytogenetical analysis
Barley (Hordeum vulgare L., 2n = 14, Fig. 2b) is one of the world’s oldest and most often grown cultivated crop plant. Its success and popularity for agriculture is based on its high adaptive capacity to withstand abiotic stress. Because of its innate ability to endure drought, fungal infection and salinity, barley is a model plant; thus, it is an ideal model organism for in biological research to study plant stress responses. This study used barley (H. vulgare L. cv. Bulbul-89) seeds to explore the cytogenetic response of l-carnitine (C7H15NO3, MA: 161.2 g/mol) in salt stress conditions.
Three biological replicates of each twenty-five uniformly sized barley seeds were prepared for each application by pre-treating with 1 mM of l-carnitine or distilled water (negative control), then leaving them at room temperature for 24 h. Then the solutions were removed and the seeds were vacuum-dried83. For each application, the seeds were put into 10 cm petri dishes and covered with two sheets of filter paper; sheets were moistened with 10 ml of distilled water (0 M salt) and the others with one of the predetermined concentrations of salt (0.25 M, 0.30 M or 0.35 M). To allow the seeds to germinate, the petri dishes were incubated at 20 ± 1 °C for 7 days. The root tips of the germinated seeds were cut off once they were 1–1.5 cm long, and they were pre-treated for 4 hours with paradichlorobenzene. The root tips were fixed in Carnoy solution [(ethanol (99%): glacial acetic acid (3:1)] for 24 h. Then they were stored at 4 °C in 70% ethanol until required. The root tips were hydrolyzed for 17 min. using 1 N HCl and stained using the Feulgen protocol for at least 1 h and before squashing the tips on slides with a little 45% acetic acid. At least 3.000 cells per treatment (1.000 per slide) were evaluated, and the mitotic index, phase indices (I) of dividing cells and chromosome aberrations were scored. To calculate the mitotic index (%), the number of cells undergoing mitotic division was divided by the total number of cells × 10084. For a more detailed evaluation of cell division, we calculated the indices (I) of the separate phases, prophase (IP), metaphase (IM), anaphase (IA) and telophase (IT). In accordance with Ivanova et al.85 the following formula was used for the phase indices:
The effects of l-carnitine and the cytotoxicity of salt stress were determined by scoring the number of aberrant cells by the total number of divided cells. The percentage of aberrations was calculated as follows:
An Olympus CX- 41 research microscope (100X objective) was used to observe the chromosome structure; they were photographed using a C–5060 WZ camera.
Commet assay
Microscope slides were pre-heated and coated with 1% normal melting point agarose and dehydrated at room temperature. The meristematic root tips of barley seedlings were chopped in 600 μl nucleus isolation buffer (4 mM MgCl2.6H2O, 0.5% TritonTMX-100 and 0.2 M Tris, pH 7.5) for 30 s. The buffer containing nuclei was transferred to a clean microcentrifuge tubes for each treatment and the tubes were centrifuged at 12,000 rpm for 15 min. at 4 °C. Following centrifugation, 50 μl of suspension of nuclei were placed on each slide mixed with 50 μl 1% low melting point agarose at 55 °C, covered with 20 × 50 mm cover glass and stated on ice for 5 min. Then, the samples were subjected to electrophoresis buffer in high salt solution (300 mM NaOH, 1 mM EDTA, pH > 13) for 30 min. at 4 °C. After that, they were exposed to 25 V for 20 min. and 300 mA and cooled on ice for 5 min. For neutrolization step, the slides were kept in 100 mM Tris-HCl for 5 min. Each slide stained with 70 μl ethidium bromide solution and kept at 4 °C for 5 min. to photograph the DNA damage on nuclei under fluorescence microscope on 200 cells/comets for each concentration using OpenComet v1.3.1 Software.
Statistical analysis
To compare the samples from the untreated group (control) with those pre-treated with l-carnitine (1 mM), ANOVA analysis was performed using MiniTab 1786 and SPSS 2287 software. The non-parametric Kruskal–Wallis test and Duncan’s multiple range test was used to identify differences between each group; the level of significance was set at p ≤ 0.0588.
Ethical approval and informed consent
Not applicable: This study does not directly involve humans or animals. Plant collection permits were not required because seed samples are commercial cultivars which can be purchased and no species are endangered or threatened.
