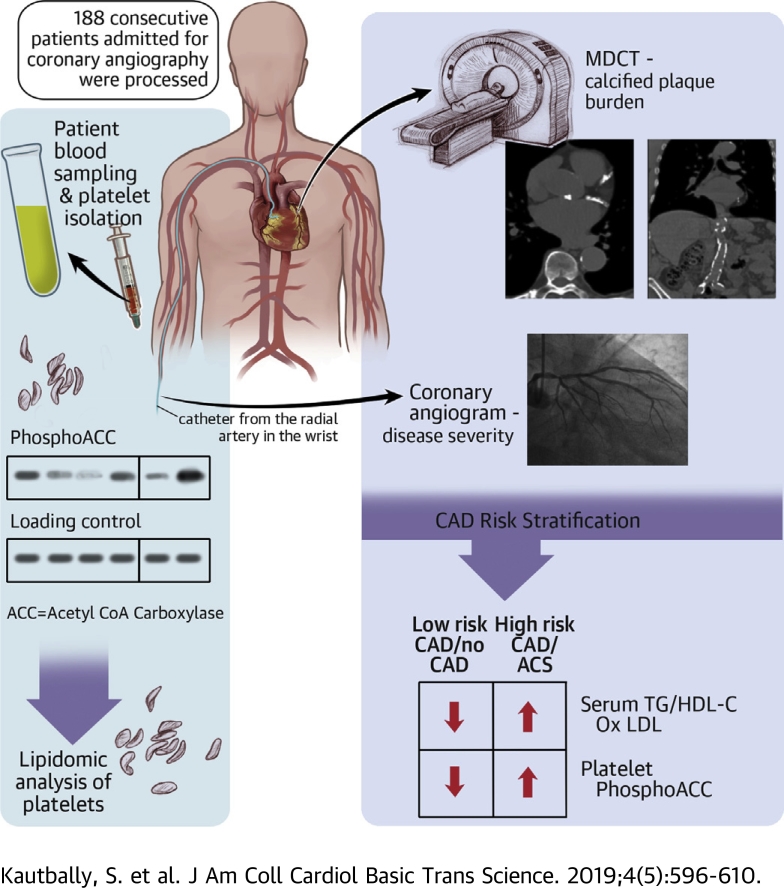
Visual Abstract
Key Words: AMPK, acetyl-CoA carboxylase, coronary artery disease, lipidomics, platelet
Abbreviations and Acronyms: ACC, acetyl-CoA carboxylase; AMPK, adenosine monophosphate–activated protein kinase; AoC, extra-coronary calcification score; AU, arbitrary units; CAC, coronary artery calcification; CAD, coronary artery disease; oxLDL, oxidized low-density lipoprotein; phosphoACC, acetyl-CoA carboxylase phosphorylation on serine 79; S-CAD, stable coronary artery disease; TG, triglyceride
Highlights
-
•
Platelet phosphoACC is a marker for risk stratification in suspected CAD patients. It identifies high-risk CAD patients and correlates with severity of coronary artery calcification.
-
•
The triglycerides/high-density lipoprotein cholesterol ratio is strongly associated with increased phosphoACC in circulating platelets. PhosphoACC is a metabolic signature of the platelet-proatherogenic lipid interplay in CAD patients.
-
•
Phosphorylation and inhibition of acetyl-CoA carboxylase impacts platelet lipid content by down-regulating triglycerides lipid species.
Summary
Adenosine monophosphate–activated protein kinase (AMPK) acetyl-CoA carboxylase (ACC) signaling is activated in platelets by atherogenic lipids, particularly by oxidized low-density lipoproteins, through a CD36-dependent pathway. More interestingly, increased platelet AMPK–induced ACC phosphorylation is associated with the severity of coronary artery calcification as well as acute coronary events in coronary artery disease patients. Therefore, AMPK–induced ACC phosphorylation is a potential marker for risk stratification in suspected coronary artery disease patients. The inhibition of ACC resulting from its phosphorylation impacts platelet lipid content by down-regulating triglycerides, which in turn may affect platelet function.
Platelets are key players in atherothrombosis. In acute coronary syndrome (ACS), coagulation cascade activation upon plaque rupture leads to thrombin generation (ThG), a crucial platelet agonist enhancing the formation of platelet-rich thrombi (1). The association between increased ThG and ischemic risk in coronary artery disease (CAD) patients renders the coagulation cascade an interesting therapeutic target 2, 3.
We previously established the adenosine monophosphate–activated protein kinase (AMPK) to be crucial for platelet activation. In human platelets, thrombin is the major agonist leading to AMPK activation through a calcium-dependent mechanism (4). Once activated, AMPK contributes to platelet secretion, platelet aggregation, and clot retraction by controlling the actin cytoskeleton. AMPK activation likewise leads to phosphorylation of acetyl-CoA carboxylase (ACC) on serine 79 (phosphoACC), its bona-fide substrate, typically used as a marker of AMPK activation in cells and tissues, including platelets (4). It is tempting to speculate that ThG affects platelet AMPK signaling, resulting in increased phosphoACC in CAD. However, although thrombin is crucial for thrombus formation at the plaque rupture site in ACS, its impact on circulating platelets remains unclear. In CAD patients, the atherogenic environment influences platelet biology and reactivity, mainly through CD36 5, 6. Oxidized low-density lipoprotein (oxLDL) binds to CD36, inducing platelet activation and shape changes via a calcium-dependent mechanism (7). Yet, other factors besides thrombin may affect AMPK-ACC signaling in circulating platelets of CAD patients.
ACC is the first committed enzyme of the fatty acid biosynthesis pathway, while its phosphorylation on serine 79 by AMPK inhibits its activity (8). We demonstrated that AMPK-ACC signaling is a key pathway in controlling platelet lipid content, thereby modulating platelet function and thrombus formation (9). However, ACC contribution to platelet lipid metabolism in CAD, in which atherogenic lipids interact with circulating platelets (10), remains unexplored.
Here, we report platelet phosphoACC as a potential risk stratification marker in suspected CAD patients. In consecutive patients admitted for coronary angiography, phosphoACC was significantly increased in circulating platelets of CAD patients and highly associated with acute coronary events. We identified an interplay between platelets and lipids, with oxLDL as a central contributor to increased platelet phosphoACC. Interestingly, the lipidomic data show that sustained phosphoACC regulates triglyceride (TG) lipids in circulating platelets of CAD patients.
Methods
Methods (including experimental dataset) and reagents are described in the Supplemental Appendix.
Clinical cohort
Study design
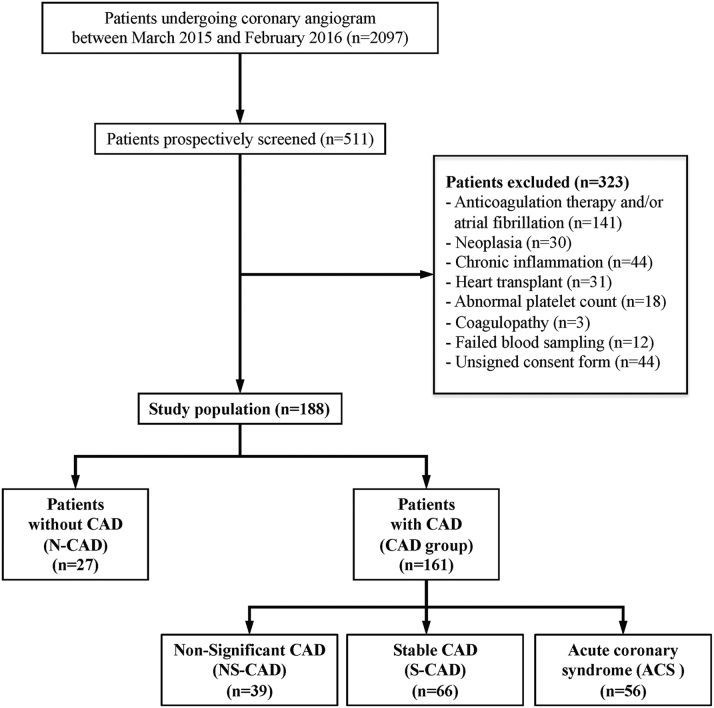
From March 2015 to February 2016, 188 consecutive patients admitted for coronary angiography were included in the ACCTHEROMA (prospective evaluation of Acetyl-CoA Carboxylase phosphorylation state in platelets as a marker of atherothrombotic coronary and extra-coronary artery disease) study (NCT03034148), with at least 2 patients prospectively screened per day, regardless of the indication for angiography. Based on indication and coronary angiography results analyzed by 2 experienced cardiologists, patients were classified into 4 groups. Patients undergoing angiography for chest pain or valvular disease investigation with normal coronary vessels were classed as non-CAD (N-CAD) (reference population). The presence of at least 1 plaque with <50% luminal stenosis was classed as nonsignificant CAD (NS-CAD). Patients presenting with stable disease with significant stenosis (>50%) were classed as stable CAD (S-CAD). ACS comprised unstable angina (n = 30), non–ST-segment elevation myocardial infarction (n = 22), and ST-segment elevation myocardial infarction (n = 4). Further details on patient classifications are provided in the Supplemental Appendix and study flowchart in Figure 1. The study was approved by the institutional ethics committee (2015/08JAN/010) and complied with the Declaration of Helsinki and good clinical practice guidelines. All participants provided written informed consent.
