Abstract
Background
A subset of horses deficient in alpha‐tocopherol (α‐TP) develop muscle atrophy and vitamin E‐responsive myopathy (VEM) characterized by mitochondrial alterations in the sacrocaudalis dorsalis medialis muscle (SC).
Objectives
To quantify muscle histopathologic abnormalities in subclinical α‐TP deficient horses before and after α‐TP supplementation and compare with retrospective (r)VEM cases.
Animals
Prospective study; 16 healthy α‐TP‐deficient Quarter Horses. Retrospective study; 10 retrospective vitamin E‐responsive myopathy (rVEM) cases .
Methods
Blood, SC, and gluteus medius (GM) biopsy specimens were obtained before (day 0) and 56 days after 5000 IU/450 kg horse/day PO water dispersible liquid α‐TP (n = 8) or control (n = 8). Muscle fiber morphology and mitochondrial alterations were compared in samples from days 0 and 56 and in rVEM cases.
Results
Mitochondrial alterations more common than our reference range (<2.5% affected fibers) were present in 3/8 control and 4/8 treatment horses on day 0 in SC but not in GM (mean, 2.2; range, 0%‐10% of fibers). Supplementation with α‐TP for 56 days did not change the percentage of fibers with mitochondrial alterations or anguloid atrophy, or fiber size in GM or SC. Clinical rVEM horses had significantly more mitochondrial alterations (rVEM SC, 13% ± 7%; GM, 3% ± 2%) and anguloid atrophy compared to subclinical day 0 horses.
Conclusions and Clinical Importance
Clinically normal α‐TP‐deficient horses can have mitochondrial alterations in the SC that are less severe than in atrophied VEM cases and do not resolve after 56 days of α‐TP supplementation. Preventing α‐TP deficiency may be of long‐term importance for mitochondrial viability.
Keywords: atrophy, mitochondria, myopathy, skeletal muscle, vitamin E
Abbreviations
- 3D
3‐dimensional
- C
control group
- EM
electron microscopy
- EMND
equine motor neuron disease
- eNAD
equine neuroaxonal dystrophy
- GM
gluteus medius
- HE
hematoxylin and eosin
- NADH‐TR
nicotinamide adenine dinucleotide tetrazolium reductase
- rVEM
retrospective vitamin E‐responsive myopathy
- SC
sacrocaudalis dorsalis medialis muscle
- SDH
succinate dehydrogenase
- Tx
α‐TP treatment group
- VEM
vitamin E‐responsive myopathy
- α‐TP
alpha‐tocopherol
1. INTRODUCTION
Vitamin E is an essential fat‐soluble vitamin that prevents cyclic propagation of lipid peroxidation and stabilizes membranes and with lesser known functions in cellular transport, cell signaling, and gene transcription.1, 2 Vitamin E comprises both tocopherols (saturated) and tocotrienols (unsaturated) with the most biologically active isoform, alpha‐tocopherol (α‐TP), often administered as a dietary supplement in synthetic or highly bioavailable natural formulations.1, 3, 4
Dietary deficiency of α‐TP in horses usually arises from a lack of access to fresh grass.5, 6 Rigorous competition schedules, overcrowding, overgrazing, and drought conditions limit pasture access and therefore increase the risk of α‐TP deficiency. Alpha‐TP concentrations in hay begin to decrease after a few months of storage, and concentrates for horses are fortified with the less bioavailable synthetic α‐TP.7, 8 Thus, over time, horses without pasture access are at risk for α‐TP deficiency. Serum α‐TP >2 μg/mL often is used as the reference range, but most grazing horses maintain serum α‐TP concentrations between 3 and 7 μg/mL.6
Diseases known to be associated with α‐TP deficiency include the upper motor neuron disorders equine neuroaxonal dystrophy (eNAD)/equine degenerative myeloencephalopathy and the lower motor neuron disorder equine motor neuron disease (EMND).6, 9, 10 The reason why specific clinical signs and localized histopathology develop in some horses with α‐TP deficiency and not others is unclear. Potential explanations include the timing and duration of α‐TP deficiency, genetic predisposition, and other environmental or nutritional factors.10, 11, 12, 13, 14 For example, prerequisites for development of eNAD appear to be a genetic predisposition as well as temporal exposure to low amounts of α‐TP as foals.11, 13 In contrast, years of α‐TP deficiency may be required for development of EMND.5 Many horses with low serum α‐TP concentration, however, do not appear to develop neuromuscular disease, leaving owners to debate the necessity of providing α‐TP supplementation for their horses.
Recently, a myopathy has been described in adult horses, which is responsive to α‐TP called vitamin E‐responsive myopathy (VEM).15 Similar to EMND, VEM presents as an insidious onset of poor performance, muscular weakness, and grossly apparent muscle atrophy.15 Distinct from EMND, mitochondrial stains of the sacrocaudalis dorsalis medialis muscle (SC) from horses with VEM show a hallmark “moth‐eaten” staining pattern in muscle fibers and a preponderance of anguloid rather than the angular muscle fiber atrophy seen in EMND.15, 16 Anguloid atrophied fibers are common with myogenic atrophy and characterized by concavity on ≥1 sides. Angular atrophy is common with motor nerve damage and is characterized by a smaller fiber size with pronounced triangular shape.17 In contrast to the progressive nature of EMND, horses with VEM often regain muscle mass and return to their previous level of performance within 3 months of α‐TP supplementation.5, 6, 15 Vitamin E‐responsive myopathy could be either a precursor to EMND or potentially a separate myopathic disorder characterized by grossly apparent muscle atrophy.
Determination of whether apparently healthy horses with α‐TP deficiency have subclinical evidence of VEM or EMND in skeletal muscle would inform horse owners of potential benefits to providing α‐TP supplementation. We hypothesized that myogenic atrophy and mitochondrial alterations would be present in the SC muscle of apparently healthy horses deficient in α‐TP and that lesions would be ameliorated by 8 weeks of α‐TP supplementation. The first aim of our prospective study was to quantify histopathologic alterations in SC and gluteus medius (GM) of α‐TP‐deficient horses that had no apparent signs of neuromuscular disease. The second aim was to determine if α‐TP supplementation over 8 weeks would ameliorate any histopathologic alterations and increase muscle volume. The final aim was to compare the severity of SC and GM histopathology between horses in the prospective study with a retrospective group of horses with VEM that had clinical signs of muscle atrophy, weakness, and potentially altered gait.