Acknowledgements
The cytogenetic and statistical analysis was performed in Department of Molecular Biology and Genetics at Burdur Mehmet Akif Ersoy University. However, this research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contributions
S.O.-B. designed the study, conducted preliminary experiments, performed seed germination analysis, cell division analysis, conducted the statistical analyses. The author contributed to the preparation of the manuscript, read and approved the final version of the manuscript.
Competing interests
The author declares no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yu J, Ye J, Liu X, Han Y, Wang C. Protective effect of L‐carnitine against H2O2-induced neurotoxicity in neuroblastoma (SH-SY5Y) cells. Neurol. Res. 2011;33:708–716. doi: 10.1179/1743132810Y.0000000028. [DOI] [PubMed] [Google Scholar]
- 2.Bieber LL. Carnitine. Annu. Rev. Biochem. 1988;57:261–283. doi: 10.1146/annurev.bi.57.070188.001401. [DOI] [PubMed] [Google Scholar]
- 3.Pekala J, et al. L-carnitine metabolic functions and meaning in humans life. Curr. Drug. Metab. 2011;12:667–678. doi: 10.2174/138920011796504536. [DOI] [PubMed] [Google Scholar]
- 4.Rippa S, Zhao Y, Merlier F, Charrier A, Perrin Y. The carnitine biosynthetic pathway in Arabidopsis thaliana shares similar features with the pathway of mammals and fungi. Plant Physiol. Biochem. 2012;60:109–114. doi: 10.1016/j.plaphy.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Botham, K. M. & Mayes, P. A. Lipids of physiologic significance. (eds Murray, R. K., Granner, D. K., Mayes, P. A. & Rodwell, V. W.) 160–171 (Stamford, 2000).
- 6.Bourdin B, Adenier H, Perrin Y. Carnitine is associated with fatty acid metabolism in plants. Plant Physiol. Biochem. 2007;45:926–931. doi: 10.1016/j.plaphy.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Li H, et al. Particulate matter exposure and stress hormone levels: a randomized, double-blind, crossover trial of air purification. Circulation. 2017;136:618–627. doi: 10.1161/CIRCULATIONAHA.116.026796. [DOI] [PubMed] [Google Scholar]
- 8.Bremer J. Carnitine metabolism and functions. Physiol. Rev. 1983;63:1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- 9.Zammit VA. Carnitine palmitoyltransferase 1: central to cell function. IUBMB. Life. 2008;60:347–354. doi: 10.1002/iub.78. [DOI] [PubMed] [Google Scholar]
- 10.Gülçin I. Antioxidant and antiradical activities of l-carnitine. Life Sci. 2006;78(8):803–811. doi: 10.1016/j.lfs.2005.05.103. [DOI] [PubMed] [Google Scholar]
- 11.Kremser K, Kremser-Jezik M, Singh I. Effect of hypoxiareoxygenation on peroxisomal functions in cultured human skin fibroblasts from control and Zellweger syndrome patients. Free Rad. Res. 1995;22:39–46. doi: 10.3109/10715769509147526. [DOI] [PubMed] [Google Scholar]
- 12.Brown GC. Nitric oxide and mitochondrial respiration. Biochim. Biophys. Acta. 1999;1411:351–369. doi: 10.1016/S0005-2728(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 13.Binienda ZK, Ali SF. Neuroprotective role of l-carnitine in the 3-nitropropionic acid induced neurotoxicity. Toxicol. Lett. 2001;125(1–3):67–73. doi: 10.1016/S0378-4274(01)00415-5. [DOI] [PubMed] [Google Scholar]
- 14.Wood, C., Masterson, C. & Thomas, D. R. Carnitine-dependent fatty acyl transport in plant cells. (eds Lambers, H., Van Der Plas, H. L. W.) Molecular, biochemical and physiological aspects of plant respiration 195–207 (Campaign, 1992b).
