Abstract
Proton-pump inhibitors, PPIs, are considered effective therapy for stomach acid suppression due to their irreversible inhibition of the hydrogen/potassium pump in the gastric parietal cells. They are widely prescribed and are considered safe for over-the-counter use. Recent studies have shown an association between PPI use and Alzheimer dementia, while others have disputed that connection. We analyzed over ten million United States Food and Drug Administration Adverse Event Reporting System reports, including over forty thousand reports containing PPIs, and provided evidence of increased propensity for memory impairment among PPI reports when compared to histamine-2 receptor antagonist control group. Furthermore, we found significant associations of PPI use with a wide range of neurological adverse reactions including, migraine, several peripheral neuropathies, and visual and auditory neurosensory abnormalities.
Subject terms: Adverse effects, Alzheimer's disease, Peripheral neuropathies
Introduction
Proton pump inhibitors (PPIs) are drugs commonly used in treatment of acid-related disorders including gastroesophageal reflux disease, Helicobacter Pylori induced gastric ulcers, duodenal ulcer, erosive esophagitis, and Zollinger-Ellison syndrome1,2. Treatment of acid-related disorders includes antacids, PPIs, and histamine-2 receptor antagonists (H2RAs)3. The PPIs are preferred over the H2RAs because of their superior efficacy due to their irreversible inhibition of the H+/K+ ATPase4,5. National Health and Nutrition Examination Survey (NHANES) revealed a rise in the number of PPI prescriptions (2.9–7.8%) among 40–64 year old individuals from 1999 to 20126. NHANES did not account for over-the-counter (OTC) PPI intake. The class of PPI drugs includes six Food and Drug Administration (FDA) approved medications such as rabeprazole, lansoprazole, pantoprazole, esomeprazole, omeprazole, and dexlansoprazole. The high number of the PPI prescriptions, their OTC availability, and the increased likelihood of long-term use have raised concerns over unexpected adverse reactions (ADRs). It was demonstrated that the PPI pharmacology may not be limited to local inhibition of H-K-ATPase pump in parietal cells in the stomach7,8.
Common ADRs of PPIs, observed in clinical trials, include diarrhea, nausea, vomiting, flatulence, and headache9–12. Serious ADRs include breathing difficulty, rash, facial swelling, and throat tightness9–12. Recent studies revealed growing evidence of association with electrolyte abnormalities13,14 kidney injury15,16, bone fractures17, Clostridium difficile-associated diarrhea18, Alzheimer disease (AD)19, and non-AD type dementia19,20. However, other studies were not able to confirm the association between PPI use and a greater risk of dementia of both AD or non-AD type21,22.
Dementia associated with AD has a substantial impact on the quality of life of the patients and their caregivers23,24 and on the healthcare costs25,26. AD is considered the third most costly disease in the United States, with the costs being primarily associated with long-term care in nursing facilities27.
The current lack of consensus on PPI association with AD and non-AD type dementia warranted further investigation and analysis of other neurological outcomes. In our study, we performed an analysis of the FDA Adverse Event Reporting System database (FAERS/AERS) and identified significant increases of AD and non-AD dementia reports along with increased association with other types of memory impairment in PPI patients. Additionally, we found a significant increase in wide variety of peripheral neurological and neuropathic adverse events, as well as visual and auditory impairment ADRs.
Results
PPI “monotherapy” - neurological and neurosensory ADRs
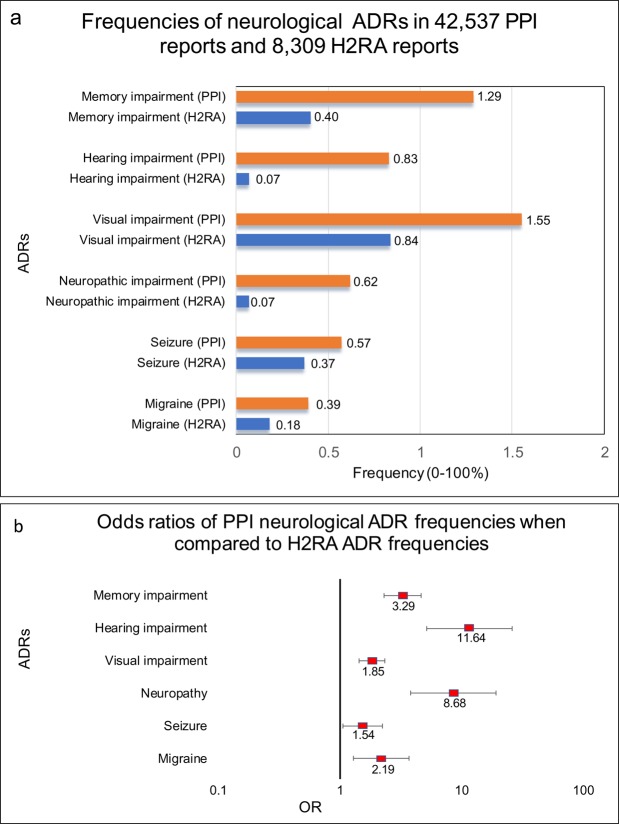
Reports in which PPIs were administered with no reported concurrent medications had a significant increase in memory impairment ADRs in comparison with H2RAs reports (OR 3.28, 95% CI [2.31, 4.67]) (Fig. 1b, Table 1). The outcomes included memory impairment, amnesia, dementia of the AD type, and non-AD dementia. Surprisingly, the H2RA cohort (n = 8,309) had three out of four ADRs listed above, but had no single report of dementia of the AD type (Table 1) while the PPI cohort (n = 42,537) had as many as 80 reports of the AD dementia. Interestingly, the auditory and visual ADRs followed a similar trend, with ORs being (11.64 [5.20, 26.11]) and (1.85 [1.44, 2.37]) respectively (Fig. 1b Tables 2 and 3). Neuropathic/neurological impairment ADR frequencies were also increased in the described above PPI cohort (8.68 [3.86, 19.49]) (Fig. 1b, Table 4). These included cranial and peripheral neuropathies, sciatica, and nerve injury as well as other neuropathic ADRs (Table 4). There was a small but significant increase in reported seizures (1.54 [1.06, 2.24]) (Fig. 1b and Table 5) and a significant increase in migraine reports in the PPI cohort (2.19 [1.29, 3.72]) (Fig. 1b and Table 6).