2. MATERIALS AND METHODS
2.1. Prospective study
2.1.1. Horses
Sixteen unfit Quarter Horse types owned by the University of California, Davis, that had no evidence of neuromuscular disease, were included in the study. Horses were housed at an accredited facility and were cared for according to the guidelines of the Animal Use and Care Committees at the University of California, Davis, and Michigan State University. Horses had been maintained in dry lots and fed grass hay with no additional supplementation for 5 months before the study. Before the trial, serum α‐TP concentrations were measured and horses were selected for inclusion if they had concentrations <3 μg/mL.6 Fifteen of 16 horses had serum α‐TP < 2 μg/mL, with 1 horse at 2.2 μg/mL. Horses were divided into 2 groups based on sex and pretrial serum α‐TP concentrations: untreated control (C; 3 geldings, 5 mares; 12.4 ± 3.1 years of age; enrollment serum α‐TP, 0.72 ± 0.65 μg/mL) and α‐TP treatment (Tx; 3 geldings, 5 mares; 11.9 ± 2.5 years of age; serum α‐TP, 0.52 ± 0.36 μg/mL). Serum α‐TP <3 μg/mL was confirmed on day 0 for all enrolled horses.
2.1.2. Study design
On day 0, blood samples were collected, horses were weighed and received physical and neurologic examinations, a 3‐dimensional (3D) infrared scan for body volume was performed, and muscle biopsy specimens were obtained from SC and GM.18 Between days 0 and 56, horses were housed in dry lots and fed 1st cutting grass hay (1.5%‐2% of body weight), and 0.5 kg/day of alfalfa pellets (Harlan Feed Alfalfa Pellets, Woodland, California) using over‐the‐nose feed bags. Horses in the Tx group received 5000 IU/day liquid water‐dispersible natural form of vitamin E with stereoisomer RRR‐α‐TP (Nano‐E, Kentucky Equine Research, Versailles, Kentucky) top‐dressed onto the alfalfa pellets. When all the pellets were consumed, feed bags were removed. Weekly serum samples and a day 28 GM muscle biopsy specimen were obtained but not analyzed in the present study. On day 56, the same day 0 sampling protocol was repeated.
2.1.3. 3‐Dimensional scanning
Three‐dimensional scanning was performed by the same individual (A. B.) as previously described to determine the volume of muscle over the caudal thorax and hindquarters termed “torso.”18 Scans were performed in duplicate with <3.5% error between duplicate scans. Briefly, the tail was wrapped and a lunging surcingle was fitted into the natural girth groove to define the cranial margin of the torso. Xylazine hydrochloride (0.3‐0.4 mg/kg IV) was administered to horses that were reluctant to stand still. An Occipital Structure Sensor (Structure v1.9, Occipital, Inc, Boulder, Colorado) was attached to an iPad Air 2 running the Structure application (Structure v1.9, Occipital, Inc) and linked to a laptop computer that ran the Skanect scanning program (Skanect Pro v1.9 [Win64], Occipital, San Francisco, California). Horses were scanned while the operator with the handheld scanner moved around the horse, which was standing squarely. Object files for each scan were exported from Skanect and imported into the Meshmixer program (Version 3.3.15, Autodesk, Inc, San Francisco, California). The horse's torso then was isolated in each scan by cropping. The torso was delineated cranially by the surcingle and ventrally by a plane drawn parallel to the floor from the junction of the flank and stifle to the surcingle. Torso volume was calculated for each horse on days 0 and 56 as a representation of muscle mass. To ensure volume changes were not the result of increased fat deposition, measurement of SC adipose tissue depth was performed at days 0 and 56 using an ultrasound examination (TeraVet 3000; Teratech Corporation, Burlington, Massachusetts) at a site 5 cm to the left and right of the root of the tail. A linear 6‐MHz probe (Terrason, Teratech Corporation) was oriented transversely, and fat depth was measured from the skin surface to the ventral limit of the SC adipose tissue. Right and left values were averaged.
2.1.4. Serum samples
Jugular venous blood samples were collected into vacutainer, immediately placed on ice and protected from sunlight. Samples were collected just before the scheduled time for supplement administration. Serum was separated within 6 hours and stored at −80°C until analyzed at the conclusion of the study. Alpha‐tocopherol analysis was performed at the University of California, Davis, using high‐performance liquid chromatography as previously described.11
2.1.5. Muscle biopsies
Horses were sedated with xylazine (0.5‐1.1 mg/kg IV) or detomidine (0.005‐0.02 mg/kg IV). Subcutaneous muscle biopsy specimens were obtained using an open surgical technique alternating sides for days 0 and 56. A site 1 cm abaxial to dorsal midline extending 6 cm cranially from the tail head was clipped, surgically sterilized, and 4 mL of 2% lidocaine was injected SC. A 4‐cm incision was made in the skin and fascia, which then were retracted to expose the underlying muscle. A 2‐cm cube of muscle was removed and SC tissue and skin sutured. Horses were given 1.1 mg/kg IV flunixin meglumine after the biopsy.
Gluteus medius biopsies were performed as previously described using a modified Bergstrom needle at a site approximately 8 cm caudal to the tuber coxae, along a line from the top of the tuber coxae to the tail head.15, 19 Samples for histopathology were obtained from the right GM on day 0, a second sample that was snap frozen for future molecular analyses was obtained on day 28 from the left GM and, on day 56, samples for histopathology were obtained from the right GM. Samples for muscle histopathology were frozen in isopentane suspended in liquid nitrogen within 4 hours of sampling. Samples for electron microscopy (EM) were placed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer.
2.1.6. Muscle histopathology and morphometry
Seven micron‐thick cross‐sections of SC and GM muscle were stained with hematoxylin and eosin (HE), modified Gomori Trichrome, succinate dehydrogenase (SDH), nicotinamide adenine dinucleotide tetrazolium reductase (NADH‐TR), and myosin adenosine triphosphatase with preincubation at pH 4.4.20 One researcher (S.J.V.) masked to sample identity including time and treatment conducted the histopathologic evaluations.
A minimum of 150 muscle fibers (range, 150‐378) per muscle biopsy specimen were examined to determine the number of muscle fibers with “moth‐eaten” staining, aggregates of subsarcolemmal mitochondria, anguloid atrophy, and angular atrophy using 10× images of NADH‐TR stains. The presence of “moth‐eaten” fibers was confirmed by examination of SDH stains. Percentages of fibers for each variable were calculated and mitochondrial alterations compared to the reference range (<2.5% mitochondrial alterations). The reference range used by the Neuromuscular Diagnostic Laboratory at Michigan State University represents the mean ± 2 standard deviations for mitochondrial alterations in 6 SC samples previously taken from healthy Quarter Horses with normal serum and muscle α‐TP concentrations. The reference population included 4 geldings and 2 mares with an average age of 8 ± 2 years.
