Abstract
Lung cancer is the leading cause of cancer death in both sexes worldwide and has a predicted 5-year survival rate of <20%. Immunotherapy targeting immune checkpoints such as the programmed death 1 (PD-1) signaling pathway has led to a shift of paradigm in the treatment of advanced non–small-cell lung cancer (NSCLC) but remains without effect in ∼80% of patients. Accumulating evidence suggests that several immunosuppressive mechanisms may work together in NSCLC. The contribution and cooperation between different immunosuppressive mechanisms in NSCLC remain unknown. Recently, the CD39-adenosine pathway has gained increasing attention as a crucial immunosuppressive mechanism and possible target for immunotherapy. Immune cells were extracted from lung and tumor tissue after lung resection in 12 patients by combined enzymatic and mechanical tissue disaggregation. A multiparameter flow cytometry panel was established to investigate the expression and coexpression of CD39 and PD-1 on key lymphocyte subtypes. Frequencies of CD39+, PD-1+, and CD39+/PD-1+cells were higher among both CD4+ and CD8+ T cells isolated from NSCLC tumor tissue than in T cells from normal lung tissue. Similarly, the frequency of FoxP3+ CD4+ T cells (Tregs) was highly significantly elevated in tumor tissue compared to adjacent lung tissue. The consistent upregulation of CD39 on immune cells in tumor microenvironment indicates that the CD39 signaling pathway may, in addition to the PD-1 pathway, represent another important mechanism for tumor-induced immunosuppression in NSCLC. In addition, the present study indicates that a comprehensive immune response profiling with flow cytometry may be both feasible and clinically relevant.
Introduction
Lung cancer is the second most common cancer in both men and women, and the leading cause of cancer death in both sexes, accounting for more than 1 million deaths worldwide in 2012 [1]. Non–small-cell lung carcinoma (NSCLC) accounts for >85% of cases and has a predicted 5-year survival rate of <20% [2].
NSCLC was considered a poorly immunogenic malignancy until 2012 [3], when the efficacy of an immune checkpoint inhibitor blocking the programmed death 1 (PD-1) signaling pathway in NSCLC was reported [4]. This unanticipated finding led to a shift of paradigm in the treatment of advanced NSCLC, and immunotherapy has become a fourth pillar in the therapeutic approach, in addition to surgery, radiation and chemotherapy [5]. Still, immunotherapy remains without effect in ∼80% of unselected patients with NSCLC, and biomarkers to guide selection of patients remain highly needed [6].
CD4+ and CD8+ T cells are effector cells of the adaptive immune system and fundamental in the antitumor immune response. Tumor-specific CD4+ T helper (Th) cells are activated by immunogenic signals from antigen-presenting cells, including dendritic cells, macrophages, and B cells in the tumor microenvironment (TME). Activated effector CD4+ T cells maintain and bolster the adaptive antitumor immune response by interaction with antigen-specific cytotoxic CD8+ T cells [5]. CD4+FoxP3+ regulatory T cells (Treg) suppress antigen-specific effector T cell responses via several direct and indirect mechanisms and play a pivotal role in cancer immunosuppression [7]. In addition, activation of adaptive immune cells can be regulated by a variety of inhibitory signaling molecules expressed on various immune cells. These regulatory circuits are considered immune checkpoint pathways and primarily contribute to maintenance of self-tolerance and regulation of immune responses and are particularly important in preventing organ damage during chronic infections such as HIV and hepatitis C virus (HCV). However, they can also be "hijacked" or exaggerated by tumors leading to evasion of the adaptive antitumor immune response [8,9]. Various tumor immune escape mechanisms are mediated by immune cells that have been polarized in the TME towards immunosuppressive instead of proinflammatory properties [10].
The PD-1 signaling pathway constitutes a major immunosuppressive mechanism in the TME. PD-1 expression is a marker of reversible T-cell exhaustion, and PD-1 may be upregulated on tumor-infiltrating T cells because of persistent antigenic exposure in the TME [[11], [12], [13]], making T cells ineffective in controlling tumor cell expansion. Therapies targeting PD-1 and its ligand PD-L1 may represent a game changer in treatment of advanced NSCLC [14]. PD-L1 expression in lung cancer tissues has been measured by immunohistochemistry (IHC) in clinical trials, but the use of PD-L1 as a predictive biomarker has several limitations and remains controversial [[15], [16], [17]]. In addition, standardization of available PD-L1 IHC tests is currently lacking [18].
Extracellular adenosine triphosphate (ATP) released from dead, decaying, or stressed cells is one of the major biochemical constituents of the TME and was recently discovered to play a role in generating tumor immunosuppression [19]. The ectonucleotidases CD39 and CD73 are expressed on immune cells as well as on stromal cells and degrade extracellular ATP via adenosine monophosphate to adenosine, CD39 being the rate-limiting enzyme in the cascade [20]. In a study from 2006, adenosine was shown to suppress T-cell proliferation and effector functions by stimulating the A2A receptor on T cells, and the adenosine pathway was proposed as a target for cancer immunotherapy [21]. More recently, extracellular adenosine was recognized as one of the most potent immunosuppressive factors in the TME [19,22], and this pathway has emerged as a one of the key metabolic pathways that regulate the antitumor immune response in various types of cancers [9,23]. So far little is known of the expression levels of CD39 on intratumoral T cells in NSCLC [24].