Figure 1.
FAERS-reported frequencies and odds ratios of neurological/neurosensory adverse drug reactions. (a) Frequencies of neurological/neurosensory adverse events for patients in FAERS/AERS who took PPIs (n = 42,537) and H2RAs (n = 8,309). (b) Odds ratios were calculated comparing adverse event frequencies of PPI and H2RA patients. Ranges represent 95% confidence intervals (95% CI) (see Methods). A logarithmic X-axis shows odds ratios and their confidence intervals.
Table 1.
Types and numbers of memory impairment (Memory impairment, amnesia, Alzheimer dementia, non-AD type dementia) related ADRs for patients on PPIs (n = 42,537) and H2RAs (n = 8,309).
| Adverse drug reaction | PPI+ | H2RA+ |
|---|---|---|
| (n = 42,537) | (n = 8,309) | |
| memory impairment | 246 | 11 |
| amnesia | 152 | 11 |
| dementia, Alzheimer type | 80 | 0 |
| dementia, non-AD type | 72 | 11 |
| total memory impairment ADRs | 550 out of 42,537 | 33 out of 8,309 |
| odds ratio (95% CI) [p value] | 3.29 (2.31 to 4.67) [p < 0.0001] |
Odds ratios were calculated from adverse event frequencies files.
Table 2.
Types and numbers of hearing impairment related ADRs (hypoacusis, impaired hearing, deafness, unilateral deafness, sudden hearing loss etc.) for patients on PPIs (n = 42,537) and H2RAs (n = 8,309).
| Adverse drug reaction | PPI+ | H2RA+ |
|---|---|---|
| (n = 42,537) | (n = 8,309) | |
| hypoacusis | 134 | 2 |
| impaired hearing | 127 | 0 |
| deafness | 59 | 4 |
| deafness unilateral | 20 | 0 |
| sudden hearing loss | 12 | 0 |
| deafness transitory | 1 | 0 |
| deafness neurosensory | 1 | 0 |
| deafness bilateral | 1 | 0 |
| total hearing impairment ADRs | 355 out of 42,537 | 6 out of 8,309 |
| odds ratio (95% CI) [p value] | 11.64 (5.20 to 26.11) [p < 0.0001] |
Odds ratios were calculated from adverse event frequencies. ADRs reported as listed in the FAERS/AERS files.
Table 3.
Reports containing ADRs related to visual impairment (visual impairment, blurred vision, blindness, reduced visual acuity, unilateral blindness etc.) for patients on PPIs (n = 42,537) and H2RAs (n = 8,309).
| Adverse drug reaction | PPI+ | H2RA+ |
|---|---|---|
| (n = 42,537) | (n = 8,309) | |
| visual impairment | 205 | 18 |
| vision blurred | 204 | 33 |
| blindness | 94 | 6 |
| visual acuity reduced | 82 | 5 |
| blindness unilateral | 34 | 1 |
| visual field defect | 18 | 1 |
| visual disturbance | 11 | 5 |
| blindness transient | 7 | 1 |
| night blindness | 2 | 0 |
| sudden visual loss | 1 | 0 |
| total visual impairment ADRs | 658 out of 42,537 | 70 out of 8,309 |
| odds ratio (95% CI) [p value] | 1.85 (1.44 to 2.37) [p < 0.0001] |
Odds ratios and 95% confidence intervals were calculated from adverse event frequencies and numbers of reports. The ADRs terms are taken directly from the FAERS/AERS files.
Table 4.
ADRs related to neurological/neuropathic impairment (neuropathy, peripheral, nerve injury, nerve compression, sciatica, neuralgia, polyneuropathy, optic neuritis, hyperreflexia, peripheral sensory neuropathy etc.) for patients on PPIs (n = 42,537) and H2RAs (n = 8,309).