Muscle fiber compositions were determined for SC and GM muscle samples by typing at least 250 fibers that stained for type 1, type 2A, and type 2X in ΑTPase stains. The cross‐sectional area of muscle fibers was measured using Image J software (https://imagej.nih.gov/ij/) to outline the sarcolemma of >100 fibers of each type (if present) that were in precise cross section, avoiding any obliquely sectioned fibers. Mean areas were calculated for each fiber type, and the mean area of all fibers examined per sample also was calculated.
2.1.7. Electron microscopy
Seven randomly selected SC samples from day 56, including 3 C and 4 Tx horses, were subjectively evaluated by EM for the presence of lipopigment, lipofuscin, dense bodies, honeycomb tubular structures, mitochondrial size and shape, myofibril size, and myofibril density.
2.2. Retrospective study
2.2.1. Retrospective VEM case selection
The database of the Neuromuscular Diagnostic Laboratory at Michigan State University was searched from December 2018 and dating backward to January 2016 to identify 10 cases of VEM with SC muscle samples submitted to the Neuromuscular Diagnostic laboratory that were well preserved, in cross section, and had minimal artifact from shipping. Seven of the 10 rVEM horses had concurrent GM (n = 5) or semimembranosus (SM; n = 2) muscle samples submitted, and these also were evaluated.
2.2.2. VEM histopathology
Slides of SC and GM or, if submitted, SM muscles were evaluated for the percentage of fibers with histopathologic changes in the same manner as for the prospective trial with a range of 164 to 277 fibers scored. The person scoring each retrospective clinical sample was blinded to the details of the case. Comparisons of the percentage of fibers with mitochondrial alterations and atrophy in the rVEM cases were made using values for all prospective horses on day 0. Two rVEM horses had SC and GM samples frozen within 4 hours of sampling, and fiber sizes were determined for these cases. Fiber sizes were not assessed in shipped samples because they develop artifactual fiber swelling.21 Electron microscopy was performed on SC from 1 rVEM case that had been immediately placed in glutaraldehyde.
2.3. Statistical analysis
Data were evaluated for normal distribution using the Kolmogorov‐Smirnov test. In the prospective study, data for age, body weight, torso volume, serum α‐TP and SC muscle were normally distributed. Log10 transformation was performed on tail head fat depth and GM type 2X fiber size because data were not normally distributed. Data that were expressed as proportions and not normally distributed (GM anguloid atrophy, GM mitochondrial aggregation) was transformed using the arcsine of the square root of the proportion before analysis. One prospective muscle biopsy specimen from the SC and 1 from GM muscle could not be analyzed because of excessive freeze artifact. The effect of time (day 0 versus day 56) within Tx and C groups, respectively, was evaluated using a mixed effects model, with time and treatment as fixed variables and horse as the random variable. Post hoc testing was performed using a Sidak's multiple comparison test and P was set at <.05.
For retrospective data, an unpaired Student's t test (normally distributed data; subsarcolemmal aggregates, percentage mitochondrial change, anguloid atrophy) or Mann‐Whitney test (nonnormally distributed data; “moth‐eaten” fibers and angular atrophy) was used to compare data from rVEM cases with day 0 prospective horses using a Bonferroni corrected P < .01 (n = 5 variables). Statistical analysis was performed using GraphPad Prism version 8.0 for Windows (GraphPad, San Diego, California).
3. RESULTS
3.1. Prospective study
3.1.1. Horses
No significant difference was found in the age or sex of horses in C and Tx groups. All horses had normal physical and neurologic examination findings on days 0 and 56 and subjectively appeared to have normal muscle mass.
3.1.2. Serum α‐TP
Mean serum α‐TP concentrations did not change between day 0 (1.46 ± 0.63 μg/mL) and day 56 (1.29 ± 0.67 μg/mL) for the C group (reference range, > 3 μg/mL).6 In contrast, serum α‐TP concentrations increased significantly in the Tx group (day 0, 1.17 ± 0.34 μg/mL; range, 0.70‐1.71 μg/mL; day 56, 2.56 ± 0.54 μg/mL; range, 1.70‐3.39 μg/mL; P < .0001) with only 1 horse still having a concentration < 2.0 μg/mL.
3.1.3. Body weight and volume
Weight did not change significantly between days 0 and 56 for C (day 0, 548 ± 56 kg; day 56, 563 ± 40 kg) or Tx groups (day 0, 522 ± 50 kg; day 56, 540 ± 50 kg). Tail head fat depth did not change significantly between days 0 and 56 for either C (day 0, 1.66 ± 0.28 cm; day 56, 1.90 ± 0.62 cm) or Tx groups (day 0, 1.71 ± 0.66 cm; day 56, 1.71 ± 0.70 cm). The mean torso volume did not change between days 0 and 56 for either the C (day 0, 0.2823 ± 0.0228 m3; day 56, 0.2874 ± 0.0242 m3) or Tx groups (day 0, 0.2679 ± 0.0215 m3; day 56, 0.2723 ± 0.0219 m3).
3.1.4. Sacrocaudalis muscle histopathology
A mean of 231 ± 37 SC fibers were evaluated. Mitochondrial alterations in amounts above the upper limit of the established reference range (2.5% of fibers) were present in the SC samples at day 0 in 3 of 8 C horses (4.1%, 4.5%, and 5.8% of fibers) and 4 of 8 C horses at day 56 (3.4%, 5.8%, 6.5%, and 8.5% of fibers; Table 1; Figure 1A‐D). For the Tx group, the same 3 horses had mitochondrial alterations above the reference range at days 0 and 56 (day 0: 2.5%, 3.5%, and 3.9% of fibers; day 56: 3.1%, 3.1%, and 9.8% of fibers). Between days 0 and 56, no significant differences were found in the percentages of “moth‐eaten” fibers, fibers with aggregates of subsarcolemmal mitochondria, or anguloid atrophied fibers in either the C or Tx groups (Table 1). The number of angular atrophied fibers was very small, and significantly fewer angular atrophied fibers were found in the control group on day 56 (0.0) compared to day 0 (0.3%; P = .04; Table 1).
Table 1.