Several immunosuppressive mechanisms may work together in the TME [19], and targeting one immune checkpoint may not be sufficient to relieve tumor-induced immunosuppression. There are several clinical trials ongoing that use a combination of different checkpoint inhibitors in immunotherapy treatment of NSCLC [25]. However, the cooperation between different immunosuppressive mechanisms in the TME in NSCLC remains largely unknown.
In this study, we investigated the expression of CD39 and PD-1 in key immune cell subtypes in the TME of patients with NSCLC. The objective was to provide an immune cell profile in the tumor after lung resection in patients. Using a method of combined mechanical and enzymatic tissue disintegration, we isolated immune cells from NSCLC tumors and healthy tissue, phenotyped immune cell subsets, and investigated checkpoint marker expression. Our results expand the knowledge of the immunosuppressive mechanisms induced in the NSCLC TME and might also contribute to optimizing and personalizing immunotherapy in patients with NSCLC, with the aim to select patients that will benefit from immunotherapy.
Materials and Methods
Study Population
Twelve patients with NSCLC who underwent thoracic surgery with curative intention between February 2017 and March 2018 were included in the study. Inclusion criteria were subsolid or solid tumors with a minimum size of 20 mm in diameter as estimated by thoracic computed tomographic scan. The tumors were histologically classified and subtyped according to the 2015 World Health Organization classification of lung tumors [26]. None of the patients had a previous lung cancer diagnosis, and all were treatment naïve. Patient characteristics were as follows: sex (male/female, 8/4); median age 74 years (range, 56–83); five patients had squamous cell carcinomas (SCCs), and seven patients had adenocarcinomas. Written informed consent was obtained from all subjects, and the study was approved by the regional ethics committee (Ref.nr.: 2010/1939).
For characteristics of the study population, see Table 1.
Table 1.
Basic Characteristics of the Study Population
| Patient | Age | Smoker | Tumour | Subtype | PDL1 | pTNM | Stadium | Relapse |
|---|---|---|---|---|---|---|---|---|
| 1 | 71–75 | Former | AC | Acinary | 0 | pT2bN1M0 | IIB | No |
| 2 | 76–80 | Former | AC | Invasive mucinous | 0 | pTT2aN0M0 | IB | Yes |
| 3 | 71–75 | Former | AC | Micropapillary | 1 | pT1cN0M0 | IA3 | No |
| 4 | 71–75 | Current | SCC | Keratinizing SCC | 15 | pT3pN0M0 | IIB | No |
| 5 | 71–75 | Current | AC | Acinary | 30 | pT1cN0M1a | IVA | Yes |
| 6 | 66–70 | Never | AC | Acinary | 0 | pT3N1M0 | IIIA | Yes |
| 7 | 81–85 | Former | SCC | Nonkeratinizing SCC | 0 | pT2aN1M0 | IIB | Yes |
| 8 | 71–75 | Former | SCC | Nonkeratinizing SCC | 0 | pT3N0M0 | IIB | No |
| 9 | 81–85 | Former | SCC | Keratinizing SCC | 0 | pT2aN0M0 | IB | No |
| 10 | 71–75 | Former | AC | Micropapillary | 0 | pT3N2M0 | IIIB | No |
| 11 | 76–80 | Former | SCC | Keratinizing SCC | 0 | pT2bN0M0 | IIA | Yes |
| 12 | 56–60 | Current | AC | Cribriform | 0 | pT2aN2M0 | IIIA | No |
F: female; M: male; AC: adenocarcinoma; SCC: squamous cell carcinoma; pTNM and Stadium: histopathologic TNM Classification of Malignant Tumours (8th edition); Relapse: After a minimum of 12 months of observation after surgery.
Isolation of Mononuclear Cells From Tumor and Lung Tissue
Video-assisted thoracic surgery was performed in eight and open thoracic surgery in four patients with NSCLC. After surgery, the lung lobe was immediately placed on ice and transported to the laboratory for processing. The pathologist obtained tissue samples, typically 1–3 cm3, from the tumor tissue and from macroscopically normal lung tissue ≥3 cm from tumor within 30 min of the lobectomy. Immune cells were extracted from tissues by optimized combined enzymatic and mechanical disaggregation, as described by Quatromoni et al. [27]. In brief, tumor and lung tissue samples were rinsed with serum-free HyClone DMEM/F12 media (Thermo Fisher Scientific, Waltham, MA, US) and sliced into 1–2 mm3 pieces by sterile microdissection. The tissue fragments were then incubated at 37 °C on an orbital shaker with a speed of 85 rpm for 45 min, pipetted for 5–10 min, and then shaken for another 30–50 min in HyClone Leibovitz L-15 media (Thermo Fisher Scientific) supplemented with the enzymes collagenases I (170 mg/L), II (56 mg/L), IV (170 mg/L), DNase I (25 mg/L), and elastase (25 mg/L) (all Worthington Biochemical, Lakewood, NJ, US) as described [27]. The resulting cell suspension was filtered through a 70-μm cell strainer (BD Biosciences, San Jose, CA, US) and centrifuged for 5 min at 300 g. The cell pellet was washed in calcium-free phosphate-buffered saline and resuspended in cell culture medium (HyClone DMEM/F12 supplemented with 10% fetal calf serum (FCS)). Mononuclear cells were isolated by density gradient centrifugation at 600 g for 30 min without brakes at room temperature (Lymphoprep, STEMCELL technologies, Cambridge, UK). Contaminating red blood cells were lysed in 1 × BD Pharm Lyse (BD Biosciences). Cells were washed twice and resuspended in cell culture medium for further analysis.