Figure 1.
Flowchart of the Study Population
Patients included in the ACCTHEROMA study. Classification based on clinical presentation and angiographic data. ACCTHEROMA = prospective evaluation of Acetyl-CoA Carboxylase phosphorylation state in platelets as a marker of atherothrombotic coronary and extra-coronary artery disease; ACS = acute coronary syndrome; CAD = coronary artery disease; N-CAD = non–coronary artery disease; NS-CAD = nonsignificant coronary artery disease; S-CAD = significant coronary artery disease.
Blood sampling and phosphoACC analysis
All patients had been fasting for at least 6 h before angiography, except for 4 ST-segment elevation myocardial infarction patients. Blood samples drawn from the arterial sheath were collected in citrated tubes before any drug administration in the catheterization laboratory, including heparin. In a subgroup of patients (n = 8) undergoing right heart catheterization in addition to angiography, a venous blood sample was collected from the femoral access site to compare the level of ACC phosphorylation in arterial and venous blood simultaneously. All samples were immediately processed for platelet isolation. Using flow cytometry, we verified that platelet preparations did not contain any leukocytes (Supplemental Figure 1). Platelets were lysed in Laemmli buffer before phosphoACC analysis by Western blotting. A standard positive control for phosphoACC was prepared with washed platelets isolated from a healthy volunteer and stimulated with a high dose of thrombin (0.5 U/ml) for 2 min. This standard positive control was used for all the Western blots, placed 4 times on each gel to validate the signal reproducibility. For each patient, band intensities were normalized to corresponding loading controls (gelsolin) on the same gel. The normalized phosphoACC value was compared with the standard positive control. Western blot analyses were confirmed by electrochemiluminescence immunoassay (Meso Scale Diagnostics, Rockville, Maryland).
Multidetector computed tomography
Thoracoabdominal multidetector computed tomography (MDCT) was performed in a subgroup of patients (n = 68) to assess calcified plaque burden. Arbitrarily, the first and third patient on the list for planned coronary angiogram underwent MDCT for calcium scoring. MDCT was done just before coronary angiogram. Scans were taken with a 256-slice multidetector-row CT scanner (Brilliance iCT 256, Philips Healthcare, Cleveland, Ohio) with 3.0-mm slice collimation, 120-kV tube voltage, and 100-mAs tube current using a prospectively gated “step and shoot” protocol. Coronary artery calcification (CAC) was expressed by means of the Agatston score using calcium scoring software (Philips Healthcare) with a threshold of 130 Hounsfield units. The degree of CAC was classified as mild (Agatston score <100), moderate (between 100 and 400), or severe (>400) (11). An extracoronary calcification score (aorta calcification [AoC]) was measured from the aortic root (excluding the aortic valve) to the common iliac artery in all patients. The AoC score was divided into tertiles for analysis.
Platelet lipidomics
To characterize the phosphoACC impact on regulating platelet lipid homeostasis, we performed a quantitative lipidomic study on 31 samples from patients with the lowest (n = 12) and highest (n = 19) platelet phosphoACC values. Lipids were extracted from a platelet pellet by the methyl-tert-butylether method and analyzed using Lipidyzer, a direct infusion-tandem mass spectrometry (DI-MS/MS)–based platform (Sciex, Redwood City, California).
Statistical analysis
Clinical cohort
Analyses were conducted using SPSS version 24 (IBM Corporation, Armonk, New York). Continuous variables were expressed as mean ± SD or median (interquartile range [IQR]) depending on data distribution, and categorical variables were expressed as number and percentage. CAC and AoC scores, D-dimers, high-sensitivity C-reactive protein, and TG and high-density lipoprotein-cholesterol (HDL-C) ratio were normalized by log-10 transformation. Categorical variables were analyzed using the chi-square test or Fisher’s exact test and continuous variables were analyzed using an unpaired Student's t-test or the Mann-Whitney U test, as appropriate. Data were subjected to the Kolmogorov-Smirnov normality test and Bartlett’s test for homogeneity of variance. Group comparisons were made using either 1-way analysis of variance with the F test (Bonferroni correction) or Kruskal-Wallis test. Correlations were presented as Pearson (Rp) or Spearman (Rs) coefficients. Multivariable logistic regression analysis (backward elimination) included variables with p value < 0.05 on univariable analysis, with odds ratio (OR) and 95% confidence interval (CI) calculated to determine independent factors associated with ACS. With the receiver-operating characteristic curve, we determined a threshold phosphoACC value for CAD by maximizing sensitivity and specificity. C-statistics were used to describe diagnostic discrimination. The prognostic value of platelet phosphoACC for ischemic outcomes, including cardiovascular death and recurrent myocardial infarction/revascularization procedures, was assessed during patient follow-up. Multivariable Cox regression analysis was used to identify independent predictors for events. Hazard ratios with 95% CI are presented. Event-free survival according to platelet phosphoACC levels was computed using the Kaplan-Meier method.
Lipidomics
Lipid species concentrations obtained from DI-MS/MS (Lipidyzer) were analyzed using R software version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria) according to the following bioinformatics pipeline. Missing DI-MS/MS data were imputed using probabilistic principal component analysis from pcaMethods Bioconductor package (12). The data were normalized using total lipid abundance, with a log-2 transformation applied to normalized concentrations. The limma Bioconductor package was used to build a multivariable regression model for each lipid species with predictors including platelet phosphoACC, aspirin intake, diabetes, and plasma TG levels. Fold-change estimates and corresponding p values were derived from regression models for each lipid species and each predictor. To control for multiple testing, all p values were further adjusted for Benjamini-Hochberg false discovery rate, with a false discovery rate <0.05 considered statistically significant (13). Considering that 865 lipid species belong to 11 lipid classes, namely cholesterol ester, diacylglycerol, free fatty acid, lysophosphatidylcholine, lysophosphatidylethanolamine, phosphatidylcholine, phosphatidylethanolamine, plasmenyl phosphatidylethanolamine, plasmalogen phosphatidylethanolamine, sphingomyelin, and TG, lipid class enrichment analysis was performed using Fisher’s exact tests to identify lipid classes with a high proportion of differentially regulated lipid species.
Results
Population baseline characteristics and global atherosclerotic calcified plaque burden
The study population comprised 188 individuals (70% men, 65 ± 12 years) admitted for coronary angiography. They were divided into 4 groups according to clinical presentation and coronary anatomy (Table 1). The N-CAD group included 27 (14.4%) patients with normal coronary arteries, with about 41% undergoing angiography for valvular disease. In the CAD group (n = 161), 122 (76%) patients had significant coronary stenosis above 50%, 46% of whom had ACS. Most ACS patients (93%) were taking aspirin at enrollment, with only 36% of them on dual antiplatelet therapy, as most unstable angina and non–ST-segment elevation myocardial infarction patients did not receive P2Y12 inhibitors before angiography. Accordingly, mean platelet reactivity assessed using the Multiplate Analyzer (Roche Diagnostics International Ltd., Rotkreuz, Switzerland) showed decreased platelet aggregation in response to arachidonic acid in ACS patients. Laboratory parameters revealed significantly increased platelet counts in CAD patients, yet within the normal range. As previously reported (2), our data confirmed a significant increase in ThG, assessed by D-dimer levels, in ACS patients.
Table 1.