The percentage of fibers with histopathologic features of moth‐eaten fibers, subsarcolemmal aggregates of mitochondria (subsarc. mitochondria), all mitochondrial alterations (sum of moth‐eaten + subsarcolemmal aggregates; mitochondrial alterations), anguloid and angular atrophy in the sacrocaudalis dorsalis medialis and gluteus medius muscles in control horses (C) and horses treated with 5000 IU/day of water dispersible α‐TP (Tx) at day 0 and day 56 of the treatment trial
| Group | N | Moth eaten (%) | Subsarc. Mitochondria (%) | Mitochondrial alterations (%) | Anguloid atrophy (%) | Angular atrophy (%) |
|---|---|---|---|---|---|---|
| Sacrocaudalis | ||||||
| Reference | 6 | 0.0 ± 0.0 | 0.9 ± 0.8 | 0.9 ± 0.8 | 4.3 ± 1.6 | 0.0 ± 0.0 |
| C day 0 | 8 | 1.4 ± 1.2 | 1.2 ± 0.9 | 2.6 ± 1.9 | 4.1 ± 3.2 | 0.3 ± 0.3a |
| C day 56 | 8 | 1.7 ± 2.2 | 1.1 ± 1.1 | 3.2 ± 3.3 | 4.0 ± 2.6 | 0.0 ± 0.0 |
| Tx day 0 | 8 | 1.1 ± 0.8 | 1.0 ± 0.6 | 2.1 ± 1.2 | 7.5 ± 2.7 | 0.4 ± 0.3 |
| Tx day 56 | 8 | 1.5 ± 2.6 | 1.2 ± 0.9 | 2.7 ± 3.1 | 7.2 ± 2.5 | 0.2 ± 0.3 |
| Gluteal muscle | ||||||
| C day 0 | 8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.5 ± 1.5 | 0.1 ± 0.2a |
| C day 56 | 8 | 0.0 ± 0.0 | 1.5 ± 2.0 | 1.5 ± 2.0 | 9.5 ± 11.2 | 6.3 ± 9.4 |
| Tx day 0 | 8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.7 ± 1.2 | 0.0 ± 0.0 |
| Tx day 56 | 8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 3.0 ± 1.9 | 1.8 ± 3.4 |
A significant difference between C day 0 and C day 56.
Figure 1.
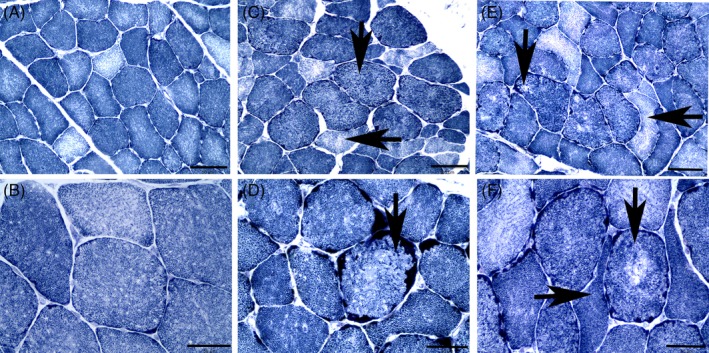
Cross sections of sacrocaudalis dorsalis medialis muscle stained with NADH‐TR. Bar in (A, C, E) indicated 100 μm. Bar in (B, D, F) indicates 50 μm. A, Muscle fibers from a day 0 subclinical α‐TP‐deficient horse showing a normal staining pattern (×20). B, Higher power view of the normal staining pattern of myofibers (×40). C, Muscle fibers from a day 0 subclinical α‐TP‐deficient horse containing moth‐eaten fibers (vertical arrow) and anguloid atrophied fibers (horizontal arrow) (×20). D, Higher power view showing a moth‐eaten fiber (vertical arrow) and fibers with subsarcolemmal mitochondrial aggregates (×40). E, Cross section of muscle from a retrospective VEM case showing a larger number of fibers with a more marked moth‐eaten staining pattern (vertical arrow) and anguloid atrophied fibers (horizontal arrow) (×20). F, Higher power view of a moth‐eaten fiber with central pallor (vertical arrow) and more marked anguloid atrophy (horizontal arrow) in a retrospective VEM case (×40). NADH‐TR, nicotinamide adenine dinucleotide tetrazolium reductase; VEM, vitamin E‐responsive myopathy
3.1.5. Gluteus medius muscle histopathology
A mean of 166 ± 11 GM fibers were morphologically evaluated. No fibers had a “moth‐eaten” appearance or subsarcolemmal mitochondrial aggregates at day 0, and no significant change in mitochondrial alterations occurred between days 0 and 56 in either C or Tx horses (Table 1). Fewer than 5% of muscle fibers showed anguloid atrophy at day 0 (Table 1), and no significant change occurred in the percentage of anguloid atrophied fibers for either C or TX groups by day 56 (Table 1). A significant increase occurred in angular atrophied fibers between days 0 and 56 in the C (P = .01) but not the Tx group (P = .25; Table 1). In 3 C and 4 Tx horses on day 56, repeat biopsy samples of the right GM had focal fascicles with pronounced anguloid and angular atrophied type 1, type 2A, and type 2X fibers (Figure S1). These fibers had peripherally located nuclei in HE‐stained sections, and some fibers had subsarcolemmal mitochondrial aggregates (Figure S1). The focal extent of atrophy resembled neurogenic atrophy that could have arisen from damage to a nerve branch during the previous biopsy of the right GM. Images obtained to measure muscle fiber areas included these focal areas of atrophy.
3.1.6. Muscle fiber type composition and morphometry
The SC muscle had a high proportion of large type 1 muscle fibers (>86%) with few type 2A fibers and rare type 2X fibers (Table 2) with no significant differences between days 0 and 56. In contrast, the GM muscle had a high proportion of type 2A and 2X fibers and, on average, <16% type 1 fibers (Table 2). The fiber type compositions and cross‐sectional areas of muscle fibers in the SC and GM muscle did not differ significantly between days 0 and 56 in either C or Tx groups (Table 2).
Table 2.