- 15.Burgess N, Thomas DR. Carnitine acetyltransferase in pea cotyledon mitochondria. Planta. 1986;167(1):58–65. doi: 10.1007/BF00446369. [DOI] [PubMed] [Google Scholar]
- 16.Gerbling H, Gerhardt B. Carnitine-acyltransferase activity of mitochondria from mung-bean hypocotyls. Planta. 1988;174(1):90–93. doi: 10.1007/BF00394878. [DOI] [PubMed] [Google Scholar]
- 17.Thomas MD, Langston-Unkefer PJ, Uchytil TF, Durbin RD. Inhibition of glutamine synthetase from pea by tabtoxinine-p-lactam. Plant Physiol. 1983;71:912–915. doi: 10.1104/pp.71.4.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaren I, Wood C, Jalil MNH, Yong BCS, Thomas DR. Carnitine acyltransferases in chloroplasts of Pisum sativum L. Planta. 1985;163:197. doi: 10.1007/BF00393506. [DOI] [PubMed] [Google Scholar]
- 19.Schwabedissen-Gerbling H, Gerhardt B. Purification and characterization of carnitine acyltransferase from higher plant mitochondria. Phytochemistry. 1995;39:36–43. doi: 10.1016/0031-9422(95)95267-X. [DOI] [Google Scholar]
- 20.Masterson C, Wood C. Pea chloroplast carnitine acetyltransferase. Proc. Biol. Sci. 2000;267:1–6. doi: 10.1098/rspb.2000.0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masterson C, Wood C. Mitochondrial oxidation of fatty acids in higher plants. Physiol. Plant. 2000;109:217–224. doi: 10.1034/j.1399-3054.2000.100301.x. [DOI] [Google Scholar]
- 22.Kuiper PJC. Environmental changes and lipid metabolism of higher plants. Physiol. Plant. 1985;64:118–122. doi: 10.1111/j.1399-3054.1985.tb01221.x. [DOI] [Google Scholar]
- 23.Mansour MMF. Changes in cell permeability and lipid content in wheat roots induced by NaCl. Biol. Plant. 1995;37(1):143–145. doi: 10.1007/BF02913010. [DOI] [Google Scholar]
- 24.Xiong L, Lee H, Ishitani M, Zhu JK. Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J. Biol. Chem. 2002;8(277):8588–8596. doi: 10.1074/jbc.M109275200. [DOI] [PubMed] [Google Scholar]
- 25.Panter RA, Mudd JB. Some aspects of carnitine metabolism in avocado(Persea americana) Biochem. J. 1973;134:655–658. doi: 10.1042/bj1340655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas DR, et al. The synthesis of palmitoylcarnitine by etio-chloroplasts of greening barley leaves. Planta. 1982;154:60–65. doi: 10.1007/BF00385497. [DOI] [PubMed] [Google Scholar]
- 27.Wood, C., Masterson, C. & Thomas, D. R. The role of carnitine in plant cell metabolism. (ed. Tobin, A. K.) Plant organelles: compartmentation of metabolism in photosynthetic cells 229–263 (Cambridge, 1992a).
- 28.Masterson C, Wood C. Influence of mitochondrial beta-oxidation on early pea seedling development. New Phytol. 2009;181:832–84. doi: 10.1111/j.1469-8137.2008.02717.x. [DOI] [PubMed] [Google Scholar]
- 29.Charrier A, et al. The effect of carnitine on Arabidopsis development and recovery in salt stress conditions. Planta. 2012;235:123–135. doi: 10.1007/s00425-011-1499-4. [DOI] [PubMed] [Google Scholar]
- 30.Munns R. Genes and salt tolerance: bringing them together. New Phytol. 2005;167(3):645–663. doi: 10.1111/j.1469-8137.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 31.Rozema J, Flowers T. Ecology: crops for a salinized world. Science. 2008;322(5907):1478–1480. doi: 10.1126/science.1168572. [DOI] [PubMed] [Google Scholar]
- 32.FAO. Land and Plant Nutrition Management Service. http://www.fao.org/ag/AGL/public.stm/ (2008).