Baseline Characteristics of the ACCTHEROMA Cohort
| Overall Population (N = 188) | NCAD (n = 27) | CAD |
p Value | |||
|---|---|---|---|---|---|---|
| NS-CAD (n = 39) | S-CAD (n = 66) | ACS (n = 56) | ||||
| Clinical characteristics | ||||||
| Age, yrs | 65 ± 12 | 59 ± 9 | 68 ± 11 | 66 ± 12 | 66 ± 14 | 0.029∗ |
| Male | 131 (69.7) | 15 (55.6) | 23 (59.0) | 48 (72.7) | 45 (80.4) | 0.046 |
| BMI, kg/m2 | 27.6 ± 4.9 | 27.4 ± 4.0 | 28.0 ± 5.3 | 27.4 ± 5.5 | 27.6 ± 4.2 | 0.94 |
| Hypertension | 119 (63.3) | 8 (29.6) | 21 (53.8) | 52 (78.8) | 38 (67.9) | <0.001 |
| Smoking | 104 (55.3) | 12 (44.4) | 17 (43.6) | 39 (59.1) | 36 (64.3) | 0.13 |
| Diabetes | 43 (22.9) | 1 (3.7) | 9 (23.1) | 14 (21.2) | 19 (33.9) | 0.022 |
| Prior history of CAD | 62 (33.0) | 0 (0) | 0 (0) | 36 (54.5) | 26 (46.4) | <0.001 |
| MI | 35 (18.6) | 0 (0) | 0 (0) | 19 (28.8) | 16 (28.6) | <0.001 |
| PCI | 47 (25.0) | 0 (0) | 0 (0) | 26 (39.4) | 21 (37.5) | <0.001 |
| CABG | 16 (8.5) | 0 (0) | 0 (0) | 10 (15.2) | 6 (10.7) | 0.017 |
| Aortic valvular disease | 34 (18.1) | 4 (14.8) | 14 (35.9) | 16 (24.2) | 0 (0) | <0.001 |
| Mitral valvular disease | 12 (6.4) | 7 (25.9) | 3 (7.7) | 2 (3.0) | 0 (0) | <0.001 |
| Biological | ||||||
| Hemoglobin, g/dl | 14.1 ± 1.6 | 13.7 ± 1.3 | 14.1 ± 1.5 | 14.2 ± 1.4 | 14.0 ± 1.9 | 0.62 |
| Fasting glycemia, mg/dl | 100 (92–114) | 98 (92–105) | 99 (91–123) | 101 (93–108) | 105 (95–134) | 0.61 |
| Creatinine, mg/dl | 1.0 (0.8–1.1) | 0.9 (0.8–1.1) | 1.0 (0.8–1.0) | 1.0 (0.9–1.2) | 0.9 (0.8–1.1) | 0.22 |
| CRI | 22 (11.7) | 2 (7.4) | 3 (7.7) | 12 (18.2) | 5 (8.9) | 0.24 |
| Non-HDL, mg/dl | 120 ± 45 | 124 ± 49 | 123 ± 39 | 112 ± 45 | 127 ± 46 | 0.32 |
| HDL, mg/dl | 50.4 ± 15.3 | 53.8 ± 15.6 | 56.5 ± 17.8 | 50.2 ± 13.6 | 44.7 ± 13.4 | 0.002† |
| TG, mg/dl | 102 (76–152) | 84 (66–132) | 95 (78–140) | 95 (75–146) | 126 (91–161) | 0.017‡ |
| hsCRP, mg/l | 1.4 (0.6–3.4) | 0.9 (0.5–2.1) | 1.2 (0.6–3.0) | 1.3 (0.5–3.6) | 1.7 (0.8–5.6) | 0.13 |
| Platelet count (×103)/μl | 244 ± 61 | 211 ± 38 | 268 ± 65 | 248 ± 66 | 240 ± 54 | 0.002∗§ |
| Multiplate analysis | ||||||
| ASPI test, AU∗min | 515 ± 278 | 549 ± 319 | 631 ± 258 | 507 ± 287 | 427 ± 228 | 0.004† |
| ADP test, AU∗min | 638 ± 217 | 626 ± 163 | 692 ± 132 | 643 ± 243 | 601 ± 249 | 0.24 |
| TRAP test, AU∗min | 1,101 ± 255 | 1,007 ± 258 | 1,177 ± 232 | 1,057 ± 226 | 1,146 ± 280 | 0.011∗ |
| D-dimers, ng/ml | 407 (281–686) | 282 (250–435) | 398 (288–573) | 407 (290–775) | 443 (349–706) | 0.018‡ |
| Medication | ||||||
| ACE inhibitor/ARB | 86 (45.7) | 6 (22.2) | 16 (41.0) | 43 (65.2) | 21 (37.5) | <0.001 |
| Beta-blockers | 97 (51.6) | 9 (33.3) | 15 (38.5) | 39 (59.1) | 34 (60.7) | 0.022 |
| Lipid-lowering treatment | 111 (59.0) | 13 (48.1) | 16 (41.0) | 48 (72.7) | 34 (60.7) | 0.008 |
| Aspirin | 141 (75.0) | 13 (48.1) | 21 (53.8) | 55 (83.3) | 52 (92.9) | <0.001 |
| Dual antiplatelet therapy | 30 (16.0) | 0 (0) | 0 (0) | 12 (18.2) | 20 (35.7) | <0.001 |
| Clopidogrel | 15 (8.0) | 0 (0) | 0 (0) | 6 (9.1) | 9 (16.1) | 0.013 |
| Ticagrelor | 13 (6.9) | 0 (0) | 0 (0) | 3 (4.5) | 10 (17.9) | <0.001 |
| Prasugrel | 4 (2.1) | 0 (0) | 0 (0) | 3 (4.5) | 1 (1.8) | 0.35 |
Values are mean ± SD, n (%), or median (interquartile range).
ACCTHEROMA = prospective evaluation of Acetyl-CoA Carboxylase phosphorylation state in platelets as a marker of atherothrombotic coronary and extra-coronary artery disease; ACE = angiotensin-converting enzyme; ACS = acute coronary syndrome; ARB = angiotensin receptor blocker; ASPI = aspirin channel; AU = arbitrary units; BMI = body mass index; CABG = coronary artery bypass grafting; CAD = coronary artery disease; CRI = chronic renal insufficiency; hsCRP = high-sensitivity C-reactive protein; MI = myocardial infarction; NCAD = non–coronary artery disease; NS-CAD = nonsignificant coronary artery disease; PCI = percutaneous coronary intervention; S-CAD = significant coronary artery disease; TG = triglycerides; TRAP = thrombin receptor activating peptide.
Pairwise significant difference (p < 0.05) between NS-CAD and NCAD.
Pairwise significant difference (p < 0.05) between ACS and NS-CAD.
Pairwise significant difference (p < 0.05) between ACS and N-CAD.
Pairwise significant difference (p < 0.05) between S-CAD and NCAD.
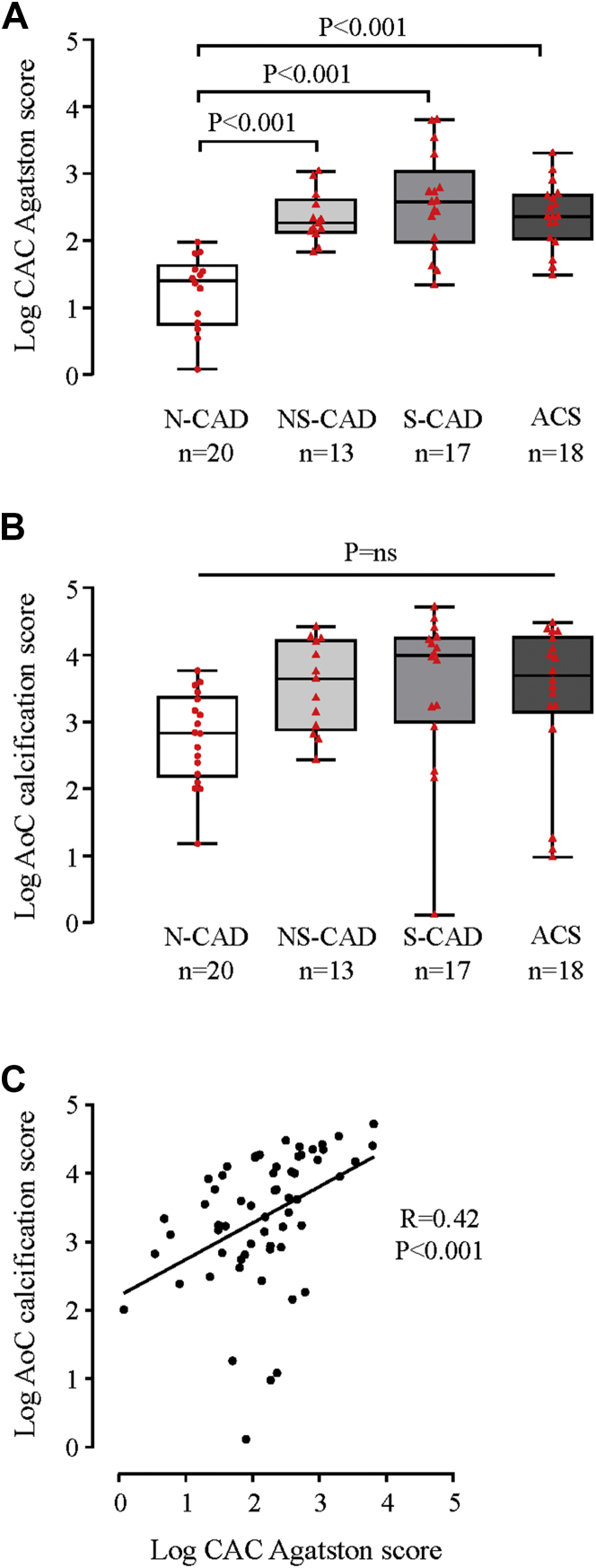
Thoracoabdominal MDCT with prospective electrocardiography gating aimed to evaluate calcified plaque burden, including coronary (CAC Agatston) and extracoronary (AoC) calcification scores. We confirmed that N-CAD patient (reference population) classification based on angiographic data was consistent with low CAC Agatston scores (median 7.0 [interquartile range: 0.0 to 33.9]) (Figure 2A). Although N-CAD patients had low CAC Agatston scores, their AoC scores were fairly high (median 543 [interquartile range: 107 to 2,001]) (Figure 2B). Accordingly, we observed a modest correlation between CAC and AoC calcification scores (Rp = 0.42; p < 0.001), indicating a heterogeneous atherosclerotic development or arterial calcifications (Figure 2C).