Sacrocaudalis dorsalis medialis (SC) and gluteus medius (GM) muscle fiber type composition and cross‐sectional areas in control horses (C) and horse treated with 5000 IU/day of water dispersible α‐TP (Tx) for 56 days
| Group | N | Type 1 (%) | Type 2A (%) | Type 2X (%) | Area | |||
|---|---|---|---|---|---|---|---|---|
| Type 1 (μm2) | Type 2A (μm2) | Type 2X (μm2) | All fibers (μm2) | |||||
| Sacrocaudalis | ||||||||
| C day 0 | 8 | 90 ± 14 | 10 ± 14 | 5222 ± 1719 | 5807 ± 3156 | 5239 ± 1744 | ||
| C day 56 | 8 | 86 ± 10 | 14 ± 10 | 5683 ± 899 | 4223 ± 1866 | 5514 ± 995 | ||
| Tx Day 0 | 8 | 87 ± 11 | 13 ± 1 | 4686 ± 957 | 3219 ± 1184 | 4563 ± 1012 | ||
| Tx Day 56 | 8 | 85 ± 6 | 15 ± 6 | 5544 ± 980 | 4548 ± 1837 | 5414 ± 837 | ||
| GM | ||||||||
| C day 0 | 8 | 12 ± 5 | 19 ± 6 | 69 ± 10 | 2054 ± 584 | 2555 ± 454 | 5085 ± 1033 | 3233 ± 801 |
| C day 56 | 8 | 10 ± 2 | 18 ± 7 | 72 ± 6 | 1843 ± 802 | 2121 ± 671 | 3988 ± 884 | 2738 ± 648 |
| Tx day 0 | 8 | 16 ± 8 | 26 ± 10 | 60 ± 14 | 2268 ± 561 | 3270 ± 1036 | 5388 ± 1876 | 3354 ± 777 |
| Tx day 56 | 8 | 13 ± 3 | 24 ± 8 | 63 ± 10 | 1930 ± 534 | 2550 ± 493 | 5583 ± 1303 | 2651 ± 836 |
No significant differences were identified.
3.1.7. Electron microscopy
Samples for EM included 2 C and 3 Tx horses that did not have light microscopic mitochondrial alterations affecting >2.5% of fibers and 1 C and 1 Tx horse with light microscopic mitochondrial alterations affecting ≥2.5% of fibers. Granular lipopigment and lipofuscin and lysosomes laden with lipofuscin were present in all seven SC samples of C and Tx horses (Figure 2A, B). Mitochondria in 2 of 4 Tx and 2 of 3 C samples were small and present in large numbers under a scalloped sarcolemma (Figure 2B). Degenerating mitochondrial cristae were observed in 1 Tx and 1 C sample. One Tx sample with 2.4% mitochondrial alterations contained dense bodies and honeycomb‐shaped tubular structures (Figure 2C).
Figure 2.
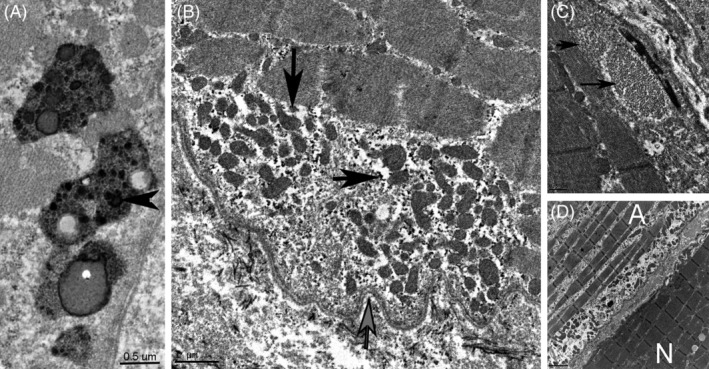
Electron microscopy. A, Four lysosomes laden with lipofuscin (horizontal arrow) and lipid droplets in a prospective day 56 horse treated with α‐TP (bar = 0.5 μm). B, Accumulation of lipopigment, (horizontal arrow) and pleomorphic small mitochondria (vertical arrow) under a scalloped sarcolemma (gray vertical arrow) in a day 56 horse treated with α‐TP (bar = 1 μm). C, Honeycomb‐like tubular structures (arrows) under the sarcolemma of a day 56 horse treated with α‐TP (bar = 0.5 μm). D, Longitudinal segments of two adjacent myofibers, one normal (N) and one atrophied (A) in a retrospective VEM horse. The atrophied fiber contains thin myofibrils with gaps between myofibrils and small subsarcolemmal mitochondria aggregating under a scalloped sarcolemma (bar = 2 μm). α‐TP, alpha‐tocopherol; VEM, vitamin E‐responsive myopathy
3.2. Retrospective VEM cases
3.2.1. Horses
Of the 10 VEM cases that met our inclusion criteria, 7 different breeds were represented, and included Belgian Warmblood (n = 2), Quarter Horse (n = 2), Paint (n = 1), Arabian (n = 1), Clydesdale Cross (n = 1), Rocky Mountain Horse (n = 1), and mixed breed (n = 2). There were 6 geldings and 4 mares with a mean age of 11.7 ± 3.5 years (range, 6‐15 years of age). Serum creatine kinase activities reported for 5 of 10 horses were all in the normal range, with a mean of 169 ± 60 U/L (reference, 82‐303 U/L). Serum α‐TP concentrations were reported for 9 of 10 horses, with a mean of 2.3 ± 1.1 μg/mL (range, 0.8‐3.9 μg/mL). Clinical signs in the rVEM horses included muscle atrophy, fasciculations, weakness and, in 3 horses, a gait abnormality resembling mild shivers or stringhalt. Muscle mass was reported (n = 3) or directly observed by investigators (n = 5) to be decreased in 8 horses. In 1 horse, a gait abnormality and trembling were the primary presentating findings, and in 1 horse poor performance was the primary complaint. Recommendations were to treat with 5000 IU/day of water‐dispersible RRR α‐TP, and follow‐up emails were received in April 2019 for 9 of 10 cases. All owners felt that horses had normal muscle mass on follow‐up, with the exception of 1 horse that was euthanized because of suspensory ligament desmitis without ever having received vitamin E.
3.2.2. Sacrocaudalis muscle histopathology
A mean of 221 ± 34 SC fibers were scored for rVEM horses. Histopathologic lesions were more pronounced in the SC muscle of rVEM horses than in the day 0 prospective subclinical horses (Figures 1E, F and 3). A significantly higher percentage of muscle fibers in rVEM horses had “moth‐eaten” staining, mitochondrial aggregates, total mitochondrial alterations, and anguloid atrophy compared to prospective day 0 samples (Figure 3A‐C). Angular atrophied fibers were rare at <3% of fibers in rVEM horses with no significant difference in percentage of angular atrophied fibers compared to day 0 prospective horses (Figure 3D).
Figure 3.
