Abstract
Background
Heart disease is an important cause of morbidity and mortality in cats, but there is limited evidence of the benefit of any medication.
Hypothesis
The angiotensin‐converting enzyme inhibitor benazepril would delay the time to treatment failure in cats with heart disease of various etiologies.
Animals
One hundred fifty‐one client‐owned cats.
Methods
Cats with heart disease, confirmed by echocardiography, with or without clinical signs of congestive heart failure, were recruited between 2002 and 2005 and randomized to benazepril or placebo in a prospective, multicenter, parallel‐group, blinded clinical trial. Benazepril (0.5‐1.0 mg/kg) or placebo was administered PO once daily for up to 2 years. The primary endpoint was treatment failure. Analyses were conducted separately for all‐cause treatment failure (main analysis) and heart disease‐related treatment failure (supportive analysis).
Results
No benefit of benazepril versus placebo was detected for time to all‐cause treatment failure (P = .42) or time to treatment failure related to heart disease (P = .21). Hazard ratios (95% confidence interval [CI]) from multivariate analysis for benazepril compared with placebo were 1.00 (0.57‐1.74) for all‐cause failure, and 0.99 (0.50‐1.94) for forward selection and 0.93 (0.48‐1.81) for bidirectional selection models for heart disease‐related failure. There were no significant differences between groups over time after administration of the test articles in left atrium diameter, left ventricle wall thickness, quality of life scores, adverse events, or plasma biochemistry or hematology variables.
Conclusions and Clinical Relevance
Benazepril was tolerated well in cats with heart disease, but no evidence of benefit was detected.
Keywords: ACE inhibitor, survival, treatment failure
Abbreviations
- ACE
angiotensin‐converting enzyme
- ACEI
angiotensin‐converting enzyme inhibitor
- CHF
congestive heart failure
- CI
confidence interval
- CPH
Cox proportional hazards
- DCM
dilated cardiomyopathy
- FS
fractional shortening
- HCM
hypertrophic cardiomyopathy
- HOCM
hypertrophic obstructive cardiomyopathy
- HR
hazard ratio
- LA
left atrium
- LV
left ventricle
- NRS
numerical rating scale
- RAAS
renin‐angiotensin‐aldosterone system
- RCM
restrictive cardiomyopathy
- UCM
unclassified cardiomyopathy
- WBC
white blood cell
1. INTRODUCTION
Heart disease is an important source of morbidity and mortality in cats. A variety of medications have been recommended historically and used in cats with heart disease, including angiotensin‐converting enzyme (ACE) inhibitors (ACEIs), aspirin, beta blockers, clopidogrel, calcium channel blockers, diuretics, and more recently pimobendan.1, 2, 3, 4, 5 However, to our knowledge, the benefit of any pharmaceutical agent has not been demonstrated in well‐controlled prospective clinical trials in cats with heart disease.
At the time of the initiation of the study in 2002, the state of knowledge was that positive effects of ACEIs on clinical signs and survival had been demonstrated in humans6, 7 and dogs8, 9, 10 with congestive heart failure (CHF). In cats, the ACEIs enalapril and benazepril decreased left ventricular wall thickness of cats with hypertrophic cardiomyopathy (HCM), but the studies were not well‐controlled.11, 12 In addition, there is increased expression of renin in the kidneys of cats with HCM examined at necropsy, consistent with activation of the renin‐angiotensin‐aldosterone system (RAAS).13
Based on the evidence available at the time of starting the study, it was hypothesized that the ACEI benazepril might have beneficial effects in cats with heart disease of various etiologies, and not restricted to cases of HCM.
The main hypothesis of the study was that, compared to placebo, benazepril would delay the time to treatment failure in cats with heart disease of various etiologies.
2. MATERIALS AND METHODS
The study was a prospective, multicenter, parallel‐group, randomized, and blinded clinical trial comparing benazepril with placebo in cats with heart disease. The study was conducted in compliance with the Procedures and Principles of Good Clinical Practice (VICH GL9, CVMP:VICH/595/98, 2000), and after approval by company internal review procedures and the respective regulatory authorities in each country taking into account animal welfare and ethical guidelines. All owners had to give their written informed consent before the start of the study.
The manuscript was prepared after consultation of the CONSORT statement for reporting of randomized clinical trials.14
2.1. Study design
All cats were client‐owned animals and therefore were fed, housed, and managed at home as pets. Owners were requested, as far as possible, not to change the home management of their cats during the trial. On day 0 (baseline), case history, demographic variables, and results of the owner assessment and the investigator (veterinarian) clinical examination were recorded. The cats were scheduled to be examined by the investigator again at weeks 5 (range, ±1), 13 (±1), 26 (±2), and 52 (±4) weeks, and in some cases also at 78 (±4) and 104 (±4) weeks. The maximum treatment and follow‐up time was initially 1 year, but was extended to 2 years during the study. Systemic systolic blood pressure measured via Doppler sphygmomanometry, 2‐dimensional and M‐mode echocardiography, electrocardiography, and thoracic radiography were scheduled to be performed at day 0 and weeks 13, 26, and 52 (plus 78 and 104 weeks in some cats). No sedation was permitted for 24 hours before an echocardiogram. In order to optimize consistency, investigators were trained on the techniques used in the study, including echocardiography which followed published methods.15, 16, 17 Blood samples for routine clinical chemistry and hematology were taken at each visit.
2.2. Selection of cats
Cats were recruited according to the following inclusion criteria:
Cats with heart disease requiring treatment. This included cases with CHF, and cats without CHF but which had heart disease and structural changes to the heart that, in the opinion of the investigator, required treatment. Animals were selected according to the investigator's clinical judgment confirmed by complementary analyses, notably echocardiography. Underlying cardiac diseases permitted were: HCM; hypertrophic obstructive cardiomyopathy (HOCM), that is, outflow obstruction was not an exclusion criterion; dilated cardiomyopathy (DCM); restrictive cardiomyopathy (RCM); unclassified cardiomyopathy (UCM); valvular disease, for example, endocardiosis (but not endocarditis); and congenital defects not correctable surgically or that could lead to CHF, for example, mitral dysplasia or aortic stenosis. The classification of cases to HCM, HOCM, DCM, RCM, and UCM was made according to published criteria.16, 17
Body weight ≥1.25 to ≤10 kg, to allow dosing with tablets containing 2.5 mg benazepril hydrochloride.
Exclusion criteria for admission were:
Congenital heart disease that should be treated surgically, for example, patent ductus arteriosus, peritoneo‐pericardial diaphragmatic hernia, or pulmonic stenosis.
Congenital cyanotic heart disease.
Diabetes mellitus (glycosuria or elevated fructosamine >341 μm/L or both).
Endocarditis or primary pericardial disease.
Confirmed heartworm infection (cats were required to be tested in heartworm endemic areas).
Hyperthyroidism (presence of relevant clinical signs or total plasma thyroxine >55 nmol/L or both).
Pulmonary or systemic arterial thrombo‐embolism (intracardiac thrombi were permitted).