- 33.Gupta B, Huang B. Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int. J. Genomics. 2014;24:701596. doi: 10.1155/2014/701596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huflejt ME, et al. Changes in membrane lipid composition during saline growth of the fresh water cyanobacterium Synechococcus 6311. Plant Physiol. 1990;94(4):1512–1521. doi: 10.1104/pp.94.4.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandit PR, Fulekar MH, Karuna MSL. Effect of salinity stress on growth, lipid productivity, fatty acid composition, and biodiesel properties in Acutodesmus obliquus and Chlorella vulgaris. Environ. Sci. Pollut. Res. Int. 2017;24(15):13437–13451. doi: 10.1007/s11356-017-8875-y. [DOI] [PubMed] [Google Scholar]
- 36.Fuhrmann M, Delisle L, Petton B, Corporeau C, Pernet F. Metabolism of the Pacific oyster, Crassostrea gigas, is influenced by salinity and modulates survival to the Ostreid herpes virus OsHV-1. Biology Open. 2018;7:bio028134. doi: 10.1242/bio.028134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Los DA, Murata N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta. 2004;1666(1-2):142–157. doi: 10.1016/j.bbamem.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Zarrouk M, Marzouk B, Ben-Miled D, Cherif A. Oil accumulation in olives and effect of salt on their composition. Olivae. 1996;61:41–45. [Google Scholar]
- 39.Shaya-Khmetova, I., Rakova, N. & Polimbetova, F. Effect of NaCl salinity and nitrogen form on fatty acid composition of wheat roots. Root demographics and their efficiencies in sustainable agriculture, grasslands and forest ecosystems. Developments in Plant and Soil Sciences (ed. Box, J. E.) 493–498 (Dordrecht, 1998).
- 40.Parks GE, Ditrich MA, Schumaker KS. Increased vacuolar Na /H exchange activity in S alicornia bigelovii Torr. in response to NaCl. J. Exp. Bot. 2002;53:1055–1065. doi: 10.1093/jexbot/53.371.1055. [DOI] [PubMed] [Google Scholar]
- 41.Giri J. Glycinebetaine and abiotic stress tolerance in plants. Plant Signal Behav. 2011;6(11):1746–1751. doi: 10.4161/psb.6.11.17801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsydendambaev VD, Ivanova TV, Khalilova LA. Fatty acid composition of lipids in vegetative organs of the halophyte Suaeda altissima under different levels of salinity. Russ. J. Plant Physiol. 2013;60:661–671. doi: 10.1134/S1021443713050142. [DOI] [Google Scholar]
- 43.Fraenkel G. Effect and distribution of vitamin BT. Arch. Biochem. Biophys. 1951;34:457–464. doi: 10.1016/0003-9861(51)90026-4. [DOI] [PubMed] [Google Scholar]
- 44.van Vlies N, et al. Characterization of carnitine and fatty acid metabolism in the long-chain acyl-CoA dehydrogenase deficient mouse. Biochem. J. 2005;387:185–193. doi: 10.1042/BJ20041489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panter RA, Mudd JB. Carnitine levels in some higher plants. FEBS Lett. 1969;5:169–170. doi: 10.1016/0014-5793(69)80322-4. [DOI] [PubMed] [Google Scholar]
- 46.Franken J, Kroppenstedt S, Swiegers JH, Bauer FF. Carnitine and carnitine acetyltransferases in the yeast Saccharomyces cerevisiae: a role for carnitine in stress protection. Curr. Genet. 2008;53:347–360. doi: 10.1007/s00294-008-0191-0. [DOI] [PubMed] [Google Scholar]
- 47.Franken J, Bauer FF. Carnitine supplementation has protective and detrimental effects in Saccharomyces cerevisiae that are genetically mediated. FEMS. Yeast. Res. 2010;10(3):270–281. doi: 10.1111/j.1567-1364.2010.00610.x. [DOI] [PubMed] [Google Scholar]
- 48.Kempf B, Bremer E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 1998;170:319–330. doi: 10.1007/s002030050649. [DOI] [PubMed] [Google Scholar]
- 49.Canovas D, et al. Racemisation of d(+)-carnitine into l(−)-carnitine by Escherichia coli strains. Process. Biochem. 2003;39:287–293. doi: 10.1016/S0032-9592(03)00080-3. [DOI] [Google Scholar]