Mitogen Stimulation of Mononuclear Cells
Mononuclear cells isolated from the tumors and from macroscopically normal tissue were incubated at 37 °C and 5% CO2 for 4 hours in Roswell Park Memorial Institute medium (Sigma-Aldrich Corp., St. Louis, US) supplemented with 10% fetal calf serum (Thermo Fisher Scientific) in the presence or absence of phorbol-12-myristate-13-acetate (Sigma-Aldrich) at 50 ng/ml and ionomycin 1 μg/ml, in the presence of Protein Transport Inhibitor Cocktail (500X) (eBioscience, Thermo Fisher Scientific).
Antibody Staining and Flow Cytometry
For surface antigen staining, the mononuclear cells were incubated for 15 min with fluorescence-labelled monoclonal antibodies: BV421-CD206, BV421-PD-1, BV510-CD45, BV605-CD8, BV711-CD25, BV711-CD19, BV785-CD3, BV785-CD274, FITC-CD4, PerCP-Cy5.5-CD8, PerCP-Cy5.5-HLA-DR (all from BD Biosciences), PE-CF594-CD4, PE-CF594-CD366 (BioLegend, San Diego, CA, US), PE-Cy7-CD39 (eBioscience), AF647-PD-1, AF700-CD16 (BD Biosciences), and eFluor780-Fixable Viability dye (eBioscience). Intracellular staining with BV605-IFN-γ (BD Biosciences), AF488-IL17A, and AF647-FoxP3 (BD Biosciences) was performed after fixation and permeabilization with the BD FoxP3 buffer set (BD Biosciences). For interferon gamma (IFN-γ) and interleukin (IL)-17, unstimulated cells were used as negative controls for gating. For PD-1, a separate tube without anti-PD-1 staining was used (Fluorescence Minus One) as control for gating.
A minimum of 100 000 and 200 000 cells for surface and intracellular antigens, respectively, were acquired on a BD LSR II flow cytometer (BD Biosciences). Gating and visualization were performed in R Bioconductor (packages: flowCore, openCyto, flowWorkspace, and ggcyto). Lymphocytes were identified as viable CD45+ Side Scatter (SSc)low and Forward Scatter (FSc)low. T cells were identified as CD3+ lymphocytes. CD4+ Th and CD8+ T cell subsets were identified from CD3+ T cells and analysed for expression of further markers or cytokine production. B cells were identified as CD19+ CD3- lymphocytes. We also identified the CD16+ subset of natural killer (NK) cells as CD3−CD19−-CD16+ lymphocytes, which is reported to be the dominating NK cell subset in peripheral blood and normal lung tissue [28]. CD56 was not part of our flow cytometry panel, thus CD16−CD56+ NK cells were not identified in our experimental setup. Macrophages were identified as viable CD45+ SSchigh cells with high autofluorescence and displayed high expression of CD206. In normal human lung, CD206 is ubiquitously expressed in macrophage populations [29,30].
Statistical Methods
Group comparison for continuous and categorical data was performed by Wilcoxon signed-rank sum test or Mann-Whitney U Test and Pearson chi-squared test, respectively. Spearman's rank correlation test was used to determine correlation. Statistical analyses were performed in R: A Language and Environment for Statistical computing (R Core Team, R Foundation for Statistical Computing, Vienna, Austria). A P value of <0.05 was considered to be statistically significant.
Results
Tumor-infiltrating T Cells in NSCLC Display Augmented Expression and Coexpression of PD-1 and CD39
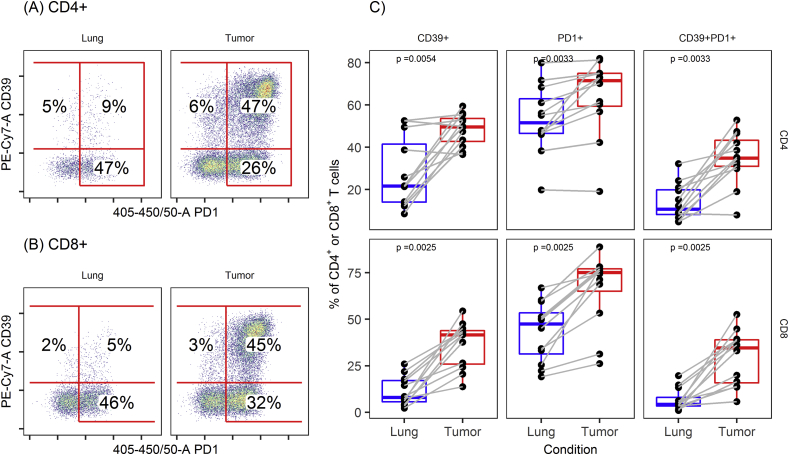
PD-1 was highly expressed on CD4+ and CD8+ T cells from both tumor and macroscopically normal lung tissue from the same patients, although significantly higher in tumor T cells [median (IQR): 71.5% (59.5–75.0) versus 51.6% (46.6–63.0) on CD4+ T cells, P < 0.01 and 75.2% (65.0–77.1) versus 47.5% (31.3–53.4) on CD8+ T cells, P < 0.01, respectively].