Systemic hypertension (arterial systolic blood pressure >170 mm Hg). Blood pressure was measured by Doppler sphygmomanometry, with a minimum of 3 consistent recordings in a visibly calm cat.
Animals intended for breeding or known to be pregnant or lactating.
Cats could be removed from the study after admission because of the occurrence of the criteria listed below:
Hyperthyroidism.
Persistent and unacceptably high heart rate, in the clinician's opinion (no threshold value was defined).
Need for treatment not permitted in the protocol.
Repeated thoracocentesis.
Ventricular arrhythmia requiring treatment.
Noncompliance with the protocol sufficient to affect the outcome of the study, including failure to administer the test items.
2.3. Randomization
After inclusion in the study at day 0, cats were allocated by permuted block randomization to 1 of the 2 treatment groups in a 1:1 ratio, with a block size of 4. Separate randomization lists for each investigator were generated by the statistician using randomization software.
2.4. Test items
Benazepril was administered as the hydrochloride salt at a minimum dosage of 0.5 mg/kg (target range of 0.5 to 1 mg/kg) once daily in the form of palatable divisible tablets containing 2.5 mg benazepril hydrochloride (Fortekor 2.5 mg, Elanco Animal Health, Huningue, France). The placebo tablets had the same appearance and composition as the benazepril tablets, except that benazepril hydrochloride was replaced by lactose. The benazepril and placebo tablets were packed into identical bottles, which were labeled A‐D, with 2 codes for each of the items. Investigators and owners (as well as all study personnel except the sponsor representative and statistician) were therefore blinded to the test item administered. The blinding code was not broken in the study.
Owners were instructed to administer the test items, as far as possible, at the same time each day. The test items could be administered with or without food.
The dosage of benazepril hydrochloride tested (minimum 0.5 mg/kg) was the same as registered in many countries including the European Union for the management of proteinuria in cats with chronic kidney disease.
The number of tablets dispensed to and returned by the owner for each cat was checked at each visit. Daily treatment compliance and ease of administration of the tablets were not assessed.
2.5. Concomitant medication
All cats with DCM were required to be fed taurine supplementation.
Three drugs with an effect on the cardiovascular system were permitted as concomitant medication: aspirin (at a target dosage of 10 mg/kg/week PO), digoxin (only in cats with DCM as an inotrope at a target dosage of 0.01 mg/kg PO on alternate days), and furosemide (dosed to effect, no guidelines or limits were defined, PO but not parenteral). Usage and dosages were decided by the investigator as required, but were not analyzed. Other drugs with a potential action on the cardiovascular system in cats were not permitted during the study or within 3 days before inclusion at day 0; including open‐label prescription of ACEIs, beta blockers, calcium channel blockers, diuretics (other than furosemide PO), nitroglycerin, and pimobendan.
Other concomitant medications or therapies were permitted provided that they had no relevant action on the cardiovascular system or would not interfere with the objectives of the study, or both, for example, antibiotics or antiparasite products were allowed.
2.6. Efficacy assessments
The primary endpoint was treatment failure and was defined prospectively in the protocol. Analyses were conducted separately for all‐cause treatment failure (defined as the main analysis) and for heart disease‐related treatment failure (defined as supportive). Heart disease‐related treatment failure (a composite of several sub‐endpoints) was defined as death or euthanasia or withdrawal of the cat from the study because of worsening of clinical condition (all related to heart disease), persistent and unacceptably high heart rate (no threshold was defined), repeated thoracocentesis, or ventricular arrhythmia requiring treatment. All‐cause treatment failure (also a composite) included the heart disease‐related treatment failure reasons plus additionally death or euthanasia or withdrawal of the cat from the study (all for reasons other than heart disease), development of hyperthyroidism, or occurrence of a serious adverse event resulting in the removal of the cat from the study.
The secondary endpoints defined prospectively in the protocol were the quality of life of the cat assessed by the owner (rated as normal, medium, poor, or very poor) and the left atrium (LA) diameter and left ventricle (LV) wall dimension on echocardiography.16, 17
In addition, investigators assessed the following variables subjectively using present/absent criteria or 4‐point numerical rating scales (NRS; eg, absent, mild, moderate, or severe): ascites and peripheral edema on palpation; LA dilation, cardiomegaly, pleural effusion, and pulmonary edema evident on thoracic radiography (right lateral recumbent view with or without additional dorsal‐ventral view); cardiac and respiratory rate; dyspnea; fluid sounds on pulmonary auscultation; gallop sound or murmur audible on cardiac auscultation; and cyanotic or pale mucous membranes. The owners assessed the following variables using 3‐ or 4‐point subjective NRS: appetite, cough, dyspnea, level of activity, time spent grooming, and ease of administration of the test items.
2.7. Safety assessments
The safety of the test items was assessed from the investigator clinical examinations, adverse events reported by the owner, and results of the clinical chemistry and hematology variables.
2.8. Statistical analysis
All statistical tests were performed using commercial software (SAS Software, Version 9.2, Cary, North Carolina). Reported P values are 2‐tailed with P < .05 defined as significant. Baseline data were compared between groups using analysis of variance for parametric data and Fisher's exact test for frequency data.
Time to event analysis was conducted separately for all‐cause treatment failure and for heart disease‐related treatment failure (defined earlier). Cats lost from the study for a reason not defined in the protocol, or still alive when the study was terminated by the sponsor, were included in the analysis up until the last time point at which they remained in the study and were thereafter censored. The Kaplan‐Meier method was used to estimate the median and 95% confidence interval (CI) time to event, and to generate time to event graphs. The log rank test with right censoring was used to compare times to event between groups.
Univariate Cox proportional hazards (CPH) model analysis with right censoring was performed for each variable to determine the association between that variable (at baseline) and time to event; the hazards ratio (HR) and 95% CI were calculated.
The database contained a total of 53 baseline variables from 151 cats, and only 62 cats reached the all‐cause failure endpoint. In order to reduce the risk of overparameterization in the multivariate analysis, variables were selected for analysis as follows:
Heart disease was classified as either congenital or noncongenital in origin. Noncongenital cardiomyopathy cases were originally classified as HCM, HOCM, DCM, RCM, or UCM; however, cases were finally not analyzed according to these subgroups.
For echocardiogram variables in which values were available from 2 different techniques, the variable with data from the highest number of cats was selected. The LA diameter was assessed on both the short and long axis views, and the end diastolic mean LV diameter, LV free wall, and LV septum dimensions were based on the 2‐dimensional and M‐mode.16, 17
Correlations for clinical chemistry and hematology variables were investigated using Spearman rank correlation, and only 1 of the highly correlated variables was selected. Hematocrit was highly correlated with red cell count (the correlation coefficient ρ = .79) and hemoglobin concentration (ρ = .91), therefore hematocrit was selected. Alkaline phosphatase and alanine aminotransferase activities were highly correlated (ρ = .70), therefore the former was selected.
Clinical signs (presence or absence) of reduced appetite, ascites, crackles, dyspnea, pleural effusion, pulmonary edema, and poor respiratory character were summarized into a single category, the presence or absence of CHF signs.