| Adverse drug reaction | PPI+ | H2RA+ |
|---|---|---|
| (n = 42,537) | (n = 8,309) | |
| neuropathy peripheral | 74 | 4 |
| nerve injury | 38 | 0 |
| nerve compression | 23 | 1 |
| sciatica | 21 | 0 |
| neuralgia | 14 | 0 |
| polyneuropathy | 12 | 0 |
| optic neuritis | 8 | 0 |
| hyperreflexia | 7 | 0 |
| peripheral sensory neuropathy | 5 | 0 |
| IV-th nerve paralysis | 5 | 0 |
| VII-th nerve paralysis | 5 | 0 |
| autonomic neuropathy | 4 | 0 |
| peroneal nerve palsy | 4 | 0 |
| neurodegenerative disorder | 3 | 0 |
| areflexia | 3 | 0 |
| neurological symptom | 3 | 0 |
| optic ischaemic neuropathy | 3 | 0 |
| neuromyopathy | 3 | 0 |
| peripheral nerve injury | 3 | 0 |
| sciatic nerve injury | 3 | 1 |
| nerve degeneration | 2 | 0 |
| trigeminal neuralgia | 2 | 0 |
| cranial nerve disorder | 2 | 0 |
| neuritis | 2 | 0 |
| VI-th nerve paralysis | 2 | 0 |
| peripheral sensorimotor neuropathy | 2 | 0 |
| vagus nerve disorder | 1 | 0 |
| optic neuropathy | 1 | 0 |
| optic nerve injury | 1 | 0 |
| nerve root compression | 1 | 0 |
| nerve conduction studies abnormal | 1 | 0 |
| radial nerve palsy | 1 | 0 |
| neuropathy | 1 | 0 |
| neuropathic arthropathy | 1 | 0 |
| neurogenic bladder | 1 | 0 |
| motor neuron disease | 1 | 0 |
| hyporeflexia | 1 | 0 |
| CIDP | 1 | 0 |
| total neuropathic/neurological impairment ADRs | 265 out of 42,537 | 6 out of 8,309 |
| odds ratio (95% CI) [p value] | 8.68 (3.86 to 19.49) [p < 0.0001] |
Abbreviations: CIDP - chronic inflammatory demyelinating polyradiculoneuropathy. Odds ratios and 95% confidence intervals were calculated from adverse event frequencies and numbers of reports. The ADRs terms are taken directly from the FAERS/AERS files.
Table 5.
Types and numbers of seizure related ADRs (convulsion, seizure, epilepsy, grand mal convulsion, status epilepticus etc.) for patients on PPIs (n = 42,537) and H2RAs (n = 8,309).
| Adverse drug reaction | PPI+ | H2RA+ |
|---|---|---|
| (n = 42,537) | (n = 8,309) | |
| convulsion | 142 | 18 |
| seizure | 44 | 1 |
| epilepsy | 24 | 5 |
| grand mal convulsion | 10 | 2 |
| status epilepticus | 9 | 2 |
| petit mal epilepsy | 5 | 1 |
| hypocalcaemic seizure | 4 | 0 |
| generalized tonic clonic seizure | 2 | 0 |
| partial seizures | 1 | 2 |
| hyperglycaemic seizure | 1 | 0 |
| juvenile myoclonic epilepsy | 1 | 0 |
| clonic convulsion | 1 | 0 |
| total seizure ADRs | 244 out of 42,537 | 31 out of 8,309 |
| odds ratio (95% CI) [p value] | 1.54 (1.06 to 2.24) [p = 0.0237] |
Odds ratios were calculated from adverse event frequencies. ADRs reported as listed in FAERS/AERS files.
Table 6.
ADRs related to migraine (migraine, migraine with aura, ophthalmoplegic migraine) for patients on PPIs (n = 42,537) and H2RAs (n = 8,309).
| Adverse drug reaction | PPI+ | H2RA+ |
|---|---|---|
| (n = 42,537) | (n = 8,309) | |
| migraine | 163 | 14 |
| migraine with aura | 4 | 1 |
| ophthalmoplegic migraine | 1 | 0 |
| total migraine ADRs | 168 out of 42,537 | 15 out of 8,309 |
| Odds ratio (95% CI) [p value] | 2.19 (1.29 to 3.72) [p = 0.0036] |
Odds ratios and 95% confidence intervals were calculated from adverse event frequencies and numbers of reports. The ADRs terms are taken directly from the FAERS/AERS files.
Methods
FDA adverse event reporting system
The FDA Adverse Event Reporting System (FAERS/AERS) was created by the FDA to document medication error reports, product quality complaints, and medication adverse events. Physicians, pharmacists, other healthcare providers, patients, and legal representatives submit the drug-related adverse event reports through MedWatch on a voluntary basis. If the adverse event is reported to the manufacturer, the manufacturer is required by law to forward the report to the FDA FAERS system.
At the time of data collection for the study, the FAERS/AERS contained over 10.3 million reports from January 2004 to March 2018. Both FAERS and AERS data sets are available online at: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm082193.htm.
All data analysis methods and procedures were carried out in accordance with existing guidelines and regulations. Since only the publicly available data were used in the study, and the FDA data sets used had been reviewed and released, no additional institutional and/or licensing committee approval was warranted.
Combining and normalizing the FAERS/AERS reports
FAERS/AERS contains reports from the United States and other countries with their respective specific demographic formats and medication brand/generic names. Before the data collection and analysis online drug databases were utilized to create a dictionary with all of the varieties of drug brand names to translate them into generic names. FAERS/AERS quarterly report sets of seven files vary and their format had been changed at some point. To make the data sets more homogeneous, each quarterly report set of files was downloaded in dollar-separated text format (.txt) and modified to standardize the fields. Missing columns in the FAERS/AERS data set were added with no values to create a standard data table with over 10.3 million adverse event reports.