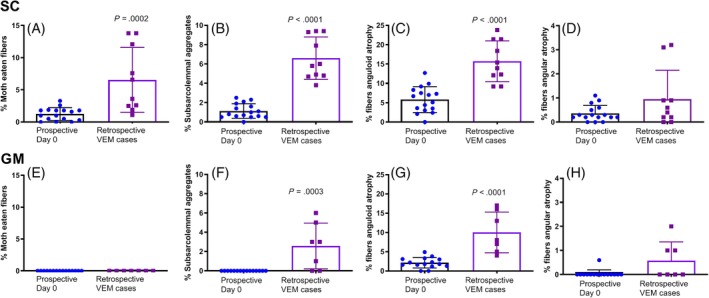
The percentage of fibers in the sacrocaudalis dorsalis medialis (SC) of prospective subclinical α‐TP‐deficient horses on day 0 and in clinical rVEM horses with histological features of (A) moth‐eaten fibers; (B) subsarcolemmal mitochondrial aggregates; (C) anguloid fibers; and (D) angular fibers. The percentage of fibers in the gluteus medius (GM) muscle of prospective subclinical α‐TP‐deficient horses on day 0 and in clinical rVEM horses with histological features of (E) moth‐eaten fibers; (F) subsarcolemmal mitochondrial aggregates; (G) anguloid fibers; and (H) angular fibers. α‐TP, alpha‐tocopherol; rVEM, responsive vitamin E‐responsive myopathy
3.2.3. GM/SM muscle histopathology
A mean of 221 ± 30 GM fibers were scored for rVEM horses. “Moth‐eaten” fibers were not present in the GM muscle of any rVEM horses (Figures 3C, E and 4). Significantly more mitochondrial aggregates, mitochondrial alterations, and anguloid atrophied fibers were present in rVEM horses compared to day 0 prospective subclinical horses (Figure 3F, G). The percentage of angular atrophied fibers did not differ between VEM and day 0 horses (Figure 3H).
Figure 4.
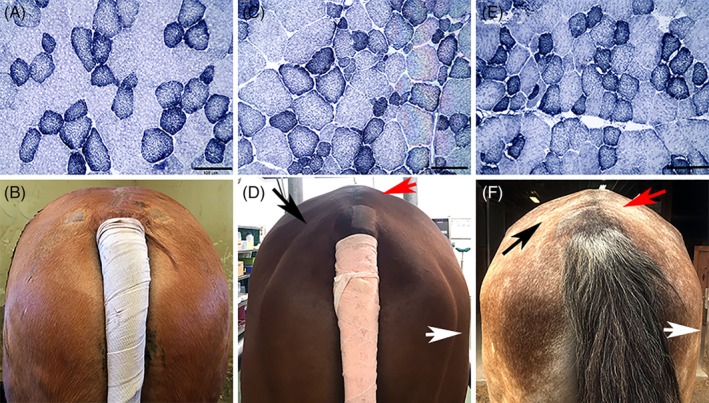
Cross sections of gluteal muscle stained with NADH‐TR (bar = 100 μm) and view of the hindquarter muscling of the respective horse. A, Muscle fibers with a normal mosaic staining pattern of oxidative and non‐oxidative fibers in a prospective α‐TP‐deficient horse on day 56 (×20). B, Normal muscling of the same Quarter Horse as in (A). C, Increased fiber size variation apparent in the gluteal muscle of a retrospective VEM horse (×20). D, Note the atrophy of the biceps femoris (white arrow), middle (red arrow), and superficial (black arrow) gluteal muscles in the same horse as shown in (C). E, Mild subsarcolemmal mitochondrial aggregates, anguloid atrophy, and smaller cross‐sectional area of gluteal muscle fibers in another retrospective VEM horse (×20). F, Note the atrophy of the biceps femoris (white arrow), middle (red arrow), and superficial (black arrow) gluteal muscles in the horse represented in (E). NADH‐TR, nicotinamide adenine dinucleotide tetrazolium reductase; α‐TP, alpha‐tocopherol; VEM, vitamin E‐responsive myopathy
3.2.4. Muscle fiber composition and cross‐sectional areas
For the 2 rVEM horses with samples frozen soon after biopsy (Figure 4), fiber compositions in SC were 83% and 75% in type 1 and 17% and 25% in type 2A. Compared to day 0 horses (5012 ± 1421 μm2; Figure 4A, B), the overall SC muscle fiber cross‐sectional areas for these 2 rVEM horses were 6% to 9% smaller (4783 and 4544 μm2; Figure 4C‐F). The GM fiber type compositions were 17% and 11% in type 1; 40% and 21% in type 2A; and 43% and 69% in type 2X, respectively, in the 2 rVEM horses. Compared to day 0 horses (3520 ± 764 μm2), the overall GM muscle fiber cross‐sectional areas in the 2 rVEM horses were similar for 1 rVEM horse (Figure 4C; 3544 μm2) and 20% smaller in the other rVEM horse (Figure 4E; 2752 μm2).
3.2.5. Electron microscopy
In the 1 rVEM SC sample processed, granular lipopigment and deposits of lipofuscin were evident in numerous fibers. Many muscle fibers were atrophic with sparse numbers of thin myofibrils creating expanded gaps between myofibrils (Figure 2D). Membranous whirls and accumulations of small pleomorphic mitochondria were evident under the sarcolemma, which often had a scalloped appearance (Figure 2D).
4. DISCUSSION
In our study, light microscopic alterations in mitochondrial staining were present in SC muscle fibers of subclinical α‐TP‐deficient horses. Mitochondrial alterations were more abundant in clinically affected rVEM horses, which had 5 times more fibers with mitochondrial alterations (range, 6%‐23%) compared to subclinical α‐TP‐deficient horses (day 0: range, 0%‐6% of fibers). Mitochondrial alterations consisted of subsarcolemmal aggregates of mitochondria and a “moth‐eaten” staining pattern in muscle fibers. Mitochondrial alterations were restricted to the SC muscle of horses with subclinical α‐TP deficiency, but subsarcolemmal aggregates of mitochondria were present in the SC and GM/SM of rVEM horses. “Moth‐eaten” fibers, which represent multifocal zones that lack mitochondria,22 only were observed in the SC muscle of subclinical and clinical α‐TP‐deficient horses. The reason why the SC muscle most readily displays mitochondrial staining abnormalities with α‐TP deficiency could be related to the predominance of oxidative type 1 fibers in the SC (67%‐100%) but not the GM (4%‐22%) muscle. Our results concur with those of previous studies that showed that the SC muscle is most likely to show histopathologic lesions associated with α‐TP deficiency.15, 16 Our results provide additional information indicating that mitochondrial alterations can be present in <6% of fibers of SC muscle with subclinical α‐TP deficiency, whereas mitochondrial alterations in ≥6% of SC fibers appear to be associated with clinical evidence of grossly apparent muscle atrophy.