Application of the selection methods described above led to a smaller subset of 24 variables, which were included in the univariate CPH analysis (Tables 4 and 6).
For the multivariate analysis, both forward selection and bidirectional selection approaches were used. Models were assessed with or without forcing treatment into the model; although treatment was not significant in the univariate analysis, assessment of treatment was the primary objective of the study. For forward selection, no variables were selected initially, and candidate variables were tested and added if they improved the fit of the model (using F tests). Once a variable entered the model, it was retained. For bidirectional selection, the 5 variables with the lowest P values from the univariate analysis (Tables 4 and 6) were selected initially (with or without treatment), and the effect of adding or subtracting the other variables was tested (using F tests). Introduction of a maximum of 6 variables initially into the bidirectional selection models complies with the recommendation to have a minimum of 10 event cases per variable.18
As a final validation of the multivariate CPH models, the best model approach was employed and confirmed the choice of variables selected by the forward and bidirectional selection approaches.
The interaction was analyzed between each of the failure endpoints and the presence or absence at baseline of LA diameter >16 mm (reflecting probable LA enlargement), congenital heart disease, presence of CHF signs, and presence of dyspnea plus poor quality of life (representing more severe signs).
Echocardiography, clinical chemistry, and hematology variables were analyzed longitudinally using repeated measures analysis of covariance, with baseline as the covariate, and treatment, visit, and treatment/time interaction as effects. Data were transformed as appropriate to give the best approximation of a normal distribution.
Scores for clinical signs at each time point and frequencies of adverse events in the 2 groups after day 0 were compared with Fisher's exact test.
A minimum of 100 cats was planned in the protocol, as it was calculated that 50 cats per group would provide 74% power with a 25% difference between groups for the frequency of occurrence of the primary endpoint. At least 80% power required an estimated 58 cats per group. Insufficient data were available before the study, however, to allow reliable sample size and power estimations.
3. RESULTS
The number of cats screened was not recorded, but 151 cats were enrolled into the study at 23 centers (median 5, range 1‐27 cats per center) between October 2002 and March 2005, and treated up to January 2006. Data from all 151 cats were included in the statistical analysis, which can therefore be considered an “all‐randomized animal” or “intent‐to‐treat” analysis data set; 77 received benazepril and 74 received placebo. No separate “per‐protocol” analysis was conducted.
Baseline and demographic data are shown in Table 1, and the frequency of echocardiographic diagnoses in Table 2. A total of 93 cats (62%) had clinical signs of CHF, whereas 58 (38%) were asymptomatic. The frequency of congenital disorders (P = .009) and mean white blood cell (WBC) counts (P = .04) were significantly higher in the group of cats randomized to receive benazepril. A flowchart with study outcomes is shown in Figure 1.
Table 1.
Baseline and demographic data
| Variable | Benazepril (n = 77) | Placebo (n = 74) | P value |
|---|---|---|---|
| Age (years) | 7.8 (4.1) | 8.0 (4.1) | .74 |
| Sex male:female | 59:17 | 54:19 | .70 |
| Congenital disorder yes: no | 14:63 | 3:71 | .009 |
| Fractional shortening (%) | 45.3 (16.0) | 44.2 (16.7) | .68 |
| LA diameter (short axis view, mm) | 17.2 (5.5) | 16.5 (4.7) | .39 |
| End diastolic LV diameter (M‐mode, mm) | 16.2 (6.0) | 16.5 (13.7) | .85 |
| End diastolic LV free wall thickness (M‐mode, mm) | 6.98 (2.1) | 7.57 (7.8) | .54 |
| End diastolic LV septum thickness (M‐mode, mm) | 5.70 (1.7) | 6.92 (8.1) | .22 |
| Hematocrit (%) | 39.7 (6.8) | 40.2 (6.1) | .59 |
| White blood cells (109/L) | 12.6 (5.9) | 10.8 (4.2) | .04 |
| Plasma albumin (g/L) | 34.2 (3.9) | 34.9 (3.3) | .22 |
| Plasma calcium (mmol/L) | 2.46 (0.19) | 2.48 (0.19) | .52 |
| Plasma creatinine (μmol/L) | 151.9 (56.8) | 144.4 (37.4) | .35 |
| Plasma phosphate (mmol/L) | 1.70 (0.44) | 1.60 (1.39) | .14 |
| Plasma potassium (mmol/L) | 4.54 (1.2) | 4.70 (1.1) | .41 |
| Plasma sodium (mmol/L) | 152.7 (4.8) | 153.8 (4.1) | .14 |
| Plasma urea (g/L) | 0.72 (0.31) | 0.65 (0.21) | .14 |
| Plasma alkaline phosphatase (U/L) | 30.4 (19.9) | 34.9 (36.4) | .35 |
| CHF signs present:absent | 53:24 | 40:34 | .07 |
| Arrhythmia present:absent | 14:63 | 6:68 | .09 |
| Gallop sound present:absent | 17:60 | 18:56 | .85 |
| Level of activity reduced:not reduced | 66:11 | 64:10 | 1.0 |
| Quality of life poor:good | 28:49 | 24:50 | .73 |
Notes: P values <.05 are shown in bold. Data are mean (SD) or frequency. P values were calculated by analysis of variance for data reported as mean (SD) and Fisher's exact test for frequency data.
Abbreviations: CHF, congestive heart failure; LA, left atrium; LV, left ventricle.
Table 2.
Main echocardiographic diagnosis at baseline
| Echocardiographic diagnosis | Benazepril (n = 77) | Placebo (n = 74) | P value |
|---|---|---|---|
| Hypertrophic cardiomyopathy | 28 | 33 | .32 |
| Hypertrophic obstructive cardiomyopathy | 12 | 19 | .16 |
| Restrictive cardiomyopathy | 10 | 4 | .16 |
| Unclassified cardiomyopathy | 8 | 6 | .78 |
| Dilated cardiomyopathy | 4 | 7 | .36 |
| Mitral valve dysplasia | 4 | 1 | .37 |
| Aortic stenosisa | 3 | 0 | .25 |
| Ventricular septal defect plus mitral or tricuspid valve dysplasia | 3 | 0 | .25 |
| Ventricular septal defect | 2 | 2 | 1.0 |
| Atrial septal defect | 1 | 0 | 1.0 |
| Mitral valve regurgitation | 1 | 0 | 1.0 |
| Tricuspid valve insufficiency | 0 | 1 | .49 |
| Not recorded | 1 | 1 | 1.0 |
Notes: Data are number of cases in each group at baseline. P values were calculated with Fisher's exact test.
One case of hypertrophic cardiomyopathy in the benazepril group had in addition aortic stenosis.
Figure 1.