Study outcomes
There were 20,317 uniquely worded ADRs reported to FAERS and AERS. ADRs were grouped into generalized categories of study outcomes: (1) memory impairment (memory impairment, amnesia, dementia Alzheimer type, dementia), (2) neuropathy (neuropathy peripheral, nerve injury, nerve compression, sciatica, neuralgia, polyneuropathy, optic neuritis, hyperreflexia, peripheral sensory neuropathy, IV-th nerve paralysis, VII-th nerve paralysis, autonomic neuropathy, peroneal nerve palsy, neurodegenerative disorder, areflexia, neurological symptom, optic ischaemic neuropathy, neuromyopathy, peripheral nerve injury, sciatic nerve injury, nerve degeneration, trigeminal neuralgia, cranial nerve disorder, neuritis, VI-th nerve paralysis, peripheral sensorimotor neuropathy, vagus nerve disorder, optic neuropathy, optic nerve injury, nerve root compression, nerve conduction studies abnormal, radial nerve palsy, neuropathy, neuropathic arthropathy, neurogenic bladder, motor neuron disease, hyporeflexia, and chronic inflammatory demyelinating polyradiculoneuropathy), (3) visual impairment (visual impairment, vision blurred, blindness, visual acuity reduced, blindness unilateral, visual field defect, visual disturbance, blindness transient, night blindness, and sudden visual loss), (4) hearing impairment (hypoacusis, impaired hearing, deafness, deafness unilateral, sudden hearing loss, deafness transitory, deafness neurosensory and deafness bilateral),(5) migraine (migraine, migraine with aura, and ophthalmoplegic migraine), and (6) seizure (convulsion, seizure, epilepsy, grand mal convulsion, status epilepticus, petit mal epilepsy, hypocalcemic seizure, generalized tonic-clonic seizure, partial seizures, hyperglycemic seizure, juvenile myoclonic epilepsy, and clonic convulsion).
Cohort choice
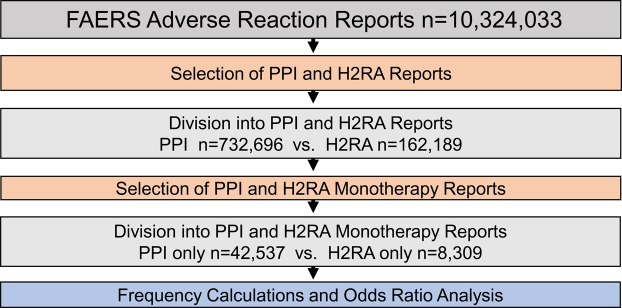
For the PPI cohort (n = 732,696), out of 10,324,033 records, reports where rabeprazole, lansoprazole, pantoprazole, omeprazole and esomeprazole and dexlansoprazole were selected excluding reports with ranitidine, famotidine, cimetidine, and nizatidine concurrent use. For the H2RA cohort (n = 162,189), reports where ranitidine, famotidine, cimetidine, and nizatidine were used, excluding concurrent PPI use. The cohorts were further narrowed down to “monotherapy” reports. This assignment was defined when a submitted report contained a single PPI or a single H2RA medication. For that reason, any confounding concurrent medications and associated comorbidities were not applicable. Resulting cohorts consisted of 42,537 PPI reports and 8,309 H2RA reports (Fig. 2). ADR frequencies for both cohorts were calculated, and odds ratio analysis was performed by dividing their relative ADR frequencies and calculating the 95% confidence intervals of the OR values. The cohort choice was validated by strongly overlapping distributions of demographic parameters (Tables 7 and 8).
Figure 2.
Legend: Flowchart of inclusion, exclusion and cohort selection for adverse event comparison between PPI and H2RA”monotherapy” reports.
Table 7.
Reported patient demographics in PPI and H2RA “monotherapy” cohorts.
| Sex | PPI reports (n = 42,537) | Frequency (%) | H2RA reports (n = 8,309) | Frequency (%) | P-value | % Difference |
|---|---|---|---|---|---|---|
| Female | 25116 | 59.69 | 4579 | 57.68 | <0.001 | 2.01 |
| Male | 12000 | 28.52 | 2710 | 34.14 | <0.001 | 5.62 |
| Unreported | 4963 | 11.79 | 650 | 8.19 | <0.001 | 3.61 |
| Age difference | ||||||
| Mean age, years (SD) | 58.3 (15.9) | 55.6 (20.1) | <0.001 | 2.7 | ||
| Median age | 58.6 | 59.7 | <0.001 | 1.1 | ||
| Unreported (%) | 45.4 | 55.1 | ||||
Table 8.
PPI and H2RA “monotherapy” report frequencies by country of origin.