- 50.Angelidis AS, Smith LT, Hoffman LM, Smith GM. Identification of opuC as a chill-activated and osmotically activated carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 2002;68:2644–2650. doi: 10.1128/AEM.68.6.2644-2650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cotton LM, Rodriguez CM, Suzuki K, Orgebin-Crist MC, Hinton BT. Organic cation/carnitine transporter, OCTN2, transcriptional activity is regulated by osmotic stress in epididymal cells. Mol. Reprod. Dev. 2010;77:114–125. doi: 10.1002/mrd.21122. [DOI] [PubMed] [Google Scholar]
- 52.Jacques F, Rippa S, Perrin Y. Physiology of l-carnitine in plants in light of the knowledge in animals and microorganisms. Plant Sci. 2018;274:432–440. doi: 10.1016/j.plantsci.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 53.Benjamin JJ, Lucini L, Jothiramshekar S, Parida A. Metabolomic insights into the mechanisms underlying tolerance to salinity in different halophytes. Plant Physiol. Biochem. 2019;135:528–545. doi: 10.1016/j.plaphy.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Rojas E, et al. Mitotic index and cell proliferation kinetics for the identification of antineoplastic activity. Anticancer Drugs. 1993;4:637–640. doi: 10.1097/00001813-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Gadano A, Gurni A, Lopez P, Ferraro G, Carballo M. In vitro genotoxic evaluation of the medicinal plant Chenopodium ambrosioides L. J. Ethnopharmacol. 2002;81:11–16. doi: 10.1016/S0378-8741(01)00418-4. [DOI] [PubMed] [Google Scholar]
- 56.Santoro A, et al. L-carnitine protects mammalian cells from chromosome aberrations but not from inhibition of cell proliferation induced by hydrogen peroxide. Mut. Res. 2005;587:16–25. doi: 10.1016/j.mrgentox.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L, et al. Cotton GhMKK5 affects disease resistance, induces HR-like cell death, and reduces the tolerance to salt and drought stress in transgenic Nicotiana benthamiana. J. Exp. Bot. 2012;63(10):3935–3951. doi: 10.1093/jxb/ers086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alzahrani HAS. Protective effect of l-carnitine against acrylamide-induced DNA damage in somatic and germ cells of mice. Saudi J. Biol. Sci. 2011;18(1):29–36. doi: 10.1016/j.sjbs.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Surai PF. Antioxidant Action of Carnitine: Molecular mechanisms and practical applications. EC. Veterinary Science. 2015;2.1:66–84. [Google Scholar]
- 60.Wang W, Vinocur B, Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003;218:1–14. doi: 10.1007/s00425-003-1105-5. [DOI] [PubMed] [Google Scholar]
- 61.Bartels D, Sunkar R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005;24:23–58. doi: 10.1080/07352680590910410. [DOI] [Google Scholar]
- 62.Munns R. Utilizing genetic reserves to enhance productivity of salt prone land CAB Rev: Prospective in agriculture. Veterinary Sci. Nutr. Nat. Res. 2006;2(009):11. [Google Scholar]
- 63.Muchate NS, Nikalje GC, Rajurkar NS, Suprasanna P, Nikam TD. Plant salt stress: adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot. Rev. 2016;82:371–406. doi: 10.1007/s12229-016-9173-y. [DOI] [Google Scholar]
- 64.Kumari A, Parida AK. Metabolomics and network analysis reveal the potential metabolites and biological pathways involved in salinity tolerance of the halophyte Salvadora persica. Environ. Exper. Bot. 2018;148:85–99. doi: 10.1016/j.envexpbot.2017.12.021. [DOI] [Google Scholar]
- 65.Paranychianakis NV, Chartzoulakis KS. Irrigation of Mediterranean crops with saline water: >From physiology to management practices. Agric. Ecosyst. Environ. 2005;106:171–187. doi: 10.1016/j.agee.2004.10.006. [DOI] [Google Scholar]
- 66.Anuradha S, Rao SSR. Effect of brassinosteroids on salinity stress induced inhibition of seed germination and seedling growth of rice(Oryza sativa L.) Plant Growth Regul. 2001;33:151–153. doi: 10.1023/A:1017590108484. [DOI] [Google Scholar]
- 67.Rodríguez-Eugenio, N., McLaughlin, M. & Pennock, D. Soil Pollution: a hidden reality. FAO. 142 (Rome, 2018).