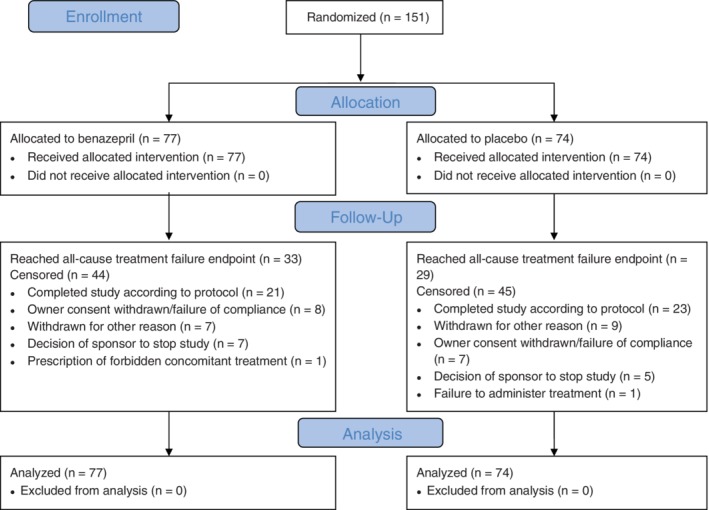
Flowchart showing outcomes of the cats enrolled in the study. The CONSORT (2010) template was used.10 The reasons for treatment failure are provided in Table 3
Benazepril hydrochloride at a mean dosage of 0.70 mg/kg (range 0.50 to 1.0 mg/kg) or placebo was administered once daily for up to 2 years.
3.1. Time to event analysis
Visual examination of the Kaplan‐Meier plots indicated no violation of the proportional hazards assumption (Figures 2 and 3). There were no clinically relevant differences in conclusions from the time to event analysis of all‐cause or heart disease‐related treatment failure.
Figure 2.
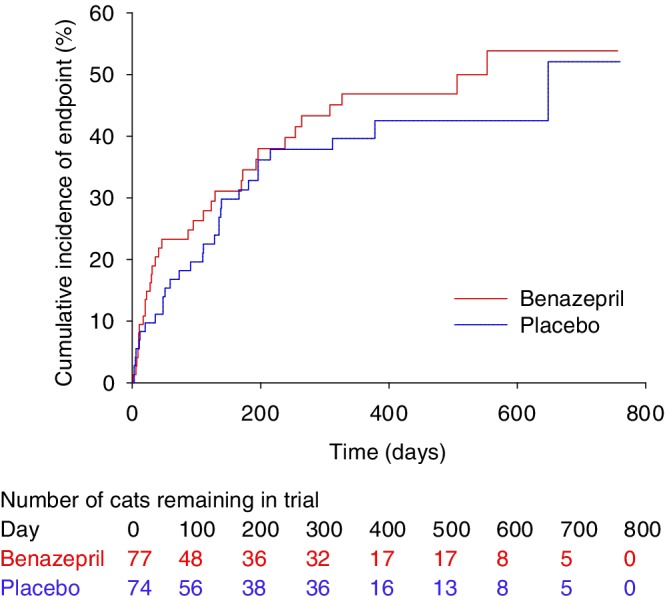
Kaplan‐Meier plot of the cumulative incidence over time of cats reaching the all‐cause treatment failure endpoint. The number of cats reaching the endpoint/total was 33/77 in the benazepril and 29/74 in the placebo group. The median time to event was 553 days in the benazepril and 648 days in the placebo group. P = .42 with the log rank test
Figure 3.
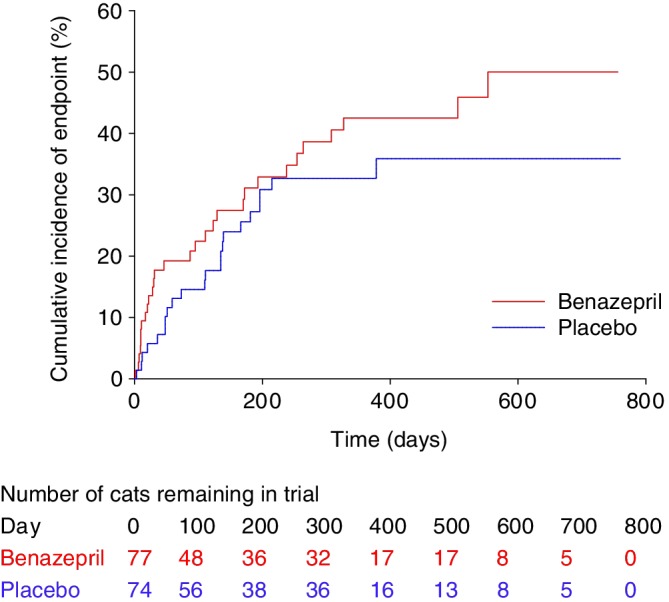
Kaplan‐Meier plot of the cumulative incidence over time of cats reaching the heart disease‐related treatment failure endpoint. The number of cats reaching the endpoint/total was 29/77 in the benazepril and 22/74 in the placebo group. The 25% interquartile for time to event was 123 days in the benazepril and 166 days in the placebo group. P = .21 with the log rank test
3.2. All‐cause treatment failure
In the benazepril group, a total of 33 cats reached the all‐cause treatment failure endpoint and 44 were censored. In the placebo group, a total of 29 cats reached the endpoint and 45 were censored. The proportion of cats reaching the endpoint (benazepril 43%, placebo 39%) or censored was similar in both groups, as were the reasons for failure (Table 3) and censoring (Figure 1).
Table 3.
Number of cases reaching the defined treatment failure endpoints
| Reason | Benazepril (n = 77) | Placebo (n = 74) | P value |
|---|---|---|---|
| Related to heart disease | |||
| Death of cat related to heart disease | 10 | 5 | .28 |
| Euthanasia of cat related to heart disease | 10 | 4 | .16 |
| Cat withdrawn from study because of worsening of clinical condition related to heart disease | 7 | 11 | .32 |
| Repeated thoracocentesis | 2 | 1 | 1.0 |
| Ventricular arrhythmia requiring treatment | 0 | 1 | .49 |
| Persistent and unacceptably high heart rate | 0 | 0 | 1.0 |
| Total (related to heart disease) | 29 | 22 | .39 |
| Other reasons | |||
| Cat withdrawn from study for reason other than heart disease | 3 | 2 | 1.0 |
| Development of hyperthyroidism | 1 | 2 | .62 |
| Death of cat for reason other than heart disease | 0 | 3 | .12 |
| Euthanasia of cat for reason other than heart disease | 0 | 0 | 1.0 |
| Cat withdrawn from study because of serious adverse event | 0 | 0 | 1.0 |
| Total (other) | 4 | 7 | .36 |
| Total (all cause, related to heart disease + other) | 33 | 29 | .74 |
Note: P values were calculated with Fisher's exact test.
For all‐cause treatment failure, there was no effect of treatment (benazepril versus placebo) on time to event with P = .42 in the log rank test (Figure 2) and univariate CPH model (Table 4). The median (95% CI) time to event was 553 (196‐infinity) days with benazepril and 648 (215‐infinity) days with placebo.
Table 4.