| Country | No. of PPI reports | Frequency % | No. of H2RA reports | Frequency % | % difference | >1% Different |
|---|---|---|---|---|---|---|
| United States | 37139 | 88.26 | 6928 | 87.27 | 0.99 | |
| Great Britain | 1122 | 2.67 | 177 | 2.23 | 0.44 | |
| Japan | 472 | 1.12 | 382 | 4.81 | 3.69 | * |
| Germany | 357 | 0.85 | 24 | 0.3 | 0.55 | |
| France | 330 | 0.78 | 29 | 0.37 | 0.42 | |
| Canada | 309 | 0.73 | 22 | 0.28 | 0.46 | |
| Italy | 312 | 0.74 | 39 | 0.49 | 0.25 | |
| Brazil | 282 | 0.67 | 3 | 0.04 | 0.63 | |
| Turkey | 173 | 0.41 | 13 | 0.16 | 0.25 | |
| Australia | 151 | 0.36 | 7 | 0.09 | 0.27 | |
| China | 149 | 0.35 | 9 | 0.11 | 0.24 | |
| Denmark | 143 | 0.34 | 4 | 0.05 | 0.29 | |
| Spain | 138 | 0.33 | 13 | 0.16 | 0.16 | |
| Nederlands | 103 | 0.24 | 34 | 0.43 | 0.18 | |
| Sweden | 52 | 0.12 | 6 | 0.08 | 0.05 | |
| Singapore | 48 | 0.11 | 9 | 0.11 | 0 | |
| Belgium | 41 | 0.1 | 9 | 0.11 | 0.02 | |
| New Zealand | 42 | 0.1 | 3 | 0.04 | 0.06 | |
| Chile | 28 | 0.07 | 3 | 0.04 | 0.03 | |
| India | 15 | 0.04 | 15 | 0.19 | 0.15 | |
| Costa Rica | 30 | 0.07 | 0 | 0 | 0.07 | |
| Unknown | 198 | 0.47 | 45 | 0.57 | 0.1 |
Statistical analysis
Descriptive statistics
Frequencies for each studied side effect (Fig. 1a) was calculated by the equation:
| 1 |
Comparative Statistics
ADR report rates were compared via the Odds Ratio (OR) analysis for Fig. 1b and Tables 1–6 using the following equations:
| 2 |
where
Number of cases in exposed group with an adverse event.
Number of cases in exposed group with no adverse event.
Number of cases in control group with the adverse event.
Number of cases in control group with no adverse event.
| 3 |
Standard Error of Log Odds Ratio;
| 4 |
95% Confidence Interval;
| 5 |
Discussion
In our study, we analyzed a large number of ADR reports concerning patients taking only a single treatment of either a PPI and an H2RA drug using the FAERS/AERS databases, and quantified the association between PPI exposure and memory impairments, a wide range of neuropathies, visual and auditory impairments, migraines, and seizures.
Memory impairment related ADRs
Using data from the German Study on Aging, Cognition and Dementia in Primary Care Patients 75 years and older, Haenisch et al. found that PPIs users were at increased risk of dementia (Hazard Ratio (HR) 1.38, 95% CI, [1.04–1.83]) and AD (HR, 1.44, 95% CI, [1.01, 2.06]) compared to non-users19. A later study lead by Gomm et al. analyzed data from the German statutory health insurer, Allgemeine Ortskrankenkassen (AOK) and observed a significantly increased risk of dementia (HR, 1.44, 95% CI, [1.36, 1.52], p < 0.001) in older adults using PPIs20. In contrast, Goldstein et al.21 analyzed data from Tertiary Academic Alzheimer Disease Centers and found that continuous and intermittent PPIs use was not associated with increased risk of dementia or AD21. In our study, we were not able to quantify the risk of AD-type dementia by itself since the H2RA cohort had zero reports of AD-type dementia and the PPI cohort had eighty reports. We included AD-type dementia in the generalized memory impairment cohort which included AD and non-AD type dementia, memory impairment and amnesia. We found a significant increase in memory impairment ADRs (OR 3.28, 95% CI [2.31, 4.67]) in the PPI cohort (Fig. 1b, Table 1). Memory impairment ADRs were helpful to capture possible early symptoms leading to dementia.
Neuropathies
No large-scale clinical study has previously established a direct correlation of PPI use with neuropathies, including peripheral and poly-neuropathy, nerve injury and compression, sciatica, and neuralgia. Surprisingly, we identified a significant increase of neuropathy reports in patients taking only PPIs when compared to patients taking only H2RAs (OR 8.68, 95% CI [3.86, 19.49]). This observation was statistically significant, and clinically relevant. While our study did not address the molecular mechanism of action leading to neuropathies, a possible mechanism may involve Vitamin B12. An increased gastric pH level has been found to correlate with decreased Vitamin B12 levels28–30. In turn, B12 deficiency has been associated with reversible peripheral neuropathy and spinal cord degeneration and mental status alteration31,32. There are not many case reports depicting a direct correlation of PPI use with peripheral neuropathies33,34. While the direct correlation with PPI use and peripheral neuropathies had been noticed in a few case studies, we established an over eight-fold increase in broader range of neuropathy reports in a cohort of 42,537 PPI patients when compared to 8,309 H2RA patients.
Hearing impairment
While a recent prospective study established an association between hearing loss with gastroesophageal reflux disease (GERD)35, no correlation with a specific antacid drug class was established. Furthermore, the study associated the hearing loss with the disease itself and suggested that PPI use may be beneficial for auditory ADRs. In other studies, the association between GERD and hearing problems was explained by the mid ear exposure to gastric acid in young patients with otitis media36,37. Contrary to these findings, our analysis showed a significant increase in risk of hearing impairment (OR 11.64 95% CI [5.20, 26.11]) specific to PPI-containing reports. This observation could not be explained by gastroesophageal disorders alone. Moreover, if this assumption were true, the hearing impairment risk would have been expected to be higher in the H2RA cohort since PPIs have superior efficacy in pH control. Thus, our finding supports a possible different mechanism in which the hearing impairment ADRs may be affected by the drug treatment and not the disease alone.