The fractions of CD4+ and CD8+ T cells expressing CD39 were also significantly higher in tumor than in lung tissue [median (IQR): 50.0% (42.7–53.6) versus 21.7% (14.1–41.5) on CD4+, P < 0.01 and 41.7% (25.9–43.9) versus 8.0% (5.6–17.1) on CD8+, P < 0.01, respectively].
Frequencies of CD39+PD-1+ double positive CD4+ and CD8+ T cells were also consistently and highly significantly higher in T cells isolated from NSCLC tumor tissue than those from normal lung tissue [median (IQR): 34.9% (31.0–43.3) versus 10.7% (8.2–19.9) on CD4, P < 0.01 and 34.6% (15.9–39.0) versus 4.3% (3.4–8.0) for CD8, P < 0.01, respectively].
Flow cytometry dot plots displaying CD39 and PD-1 expression on CD4+ (a) and CD8+ (b) T cells from a representative tumor and lung tissue as well as data quantification from all patients (c) are shown in Figure 1.
Figure 1.
CD39 and PD-1 on CD4+and CD8+T cells in NSCLC. A representative example of flow cytometry data. Expression and coexpression of PD-1 and CD39 were significantly higher in (A) CD4+ and (B) CD8+ T cells from tumor tissue than from adjacent normal lung. (C) Boxplots showing expression and coexpression of CD39 and PD-1 on CD4+ and CD8+ T cells in paired samples from tumor tissue and in adjacent normal lung (Wilcoxon signed-rank sum test).
Tumor-infiltrating FoxP3+ CD4+ T Regulatory Cells Express Both CD39 and PD-1
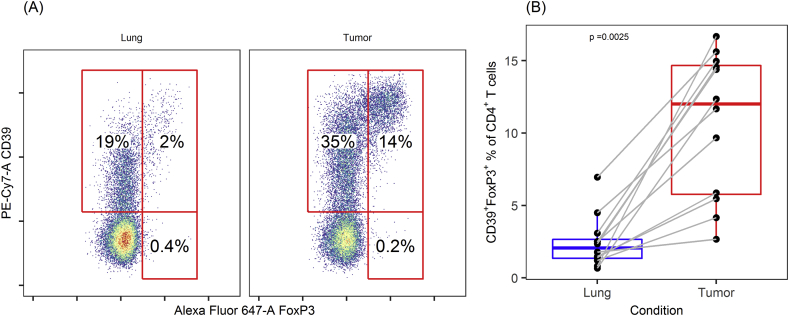
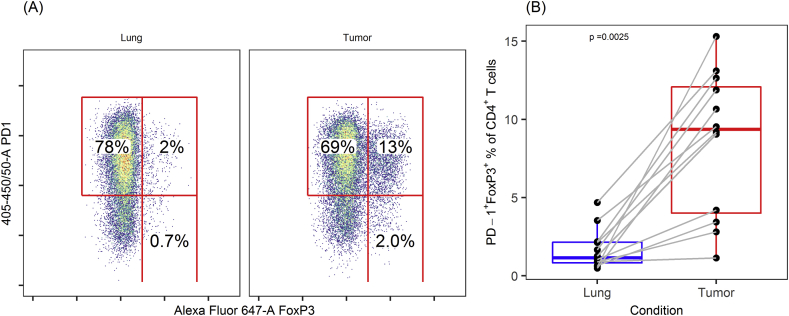
The FoxP3+ fraction of CD4+ T cells (Treg) was highly significantly elevated in tumor compared with cells from adjacent lung tissue [median (IQR): 12.8% (6.1–15.8) versus 2.3% (1.6–3.6), P < 0.01]. The vast majority of Tregs in both tumor and lung expressed CD39 (Figure 2), although the fraction of Tregs expressing CD39 was significantly higher in tumor than in such cells from adjacent lung tissue [median (IQR): 94.2% (92.0–97.3) versus 85.4% (73.1–90.5), P < 0.01]. PD-1 was commonly expressed by Tregs in both groups, but PD-1+ fractions were significantly larger in tumor Tregs [median (IQR): 74.4% (60.8–82.0) versus 53.2% (43.4–66.0), P < 0.01] (see Supplementary Figure 1).
Figure 2.
CD39 and FoxP3 expression in CD4+T cells in NSCLC. Flow cytometry data from (A) a representative patient and (B) all samples, showing increased fraction of CD39+ FoxP3+ in CD4+ T cells from tumor compared with adjacent normal lung tissue. Numbers displayed are CD39+ FoxP3+ fractions of CD4+ T cells.