Results of univariate Cox proportional hazard analysis for the association among baseline variables, and treatment, and the risk of reaching the all‐cause treatment failure endpoint (n = 151 cats)
| Variable | Hazard ratio | 95% confidence interval | P value | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Treatment (benazepril versus placebo) | 1.23 | 0.75 | 2.02 | .42 |
| Age (years) | 1.05 | 0.99 | 1.12 | .08 |
| Sex (male versus female) | 1.22 | 0.65 | 2.24 | .53 |
| Congenital disorder (versus acquired) | 0.83 | 0.38 | 1.83 | .65 |
| Fractional shortening (%) | 0.97 | 0.95 | 0.99 | <.001 |
| LA diameter (short axis view, mm) | 1.13 | 1.08 | 1.18 | <.001 |
| End diastolic LV diameter (M‐mode, mm) | 1.00 | 0.98 | 1.02 | .76 |
| End diastolic LV free wall thickness (M‐mode, mm) | 0.82 | 0.70 | 0.96 | .02 |
| End diastolic LV septum thickness (M‐mode, mm) | 0.76 | 0.63 | 0.91 | .003 |
| Hematocrit (%) | 0.96 | 0.93 | 1.00 | .05 |
| White blood cells (109/L) | 1.07 | 1.03 | 1.11 | <.001 |
| Plasma albumin (g/L) | 0.94 | 0.87 | 1.02 | .11 |
| Plasma calcium (mmol/L) | 1.17 | 0.31 | 4.47 | .82 |
| Plasma creatinine (μmol/L) | 1.01 | 1.00 | 1.01 | .004 |
| Plasma phosphate (mmol/L) | 1.10 | 0.62 | 1.95 | .76 |
| Plasma potassium (mmol/L) | 1.14 | 0.91 | 1.44 | .25 |
| Plasma sodium (mmol/L) | 0.98 | 0.93 | 1.05 | .60 |
| Plasma urea (g/L) | 3.06 | 1.64 | 5.72 | <.001 |
| Plasma alkaline phosphatase (U/L) | 0.99 | 0.98 | 1.01 | .33 |
| CHF signs present (versus absent) | 3.77 | 2.00 | 7.11 | <.001 |
| Arrhythmia present (versus absent) | 2.78 | 1.55 | 4.99 | <.001 |
| Gallop sound present (versus absent) | 1.69 | 0.97 | 2.97 | .07 |
| Level of activity reduced (versus not reduced) | 3.85 | 1.21 | 12.29 | .02 |
| Quality of life poor (versus good) | 2.37 | 1.44 | 3.91 | <.001 |
Notes: P values <.05 are shown in bold. Variables were analyzed as continuous unless stated.
Abbreviations: CHF, congestive heart failure; LA, left atrium; LV, left ventricle.
Results of the univariate CPH analysis of the selected baseline variables are shown in Table 4. Variables significantly (P < .05) associated with HRs >1 were: LA diameter; WBC, plasma creatinine and plasma urea; presence of arrhythmias and CHF signs; and reduced level of activity and poor quality of life. Variables significantly (P < .05) associated with HRs <1 were: fractional shortening (FS), end diastolic LV free wall thickness, and interventricular septum thickness.
The 5 variables (those with the lowest P values from the univariate analysis) included in the bidirectional selection multivariate CPH model were: LA diameter, CHF signs, FS, WBC, and plasma urea. The forward selection and bidirectional selection models gave identical results in the multivariate analysis with 2 variables being significant (P < .05); LA diameter and presence of CHF signs (Table 5). Treatment was not significant (P = 1.0).
Table 5.
Results from the multivariate Cox proportional hazard analysis for the association among baseline variables, and treatment, and the risk of reaching the all‐cause treatment failure endpoint (n = 151 cats)
| Variable | Hazard ratio | 95% confidence interval | P value | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Treatment (benazepril versus placebo) | 1.00 | 0.57 | 1.74 | 1.0 |
| LA diameter (short axis view, mm) | 1.08 | 1.03 | 1.14 | .004 |
| CHF signs present (versus absent) | 2.70 | 1.22 | 5.96 | .01 |
Notes: P values <.05 are shown in bold. Identical results were obtained with the forward selection and bidirectional selection model fitting approaches.
Abbreviation: CHF, congestive heart failure; LA, left atrium.
There was no significant interaction between the effect of treatment on the risk of all‐cause treatment failure and the presence or absence of a congenital disorder (P = .46), LA diameter >16 or ≤16 mm (P = .24), CHF signs present or absent (P = .57), or presence of dyspnea plus poor quality of life (P = .22) (details not shown).
3.3. Heart disease‐related treatment failure
In the benazepril group, a total of 29 cats (38%) reached the heart disease‐related treatment failure endpoint, whereas 48 were censored (Figure 1 and Table 3). In the placebo group, 22 cats (30%) reached the endpoint, whereas 52 were censored. There was no significant effect of treatment (benazepril versus placebo) on time to event with P = .21 in the log rank test (Figure 3) and univariate CPH model (Table 6). The median (95% CI) time to event was 553 (264‐infinity) days in the benazepril group, and could not be determined in the placebo group as the treatment failure rate was 36% at the last event (day 378). The 25% interquartile for time to event was 123 days in the benazepril and 166 days in the placebo group.
Table 6.
Results of univariate Cox proportional hazard analysis for the association among baseline variables, and treatment, and the risk of reaching the heart disease‐related treatment failure endpoint (n = 151 cats)
| Variable | Hazard ratio | 95% confidence interval | P value | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Treatment (benazepril versus placebo) | 1.42 | 0.82 | 2.48 | .21 |
| Age (years) | 1.03 | 0.96 | 1.10 | .45 |
| Sex (male versus female) | 1.19 | 0.61 | 2.32 | .61 |
| Congenital disorder (versus acquired) | 1.02 | 0.46 | 2.27 | .96 |
| Fractional shortening (%) | 0.96 | 0.94 | 0.98 | <.001 |
| LA diameter (short axis view, mm) | 1.16 | 1.11 | 1.22 | <.001 |
| End diastolic LV diameter (M‐mode, mm) | 1.01 | 0.99 | 1.02 | .45 |
| End diastolic LV free wall thickness (M‐mode, mm) | 0.77 | 0.64 | 0.93 | .005 |
| End diastolic LV septum thickness (M‐mode, mm) | 0.73 | 0.59 | 0.89 | .002 |
| Hematocrit (%) | 0.96 | 0.92 | 1.00 | .04 |
| White blood cells (109/L) | 1.08 | 1.04 | 1.13 | <.001 |
| Plasma albumin (g/L) | 0.92 | 0.85 | 1.00 | .05 |
| Plasma calcium (mmol/L) | 1.07 | 0.24 | 4.78 | .93 |
| Plasma creatinine (μmol/L) | 1.01 | 1.00 | 1.01 | .06 |
| Plasma phosphate (mmol/L) | 1.20 | 0.64 | 2.23 | .57 |
| Plasma potassium (mmol/L) | 1.22 | 0.97 | 1.55 | .09 |
| Plasma sodium (mmol/L) | 0.95 | 0.89 | 1.02 | .15 |
| Plasma urea (g/L) | 3.30 | 1.69 | 6.42 | <.001 |
| Plasma alkaline phosphatase (U/L) | 0.98 | 0.96 | 1.00 | .06 |
| CHF signs present (versus absent) | 6.98 | 2.97 | 16.41 | <.001 |
| Arrhythmia present (versus absent) | 3.68 | 2.01 | 6.75 | <.001 |
| Gallop sound present (versus absent) | 2.11 | 1.16 | 3.82 | .01 |
| Level of activity reduced (versus not reduced) | 3.19 | 0.99 | 10.24 | .05 |
| Quality of life poor (versus good) | 3.14 | 1.81 | 5.47 | <.001 |
Notes: P values <.05 are shown in bold. Variables were analyzed as continuous unless stated.