Figure 2.
Assessment of Coronary and Aortic Calcified Plaque by Prospective Electrocardiography-Gated Multidetector Computed Tomography
Box-plot representation of log-transformed (A) coronary artery calcification (CAC) Agatston and (B) aorta calcification (AoC) scores in the N-CAD and CAD subgroups of patients. Red dots (N-CAD, reference population) and triangles (CAD patients) represent individual values. Boxplots represent medians and corresponding whiskers represent extremes of the distribution. (C) Correlation between log-transformed CAC Agatston and AoC scores. Abbreviations as in Figure 1.
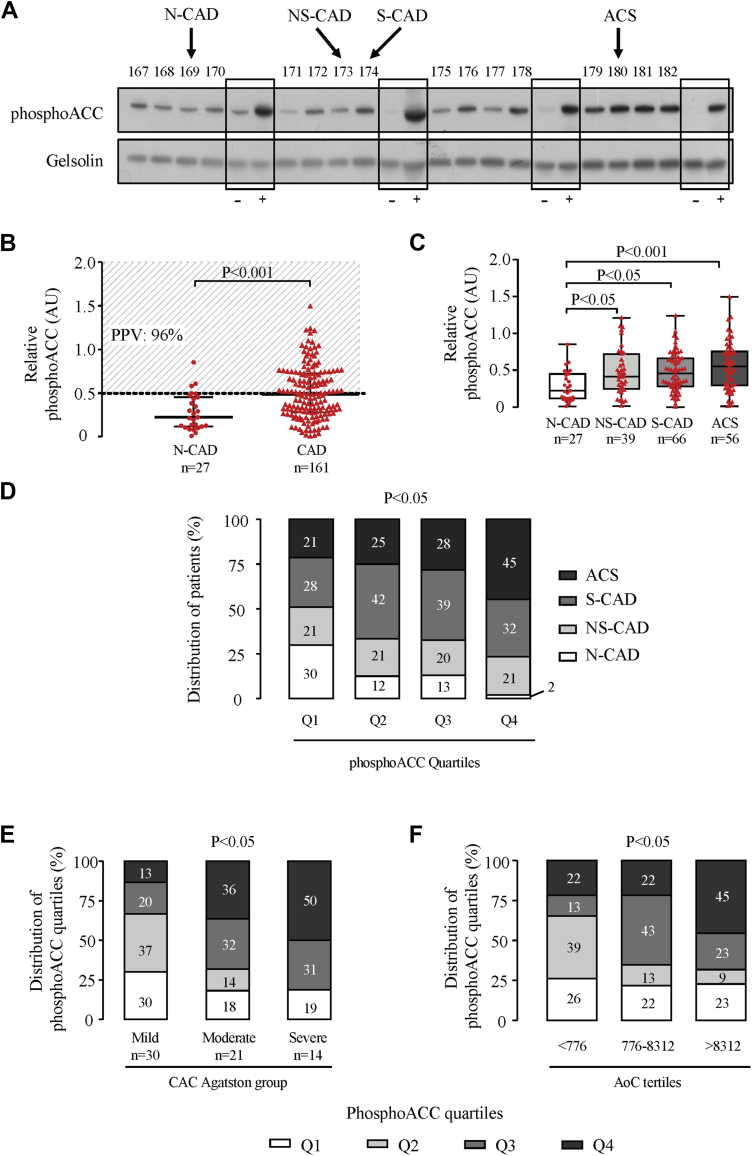
Identification of high-risk CAD patients by platelet phosphoACC
Platelet phosphoACC was studied in consecutive patients by Western blotting (Figure 3A). Quantification of band intensities showed significant increased phosphoACC in circulating platelets of CAD compared with N-CAD patients (median 0.48 [IQR: 0.29 to 0.73] vs. 0.22 [IQR: 0.11 to 0.45]; p < 0.001) (Figure 3B). Platelet phosphoACC in N-CAD patients was almost never >0.5 arbitrary units (AU), a threshold value estimated from receiver-operating characteristic analysis (Supplemental Figure 2) with a 96% positive predictive value for CAD. PhosphoACC signal was fairly similar in arterial and central venous blood in a subset of patients, undergoing left and right heart catheterization (Supplemental Figure 3). Of note, phosphorylation of protein kinase C substrates, a readout of platelet activation, was nearly undetectable in the platelets of CAD patients (Supplemental Figure 4), suggesting that phosphoACC occurred independently of platelet activation.
Figure 3.
PhosphoACC Correlates With Calcified Plaque Severity and Identifies High-Risk ACS Patients
(A) Representative Western blot of platelet acetyl-CoA carboxylase phosphorylation on serine 79 (phosphoACC) in 16 consecutive patients from the ACCTHEROMA trial, with negative control (–) corresponding to washed unstimulated platelets from healthy volunteers and positive control (+) corresponding to washed platelets from healthy volunteers stimulated with thrombin (0.5 U/ml) for 2 min. Four different controls are shown on the Western blot. (B) PhosphoACC quantification in N-CAD and CAD patients. Dotted line represents the threshold value of 0.5 arbitrary units (AU) estimated from receiver-operating characteristic curve analysis for discriminating between N-CAD and CAD patients. Positive predictive values (PPVs) of this threshold for CAD are indicated on the graph. Red dots (N-CAD, reference population) and triangles (CAD patients) represent individual values. Medians with interquartile range are presented. (C) Boxplot representation of platelet phosphoACC quantifications in N-CAD and CAD subgroups. (D) Clinical and angiographic characteristics of patients across the different quartiles of platelet phosphoACC. Distribution of platelet phosphoACC quartiles across (E) CAC Agatston score groups and (F) AoC score tertiles. The statistical differences between the groups were determined using the Mann-Whitney U test in B, Kruskal-Wallis test in C, and chi-square test in D to F. ACCTHEROMA = prospective evaluation of Acetyl-CoA Carboxylase phosphorylation state in platelets as a marker of atherothrombotic coronary and extra-coronary artery disease; M = molecular weight marker; Q = quartile; other abbreviations as in Figures 1 and 2.
These data were further confirmed by quantitative electrochemiluminescence analysis. Platelet phosphoACC was analyzed twice to test interexperiment reproducibility (intraclass correlation coefficient) and found to be high (0.90), with a bias of –0.03 (95% CI: –0.10 to 0.04) (Supplemental Figure 5A). Furthermore, phosphoACC correlated with Western blot results, confirming that it was significantly increased in circulating platelets of CAD patients (Supplemental Figures 5B and 5C).
Among the CAD subgroups, the highest platelet phosphoACC level was found in ACS patients (median 0.55 [IQR: 0.29 to 0.76]) (Figure 3C). Indeed, quartile analysis of platelet phosphoACC demonstrated that the fourth quartile included 98% of CAD patients, with a large proportion of high-risk ACS patients (45%) (chi-square test; p = 0.020) (Figure 3D). Multivariable logistic regression results coincided with this, showing that, in addition to D-dimer levels (OR: 5.7; 95% CI: 1.8 to 17.8; p = 0.003) and the TG/HDL-C ratio (OR: 7.9; 95% CI: 2.1 to 29.9; p = 0.002), platelet phosphoACC (OR: 4.8; 95% CI: 1.5 to 15.8; p = 0.009) was independently associated to ACS (Table 2).
Table 2.
Univariable and Multivariable Models of Factors Associated With ACS
| Univariable Analysis |
Multivariable Analysis |
|||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| ASCVD score | 3.61 (0.80–16.24) | 0.09 | ||
| hsCRP (log transformed) | 1.99 (1.11–3.55) | 0.020 | ||
| D-dimer (log transformed) | 3.14 (1.16–8.51) | 0.024∗ | 5.69 (1.82–17.76) | 0.003∗ |
| Non-HDL | 1.00 (0.99–1.01) | 0.23 | ||
| TG/HDL-C ratio (log transformed) | 7.57 (2.32–24.72) | 0.001∗ | 7.95 (2.11–29.90) | 0.002∗ |
| Platelet phosphoACC | 4.02 (1.39–11.56) | 0.010∗ | 4.83 (1.48–15.81) | 0.009∗ |
ASCVD = atherosclerotic cardiovascular disease; CI = confidence interval; HDL-C = high-density lipoprotein cholesterol; OR = odds ratio; phosphoACC = acetyl-CoA carboxylase phosphorylation on Ser79; other abbreviations as in Table 1.