Immune Cell Composition in NSCLC Tumor and Normal Lung Tissue
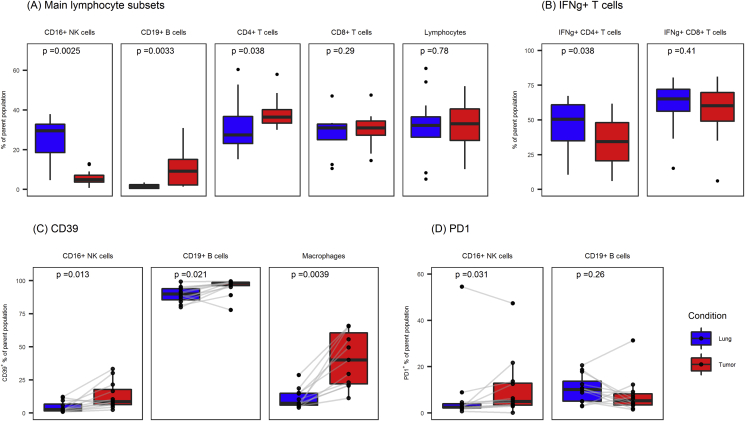
Main lymphocyte populations from lung and tumor tissue are displayed in Figure 3A. There were higher frequencies of CD19+ B cells among lymphocytes isolated from tumor than in lymphocytes from lung tissue [median (IQR): 9.2% (2.2–15.1) versus 1.3% (0.5–2.4), P < 0.01], whereas CD16+ NK cells were fewer in tumor tissue [median (IQR): 4.9% (3.6–7.1) versus 29.5% (18.5–32.8), P < 0.01]. CD4+ Th1 cells were defined as the IFN-γ+-producing CD4+ T cells after in vitro mitogen stimulation. CD4+ Th1 cell frequencies were lower in T cells isolated from tumor than in T cells isolated from lung tissue [median (IQR): 34.4% (20.5–48.1) versus 50.6% (35.0–60.8), P < 0.05], while fractions of CD8+ T cells producing IFN-γ were similar in tumor and lung (median: 60.2% versus 65.1%, P = 0.41) (Figure 3B).
Figure 3.
Fractions of main lymphocyte subsets and expression of CD39 and PD1 in B cells and NK cells in lymphocytes from lung and tumor tissue. (A) Main lymphocyte subsets as fractions of parent population (Lymphocytes are given as fractions of viable leucocytes; lymphocyte subsets are given as fractions of lymphocytes). (B) Fractions of CD4+ T cells expressing IFN-γ was lower in TILs compared with cells from lung tissue. (C) Expression of CD39 and (D) PD1 in CD16+ NK cells and CD19+ B cells from lung and tumor tissue. CD39 was consistently higher in TILs in CD16+ NK cells, B cells, and macrophages. Wilcoxon signed-rank sum test was used to calculate P-values. TILs: Tumor-infiltrating lymphocytes.
Tumor-associated Macrophages, CD19+ B Cells, and CD16+ NK Cells in TME Express CD39
We further determined the frequencies of CD39+ cells on other tumor-infiltrating immune cell subsets (Figure 3C). CD39 expression on CD206+ macrophages, CD19+ B cells, and CD16+ NK cells were analyzed in immune cells isolated from tumor or normal lung tissue. Similar to other tumor-infiltrating immune cell subsets, tumor-associated macrophages (TAM) showed more than three-fold higher fractions of CD39+ cells than lung macrophages [median (IQR): 40.1% (22.0–60.6) versus 7.4% (5.9–14.9), P < 0.01]. The mean fluorescence intensity of macrophages for CD39 was also significantly higher in tumor (P < 0.01).
Expression of CD39 was higher in both B cells [median (IQR): 98.2% (96.2–98.8) in tumor versus 90.0% (85.5–93.9) in lung, P < 0.05] and CD16+ NK cells in tumor than in such cells in lung tissue [median (IQR): 8.6% (6.3–17.7) in tumor versus 2.6% (1.5–6.7) in lung, P < 0.05] (Figure 3C).
The Relationship Between TME Phenotyping Results and Clinical Patient Data
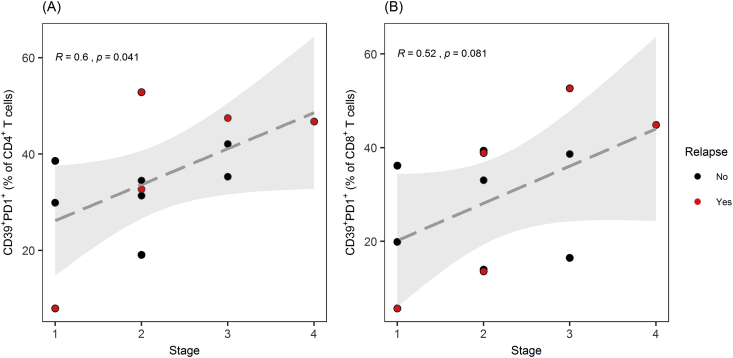
All patients were observed for 12 months after surgery and categorized according to documented recurrence of lung cancer. We then investigated if frequency of CD39, PD-1, or CD39/PD-1 coexpression on tumour-infiltrating lymphocytes (TILs) from these patients correlated to stage or recurrence of lung cancer (Figure 4). Three out of the 5 patients who relapsed had high frequencies (∼40% or more) of double-positive CD39+PD1+ cells among both the CD8+ and CD4+ TILs. From one of the relapsed patients, we obtained only a very small number of TILs due to extensive cell death in one of the relapsed patients.
Figure 4.