- 68.Zhu JK. Abiotic stress signalling and responses in plants. Cell. 2016;167(2):313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Menon SG, Goswami PC. A redox cycle within the cell cycle: ring in the old with the new. Oncogene. 2007;26(8):1101–1109. doi: 10.1038/sj.onc.1209895. [DOI] [PubMed] [Google Scholar]
- 70.Diaz Vivancos PD, et al. Recruitment of glutathione into the nucleus during cell proliferation adjusts whole-cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield. Plant J. 2010;64:825–838. doi: 10.1111/j.1365-313X.2010.04371.x. [DOI] [PubMed] [Google Scholar]
- 71.Livanos P, Galatis B, Quader H, Apostolakos P. Disturbance of reactive oxygen species homeostasis induces atypical tubulin polymer formation and affects mitosis in root-tip cells of Triticum turgidum and Arabidopsis thaliana. Cytoskeleton (Hoboken) 2012;69:1–21. doi: 10.1002/cm.20538. [DOI] [PubMed] [Google Scholar]
- 72.Tabur S, Demir K. Role of some growth regulators on cytogenetic activity of barley under salt stress. Plant Growth Regul. 2010;60:99–104. doi: 10.1007/s10725-009-9424-6. [DOI] [Google Scholar]
- 73.Marakli S, Temel A, Gozukirmizi N. Salt stress and homobrassinosteroid interactions during germination in barley roots. Not. Bot. Horti. Agrobot. Cluj. Napoca. 2014;42:446–452. doi: 10.15835/nbha4229461. [DOI] [Google Scholar]
- 74.Pekol S, Baloglu MC, Altunoglu YÇ. Evaluation of genotoxic and cytologic effects of environmental stress in wheat species with different ploidy levels. Turkish J. Biol. 2016;40:580–588. doi: 10.3906/biy-1506-6. [DOI] [Google Scholar]
- 75.Berni A, et al. L-carnitine enhances resistance to oxidative stress by reducing DNA damage in Ataxia telangiectasia. cells. Mut. Res. 2008;650:165–174. doi: 10.1016/j.mrgentox.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 76.Dong Y, et al. Effect of l-carnitine on DNA damage and oxidative stress in maintenance hemodialysis patients with hepatitis C virus infection in East China. Int. J. Clin. Exp.Med. 2016;9(3):6100–6106. [Google Scholar]
- 77.Zakzok, F. B., Hegazy, H. M., Yosef, T. A. & Gomaa, G. M. Mitigating impact of l-carnitine against dimethoate induction of hepatic and testicular genotoxicity in rats: the role of oxidative stress, Toxin Reviews. 10.1080/15569543.2018.1522645 (2018).
- 78.Ricciardi MR, et al. Targeting the leukemia cell metabolism by the CPTIa inhibition: functional pre-clinical effects in leukemias. Blood. 2015;126:1925–1929. doi: 10.1182/blood-2014-12-617498. [DOI] [PubMed] [Google Scholar]
- 79.Melone MAB, et al. The carnitine system and cancer metabolic plasticity. Cell Death Dis. 2018;9(2):228. doi: 10.1038/s41419-018-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pacilli A, et al. Carnitine-acyltransferase system inhibition, cancer cell death, and prevention of myc-induced lymphomagenesis. J. Natl. Cancer Inst. 2013;105(7):489–498. doi: 10.1093/jnci/djt030. [DOI] [PubMed] [Google Scholar]
- 81.Missihoun TD, et al. Overexpression of ALDH10A8 and ALDH10A9 genes provides insight into their role in glycine betaine synthesis and affects primary metabolism in Arabidopsis thaliana. Plant Cell Physiol. 2015;56(9):1798–807. doi: 10.1093/pcp/pcv105. [DOI] [PubMed] [Google Scholar]
- 82.Zhang J, Nishimura N, Okubo A, Yamazaki S. Development of an analytical method for the determination of betaines in higher plants by capillary electrophoresis at low pH. Phytochem. Anal. 2002;13:189–194. doi: 10.1002/pca.639. [DOI] [PubMed] [Google Scholar]
- 83.Braun JW, Khan AA. Alleviation of salinity and high temperature stress by plant growth regulators permeated into lettuce seeds via acetone. J. Am. Soc. Hortic. Sci. 1976;101:716–721. [Google Scholar]
- 84.Sehgal R, Roy S, Kumar VL. Evaluation of cytotoxic potential of latex of Calotropis procera and podophyllotoxin in Allium cepa. Biocell. 2006;30(1):9–13. [PubMed] [Google Scholar]
- 85.Ivanova E, Staikova T, Velcheva I, Kostadinovn K. Somatostatic effect of heavy metal contaminated waters in the region of the town of Panagjurishte. Bulgaria J. Envir. Protect. Sci. 2003;4(2):284–287. [Google Scholar]
- 86.Minitab 17 Statistical software [Computer software] by Minitab Inc (2010).
- 87.IBM Corp. Released. IBM SPSS statistics for Windows version 22.0. Armonk, IBM Corp. New York (2013).
- 88.Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11:1. doi: 10.2307/3001478. [DOI] [Google Scholar]