Statistical significance when p value < 0.05 in univariable and multivariable analysis.
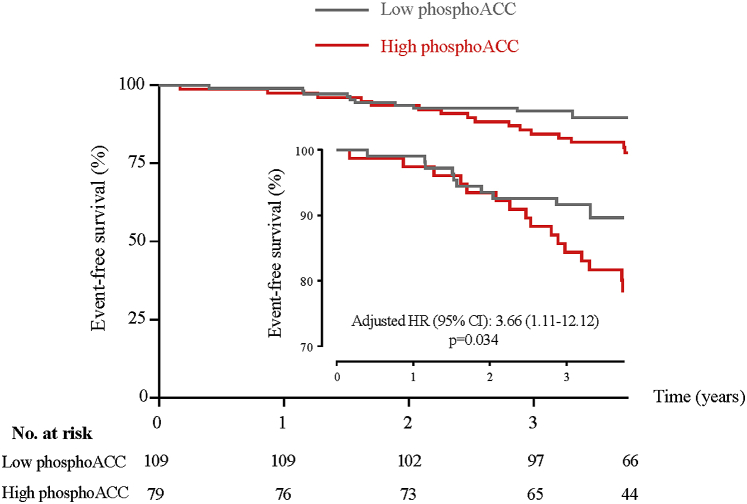
Prognostic value of platelet phosphoACC levels was assessed for ischemic events (cardiovascular death, recurrent myocardial infarction, and revascularization procedures) during a mean follow-up of 3.7 ± 0.4 years. In our cohort, 28 (14.8%) patients experienced with ischemic events. Multivariable Cox regression analysis identified increased platelet phosphoACC as an independent predictor of events (hazard ratio: 3.7; 95% CI: 1.1 to 12.1; p = 0.034), in addition to previous history of MI (hazard ratio: 3.2; 95% CI: 1.4 to 7.3; p = 0.006) and atherosclerotic cardiovascular disease score (hazard ratio: 10.1; 95% CI: 1.9 to 54.0; p = 0.007) (Supplemental Table 1). Kaplan-Meier event-free survival curves confirmed that patients with high phosphoACC levels (above 0.5 AU) had a higher events rate compared with low phosphoACC levels (log-rank test; p = 0.036) (Figure 4).
Figure 4.
Kaplan-Meier Curves for Ischemic Events According to Platelet PhosphoACC Levels
Ischemic events include cardiovascular death, recurrent myocardial infarction and revascularization procedures. Low and high phosphoACC defined according to threshold of 0.5 AU. CI = confidence interval; HR = hazard ratio; other abbreviations as in Figures 1 and 3.
Relationship between platelet phosphoACC, calcified plaque burden, and ThG markers
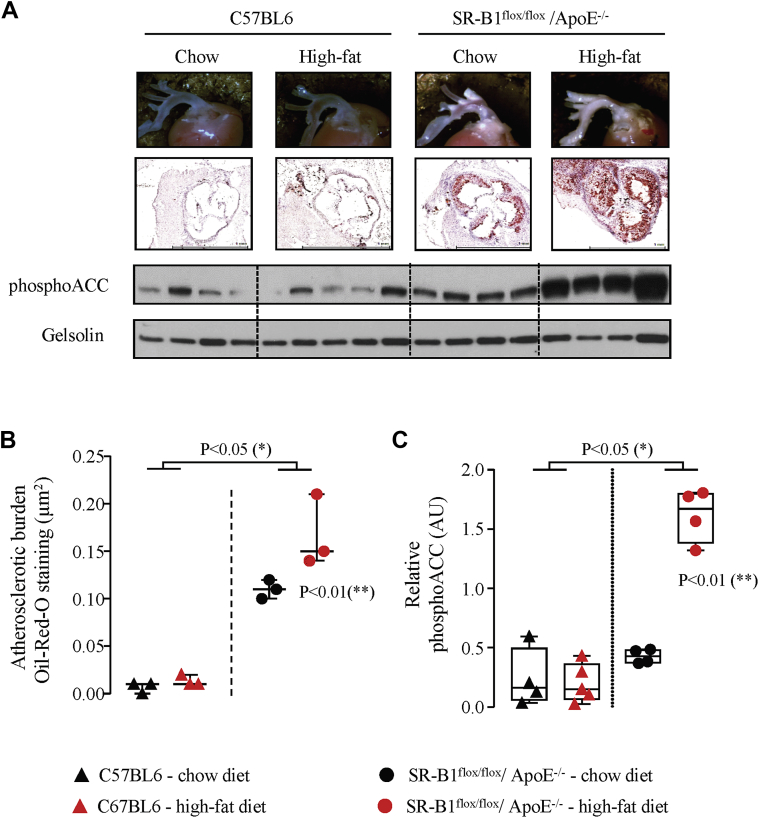
In patients who underwent MDCT, platelet phosphoACC, in addition to age and D-dimer levels, was independently associated to coronary calcification severity (global R2 = 0.47; p < 0.001) (Supplemental Table 2). Accordingly, as depicted in Figure 3E, 50% of patients with high phosphoACC (fourth quartile) exhibited severe CAC Agatston scores (chi-square test; p = 0.023). Similar results were obtained with the AoC score (Figure 3F). Altogether, these findings highlight the potential of platelet phosphoACC for identifying disease severity and high-risk CAD patients. We next confirmed such observations in an animal model developing spontaneous atherosclerosis, which is enhanced by a Western diet, the SR-B1flox/flox/ApoE–/– hypercholesterolemic mice (Figures 5A and 5B). As in human platelets, phosphoACC levels drastically increased with the severity of atherosclerotic plaque burden (Figure 5C).
Figure 5.
Increased Platelet PhosphoACC in Atherosclerotic Mice
(A–C) Female SR-B1flox/flox/ApoE–/–(dot) and control C57BL6 (triangle) mice received either chow diet for 24 weeks (black symbols) or 12 weeks chow diet followed by 12 weeks Western diet (red symbols) before sacrifice. Atherosclerotic burden was evaluated by (A, B) Oil-Red-O staining of the aortic root and (A, C) platelet phosphoACC by Western blot. (A) The top 2 panels show representative pictures of the aortic root (top) and of Oil-Red-O staining after cross section of the aortic root. Scale bar = 1 mm (bottom). The bottom 2 panels show representative Western blot of platelet phosphoACC. Gelsolin was used as loading control. Quantifications of (B) Oil-Red-O staining and (C) phosphoACC are shown. Data are represented as (B) medians with interquartile range or (C) boxplot. Single asterisk denotes statistical difference between C57BL6 and SR-B1flox/flox/ApoE–/–. Double asterisk denotes statistical differences between SR-B1flox/flox/ApoE–/– under high-fat diet compared with all other groups. Abbreviations as in Figures 1 and 3.
ThG markers correlated with the severity of calcified plaque, given that D-dimer levels (chi-square test; p = 0.014), thrombin antithrombin complexes (chi-square test; p = 0.036), or fragments 1.2 (chi-square test; p = 0.004) had a significant positive association with the severity of both CAC Agatston (Supplemental Figure 6A) and AoC score (Supplemental Figure 6B). However, we did not find any correlation between ThG markers (D-dimer levels) and platelet phosphoACC across the entire population (Supplemental Figure 6C), although thrombin is a major agonist leading to increased phosphoACC in platelets in vitro. Therefore, other factors besides thrombin should contribute to platelet phosphoACC in atherosclerosis.