TILs expressing CD39 and PD-1 and relapse of NSCLC. (A) Fractions of tumor-infiltrating CD4+ T cells coexpressing CD39 and PD1 correlated significantly with clinical stage in patients with NSCLC. A trend towards correlation was found in fractions of tumor-infiltrating CD8+ T cells coexpressing CD39 and PD1. (B) Spearman's rank correlation test was used to determine correlation. TILs: Tumor-infiltrating lymphocytes. Patients with relapse of NSCLC within the first 12 months after surgery are displayed in red points.
Discussion
This study provides novel information about coexpression of the ectonucleotidase CD39 and the checkpoint molecule PD-1 on tumor-infiltrating immune cells in NSCLC, indicating that adenosine and PD1 signaling pathways for immunosuppression and tumor immune-escape may be acting simultaneously. Expression of these key immunosuppression markers was found to be much higher in both CD4+ and CD8+ T cells from tumor than in such cells from adjacent normal lung tissue. CD39 was also upregulated on a variety of other immune cells, including CD16+ NK cells, B cells, and macrophages. Furthermore, CD39+ FoxP3+ regulatory T cells were highly enriched in the TME. The consistent upregulation of CD39 on immune cells in TME indicate that the CD39 signaling pathway may, in addition to the PD-1 pathway, represent another important mechanism for tumor-induced immunosuppression in NSCLC.
Few reports exist on expression of CD39 on TILs in NSCLC [[31], [32], [33]]. O'Brien et al. performed a thorough analysis of TIL function in NSCLC and reported increased expression of CD39 in CD8+ TILs compared with lung tissue-resident lymphocytes [32]. Interestingly, they did not find any correlation between TIL hypofunction and expression of CD39 or PD-1. Simoni et al. reported on expression of CD39 on CD8+ TILs in human lung and colorectal cancer assessed by mass cytometry [33]. They did not report fractions in normal lung tissue. Interestingly, transcriptomic profiling of CD39+ CD8+ TILs revealed enrichment in genes related to cell proliferation and exhaustion. In addition to confirming the data on CD39 expression from these studies, the present study present novel data on CD39 on CD4+ TILs and PD1/CD39 coexpression. Elevated CD39 expression has been associated with poor outcome and advanced state in gastric cancer [34], hepatocellular carcinoma [35], and chronic lymphocytic leukemia [36]. To our knowledge, there are no reports on coexpression of CD39 and PD-1 in NSCLC.
Lizotte et al. demonstrated increased fraction of PD-1+ CD4+ and CD8+ TILs in NSCLC compared to normal lung by flow cytometry [37]. In line with our results, this study reported higher fractions of CD19+ B cells (6.2 ± 1% of total live cells) and FoxP3+ CD4+ Treg cells in samples from tumor than in normal lung, while NK cells were rarer in tumor. Another recent study investigated the expression of PD-1 and PD-L1 in TILs by flow cytometry [38] and reported higher fractions of PD-1+ CD8+ T cells in tumor than in normal lung, which is consistent with our findings.
In a recent meta-analysis on tumor-infiltrating immune cells in NSCLC using IHC, high levels of NK cells, M1 macrophages, and CD8+ T cells were associated with favorable prognosis, whereas M2 macrophages and regulatory T cells predicted worse prognosis [39]. Accumulation of regulatory T cells in the TME was also associated with disease recurrence after surgery in NSCLC in another study [40]. However, few studies have investigated immune cell subsets and markers of immunosuppression in the NSCLC TME by flow cytometry.
ATP is released in high amounts to the TME from dying cells and contributes to inflammation. Adenosine on the other hand exerts profound immune suppressive effects, skewing T cells, macrophages, and Dendritic cells (DCs) to suppressive phenotypes and stabilizing immune-suppressive Tregs [9]. CD39 is expressed on a variety of tumor-infiltrating immune cells and some tumor cells [9], and can be upregulated due to hypoxia [9]. Increased expression of CD39 leads to augmented concentration of adenosine in the TME. Our finding of a trend towards higher fractions of CD39+PD-1+ CD4+ and CD8+ TILs in more advanced stage in NSCLC is coherent with reports on expression of CD39 linked to poor prognosis in several human cancers [9,22].
Increased fractions of Tregs and B cells and increased expression of CD39 on both cell subsets as found in our study are further indicative of a primarily immunosuppressive TME in NSCLC. Intriguingly, CD39 is induced in a positive feedback loop by adenosine signaling via its receptor in Tregs, resulting in strong immunoregulatory activity [9,41]. Tregs from TILs in NSCLC substantially suppressed the induction of cytotoxic T lymphocytes against autologous tumor cells in one study [42]. In several human cancers, high infiltration of Tregs has been associated with poor prognosis [39,43]. Accordingly, Tregs may represent an important target for NSCLC antitumor immunotherapy [43,44]. B cells are potent APCs and can regulate the phenotype of TAM [[45], [46], [47]], resulting in suppression of cytotoxic T cells [12,48], possibly contributing to the immunosuppression of the TME in NSCLC.
With the increasing options for immunotherapy, and possibly combinational regimes, an immune response profile in NSCLC may provide a means to target immunotherapy to the specific immunosuppressive mechanism(s) active in the individual patient and tumor.