The basis for mitochondrial alterations with α‐TP deficiency is likely lipid peroxidation of mitochondrial membranes because of an inability of α‐TP‐deficient muscle to scavenge peroxyl radicals faster than they can react with lipids or proteins.23 The mitochondrial electron transport chain is the major source of reactive oxygen species in skeletal muscle, and α‐TP is the primary antioxidant found in the lipid bilayer.1, 24 Membrane damage in the SC muscle of horses in the present study was evident on EM as membranous whirls, dense bodies, and honeycomb‐like elements, all features previously described in α‐TP‐deficient rats.25 Mitochondrial membrane damage decreases the stability of mitochondrial membranes, which results in a significantly decreased metabolic rate, reflected by decreased NADH and FADH2 utilization and decreased activity of respiratory chain complexes.25 Decreased oxidative capacity with α‐TP deficiency could account for some of the reported muscle weakness and decrease in exercise performance in VEM horses.
An additional feature of the SC muscle in all subclinical and clinical rVEM horses examined was the presence of lipofuscin, a recognized feature in the retina, central nervous system, gastrointestinal tract, and liver of EMND α‐TP‐deficient horses.5 Lipofuscin is oxidized protein and lipid, and its accumulation in cells is both age‐ and oxidative stress‐dependent.26 The accumulation of lipofuscin in subclinical α‐TP‐deficient horses with or without abnormal mitochondrial staining may represent oxidative damage occurring at an early stage of α‐TP deficiency in horses. Because lipofuscin accumulates as cells age, we could not rule out the possibility that lipofuscin was present merely as a reflection of age. Lipofuscin itself can become a pro‐oxidant because of its ability to incorporate redox‐active metals such as iron.26 As lipofuscin accumulates in lysosomes, it triggers a cycle of progressive accumulation of protein oxidation products that impair autophagocytic capacity.26 Impairment of mitophagy in muscle cells, which are post‐mitotic and therefore unable to permanently remove cellular debris, could explain the persistent alterations in mitochondrial staining in day 56 samples. Whether a longer duration of Tx or a different dose of α‐TP would have resulted in normal mitochondrial morphology was not established. The potentially irreversible mitochondrial damage that was present in subclinical and clinically affected VEM horses suggests that assessing α‐TP concentrations and preventing α‐TP deficiency in clinically normal horses may be advantageous to preserve oxidative muscle fiber function.
Acute or chronic onset of grossly apparent muscle atrophy is a hallmark of clinical VEM that impacts many different breeds including Quarter Horses used in the prospective study.15 The presence of grossly apparent muscle atrophy was associated with progressive atrophic alteration in fiber shapes. Horses with clinical VEM in our study had significantly more anguloid atrophied fibers than did subclinical day 0 α‐TP‐deficient horses. For example, rVEM horses had anguloid atrophy in 16% of SC and 10% of GM fibers, whereas at day 0, subclinical α‐TP‐deficient horses had a mean of 6% SC and 2% GM fibers with anguloid atrophy. Anguloid atrophy previously has been reported with VEM, and anguloid atrophy is most characteristic of myogenic rather than neurogenic disorders.15, 27 Angular atrophy was rare in our study, with an average of <0.5% of fibers in SC and GM muscles of day 0 α‐TP‐deficient horses showing angular atrophy and an average of 0.9% of SC and 0.5% of GM fibers having angular atrophy in rVEM horses. Angular atrophy was found in focal regions of some of the day 56 GM specimens that were not present in day 0 GM biopsy specimens. This finding appeared to be a result of obtaining day 56 biopsy specimens on the same side, and too close to the site of day 0 specimens, apparently damaging focal nerve branches. Smaller fibers at this site also could have been a result of regeneration, but this was less likely based on the absence of large internalized myonuclei and basophilia typical of regeneration.22
In general, grossly apparent muscle atrophy occurs when the physiological balance between protein synthesis and breakdown is disrupted. This balance has been proposed to be redox‐sensitive with 1 mechanism for atrophy occurring when oxidative stress induces both expression and activity of components of the ubiquitin‐proteasome‐dependent proteolytic pathway.2, 24 The trigger for muscle atrophy in the rVEM horses could be related to the extent of oxidative stress, the total balance of other antioxidants such as glutathione, thioredoxins, catalase, and superoxide dismutase, and the duration of antioxidant deficiency in skeletal muscle.23 In mouse models of disuse atrophy, supplementation with α‐TP has been shown to downregulate genes involved in muscle proteolysis such as μ calpain, caspases‐3, ‐9, and ‐12, and 2 atrophy‐related ubiquitin ligases (MuRF1 and MAFbx).28 Thus, in horses with gross muscle atrophy, α‐TP supplementation likely reestablishes normal muscle mass by inhibition of proteolysis. In our study, the lack of an increase in skeletal muscle volume or muscle fiber cross‐sectional areas with α‐TP supplementation of normal‐appearing α‐TP‐deficient horses supports the view that α‐TP inhibits proteolysis rather than enhancing muscle protein synthesis.
In conclusion, mitochondrial alterations in <6% of SC fibers are evident in apparently healthy α‐TP‐deficient horses and do not resolve after 56 days of 5000 IU/day of α‐TP supplementation. Grossly apparent muscle atrophy in clinical VEM occurred in horses with ≥6% (mean 13%) of SC fibers showing alterations on mitochondrial stains. Serum α‐TP deficiency appears to be associated with persistent mitochondrial alterations that, once beyond a certain threshold, are associated with grossly apparent muscle atrophy.
CONFLICT OF INTEREST DECLARATION
The vitamin E supplement (Nano E) used in this trial was provided by Kentucky Equine Research, Versailles, KY at no cost to the study. Kentucky Equine Research did not participate in the design of the study, review the results or have input into the writing of this paper.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study was approved by the Animal Use and Care Committees of the University of California, Davis.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Supplemental Figure 1. A. Cross‐section of gluteal muscle from a control horse with subclinical α tocopherol deficiency taken on day 56. Note the angular atrophy of numerous muscle fibers that have small subsarcolemmal myonuclei. (hematoxylin and eosin 20X) B. Serial section of the same muscle sample in A showing angular atrophy of type 1 (lower arrow black myofiber) and type 2X (upper arrow brown myofiber) muscle fibers, typical features of neurogenic atrophy.
ACKNOWLEDGMENT
Authors thank Kentucky Equine Research for providing the vitamin E used in this trial. An abstract of this subject was presented at the 2019 ACVIM Forum, Phoenix, AZ.