Contribution of inflammation and atherogenic oxLDL to phosphoACC in platelets
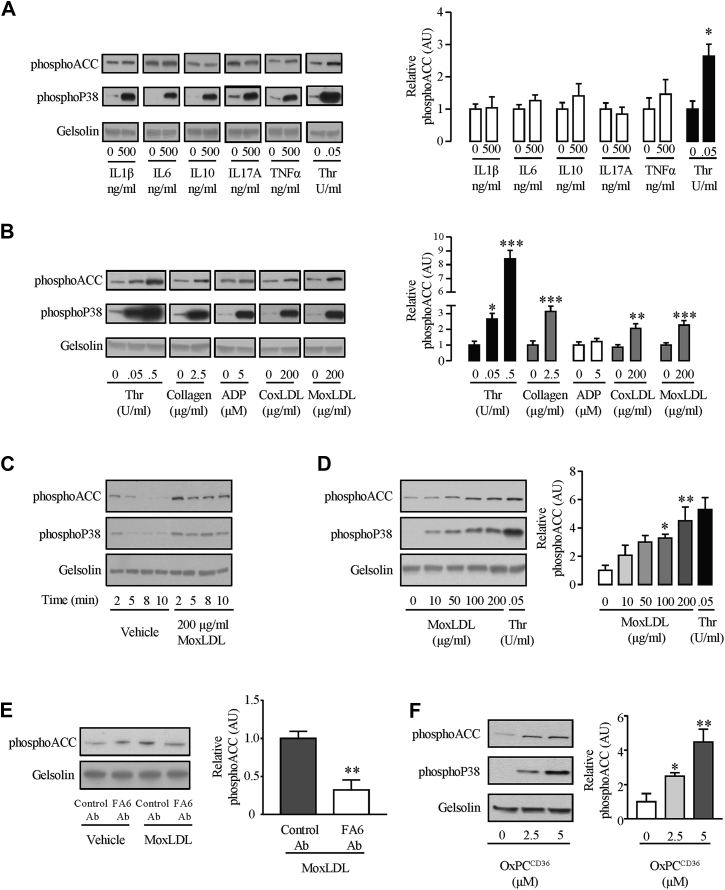
As inflammatory cytokines may affect platelets (14) and are established circulating actors of atherosclerosis, we explored whether interleukin-1beta, interleukin-6, interleukin-10, interleukin-17A, and tumor necrosis factor-alpha impacted phosphoACC level in washed platelets from healthy volunteers. Although their respective receptors are present in human platelets (15), none of these cytokines affected platelet phosphoACC (Figure 6A).
Figure 6.
Oxidized LDLs Induce Platelet phosphoACC in a CD36-Dependent Manner
Washed platelets (4.0 × 108/ml) from healthy volunteers were (A) treated with the following selected cytokines (interleukin-1beta [IL1β], IL-6, IL-10, IL-17A, and tumor necrosis factor alpha [TNFα]) and (B) stimulated with thrombin (Thr), collagen, adenosine diphosphate (ADP), copper-oxidized low-density lipoprotein (coxLDL) or myeloperoxidase-oxidized low-density lipoprotein (moxLDL) before lysis. (C) Time course and (D) dose-response curve of effect of moxLDL on phosphoACC. (E) Platelets were pretreated with 0.2 U/ml anti-CD36 antibody (FA6-152) (FA6 Ab) or an isotype control (control Ab) for 15 min before stimulation with moxLDL. (F) Platelets were stimulated with varying concentrations of a specific CD36 ligand (OxPCCD36) for 5 min before lysis. All experiments were carried out at least 4 times (biological replicates). Thr-stimulated platelets were used as a positive control. Gelsolin was the loading control. Representative Western blots are shown, with quantification of Western blots represented in the right-hand panels. Data are expressed as mean ± SEM. Significance was determined by (A, B, E) 2-tailed Student’s t-test or (B [Thr], D, F) 1-way analysis of variance with Bonferroni post hoc analysis. *p < 0.05, **p < 0.01, ***p < 0.001 compared with unstimulated platelets. Ab = antibody; OxPCCD36 = oxidized choline glycerophospholipids; other abbreviations as in Figure 3.
We continued our analysis to identify biological mediators of increased platelet phosphoACC in the CAD group (above 0.5 AU) (Figure 3B). Whereas high-sensitivity C-reactive protein and ThG markers were not associated with increased phosphoACC, we identified a striking positive association between TG/HDL-C and platelet phosphoACC, suggesting atherogenic lipids to affect phosphoACC in circulating platelets (OR: 4.0; 95% CI: 1.3 to 12.6; p = 0.019) (Table 3). TG/HDL-C ratio, a well-known atherogenic marker, indicates LDL particle size (16), while TGs were shown to correlate with plasma oxLDL levels (17). In our cohort of patients, TG/HDL-C ratio correlated with oxLDL levels (Rp = 0.23; p = 0.002). We thus studied the effect of oxLDL on AMPK-ACC signaling. PhosphoACC was assessed in platelets from healthy volunteers treated with copper-oxidized LDL and myeloperoxidase-oxidized LDL. Both induced a time- and dose-dependent increase in platelet phosphoACC (Figures 6B to 6D, Supplemental Figure 7A). Myeloperoxidase-oxidized LDL-induced phosphoACC was prevented by preincubating platelets with a blocking anti-CD36 antibody (Figure 6E). Accordingly, oxidized choline glycerophospholipids (OxPCCD36), a specific high-affinity ligand for CD36, increased platelet phosphoACC (Figure 6F).
Table 3.
Factors Associated With Increased Platelet phosphoACC in CAD Patients (>0.5 AU Threshold)
| OR (95% CI) | p Value | |
|---|---|---|
| Aspirin treatment | 1.16 (0.54–2.51) | 0.70 |
| DAPT | 1.50 (0.68–3.34) | 0.32 |
| D-dimer (log transformed) | 0.62 (0.20–1.94) | 0.41 |
| hsCRP (log transformed) | 1.00 (0.58–1.71) | 0.99 |
| Non-HDL | 1.00 (0.99–1.01) | 0.53 |
| TG | 1.00 (1.00–1.01) | 0.048∗ |
| HDL | 0.97 (0.96–1.00) | 0.043∗ |
| TG/HDL-C ratio (log transformed) | 3.97 (1.25–12.61) | 0.019∗ |
Lipidomic profiling of circulating platelets and metabolic regulation of intraplatelet TG levels by phosphoACC in CAD patients
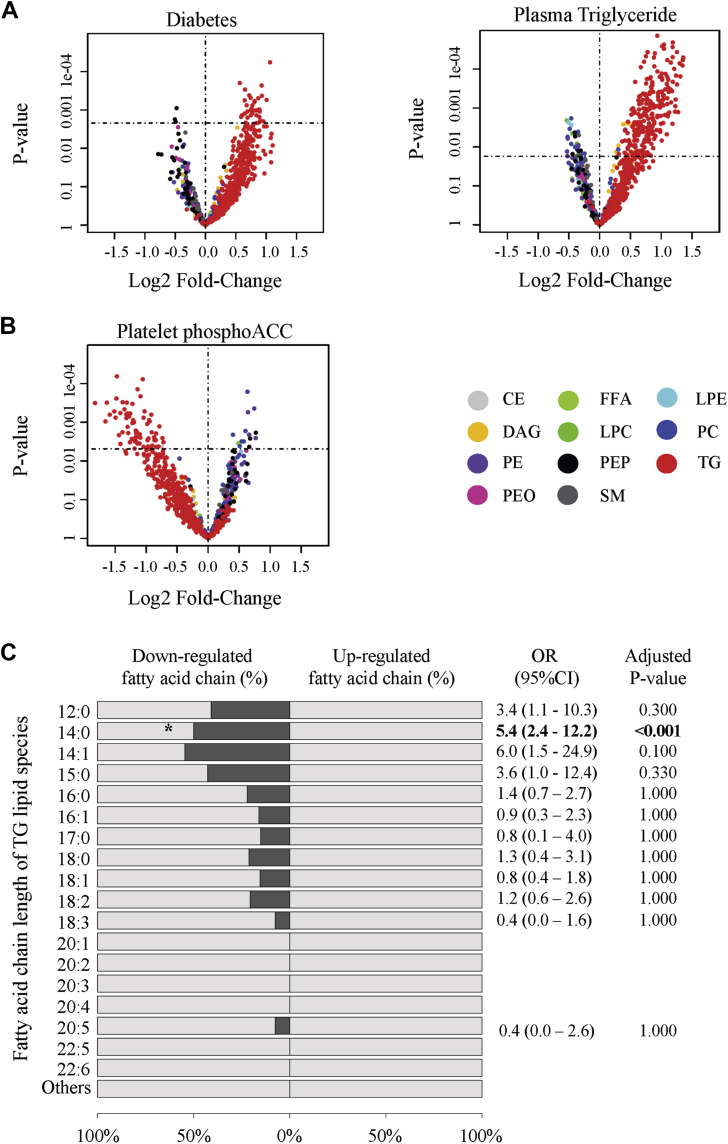
We investigated the impact of ACC phosphorylation or inhibition on platelet lipid composition through unbiased lipidomic analysis. To this end, we selected platelet lysates from patients displaying the lowest and highest phosphoACC levels. Baseline characteristics of these patients are provided in Supplemental Table 3. Multivariable regression analysis first showed that diabetes and plasma TG levels were independently associated with up-regulation of intraplatelet TG lipid species. Of the 490 TG lipid species detected within platelets, 253 (52%) were significantly up-regulated (Figure 7A) when plasma TG levels increased. Contrarily, increased phosphoACC was independently associated with significant down-regulation of 66 (14%) intraplatelet TGs (Figure 7B, Supplemental Table 4). This was confirmed by lipid class enrichment analysis revealing a significant proportion of down-regulated lipid species belonging to TG class in platelets of patients with high phosphoACC levels (OR: 27.0; 95% CI: 7.1 to 228.3; p < 0.001) (Supplemental Figure 8). Furthermore, we characterized changes in fatty-acid-chain composition of TGs. Increased platelet phosphoACC was associated with down-regulation of TGs containing 14 carbons (C14:0, myristic acid) (Figure 7C). These findings support that circulating platelets interact with the hyperlipidemic environment in atherosclerosis and, more importantly, that ACC phosphorylation or inhibition affects endogenous platelet lipid content, by regulating TG lipid species and their fatty-acid-chain composition.