This study has some limitations. First, the number of patients is limited, which may moderate the confidence in our results regarding the TME immune cell profile of NSCLC. Furthermore, we included patients with both adenocarcinoma and SCC, tumors that might have dissimilar patterns of antitumor immune responses. However, our results do not indicate any difference in our major findings between patients with adenocarcinoma and SCC.
In conclusion, our results suggest that further studies on the CD39 CD73 adenosine pathway should be undertaken, as it may be an important mechanism for tumor-induced immune suppression in NSCLC. The present study supports the feasibility of immune response profiling in the TME by flow cytometry. A comprehensive study on the immune response profile in NSCLC investigated in relation to recurrence in a greater number of patients is warranted. In the future, information from profiling of the TME and the antitumor immune response may be used to tailor immunotherapy in selected patients suffering from recurrent disease or possibly as adjuvant immunotherapy after surgery [49].
Author contributions statement
A.T., M.H., and A.-M.S. contributed conception and design of the study; S.G.F.W. obtained lung lobe and tumor tissue samples; A.T. and M.H. performed the laboratory work and flow cytometry; A.T. performed the statistical analysis; A.T. wrote the first draft of the manuscript; M.H. and S.G.F.W. wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding for the research reported in this manuscript
The study was funded by the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology (NTNU) (ref. 2014/23203), St. Olavs University Hospital research funding 2014 and 2016 and the Research Council of Norway through its Centres of Excellence Scheme (project number 223255/F50).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
Assistance provided by Hanne Hella, Anne Elanor Kristensen, and colleagues at the laboratory in the Department of Clinical and Molecular Medicine, Norwegian University of Science and Technology, was greatly appreciated, and the unit's expertise in flow cytometry has been pivotal to this study. The authors are very grateful for the excellent cooperation with Kjetil Sundt and the Department of Cardiothoracic Surgery at St. Olavs Hospital, for unfailingly and without delay supplying the resected lung lobes immediately after surgery.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2019.09.003.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Supplementary Figure 1.
PD-1 and FoxP3 expression in CD4+T cells in NSCLC. Flow cytometry data from (A) a representative patient and (B) all samples, showing increased fraction of PD-1+ FoxP3+ in CD4+ T cells from tumor compared to adjacent normal lung tissue. Numbers displayed are PD-1+ FoxP3+ fractions of CD4+ T cells.
References
- 1.Torre L.A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14(8):535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herzberg B., Campo M.J., Gainor J.F. Immune checkpoint inhibitors in non-small cell lung cancer. The Oncologist. 2017;22(1):81–88. doi: 10.1634/theoncologist.2016-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian S.L. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin A. Mechanisms of immune evasion and current status of checkpoint inhibitors in non-small cell lung cancer. Cancer Med. 2016;5(9):2567–2578. doi: 10.1002/cam4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grigg C., Rizvi N.A. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? J Immunother Cancer. 2016;4:48. doi: 10.1186/s40425-016-0153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roychoudhuri R., Eil R.L., Restifo N.P. The interplay of effector and regulatory T cells in cancer. Curr Opin Immunol. 2015;33:101–111. doi: 10.1016/j.coi.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Munn D.H., Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Curr Opin Immunol. 2016;39:1–6. doi: 10.1016/j.coi.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leone R.D., Emens L.A. Targeting adenosine for cancer immunotherapy. J Immunother Cancer. 2018;6(1):57. doi: 10.1186/s40425-018-0360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banat G.A. Immune and inflammatory cell composition of human lung cancer stroma. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0139073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wherry E.J., Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joyce J.A., Fearon D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348(6230):74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 13.Sharpe A.H., Pauken K.E. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2017;18:153. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 14.Ji M. PD-1/PD-L1 pathway in non-small-cell lung cancer and its relation with EGFR mutation. J Transl Med. 2015;13:5. doi: 10.1186/s12967-014-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takada K. The significance of the PD-L1 expression in non-small-cell lung cancer: trenchant double swords as predictive and prognostic markers. Clin Lung Cancer. 2018;19(2):120–129. doi: 10.1016/j.cllc.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Teixidó C. Assays for predicting and monitoring responses to lung cancer immunotherapy. Cancer Biol Med. 2015;12(2):87–95. doi: 10.7497/j.issn.2095-3941.2015.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guibert N., Mazieres J. Nivolumab for treating non-small cell lung cancer. Expert Opin Biol Ther. 2015;15(12):1789–1797. doi: 10.1517/14712598.2015.1114097. [DOI] [PubMed] [Google Scholar]
- 18.Udall M. PD-L1 diagnostic tests: a systematic literature review of scoring algorithms and test-validation metrics. Diagn Pathol. 2018;13(1):12. doi: 10.1186/s13000-018-0689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Virgilio F. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer. 2018 Oct;18(10):601–618. doi: 10.1038/s41568-018-0037-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhao H. What else can CD39 tell us? Front Immunol. 2017;8:727. doi: 10.3389/fimmu.2017.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohta A. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. 2006;103(35):13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vijayan D. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17(12):765. doi: 10.1038/nrc.2017.110. [DOI] [PubMed] [Google Scholar]