Abbreviations: CHF, congestive heart failure; LA, left atrium; LV, left ventricle.
Results of the CPH univariate analysis of the selected baseline variables are shown in Table 6. Variables significantly (P < .05) associated with HRs >1 were: LA diameter; WBC; plasma urea; presence of CHF signs, arrhythmias and gallop sound; and poor quality of life. Variables significantly (P < .05) associated with HRs <1 were: FS, end diastolic LV free wall and septum thickness, hematocrit, and plasma albumin.
The 5 variables (those with the lowest P values from the univariate analysis) included in the bidirectional selection multivariate CPH model were: LA diameter, FS, CHF signs, arrhythmias, and quality of life.
In the multivariate CPH analysis (Table 7), FS, plasma sodium, presence of CHF signs, and arrhythmia were significant in both the forward and bidirectional selection models (P < .05). Treatment was not significant in either model (P ≥ .84). Left atrium diameter and plasma potassium were significant in initial iterations of the forward selection model, and were therefore retained, but were not significant in the final model (Table 7).
Table 7.
Results from the multivariate Cox proportional hazard analysis for the association among baseline variables, and treatment, and the risk of reaching the heart disease‐related treatment failure endpoint (n = 151 cats)
| Model fitting approach/variable | Hazard ratio | 95% confidence interval | P value | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Forward selection | ||||
| Treatment (benazepril versus placebo) | 0.99 | 0.50 | 1.94 | .97 |
| Fractional shortening (%) | 0.97 | 0.95 | 0.99 | .007 |
| LA diameter (short axis view, mm) | 1.04 | 0.97 | 1.11 | .29 |
| Plasma potassium (mmol/L) | 1.19 | 0.97 | 1.48 | .10 |
| Plasma sodium (mmol/L) | 0.92 | 0.87 | 0.98 | .008 |
| CHF signs present (versus absent) | 5.10 | 1.66 | 15.67 | .004 |
| Arrhythmia present (versus absent) | 2.84 | 1.29 | 6.26 | .009 |
| Bidirectional selection | ||||
| Treatment (benazepril versus placebo) | 0.93 | 0.48 | 1.81 | .84 |
| Fractional shortening (%) | 0.96 | 0.95 | 0.98 | <.001 |
| Plasma sodium (mmol/L) | 0.92 | 0.86 | 0.98 | .008 |
| CHF signs present (versus absent) | 5.34 | 1.83 | 15.60 | .002 |
| Arrhythmia present (versus absent) | 3.52 | 1.71 | 7.26 | <.001 |
Note: P values <.05 are shown in bold.
Abbreviations: CHF, congestive heart failure; LA, left atrium.
There was no significant interaction between the effect of treatment on risk of treatment failure and the presence or absence of congenital disorder (P = .37), LA diameter >16 or ≤16 mm (P = .32), CHF signs present or absent (P = .59), or presence of dyspnea plus poor quality of life (P = .41) (data not shown).
3.4. Clinical signs
There were no significant differences between the 2 groups after administration of the test items (ie, at weeks 5‐104) for presence/absence or severity of score for any of the clinical signs, including the quality of life (data not shown).
3.5. Echocardiography
There were no significant differences between the 2 groups after administration of the test items (weeks 13‐104) in LA diameter or LV free wall thickness (data not shown).
3.6. Safety
The number of cats with at least 1 reported adverse event was 42 (77%) with benazepril and 35 (74%) with placebo (P = .42, Fisher's exact test). The frequency of individual adverse events was similar in both groups; results for events occurring twice or more in either group are shown in Table 8. There were no significant differences between the 2 groups after administration of the test items (weeks 5‐104) for any clinical chemistry or hematology variable (data not shown).
Table 8.
Frequency of reported adverse events occurring in two or more cats in either group
| Reported adverse event | Benazepril (n = 77) | Placebo (n = 74) | P value |
|---|---|---|---|
| Dyspnea | 6 | 4 | .75 |
| Vomiting | 2 | 6 | .16 |
| Abscess | 2 | 1 | 1.0 |
| Syncope | 2 | 1 | 1.0 |
| Tachypnea | 2 | 1 | 1.0 |
| Weakness | 2 | 1 | 1.0 |
| Cystitis | 2 | 0 | .50 |
| Diarrhea | 2 | 0 | .50 |
| Skin infection | 1 | 2 | .62 |
| Collapse | 0 | 2 | .24 |
| Ocular discharge | 0 | 2 | .24 |
Note: P values were calculated with Fisher's exact test.
4. DISCUSSION
The main result of this study is that we did not detect any beneficial effect of the ACEI benazepril on the primary endpoint of treatment failure, or on the secondary echocardiography or quality of life endpoints, in cats with heart disease. Benazepril was tolerated well, with no differences compared to placebo in the frequency of reported adverse events or results of plasma chemistry or hematology variables.
The primary endpoint of the study was treatment failure, which was defined prospectively in the protocol and analyzed using time to event techniques. All‐cause treatment failure was defined as the main analysis, as it is relevant clinically and in addition maximized the proportion of cats reaching a defined endpoint (41%) rather than being censored (59%). Nevertheless, similar results were found, including no detected benefit of benazepril, when treatment failure related to heart disease was analyzed.