Figure 7.
PhosphoACC Regulates TG Lipid Species in Platelets of CAD Patients
(A, B) Volcano plot representations of the 865 lipid species detected in platelets by lipidomic profiling. Log fold-changes and p values were calculated from the multivariate regression model. Lipids above the horizontal dotted line were up- or down-regulated with significant adjusted p values. Relationship among (A) diabetic status and plasma triglyceride (TG) levels and (B) platelet phosphoACC and intraplatelet lipid species content. (C) Class enrichment analysis of fatty-acid-chain constituents of TG lipid species in platelets with respect to increased phosphoACC. Bars (dark gray) represent the percentage of down-regulated fatty acid-containing TG. Odds ratio (OR) and adjusted p values derived from Fisher’s exact test are shown. CE = cholesterol ester; CI = confidence interval; DAG = diacylglycerol; FFA = free fatty acid; LPC = lysophosphatidylcholine; LPE = lysophosphatidylethanolamine; OR = odds ratio; PC = phosphatidylcholine; PE = phosphatidylethanolamine; PEO = plasmenyl phosphatidylethanolamine; PEP = plasmalogen phosphatidylethanolamine; SM = sphingomyelin; other abbreviations as in Figure 3.
Discussion
This study highlights that platelet phosphoACC is a risk marker in patients with suspected CAD and provides additional evidence of an interplay between platelets and lipids in atherosclerosis. We demonstrate a clear relationship between platelet phosphoACC and CAC Agatston score severity, and acute coronary events in CAD. In a molecular point of view, oxLDL participates in increasing phosphoACC in circulating platelets via a CD36-dependent pathway. ACC phosphorylation/inhibition impacts endogenous platelet lipid synthesis by regulating TG lipid species.
Platelet phosphoACC levels and risk assessment
Despite the LDL cholesterol–lowering drugs and platelet inhibitors currently available, substantial residual risk remains (18), and many patients should be given more aggressive treatment 3, 19. These patients must be identified by measurable risk markers. Our study brings platelet phosphoACC to the fore as a marker for identifying high-risk patients. More importantly, higher phosphoACC states were detected in platelets of ACS patients, even if treated with aspirin, P2Y12 inhibitors, or statins. The long-term influence of treatments on platelet phosphoACC remains to be determined in this high-risk population.
Numerous studies have reported that high on-treatment platelet reactivity and platelet activation indices reveal high-risk patients in CAD population (20). Particularly, increased circulating activated platelets, evidenced by monocyte- or neutrophil-platelet aggregates or P-selectin expression, can be detected in high-risk patients versus normal subjects. However, this increase is marginal and involves a small proportion of the total platelet pool 21, 22, 23. In our study, AMPK-ACC signaling activation was observed in circulating platelets. The absence of protein kinase C substrate phosphorylation, a sign of platelet activation (24), supports the theory that increased phosphoACC and platelet activation are unrelated. Even if thrombin is the principal agonist of platelet AMPK activation ex vivo (4), we found no association between ThG markers and phosphoACC. Therefore, platelet phosphoACC is not caused by thrombin-induced platelet activation in patients. Thus, our findings suggest that platelets of high-risk CAD patients exhibit altered metabolic phenotypes characterized by activation of AMPK-ACC signaling, independent of platelet activation state.
Lipid-platelet interaction
Changes in LDL phenotypes, such as tendency to aggregate, oxidative status, or size and density, influence cardiovascular risk and can determine residual post-treatment risk 25, 26. In line with previous studies (10), our work further supports that this atherogenic environment influences platelet phenotype in CAD. A novel finding of our study is the significant positive correlation between platelet phosphoACC and TG/HDL-C ratio, a marker of a proatherogenic lipid profile, including small LDL particles known to be more oxidizable 16, 17. Based on our data, oxLDL mediates, at least in part, the phosphoACC increase in CAD patients. PhosphoACC in response to oxLDL occurs downstream of CD36. Numerous in vitro studies suggested that oxLDL binds to platelet CD36 promoting intracellular signals, including the recruitment of Src family kinases Fyn and Lyn (27), cytoskeleton rearrangement (7), and nicotinamide adenine dinucleotide phosphate oxidase activation (28). Interestingly, an increased binding of oxLDL on platelets has been reported in patients with ACS, in line with our observation (29). Additionally, CD36 is an established receptor for platelet interaction with atherogenic environments, and CD36 expression variability may determine platelet reactivity and thrombotic risk 5, 6.
ACC and platelet lipid content
Given the central role of ACC in lipid biosynthesis, we performed lipidomic profiling to investigate the impact of its phosphorylation/inhibition on lipid content. Plasma TG levels were associated with an up-regulation of TG lipid species in platelets of CAD patients, highlighting the strong interaction between platelets and plasma lipids. Our results corroborate recent data revealing platelet lipid content to be altered in ACS patients (10). We further demonstrated that increased platelet phosphoACC in high-risk CAD patients is associated with platelet TG down-regulation, reflecting the inhibitory impact of phosphoACC on platelet lipid synthesis. Similar findings were reported in hepatocytes, where liver-specific pharmacological inhibition of ACC lowered lipogenesis and reduced liver TG (30). The down-regulation of fatty acid chains containing 14 carbons (C14:0, myristic acid) provides further evidence that endogenous platelet lipid synthesis is altered upon ACC phosphorylation. In line with our data, ACC inhibition in cancer cells decreases de novo fatty acid synthesis, leading to a reduction in fatty acids containing 14 to 18 carbons (31). To our knowledge, this is the first study to report the impact of ACC phosphorylation/inhibition on the regulation of lipid content in circulating platelets of CAD patients.
Study limitations
First, phosphoACC was determined using immunoblotting in whole platelet lysates. Given the circulating platelets’ heterogeneity (32), it is still unclear whether increased phosphoACC occurs in the entire platelet population or in a subgroup only. Moreover, in this setting, platelets must be purified before phosphoACC is measured, and quantitative assessment of phosphoACC remains challenging. However, we employed a more quantitative method, electrochemiluminescence immunoassay, to confirm the phosphoACC increase. Second, the ACCTHEROMA trial was a single-center trial, with a limited patient number. This sample size allowed us to fully address our primary endpoint (i.e., increase in phosphoACC in CAD patients). However, despite this limited number of patients, we identified phosphoACC as an independent predictor for adverse cardiac events and more particularly ischemic events, during follow-up, reinforcing the link between phosphoACC and disease severity. Multicenter studies may be required to validate the prognostic impact of increased phosphoACC in peripheral blood and determine signal variability over time, as with LDL cholesterol (25).
Conclusions
Our study identifies platelet phosphoACC as a risk stratification marker that reflects the interaction between proatherogenic lipids and circulating platelets. In CAD patients, phosphoACC contributed to regulating endogenous lipid synthesis. We recently provided new insights into AMPK-ACC signaling’s key role in regulating platelet lipid composition and function using a genetic mouse model. The absence of phosphoACC (and inhibition) by AMPK in platelets resulted in increased phospholipid content, with enhanced platelet reactivity and thrombus formation. It is tempting to speculate that increased phosphoACC is a counter-regulatory mechanism limiting lipogenesis and platelet reactivity in CAD patients within a proatherogenic environment.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The circulating platelets of high-risk CAD patients exhibit altered metabolic phenotypes characterized by phosphorylation and inhibition of ACC, the first committed enzyme of the fatty acid biosynthesis pathway. Platelet phosphoACC is thus a potential marker for risk stratification in suspected CAD patients, indicating an interplay between platelets and atherogenic lipids.
TRANSLATIONAL OUTLOOK 1: Additional studies are needed to further define the role of endogenous lipogenesis in the control of platelet function.
TRANSLATIONAL OUTLOOK 2: Large multicenter studies must investigate whether individual platelet phenotypes, including phosphoACC, influences major adverse outcomes in CAD.
Acknowledgments
The authors wish to thank professors Patrick Chenu, Olivier Gurné, and Jean Renkin for their support in collecting blood samples upon coronary angiography.