- 23.Li J. CD39/CD73 upregulation on myeloid-derived suppressor cells via TGF-beta-mTOR-HIF-1 signaling in patients with non-small cell lung cancer. OncoImmunology. 2017;6(6) doi: 10.1080/2162402X.2017.1320011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmid S. Altered purinergic signaling in the tumor associated immunologic microenvironment in metastasized non-small-cell lung cancer. Lung Cancer. 2015;90(3):516–521. doi: 10.1016/j.lungcan.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Chae Y.K. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC) J Immunother Cancer. 2018;6:39. doi: 10.1186/s40425-018-0349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Travis W.D. WHO classification of tumours of the lung, pleura, thymus and heart/ In: Travis William D., Brambilla Elisabeth, Burke Allen P., Marx Alexander, Nicholson Andrew G., editors. World Health Organization classification of tumours. 4th ed. International Agency for Research on Cancer; Lyon: 2015. seventh. 2015. [DOI] [PubMed] [Google Scholar]
- 27.Quatromoni J.G. An optimized disaggregation method for human lung tumors that preserves the phenotype and function of the immune cells. J Leukoc Biol. 2015;97(1):201–209. doi: 10.1189/jlb.5TA0814-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruno A. The proangiogenic phenotype of natural killer cells in patients with non-small cell lung cancer. Neoplasia (New York, N.Y.) 2013;15(2):133–142. doi: 10.1593/neo.121758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bharat A. Flow cytometry reveals similarities between lung macrophages in humans and mice. Am J Respir Cell Mol Biol. 2016;54(1):147–149. doi: 10.1165/rcmb.2015-0147LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitsi E. Human alveolar macrophages predominately express combined classical M1 and M2 surface markers in steady state. Respir Res. 2018;19(1):66. doi: 10.1186/s12931-018-0777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thelen M. CD39 expression defines cell exhaustion in tumor-infiltrating CD8+ T cells—letter. Cancer Res. 2018;78(17):5173. doi: 10.1158/0008-5472.CAN-18-0873. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien S.M. Function of human tumor-infiltrating lymphocytes in early-stage non-small cell lung cancer. Cancer Immunol Res. 2019;7(6):896–909. doi: 10.1158/2326-6066.CIR-18-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simoni Y. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557(7706):575–579. doi: 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 34.Cai X.Y. High expression of CD39 in gastric cancer reduces patient outcome following radical resection. Oncol Lett. 2016;12(5):4080–4086. doi: 10.3892/ol.2016.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai X.Y. Overexpression of CD39 in hepatocellular carcinoma is an independent indicator of poor outcome after radical resection. Medicine (Baltim) 2016;95(40):e4989. doi: 10.1097/MD.0000000000004989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perry C. Increased CD39 expression on CD4(+) T lymphocytes has clinical and prognostic significance in chronic lymphocytic leukemia. Ann Hematol. 2012;91(8):1271–1279. doi: 10.1007/s00277-012-1425-2. [DOI] [PubMed] [Google Scholar]
- 37.Lizotte P.H. Multiparametric profiling of non-small-cell lung cancers reveals distinct immunophenotypes. JCI Insight. 2016;1(14) doi: 10.1172/jci.insight.89014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu S.P. Stromal PD-L1-positive regulatory T cells and PD-1-positive CD8-positive T cells define the response of different subsets of non-small cell lung cancer to PD-1/PD-L1 blockade immunotherapy. J Thorac Oncol. 2018;13(4):521–532. doi: 10.1016/j.jtho.2017.11.132. [DOI] [PubMed] [Google Scholar]
- 39.Soo R.A. Prognostic significance of immune cells in non-small cell lung cancer: meta-analysis. Oncotarget. 2018;9(37):24801–24820. doi: 10.18632/oncotarget.24835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petersen R.P. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107(12):2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 41.Bono M.R. CD73 and CD39 ectonucleotidases in T cell differentiation: beyond immunosuppression. FEBS Lett. 2015;589(22):3454–3460. doi: 10.1016/j.febslet.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 42.Shigematsu Y. Immunosuppressive effect of regulatory T lymphocytes in lung cancer, with special reference to their effects on the induction of autologous tumor-specific cytotoxic T lymphocytes. Oncol Lett. 2012;4(4):625–630. doi: 10.3892/ol.2012.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeuchi Y., Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. 2016;28(8):401–409. doi: 10.1093/intimm/dxw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ke X. Non-small-cell lung cancer-induced immunosuppression by increased human regulatory T cells via Foxp3 promoter demethylation. Cancer Immunol Immunother. 2016;65(5):587–599. doi: 10.1007/s00262-016-1825-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biswas S.K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 46.Ohri C.M. Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J. 2009;33(1):118–126. doi: 10.1183/09031936.00065708. [DOI] [PubMed] [Google Scholar]
- 47.Shien K., Papadimitrakopoulou V.A., Wistuba Predictive biomarkers of response to PD-1/PD-L1 immune checkpoint inhibitors in non-small cell lung cancer. Lung Cancer. 2016;99:79–87. doi: 10.1016/j.lungcan.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarvaria A., Madrigal J.A., Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol. 2017;14(8):662–674. doi: 10.1038/cmi.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Owen D., Chaft J.E. Immunotherapy in surgically resectable non-small cell lung cancer. J Thorac Dis. 2018;10(Suppl 3):S404–S411. doi: 10.21037/jtd.2017.12.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.