Bookbinder L, Finno CJ, Firshman AM, et al. Impact of alpha‐tocopherol deficiency and supplementation on sacrocaudalis and gluteal muscle fiber histopathology and morphology in horses. J Vet Intern Med. 2019;33:2770–2779. 10.1111/jvim.15643
Funding information Endowment of the Mary Anne McPhail Dressage Chair in Equine Sports Medicine; Foundation for the National Institutes of Health, Grant/Award Numbers: K05OD015134, L40 TR001136.; University of California Davis Center for Equine Health; Michigan State University, EquineFreeman Fund
REFERENCES
- 1. Herrera E, Barbas C. Vitamin E: action, metabolism and perspectives. J Physiol Biochem. 2001;57:43‐56. [PubMed] [Google Scholar]
- 2. Galli F, Azzi A. Present trends in vitamin E research. Biofactors. 2010;36:33‐42. [DOI] [PubMed] [Google Scholar]
- 3. Siciliano PD, Parker AL, Lawrence LM. Effect of dietary vitamin E supplementation on the integrity of skeletal muscle in exercised horses. J Anim Sci. 1997;75:1553‐1560. [DOI] [PubMed] [Google Scholar]
- 4. Pagan JD. Form and source of tocopherol affects vitamin E status in thoroughbred horses. Pferdeheilkunde. 2005;21:101‐102. [Google Scholar]
- 5. Divers TJ, Cummings JE, de Lahunta A, et al. Evaluation of the risk of motor neuron disease in horses fed a diet low in vitamin E and high in copper and iron. Am J Vet Res. 2006;67:120‐126. [DOI] [PubMed] [Google Scholar]
- 6. Finno CJ, Valberg SJ. A comparative review of vitamin E and associated equine disorders. J Vet Intern Med. 2012;26:1251‐1266. [DOI] [PubMed] [Google Scholar]
- 7. Bruhn JC, Oliver JC. Effect of storage on tocopherol and carotene concentrations in Alfalfa Hay. J Dairy Sci. 1978;61:980‐982. [Google Scholar]
- 8. Lewis LD. Minerals for horses Equine Clinical Nutrition: Feeding and Care. Topeka, KS: Williams and Wilkins; 1995:41. [Google Scholar]
- 9. Burns EN, Finno CJ. Equine degenerative myeloencephalopathy: prevalence, impact, and management. Vet Med. 2018;9:63‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mohammed HO, Divers TJ, Summers BA, et al. Vitamin E deficiency and risk of equine motor neuron disease. Acta Vet Scand. 2007;49:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aleman M, Finno CJ, Higgins RJ, et al. Evaluation of epidemiological, clinical, and pathological features of neuroaxonal dystrophy in quarter horses. J Am Vet Med Assoc. 2011;239:823‐833. [DOI] [PubMed] [Google Scholar]
- 12. Finno CJ, Famula T, Aleman M, et al. Pedigree analysis and exclusion of alpha‐tocopherol transfer protein (TTPA) as a candidate gene for neuroaxonal dystrophy in the American quarter horse. J Vet Intern Med. 2013;27:177‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finno CJ, Estell KE, Katzman S, et al. Blood and cerebrospinal fluid alpha‐tocopherol and selenium concentrations in neonatal foals with neuroaxonal dystrophy. J Vet Intern Med. 2015;29:1667‐1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mohammed HO, Cummings JF, Divers TJ, et al. Epidemiology of equine motor neuron disease. Vet Res. 1994;25:275‐278. [PubMed] [Google Scholar]
- 15. Bedford HE, Valberg SJ, Firshman AM, et al. Histopathologic findings in the sacrocaudalis dorsalis medialis muscle of horses with vitamin E‐responsive muscle atrophy and weakness. J Am Vet Med Assoc. 2013;242:1127‐1137. [DOI] [PubMed] [Google Scholar]
- 16. Valentine BA, de Lahunta A, George C, et al. Acquired equine motor neuron disease. Vet Pathol. 1994;31:130‐138. [DOI] [PubMed] [Google Scholar]
- 17. Goetz CJ. Body and tissue analysis Textbook of Clinical Neurology. Philadelphia PA: Saunders Elsevier; 2007:515. [Google Scholar]
- 18. Valberg SJM AJ, Firshman AM, Bookbinder L, Katzman SA, Finno CJ. 3 dimensional photonic scans for measuring body volume and muscle development in the standing horse. PLoS One. 2019; In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lindholm A, Piehl K. Fibre composition, enzyme activity and concentrations of metabolites and electrolytes in muscles of standardbred horses. Acta Vet Scand. 1974;15:287‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cumming WJKF JJ, Hudgson P, Mahon M. Color Atlas of Muscle Pathology. London, UK: Mosby‐Wolfe; 1994. [Google Scholar]
- 21. Stanley RL, Maile C, Piercy RJ. Storage‐associated artefact in equine muscle biopsy samples. Equine Vet J. 2009;41:82‐86. [DOI] [PubMed] [Google Scholar]
- 22. Engel AG. Basic reactions of muscle In: Engel AGF‐A C, ed. Myology. 2nd ed. New York: McGraw‐Hill; 1994:861‐862. [Google Scholar]
- 23. Schneider C. Chemistry and biology of vitamin E. Mol Nutr Food Res. 2005;49:7‐30. [DOI] [PubMed] [Google Scholar]
- 24. Bonetto A, Penna F, Muscaritoli M, et al. Are antioxidants useful for treating skeletal muscle atrophy? Free Radic Biol Med. 2009;47:906‐916. [DOI] [PubMed] [Google Scholar]
- 25. Thomas PK, Cooper JM, King RH, et al. Myopathy in vitamin E deficient rats: muscle fibre necrosis associated with disturbances of mitochondrial function. J Anat. 1993;183(Pt 3):451‐461. [PMC free article] [PubMed] [Google Scholar]
- 26. Brunk UT, Terman A. Lipofuscin: mechanisms of age‐related accumulation and influence on cell function. Free Radic Biol Med. 2002;33:611‐619. [DOI] [PubMed] [Google Scholar]
- 27. Valberg SJ. Spinal muscle pathology. Vet Clin North Am Equine Pract. 1999;15:87‐96. vi‐vii. [DOI] [PubMed] [Google Scholar]
- 28. Servais S, Letexier D, Favier R, et al. Prevention of unloading‐induced atrophy by vitamin E supplementation: links between oxidative stress and soleus muscle proteolysis? Free Radic Biol Med. 2007;42:627‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. A. Cross‐section of gluteal muscle from a control horse with subclinical α tocopherol deficiency taken on day 56. Note the angular atrophy of numerous muscle fibers that have small subsarcolemmal myonuclei. (hematoxylin and eosin 20X) B. Serial section of the same muscle sample in A showing angular atrophy of type 1 (lower arrow black myofiber) and type 2X (upper arrow brown myofiber) muscle fibers, typical features of neurogenic atrophy.