Visual impairment
There have been many published case reports of visual impairment, including blurred vision38, ocular damage, optic neuropathy and blindness, associated with PPI use39,40. However, a retrospective cohort study of 94,063 patients, did not find an association between patients who took omeprazole or H2RAs (cimetidine, famotidine, nizatidine, ranitidine) and visual impairment41. In our analysis we divided the treated patients into two groups, compared their ADRs (42,537 PPI reports vs. 8,309 H2RA reports) and found an increased risk of visual impairment in patients taking PPIs (OR 1.85, 95% CI [1.44, 2.37]). This large-scale data analysis supports the initial case studies and provides a possible explanation the negative results of García Rodríguez et al. study in which PPI- and H2RA-treated patients were in a mixed cohort.
Migraine
Associations between PPI use and headache were established in the clinical trials of all the approved PPIs9, but there were no reports specific to migraine which is associated with a higher burden on quality of life42,43.
The headache association was confirmed in a crossover study conducted in Taiwan where lansoprazole and esomeprazole use increased headache incidence (OR, 1.20, 95% CI, [1.07,1.35], P < 0.002)44. However, according to our findings the headache effect may be common to both treatment classes, since there was no significant difference in headache reports between PPI and H2RA cohorts. Surprisingly the migraine report frequencies were different between the cohorts. We observed a significant increase in migraine reports in the PPI monotherapy cohort (OR 2.19, 95% CI [1.29, 3.72]). To our knowledge this is the first study showing association between PPI use and risk of migraine.
Seizures, convulsions, and epilepsy
An earlier observational cohort study found no association between PPI use and seizure risk in the overall population or in patients with epilepsy. In contrast, the study did find an increased risk of seizures in H2RA users45. On the other hand, in a few case reports the PPIs were found to be associated with seizures, and the authors attributed the seizure to PPI induced hypomagnesemia and hypocalcemia (electrolyte abnormalities known to influence neuronal function)46–48. Our analysis confirmed a small but significant association of PPI use with seizure-related ADRs (OR 1.54 95% CI [1.06, 2.24]) (Fig. 1b and Table 5).
Practical implications
The observed risk of memory impairment, neuropathy, hearing and visual impairment, seizures as well as migraines with PPIs warrant a more careful consideration when selecting this class of medications for patients who already have or may be at high risk for these adverse effects, in particular, if the treatment exceeds the recommended duration limit of two or three weeks. Given these ADR observations and the availability of alternatives to PPIs for related conditions, the risk versus benefit should be carefully considered before initiating a PPI. When clinically indicated, PPIs should only be used for the duration recommended by FDA and not exceed it. The latter may be difficult to enforce because of the OTC availability of some PPIs. Additionally, other medications that may exacerbate these ADRs should be avoided when patients are prescribed PPIs. Long-term use of PPIs beyond eight weeks can be considered in individual cases as long as the benefits continue to outweigh the risks. During PPI treatment, patients should be educated about potential ADRs and advised to contact the prescriber if they suspect any of the ADRs listed above.
Conclusion
This is the first large-scale postmarketing study to show significant association between PPI monotherapy and neurological and neurosensory ADRs. Further prospective clinical trials should evaluate the neurological and sensory ADRs. In the meantime, caution and awareness of these potential ADRs are recommended. H2RAs and other treatment modalities may be considered in patients at high risk for developing memory impairment, neuropathy, hearing and visual impairment, or migraines.
Study limitations
Since FAERS and AERS reporting is voluntary, the data set represents only a subset of actual cases and therefore the FAERS/AERS ADR frequencies should not be confused with absolute population incidences. FAERS/AERS reporting can be biased by newsworthiness, and scientific and legal variables49,50. Recent studies have shown that some adverse events in the FDA database are significantly underreported49,51. An acceptable way to deal with this issue was to use Odds Ratios with 95% CI between two cohorts instead of relying on ADR frequencies in a single cohort. Other limitations stem from the occasionally missing demographic variables, treatment dose and duration, and lack of comprehensive medical record data.
Although the study design was modeled to exclude potential confounding factors by selecting monotherapy reports, some concurrent medications and comorbidities may be also underreported, which in turn may affect the PPI and H2RA cohort composition and statistical analysis. Despite the fact that in clinical and community practice medicine reconciliation is becoming increasingly implemented, accounting for over-the-counter medication and supplement use still remains a significant unknown variable as it relies on patient self-reporting. This variable is a limitation at all the levels of clinical research from case studies to most controlled clinical trials. This is an association study where the physiological mechanism of the ADRs cannot be derived from the records. The causality of the ADRs cannot be inferred from association alone.
Acknowledgements
We thank Chris Edwards and Da Shi for contributions to processing the FAERS/AERS data files and supporting the computer environment. We also thank Dr. Isaac V. Cohen for useful discussions.
Author contributions
T.M. performed the experiments, R.A., R.S.A., K.C.L., S.A. and T.M. designed the study and, R.A., R.S.A., K.C.L., S.A. and T.M. drafted the manuscript and reviewed the final version. R.A. processed the data set.