The relatively low number of cats reaching the defined treatment failure endpoint (41% for all‐cause failure and 34% for heart disease‐related treatment failure) versus being censored is a weakness of the study, as fewer event cases lowers the power and reliability of the statistical analysis. Relatively low event rates occur in ACEI studies in dogs.9, 10 Approximately equal numbers of censored and event cases occurred in a 5 year study in cats with preclinical HCM.19
Further limitations to the study are discussed below. First, the cats recruited had a wide range of etiologies of heart disease and stages of progression of disease, and included both asymptomatic and symptomatic cases. These cases were recruited as the hypothesis for the study was that an ACEI might have beneficial effects in many of these cats. However, the relatively high proportion of asymptomatic cats (58 of 151 cats, 38%), and high variability between cases, could well have masked possible benefits of benazepril in certain subgroups. The inclusion criteria permitted inclusion of cats without CHF that, in the opinion of the investigator, “required treatment” with no further definition or guideline. We conclude that, in hindsight, this approach contributed to too many cats without CHF being recruited. Second, it was not confirmed in all cats that clinical signs attributed to CHF, for example dyspnea or low appetite, were primarily caused by heart disease rather than concomitant noncardiac disorders. Third, although allocation to groups was randomized, the 2 groups were not matched perfectly at baseline; the group allocated to receive benazepril having significantly more frequent congenital disorders and higher WBC counts and numerically, although not significantly, more frequent arrhythmias (P = .09), clinical signs of CHF (P = .07), and RCM (P = .16). This is the probable reason why, after treatment, cats receiving benazepril had numerically (but not significantly) higher frequencies of death and euthanasia related to heart disease (Table 3) and shorter times to events (Figures 1 and 2) compared with the placebo group. Results from the multivariate CPH models, in which differences in baseline variables should be accounted for, show that the HRs for treatment effect were less or equal to 1, consistent with no negative effect of benazepril. Fourth, management and clinical assessment of cats were not highly standardized between investigators, and numbers of cases varied widely between centers (range 1‐27) with 14 of the 23 centers contributing 5 or fewer cases. Fifth, we have no proof that adequate dosing or inhibition of the RAAS was achieved in the benazepril‐treated cats. We are confident that the dosage of benazepril tested (range 0.5‐1.0 mg/kg) was sufficiently high as it has been shown previously to effectively inhibit the RAAS in cats, as assessed by inhibition of plasma ACE activity in healthy cats,20 normalization of glomerular capillary pressure in cats with experimental renal insufficiency21 and reduction of proteinuria in cats with chronic kidney disease.22 However, we have no information that the cats in our study were dosed correctly with the test items, and did not monitor blood benazepril concentrations or measure biomarkers (eg, plasma ACE activity), which could have indicated adequate intake. Finally, the study is old; the cats were treated from 2002 to 2006 and the manuscript was only completed in 2016. The main reason the manuscript was delayed was that it was judged initially that the study might not be worth publishing with (exclusively) univariate time to event analysis and the limitations discussed above. Later, after awareness of the importance of publishing negative results had increased, it took several years to agree on variable selection and perform valid multivariate time to event analysis.
The study had important flaws; the main motivation for publication is disclosure of the (negative efficacy) results, but the results do contribute knowledge on the natural history of cats with heart disease. Despite the limitations of the study, the results suggest that the ACEI benazepril at the tested dosage has no or at best only a small benefit on time to treatment failure in many cats with heart disease. Data published before and after completion of the study do not provide strong evidence for a benefit of ACEIs in cats with HCM. Evidence of RAAS activation in cats with HCM is weak; renin expression is increased in kidneys of cats with HCM at necropsy13 and abnormally high plasma aldosterone concentrations occur in approximately half of Maine Coon cats with asymptomatic HCM.23 Although the ACEIs enalapril and benazepril had positive effects on echocardiography variables in small not well‐controlled studies of cats with HCM,11, 12 the ACEI ramipril and the aldosterone antagonist spironolactone did not improve diastolic function or left ventricular mass in Maine Coon cats with asymptomatic HCM.23, 24 No significant differences between effects of benazepril and diltiazem occurred in cats with HCM.25 Interim results of a prospective field study in cats with diastolic heart failure including the ACEI enalapril were published as an abstract in 2003.26
The study was designed to provide 74% power with a 25% difference between groups for the primary endpoint with a minimum of 100 cats recruited. Although more cases were recruited, the power of the study was impaired by the relatively low number of cats (n = 62) which reached the defined failure endpoints rather than being censored. Post hoc power analysis confirmed that with 75 cats per group, as recruited, 80% power would be achieved if the difference between groups was at least 25% (30/75 for benazepril versus 49/75 for placebo). The lack of observed significant differences between groups therefore does not exclude the possibility of a smaller effect of benazepril or effects in certain subgroups of cats, such as those presented in CHF.
Although it was not 1 of the predefined objectives of the study, we were able to determine some prognostic variables for cats with heart disease. In the CPH multivariate analysis, LA diameter and presence of CHF signs were significantly associated with increased risk to reach the all‐cause treatment failure endpoint. For the heart disease‐related treatment failure endpoint, FS, plasma sodium, and the presence of arrhythmias and CHF signs were significant. These results are consistent with previous retrospective studies in cats with HCM, which identified age, LA dysfunction, LA enlargement, low LV systolic function, LV hypertrophy, and presence of CHF signs at diagnosis as risk factors in cats with HCM.19, 27, 28, 29, 30, 31, 32
5. CONCLUSIONS
The ACEI benazepril was tolerated well in cats with heart disease, but we found no evidence of benefit. In view of the limitations of the study, however, it is not possible to make a definitive conclusion that ACEIs have no beneficial actions in all etiologies and stages of heart disease in cats. Nevertheless, there was no significant interaction between the effect of treatment with benazepril on the risk of treatment failure for any of the analyses, including the presence or absence of CHF signs. Further adequately designed and powered studies are recommended, including in selected subgroups of cats with specific etiologies of heart disease.
CONFLICT OF INTEREST DECLARATION
The study was supported by Novartis Animal Health, now owned by Elanco Animal Health, which manufactures and distributes benazepril hydrochloride tablets (Fortekor). J.N.K. and W.S. are employed by Elanco Animal Health, and G.S. is retired from Novartis Animal Health. The following investigators received payment from Novartis and/or Elanco in the past 5 years for consultancy and/or speaking engagements: S.M.A.C., V.C., C.J.L.L., C.M., and M.A.S. The other authors declare no conflict of interest. S.T.S. serves as Associate Editor for the Journal of Veterinary Internal Medicine. He was not involved in review of this manuscript.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Since the period during which the data were acquired ran from 2002‐2006, we cannot confirm that there was no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study was conducted in compliance with the Procedures and Principles of Good Clinical Practice (VICH GL9, CVMP: VICH/595/98, 2000), and after approval by company internal review procedures and the respective regulatory authorities in each country taking into account animal welfare and ethical guidelines. All owners had to give their written informed consent before the start of the study.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
The authors thank the other investigators including Michael Stafford‐Johnson and Liam Atkinson for managing clinical cases; and Fabienne Brovedani and Philippe Gruet (France), Katje Funke and Klaus Jantzen (Germany), and Deborah Alexander and Katherine Illsley (United Kingdom) for assistance managing or monitoring the study.