Footnotes
This work was supported by grants from the Fonds National de la Recherche Scientifique et Médicale (FNRS) (Belgium) and Louvain Foundation (Louvain-La-Neuve, Belgium), and by unrestricted grants from Bayer and AstraZeneca (Belgium). The Division of Cardiology at Cliniques universitaires Saint-Luc, Belgium, has received unrestricted research grants from AstraZeneca, Bayer Healthcare, and Daiichi-Sankyo (Belgium). Drs. Kautbally and Lepropre were supported by the FNRS (Belgium). Drs. Lepropre and Onselaer were supported by grants from the Salus Sanguinis Foundation (UCLouvain, Belgium). Drs. Octave and Wéra have FRIA fellowships from the FNRS (Belgium). Dr. Horman is a research associate, and Drs. Bertrand and Oury are senior research associates at the FNRS (Belgium). Dr. Pouleur is, and Dr. Beauloye was, a clinical master specialist at the FNRS (Belgium). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Mastenbroek T.G., van Geffen J.P., Heemskerk J.W., Cosemans J.M. Acute and persistent platelet and coagulant activities in atherothrombosis. J Thromb Haemost. 2015;13 Suppl 1:S272–S280. doi: 10.1111/jth.12972. [DOI] [PubMed] [Google Scholar]
- 2.Merlini P.A., Bauer K.A., Oltrona L. Persistent activation of coagulation mechanism in unstable angina and myocardial infarction. Circulation. 1994;90:61–68. doi: 10.1161/01.cir.90.1.61. [DOI] [PubMed] [Google Scholar]
- 3.Eikelboom J.W., Connolly S.J., Bosch J. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 4.Onselaer M.B., Oury C., Hunter R.W. The Ca(2+) /calmodulin-dependent kinase kinase beta-AMP-activated protein kinase-alpha1 pathway regulates phosphorylation of cytoskeletal targets in thrombin-stimulated human platelets. J Thromb Haemost. 2014;12:973–986. doi: 10.1111/jth.12568. [DOI] [PubMed] [Google Scholar]
- 5.Podrez E.A., Byzova T.V., Febbraio M. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh A., Murugesan G., Chen K. Platelet CD36 surface expression levels affect functional responses to oxidized LDL and are associated with inheritance of specific genetic polymorphisms. Blood. 2011;117:6355–6366. doi: 10.1182/blood-2011-02-338582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wraith K.S., Magwenzi S., Aburima A., Wen Y., Leake D., Naseem K.M. Oxidized low-density lipoproteins induce rapid platelet activation and shape change through tyrosine kinase and Rho kinase-signaling pathways. Blood. 2013;122:580–589. doi: 10.1182/blood-2013-04-491688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyck J.R., Kudo N., Barr A.J., Davies S.P., Hardie D.G., Lopaschuk G.D. Phosphorylation control of cardiac acetyl-CoA carboxylase by cAMP-dependent protein kinase and 5'-AMP activated protein kinase. Eur J Biochem. 1999;262:184–190. doi: 10.1046/j.1432-1327.1999.00371.x. [DOI] [PubMed] [Google Scholar]
- 9.Lepropre S., Kautbally S., Octave M. AMPK-ACC signaling modulates platelet phospholipids content and potentiates platelet function and thrombus formation. Blood. 2018;132:1180–1192. doi: 10.1182/blood-2018-02-831503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee M., Rath D., Schlotterbeck J. Regulation of oxidized platelet lipidome: implications for coronary artery disease. Eur Heart J. 2017;38:1993–2005. doi: 10.1093/eurheartj/ehx146. [DOI] [PubMed] [Google Scholar]
- 11.Greenland P., Blaha M.J., Budoff M.J., Erbel R., Watson K.E. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72:434–447. doi: 10.1016/j.jacc.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stacklies W., Redestig H., Scholz M., Walther D., Selbig J. pcaMethods--a bioconductor package providing PCA methods for incomplete data. Bioinformatics. 2007;23:1164–1167. doi: 10.1093/bioinformatics/btm069. [DOI] [PubMed] [Google Scholar]
- 13.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 14.Beaulieu L.M., Lin E., Mick E. Interleukin 1 receptor 1 and interleukin 1beta regulate megakaryocyte maturation, platelet activation, and transcript profile during inflammation in mice and humans. Arterioscler Thromb Vasc Biol. 2014;34:552–564. doi: 10.1161/ATVBAHA.113.302700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowley J.W., Oler A.J., Tolley N.D. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood. 2011;118:e101–e111. doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanak V., Munoz J., Teague J., Stanley A., Jr., Bittner V. Accuracy of the triglyceride to high-density lipoprotein cholesterol ratio for prediction of the low-density lipoprotein phenotype B. Am J Cardiol. 2004;94:219–222. doi: 10.1016/j.amjcard.2004.03.069. [DOI] [PubMed] [Google Scholar]
- 17.Harmon M.E., Campen M.J., Miller C. Associations of circulating oxidized LDL and conventional biomarkers of cardiovascular disease in a cross-sectional study of the Navajo population. PLoS One. 2016;11 doi: 10.1371/journal.pone.0143102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaasenbrood L., Boekholdt S.M., van der Graaf Y. Distribution of estimated 10-year risk of recurrent vascular events and residual risk in a secondary prevention population. Circulation. 2016;134:1419–1429. doi: 10.1161/CIRCULATIONAHA.116.021314. [DOI] [PubMed] [Google Scholar]
- 19.Sabatine M.S., Giugliano R.P., Keech A.C. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 20.Gurbel P.A., Becker R.C., Mann K.G., Steinhubl S.R., Michelson A.D. Platelet function monitoring in patients with coronary artery disease. J Am Coll Cardiol. 2007;50:1822–1834. doi: 10.1016/j.jacc.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 21.Michelson A.D., Barnard M.R., Krueger L.A., Valeri C.R., Furman M.I. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation. 2001;104:1533–1537. doi: 10.1161/hc3801.095588. [DOI] [PubMed] [Google Scholar]
- 22.Linden M.D., Furman M.I., Frelinger A.L., 3rd Indices of platelet activation and the stability of coronary artery disease. J Thromb Haemost. 2007;5:761–765. doi: 10.1111/j.1538-7836.2007.02462.x. [DOI] [PubMed] [Google Scholar]
- 23.Stellos K., Bigalke B., Stakos D., Henkelmann N., Gawaz M. Platelet-bound P-selectin expression in patients with coronary artery disease: impact on clinical presentation and myocardial necrosis, and effect of diabetes mellitus and anti-platelet medication. J Thromb Haemost. 2010;8:205–207. doi: 10.1111/j.1538-7836.2009.03659.x. [DOI] [PubMed] [Google Scholar]
- 24.Konopatskaya O., Gilio K., Harper M.T. PKCalpha regulates platelet granule secretion and thrombus formation in mice. J Clin Invest. 2009;119:399–407. doi: 10.1172/JCI34665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruuth M., Nguyen S.D., Vihervaara T. Susceptibility of low-density lipoprotein particles to aggregate depends on particle lipidome, is modifiable, and associates with future cardiovascular deaths. Eur Heart J. 2018;39:2562–2573. doi: 10.1093/eurheartj/ehy319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laufs U., Weingartner O. Pathological phenotypes of LDL particles. Eur Heart J. 2018;39:2574–2576. doi: 10.1093/eurheartj/ehy387. [DOI] [PubMed] [Google Scholar]
- 27.Chen K., Febbraio M., Li W., Silverstein R.L. A specific CD36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circ Res. 2008;102:1512–1519. doi: 10.1161/CIRCRESAHA.108.172064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magwenzi S., Woodward C., Wraith K.S. Oxidized LDL activates blood platelets through CD36/NOX2-mediated inhibition of the cGMP/protein kinase G signaling cascade. Blood. 2015;125:2693–2703. doi: 10.1182/blood-2014-05-574491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stellos K., Sauter R., Fahrleitner M. Binding of oxidized low-density lipoprotein on circulating platelets is increased in patients with acute coronary syndromes and induces platelet adhesion to vascular wall in vivo--brief report. Arterioscler Thromb Vasc Biol. 2012;32:2017–2020. doi: 10.1161/ATVBAHA.111.244707. [DOI] [PubMed] [Google Scholar]
- 30.Kim C.W., Addy C., Kusunoki J. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab. 2017;26:394–406 e6. doi: 10.1016/j.cmet.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Svensson R.U., Parker S.J., Eichner L.J. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat Med. 2016;22:1108–1119. doi: 10.1038/nm.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibrahim H., Nadipalli S., Usmani S., DeLao T., Green L., Kleiman N.S. Detection and quantification of circulating immature platelets: agreement between flow cytometric and automated detection. J Thromb Thrombolysis. 2016;42:77–83. doi: 10.1007/s11239-016-1338-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.