Competing interests
Dr. Lee is a consultant for Takeda and Otsuka America Pharmaceutical, Inc. Other authors declare no conflict of financial or non-financial interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Song, H., Zhu, J. & Lu, D. Long-term proton pump inhibitor (PPI) use and the development of gastric pre-malignant lesions. Cochrane Database Syst Rev, CD010623, 10.1002/14651858.CD010623.pub2 (2014). [DOI] [PMC free article] [PubMed]
- 2.Londong W, et al. Dose-related healing of duodenal ulcer with the proton pump inhibitor lansoprazole. Aliment Pharmacol Ther. 1991;5:245–254. doi: 10.1111/j.1365-2036.1991.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 3.Katz Philip O, Gerson Lauren B, Vela Marcelo F. Guidelines for the Diagnosis and Management of Gastroesophageal Reflux Disease. American Journal of Gastroenterology. 2013;108(3):308–328. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 4.Clissold SP, Campoli-Richards DM. Omeprazole. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in peptic ulcer disease and Zollinger-Ellison syndrome. Drugs. 1986;32:15–47. doi: 10.2165/00003495-198632010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Walan A, et al. Effect of omeprazole and ranitidine on ulcer healing and relapse rates in patients with benign gastric ulcer. N Engl J Med. 1989;320:69–75. doi: 10.1056/NEJM198901123200201. [DOI] [PubMed] [Google Scholar]
- 6.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in Prescription Drug Use Among Adults in the United States From 1999–2012. JAMA. 2015;314:1818–1831. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528–534. doi: 10.1007/s11894-008-0098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin JM, Munson K, Vagin O, Sachs G. The gastric HK-ATPase: structure, function, and inhibition. Pflugers Arch. 2009;457:609–622. doi: 10.1007/s00424-008-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prilosec (omeprazole) Label – FDA, https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019810s096lbl.pdf (2012).
- 10.Prevacid (lansoprazole) Label – FDA, https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020406s078-021428s025lbl.pdf (2012).
- 11.Nexium (esomeprazole magnesium) Label – FDA, https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022101s014021957s017021153s050lbl.pdf (2014).
- 12.Protonix (pantoprazole sodium) Label – FDA, https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020987s045lbl.pdf (2012)
- 13.Hardy P, et al. Inhibition of gastric secretion by omeprazole and efficiency of calcium carbonate on the control of hyperphosphatemia in patients on chronic hemodialysis. Artif Organs. 1998;22:569–573. doi: 10.1046/j.1525-1594.1998.06200.x. [DOI] [PubMed] [Google Scholar]
- 14.Makunts T, Cohen IV, Awdishu L, Abagyan R. Analysis of postmarketing safety data for proton-pump inhibitors reveals increased propensity for renal injury, electrolyte abnormalities, and nephrolithiasis. Scientific Reports. 2019;9:2282. doi: 10.1038/s41598-019-39335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazarus B, et al. Proton Pump Inhibitor Use and the Risk of Chronic Kidney Disease. JAMA Intern Med. 2016;176:238–246. doi: 10.1001/jamainternmed.2015.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Y, et al. Proton Pump Inhibitors and Risk of Incident CKD and Progression to ESRD. J Am Soc Nephrol. 2016;27:3153–3163. doi: 10.1681/ASN.2015121377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson C. Bone: proton-pump inhibitors and fractures. Nat Rev Endocrinol. 2012;8:625. doi: 10.1038/nrendo.2012.170. [DOI] [PubMed] [Google Scholar]
- 18.Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107:1001–1010. doi: 10.1038/ajg.2012.179. [DOI] [PubMed] [Google Scholar]
- 19.Haenisch B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265:419–428. doi: 10.1007/s00406-014-0554-0. [DOI] [PubMed] [Google Scholar]
- 20.Gomm W, et al. Association of Proton Pump Inhibitors With Risk of Dementia: A Pharmacoepidemiological Claims Data Analysis. JAMA Neurol. 2016;73:410–416. doi: 10.1001/jamaneurol.2015.4791. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein FC, et al. Proton Pump Inhibitors and Risk of Mild Cognitive Impairment and Dementia. J Am Geriatr Soc. 2017;65:1969–1974. doi: 10.1111/jgs.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Booker A, Jacob LE, Rapp M, Bohlken J, Kostev K. Risk factors for dementia diagnosis in German primary care practices. Int Psychogeriatr. 2016;28:1059–1065. doi: 10.1017/S1041610215002082. [DOI] [PubMed] [Google Scholar]
- 23.Lawton MP. Quality of life in Alzheimer disease. Alzheimer Dis Assoc Disord. 1994;8(Suppl 3):138–150. doi: 10.1097/00002093-199404000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Lawton MP, Brody EM, Saperstein AR. A controlled study of respite service for caregivers of Alzheimer’s patients. Gerontologist. 1989;29:8–16. doi: 10.1093/geront/29.1.8. [DOI] [PubMed] [Google Scholar]
- 25.Mount C, Downton C. Alzheimer disease: progress or profit? Nat Med. 2006;12:780–784. doi: 10.1038/nm0706-780. [DOI] [PubMed] [Google Scholar]