King JN, Martin M, Chetboul V, et al. Evaluation of benazepril in cats with heart disease in a prospective, randomized, blinded, placebo‐controlled clinical trial. J Vet Intern Med. 2019;33:2559–2571. 10.1111/jvim.15572
Funding information Novartis Animal Health
REFERENCES
- 1. Rishniw M, Pion PD. Is treatment of feline hypertrophic cardiomyopathy based in science or faith? A survey of cardiologists and a literature search. J Feline Med Surg. 2011;13:487‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reina‐Doreste Y, Stern JA, Keene BW, et al. Case‐control study of the effects of pimobendan on survival time in cats with hypertrophic cardiomyopathy and congestive heart failure. J Am Vet Med Assoc. 2014;245:534‐539. [DOI] [PubMed] [Google Scholar]
- 3. Gordon SG, Côté E. Pharmacotherapy of feline cardiomyopathy: chronic management of heart failure. J Vet Cardiol. 2015;17:159‐172. [DOI] [PubMed] [Google Scholar]
- 4. Côté E. Feline congestive heart failure current diagnosis and management. Vet Clin Small Anim. 2017;47:1055‐1064. [DOI] [PubMed] [Google Scholar]
- 5. Fox PR, Schober KE. Management of asymptomatic (occult) feline cardiomyopathy: challenges and realities. J Vet Cardiol. 2015;17:150‐158. [DOI] [PubMed] [Google Scholar]
- 6. CONSENSUS Trial Study Group . Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429‐1435. [DOI] [PubMed] [Google Scholar]
- 7. The SOLVD investigators . Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. New Eng J Med. 1991;325:293‐302. [DOI] [PubMed] [Google Scholar]
- 8. The COVE Study Group . Controlled clinical evaluation of enalapril in dogs with heart failure: results of the Cooperative Veterinary Enalapril Study Group. J Vet Int Med. 1995;9:243‐252. [DOI] [PubMed] [Google Scholar]
- 9. Ettinger SJ, Benitz AM, Ericsson GF, et al. Effects of enalapril maleate on survival of dogs with naturally acquired heart failure. The Long‐Term Investigation of Veterinary Enalapril (LIVE) Study Group. J Am Vet Med Assoc. 1998;213:1573‐1577. [PubMed] [Google Scholar]
- 10. The BENCH Study Group . The effect of benazepril on survival times and clinical signs of dogs with congestive heart failure: results of a multicenter, prospective, randomized, double‐blinded, placebo‐controlled, long‐term clinical trial. J Vet Cardiol. 1999;1:7‐18. [DOI] [PubMed] [Google Scholar]
- 11. Rush JE, Freeman LM, Brown DJ, Smith FW Jr. The use of enalapril in the treatment of feline hypertrophic cardiomyopathy. J Am Anim Hosp Assoc. 1998;34:38‐41. [DOI] [PubMed] [Google Scholar]
- 12. Amberger CN, Glardon O, Glaus T, et al. Effects of benazepril in the treatment of feline hypertrophic cardiomyopathy. Results of a prospective, open‐label, multicenter clinical trial. J Vet Cardiol. 1999;1:19‐26. [DOI] [PubMed] [Google Scholar]
- 13. Taugner FM. Stimulation of the renin‐angiotensin system in cats with hypertrophic cardiomyopathy. J Comp Pathol. 2001;125:122‐129. [DOI] [PubMed] [Google Scholar]
- 14.CONSORT 2010 checklist of information to include when reporting a randomised trial. www.consort-statement.org. Accessed February 10, 2015.
- 15. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med. 1993;7:247‐252. [DOI] [PubMed] [Google Scholar]
- 16. Kittleson MD, Kienle RD. Small Animal Cardiovascular Medicine. St Louis, MO: Mosby; 1998. [Google Scholar]
- 17. Fox PR, Sisson DD, Moise SN. Textbook of Canine & Feline Cardiology. Philadelphia, PA: Saunders; 1999. [Google Scholar]
- 18. Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503‐1510. [DOI] [PubMed] [Google Scholar]
- 19. Schober KE, Zientek J, Li X, Fuentes VL, Bonagura JD. Effect of treatment with atenolol on 5‐year survival in cats with preclinical (asymptomatic) hypertrophic cardiomyopathy. J Vet Cardiol. 2013;15:93‐104. [DOI] [PubMed] [Google Scholar]
- 20. King JN, Humbert‐Droz E, Maurer M. Plasma angiotensin converting enzyme activity and pharmacokinetics of benazepril and benazeprilat in cats after single and repeated oral administration of benazepril.HCl. J Vet Pharmacol Ther. 1999;22:360‐367. [DOI] [PubMed] [Google Scholar]
- 21. Brown SA, Brown CA, Jacobs G, Stiles J, Hendi RS, Wilson S. Effects of the angiotensin converting enzyme inhibitor benazepril in cats with induced renal insufficiency. Am J Vet Res. 2001;62:375‐383. [DOI] [PubMed] [Google Scholar]
- 22. King JN, Gunn‐Moore DA, Tasker S, Gleadhill A, Strehlau G, Benazepril in Renal Insufficiency in Cats Study Group . Tolerability and efficacy of benazepril in cats with chronic kidney disease. J Vet Intern Med. 2006;20:1054‐1064. [DOI] [PubMed] [Google Scholar]
- 23. MacDonald KA, Kittleson MD, Larson RF, et al. The effect of ramipril on left ventricular mass, myocardial fibrosis, diastolic function, and plasma neurohormones in Maine Coon cats with familial hypertrophic cardiomyopathy without heart failure. J Vet Intern Med. 2006;20:1093‐1105. [DOI] [PubMed] [Google Scholar]
- 24. MacDonald KA, Kittleson MD, Kass PH. Effect of spironolactone on diastolic function and left ventricular mass in Maine Coon cats with familial hypertrophic cardiomyopathy. J Vet Intern Med. 2008;22:335‐341. [DOI] [PubMed] [Google Scholar]
- 25. Taillefer M, Di Fruscia R. Benazepril and subclinical feline hypertrophic cardiomyopathy: a prospective, blinded, controlled study. Can Vet J. 2006;47:437‐445. [PMC free article] [PubMed] [Google Scholar]
- 26. Fox PR, The Multi‐Center Feline Chronic Heart Failure Study Group . Prospective, double‐blinded, multicenter evaluation of chronic therapies for feline diastolic heart failure: interim analysis. J Vet Int Med. 2003;17:398‐399. [Google Scholar]
- 27. Atkins CE, Gallo AM, Kurzman ID, Cowen P. Risk factors, clinical signs, and survival in cats with a clinical diagnosis of idiopathic hypertrophic cardiomyopathy: 74 cases (1985‐1989). J Am Vet Med Assoc. 1992;201:613‐618. [PubMed] [Google Scholar]
- 28. Fox PR, Liu SK, Maron BJ. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy. An animal model of human disease. Circulation. 1995;92:2645‐2651. [DOI] [PubMed] [Google Scholar]
- 29. Rush JE, Freeman LM, Fenollosa NK, Brown DJ. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999). J Am Vet Med Assoc. 2002;220:202‐207. [DOI] [PubMed] [Google Scholar]
- 30. Peterson EN, Moise NS, Brown CA, Erb HN, Slater MR. Heterogeneity of hypertrophy in feline hypertrophic heart disease. J Vet Intern Med. 1993;7:183‐189. [DOI] [PubMed] [Google Scholar]
- 31. Payne JR, Borgeat K, Connolly DJ, et al. Prognostic indicators in cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2013;27:1427‐1436. [DOI] [PubMed] [Google Scholar]
- 32. Payne J, Luis Fuentes V, Boswood A, Connolly D, Koffas H, Brodbelt D. Population characteristics and survival in 127 referred cats with hypertrophic cardiomyopathy (1997 to 2005). J Small Anim Pract. 2010;51:540‐547. [DOI] [PubMed] [Google Scholar]