- 26.Castro DM, Dillon C, Machnicki G, Allegri RF. The economic cost of Alzheimer’s disease: Family or public health burden? Dement Neuropsychol. 2010;4:262–267. doi: 10.1590/S1980-57642010DN40400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dharmarajan TS, Gunturu SG. Alzheimer’s disease: a healthcare burden of epidemic proportion. Am Health Drug Benefits. 2009;2:39–47. [PMC free article] [PubMed] [Google Scholar]
- 28.Heidelbaugh JJ. Proton pump inhibitors and risk of vitamin and mineral deficiency: evidence and clinical implications. Ther Adv Drug Saf. 2013;4:125–133. doi: 10.1177/2042098613482484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valuck RJ, Ruscin JM. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults. J Clin Epidemiol. 2004;57:422–428. doi: 10.1016/j.jclinepi.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310:2435–2442. doi: 10.1001/jama.2013.280490. [DOI] [PubMed] [Google Scholar]
- 31.McCombe PA, McLeod JG. The peripheral neuropathy of vitamin B12 deficiency. J Neurol Sci. 1984;66:117–126. doi: 10.1016/0022-510X(84)90147-3. [DOI] [PubMed] [Google Scholar]
- 32.Sakly G, Hellara O, Trabelsi A, Dogui M. [Reversible peripheral neuropathy induced by vitamin B12 deficiency] Neurophysiol Clin. 2005;35:149–153. doi: 10.1016/j.neucli.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Rajabally YA, Jacob S. Neuropathy associated with lansoprazole treatment. Muscle Nerve. 2005;31:124–125. doi: 10.1002/mus.20155. [DOI] [PubMed] [Google Scholar]
- 34.Wang AK, Sharma S, Kim P, Mrejen-Shakin K. Hypomagnesemia in the intensive care unit: Choosing your gastrointestinal prophylaxis, a case report and review of the literature. Indian J Crit Care Med. 2014;18:456–460. doi: 10.4103/0972-5229.136075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin BM, et al. Prospective Study of Gastroesophageal Reflux, Use of Proton Pump Inhibitors and H2-Receptor Antagonists, and Risk of Hearing Loss. Ear Hear. 2017;38:21–27. doi: 10.1097/AUD.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCoul ED, et al. A prospective study of the effect of gastroesophageal reflux disease treatment on children with otitis media. Arch Otolaryngol Head Neck Surg. 2011;137:35–41. doi: 10.1001/archoto.2010.222. [DOI] [PubMed] [Google Scholar]
- 37.O’Reilly RC, et al. The role of gastric pepsin in the inflammatory cascade of pediatric otitis media. JAMA Otolaryngol Head Neck Surg. 2015;141:350–357. doi: 10.1001/jamaoto.2014.3581. [DOI] [PubMed] [Google Scholar]
- 38.Trevisani S, Cereda JM. Blurred vision: a rare secondary effect of proton pump inhibitors. Rev Med Suisse. 2012;8(811–812):814. [PubMed] [Google Scholar]
- 39.Kohno M, et al. Two cases of ocular damage associated with proton pump inhibitors. Nihon Shokakibyo Gakkai Zasshi. 2000;97:575–579. [PubMed] [Google Scholar]
- 40.Schönhöfer PS, Werner B, Tröger U. Ocular damage associated with proton pump inhibitors. BMJ. 1997;314:1805. doi: 10.1136/bmj.314.7097.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.García Rodríguez LA, Mannino S, Wallander MA. Ocular safety of antiulcer drugs. Lancet. 1995;345:1059–1060. doi: 10.1016/S0140-6736(95)90805-6. [DOI] [PubMed] [Google Scholar]
- 42.Malone CD, Bhowmick A, Wachholtz AB. Migraine: treatments, comorbidities, and quality of life, in the USA. J Pain Res. 2015;8:537–547. doi: 10.2147/JPR.S88207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monzón MJ, Láinez MJ. Quality of life in migraine and chronic daily headache patients. Cephalalgia. 1998;18:638–643. doi: 10.1111/j.1468-2982.1998.1809638.x. [DOI] [PubMed] [Google Scholar]
- 44.Liang JF, et al. Proton pump inhibitor-related headaches: a nationwide population-based case-crossover study in Taiwan. Cephalalgia. 2015;35:203–210. doi: 10.1177/0333102414535114. [DOI] [PubMed] [Google Scholar]
- 45.Sáez ME, et al. Risk of seizure associated with use of acid-suppressive drugs: An observational cohort study. Epilepsy Behav. 2016;62:72–80. doi: 10.1016/j.yebeh.2016.06.039. [DOI] [PubMed] [Google Scholar]
- 46.Gandhi NY, Sharif WK, Chadha S, Shakher J. A patient on long-term proton pump inhibitors develops sudden seizures and encephalopathy: an unusual presentation of hypomagnesaemia. Case Rep Gastrointest Med. 2012;2012:632721. doi: 10.1155/2012/632721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milman S, Epstein EJ. Proton pump inhibitor-induced hypocalcemic seizure in a patient with hypoparathyroidism. Endocr Pract. 2011;17:104–107. doi: 10.4158/EP10241.CR. [DOI] [PubMed] [Google Scholar]
- 48.Arulanantham N, Anderson M, Gittoes N, Ferner RE. A 63-year-old man with hypomagnesaemia and seizures. Clin Med (Lond) 2011;11:591–593. doi: 10.7861/clinmedicine.11-6-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alatawi YM, Hansen RA. Empirical estimation of under-reporting in the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS) Expert Opin Drug Saf. 2017;16:761–767. doi: 10.1080/14740338.2017.1323867. [DOI] [PubMed] [Google Scholar]
- 50.Maciejewski, M. et al. Reverse translation of adverse event reports paves the way for de-risking preclinical off-targets. Elife6, 10.7554/eLife.25818 (2017). [DOI] [PMC free article] [PubMed]
- 51.Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the FDA Adverse Event Reporting System. Int J Med Sci. 2013;10:796–803. doi: 10.7150/ijms.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]